Complement Factor B Deficiency Is Dispensable for Female Fertility but Affects Microbiome Diversity and Complement Activity
Abstract
1. Introduction
2. Results
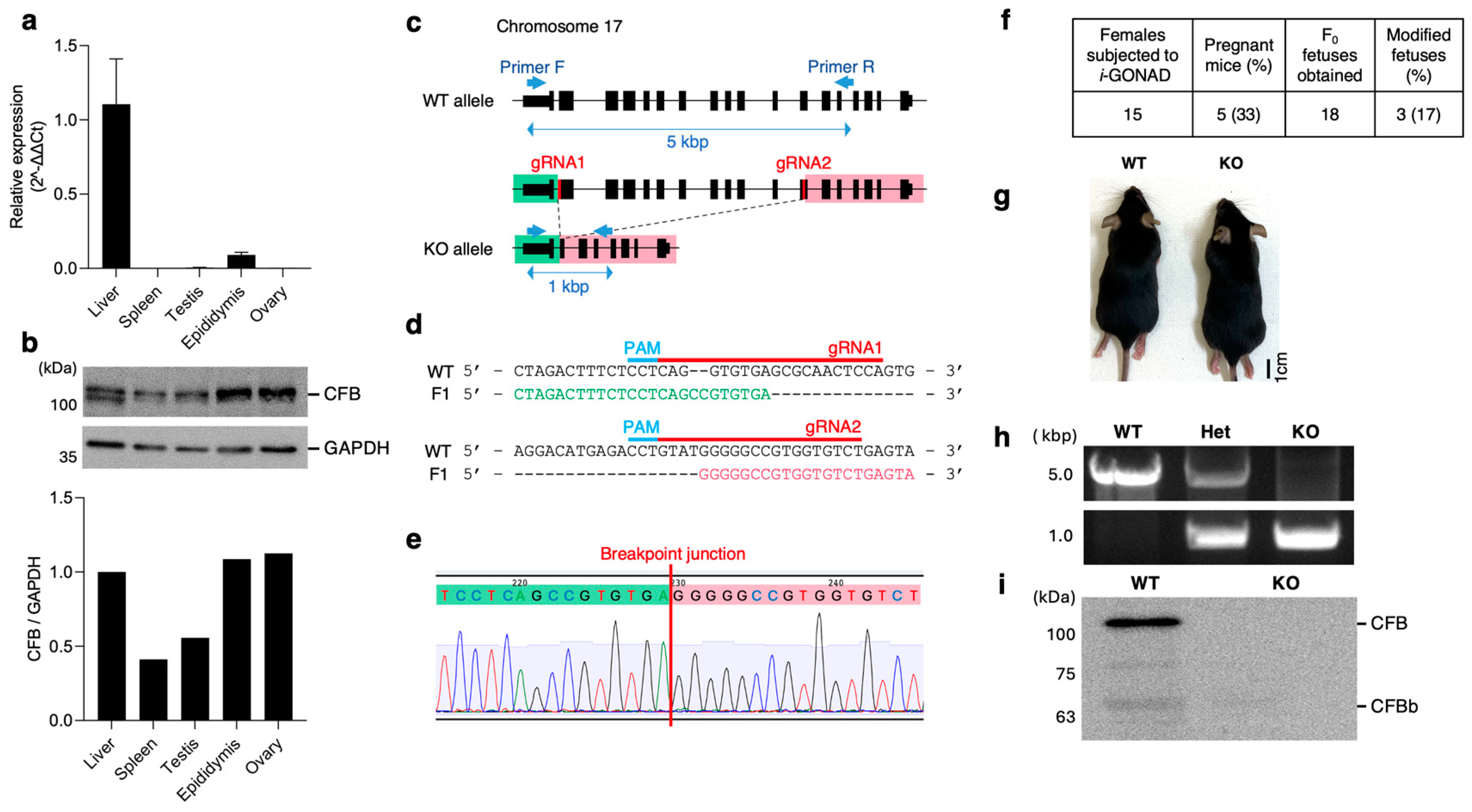
2.1. Cfb Expression in Reproductive Tissues
2.2. Generation of KO Mice
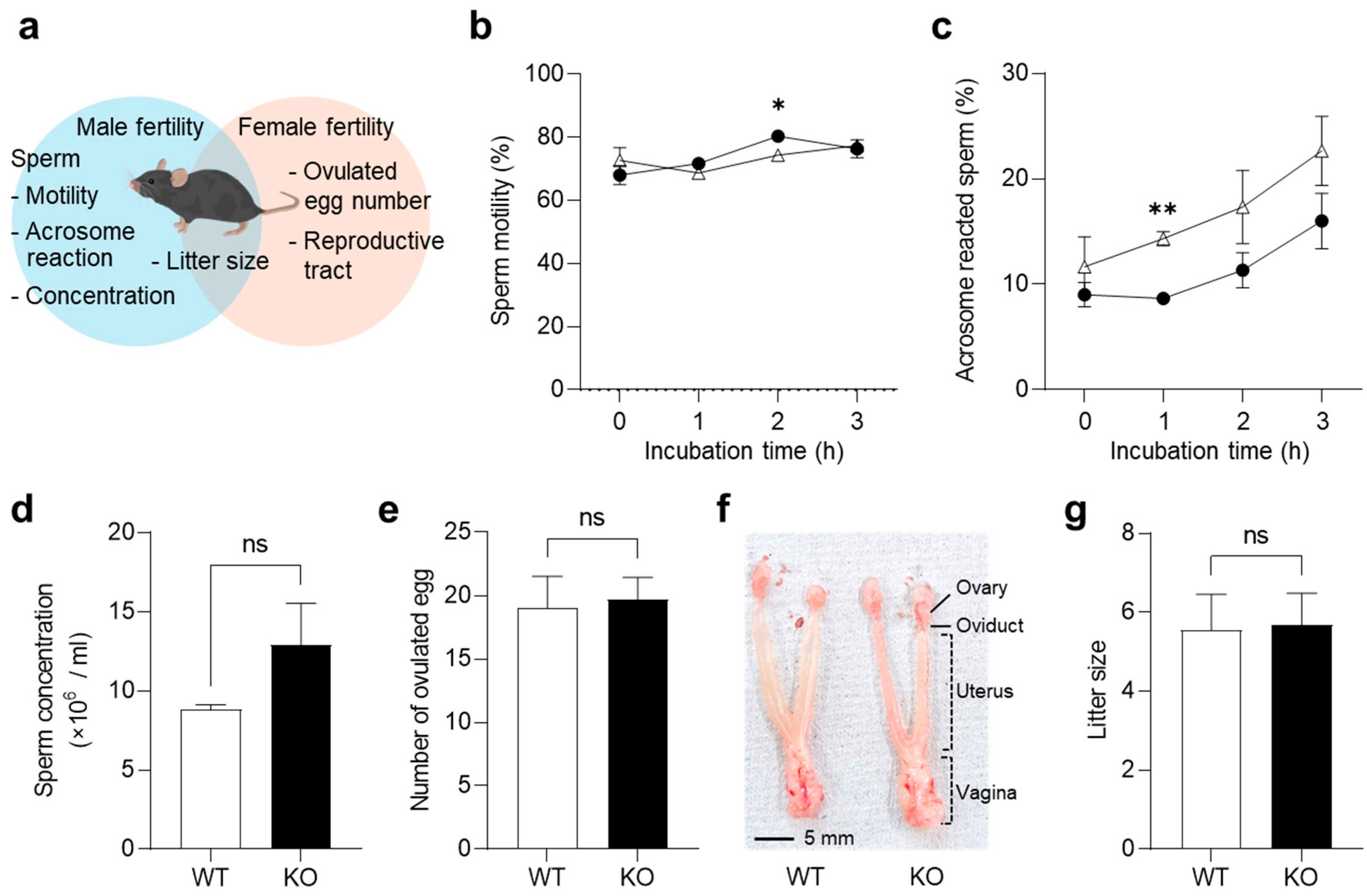
2.3. Fertility of KO Mice
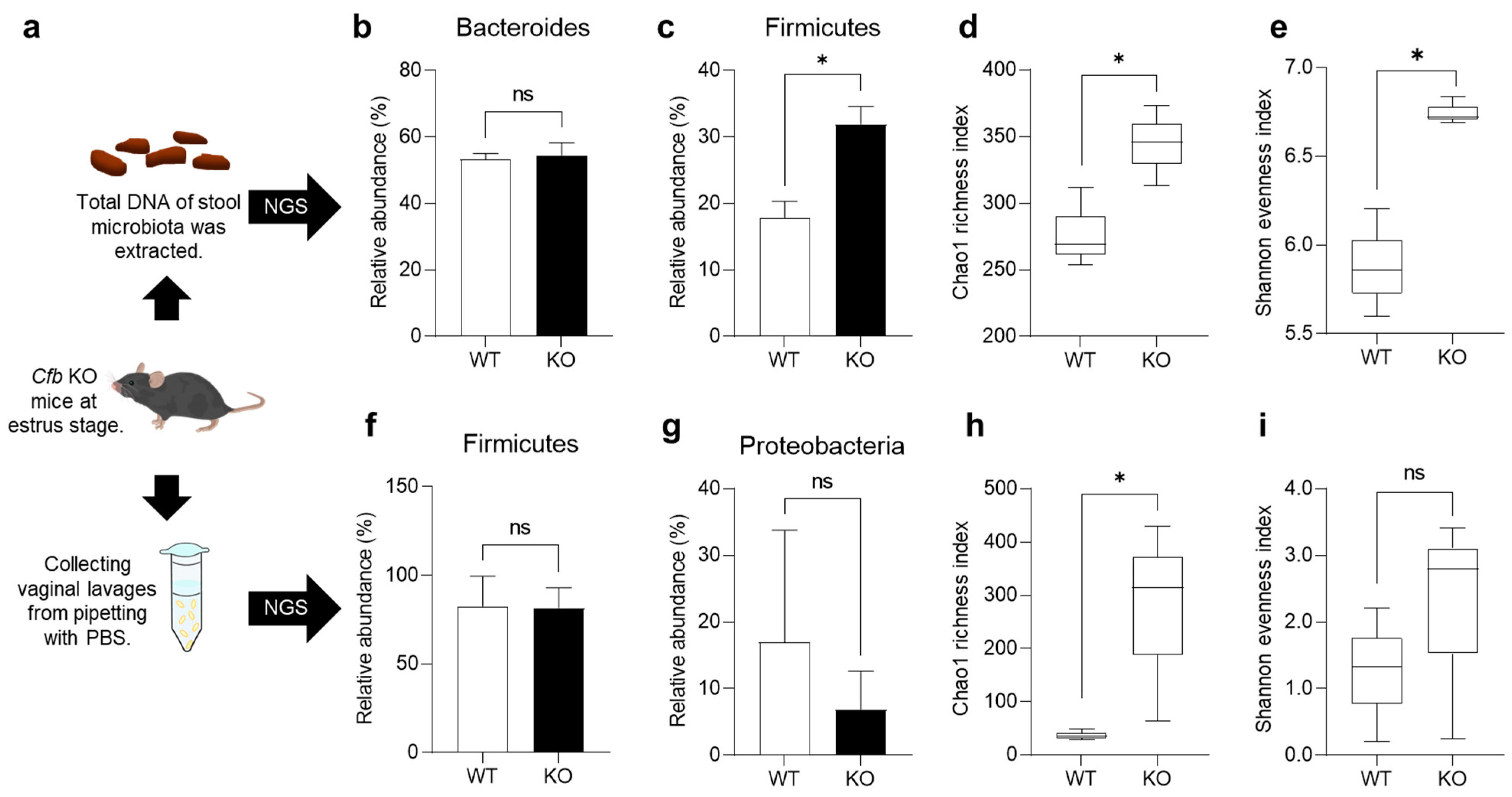
2.4. Gut and Vaginal Microbiome of KO Mice
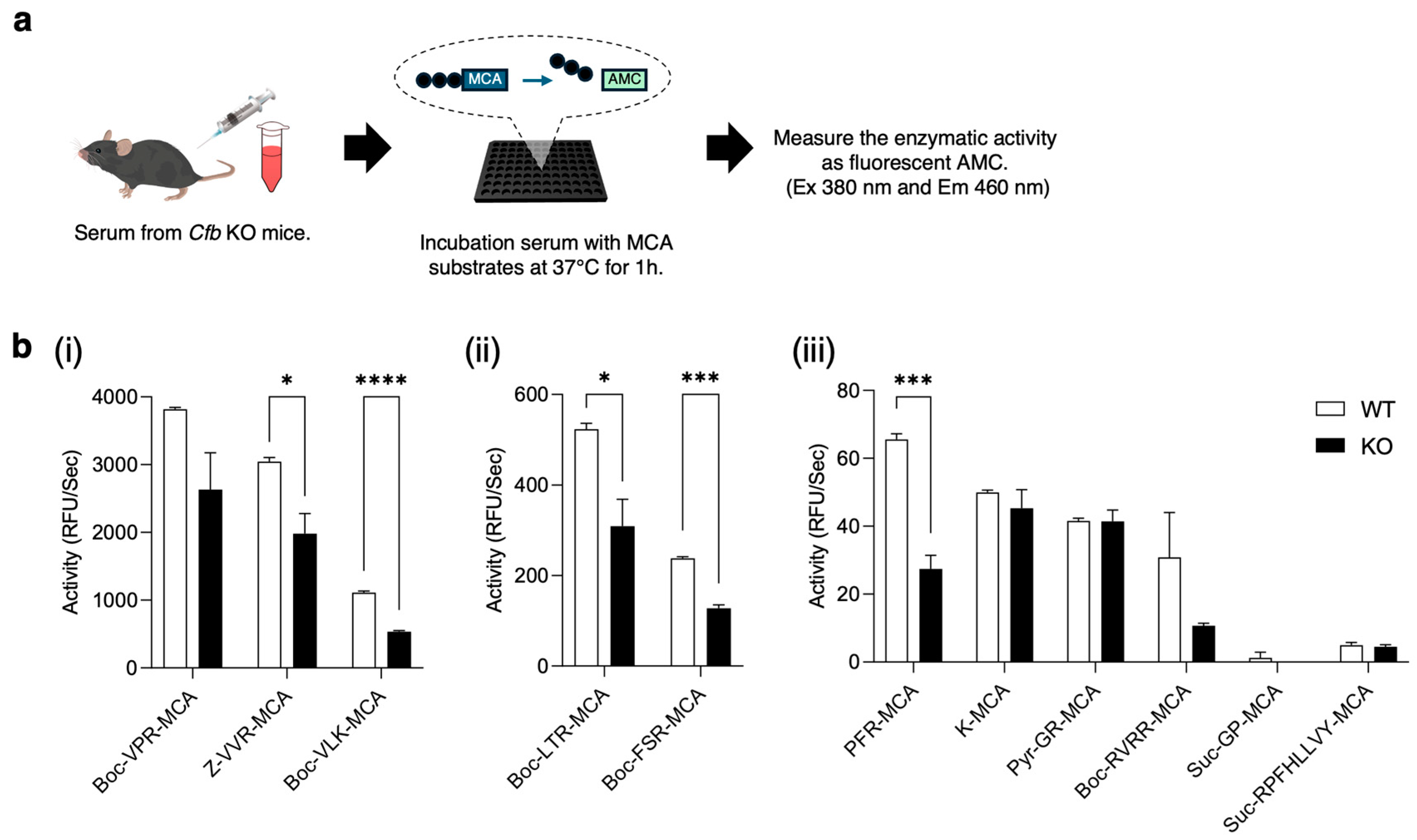
2.5. Proteolytic Activity in the Serum from KO Mice
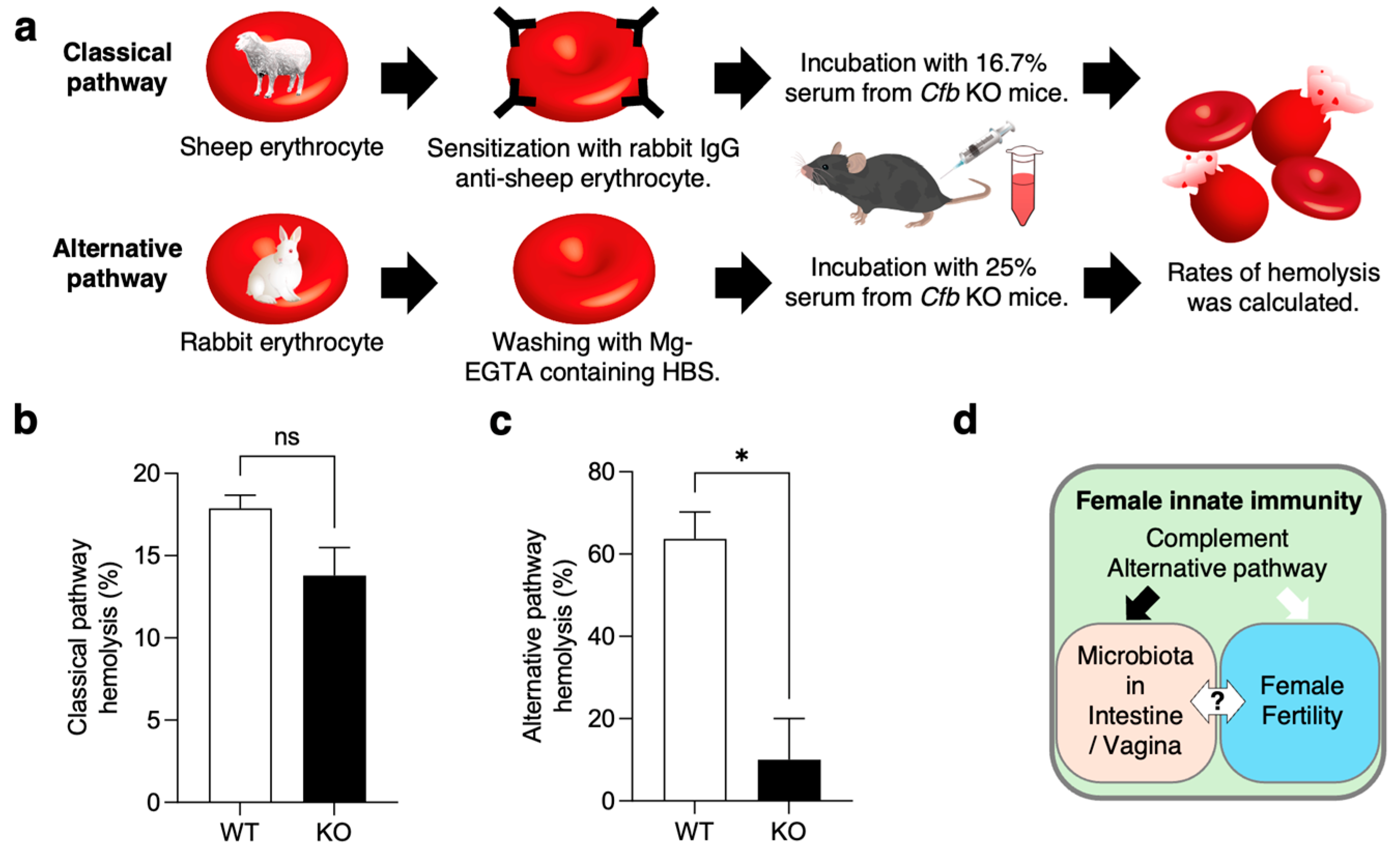
2.6. Hemolytic Assay of the Serum from KO Mice
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. qPCR
4.3. Immunoblotting
4.4. Preparation of Reagent Used for i-GONAD
4.5. i-GONAD
4.6. Genotyping and Sequencing
4.7. Mouse Fertility In Vivo and In Vitro
4.8. Analysis of Gut and Vaginal Microbiota
4.9. Enzyme Assay
4.10. Hemolytic Assay
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elvington, M.; Liszewski, M.K.; Atkinson, J.P. Evolution of the complement system: From defense of the single cell to guardian of the intravascular space. Immunol. Rev. 2016, 274, 9–15. [Google Scholar] [CrossRef]
- Galvan, M.D.; Luchetti, S.; Burgos, A.M.; Nguyen, H.X.; Hooshmand, M.J.; Hamers, F.P.T.; Anderson, A.J. Deficiency in complement C1q improves histological and functional locomotor outcome after spinal cord injury. J. Neurosci. 2008, 28, 13876–13888. [Google Scholar] [CrossRef]
- Shi, L.; Takahashi, K.; Dundee, J.; Shahroor-Karni, S.; Thiel, S.; Jensenius, J.C.; Gad, F.; Hamblin, M.R.; Sastry, K.N.; Ezekowitz, R.A.B. Mannose-binding lectin-deficient mice are susceptible to infection with Staphylococcus aureus. J. Exp. Med. 2004, 199, 1379–1390. [Google Scholar] [CrossRef] [PubMed]
- Pangburn, M.K.; Schreiber, R.D.; Müller-Eberhard, H.J. Formation of the initial C3 convertase of the alternative complement pathway. Acquisition of C3b-like activities by spontaneous hydrolysis of the putative thioester in native C3. J. Exp. Med. 1981, 154, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Pangburn, M.K.; Müller-Eberhard, H.J. Initiation of the alternative complement pathway due to spontaneous hydrolysis of the thioester of C3. Ann. N. Y. Acad. Sci. 1983, 421, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Nilsson Ekdahl, K.; Nilsson, B.; Pekna, M.; Nilsson, U.R. Generation of iC3 at the interface between blood and gas. Scand. J. Immunol. 1992, 35, 85–91. [Google Scholar] [CrossRef]
- Pekna, M.; Nilsson, L.; Nilsson-Ekdahl, K.; Nilsson, U.R.; Nilsson, B. Evidence for iC3 generation during cardiopulmonary bypass as the result of blood-gas interaction. Clin. Exp. Immunol. 1993, 91, 404–409. [Google Scholar] [CrossRef]
- Ochiel, D.O.; Fahey, J.V.; Ghosh, M.; Haddad, S.N.; Wira, C.R. Innate immunity in the female reproductive tract: Role of sex hormones in regulating uterine epithelial cell protection against pathogens. Curr. Women’s Health Rev. 2008, 4, 102–117. [Google Scholar] [CrossRef]
- Chen, C.; Song, X.; Wei, W.; Zhong, H.; Dai, J.; Lan, Z.; Li, F.; Yu, X.; Feng, Q.; Wang, Z.; et al. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat. Commun. 2017, 8, 875. [Google Scholar] [CrossRef]
- Chadchan, S.B.; Singh, V.; Kommagani, R. Female reproductive dysfunctions and the gut microbiota. J. Mol. Endocrinol. 2022, 69, R81–R94. [Google Scholar] [CrossRef]
- Wu, M.; Zheng, W.; Song, X.; Bao, B.; Wang, Y.; Ramanan, D.; Yang, D.; Liu, R.; Macbeth, J.C.; Do, E.A.; et al. Gut complement induced by the microbiota combats pathogens and spares commensals. Cell 2024, 187, 897–913.e18. [Google Scholar] [CrossRef]
- Atkinson, J.P.; Farries, T. Separation of self from non-self in the complement system. Immunol. Today 1987, 8, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Bulla, R.; Bossi, F.; Agostinis, C.; Radillo, O.; Colombo, F.; De Seta, F.; Tedesco, F. Complement production by trophoblast cells at the feto-maternal interface. J. Reprod. Immunol. 2009, 82, 119–125. [Google Scholar] [CrossRef]
- Gelber, S.E.; Brent, E.; Redecha, P.; Perino, G.; Tomlinson, S.; Davisson, R.L.; Salmon, J.E. Prevention of defective placentation and pregnancy loss by blocking innate immune pathways in a syngeneic model of placental insufficiency. J. Immunol. 2015, 195, 1129–1138. [Google Scholar] [CrossRef]
- Pierik, E.; Prins, J.R.; van Goor, H.; Dekker, G.A.; Daha, M.R.; Seelen, M.A.J.; Scherjon, S.A. Dysregulation of complement activation and placental dysfunction: A potential target to treat preeclampsia? Front. Immunol. 2019, 10, 3098. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, A.; Nagaishi, S.; Kondo, T.; Okada, T.; Kusakabe, K.T. Regulation of complement activity via the alternative pathway in placentas of mouse spontaneous abortions. J. Vet. Med. Sci. 2010, 72, 1375–1377. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Matsumoto, M.; Fukuda, W.; Circolo, A.; Goellner, J.; Strauss-Schoenberger, J.; Wang, X.; Fujita, S.; Hidvegi, T.; Chaplin, D.D.; Colten, H.R. Abrogation of the alternative complement pathway by targeted deletion of murine factor B. Proc. Natl. Acad. Sci. USA 1997, 94, 8720–8725. [Google Scholar] [CrossRef]
- Ohtsuka, M.; Sato, M.; Miura, H.; Takabayashi, S.; Matsuyama, M.; Koyano, T.; Arifin, N.; Nakamura, S.; Wada, K.; Gurumurthy, C.B. i-GONAD: A robust method for in situ germline genome engineering using CRISPR nucleases. Genome Biol. 2018, 19, 25. [Google Scholar] [CrossRef]
- Hasegawa, M.; Yada, S.; Liu, M.Z.; Kamada, N.; Muñoz-Planillo, R.; Do, N.; Núñez, G.; Inohara, N. Interleukin-22 regulates the complement system to promote resistance against pathobionts after pathogen-induced intestinal damage. Immunity 2014, 41, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Bassis, C.M.; Kennedy, E.; Yoest, K.E.; Becker, J.B.; Bell, J.; Berger, M.B.; Bruns, T.M. The rodent vaginal microbiome across the estrous cycle and the effect of genital nerve electrical stimulation. PLoS ONE 2020, 15, e0230170. [Google Scholar] [CrossRef]
- Matějková, T.; Dodoková, A.; Kreisinger, J.; Stopka, P.; Stopková, R. Microbial, proteomic, and metabolomic profiling of the estrous cycle in wild house mice. Microbiol. Spectr. 2024, 12, e0203723. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Kim, J.E.; Lee, S.J.; Gong, J.E.; Son, H.J.; Hong, J.T.; Hwang, D.Y. Dysbiosis of fecal microbiota from Complement 3 knockout mice with constipation phenotypes contributes to development of defecation delay. Front. Physiol. 2021, 12, 650789. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.P.; Chen, W.C.; Cheng, C.M.; Shen, C.J. Vaginal pH value for clinical diagnosis and treatment of common vaginitis. Diagnostics 2021, 11, 1996. [Google Scholar] [CrossRef] [PubMed]
- Mejia, M.E.; Mercado-Evans, V.; Zulk, J.J.; Ottinger, S.; Ruiz, K.; Ballard, M.B.; Fowler, S.W.; Britton, R.A.; Patras, K.A. Vaginal microbial dynamics and pathogen colonization in a humanized microbiota mouse model. NPJ Biofilms Microbiomes 2023, 9, 87. [Google Scholar] [CrossRef] [PubMed]
- Vrbanac, A.; Riestra, A.M.; Coady, A.; Knight, R.; Nizet, V.; Patras, K.A. The murine vaginal microbiota and its perturbation by the human pathogen group B Streptococcus. BMC Microbiol. 2018, 18, 197. [Google Scholar] [CrossRef]
- Miao, J.; Willems, H.M.E.; Peters, B.M. Exogenous reproductive hormones nor Candida albicans colonization alter the near neutral mouse vaginal pH. Infect. Immun. 2021, 89, e00550-20. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.; Bennett, P.R.; Lee, Y.S.; Kundu, S.; Teoh, T.G.; Adan, M.; Ahmed, S.; Brown, R.G.; David, A.L.; Lewis, H.V.; et al. Microbial-driven preterm labour involves crosstalk between the innate and adaptive immune response. Nat. Commun. 2022, 13, 975. [Google Scholar] [CrossRef] [PubMed]
- Livson, S.; Virtanen, S.; Lokki, A.I.; Holster, T.; Rahkonen, L.; Kalliala, I.; Nieminen, P.; Salonen, A.; Meri, S. Cervicovaginal complement activation and microbiota during pregnancy and in parturition. Front. Immunol. 2022, 13, 925630. [Google Scholar] [CrossRef]
- Morgan, B.P.; Gasque, P. Extrahepatic complement biosynthesis: Where, when and why? Clin. Exp. Immunol. 1997, 107, 1–7. [Google Scholar] [CrossRef]
- Grossman, T.R.; Hettrick, L.A.; Johnson, R.B.; Hung, G.; Peralta, R.; Watt, A.; Henry, S.P.; Adamson, P.; Monia, B.P.; McCaleb, M.L. Inhibition of the alternative complement pathway by antisense oligonucleotides targeting complement factor B improves lupus nephritis in mice. Immunobiology 2016, 221, 701–708. [Google Scholar] [CrossRef]
- Li, D.; Zou, L.; Feng, Y.; Xu, G.; Gong, Y.; Zhao, G.; Ouyang, W.; Thurman, J.M.; Chao, W. Complement factor B production in renal tubular cells and its role in sodium transporter expression during polymicrobial sepsis. Crit. Care Med. 2016, 44, e289–e299. [Google Scholar] [CrossRef]
- Lu, Q.; Hou, Q.; Cao, K.; Sun, X.; Liang, Y.; Gu, M.; Xue, X.; Zhao, A.Z.; Dai, C. Complement factor B in high glucose-induced podocyte injury and diabetic kidney disease. JCI Insight 2021, 6, e147716. [Google Scholar] [CrossRef] [PubMed]
- Coan, P.M.; Barrier, M.; Alfazema, N.; Carter, R.N.; Marion de Procé, S.; Dopico, X.C.; Garcia Diaz, A.; Thomson, A.; Jackson-Jones, L.H.; Moyon, B.; et al. Complement factor B is a determinant of both metabolic and cardiovascular features of metabolic syndrome. Hypertension 2017, 70, 624–633. [Google Scholar] [CrossRef]
- He, C.; Wang, X.; Jiang, B.; Zhu, M.; Zhang, H.; Duan, Y.; Li, Y. Complement factor B (CFB) inhibits the malignant progression of lung adenocarcinoma by downregulating the Ras/MAPK signaling pathway. Arch. Biochem. Biophys. 2024, 760, 110130. [Google Scholar] [CrossRef]
- Taskintuna, K.; Bhat, M.A.; Shaikh, T.; Hum, J.; Golestaneh, N. Sex-dependent regulation of retinal pigment epithelium and retinal function by Pgc-1α. Front. Cell. Neurosci. 2024, 18, 1442079. [Google Scholar] [CrossRef] [PubMed]
- Schnabolk, G.; Coughlin, B.; Joseph, K.; Kunchithapautham, K.; Bandyopadhyay, M.; O’Quinn, E.C.; Nowling, T.; Rohrer, B. Local production of the alternative pathway component factor B is sufficient to promote laser-induced choroidal neovascularization. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1850–1863. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.N.; Barth, J.L.; Jafri, S.; Rumschlag, J.A.; Jenkins, T.R.; Atkinson, C.; Lang, H. Complement factor B is essential for the proper function of the peripheral auditory system. Front. Neurol. 2023, 14, 1214408. [Google Scholar] [CrossRef]
- Palta, S.; Saroa, R.; Palta, A. Overview of the coagulation system. Indian J. Anaesth. 2014, 58, 515–523. [Google Scholar] [CrossRef]
- Amara, U.; Flierl, M.A.; Rittirsch, D.; Klos, A.; Chen, H.; Acker, B.; Brückner, U.B.; Nilsson, B.; Gebhard, F.; Lambris, J.D.; et al. Molecular intercommunication between the complement and coagulation systems. J. Immunol. 2010, 185, 5628–5636. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.; Weymann, A.; Hartman, E.; Turmelle, Y.; Carroll, M.; Thurman, J.M.; Holers, V.M.; Hourcade, D.E.; Rudnick, D.A. Evidence for non-traditional activation of complement factor C3 during murine liver regeneration. Mol. Immunol. 2008, 45, 3125–3132. [Google Scholar] [CrossRef]
- Irmscher, S.; Döring, N.; Halder, L.D.; Jo, E.A.H.; Kopka, I.; Dunker, C.; Jacobsen, I.D.; Luo, S.; Slevogt, H.; Lorkowski, S.; et al. Kallikrein cleaves C3 and activates complement. J. Innate Immun. 2018, 10, 94–105. [Google Scholar] [CrossRef] [PubMed]
- de Boer, E.C.; Thielen, A.J.; Langereis, J.D.; Kamp, A.; Brouwer, M.C.; Oskam, N.; Jongsma, M.L.; Baral, A.J.; Spaapen, R.M.; Zeerleder, S.; et al. The contribution of the alternative pathway in complement activation on cell surfaces depends on the strength of classical pathway initiation. Clin. Transl. Immunol. 2023, 12, e1436. [Google Scholar] [CrossRef] [PubMed]
- Kawano, N.; Yoshida, M. Semen-coagulating protein, SVS2, in mouse seminal plasma controls sperm fertility. Biol. Reprod. 2007, 76, 353–361. [Google Scholar] [CrossRef]
- Barrett, A.J. Fluorimetric assays for cathepsin B and cathepsin H with methylcoumarylamide substrates. Biochem. J. 1980, 187, 909–912. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sunamoto, M.; Morohoshi, K.; Sato, B.; Mihashi, R.; Inui, M.; Yamada, M.; Miyado, K.; Kawano, N. Complement Factor B Deficiency Is Dispensable for Female Fertility but Affects Microbiome Diversity and Complement Activity. Int. J. Mol. Sci. 2025, 26, 1393. https://doi.org/10.3390/ijms26031393
Sunamoto M, Morohoshi K, Sato B, Mihashi R, Inui M, Yamada M, Miyado K, Kawano N. Complement Factor B Deficiency Is Dispensable for Female Fertility but Affects Microbiome Diversity and Complement Activity. International Journal of Molecular Sciences. 2025; 26(3):1393. https://doi.org/10.3390/ijms26031393
Chicago/Turabian StyleSunamoto, Manato, Kazunori Morohoshi, Ban Sato, Ryo Mihashi, Masafumi Inui, Mitsutoshi Yamada, Kenji Miyado, and Natsuko Kawano. 2025. "Complement Factor B Deficiency Is Dispensable for Female Fertility but Affects Microbiome Diversity and Complement Activity" International Journal of Molecular Sciences 26, no. 3: 1393. https://doi.org/10.3390/ijms26031393
APA StyleSunamoto, M., Morohoshi, K., Sato, B., Mihashi, R., Inui, M., Yamada, M., Miyado, K., & Kawano, N. (2025). Complement Factor B Deficiency Is Dispensable for Female Fertility but Affects Microbiome Diversity and Complement Activity. International Journal of Molecular Sciences, 26(3), 1393. https://doi.org/10.3390/ijms26031393




