Abstract
Male factors may be present in up to 50–70% of infertile couples and the prevalence of male infertility accounts for 20–30% of infertility cases. Understanding the mechanisms and causes behind male infertility remains a challenge, but new diagnostic tools such as DNA fragmentation might aid in cases where the routine semen analysis is insufficient. DNA fragmentation, which refers to damages or breaks of the genetic material of the spermatozoa, is considered one of the main causes of male infertility due to impaired functional capability of sperm. The aim of the present narrative review is to investigate and enlighten the potential correlation between DNA fragmentation and male infertility parameters such as the seminal profile and the reproductive outcomes. Comprehensive research in PubMed/Medline and Scopus databases was conducted and 28 studies were included in the present review. Fourteen studies provided data regarding the impact of DNA fragmentation and seminal parameters and showed a correlation of significantly lower sperm count, lower concentration, motility, and abnormal morphology with an increased DNA fragmentation index (DFI). Similarly, 15 studies provided data regarding the impact of DFI on reproductive outcomes. Two studies showed higher aneuploidy rates with higher DFI values, and seven studies showed significantly lower pregnancy rates and live birth rates with higher DFI values. Ultimately, the studies included in this review highlight, collectively, the importance of measuring sperm DFI in the assessment of male infertility. Further studies are needed to explore the effectiveness of interventions aiming to reduce DFI levels.
1. Introduction
Infertility is defined as the inability to conceive after one year of regular unprotected sexual intercourse [1]. It is estimated that 10–15% of couples and around 50–80 million couples worldwide are affected. While about half of infertility cases are attributed to female factors, male factors contribute to 20–30%, and another 20–30% result from common causes affecting both partners [2,3]. Recent data suggest that male factors may be present in up to 50–70% of infertility cases, although this wide range may not reflect global prevalence due to different data collection methods and cultural influences [4,5].
Understanding the mechanisms behind male infertility remains a significant challenge [6]. Currently, semen analysis is still a laboratory technique considered the gold standard for attempting to identify and evaluate male infertility [7]. This technique is based on the principle that infertility can often be triggered by several factors that alter seminal parameters, including sperm motility, morphology, liquefaction time, seminal volume, sperm concentration, and sperm motility [8]. In 2021, the World Health Organization (WHO) revised the published guidance and referred to seminal fluid parameters as “useful parameters”, indicating the imperfect association between the parameters and actual male infertility [9]. The latter led to other semen evaluating tools to explore further the field of unexplained male infertility. These tests include the evaluation of anti-sperm antibodies, sperm hyperactivation, acrosomal reaction, penetration in the zona pellucida, and sperm DNA fragmentation [10].
DNA fragmentation refers to damages or breaks of the genetic material of the spermatozoa, and as mentioned before, routine semen analysis is unable to estimate it. These damages can be caused by either intrinsic factors such as increased oxidative stress and defective maturation or external factors such as previous chemotherapy, smoking, higher temperature in the scrotum, and endocrine-disrupting compounds [11,12]. DNA fragmentation is considered one of the main causes of male infertility due to impaired functional capability of sperm [13]. Its negative effect on fertility also extends to assisted reproduction because spermatozoa with impaired DNA are able to fertilize an oocyte and, consequently, the limited repair mechanisms in the embryo [14]. Hence, high DNA fragmentation has been associated with negative reproductive outcomes and failure to reach the blastocyst stage in embryos created either in vivo or in vitro [15,16]. However, regardless of the increasing literature on the impact of sperm DNA fragmentation measured by the DNA fragmentation index (DFI), major societies such as the European Society of Human Reproduction and Embryology (ESHRE), in their recent guidance, either oppose DFI testing or consider it in the context of research [17].
The aim of the present narrative review is to investigate and enlighten the potential correlation between DNA fragmentation and male infertility parameters such as the seminal profile and the reproductive outcomes.
2. Literature Research
Comprehensive literature research was conducted across two major databases: Pubmed/Medline (2013–2024) and Scopus (2013–2024). Our research was limited to the last decade due to the implementation of newer techniques in measuring the DNA fragmentation index. The search terms used included “male”, “infertility”, “DNA Fragmentation”, “DNA Fragmentation Index”, “DFI”, and “Assisted Reproduction” with the administration of Boolean operators (OR, AND) combined with those keywords either used as presented, separately, or in combination. Two authors (AP and EM) conducted the literature search and abstract selection independently. The content of full-text publications that were eligible was further assessed. A third reviewer, S.S., was responsible for making the final decision on a study if the study was selected by only one reviewer. Additionally, the “snowball literature searching method” was applied to identify further relevant sources from the reference lists of selected articles.
All original articles on research conducted in the last decade in humans and written in English, with the main subject of the investigation being the role of sperm DNA fragmentation in male fertility, were included in this review. Similarly, secondary studies, such as reviews, systematic reviews and meta-analyses, articles written in another language than English, studies conducted in animal models, and studies referring to the effect of specific treatments on DFI, were excluded.
Regarding the quality assessment and the risk of bias assessment of the included studies, a critical evaluation of each study’s sample (size of the sample), methodology (study design and compared groups), outcome presentation (clarity and relevance of reporting outcomes), and confounding factors (potential biases and different methods of estimating DNA fragmentation) was performed. This critical evaluation helped with the interpretation of each study’s outcomes and the better presentation of our results and discussion on the effect of DNA fragmentation on male infertility. A formal risk of bias and quality assessment was not performed due to the narrative nature of this review.
From the initial research, 679 articles were collected via PubMed/Medline and Scopus databases. A total of 472 were screened by title and abstract and 56 underwent full-text assessment. Ultimately, 28 articles were suitable for providing information in this literature review. The study selection process is depicted in Figure 1.
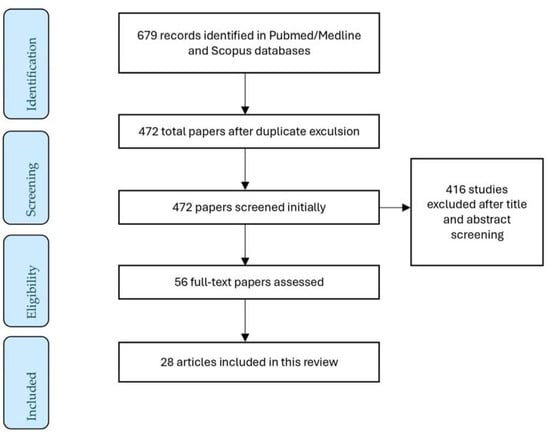
Figure 1.
A flow diagram of the study selection process.
3. Impact of DFI on Seminal Parameters
Regarding the impact of sperm DNA fragmentation on seminal parameters, 14 studies have been identified. Two studies demonstrated no significant correlation between DNA fragmentation index values and conventional seminal parameters [18,19]. The remaining 12 studies showed at least one negatively affected seminal parameter. More specifically, six studies demonstrated a significant correlation between higher values of DNA fragmentation and lower values of lower seminal volume or concentration [20,21,22,23,24,25]. Zhang et al. showed significantly lower sperm concentration values, even for the study group with a DFI between 20% and 30% [24]. The same negative effect of DNA fragmentation has also been indicated by Green et al. at the threshold of 15% [23], showing that concentration and volume are among the first parameters to be affected, even at low DFI values.
Regarding motility, six studies showed a statistically significant association of higher DFI values with lower motility [21,22,23,26,27,28]. Lastly, six studies showed a significant association between DNA fragmentation and sperm morphology [20,25,27,29,30,31]. It is worth noting that teratozoospermia was the most statistically significant finding in the majority of the studies, with p values of 0.001. The sample and the main outcome of each included study are presented in Table 1.

Table 1.
Included studies regarding association of DNA fragmentation and seminal parameters.
4. Impact of DNA Fragmentation on Reproductive Outcomes
Regarding the impact of sperm DNA fragmentation on embryo kinetics, quality, and assisted reproduction outcomes, 15 studies have been identified. Five studies provide data regarding embryo kinetics and euploid status [32,33,34,35,36]. Only one study by Sun et al. showed no significant correlation between the DFI values and embryo aneuploidy [34]. It is worth mentioning that the cut-off selected by the authors was a DFI over and under 30% for group allocation. One study by Wdowiak et al. showed that lower DNA fragmentation was associated with faster embryo development to the blastocyst stage [36], and one study demonstrated that DFI values were inversely correlated with blastocyst viability status [35]. Lastly, two studies showed statistically significant higher aneuploidy rates with higher DNA fragmentation index values [32,33].
Five studies report data on fertilization rate as an outcome [32,34,37,38,39]. Interestingly, three of them report no significant difference in the fertilization rate among high- and low-DFI groups [34,37,38], and two studies report significantly lower fertilization rates [32,39]. It is worth mentioning that from the latter two studies, the study by Wang et al. demonstrated significantly lower fertilization rates only in the IVF comparison group and not in the ICSI group [32].
On the key clinical outcomes of pregnancy rate and live birth rate, our study included data from ten studies [10,32,34,37,39,40,41,42,43,44]. Three studies report no significant differences regarding the pregnancy rate [34,37,43]. However, in the study by Omrani et al., there was no reported pregnancy in the high-DFI group, and the result of no correlation between the DFI and pregnancy rate was based on the comparison of the control group with the moderate-DFI group [43]. The remaining seven studies report significantly higher pregnancy and live birth rate results with lower DFI values [10,32,39,40,41,42,44]. In the three studies with subgroups undergoing IVF and ICSI, a higher pregnancy rate was observed in the ICSI group for the same DFI values [41,42,44]. The sample and the main outcome of each included study in this section are presented in Table 2.

Table 2.
Included studies regarding impact of DNA fragmentation on embryo kinetics, quality, and assisted reproduction outcomes.
5. Discussion
The studies examined in the section on the impact of the DNA fragmentation index (DFI) on male infertility and sperm quality underscore the significance of sperm DNA fragmentation as a critical factor in male infertility. They also confirm the relationship between the DFI and the reduced likelihood of successful conception, higher rates of miscarriage, and embryo development issues. Furthermore, they reveal that advanced age and human habits such as smoking and alcohol consumption are associated with higher DFI levels [20]. These findings represent significant strides toward understanding male infertility and developing new approaches for diagnosing and treating fertility issues. Recent studies examined the genetic and environmental influences on the DFI and their impact on male infertility [45,46]. Their findings underscore the importance of identifying genetic factors affecting the DFI and their interaction with environmental factors such as age and dietary habits. Additionally, they found that genetic mutations can contribute to increased sperm DNA fragmentation, thereby affecting male fertility. Ogawa et al. demonstrated that a balanced diet containing micronutrient antioxidants can reduce oxidative stress and the DFI and consequently improve sperm function and the outcomes of in vitro fertilization (IVF)/Intracytoplasmic Sperm Injection (ICSI)–Embryo Transfer (ET) cycles [47].
Combining the findings from the studies included in this review, several conclusions can be drawn about the effect of the sperm DNA fragmentation index on embryo development, pregnancy outcomes, and assisted reproduction technology outcomes. Regarding the dynamics of embryonic development, it is found that lower levels of the DFI in sperm were associated with faster fetal morphokinetic parameters after ICSI. More specifically, the low-DFI group reached the blastocyst stage faster, and the study results suggest that lowering the DFI may positively affect embryonic development and may be predictive of pregnancy outcomes [36]. As far as implantation and pregnancy rates are concerned, high DFI levels negatively impact pregnancy rates after the implementation of assisted reproduction techniques. In the cohort study by Zhang et al., higher values of the DFI were associated with statistically significant lower pregnancy rates after IVF and ICSI cycles [44]. Similarly, in another study, the use of semen with lower DFI levels in ICSI cycles resulted in higher clinical pregnancy rates, lower miscarriage rates, and higher live birth rates compared to high-DFI semen, and therefore, the selection of low-DFI sperm results in better assisted reproductive technology (ART) outcomes [10].
Spermatogenesis is related to different cellular procedures in which a high number of genes are involved [48,49,50]. Different studies in humans revealed that genetic variants are closely related to spermatogenesis disorders. Such variants may be detected in endocrine-related genes (Gonadotropin-Releasing Hormone—GnRH, Follicle-Stimulating Hormone—FSH, Luteinizing Hormone—LH, Follicle-Stimulating Hormone Receptor—FSHR, Luteinizing Hormone Receptor—LHR), gonadal development-related genes (Azoospermia Factors—AZF, Wilms tumor gene 1—WT1, PR/SET domain 1—PRDM1, Stoltzfus blood group—SF), and meiosis-related genes (mutL homolog 1—MLH1, interferon regulatory factor 1—IRF1, PR/SET domain 9—PRDM9, SPO11 initiator of meiotic double strand breaks—SPO11), suggesting a possible interaction between reproductive-related genes and the DFI [51,52,53,54,55]. Additionally, it has been shown from numerous experiments that the expression of MicroRNAs (miRNAs) plays an indispensable role in spermatogenesis, affecting male infertility and the DFI [56,57,58]. Moreover, it was reported that interferon regulatory factor 1 (IRF1), a member of the interferon regulatory factor (IRF) family, is directly targeted by miR-383, which, by regulating interferon in cell apoptosis and the cell cycle, is involved in testicular spermatogenesis and the DFI [59]. In another study, the role of miRNAs in infertile males has been explored in different groups of fertile and infertile men, and the results indicate that the expression of miR-34c in the moderate oligoasthenoteratozoospermic and non-obstructive azoospermia groups was significantly elevated, correlating the aforementioned miRNA with the DFI [60]. Last but not least is the role of protamines, arginine-rich nuclear proteins that play a crucial role in the compaction of DNA, particularly in sperm cells during spermatogenesis. Protamines replace histones during the final stages of spermatogenesis, enabling a more condensed and stable chromatin structure that protects the genetic material from damage [61]. However, insufficient protamination or improper protamine−DNA binding can lead to incomplete chromatin condensation, making the DNA more vulnerable to oxidative stress and can result in increased DNA fragmentation [62,63,64].
Furthermore, regarding the correlation of the DFI with sperm parameters and quality, there is a clear negative correlation. Higher DFI levels were associated with lower motility [28]. Moreover, there are data that suggest a connection between body mass index (BMI) and sperm quality. A higher BMI has been associated with lower sperm count and motility by several studies. The fertilization rate is also declining with higher body mass index [65]. Abnormal sperm morphology has been also linked with obesity, leading to reduced semen ability to penetrate the ovum [66,67]. Oxidative stress is associated with DNA fragmentation and sperm cell damage [65]. Lastly, reduced progressive motility is also associated with higher DFI values and lower sperm mitochondrial concentration [68]. The same negative impact of increased DFI levels has also been observed in infertile males with increased static redox potential (sORP). More specifically, higher levels of sORP have a positive correlation with immotility percentage and a negative correlation with total motility [69].
A major limitation in the extrapolation of the results included and the universal utilization of standardized DFI values is the wide variety of techniques used for estimating the DFI. According to the WHO, the laboratory techniques for measuring the DFI are as follows: the TUNEL assay (terminal deoxynucleotidyl transferase (dUTP), nick end labeling, the single-cell gel electrophoresis assay (Comet Assay), the SCD assay (sperm chromatin dispersion test), and SCSA (acridine orange flow cytometry). The TUNEL assay involves the incorporation of biotinylated dUTP at 3′ ends of DNA strand breaks and may detect single- and double-strand breaks; the labeled bases can be quantified by using a fluorescent microscope or flow cytometry [70]. TUNEL is a sensitive and reliable technique, with minimal inter-observer variability, but requires expensive equipment and personnel training [71]. Consistent with the results of Agarwal et al., the TUNEL assay seems to be the most commonly utilized [72]. The Comet assay is an electrophoretic technique using the principle that smaller fragmented DNA will migrate faster than intact DNA [73]. The Comet assay is very sensitive, as it may be performed in very low sperm counts. It is also reproducible but demands an experienced observer [74]. The SCD assay is an indirect method for measuring SDF, as it depends on the susceptibility of chromatin to denaturation after the application of an acid treatment [75]. It is a simple assay which does not require the use of fluorescence and can evaluate subpopulations of degraded spermatozoa [71], but it has been criticized for high inter-observer variability [74]. Lastly, SCSA is a well-described and commonly utilized test. This technique uses acridine orange staining, which by binding to denatured or intact DNA will generate different fluorescence signals. The analysis is made using a flow cytometer, allowing for the simultaneous examination of a large number of cells. It is considered the most statistically robust and reproducible method but requires expensive equipment and personnel training [71].
6. Conclusions
In conclusion, the studies included in this review highlight collectively the importance of measuring the sperm DNA fragmentation index (DFI) in the assessment of male infertility, sperm quality, natural conception outcomes, and assisted reproductive technologies (ARTs). Sperm DNA fragmentation appears to influence pregnancy outcomes and the success rates of fertilization. Research investigating sperm DNA fragmentation in relation to recurrent pregnancy loss consistently shows its association with increased miscarriage probabilities. Moreover, studies examining sperm DNA fragmentation prior to fertility treatments such as IUI and IVF demonstrate links with higher pregnancy rates and live birth percentages. Nevertheless, the overall impact of sperm DNA fragmentation on pregnancy outcomes and male infertility in general requires further investigation. Lower levels of the DFI are associated with improved development of healthy embryos and higher pregnancy rates, particularly in ICSI cycles. Understanding the impact of the DFI on sperm functionality and its predictive value for outcomes in both assisted and natural reproduction could optimize fertility treatments. Longitudinal studies are necessary to evaluate changes in DFI levels over time, considering factors such as age, lifestyle, environmental exposures, and medical interventions. They would provide insights into the natural progression of the DFI and its impact on fertility outcomes.
7. Future Directions
Additionally, longitudinal studies are proposed to explore the effectiveness of interventions aimed at reducing DFI levels, such as changes in daily habits and lifestyle (diet, exercise, stress reduction), antioxidant supplements, and therapies. Various antioxidants such as vitamin C, vitamin E, Glutathione, Coenzyme Q10, and polyphenols, have been studied in the context of DNA fragmentation. Each of these antioxidants aims to mitigate oxidative damage and prevent DNA fragmentation through various mechanisms, and an increasing number of studies provide compelling evidence regarding their potential benefits for male infertility. Assessing the effects of these interventions on fertility outcomes could aid in developing targeted therapeutic strategies. Furthermore, developing new diagnostic techniques for assessing sperm DNA fragmentation, including advanced imaging techniques, biomarker tests, and genomic approaches, would constitute an additional proposal for future research in the field. These techniques could offer greater sensitivity compared to traditional DFI measurement tests. Moreover, exploring the utility of combining the DFI with other sperm biomarkers, such as sperm DNA integrity tests, mitochondrial function tests, and RNA tests, could enhance the accuracy of predicting fertility outcomes.
Author Contributions
Conceptualization, S.S. and A.P.; methodology, A.P., E.M. and D.M.; validation, A.Z. (Athanasios Zikopoulos), K.L., T.K., E.N. and A.G.; formal analysis, D.S. and C.C.; data curation, C.S.; writing—original draft preparation, S.S., A.P. and E.M.; writing—review and editing, D.M., A.Z. (Athanasios Zikopoulos), K.L., T.K., E.N., D.S., C.C., C.S., A.G., A.Z. (Athanasios Zachariou), P.C., P.P., E.D. and P.D.; visualization, A.P.; supervision, A.Z. (Athanasios Zachariou), P.C., P.P. and E.D.; project administration, P.D. All authors have read and agreed to the published version of the manuscript.
Funding
This study received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Group, E.C.W. A prognosis-based approach to infertility: Understanding the role of time. Hum. Reprod. 2017, 32, 1556–1559. [Google Scholar] [CrossRef]
- Potiris, A.; Perros, P.; Drakaki, E.; Mavrogianni, D.; Machairiotis, N.; Sfakianakis, A.; Karampitsakos, T.; Vrachnis, D.; Antonakopoulos, N.; Panagopoulos, P.; et al. Investigating the Association of Assisted Reproduction Techniques and Adverse Perinatal Outcomes. J. Clin. Med. 2024, 13, 328. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Singh, A.K. Trends of male factor infertility, an important cause of infertility: A review of literature. J. Hum. Reprod. Sci. 2015, 8, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Mulgund, A.; Hamada, A.; Chyatte, M.R. A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 2015, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Vander Borght, M.; Wyns, C. Fertility and infertility: Definition and epidemiology. Clin. Biochem. 2018, 62, 2–10. [Google Scholar] [CrossRef]
- Babakhanzadeh, E.; Nazari, M.; Ghasemifar, S.; Khodadadian, A. Some of the Factors Involved in Male Infertility: A Prospective Review. Int. J. Gen. Med. 2020, 13, 29–41. [Google Scholar] [CrossRef]
- Cooper, T.G.; Noonan, E.; von Eckardstein, S.; Auger, J.; Baker, H.W.; Behre, H.M.; Haugen, T.B.; Kruger, T.; Wang, C.; Mbizvo, M.T.; et al. World Health Organization reference values for human semen characteristics. Hum. Reprod. Update 2010, 16, 231–245. [Google Scholar] [CrossRef]
- Sunder, M.; Leslie, S.W. Semen Analysis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Boitrelle, F.; Shah, R.; Saleh, R.; Henkel, R.; Kandil, H.; Chung, E.; Vogiatzi, P.; Zini, A.; Arafa, M.; Agarwal, A. The Sixth Edition of the WHO Manual for Human Semen Analysis: A Critical Review and SWOT Analysis. Life 2021, 11, 1368. [Google Scholar] [CrossRef]
- Esteves, S.C.; Sanchez-Martin, F.; Sanchez-Martin, P.; Schneider, D.T.; Gosalvez, J. Comparison of reproductive outcome in oligozoospermic men with high sperm DNA fragmentation undergoing intracytoplasmic sperm injection with ejaculated and testicular sperm. Fertil. Steril. 2015, 104, 1398–1405. [Google Scholar] [CrossRef]
- Panagopoulos, P.; Mavrogianni, D.; Christodoulaki, C.; Drakaki, E.; Chrelias, G.; Panagiotopoulos, D.; Potiris, A.; Drakakis, P.; Stavros, S. Effects of endocrine disrupting compounds on female fertility. Best. Pract. Res. Clin. Obs. Gynaecol. 2023, 88, 102347. [Google Scholar] [CrossRef]
- Agarwal, A.; Majzoub, A.; Baskaran, S.; Panner Selvam, M.K.; Cho, C.L.; Henkel, R.; Finelli, R.; Leisegang, K.; Sengupta, P.; Barbarosie, C.; et al. Sperm DNA Fragmentation: A New Guideline for Clinicians. World J. Mens. Health 2020, 38, 412–471. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, T.; Assumpcao, M. Sperm DNA fragmentation: Causes and identification. Zygote 2020, 28, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, D.; Alvarez, J.G. Sperm DNA fragmentation: Mechanisms of origin, impact on reproductive outcome, and analysis. Fertil. Steril. 2010, 93, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Seli, E.; Gardner, D.K.; Schoolcraft, W.B.; Moffatt, O.; Sakkas, D. Extent of nuclear DNA damage in ejaculated spermatozoa impacts on blastocyst development after in vitro fertilization. Fertil. Steril. 2004, 82, 378–383. [Google Scholar] [CrossRef]
- Borini, A.; Tarozzi, N.; Bizzaro, D.; Bonu, M.A.; Fava, L.; Flamigni, C.; Coticchio, G. Sperm DNA fragmentation: Paternal effect on early post-implantation embryo development in ART. Hum. Reprod. 2006, 21, 2876–2881. [Google Scholar] [CrossRef]
- Guideline Group on Unexplained, I.; Romualdi, D.; Ata, B.; Bhattacharya, S.; Bosch, E.; Costello, M.; Gersak, K.; Homburg, R.; Mincheva, M.; Norman, R.J.; et al. Evidence-based guideline: Unexplained infertility. Hum. Reprod. 2023, 38, 1881–1890. [Google Scholar] [CrossRef]
- Xie, D.; Lu, C.; Zhu, Y.; Zhu, S.; Yang, E.J.; Jin, X. Analysis on the association between sperm DNA fragmentation index and conventional semen parameters, blood microelements and seminal plasma ROS in male patients with infertility. Exp. Ther. Med. 2018, 15, 5173–5176. [Google Scholar] [CrossRef]
- Boushaba, S.; Belaaloui, G. Sperm DNA fragmentation and standard semen parameters in algerian infertile male partners. World J. Mens. Health 2015, 33, 1–7. [Google Scholar] [CrossRef]
- Akhavizadegan, H.; Yamini, N.; Musavi, A.M.; Moradi, M.; Khatami, F. Sperm DNA Fragmentation Index in Abortion or in Vitro Fertilization Failure in Presence of Normal Semen Analysis. Prague Med. Rep. 2023, 124, 166–171. [Google Scholar] [CrossRef]
- Wang, Q.X.; Wang, X.; Yu, M.Y.; Sun, H.; Wang, D.; Zhong, S.P.; Guo, F. Random sperm DNA fragmentation index is not associated with clinical outcomes in day-3 frozen embryo transfer. Asian J. Androl. 2022, 24, 109–115. [Google Scholar] [CrossRef]
- Antonouli, S.; Papatheodorou, A.; Panagiotidis, Y.; Petousis, S.; Prapas, N.; Nottola, S.A.; Palmerini, M.G.; Macchiarelli, G.; Prapas, Y. The impact of sperm DNA fragmentation on ICSI outcome in cases of donated oocytes. Arch. Gynecol. Obs. 2019, 300, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Green, K.A.; Patounakis, G.; Dougherty, M.P.; Werner, M.D.; Scott, R.T., Jr.; Franasiak, J.M. Sperm DNA fragmentation on the day of fertilization is not associated with embryologic or clinical outcomes after IVF/ICSI. J. Assist. Reprod. Genet. 2020, 37, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, J.; Liang, Z.; Wu, J.; Li, L.; Chen, C.; Jin, F.; Tian, Y. Sperm DNA fragmentation and male fertility: A retrospective study of 5114 men attending a reproductive center. J. Assist. Reprod. Genet. 2021, 38, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.C.; Jing, J.; Chen, L.; Ge, Y.F.; Feng, R.X.; Liang, Y.J.; Yao, B. Analysis of human sperm DNA fragmentation index (DFI) related factors: A report of 1010 subfertile men in China. Reprod. Biol. Endocrinol. 2018, 16, 23. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, J.; Cheng, Z.; Wang, C.; Feng, Y. Mean number of DNA breakpoints: Illuminating sperm DNA integrity and in vitro fertilization outcomes. Fertil. Steril. 2024, 121, 264–270. [Google Scholar] [CrossRef]
- Le, M.T.; Nguyen, T.A.T.; Nguyen, H.T.T.; Nguyen, T.T.T.; Nguyen, V.T.; Le, D.D.; Nguyen, V.Q.H.; Cao, N.T. Does sperm DNA fragmentation correlate with semen parameters? Reprod. Med. Biol. 2019, 18, 390–396. [Google Scholar] [CrossRef]
- Vinnakota, C.; Cree, L.; Peek, J.; Morbeck, D.E. Incidence of high sperm DNA fragmentation in a targeted population of subfertile men. Syst. Biol. Reprod. Med. 2019, 65, 451–457. [Google Scholar] [CrossRef]
- Ferrigno, A.; Ruvolo, G.; Capra, G.; Serra, N.; Bosco, L. Correlation between the DNA fragmentation index (DFI) and sperm morphology of infertile patients. J. Assist. Reprod. Genet. 2021, 38, 979–986. [Google Scholar] [CrossRef]
- Jakubik-Uljasz, J.; Gill, K.; Rosiak-Gill, A.; Piasecka, M. Relationship between sperm morphology and sperm DNA dispersion. Transl. Androl. Urol. 2020, 9, 405–415. [Google Scholar] [CrossRef]
- Hosseinifar, H.; Yazdanikhah, S.; Modarresi, T.; Totonchi, M.; Sadighi Gilani, M.A.; Sabbaghian, M. Correlation between sperm DNA fragmentation index and CMA3 positive spermatozoa in globozoospermic patients. Andrology 2015, 3, 526–531. [Google Scholar] [CrossRef]
- Wang, Q.; Gu, X.; Chen, Y.; Yu, M.; Peng, L.; Zhong, S.; Wang, X.; Lv, J. The effect of sperm DNA fragmentation on in vitro fertilization outcomes of unexplained infertility. Clinics 2023, 78, 100261. [Google Scholar] [CrossRef] [PubMed]
- Asgari, F.; Gavahi, A.; Karimi, M.; Vatannejad, A.; Amjadi, F.; Aflatoonian, R.; Zandieh, Z. Risk of embryo aneuploidy is affected by the increase in sperm DNA damage in recurrent implantation failure patients under ICSI-CGH array cycles. Hum. Fertil. 2022, 25, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.C.; Zhang, Y.; Li, H.T.; Liu, X.M.; Yi, D.X.; Tian, L.; Liu, Y.X. Sperm DNA fragmentation index, as measured by sperm chromatin dispersion, might not predict assisted reproductive outcome. Taiwan. J. Obs. Gynecol. 2018, 57, 493–498. [Google Scholar] [CrossRef]
- Tello-Mora, P.; Hernandez-Cadena, L.; Pedraza, J.; Lopez-Bayghen, E.; Quintanilla-Vega, B. Acrosome reaction and chromatin integrity as additional parameters of semen analysis to predict fertilization and blastocyst rates. Reprod. Biol. Endocrinol. 2018, 16, 102. [Google Scholar] [CrossRef]
- Wdowiak, A.; Bakalczuk, S.; Bakalczuk, G. The effect of sperm DNA fragmentation on the dynamics of the embryonic development in intracytoplasmatic sperm injection. Reprod. Biol. 2015, 15, 94–100. [Google Scholar] [CrossRef]
- Dar, S.; Grover, S.A.; Moskovtsev, S.I.; Swanson, S.; Baratz, A.; Librach, C.L. In vitro fertilization-intracytoplasmic sperm injection outcome in patients with a markedly high DNA fragmentation index (>50%). Fertil. Steril. 2013, 100, 75–80. [Google Scholar] [CrossRef]
- AmirJannati, N.; Mohazzab, A.; Fathalian, M.; Akhavizadegan, H. Comparison of Embryological Results of Microinjection in Two Groups of Men with and without Requesting Sperm DNA Fragmentation Index Measurement. Biomed. Res. Int. 2024, 2024, 6769510. [Google Scholar] [CrossRef]
- Bibi, R.; Jahan, S.; Razak, S.; Hammadeh, M.E.; Almajwal, A.; Amor, H. Protamines and DNA integrity as a biomarkers of sperm quality and assisted conception outcome. Andrologia 2022, 54, e14418. [Google Scholar] [CrossRef]
- Rex, A.S.; Wu, C.; Aagaard, J.; Fedder, J. DNA Fragmentation in Human Spermatozoa and Pregnancy Rates after Intrauterine Insemination. Should the DFI Threshold Be Lowered? J. Clin. Med. 2021, 10, 1310. [Google Scholar] [CrossRef]
- Malic Voncina, S.; Stenqvist, A.; Bungum, M.; Schyman, T.; Giwercman, A. Sperm DNA fragmentation index and cumulative live birth rate in a cohort of 2,713 couples undergoing assisted reproduction treatment. Fertil. Steril. 2021, 116, 1483–1490. [Google Scholar] [CrossRef]
- Siddhartha, N.; Reddy, N.S.; Pandurangi, M.; Muthusamy, T.; Vembu, R.; Kasinathan, K. The Effect of Sperm DNA Fragmentation Index on the Outcome of Intrauterine Insemination and Intracytoplasmic Sperm Injection. J. Hum. Reprod. Sci. 2019, 12, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Al Omrani, B.; Al Eisa, N.; Javed, M.; Al Ghedan, M.; Al Matrafi, H.; Al Sufyan, H. Associations of sperm DNA fragmentation with lifestyle factors and semen parameters of Saudi men and its impact on ICSI outcome. Reprod. Biol. Endocrinol. 2018, 16, 49. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhu, L.L.; Jiang, H.S.; Chen, H.; Chen, Y.; Dai, Y.T. Predictors of pregnancy outcome for infertile couples attending IVF and ICSI programmes. Andrologia 2016, 48, 874–881. [Google Scholar] [CrossRef]
- Panner Selvam, M.K.; Ambar, R.F.; Agarwal, A.; Henkel, R. Etiologies of sperm DNA damage and its impact on male infertility. Andrologia 2021, 53, e13706. [Google Scholar] [CrossRef]
- Derbel, R.; Sellami, H.; Sakka, R.; Ben Slima, A.; Mkaddem, I.; Gdoura, R.; McElreavey, E.; Ammar-Keskes, L. Relationship between nuclear DNA fragmentation, mitochondrial DNA damage and standard sperm parameters in spermatozoa of infertile patients with leukocytospermia. J. Gynecol. Obs. Hum. Reprod. 2021, 50, 102101. [Google Scholar] [CrossRef]
- Ogawa, S.; Ota, K.; Nishizawa, K.; Shinagawa, M.; Katagiri, M.; Kikuchi, H.; Kobayashi, H.; Takahashi, T.; Yoshida, H. Micronutrient Antioxidants for Men (Menevit((R))) Improve Sperm Function by Reducing Oxidative Stress, Resulting in Improved Assisted Reproductive Technology Outcomes. Antioxidants 2024, 13, 635. [Google Scholar] [CrossRef]
- Linn, E.; Ghanem, L.; Bhakta, H.; Greer, C.; Avella, M. Genes Regulating Spermatogenesis and Sperm Function Associated With Rare Disorders. Front. Cell. Dev. Biol. 2021, 9, 634536. [Google Scholar] [CrossRef]
- Cheung, S.; Parrella, A.; Rosenwaks, Z.; Palermo, G.D. Genetic and epigenetic profiling of the infertile male. PLoS ONE 2019, 14, e0214275. [Google Scholar] [CrossRef]
- Stavros, S.; Mavrogianni, D.; Papamentzelopoulou, M.; Basamakis, E.; Khudeir, H.; Psarris, A.; Drakakis, P. Association of Tumor Necrosis Factor-alpha -308G>A, -238G>A and -376G>A polymorphisms with recurrent pregnancy loss risk in the Greek population. Fertil. Res. Pract. 2021, 7, 9. [Google Scholar] [CrossRef]
- Jing, R.; Zhang, H.; Kong, Y.; Li, K.; Dong, X.; Yan, J.; Han, J.; Feng, L. Different functions of biogenesis of lysosomal organelles complex 3 subunit 1 (Hps1) and adaptor-related protein complex 3, beta 1 subunit (Ap3b1) genes on spermatogenesis and male fertility. Reprod. Fertil. Dev. 2019, 31, 972–982. [Google Scholar] [CrossRef]
- Riel, J.M.; Yamauchi, Y.; Ruthig, V.A.; Malinta, Q.U.; Blanco, M.; Moretti, C.; Cocquet, J.; Ward, M.A. Rescue of Sly Expression Is Not Sufficient to Rescue Spermiogenic Phenotype of Mice with Deletions of Y Chromosome Long Arm. Genes 2019, 10, 133. [Google Scholar] [CrossRef] [PubMed]
- Guran, T.; Yesil, G.; Turan, S.; Atay, Z.; Bozkurtlar, E.; Aghayev, A.; Gul, S.; Tinay, I.; Aru, B.; Arslan, S.; et al. PPP2R3C gene variants cause syndromic 46,XY gonadal dysgenesis and impaired spermatogenesis in humans. Eur. J. Endocrinol. 2019, 180, 291–309. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.; Nie, H.; Meng, L.; Yuan, S.; He, W.; Luo, A.; Li, H.; Li, W.; Du, J.; Lu, G.; et al. Identification of DNAH6 mutations in infertile men with multiple morphological abnormalities of the sperm flagella. Sci. Rep. 2019, 9, 15864. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sha, Y.W.; Wang, W.T.; Cui, Y.Q.; Chen, J.; Yan, W.; Hou, X.T.; Mei, L.B.; Yu, C.C.; Wang, J. Novel IFT140 variants cause spermatogenic dysfunction in humans. Mol. Genet. Genom. Med. 2019, 7, e920. [Google Scholar] [CrossRef]
- Yang, P.; Chen, D.; Wang, Y.X.; Zhang, L.; Huang, L.L.; Lu, W.Q.; Zeng, Q. Mediation of association between polycyclic aromatic hydrocarbon exposure and semen quality by spermatogenesis-related microRNAs: A pilot study in an infertility clinic. J. Hazard. Mater. 2020, 384, 121431. [Google Scholar] [CrossRef]
- Zamore, P.D.; Haley, B. Ribo-gnome: The big world of small RNAs. Science 2005, 309, 1519–1524. [Google Scholar] [CrossRef]
- Godia, M.; Estill, M.; Castello, A.; Balasch, S.; Rodriguez-Gil, J.E.; Krawetz, S.A.; Sanchez, A.; Clop, A. A RNA-Seq Analysis to Describe the Boar Sperm Transcriptome and Its Seasonal Changes. Front. Genet. 2019, 10, 299. [Google Scholar] [CrossRef]
- Lian, J.; Tian, H.; Liu, L.; Zhang, X.S.; Li, W.Q.; Deng, Y.M.; Yao, G.D.; Yin, M.M.; Sun, F. Downregulation of microRNA-383 is associated with male infertility and promotes testicular embryonal carcinoma cell proliferation by targeting IRF1. Cell. Death Dis. 2010, 1, e94. [Google Scholar] [CrossRef]
- Rahbar, S.; Novin, M.G.; Alizadeh, E.; Shahnazi, V.; Pashaei-Asl, F.; AsrBadr, Y.A.; Farzadi, L.; Ebrahimie, E.; Pashaiasl, M. New insights into the expression profile of MicroRNA-34c and P53 in infertile men spermatozoa and testicular tissue. Cell. Mol. Biol. 2017, 63, 77–83. [Google Scholar] [CrossRef]
- Nowicka-Bauer, K.; Nixon, B. Molecular Changes Induced by Oxidative Stress that Impair Human Sperm Motility. Antioxidants 2020, 9, 134. [Google Scholar] [CrossRef]
- Iommiello, V.M.; Albani, E.; Di Rosa, A.; Marras, A.; Menduni, F.; Morreale, G.; Levi, S.L.; Pisano, B.; Levi-Setti, P.E. Ejaculate oxidative stress is related with sperm DNA fragmentation and round cells. Int. J. Endocrinol. 2015, 2015, 321901. [Google Scholar] [CrossRef] [PubMed]
- Xavier, M.J.; Nixon, B.; Roman, S.D.; Aitken, R.J. Improved methods of DNA extraction from human spermatozoa that mitigate experimentally-induced oxidative DNA damage. PLoS ONE 2018, 13, e0195003. [Google Scholar] [CrossRef] [PubMed]
- Xavier, M.J.; Nixon, B.; Roman, S.D.; Scott, R.J.; Drevet, J.R.; Aitken, R.J. Paternal impacts on development: Identification of genomic regions vulnerable to oxidative DNA damage in human spermatozoa. Hum. Reprod. 2019, 34, 1876–1890. [Google Scholar] [CrossRef] [PubMed]
- Palmer, N.O.; Bakos, H.W.; Fullston, T.; Lane, M. Impact of obesity on male fertility, sperm function and molecular composition. Spermatogenesis 2012, 2, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Darand, M.; Salimi, Z.; Ghorbani, M.; Sadeghi, N.; Babaie, S.; Hosseinzadeh, M. Obesity is associated with quality of sperm parameters in men with infertility: A cross-sectional study. Reprod. Health 2023, 20, 134. [Google Scholar] [CrossRef]
- Wang, E.Y.; Huang, Y.; Du, Q.Y.; Yao, G.D.; Sun, Y.P. Body mass index effects sperm quality: A retrospective study in Northern China. Asian J. Androl. 2017, 19, 234–237. [Google Scholar] [CrossRef]
- Moustakli, E.; Zikopoulos, A.; Skentou, C.; Bouba, I.; Tsirka, G.; Stavros, S.; Vrachnis, D.; Vrachnis, N.; Potiris, A.; Georgiou, I.; et al. Sperm Mitochondrial Content and Mitochondrial DNA to Nuclear DNA Ratio Are Associated with Body Mass Index and Progressive Motility. Biomedicines 2023, 11, 3014. [Google Scholar] [CrossRef]
- Potiris, A.; Voitse, A.; Mavrogianni, D.; Machairiotis, N.; Drakaki, E.; Papamentzelopoulou, M.; Karampitsakos, T.; Zikopoulos, A.; Evgeni, E.; Drakakis, P.; et al. Association of GSTM1 Polymorphism and Redox Potential with Idiopathic Male Infertility. J. Clin. Med. 2023, 12, 6775. [Google Scholar] [CrossRef]
- Sharma, R.; Iovine, C.; Agarwal, A.; Henkel, R. TUNEL assay-Standardized method for testing sperm DNA fragmentation. Andrologia 2021, 53, e13738. [Google Scholar] [CrossRef]
- Farkouh, A.; Salvio, G.; Kuroda, S.; Saleh, R.; Vogiatzi, P.; Agarwal, A. Sperm DNA integrity and male infertility: A narrative review and guide for the reproductive physicians. Transl. Androl. Urol. 2022, 11, 1023–1044. [Google Scholar] [CrossRef]
- Agarwal, A.; Farkouh, A.; Saleh, R.; Hamoda, T.A.A.; Salvio, G.; Boitrelle, F.; Harraz, A.M.; Ghayda, R.A.; Kavoussi, P.; Gul, M.; et al. Technical Aspects and Clinical Limitations of Sperm DNA Fragmentation Testing in Male Infertility: A Global Survey, Current Guidelines, and Expert Recommendations. World J. Mens. Health 2024, 42, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Cortes-Gutierrez, E.I.; Lopez-Fernandez, C.; Fernandez, J.L.; Davila-Rodriguez, M.I.; Johnston, S.D.; Gosalvez, J. Interpreting sperm DNA damage in a diverse range of mammalian sperm by means of the two-tailed comet assay. Front. Genet. 2014, 5, 404. [Google Scholar] [CrossRef][Green Version]
- Cho, C.L.; Agarwal, A.; Majzoub, A.; Esteves, S.C. Clinical utility of sperm DNA fragmentation testing: Concise practice recommendations. Transl. Androl. Urol. 2017, 6, S366–S373. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, J.L.; Muriel, L.; Rivero, M.T.; Goyanes, V.; Vazquez, R.; Alvarez, J.G. The sperm chromatin dispersion test: A simple method for the determination of sperm DNA fragmentation. J. Androl. 2003, 24, 59–66. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).