Restoring Homeostasis: Treating Amyotrophic Lateral Sclerosis by Resolving Dynamic Regulatory Instability
Abstract
1. Introduction
2. Results
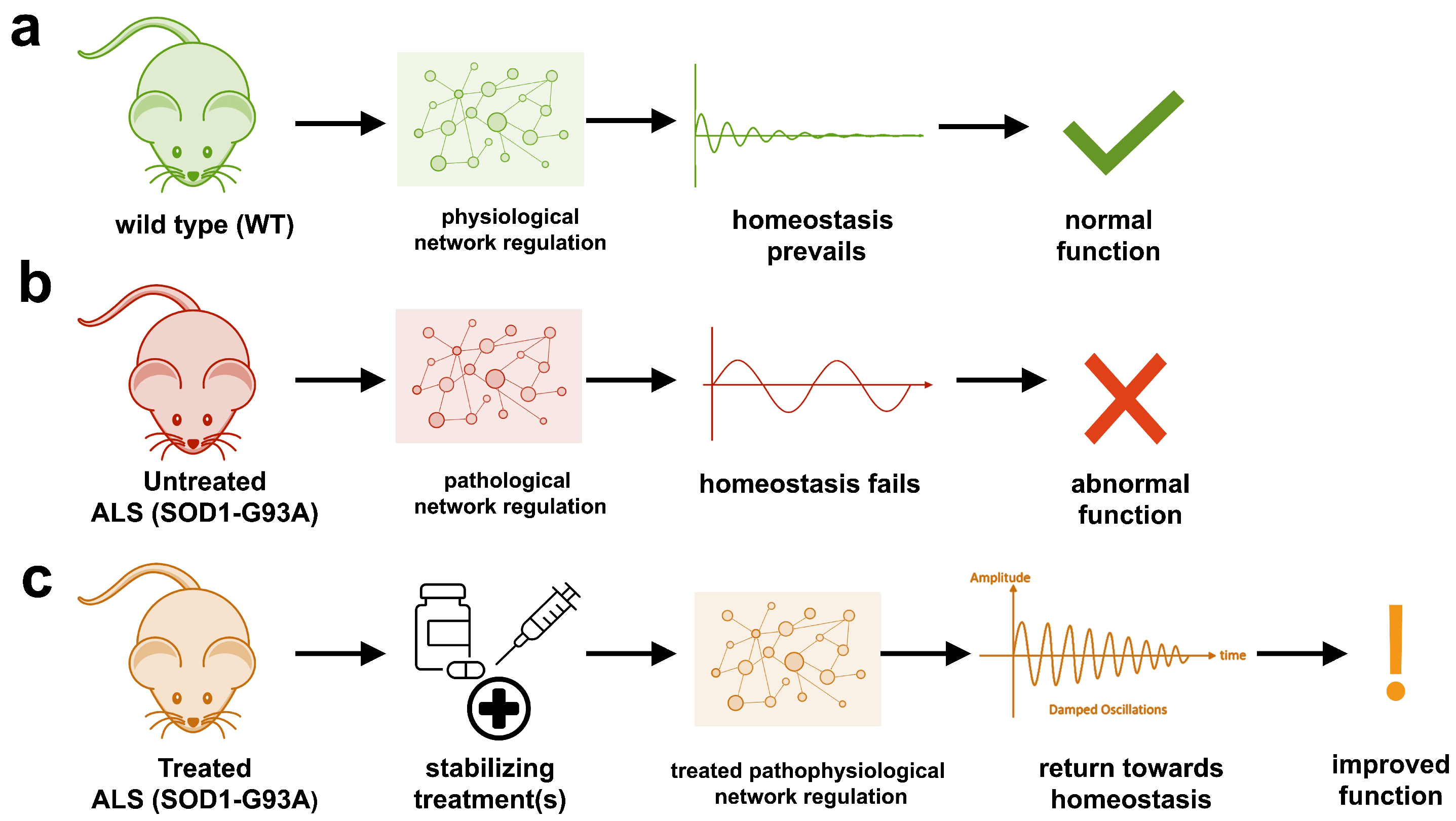
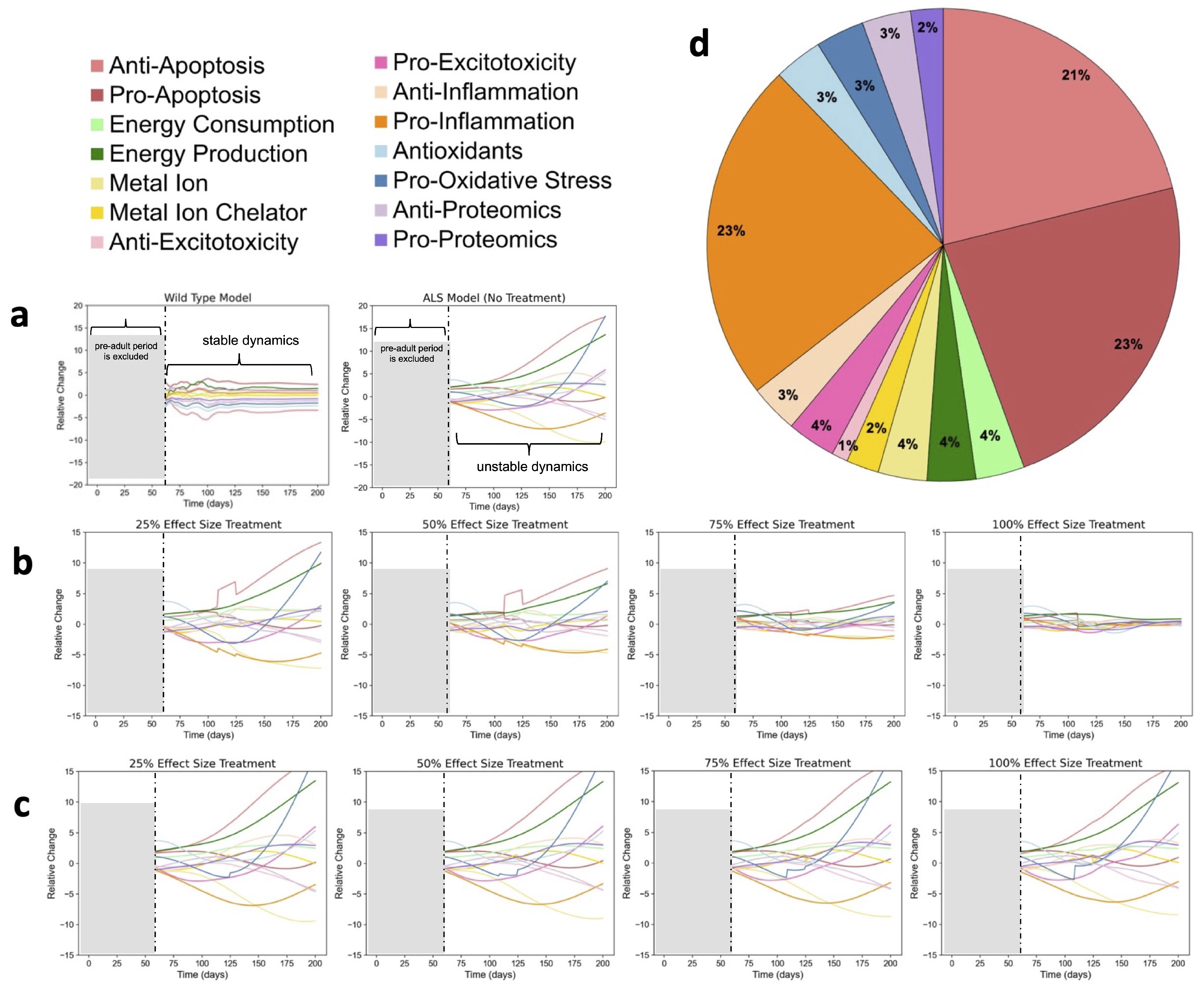
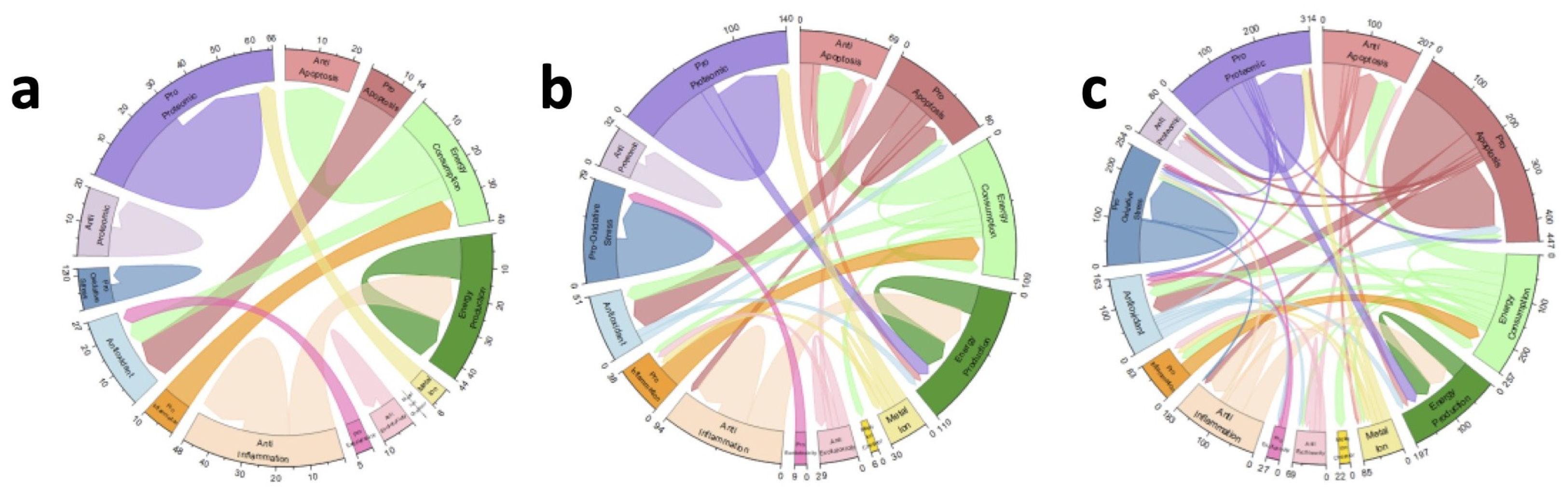
2.1. Untreated ALS Mice Have Unstable, Oscillatory Dynamics
2.2. Stabilizing Treatments Highlight Potential Targets
2.2.1. Factor-Based Stabilizing Treatments
2.2.2. Gain-Based Stabilizing Treatments
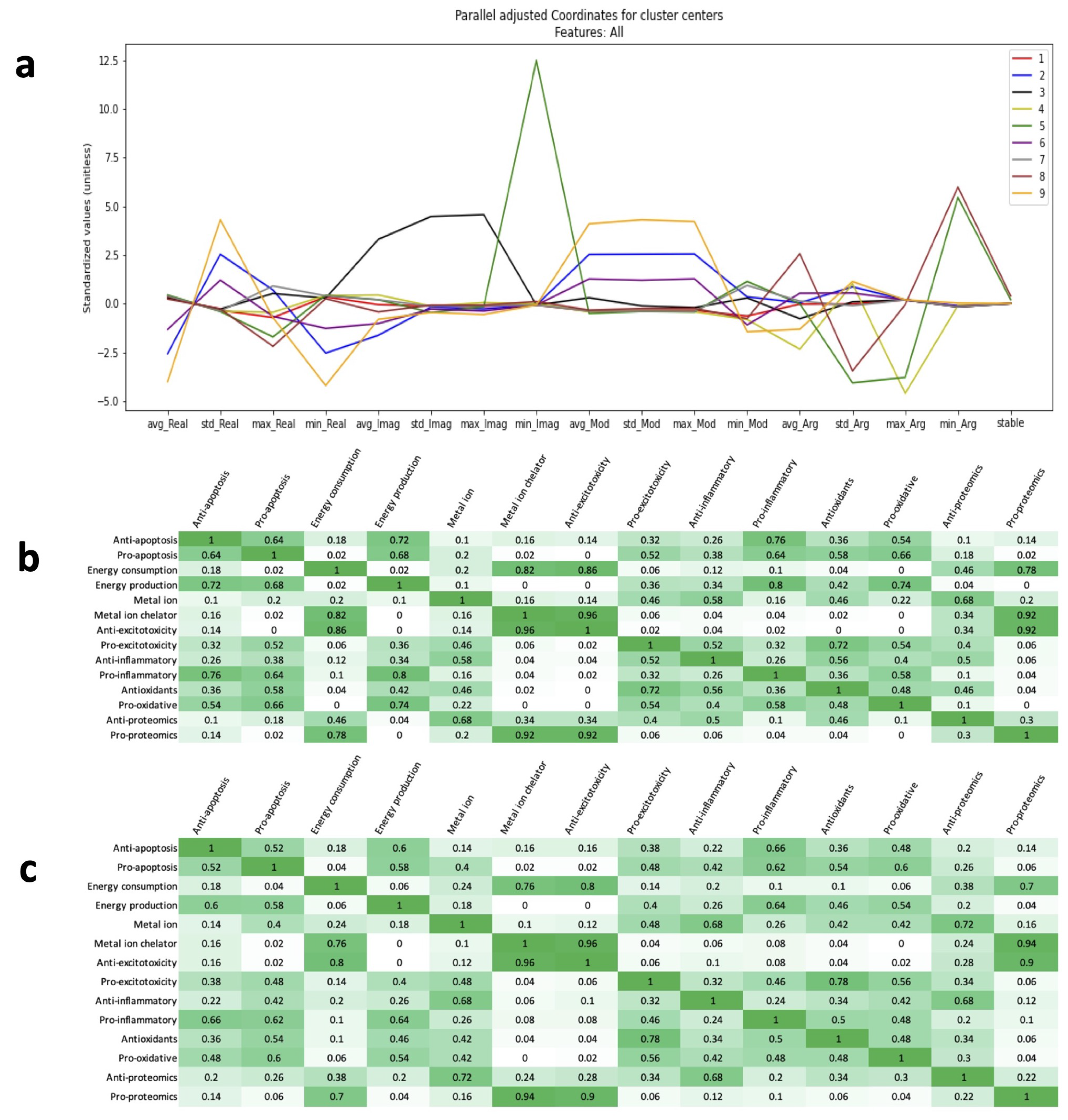
2.3. Time Series Clustering to Explore Patterns Among Re-Stabilizing Treatments
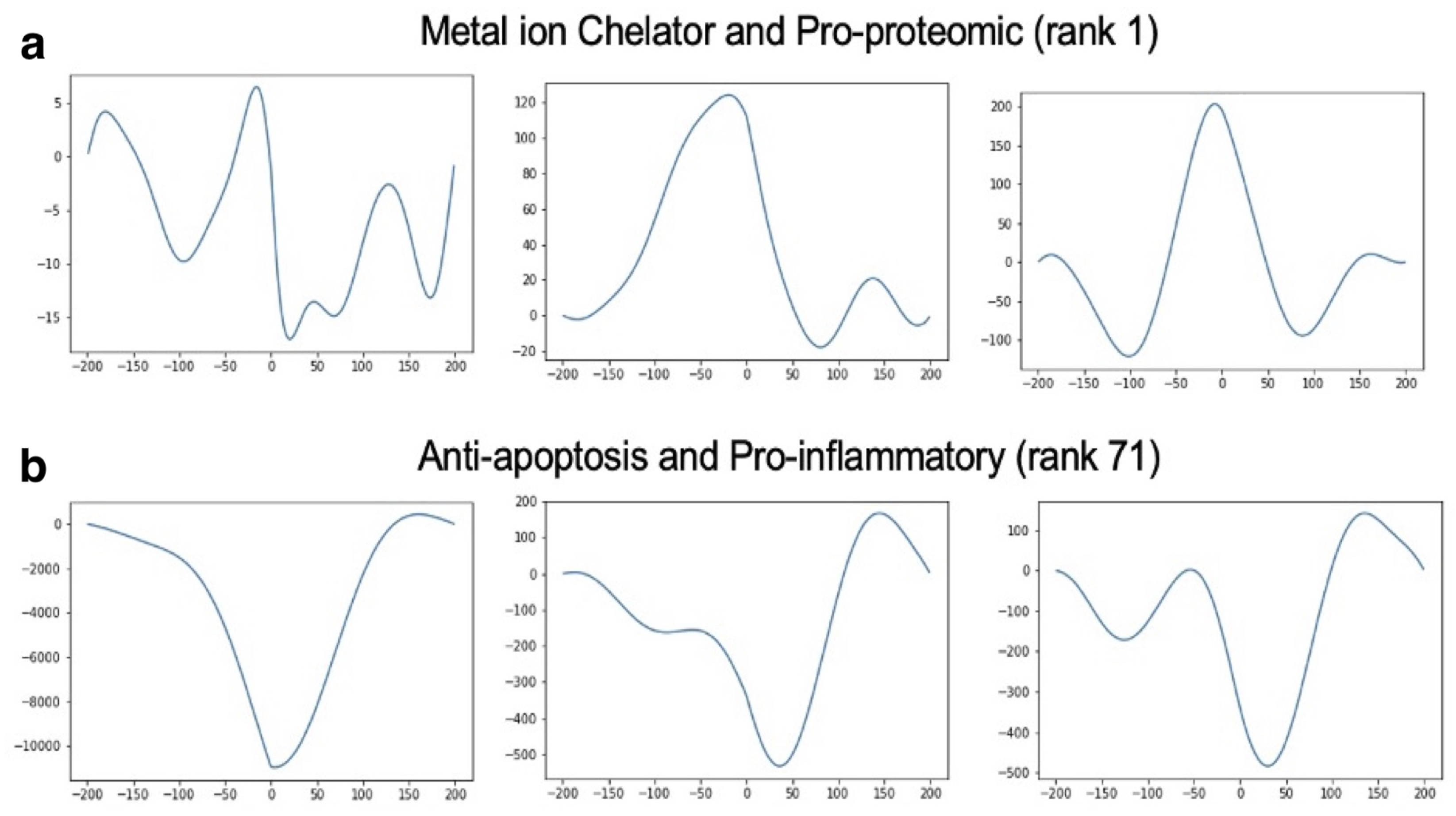
2.4. Eigenvalue Analysis of Stable Treatments
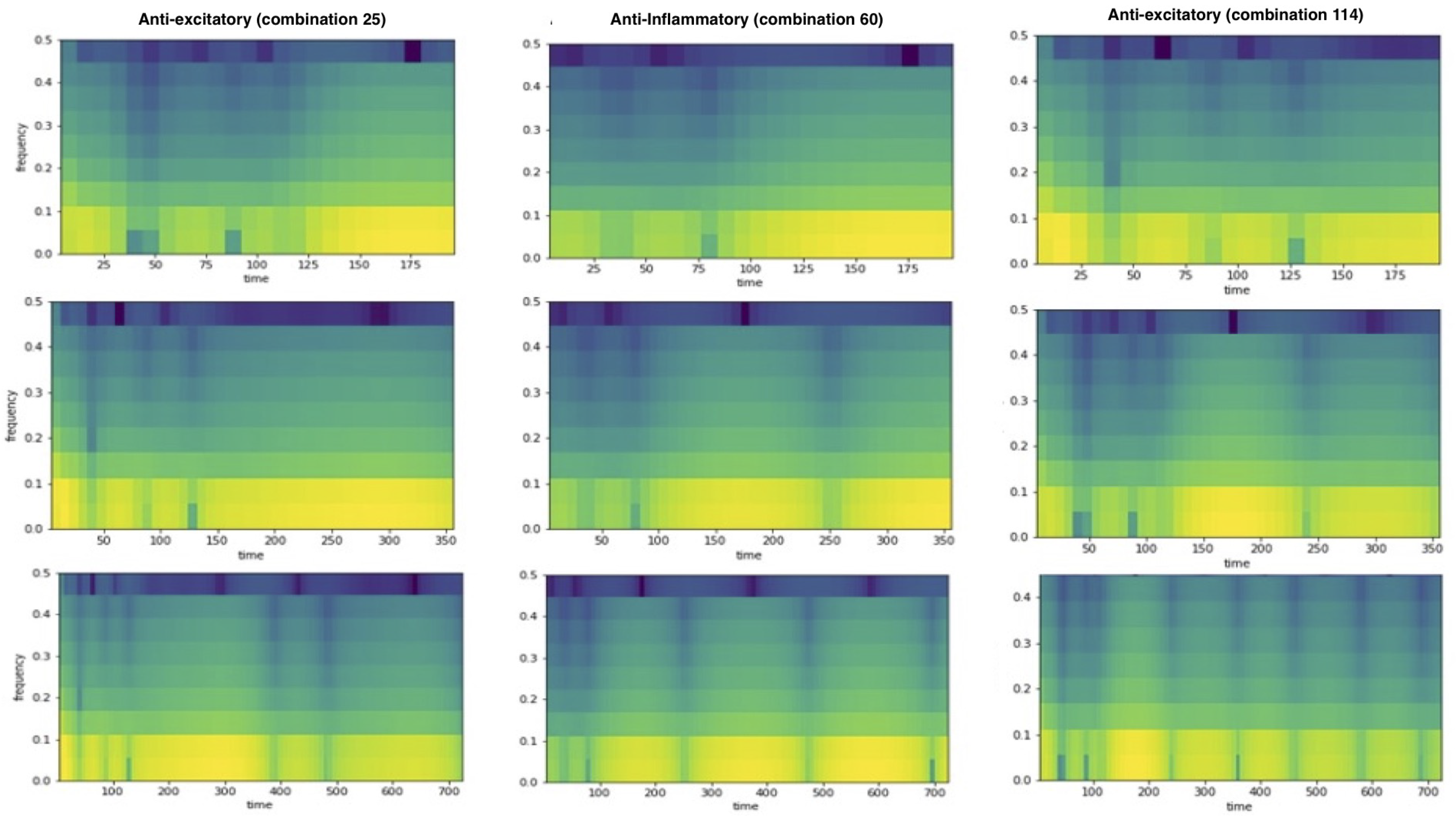
2.5. Spectrogram, Power Spectral Density, and Correlations
3. Discussion
3.1. Examination of Molecular Mechanisms Within Interactive ALS Dynamics
3.2. Model and Results Applications: Reconceptualizing ALS Therapeutic Strategies
3.3. Limitations and Future Work
4. Materials and Methods
4.1. Data Curation
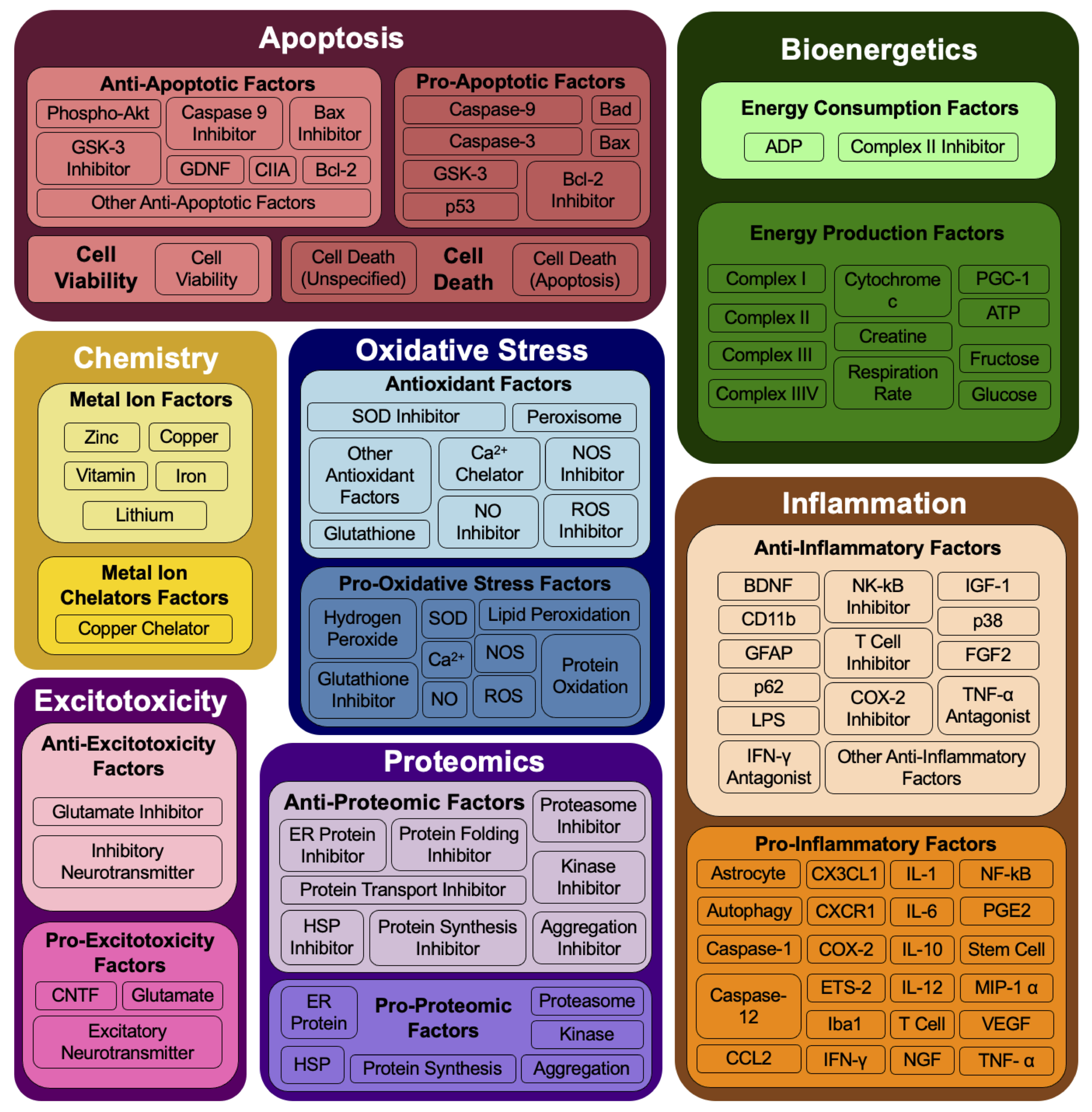
4.2. Functional Ontology to Aggregate SOD1-G93A Mechanisms
- Apoptosis: relating to or leading to cell death.
- Bioenergetics: relating to cellular processes that harvest and transform energy from cellular respiration and other metabolic processes and result in the production and utilization of energy.
- Chemistry: relating to enzymatic and metal mishandling.
- Excitotoxicity: relating to cell damage caused by the excitatory neurotransmitter.
- Inflammation: relating to the immune system.
- Oxidative stress: relating to reactive oxygen species and production of free radicals.
- Proteomics: relating to protein folding, aggregation, or degradation.
4.3. Partitioning of Data Sets
- Model construction dataset—The model construction subset exclusively included data that contained control and treated data for high copy SOD1-G93A and WT mice. The control data were obtained from experiments that did not administer treatments or processes that could change the measured outputs. The treated data included experiments that provided effects of different perturbations. The specified criteria resulted in a final model construction data pool of 2148 data points from 119 articles.
- Validation dataset—The validation dataset was an external dataset not used in model construction that contained data points that could be used to validate model output. The dataset contained peer-reviewed articles that did not meet the inclusion criteria for the model construction dataset but had quantifiable output data relevant to the evaluation of model performance. The validation dataset had 1477 data points from 180 articles.
- Disease construction dataset—The disease stage construction data subset included figures from experiments that contained systematically measured response data (i.e., rotarod, latency to fall, and body weight). This criterion provided peer-reviewed articles that included the systemic deterioration of measured mice subjects and provided a visual representation of disease progression. The disease construction dataset yielded a total of 1850 data points from 75 articles.
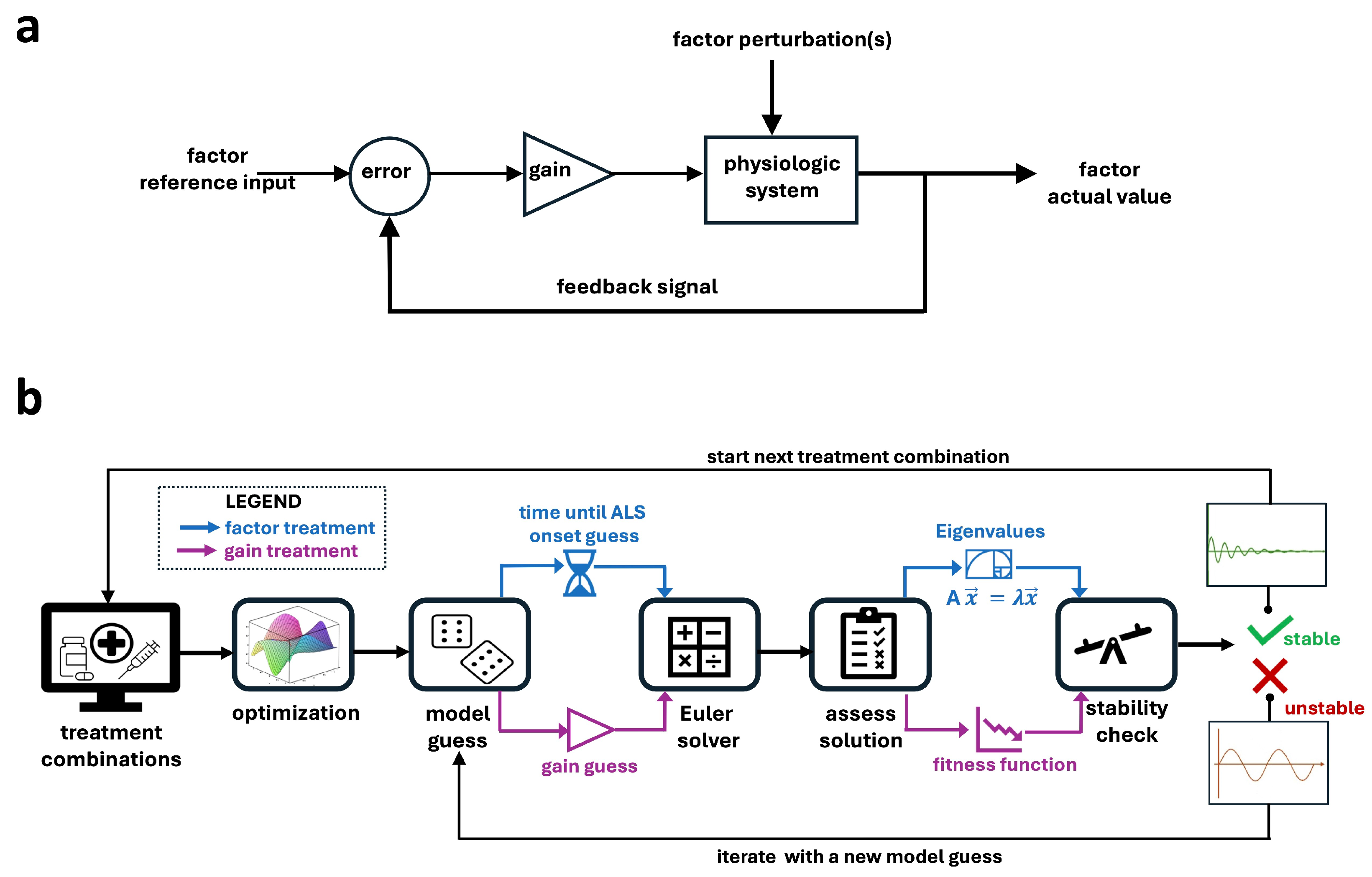
4.4. Model Construction
4.5. Combination Treatment Simulations
4.6. Factor Treatments
4.7. Gain Treatments
4.8. Post-Simulation Data Analysis
4.8.1. Stability Ranking of Factor Treatments
4.8.2. Time Series Analysis
4.8.3. Eigenvalue Analysis
4.8.4. Spectrogram, Power Spectral Density, and Correlations
5. Conclusions
- SOD1-G93A ALS transgenic mice have an unstable multifactorial regulatory network that cannot maintain normal homeostasis. The pathology dynamics, including the onset of instability and magnitude of instability, correspond to disease progression.
- SOD1-G93A ALS transgenic mice have unstable regulatory dynamics primarily due to hypervigilant regulation (e.g., too high gains) that induce oscillatory, homeostatic instability.
- Mathematical stability based on the regulatory system’s eigenvalues was used as a criterion to determine whether a treatment is likely to be successful. Successful combination treatments stabilized the underlying network physiology of SOD1-G93A ALS transgenic mice to normal or near-normal homeostasis, similar to WT mice.
- The timing and effect size of modulatory treatment combinations is critical for optimizing therapeutic success (stabilization). The top two factor pairs, metal ion chelator with pro-proteomic and energy consumption with metal ion chelator, remained consistent across all simulation lengths. However, fluctuations in phase and frequency were observed even after achieving stability.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1
| Treatment | Measure | Sign +/− | Confidence | Source Citation |
|---|---|---|---|---|
| Anti-Apoptosis | Energy Consumption | + | 5 | [60,61] |
| Anti-Apoptosis | Ion | − | 1 | N/A |
| Anti-Apoptosis | Metal Ion Chelator | + | 1 | N/A |
| Anti-Apoptosis | Anti-Excitotoxicity | − | 5 | [62,63] |
| Anti-Apoptosis | Antioxidants | + | 4 | [64,65,66] |
| Anti-Apoptosis | Anti-Proteomic | − | 2 | [67] |
| Pro-Apoptosis | Energy Consumption | − | 3 | [60,68] |
| Pro-Apoptosis | Metal ion | + | 4 | [69,70] |
| Pro-Apoptosis | Metal Ion Chelator | − | 1 | N/A |
| Pro-Apoptosis | Anti-Excitotoxicity | − | 2 | [71] |
| Pro-Apoptosis | Anti-Inflammatory | + | 2 | [72] |
| Pro-Apoptosis | Pro-Inflammatory | − | 4 | [73,74] |
| Pro-Apoptosis | Antioxidants | + | 3 | [75] |
| Pro-Apoptosis | Pro-Oxidative | − | 3 | [76,77] |
| Pro-Apoptosis | Anti-Proteomic | − | 4 | [78,79] |
| Energy Consumption | Anti-Apoptosis | + | 4 | [80,81] |
| Energy Consumption | Pro-Apoptosis | − | 4 | [82,83] |
| Energy Consumption | Energy Consumption | − | 2 | [84] |
| Energy Consumption | Energy Production | + | 4 | [85,86] |
| Energy Consumption | Metal Ion | + | 4 | [87,88] |
| Energy Consumption | Metal Ion Chelator | − | 2 | [89] |
| Energy Consumption | Anti-Excitotoxicity | + | 4 | [90,91] |
| Energy Consumption | Pro-Excitotoxicity | − | 3 | [92,93] |
| Energy Consumption | Anti-Inflammatory | + | 4 | [94,95] |
| Energy Consumption | Pro-Inflammatory | + | 4 | [96,97] |
| Energy Consumption | Pro-Oxidative | + | 4 | [98,99] |
| Energy Consumption | Anti-Proteomic | − | 2 | [100] |
| Energy Consumption | Pro-Proteomic | − | 4 | [101,102] |
| Energy Production | Metal Ion | + | 3 | [103] |
| Energy Production | Metal Ion Chelator | − | 1 | N/A |
| Energy Production | Anti-Excitotoxicity | − | 4 | [104,105] |
| Energy Production | Anti-Inflammatory | − | 2 | [106] |
| Energy Production | Antioxidants | + | 3 | [107] |
| Energy Production | Anti-Proteomic | − | 1 | N/A |
| Energy Production | Pro-Proteomic | + | 3 | [108] |
| Metal Ion | Pro-Apoptosis | + | 4 | [109,110] |
| Metal Ion | Energy Consumption | − | 4 | [111,112] |
| Metal Ion | Energy Production | − | 4 | [113,114] |
| Metal Ion | Metal Ion | + | 1 | N/A |
| Metal Ion | Metal Ion Chelator | − | 1 | N/A |
| Metal Ion | Anti-Excitotoxicity | − | 4 | [115,116] |
| Metal Ion | Anti-Inflammatory | − | 4 | [117,118] |
| Metal Ion | Antioxidants | − | 5 | [119,120] |
| Metal Ion | Anti-Proteomic | − | 4 | [121,122] |
| Metal Ion | Pro-Proteomic | − | 5 | [123,124] |
| Metal Ion Chelator | Pro-Apoptosis | + | 4 | [125,126] |
| Metal Ion Chelator | Energy Consumption | + | 4 | [127,128] |
| Metal Ion Chelator | Metal Ion Chelator | + | NA | [129,130] |
| Metal Ion Chelator | Anti-Excitotoxicity | − | 4 | [131] |
| Metal Ion Chelator | Pro-Excitotoxicity | − | 3 | [132,133] |
| Metal Ion Chelator | Anti-Inflammatory | + | 4 | [134] |
| Metal Ion Chelator | Antioxidants | + | 2 | [135] |
| Metal Ion Chelator | Anti-Proteomic | + | 3 | [136] |
| Metal Ion Chelator | Pro-Proteomic | − | 3 | [118] |
| Anti-Excitotoxicity | Pro-Apoptosis | − | 4 | [118,137] |
| Anti-Excitotoxicity | Energy Consumption | + | 4 | [138,139] |
| Anti-Excitotoxicity | Energy Production | + | 4 | [81,140] |
| Anti-Excitotoxicity | Metal Ion | + | 1 | N/A |
| Anti-Excitotoxicity | Metal Ion Chelator | − | 1 | N/A |
| Anti-Excitotoxicity | Anti-Excitotoxicity | + | 1 | N/A |
| Anti-Excitotoxicity | Anti-Inflammatory | − | 4 | [141,142] |
| Anti-Excitotoxicity | Pro-Inflammatory | − | 4 | [143,144] |
| Anti-Excitotoxicity | Antioxidants | + | 2 | [145] |
| Anti-Excitotoxicity | Pro-Oxidative | + | 2 | [146] |
| Anti-Excitotoxicity | Anti-Proteomic | − | 1 | N/A |
| Anti-Excitotoxicity | Pro-Proteomic | + | 4 | [147,148] |
| Pro-Excitotoxicity | Anti-Apoptosis | − | 4 | [149,150] |
| Pro-Excitotoxicity | Energy Consumption | − | 1 | N/A |
| Pro-Excitotoxicity | Energy Production | + | 4 | [151,152] |
| Pro-Excitotoxicity | Metal Ion | + | 2 | [153] |
| Pro-Excitotoxicity | Metal Ion Chelator | − | 1 | N/A |
| Pro-Excitotoxicity | Anti-Excitotoxicity | + | 4 | [154,155] |
| Pro-Excitotoxicity | Anti-Inflammatory | + | 3 | [156] |
| Pro-Excitotoxicity | Pro-Inflammatory | + | 5 | [157,158] |
| Pro-Excitotoxicity | Antioxidants | + | 4 | [159,160] |
| Pro-Excitotoxicity | Anti-Proteomic | + | 4 | [161,162] |
| Anti-Inflammatory | Energy Production | + | 3 | [163] |
| Anti-Inflammatory | Metal Ion | − | 3 | [164] |
| Anti-Inflammatory | Metal Ion Chelator | + | 1 | N/A |
| Anti-Inflammatory | Anti-Excitotoxicity | + | 2 | [165] |
| Anti-Inflammatory | Antioxidants | + | 4 | [166,167] |
| Anti-Inflammatory | Anti-Proteomic | − | 4 | [168,169] |
| Pro-Inflammatory | Energy Consumption | + | 4 | [170,171] |
| Pro-Inflammatory | Energy Production | + | 4 | [172,173] |
| Pro-Inflammatory | Metal Ion | − | 3 | [174] |
| Pro-Inflammatory | Metal Ion Chelator | + | 1 | N/A |
| Pro-Inflammatory | Anti-Excitotoxicity | − | 4 | [175,176] |
| Pro-Inflammatory | Anti-Proteomic | + | 1 | N/A |
| Pro-Inflammatory | Pro-Proteomic | − | 4 | [177,178] |
| Antioxidants | Energy Consumption | − | 3 | [179] |
| Antioxidants | Metal Ion | + | 1 | N/A |
| Antioxidants | Metal Ion Chelator | − | 1 | N/A |
| Antioxidants | Anti-Excitotoxicity | + | 3 | [180] |
| Antioxidants | Pro-Excitotoxicity | + | 3 | [181] |
| Antioxidants | Anti-Proteomic | + | 4 | [182,183] |
| Pro-Oxidative | Energy Consumption | − | 4 | [184] |
| Pro-Oxidative | Metal Ion Chelator | + | 3 | [185] |
| Pro-Oxidative | Anti-Excitotoxicity | + | 4 | [186] |
| Pro-Oxidative | Pro-Excitotoxicity | + | 4 | [187,188] |
| Pro-Oxidative | Anti-Proteomic | − | 4 | [189,190] |
| Anti-Proteomic | Energy Consumption | + | 3 | [191] |
| Anti-Proteomic | Metal Ion Chelator | + | 3 | [192] |
| Anti-Proteomic | Anti-Excitotoxicity | + | 3 | [193] |
| Anti-Proteomic | Anti-Inflammatory | + | 4 | [194,195] |
| Anti-Proteomic | Pro-Oxidative | − | 2 | [196] |
| Pro-Proteomic | Anti-Apoptosis | − | 4 | [197,198] |
| Pro-Proteomic | Energy Consumption | − | 4 | [52,199] |
| Pro-Proteomic | Energy Production | + | 4 | [200,201] |
| Pro-Proteomic | Metal Ion | + | 3 | [202] |
| Pro-Proteomic | Metal Ion Chelator | − | 3 | [203] |
| Pro-Proteomic | Anti-Excitotoxicity | − | 4 | [204,205] |
| Pro-Proteomic | Pro-Excitotoxicity | + | 4 | [206,207] |
| Pro-Proteomic | Pro-Inflammatory | + | 3 | [208] |
| Pro-Proteomic | Antioxidants | + | 3 | [209] |
| Pro-Proteomic | Anti-Proteomic | − | 4 | [210] |
| Pro-Proteomic | Pro-Proteomic | + | 1 | N/A |
References
- Kiernan, M.C.; Vucic, S.; Cheah, B.C.; Turner, M.R.; Eisen, A.; Hardiman, O.; Burrell, J.R.; Zoing, M.C. Amyotrophic lateral sclerosis. Lancet 2011, 377, 942–955. [Google Scholar] [CrossRef] [PubMed]
- Katyal, N.; Govindarajan, R. Shortcomings in the Current Amyotrophic Lateral Sclerosis Trials and Potential Solutions for Improvement. Front. Neurol. 2017, 8, 521. [Google Scholar] [CrossRef] [PubMed]
- Tzeplaeff, L.; Wilfling, S.; Requardt, M.V.; Herdick, M. Current State and Future Directions in the Therapy of ALS. Cells 2023, 12, 1523. [Google Scholar] [CrossRef]
- Bhandari, R.; Kuhad, A.; Kuhad, A. Edaravone: A new hope for deadly amyotrophic lateral sclerosis. Drugs Today 2018, 54, 349. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.G.; Mitchell, J.D.; Moore, D.H. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Cochrane Database Syst. Rev. 2012. [Google Scholar] [CrossRef]
- Pfohl, S.R.; Halicek, M.T.; Mitchell, C.S. Characterization of the Contribution of Genetic Background and Gender to Disease Progression in the SOD1 G93A Mouse Model of Amyotrophic Lateral Sclerosis: A Meta-Analysis. J. Neuromuscul. Dis. 2015, 2, 137–150. [Google Scholar] [CrossRef]
- Kim, R.B.; Irvin, C.W.; Tilva, K.R.; Mitchell, C.S. State of the field: An informatics-based systematic review of the SOD1-G93A amyotrophic lateral sclerosis transgenic mouse model. Amyotroph. Lateral Scler. Front. Degener. 2015, 17, 1–14. [Google Scholar] [CrossRef]
- Mitchell, C.S.; Lee, R.H. Dynamic Meta-Analysis as a Therapeutic Prediction Tool for Amyotrophic Lateral Sclerosis. In Amyotrophic Lateral Sclerosis; InTech: Houston, TX, USA, 2012. [Google Scholar] [CrossRef]
- Irvin, C.W.; Kim, R.B.; Mitchell, C.S. Seeking homeostasis: Temporal trends in respiration, oxidation, and calcium in SOD1 G93A Amyotrophic Lateral Sclerosis mice. Front. Cell. Neurosci. 2015, 9, 248. [Google Scholar] [CrossRef][Green Version]
- Hollinger, S.K.; Okosun, I.S.; Mitchell, C.S. Antecedent Disease and Amyotrophic Lateral Sclerosis: What Is Protecting Whom? Front. Neurol. 2016, 7, 47. [Google Scholar] [CrossRef]
- Mitchell, C.S.; Hollinger, S.K.; Goswami, S.D.; Polak, M.A.; Lee, R.H.; Glass, J.D. Antecedent Disease Is Less Prevalent in Amyotrophic Lateral Sclerosis. Neurodegener. Dis. 2015, 15, 109–113. [Google Scholar] [CrossRef]
- Pfohl, S.R.; Kim, R.B.; Coan, G.S.; Mitchell, C.S. Unraveling the Complexity of Amyotrophic Lateral Sclerosis Survival Prediction. Front. Neuroinform. 2018, 12, 36. [Google Scholar] [CrossRef] [PubMed]
- Bond, L.; Bernhardt, K.; Madria, P.; Sorrentino, K.; Scelsi, H.; Mitchell, C.S. A Metadata Analysis of Oxidative Stress Etiology in Preclinical Amyotrophic Lateral Sclerosis: Benefits of Antioxidant Therapy. Front. Neurosci. 2018, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.J.B.; Kittel, T.E.; Kim, R.B.; Bach, T.N.; Zhang, T.; Mitchell, C.S. Comparing therapeutic modulators of the SOD1 G93A Amyotrophic Lateral Sclerosis mouse pathophysiology. Front. Neurosci. 2023, 16, 1111763. [Google Scholar] [CrossRef]
- Huh, S.; Heckman, C.J.; Manuel, M. Time Course of Alterations in Adult Spinal Motoneuron Properties in the SOD1(G93A) Mouse Model of ALS. Eneuro 2021, 8, ENEURO.0378–20.2021. [Google Scholar] [CrossRef] [PubMed]
- Kuo, S.W.; Binder, M.D.; Heckman, C.J. Excessive Homeostatic Gain in Spinal Motoneurons in a Mouse Model of Amyotrophic Lateral Sclerosis. Sci. Rep. 2020, 10, 9049. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, N.; Bellouquid, A.; Nieto, J.; Soler, J. Complexity and mathematical tools toward the modelling of multicellular growing systems. Math. Comput. Model. 2010, 51, 441–451. [Google Scholar] [CrossRef]
- McCall, J. Genetic algorithms for modelling and optimisation. J. Comput. Appl. Math. 2005, 184, 205–222. [Google Scholar] [CrossRef]
- Jin, W.; Stokes, J.M.; Eastman, R.T.; Itkin, Z.; Zakharov, A.V.; Collins, J.J.; Jaakkola, T.S.; Barzilay, R. Deep learning identifies synergistic drug combinations for treating COVID-19. Proc. Natl. Acad. Sci. USA 2021, 118, e2105070118. [Google Scholar] [CrossRef]
- Sagiv-Barfi, I.; Czerwinski, D.K.; Levy, S.; Alam, I.S.; Mayer, A.T.; Gambhir, S.S.; Levy, R. Eradication of spontaneous malignancy by local immunotherapy. Sci. Transl. Med. 2018, 10, eaan4488. [Google Scholar] [CrossRef]
- Tan, X.; Hu, L.; Luquette, L.J.; Gao, G.; Liu, Y.; Qu, H.; Xi, R.; Lu, Z.J.; Park, P.J.; Elledge, S.J. Systematic identification of synergistic drug pairs targeting HIV. Nat. Biotechnol. 2012, 30, 1125–1130. [Google Scholar] [CrossRef]
- Mitchell, C.S.; Lee, R.H. Pathology Dynamics Predict Spinal Cord Injury Therapeutic Success. J. Neurotrauma 2008, 25, 1483–1497. [Google Scholar] [CrossRef] [PubMed]
- Bulusu, K.C.; Guha, R.; Mason, D.J.; Lewis, R.P.; Muratov, E.; Kalantar Motamedi, Y.; Cokol, M.; Bender, A. Modelling of compound combination effects and applications to efficacy and toxicity: State-of-the-art, challenges and perspectives. Drug Discov. Today 2016, 21, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Hooten, K.G.; Beers, D.R.; Zhao, W.; Appel, S.H. Protective and Toxic Neuroinflammation in Amyotrophic Lateral Sclerosis. Neurotherapeutics 2015, 12, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Murdock, B.J.; Bender, D.E.; Segal, B.M.; Feldman, E.L. The dual roles of immunity in ALS: Injury overrides protection. Neurobiol. Dis. 2015, 77, 1–12. [Google Scholar] [CrossRef]
- McCauley, M.E.; Baloh, R.H. Inflammation in ALS/FTD pathogenesis. Acta Neuropathol. 2019, 137, 715–730. [Google Scholar] [CrossRef]
- Zhao, W.; Beers, D.R.; Appel, S.H. Immune-mediated mechanisms in the pathoprogression of amyotrophic lateral sclerosis. J. Neuroimmune Pharmacol. 2013, 8, 888–899. [Google Scholar] [CrossRef]
- Tateishi, T.; Yamasaki, R.; Tanaka, M.; Matsushita, T.; Kikuchi, H.; Isobe, N.; Kira, J.I. CSF chemokine alterations related to the clinical course of amyotrophic lateral sclerosis. J. Neuroimmunol. 2010, 222, 76–81. [Google Scholar] [CrossRef]
- Guo, J.; Yang, X.; Gao, L.; Zang, D. Evaluating the levels of CSF and serum factors in ALS. Brain Behav. 2017, 7, e00637. [Google Scholar] [CrossRef]
- Shi, N.; Kawano, Y.; Tateishi, T.; Kikuchi, H.; Osoegawa, M.; Ohyagi, Y.; Kira, J.I. Increased IL-13-producing T cells in ALS: Positive correlations with disease severity and progression rate. J. Neuroimmunol. 2007, 182, 232–235. [Google Scholar] [CrossRef]
- Kuhle, J.; Lindberg, R.L.P.; Regeniter, A.; Mehling, M.; Steck, A.J.; Kappos, L.; Czaplinski, A. Increased levels of inflammatory chemokines in amyotrophic lateral sclerosis. Eur. J. Neurol. 2009, 16, 771–774. [Google Scholar] [CrossRef]
- Mitchell, R.M.; Freeman, W.M.; Randazzo, W.T.; Stephens, H.E.; Beard, J.L.; Simmons, Z.; Connor, J.R. A CSF biomarker panel for identification of patients with amyotrophic lateral sclerosis. Neurology 2009, 72, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Fiala, M.; Chattopadhay, M.; La Cava, A.; Tse, E.; Liu, G.; Lourenco, E.; Wiedau-Pazos, M. IL-17A is increased in the serum and in spinal cord CD8 and mast cells of ALS patients. J. Neuroinflamm. 2010, 7, 76. [Google Scholar] [CrossRef] [PubMed]
- Italiani, P.; Carlesi, C.; Giungato, P.; Puxeddu, I.; Borroni, B.; Bossù, P.; Boraschi, D. Evaluating the levels of interleukin-1 family cytokines in sporadic amyotrophic lateral sclerosis. J. Neuroinflamm. 2014, 11, 94. [Google Scholar] [CrossRef] [PubMed]
- Ehrhart, J.; Smith, A.J.; Kuzmin-Nichols, N.; Zesiewicz, T.A.; Jahan, I.; Shytle, R.D.; Garbuzova-Davis, S. Humoral factors in ALS patients during disease progression. J. Neuroinflamm. 2015, 12, 127. [Google Scholar] [CrossRef]
- Furukawa, T.; Matsui, N.; Fujita, K.; Nodera, H.; Shimizu, F.; Miyamoto, K.; Kaji, R. CSF cytokine profile distinguishes multifocal motor neuropathy from progressive muscular atrophy. Neurol.-Neuroimmunol. Neuroinflamm. 2015, 2, e138. [Google Scholar] [CrossRef]
- Prado, L.D.G.R.; Rocha, N.P.; de Souza, L.C.; Bicalho, I.C.S.; Gomez, R.S.; Vidigal-Lopes, M.; Teixeira, A.L. Longitudinal assessment of clinical and inflammatory markers in patients with amyotrophic lateral sclerosis. J. Neurol. Sci. 2018, 394, 69–74. [Google Scholar] [CrossRef]
- Liu, J.; Gao, L.; Zang, D. Elevated levels of IFN-γ in CSF and serum of patients with amyotrophic lateral sclerosis. PLoS ONE 2015, 10, e0136937. [Google Scholar] [CrossRef]
- Lu, C.H.; Allen, K.; Oei, F.; Leoni, E.; Kuhle, J.; Tree, T.; Malaspina, A. Systemic inflammatory response and neuromuscular involvement in amyotrophic lateral sclerosis. Neurol.-Neuroimmunol. Neuroinflamm. 2016, 3, e244. [Google Scholar] [CrossRef]
- Ngo, S.T.; Steyn, F.J.; Huang, L.; Mantovani, S.; Pfluger, C.M.M.; Woodruff, T.M.; McCombe, P.A. Altered expression of metabolic proteins and adipokines in patients with amyotrophic lateral sclerosis. J. Neurol. Sci. 2015, 357, 22–27. [Google Scholar] [CrossRef]
- Hu, Y.; Cao, C.; Qin, X.Y.; Yu, Y.; Yuan, J.; Zhao, Y.; Cheng, Y. Increased peripheral blood inflammatory cytokine levels in amyotrophic lateral sclerosis: A meta-analysis study. Sci. Rep. 2017, 7, 9094. [Google Scholar] [CrossRef]
- Chen, X.; Hu, Y.; Cao, Z.; Liu, Q.; Cheng, Y. Cerebrospinal fluid inflammatory cytokine aberrations in Alzheimer’s disease, Parkinson’s disease and amyotrophic lateral sclerosis: A systematic review and meta-analysis. Front. Immunol. 2018, 9, 2122. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Martinez, L.; Calvo, A.C.; Muñoz, M.J.; Osta, R. Are circulating cytokines reliable biomarkers for amyotrophic lateral sclerosis? Int. J. Mol. Sci. 2019, 20, 2759. [Google Scholar] [CrossRef] [PubMed]
- El-Samad, H. Biological feedback control—Respect the loops. Cell Syst. 2021, 12, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Muyderman, H.; Chen, T. Mitochondrial dysfunction in amyotrophic lateral sclerosis—A valid pharmacological target? Br. J. Pharmacol. 2014, 171, 2191–2205. [Google Scholar] [CrossRef]
- Galluzzi, L.; Morselli, E.; Kepp, O.; Kroemer, G. Targeting post-mitochondrial effectors of apoptosis for neuroprotection. Biochim. Et Biophys. Acta-Bioenerg. 2009, 1787, 402–413. [Google Scholar] [CrossRef]
- Galluzzi, L.; Kepp, O.; Kroemer, G. Mitochondria: Master regulators of danger signalling. Nat. Rev. Mol. Cell Biol. 2012, 13, 780–788. [Google Scholar] [CrossRef]
- Michels, J.; Kepp, O.; Senovilla, L.; Lissa, D.; Castedo, M.; Kroemer, G.; Galluzzi, L. Functions of BCL-X L at the interface between cell death and metabolism. Int. J. Cell Biol. 2013, 2013, 1–10. [Google Scholar] [CrossRef]
- Gao, Y.; Signore, A.P.; Yin, W.; Cao, G.; Yin, X.M.; Sun, F.; Chen, J. Neuroprotection against focal ischemic brain injury by inhibition of c-Jun N-terminal kinase and attenuation of the mitochondrial apoptosis-signaling pathway. J. Cereb. Blood Flow Metab. 2005, 25, 694–712. [Google Scholar] [CrossRef]
- Sathasivam, S.; Ince, P.G.; Shaw, P.J. Apoptosis in amyotrophic lateral sclerosis: A review of the evidence. Neuropathol. Appl. Neurobiol. 2001, 27, 257–274. [Google Scholar] [CrossRef]
- Rosen, D.R.; Siddique, T.; Patterson, D.; Figlewicz, D.A.; Sapp, P.; Hentati, A.; Brown, R.H., Jr. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 1993, 362, 59–62. [Google Scholar] [CrossRef]
- Barbour, R.L.; Ribaudo, J.; Chan, S.H. Effect of creatine kinase activity on mitochondrial ADP/ATP transport. Evidence for a functional interaction. J. Biol. Chem. 1984, 259, 8246–8251. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, Y. Protein aggregates in pathological inclusions of amyotrophic lateral sclerosis. In Amyotrophic Lateral Sclerosis; Maurer, M., Ed.; InTech: Houston, TX, USA, 2012. [Google Scholar] [CrossRef][Green Version]
- Thakore, N.J.; Lapin, B.R.; Mitsumoto, H.; Pooled Resource Open-Access Als Clinical Trials Consortium. Early initiation of riluzole may improve absolute survival in amyotrophic lateral sclerosis. Muscle Nerve 2022, 66, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Tandon, R.; Lah, J.J.; Mitchell, C.S. s-SuStaIn: Scaling subtype and stage inference via simultaneous clustering of subjects and biomarkers. In Proceedings of the Fifth Conference on Health, Inference, and Learning, New York, NY, USA, 27–28 June 2024; Pollard, T., Choi, E., Singhal, P., Hughes, M., Sizikova, E., Mortazavi, B., Chen, I., Wang, F., Sarker, T., McDermott, M., et al., Eds.; PMLR: New York, NY, USA, 2024; Volume 248, pp. 461–476. [Google Scholar]
- Raguseo, F.; Wang, Y.; Li, J.; Petrić Howe, M.; Balendra, R.; Huyghebaert, A.; Vadukul, D.M.; Tanase, D.A.; Maher, T.E.; Malouf, L.; et al. The ALS/FTD-related C9orf72 hexanucleotide repeat expansion forms RNA condensates through multimolecular G-quadruplexes. Nat. Commun. 2023, 14, 8272. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.H.; Heckman, C.J. Bistability in Spinal Motoneurons In Vivo: Systematic Variations in Rhythmic Firing Patterns. J. Neurophysiol. 1998, 80, 572–582. [Google Scholar] [CrossRef]
- Mitchell, C.S.; Cates, A.; Kim, R.B.; Hollinger, S.K. Undergraduate biocuration: Developing tomorrow’s researchers while mining today’s data. J. Undergrad. Neurosci. Educ. 2015, 14, A56–A65. [Google Scholar]
- Petitjean, F.; Ketterlin, A.; Gançarski, P. A global averaging method for dynamic time warping, with applications to clustering. Pattern Recognit. 2011, 44, 678–693. [Google Scholar] [CrossRef]
- Belzacq, A.S.; Vieira, H.L.A.; Verrier, F.; Vandecasteele, G.; Cohen, I.; Prévost, M.C.; Kroemer, G. Bcl-2 and Bax modulate adenine nucleotide translocase activity. Cancer Res. 2003, 63, 541–546. [Google Scholar]
- Manfredi, G.; Kwong, J.Q.; Oca-Cossio, J.A.; Woischnik, M.; Gajewski, C.D.; Martushova, K.; Moraes, C.T. BCL-2 improves oxidative phosphorylation and modulates adenine nucleotide translocation in mitochondria of cells harboring mutant mtDNA. J. Biol. Chem. 2003, 278, 5639–5645. [Google Scholar] [CrossRef]
- Ma, P.; Schwarten, M.; Schneider, L.; Boeske, A.; Henke, N.; Lisak, D.; Willbold, D. Interaction of Bcl-2 with the autophagy-related GABAA receptor-associated protein (GABARAP): Biophysical characterization and functional implications. J. Biol. Chem. 2013, 288, 37204–37215. [Google Scholar] [CrossRef]
- Rho, S.B.; Byun, H.J.; Kim, B.R.; Kim, I.S.; Lee, J.H.; Yoo, R.; Park, S.H. GABAA receptor-binding protein promotes sensitivity to apoptosis induced by chemotherapeutic agents. Int. J. Oncol. 2013, 42, 1807–1814. [Google Scholar] [CrossRef]
- Wilkins, H.M.; Marquardt, K.; Lash, L.H.; Linseman, D.A. Bcl-2 is a novel interacting partner for the 2-oxoglutarate carrier and a key regulator of mitochondrial glutathione. Free Radic. Biol. Med. 2012, 52, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Vlachaki, M.T.; Meyn, R.E. Astro research fellowship: The role of bcl-2 and glutathione in an antioxidant pathway to prevent radiation-induced apoptosis. Int. J. Radiat. Oncol. 1998, 42, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Rudin, C.M.; Yang, Z.; Schumaker, L.M.; VanderWeele, D.J.; Newkirk, K.; Egorin, M.J.; Cullen, K.J. Inhibition of glutathione synthesis reverses Bcl-2-mediated cisplatin resistance. Cancer Res. 2003, 63, 312–318. [Google Scholar] [PubMed]
- Zhang, Y.; Ishida, C.T.; Shu, C.; Kleiner, G.; Sanchez-Quintero, M.J.; Bianchetti, E.; Quinzii, C.M.; Westhoff, M.A.; Karpel-Massler, G.; Siegelin, M.D. Inhibition of Bcl-2/Bcl-xL and c-MET causes synthetic lethality in model systems of glioblastoma. Sci. Rep. 2018, 8, 7373. [Google Scholar] [CrossRef]
- Krüger, A.; Stier, A.; Fischbach, A.; Bürkle, A.; Hauser, K.; Mangerich, A. Interactions of p53 with poly(ADP-ribose) and DNA induce distinct changes in protein structure as revealed by ATR-FTIR spectroscopy. Nucleic Acids Res. 2019, 47, 4843–4858. [Google Scholar] [CrossRef]
- Yang, T.C.; Wu, P.C.; Chung, I.F.; Jiang, J.H.; Fann, M.J.; Kao, L.S. Cell death caused by the synergistic effects of zinc and dopamine is mediated by a stress sensor gene Gadd45b - implication in the pathogenesis of Parkinson’s disease. J. Neurochem. 2016, 139, 120–133. [Google Scholar] [CrossRef]
- Gardner, B.; Dieriks, B.V.; Cameron, S.; Mendis, L.H.S.; Turner, C.; Faull, R.L.M.; Curtis, M.A. Metal concentrations and distributions in the human olfactory bulb in Parkinson’s disease. Sci. Rep. 2017, 7, 10454. [Google Scholar] [CrossRef]
- Jebelli, J.D.; Hooper, C.; Garden, G.A.; Pocock, J.M. Emerging roles of p53 in glial cell function in health and disease. Glia 2012, 60, 515–525. [Google Scholar] [CrossRef]
- Gong, X.; Liu, A.; Ming, X.; Deng, P.; Jiang, Y. UV-induced interaction between p38 MAPK and p53 serves as a molecular switch in determining cell fate. FEBS Lett. 2010, 584, 4711–4716. [Google Scholar] [CrossRef]
- Yeung, M.C.; Lau, A.S. Tumor suppressor p53 as a component of the tumor necrosis factor-induced, protein kinase PKR-mediated apoptotic pathway in human promonocytic U937 cells. J. Biol. Chem. 1998, 273, 25198–25202. [Google Scholar] [CrossRef]
- Muret, J.; Hasmim, M.; Stasik, I.; Jalil, A.; Mallavialle, A.; Nanbakhsh, A.; Lacroix, L.; Billot, K.; Baud, V.; Thiery, J.; et al. Attenuation of soft-tissue sarcomas resistance to the cytotoxic action of TNF-α by restoring p53 function. PLoS ONE 2012, 7, e38808. [Google Scholar] [CrossRef] [PubMed]
- Vousden, K.H. Alternative fuel–another role for p53 in the regulation of metabolism. Proc. Natl. Acad. Sci. USA 2010, 107, 7117–7118. [Google Scholar] [CrossRef] [PubMed]
- Robbins, D.; Zhao, Y. Oxidative stress induced by MnSOD-p53 interaction: Pro- or anti-tumorigenic? J. Signal Transduct. 2012, 2012, 101465. [Google Scholar] [CrossRef] [PubMed]
- Ambs, S.; Hussain, S.P.; Harris, C.C. Interactive effects of nitric oxide and the p53 tumor suppressor gene in carcinogenesis and tumor progression. FASEB J. 1997, 11, 443–448. [Google Scholar] [CrossRef]
- Constantinou, C.; Bushell, M.; Jeffrey, I.W.; Tilleray, V.; West, M.; Frost, V.; Hensold, J.; Clemens, M.J. P53-induced inhibition of protein synthesis is independent of apoptosis. Eur. J. Biochem. 2003, 270, 3122–3132. [Google Scholar] [CrossRef]
- Tilleray, V.; Constantinou, C.; Clemens, M.J. Regulation of protein synthesis by inducible wild-type p53 in human lung carcinoma cells. FEBS Lett. 2006, 580, 1766–1770. [Google Scholar] [CrossRef]
- Yang, N.; Gong, F.; Sun, L.; Yang, D.; Han, X.; Ma, C.; Sun, Y. Poly (ADP-ribose) polymerase-1 binds to BCL2 major breakpoint region and regulates BCL2 expression. J. Cell. Biochem. 2010, 110, 1208–1218. [Google Scholar] [CrossRef]
- Yokoyama, T.; Fukuzumi, S.; Hayashi, H.; Nakamuta, N.; Yamamoto, Y. GABA-mediated modulation of ATP-induced intracellular calcium responses in nodose ganglion neurons of the rat. Neurosci. Lett. 2015, 584, 168–172. [Google Scholar] [CrossRef]
- Vitolo, O.V.; Ciotti, M.T.; Galli, C.; Borsello, T.; Calissano, P. Adenosine and ADP prevent apoptosis in cultured rat cerebellar granule cells. Brain Res. 1998, 809, 297–301. [Google Scholar] [CrossRef]
- Bobba, A.; Amadoro, G.; Azzariti, A.; Pizzuto, R.; Atlante, A. Extracellular ADP prevents neuronal apoptosis via activation of cell antioxidant enzymes and protection of mitochondrial ANT-1. Biochim. Biophys. Acta 2014, 1837, 1338–1349. [Google Scholar] [CrossRef]
- Fink, B.D.; Bai, F.; Yu, L.; Sheldon, R.D.; Sharma, A.; Taylor, E.B.; Sivitz, W.I. Oxaloacetic acid mediates ADP-dependent inhibition of mitochondrial complex II-driven respiration. J. Biol. Chem. 2018, 293, 19932–19941. [Google Scholar] [CrossRef] [PubMed]
- Kato, I.; Yamamoto, Y.; Fujimura, M.; Noguchi, N.; Takasawa, S.; Okamoto, H. CD38 disruption impairs glucose-induced increases in cyclic ADP-ribose, [Ca2+]i, and insulin secretion. J. Biol. Chem. 1999, 274, 1869–1872. [Google Scholar] [CrossRef] [PubMed]
- Ikehata, F.; Satoh, J.; Nata, K.; Tohgo, A.; Nakazawa, T.; Kato, I.; Kobayashi, S.; Akiyama, T.; Takasawa, S.; Toyota, T.; et al. Autoantibodies against CD38 (ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase) that impair glucose-induced insulin secretion in noninsulin- dependent diabetes patients. J. Clin. Investig. 1998, 102, 395–401. [Google Scholar] [CrossRef]
- Ahel, I.; Ahel, D.; Matsusaka, T.; Clark, A.J.; Pines, J.; Boulton, S.J.; West, S.C. Poly(ADP-ribose)-binding zinc finger motifs in DNA repair/checkpoint proteins. Nature 2008, 451, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Kunzmann, A.; Dedoussis, G.; Jajte, J.; Malavolta, M.; Mocchegiani, E.; Bürkle, A. Effect of zinc on cellular poly(ADP-ribosyl)ation capacity. Exp. Gerontol. 2008, 43, 409–414. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Opazo, C.M.; Greenough, M.A.; Bush, A.I. Copper: From neurotransmission to neuroproteostasis. Front. Aging Neurosci. 2014, 6, 143. [Google Scholar] [CrossRef]
- Liu, H.; Ma, Y.; Pagliari, L.J.; Perlman, H.; Yu, C.; Lin, A.; Pope, R.M. TNF-alpha-induced apoptosis of macrophages following inhibition of NF-kappa B: A central role for disruption of mitochondria. J. Immunol. 2004, 172, 1907–1915. [Google Scholar] [CrossRef]
- Shirasaki, T.; Aibara, K.; Akaike, N. Direct modulation of GABAA receptor by intracellular ATP in dissociated nucleus tractus solitarii neurones of rat. J. Physiol. 1992, 449, 551–572. [Google Scholar] [CrossRef]
- Herrero-Yraola, A. Regulation of glutamate dehydrogenase by reversible ADP-ribosylation in mitochondria. EMBO J. 2001, 20, 2404–2412. [Google Scholar] [CrossRef]
- Aulbach, A.; Amuzie, C. Biomarkers in Nonclinical Drug Development. In A Comprehensive Guide to Toxicology in Nonclinical Drug Development; Elsevier: Amsterdam, The Netherlands, 2017; pp. 447–471. [Google Scholar] [CrossRef]
- Dangelmaier, C.; Jin, J.; Daniel, J.L.; Smith, J.B.; Kunapuli, S.P. The P2Y1 receptor mediates ADP-induced p38 kinase-activating factor generation in human platelets. Eur. J. Biochem. 2000, 267, 2283–2289. [Google Scholar] [CrossRef]
- Plow, E.F.; Pluskota, E. MAPkinaseQuest: Novel roadway to αIIbβ3 activation. Blood 2006, 107, 851. [Google Scholar] [CrossRef][Green Version]
- Agarwal, S.; Drysdale, B.E.; Shin, H.S. Tumor necrosis factor-mediated cytotoxicity involves ADP-ribosylation. J. Immunol. 1988, 140, 4187–4192. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, A. Transcription regulation of TNF—Early response genes by poly(ADP-ribose) polymerase-1 in murine heart endothelial cells. Nucleic Acids Res. 2004, 32, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Sheffler, L.A.; Wink, D.A.; Melillo, G.; Cox, G.W. Characterization of nitric oxide–stimulated ADP-ribosylation of various proteins from the mouse macrophage cell line ANA-1 using sodium nitroprusside and the novel nitric oxide-donating compound diethylamine dinitric oxide. J. Leukoc. Biol. 1995, 57, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Beirão, P.S.; de Meis, L. ADP-activated calcium ion exchange in sarcoplasmic reticulum vesicles. Biochim. Et Biophys. Acta (BBA)-Biomembr. 1976, 433, 520–530. [Google Scholar] [CrossRef]
- Rudra, P.; Hurst-Hess, K.; Lappierre, P.; Ghosh, P. High Levels of Intrinsic Tetracycline Resistance in Mycobacterium abscessus Are Conferred by a Tetracycline-Modifying Monooxygenase. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef]
- Mateyak, M.K.; Kinzy, T.G. ADP-ribosylation of Translation Elongation Factor 2 by Diphtheria Toxin in Yeast Inhibits Translation and Cell Separation. J. Biol. Chem. 2013, 288, 24647–24655. [Google Scholar] [CrossRef]
- Chen, K.C.; Xie, H.; Cai, Y. Modes of Action of ADP-Ribosylated Elongation Factor 2 in Inhibiting the Polypeptide Elongation Cycle: A Modeling Study. PLoS ONE 2013, 8, e66446. [Google Scholar] [CrossRef][Green Version]
- Rudolf, E. Depletion of ATP and oxidative stress underlie zinc-induced cell injury. Acta Medica 2007, 50, 43–49. [Google Scholar] [CrossRef][Green Version]
- Wang, C.; Kerckhofs, K.; Van de Casteele, M.; Smolders, I.; Pipeleers, D.; Ling, Z. Glucose inhibits GABA release by pancreatic β-cells through an increase in GABA shunt activity. Am. J. Physiol.-Endocrinol. Metab. 2006, 290, E494–E499. [Google Scholar] [CrossRef]
- Bailey, S.J.; Ravier, M.A.; Rutter, G.A. Glucose-Dependent Regulation of γ-Aminobutyric Acid (GABAA) Receptor Expression in Mouse Pancreatic Islet α-Cells. Diabetes 2007, 56, 320–327. [Google Scholar] [CrossRef]
- Deldicque, L.; Theisen, D.; Bertrand, L.; Hespel, P.; Hue, L.; Francaux, M. Creatine enhances differentiation of myogenic C2C12 cells by activating both p38 and Akt/PKB pathways. Am. J. Physiol.-Cell Physiol. 2007, 293, C1263–C1271. [Google Scholar] [CrossRef]
- Liang, G.; Liao, X.; Du, G.; Chen, J. Elevated glutathione production by adding precursor amino acids coupled with ATP in high cell density cultivation ofCandida utilis. J. Appl. Microbiol. 2008, 105, 1432–1440. [Google Scholar] [CrossRef]
- Majetschak, M. Regulation of the proteasome by ATP: Implications for ischemic myocardial injury and donor heart preservation. Am. J. Physiol.-Heart Circ. Physiol. 2013, 305, H267–H278. [Google Scholar] [CrossRef]
- Formigari, A.; Gregianin, E.; Irato, P. The effect of zinc and the role of p53 in copper-induced cellular stress responses. J. Appl. Toxicol. 2013, 33, 527–536. [Google Scholar] [CrossRef]
- Zhai, Q.; Ji, H.; Zheng, Z.; Yu, X.; Sun, L.; Liu, X. Copper induces apoptosis in BA/F3? cells: Bax, reactive oxygen species, and NF?B are involved. J. Cell. Physiol. 2000, 184, 161–170. [Google Scholar] [CrossRef]
- Grauer, C.M. The Effects of Zinc Status on Hepatic Poly(ADP-ribose) Polymerase Function in Response to DNA Damage. Master’s Thesis, University of Guelph, Guelph, ON, Canada, 1997. [Google Scholar]
- Suh, S.W.; Aoyama, K.; Alano, C.C.; Anderson, C.M.; Hamby, A.M.; Swanson, R.A. Zinc Inhibits Astrocyte Glutamate Uptake by Activation of Poly(ADP-ribose) Polymerase-1. Mol. Med. 2007, 13, 344–349. [Google Scholar] [CrossRef]
- Lemire, J.; Mailloux, R.; Appanna, V.D. Zinc toxicity alters mitochondrial metabolism and leads to decreased ATP production in hepatocytes. J. Appl. Toxicol. 2007, 28, 175–182. [Google Scholar] [CrossRef]
- Dineley, K.E.; Votyakova, T.V.; Reynolds, I.J. Zinc inhibition of cellular energy production: Implications for mitochondria and neurodegeneration. J. Neurochem. 2003, 85, 563–570. [Google Scholar] [CrossRef]
- Hosie, A.M.; Dunne, E.L.; Harvey, R.J.; Smart, T.G. Zinc-mediated inhibition of GABAA receptors: Discrete binding sites underlie subtype specificity. Nat. Neurosci. 2003, 6, 362–369. [Google Scholar] [CrossRef]
- Cohen-Kfir, E.; Lee, W.; Eskandari, S.; Nelson, N. Zinc inhibition of γ-aminobutyric acid transporter 4 (GAT4) reveals a link between excitatory and inhibitory neurotransmission. Proc. Natl. Acad. Sci. USA 2005, 102, 6154–6159. [Google Scholar] [CrossRef]
- Luo, M.; Luo, P.; Zhang, Z.; Payne, K.; Watson, S.; Wu, H.; Tan, Y.; Ding, Y.; Sun, W.; Yin, X.; et al. Zinc delays the progression of obesity-related glomerulopathy in mice via down-regulating P38 MAPK-mediated inflammation. Obesity 2016, 24, 1244–1256. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Sun, S.P.; Zhu, H.S.; Jiao, X.Q.; Zhong, K.; Guo, Y.J.; Zha, G.M.; Han, L.Q.; Yang, G.Y.; Li, H.P. GABA regulates the proliferation and apoptosis of MAC-T cells through the LPS-induced TLR4 signaling pathway. Res. Vet. Sci. 2018, 118, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Omata, Y.; Salvador, G.A.; Supasai, S.; Keenan, A.H.; Oteiza, P.I. Decreased Zinc Availability Affects Glutathione Metabolism in Neuronal Cells and in the Developing Brain. Toxicol. Sci. 2013, 133, 90–100. [Google Scholar] [CrossRef]
- Trevisan, R.; Flesch, S.; Mattos, J.J.; Milani, M.R.; Bainy, A.C.D.; Dafre, A.L. Zinc causes acute impairment of glutathione metabolism followed by coordinated antioxidant defenses amplification in gills of brown mussels Perna perna. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2014, 159, 22–30. [Google Scholar] [CrossRef]
- Penttil, O.; Hurme, H.; Neuvonen, P.J. Effect of zinc sulphate on the absorption of tetracycline and doxycycline in man. Eur. J. Clin. Pharmacol. 1975, 9, 131–134. [Google Scholar] [CrossRef]
- Andersson, K.E.; Bratt, L.; Dencker, H.; Kamme, C.; Lanner, E. Inhibition of tetracycline absorption by zinc. Eur. J. Clin. Pharmacol. 1976, 10, 59–62. [Google Scholar] [CrossRef]
- Walther, U.I.; Schulze, J.; Forth, W. Inhibition of protein synthesis by zinc: Comparison between protein synthesis and RNA synthesis. Hum. Exp. Toxicol. 1998, 17, 661–667. [Google Scholar] [CrossRef]
- Alirezaei, M.; Mordelet, E.; Rouach, N.; Nairn, A.C.; Glowinski, J.; Prémont, J. Zinc-induced inhibition of protein synthesis and reduction of connexin-43 expression and intercellular communication in mouse cortical astrocytes. Eur. J. Neurosci. 2002, 16, 1037–1044. [Google Scholar] [CrossRef]
- Hileti, D.; Panayiotidis, P.; Hoffbrand, A.V. Iron chelators induce apoptosis in proliferating cells. Br. J. Haematol. 1995, 89, 181–187. [Google Scholar] [CrossRef]
- Kim, J.L.; Lee, D.H.; Na, Y.J.; Kim, B.R.; Jeong, Y.A.; Lee, S.I.; Kang, S.; Joung, S.Y.; Lee, S.Y.; Oh, S.C.; et al. Iron chelator-induced apoptosis via the ER stress pathway in gastric cancer cells. Tumor Biol. 2016, 37, 9709–9719. [Google Scholar] [CrossRef]
- Yoshida, Y.; Furuta, S.; Niki, E. Effects of metal chelating agents on the oxidation of lipids induced by copper and iron. Biochim. Et Biophys. Acta (BBA)-Lipids Lipid Metab. 1993, 1210, 81–88. [Google Scholar] [CrossRef]
- Chandel, N.S.; Maltepe, E.; Goldwasser, E.; Mathieu, C.E.; Simon, M.C.; Schumacker, P.T. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc. Natl. Acad. Sci. USA 1998, 95, 11715–11720. [Google Scholar] [CrossRef]
- Sharonova, I.N.; Vorobjev, V.S.; Haas, H.L. High-affinity copper block of GABAA receptor-mediated currents in acutely isolated cerebellar Purkinje cells of the rat. Eur. J. Neurosci. 1998, 10, 522–528. [Google Scholar] [CrossRef]
- Sharonova, I.N.; Vorobjev, V.S.; Haas, H.L. Interaction between copper and zinc at GABAA receptors in acutely isolated cerebellar Purkinje cells of the rat. Br. J. Pharmacol. 2000, 130, 851–856. [Google Scholar] [CrossRef]
- Schlief, M.L.; Craig, A.M.; Gitlin, J.D. NMDA Receptor Activation Mediates Copper Homeostasis in Hippocampal Neurons. J. Neurosci. 2005, 25, 239–246. [Google Scholar] [CrossRef]
- Kadowaki, S.; Endoh, D.; Okui, T.; Hayashi, M. Trientine, a Copper-Chelating Agent, Induced Apoptosis in Murine Fibrosarcoma Cells by Activation of the p38 MAPK Pathway. J. Vet. Med Sci. 2009, 71, 1541–1544. [Google Scholar] [CrossRef]
- Lu, J. Triethylenetetramine Pharmacology and Its Clinical Applications. Mol. Cancer Ther. 2010, 9, 2458–2467. [Google Scholar] [CrossRef]
- Buac, D.; Schmitt, S.; Ventro, G.; Rani Kona, F.; Ping Dou, Q. Dithiocarbamate-based coordination compounds as potent proteasome inhibitors in human cancer cells. Mini-Rev. Med. Chem. 2012, 12, 1193–1201. [Google Scholar] [CrossRef]
- Lambs, L.; Venturim, M.; Révérend, B.D.L.; Kozlowski, H.; Berthon, G. Metal ion-tetracycline interactions in biological fluids. J. Inorg. Biochem. 1988, 33, 193–209. [Google Scholar] [CrossRef]
- Calvert, J.G.; Simon, E.H. Effects of the Copper Chelators Diethyldithiocarbamate and Bathocuproine Sulfonate on Interferon and Its Antiviral State. J. Interferon Res. 1990, 10, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Peavy, R.D.; Chang, M.S.S.; Sanders-Bush, E.; Conn, P.J. Metabotropic Glutamate Receptor 5-Induced Phosphorylation of Extracellular Signal-Regulated Kinase in Astrocytes Depends on Transactivation of the Epidermal Growth Factor Receptor. J. Neurosci. 2001, 21, 9619–9628. [Google Scholar] [CrossRef] [PubMed]
- Sotskiĭ, O.P.; Akopian, V.P.; Zhamgarian, L.G.; Zhamgarian, A.G. Effect of GABA and pyracetam on mitochondrial ADP phosphorylation in experimental hypokinesia. Vopr. Med. Khim. 2002, 48, 485–489. [Google Scholar]
- Liu, J.; Wang, Y. Allosteric modulation of GABAA receptors by extracellular ATP. Mol. Brain 2014, 7, 6. [Google Scholar] [CrossRef]
- Jo, Y.H.; Schlichter, R. Synaptic corelease of ATP and GABA in cultured spinal neurons. Nat. Neurosci. 1999, 2, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Kelley, J.M.; Hughes, L.B.; Bridges, S.L. Does gamma-aminobutyric acid (GABA) influence the development of chronic inflammation in rheumatoid arthritis? J. Neuroinflamm. 2008, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.; Axtell, R.; Mitra, A.; Miranda, M.; Lock, C.; Tsien, R.W.; Steinman, L. Inhibitory role for GABA in autoimmune inflammation. Proc. Natl. Acad. Sci. USA 2010, 107, 2580–2585. [Google Scholar] [CrossRef]
- Cherng, S.H.; Huang, C.Y.; Kuo, W.W.; Lai, S.E.; Tseng, C.Y.; Lin, Y.M.; Tsai, F.J.; Wang, H.F. GABA tea prevents cardiac fibrosis by attenuating TNF-alpha and Fas/FasL-mediated apoptosis in streptozotocin-induced diabetic rats. Food Chem. Toxicol. 2014, 65, 90–96. [Google Scholar] [CrossRef]
- Yang, Y.; Lian, Y.T.; Huang, S.Y.; Yang, Y.; Cheng, L.X.; Liu, K. GABA and Topiramate Inhibit the Formation of Human Macrophage-Derived Foam Cells by Modulating Cholesterol-Metabolism-Associated Molecules. Cell. Physiol. Biochem. 2014, 33, 1117–1129. [Google Scholar] [CrossRef]
- Michels, L.; Schulte-Vels, T.; Schick, M.; O’Gorman, R.L.; Zeffiro, T.; Hasler, G.; Mueller-Pfeiffer, C. Prefrontal GABA and glutathione imbalance in posttraumatic stress disorder: Preliminary findings. Psychiatry Res. Neuroimaging 2014, 224, 288–295. [Google Scholar] [CrossRef]
- Tian, X.L.; Wu, X.L.; Li, Y.; Zhang, S.Q. The effect of gamma-aminobutyric acid in superoxide dismutase, peroxidase and catalase activity response to salt stress in maize seedling. Shi Yan Sheng Wu Xue Bao 2005, 38, 75–79. [Google Scholar]
- Tujioka, K.; Okuyama, S.; Yokogoshi, H.; Fukaya, Y.; Hayase, K.; Horie, K.; Kim, M. Dietary γ-aminobutyric acid affects the brain protein synthesis rate in young rats. Amino Acids 2006, 32, 255–260. [Google Scholar] [CrossRef]
- Thanapreedawat, P.; Ohsumi, M.; Hayase, K.; Yoshizawa, F.; Yokogoshi, H. Influence of GABA on Brain Protein Synthesis Mediated by the Mammalian Target on the Rapamycin Pathway. Biosci. Biotechnol. Biochem. 2013, 77, 660–662. [Google Scholar] [CrossRef]
- Schelman, W.R.; Andres, R.D.; Sipe, K.J.; Kang, E.; Weyhenmeyer, J.A. Glutamate mediates cell death and increases the Bax to Bcl-2 ratio in a differentiated neuronal cell line. Mol. Brain Res. 2004, 128, 160–169. [Google Scholar] [CrossRef]
- Pavlović, V.; Cekić, S.; Kocić, G.; Sokolović, D.; Živković, V. Effect of monosodium glutamate on apoptosis and Bcl-2/Bax protein level in rat thymocyte culture. Physiol. Res. 2007, 56, 619–626. [Google Scholar] [CrossRef]
- Parpura, V.; Fisher, E.S.; Lechleiter, J.D.; Schousboe, A.; Waagepetersen, H.S.; Brunet, S.; Baltan, S.; Verkhratsky, A. Glutamate and ATP at the Interface Between Signaling and Metabolism in Astroglia: Examples from Pathology. Neurochem. Res. 2016, 42, 19–34. [Google Scholar] [CrossRef]
- Shimamoto, K.; Lebrun, B.; Yasuda-Kamatani, Y.; Sakaitani, M.; Shigeri, Y.; Yumoto, N.; Nakajima, T. Dl-threo-β-Benzyloxyaspartate, A Potent Blocker of Excitatory Amino Acid Transporters. Mol. Pharmacol. 1998, 53, 195–201. [Google Scholar] [CrossRef]
- Xu, J.; Church, S.J.; Patassini, S.; Begley, P.; Waldvogel, H.J.; Curtis, M.A.; Faull, R.L.M.; Unwin, R.D.; Cooper, G.J.S. Evidence for widespread, severe brain copper deficiency in Alzheimer’s dementia. Metallomics 2017, 9, 1106–1119. [Google Scholar] [CrossRef]
- Ip, N.Y.; Li, Y.P.; van de Stadt, I.; Panayotatos, N.; Alderson, R.F.; Lindsay, R.M. Ciliary neurotrophic factor enhances neuronal survival in embryonic rat hippocampal cultures. J. Neurosci. 1991, 11, 3124–3134. [Google Scholar] [CrossRef]
- Salvatore, M.F.; Gerhardt, G.A.; Dayton, R.D.; Klein, R.L.; Stanford, J.A. Bilateral effects of unilateral GDNF administration on dopamine- and GABA-regulating proteins in the rat nigrostriatal system. Exp. Neurol. 2009, 219, 197–207. [Google Scholar] [CrossRef]
- Molz, S.; Decker, H.; Dal-Cim, T.; Cremonez, C.; Cordova, F.M.; Leal, R.B.; Tasca, C.I. Glutamate-induced toxicity in hippocampal slices involves apoptotic features and p38 MAPK signaling. Neurochem. Res. 2008, 33, 27–36. [Google Scholar] [CrossRef]
- Pacheco, R.; Gallart, T.; Lluis, C.; Franco, R. Role of glutamate on T-cell mediated immunity. J. Neuroimmunol. 2007, 185, 9–19. [Google Scholar] [CrossRef]
- Johnson, M.O.; Wolf, M.M.; Madden, M.Z.; Andrejeva, G.; Sugiura, A.; Contreras, D.C.; Maseda, D.; Liberti, M.V.; Paz, K.; Kishton, R.J.; et al. Distinct regulation of Th17 and Th1 cell differentiation by glutaminase-dependent metabolism. Cell 2018, 175, 1780–1795.e19. [Google Scholar] [CrossRef]
- Zanzinger, J.; Czachurski, J.; Seller, H. Neuronal Nitric Oxide Reduces Sympathetic Excitability by Modulation of Central Glutamate Effects in Pigs. Circ. Res. 1997, 80, 565–571. [Google Scholar] [CrossRef]
- Ban, K.; Sprunt, J.M.; Martin, S.; Yang, P.; Kozar, R.A. Glutamine activates peroxisome proliferator-activated receptor-γ in intestinal epithelial cells via 15-S-HETE and 13-OXO-ODE: A novel mechanism. Am. J. Physiol.-Gastrointest. Liver Physiol. 2011, 301, G547–G554. [Google Scholar] [CrossRef]
- Vornov, J.J.; Coyle, J.T. Glutamate Neurotoxicity and the Inhibition of Protein Synthesis in the Hippocampal Slice. J. Neurochem. 1991, 56, 996–1006. [Google Scholar] [CrossRef]
- Marin, P.; Nastiuk, K.L.; Daniel, N.; Girault, J.A.; Czernik, A.J.; Glowinski, J.; Nairn, A.C.; Prémont, J. Glutamate-Dependent Phosphorylation of Elongation Factor-2 and Inhibition of Protein Synthesis in Neurons. J. Neurosci. 1997, 17, 3445–3454. [Google Scholar] [CrossRef]
- Aimond, F.; Rauzier, J.M.; Bony, C.; Vassort, G. Simultaneous Activation of p38 MAPK and p42/44 MAPK by ATP Stimulates the K+ Current ITREK in Cardiomyocytes. J. Biol. Chem. 2000, 275, 39110–39116. [Google Scholar] [CrossRef]
- Ren, F.; Qian, X.H.; Qian, X.L. Astragalus polysaccharide upregulates hepcidin and reduces iron overload in mice via activation of p38 mitogen-activated protein kinase. Biochem. Biophys. Res. Commun. 2016, 472, 163–168. [Google Scholar] [CrossRef]
- Zhang, H.; Nei, H.; Dougherty, P.M. A p38 Mitogen-Activated Protein Kinase-Dependent Mechanism of Disinhibition in Spinal Synaptic Transmission Induced by Tumor Necrosis Factor-α. J. Neurosci. 2010, 30, 12844–12855. [Google Scholar] [CrossRef]
- Schild, R.; Sonnenberg-Hirche, C.; Schaiff, W.; Bildirici, I.; Nelson, D.; Sadovsky, Y. The kinase p38 Regulates Peroxisome Proliferator Activated Receptor-γ in Human Trophoblasts. Placenta 2006, 27, 191–199. [Google Scholar] [CrossRef]
- Barger, P.M.; Browning, A.C.; Garner, A.N.; Kelly, D.P. p38 Mitogen-activated Protein Kinase Activates Peroxisome Proliferator-activated Receptor α. J. Biol. Chem. 2001, 276, 44495–44501. [Google Scholar] [CrossRef]
- Croons, V.; Martinet, W.; Herman, A.G.; Timmermans, J.P.; De Meyer, G.R.Y. The Protein Synthesis Inhibitor Anisomycin Induces Macrophage Apoptosis in Rabbit Atherosclerotic Plaques through p38 Mitogen-Activated Protein Kinase. J. Pharmacol. Exp. Ther. 2009, 329, 856–864. [Google Scholar] [CrossRef]
- Zhao, T.C.; Taher, M.M.; Valerie, K.C.; Kukreja, R.C. p38 Triggers Late Preconditioning Elicited by Anisomycin in Heart: Involvement of NF-κB and iNOS. Circ. Res. 2001, 89, 915–922. [Google Scholar] [CrossRef]
- Vaculová, A.; Hofmanova, J.; Soucek, K.; Kovariková, M.; Kozubík, A. Tumor necrosis factor-alpha induces apoptosis associated with poly(ADP-ribose) polymerase cleavage in HT-29 colon cancer cells. Anticancer. Res. 2002, 22, 1635–1639. [Google Scholar]
- Agarwal, S.; Piesco, N. Poly ADP-ribosylation of a 90-kDa protein is involved in TNF-alpha-mediated cytotoxicity. J. Immunol. 1994, 153, 473–481. [Google Scholar] [CrossRef]
- Franchi, L.; Eigenbrod, T.; Núñez, G. Cutting Edge: TNF-α Mediates Sensitization to ATP and Silica via the NLRP3 Inflammasome in the Absence of Microbial Stimulation. J. Immunol. 2009, 183, 792–796. [Google Scholar] [CrossRef]
- Sánchez-Alcázar, J.A.; Ruíz-Cabello, J.; Hernández-Muñoz, I.; Pobre, P.S.; de la Torre, P.; Siles-Rivas, E.; García, I.; Kaplan, O.; Muñoz-Yagüe, M.T.; Solís-Herruzo, J.A. Tumor Necrosis Factor-α Increases ATP Content in Metabolically Inhibited L929 Cells Preceding Cell Death. J. Biol. Chem. 1997, 272, 30167–30177. [Google Scholar] [CrossRef]
- Karakochuk, C.D.; Barr, S.I.; Boy, E.; Bahizire, E.; Tugirimana, P.L.; Akilimali, P.Z.; Houghton, L.A.; Green, T.J. The effect of inflammation on serum zinc concentrations and the prevalence estimates of population-level zinc status among Congolese children aged 6–59 months. Eur. J. Clin. Nutr. 2017, 71, 1467–1470. [Google Scholar] [CrossRef]
- Pribiag, H.; Stellwagen, D. TNF-α Downregulates Inhibitory Neurotransmission through Protein Phosphatase 1-Dependent Trafficking of GABAAReceptors. J. Neurosci. 2013, 33, 15879–15893. [Google Scholar] [CrossRef]
- De Laurentiis, A.; Pisera, D.; Lasaga, M.; Díaz, M.; Theas, S.; Duvilanski, B.; Seilicovich, A. Effect of interleukin-6 and tumor necrosis factor-alpha on GABA release from mediobasal hypothalamus and posterior pituitary. Neuroimmunomodulation 2000, 7, 77–83. [Google Scholar] [CrossRef]
- Lang, C.H.; Frost, R.A.; Nairn, A.C.; MacLean, D.A.; Vary, T.C. TNF-α impairs heart and skeletal muscle protein synthesis by altering translation initiation. Am. J. Physiol.-Endocrinol. Metab. 2002, 282, E336–E347. [Google Scholar] [CrossRef]
- ZHOU, J.; FAN, S.; CAO, Y.; ZHU, M.; HAN, Y.; CAO, X.; LI, Y. Tumor necrosis factor-α suppresses the protein fractional synthesis rate of the small intestine stimulated by glutamine in rats. Exp. Ther. Med. 2014, 9, 547–552. [Google Scholar] [CrossRef][Green Version]
- Davila, J.C.; Davis, P.J.; Acosta, D. Changes in glutathione and cellular energy as potential mechanisms of papaverine-induced hepatotoxicity in vitro. Toxicol. Appl. Pharmacol. 1991, 108, 28–36. [Google Scholar] [CrossRef]
- Freitas, H.R.; de Melo Reis, R.A. Glutathione induces GABA release through P2X7R activation on Müller glia. Neurogenesis 2017, 4, e1283188. [Google Scholar] [CrossRef]
- Kaur, N.; Lu, B.; Monroe, R.; Ward, S.; Halvorsen, S. Inducers of oxidative stress block ciliary neurotrophic factor activation of Jak/STAT signaling in neurons. J. Neurochem. 2005, 92, 1521–1530. [Google Scholar] [CrossRef]
- Ernst, V.; Levin, D.H.; London, I.M. Inhibition of protein synthesis initiation by oxidized glutathione: Activation of a protein kinase that phosphorylates the α subunit of eukaryotic initiation factor 2. Proc. Natl. Acad. Sci. USA 1978, 75, 4110–4114. [Google Scholar] [CrossRef]
- Lu, S.C. Regulation of glutathione synthesis. Mol. Asp. Med. 2009, 30, 42–59. [Google Scholar] [CrossRef]
- Pozdnyakov, N.; Lloyd, A.; Reddy, V.; Sitaramayya, A. Nitric Oxide-Regulated Endogenous ADP-Ribosylation of Rod Outer Segment Proteins. Biochem. Biophys. Res. Commun. 1993, 192, 610–615. [Google Scholar] [CrossRef]
- Torres, J.; Wilson, M.T. The reactions of copper proteins with nitric oxide. Biochim. Et Biophys. Acta (BBA)-Bioenerg. 1999, 1411, 310–322. [Google Scholar] [CrossRef]
- Maddox, J.W.; Gleason, E. Nitric oxide promotes GABA release by activating a voltage-independent Ca2+ influx pathway in retinal amacrine cells. J. Neurophysiol. 2017, 117, 1185–1199. [Google Scholar] [CrossRef]
- Raju, K.; Doulias, P.T.; Evans, P.; Krizman, E.N.; Jackson, J.G.; Horyn, O.; Daikhin, Y.; Nissim, I.; Yudkoff, M.; Nissim, I.; et al. Regulation of brain glutamate metabolism by nitric oxide and S-nitrosylation. Sci. Signal. 2015, 8, ra68. [Google Scholar] [CrossRef]
- Dawson, V.L.; Dawson, T.M.; London, E.D.; Bredt, D.S.; Snyder, S.H. Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc. Natl. Acad. Sci. USA 1991, 88, 6368–6371. [Google Scholar] [CrossRef]
- Kim, Y.M.; Son, K.; Hong, S.J.; Green, A.; Chen, J.J.; Tzeng, E.; Hierholzer, C.; Billiar, T.R. Inhibition of protein synthesis by nitric oxide correlates with cytostatic activity: Nitric oxide induces phosphorylation of initiation factor eIF-2 alpha. Mol. Med. 1998, 4, 179–190. [Google Scholar] [CrossRef]
- Kolpakov, V.; Gordon, D.; Kulik, T.J. Nitric Oxide–Generating Compounds Inhibit Total Protein and Collagen Synthesis in Cultured Vascular Smooth Muscle Cells. Circ. Res. 1995, 76, 305–309. [Google Scholar] [CrossRef]
- Ballard, J.W.O.; Melvin, R.G. Tetracycline treatment influences mitochondrial metabolism and mtDNA density two generations after treatment in Drosophila. Insect Mol. Biol. 2007, 16, 799–802. [Google Scholar] [CrossRef]
- Wang, Y.J.; Jia, D.A.; Sun, R.J.; Zhu, H.W.; Zhou, D.M. Adsorption and Cosorption of Tetracycline and Copper(II) on Montmorillonite as Affected by Solution pH. Environ. Sci. Technol. 2008, 42, 3254–3259. [Google Scholar] [CrossRef]
- Bilger, M.; Heger, S.; Brann, D.W.; Paredes, A.; Ojeda, S.R. A Conditional Tetracycline-Regulated Increase in Gamma Amino Butyric Acid Production near Luteinizing Hormone-Releasing Hormone Nerve Terminals Disrupts Estrous Cyclicity in the Rat. Endocrinology 2001, 142, 2102–2114. [Google Scholar] [CrossRef]
- Sun, J.; Shigemi, H.; Tanaka, Y.; Yamauchi, T.; Ueda, T.; Iwasaki, H. Tetracyclines downregulate the production of LPS-induced cytokines and chemokines in THP-1 cells via ERK, p38, and nuclear factor-κB signaling pathways. Biochem. Biophys. Rep. 2015, 4, 397–404. [Google Scholar] [CrossRef]
- Hua, X.; Svensson, C.I.; Matsui, T.; Fitzsimmons, B.; Yaksh, T.L.; Webb, M. Intrathecal minocycline attenuates peripheral inflammation-induced hyperalgesia by inhibiting p38 MAPK in spinal microglia. Eur. J. Neurosci. 2005, 22, 2431–2440. [Google Scholar] [CrossRef]
- Kaushik, V.; Beduya, D.; Kalampokis, I.; Kohlhoff, S.; Joks, R.O.; Durkin, H.G.; Nowakowski, M. Tetracyclines tigecycline and doxycycline inhibit LPS-induced nitric oxide production by RAW 264.7 murine macrophages. (101.3). J. Immunol. 2007, 178, S200–S201. [Google Scholar] [CrossRef]
- Breitschopf, K.; Haendeler, J.; Malchow, P.; Zeiher, A.M.; Dimmeler, S. Posttranslational Modification of Bcl-2 Facilitates Its Proteasome-Dependent Degradation: Molecular Characterization of the Involved Signaling Pathway. Mol. Cell. Biol. 2000, 20, 1886–1896. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.S.; Bassik, M.C.; Suh, H.; Nishino, M.; Arroyo, J.D.; Hahn, W.C.; Korsmeyer, S.J.; Roberts, T.M. PP2A Regulates BCL-2 Phosphorylation and Proteasome-mediated Degradation at the Endoplasmic Reticulum. J. Biol. Chem. 2006, 281, 23003–23012. [Google Scholar] [CrossRef] [PubMed]
- Martıín, M.E.; Hidalgo, J.; Rosa, J.L.; Crottet, P.; Velasco, A. Effect of Protein Kinase A Activity on the Association of ADP-ribosylation Factor 1 to Golgi Membranes. J. Biol. Chem. 2000, 275, 19050–19059. [Google Scholar] [CrossRef][Green Version]
- Musi, N.; Goodyear, L.J. AMP-activated protein kinase and muscle glucose uptake. Acta Physiol. Scand. 2003, 178, 337–345. [Google Scholar] [CrossRef]
- Hayashi, T.; Hirshman, M.F.; Kurth, E.J.; Winder, W.W.; Goodyear, L.J. Evidence for 5’AMP-Activated Protein Kinase Mediation of the Effect of Muscle Contraction on Glucose Transport. Diabetes 1998, 47, 1369–1373. [Google Scholar] [CrossRef]
- Taylor, K.M.; Kille, P.; Hogstrand, C. Protein kinase CK2 opens the gate for zinc signaling. Cell Cycle 2012, 11, 1863–1864. [Google Scholar] [CrossRef]
- Xiao, Y.; Chen, D.; Zhang, X.; Cui, Q.; Fan, Y.; Bi, C.; Dou, Q.P. Molecular study on copper-mediated tumor proteasome inhibition and cell death. Int. J. Oncol. 2010, 37, 81–87. [Google Scholar] [CrossRef]
- Zemoura, K.; Benke, D. Proteasomal Degradation of γ-Aminobutyric AcidB Receptors Is Mediated by the Interaction of the GABAB2 C Terminus with the Proteasomal ATPase Rtp6 and Regulated by Neuronal Activity. J. Biol. Chem. 2014, 289, 7738–7746. [Google Scholar] [CrossRef]
- Crider, A.; Pandya, C.D.; Peter, D.; Ahmed, A.O.; Pillai, A. Ubiquitin-proteasome dependent degradation of GABAAα1 in autism spectrum disorder. Mol. Autism 2014, 5, 1–10. [Google Scholar] [CrossRef]
- Adolph, O.; Köster, S.; Räth, M.; Georgieff, M.; Weigt, H.U.; Engele, J.; Senftleben, U.; Föhr, K.J. Rapid increase of glial glutamate uptake via blockade of the protein kinase A pathway. Glia 2007, 55, 1699–1707. [Google Scholar] [CrossRef] [PubMed]
- Nisticò, R.; Florenzano, F.; Mango, D.; Ferraina, C.; Grilli, M.; Di Prisco, S.; Nobili, A.; Saccucci, S.; D’Amelio, M.; Morbin, M.; et al. Presynaptic c-Jun N-terminal Kinase 2 regulates NMDA receptor-dependent glutamate release. Sci. Rep. 2015, 5, 9035. [Google Scholar] [CrossRef] [PubMed]
- Krishna, S.; Zhong, X. Role of Diacylglycerol Kinases in T Cell Development and Function. Crit. Rev. Immunol. 2013, 33, 97–118. [Google Scholar] [CrossRef]
- Kemmerer, M.; Finkernagel, F.; Cavalcante, M.F.; Abdalla, D.S.P.; Müller, R.; Brüne, B.; Namgaladze, D. AMP-Activated Protein Kinase Interacts with the Peroxisome Proliferator-Activated Receptor Delta to Induce Genes Affecting Fatty Acid Oxidation in Human Macrophages. PLoS ONE 2015, 10, e0130893. [Google Scholar] [CrossRef] [PubMed]
- Day, L.E. Tetracycline Inhibition of Cell-Free Protein Synthesis II. Effect of the Binding of Tetracycline to the Components of the System. J. Bacteriol. 1966, 92, 197–203. [Google Scholar] [CrossRef] [PubMed]
| 5× Effect Size | 10× Effect Size | 15× Effect Size |
|---|---|---|
| Pro-proteomic | Pro-proteomic | Pro-apoptosis |
| Anti-inflammatory | Pro-oxidative | Pro-proteomic |
| Energy production | Anti-inflammatory | Pro-oxidative |
| Two-Way Treatment | ||
| Energy Consumption and Anti-Apoptosis | Energy Consumption and Anti-Apoptosis | Anti-Inflammatory and Energy Production |
| Anti-Inflammatory and Energy Production | Anti-Inflammatory and Energy Production | Energy Consumption and Anti-Apoptosis |
| Pro-Apoptosis and Antioxidant | Pro-Apoptosis and Antioxidant | Pro-Apoptosis and Antioxidant |
| Treatment Combination | Rank @ 200 Days | Rank @ 365 Days | Rank @ 730 Days |
|---|---|---|---|
| Metal Ion Chelator + Pro-Proteomics | 1 | 1 | 1 |
| Energy Consumption + Metal Ion Chelator | 2 | 2 | 2 |
| Energy Consumption + Pro-Proteomic | 3 | 5 | 3 |
| Anti-Apoptosis + Energy Production | 4 | 8 | - |
| Pro-Excitotoxicity + Pro-Inflammatory | 5 | 4 | 7 |
| Anti-Excitatory + Anti-Proteomic | 6 | 6 | 4 |
| Energy Production + Pro-Proteomic | 7 | - | - |
| Metal Ion Chelator + Anti-Proteomic | 8 | 7 | 5 |
| Energy Consumption + Anti-Excitatory | 9 | 3 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, A.J.B.; Bi, S.; Ridgeway, E.; Al-Hussaini, I.; Deshpande, S.; Krueger, A.; Khatri, A.; Tsui, D.; Deng, J.; Mitchell, C.S. Restoring Homeostasis: Treating Amyotrophic Lateral Sclerosis by Resolving Dynamic Regulatory Instability. Int. J. Mol. Sci. 2025, 26, 872. https://doi.org/10.3390/ijms26030872
Lee AJB, Bi S, Ridgeway E, Al-Hussaini I, Deshpande S, Krueger A, Khatri A, Tsui D, Deng J, Mitchell CS. Restoring Homeostasis: Treating Amyotrophic Lateral Sclerosis by Resolving Dynamic Regulatory Instability. International Journal of Molecular Sciences. 2025; 26(3):872. https://doi.org/10.3390/ijms26030872
Chicago/Turabian StyleLee, Albert J. B., Sarah Bi, Eleanor Ridgeway, Irfan Al-Hussaini, Sakshi Deshpande, Adam Krueger, Ahad Khatri, Dennis Tsui, Jennifer Deng, and Cassie S. Mitchell. 2025. "Restoring Homeostasis: Treating Amyotrophic Lateral Sclerosis by Resolving Dynamic Regulatory Instability" International Journal of Molecular Sciences 26, no. 3: 872. https://doi.org/10.3390/ijms26030872
APA StyleLee, A. J. B., Bi, S., Ridgeway, E., Al-Hussaini, I., Deshpande, S., Krueger, A., Khatri, A., Tsui, D., Deng, J., & Mitchell, C. S. (2025). Restoring Homeostasis: Treating Amyotrophic Lateral Sclerosis by Resolving Dynamic Regulatory Instability. International Journal of Molecular Sciences, 26(3), 872. https://doi.org/10.3390/ijms26030872






