Adsorption of Serum Fetuin onto Octacalcium Phosphate and Its Relation to Osteogenic Property
Abstract
1. Introduction
2. Results
2.1. Adsorption of Fetuin onto OCP
2.1.1. Adsorption Isotherms of Fetuin onto OCP Under Different Degrees of Supersaturations with Respect to OCP and HA
2.1.2. Circular Dichroism (CD) Spectra of Fetuin
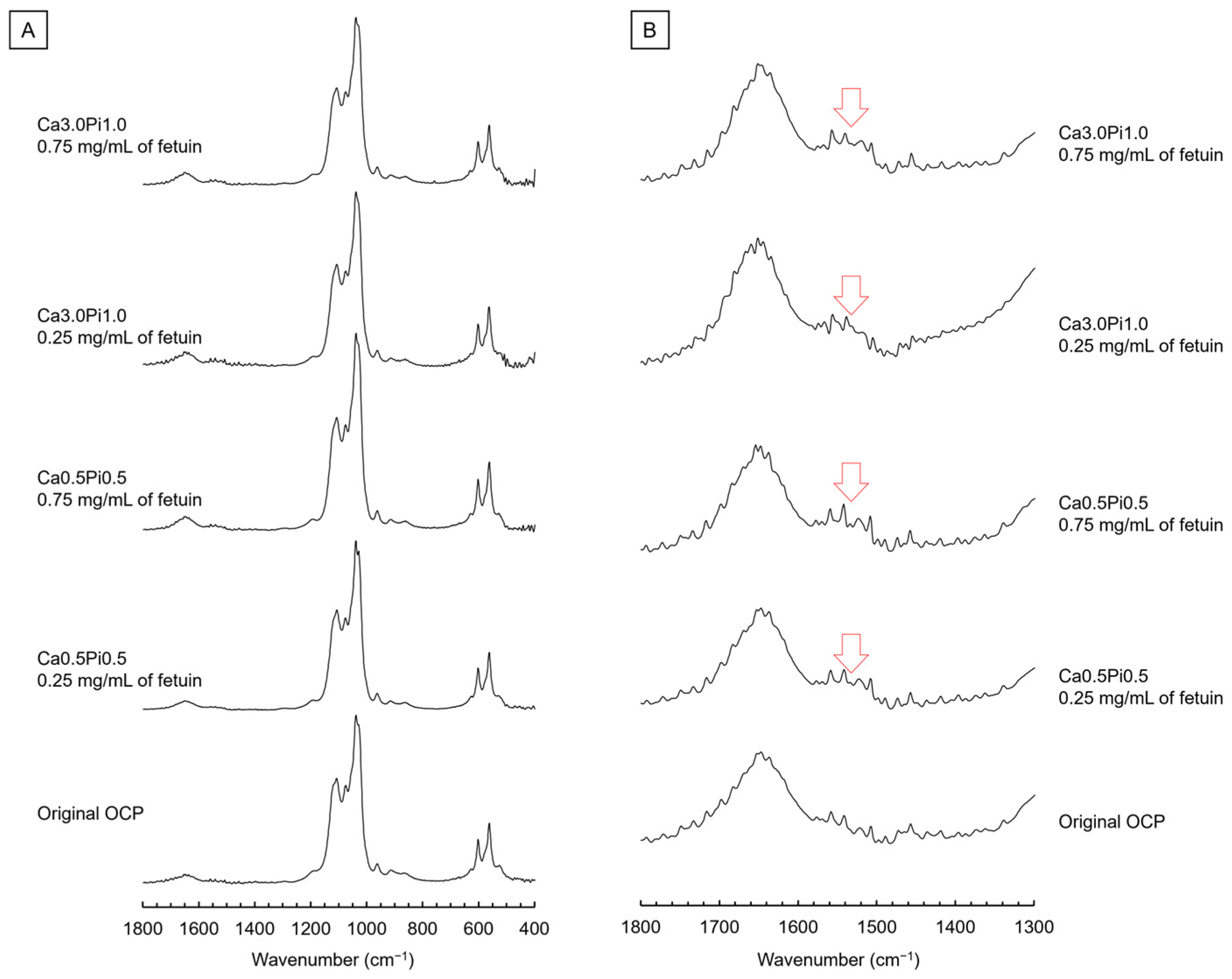
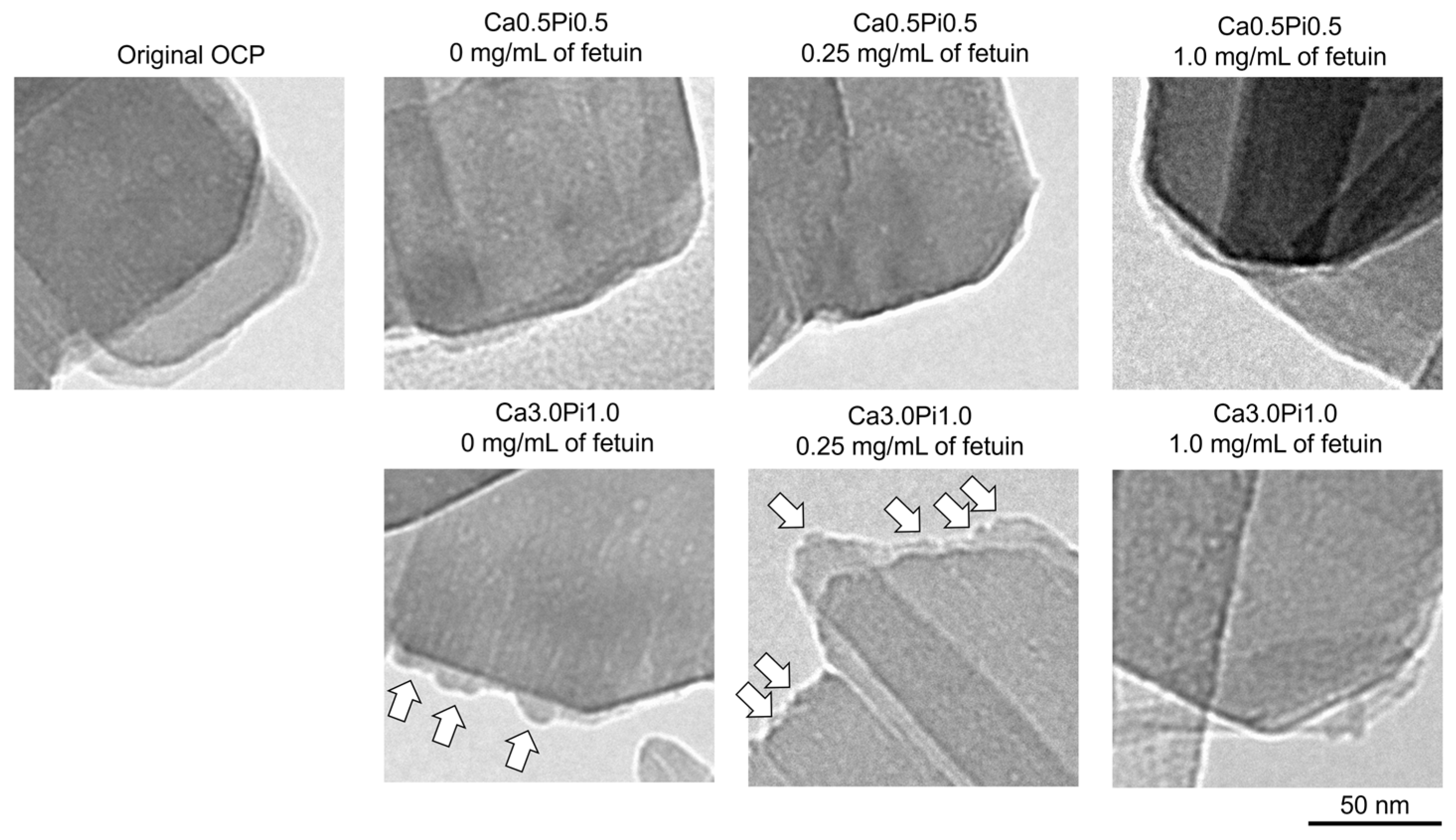
2.1.3. Fourier Transform Infrared (FTIR) Spectroscopic Analysis, Transmission Electron Microscope (TEM) Observation, and Selected Area Electron Diffraction (SAED) Analysis of OCP Before and After Fetuin Adsorption
2.2. Cell Culture Experiments
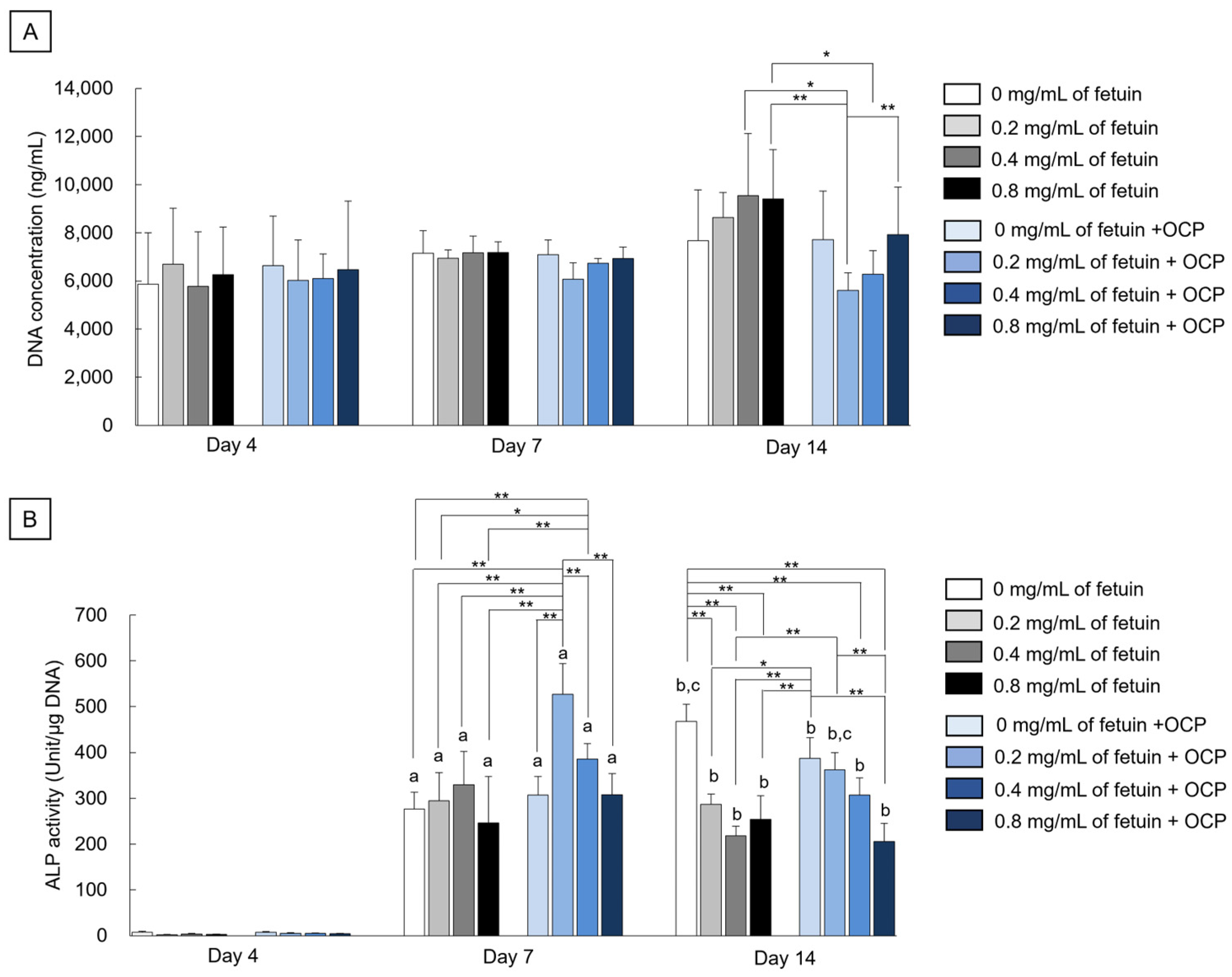
2.2.1. DNA Concentrations and Alkaline Phosphatase (ALP) Activities
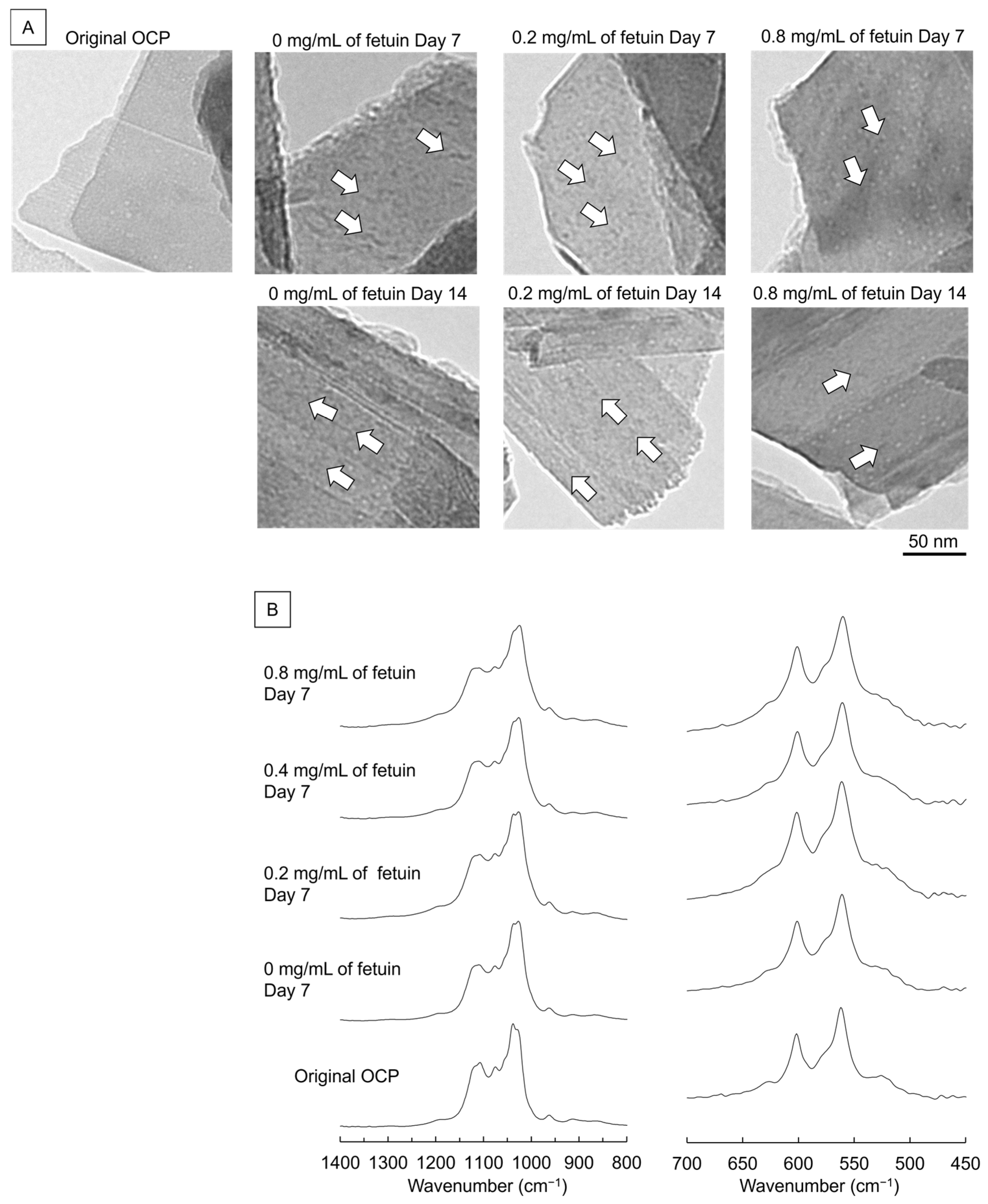
2.2.2. TEM Observation and FTIR Analysis of OCP Incubated with MSCs and Fetuin
2.2.3. Ion Concentration and DS Changes in the Culture Media
3. Discussion
4. Materials and Methods
4.1. Preparation of OCP Granules
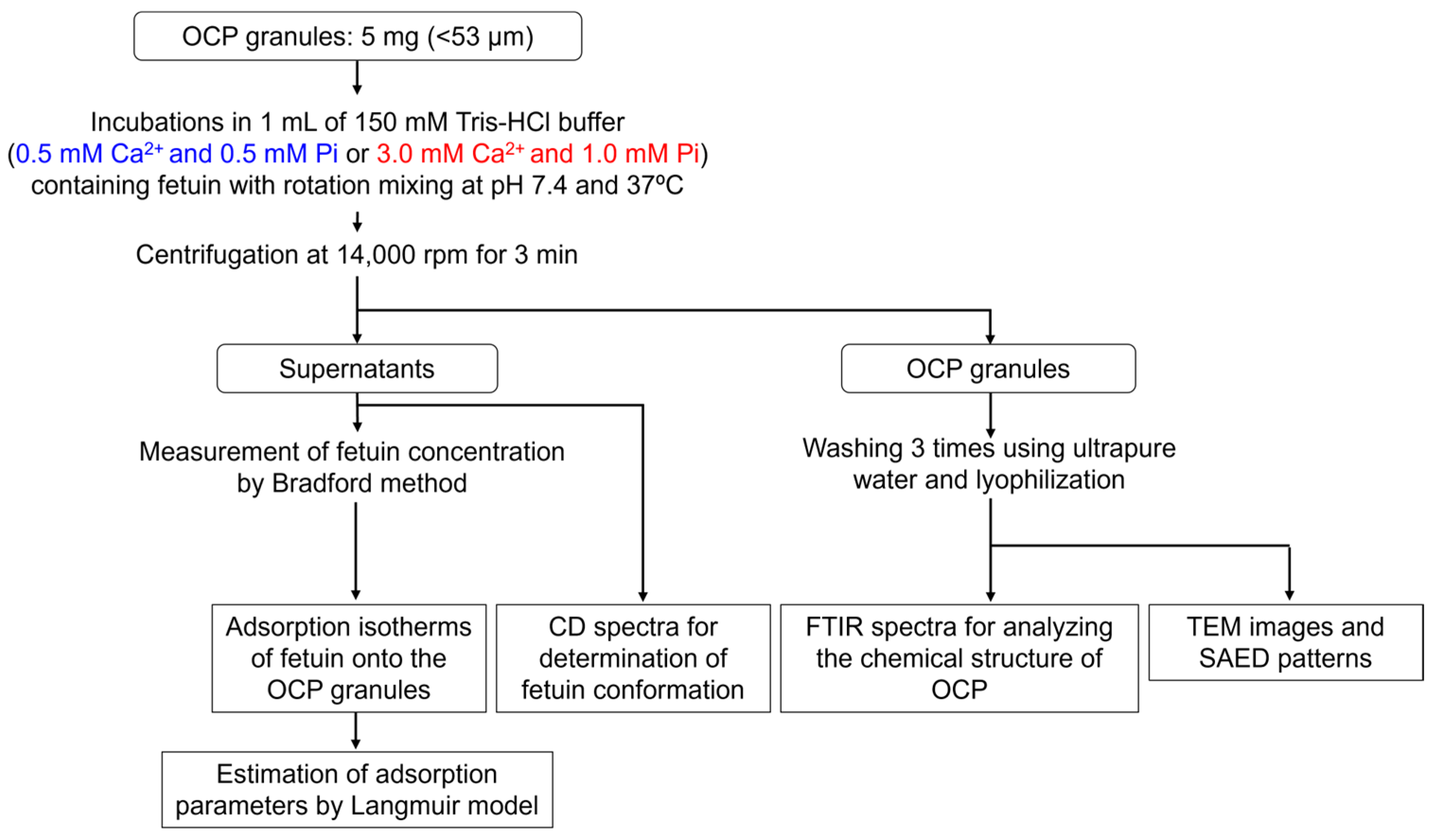
4.2. Adsorption Experiments
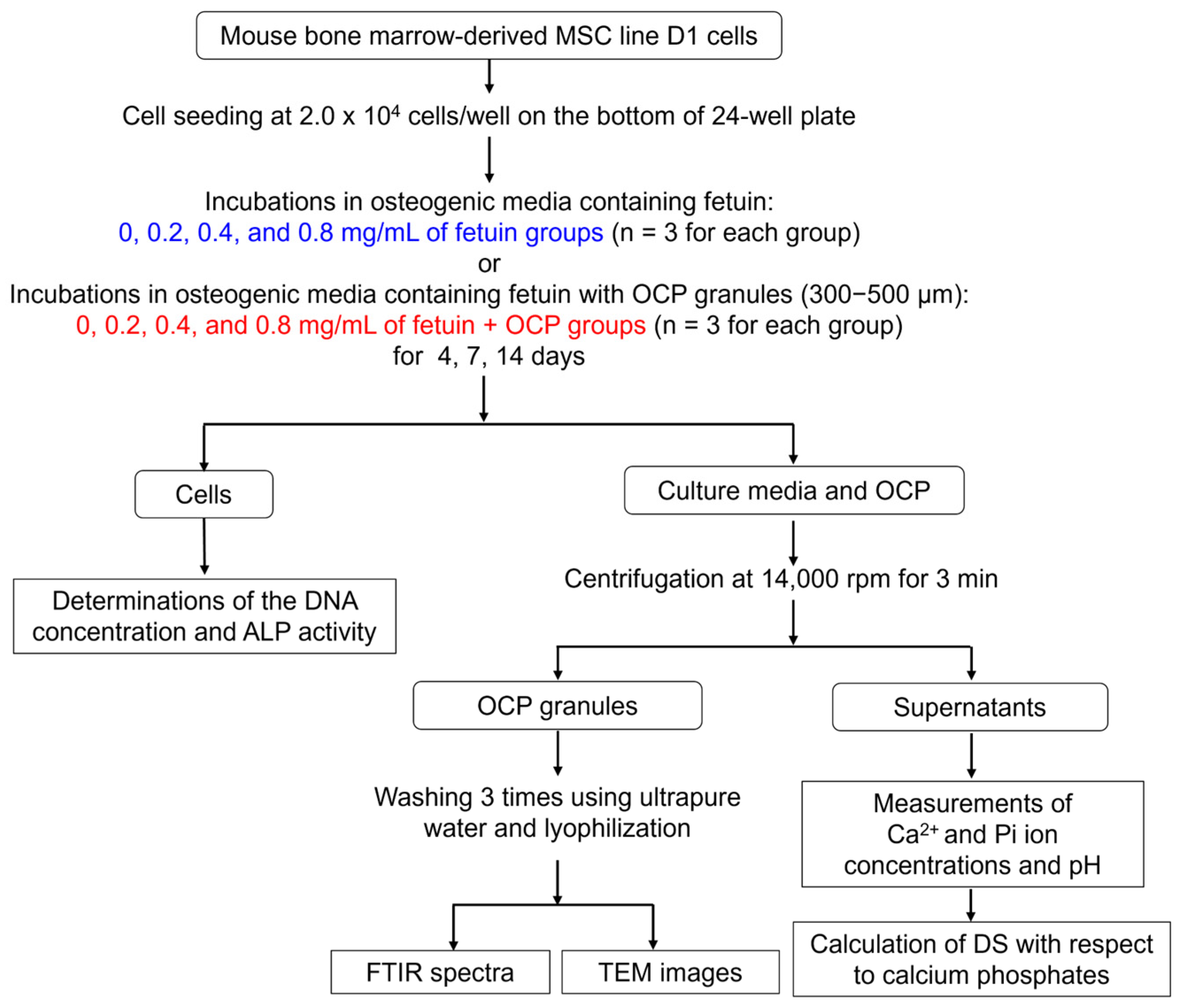
4.3. Cell Culture Experiments
4.3.1. Analysis of Cell Growth and Osteoblastic Differentiation
4.3.2. Measurement of Ion Concentrations and DS with Respect to Calcium Phosphate in Culture Medium
4.3.3. Morphological Observation and Structural Analysis of OCP After Incubations with MSCs and Fetuin
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, W.E. Crystal growth of bone mineral. Clin. Orthop. Relat. Res. 1966, 44, 205–220. [Google Scholar] [CrossRef]
- Simpson, D.R. Problems of the composition and structure of the bone minerals. Clin. Orthop. Relat. Res. 1972, 86, 260–286. [Google Scholar] [CrossRef]
- Young, R.A. Implications of atomic substitutions and other structural details in apatites. J. Dent. Res. 1974, 53, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Rey, C.; Combes, C.; Drouet, C.; Glimcher, M.J. Bone mineral: Update on chemical composition and structure. Osteoporos. Int. 2009, 20, 1013–1021. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Calcium phosphate-based osteoinductive materials. Chem. Rev. 2008, 108, 4742–4753. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Properties of osteoconductive biomaterials: Calcium phosphates. Clin. Orthop. Relat. Res. 2002, 395, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Yagi, M.; Wakisaka, Y.; Takeno, J.; Kanada, S.; Tsubouchi, T.; Hamatani, N.; Maruo, H.; Takashina, M.; Ishii, T.; Kanai, T.; et al. Dosimetric impact of stopping power for human bone porosity with dual-energy computed tomography in scanned carbon-ion therapy treatment planning. Sic Rep. 2024, 14, 17440. [Google Scholar] [CrossRef]
- Oyane, A.; Wang, X.; Sogo, Y.; Ito, A.; Tsurushima, H. Calcium phosphate composite layers for surface-mediated gene transfer. Acta Biomater. 2012, 8, 2034–2046. [Google Scholar] [CrossRef]
- Bohner, M.; Santoni, B.L.; Dobelin, N. β-tricalcium phosphate for bone substitution: Synthesis and properties. Acta Biomater. 2020, 113, 23–41. [Google Scholar] [CrossRef]
- Chow, L.C. Next generation calcium phosphate-based biomaterials. Dent. Mater. J. 2009, 28, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Komlev, V.S.; Barinov, S.M.; Bozo, I.I.; Deev, R.V.; Eremin, I.I.; Fedotov, A.Y.; Gurin, A.N.; Khromova, N.V.; Kopnin, P.B.; Kuvshinova, E.A.; et al. Bioceramics composed of octacalcium phosphate demonstrate enhanced biological behavior. ACS Appl. Mater. Interfaces 2014, 6, 16610–16620. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, O.; Shiwaku, Y.; Hamai, R. Octacalcium phosphate bone substitute materials: Comparison between properties of biomaterials and other calcium phosphate materials. Dent. Mater. J. 2020, 39, 187–199. [Google Scholar] [CrossRef]
- Brown, W.E.; Lehr, J.R.; Smith, J.P.; Frazier, A.W. Crystallography of octacalcium phosphate. J. Am. Chem. Soc. 1957, 79, 5318–5319. [Google Scholar] [CrossRef]
- Mathew, M.; Brown, W.E.; Schroeder, L.W.; Dickens, B. Crystal structure of octacalcium bis(hydrogenphosphate) tetrakis(phosphate)pentahydrate, Ca8(HPO4)2(PO4)4·5H2O. J. Crystallogr. Spectrosc. Res. 1988, 18, 235–250. [Google Scholar] [CrossRef]
- Brown, W.E.; Smith, J.P.; Lehr, J.R.; Frazier, A.W. Octacalcium phosphate and hydroxyapatite: Crystallographic and chemical relations between octacalcium phosphate and hydroxyapatite. Nature 1962, 196, 1050–1055. [Google Scholar] [CrossRef]
- Brown, W.E.; Mathew, M.; Tung, M.S. Crystal chemistry of octacalcium phosphate. Prog. Cryst. Growth Charact. 1981, 4, 59–87. [Google Scholar] [CrossRef]
- Driessens, F.C. Physiology of hard tissues in comparison with the solubility of synthetic calcium phosphates. Ann. N. Y. Acad. Sci. 1988, 523, 131–136. [Google Scholar] [CrossRef] [PubMed]
- LeGeros, R.Z.; Daculsi, G.; Orly, I.; Abergas, T.; Torres, W. Solution-mediated transformation of octacalcium phosphate (OCP) to apatite. Scanning Microsc. 1989, 3, 129–137, discussion 137-128. [Google Scholar] [PubMed]
- Tomazic, B.B.; Tung, M.S.; Gregory, T.M.; Brown, W.E. Mechanism of hydrolysis of octacalcium phosphate. Scanning Microsc. 1989, 3, 119–127. [Google Scholar]
- Suzuki, O.; Nakamura, M.; Miyasaka, Y.; Kagayama, M.; Sakurai, M. Bone formation on synthetic precursors of hydroxyapatite. Tohoku J. Exp. Med. 1991, 164, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, O.; Kamakura, S.; Katagiri, T. Surface chemistry and biological responses to synthetic octacalcium phosphate. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 77, 201–212. [Google Scholar] [CrossRef]
- Suzuki, O.; Kamakura, S.; Katagiri, T.; Nakamura, M.; Zhao, B.; Honda, Y.; Kamijo, R. Bone formation enhanced by implanted octacalcium phosphate involving conversion into Ca-deficient hydroxyapatite. Biomaterials 2006, 27, 2671–2681. [Google Scholar] [CrossRef]
- Habraken, W.J.; Tao, J.; Brylka, L.J.; Friedrich, H.; Bertinetti, L.; Schenk, A.S.; Verch, A.; Dmitrovic, V.; Bomans, P.H.; Frederik, P.M.; et al. Ion-association complexes unite classical and non-classical theories for the biomimetic nucleation of calcium phosphate. Nat. Commun. 2013, 4, 1507. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Kamakura, S.; Matsui, K.; Fukuda, M.; Takano, H.; Iino, M.; Ishikawa, S.; Kawana, H.; Soma, T.; Imamura, E.; et al. Clinical study of octacalcium phosphate and collagen composite in oral and maxillofacial surgery. J. Tissue Eng. 2020, 11, 1–115. [Google Scholar] [CrossRef]
- Hamada, S.; Mori, Y.; Shiwaku, Y.; Hamai, R.; Tsuchiya, K.; Baba, K.; Oizumi, I.; Kanabuchi, R.; Miyatake, N.; Aizawa, T.; et al. Octacalcium phosphate/gelatin composite (OCP/Gel) enhances bone repair in a critical-sized transcortical femoral defect rat model. Clin. Orthop. Relat. Res. 2022, 480, 2043–2055. [Google Scholar] [CrossRef]
- Suzuki, O.; Nakamura, M.; Miyasaka, Y.; Kagayama, M.; Sakurai, M. Maclura pomifera agglutinin-binding glycoconjugates on converted apatite from synthetic octacalcium phosphate implanted into subperiosteal region of mouse calvaria. Bone Miner. 1993, 20, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Nakajima, H.; Yamada, E.; Akahane, M.; Dohi, Y.; Ohgushi, H.; Tamai, S.; Ichijima, K. In vivo osteogenic capability of cultured allogeneic bone in porous hydroxyapatite: Immunosuppressive and osteogenic potential of FK506 in vivo. J. Bone Miner. Res. 2000, 15, 1147–1157. [Google Scholar] [CrossRef]
- Simon, P.; Gruner, D.; Worch, H.; Pompe, W.; Lichte, H.; Khassawna, E.T.; Heiss, C.; Wenisch, S.; Kniep, R. First evidence of octacalcium phosphate@osteocalcin nanocomplex as skeletal bone component directing collagen triple-helix nanofibril mineralization. Sci. Rep. 2018, 8, 13696. [Google Scholar] [CrossRef] [PubMed]
- Hoang, Q.Q.; Sicheri, F.; Howard, A.J.; Yang, D.S.C. Bone recognition mechanism of porcine osteocalcin from crystal structure. Nature 2003, 425, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Miyatake, N.; Kishimoto, K.N.; Anada, T.; Imaizumi, H.; Itoi, E.; Suzuki, O. Effect of partial hydrolysis of octacalcium phosphate on its osteoconductive characteristics. Biomaterials 2009, 30, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, Y.; Hasegawa, T.; Hongo, H.; Yamamoto, T.; Maruoka, H.; Haraguchi-Kitakamae, M.; Nakanishi, K.; Ishizu, H.; Shimizu, T.; Yoshihara, K.; et al. Phosphorylated pullulan promotes calcification during bone regeneration in the bone defects of rat tibiae. Front. Bioeng. Biotechnol. 2023, 11, 1243951. [Google Scholar] [CrossRef]
- Suzuki, O.; Hamai, R.; Sakai, S. The material design of octacalcium phosphate bone substitute: Increased dissolution and osteogenecity. Acta Biomater. 2023, 158, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, H.; Kamiie, J.; Kawakami, H.; Anada, T.; Honda, Y.; Shiraishi, N.; Kamakura, S.; Terasaki, T.; Shimauchi, H.; Suzuki, O. Proteome analysis of rat serum proteins adsorbed onto synthetic octacalcium phosphate crystals. Anal. Biochem. 2011, 418, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Triffitt, J.T.; Gebauer, U.; Ashton, B.A.; Owen, M.E.; Reynolds, J.J. Origin of plasma α2HS-glycoprotein and its accumulation in bone. Nature 1976, 262, 226–227. [Google Scholar] [CrossRef] [PubMed]
- Schinke, T.; Amendt, C.; Trindl, A.; Poschke, O.; MullerEsterl, W.; JahnenDechent, W. The serum protein α2-HS glycoprotein/fetuin inhibits apatite formation in vitro and in mineralizing calvaria cells—A possible role in mineralization and calcium homeostasis. J. Biol. Chem. 1996, 271, 20789–20796. [Google Scholar] [CrossRef] [PubMed]
- Dickson, I.R.; Poole, A.R.; Veis, A. Localisation of plasma α2HS- glycoprotein in mineralising human bone. Nature 1975, 256, 430–432. [Google Scholar] [CrossRef] [PubMed]
- Price, P.A.; Lim, J.E. The inhibition of calcium phosphate precipitation by fetuin is accompanied by the formation of a fetuin-mineral complex. J. Bone Miner. Res. 2003, 18, S197. [Google Scholar] [CrossRef] [PubMed]
- Toroian, D.; Price, P.A. The essential role of fetuin in the serum-induced calcification of collagen. Calcif. Tissue Int. 2008, 82, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Price, P.A.; Toroian, D.; Lim, J.E. Mineralization by inhibitor exclusion: The calcification of collagen with fetuin. J. Biol. Chem. 2009, 284, 17092–17101. [Google Scholar] [CrossRef]
- Puck, T.T.; Waldren, C.A.; Jones, C. Mammalian cell growth proteins. I. Growth stimulation by fetuin. Proc. Natl. Acad. Sci. USA 1968, 59, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Demetriou, M.; Binkert, C.; Sukhu, B.; Tenenbaum, H.C.; Dennis, J.W. Fetuin/α2-HS glycoprotein is a transforming growth factor-β type II receptor mimic and cytokine antagonist. J. Biol. Chem. 1996, 271, 12755–12761. [Google Scholar] [CrossRef]
- Zhao, L.M.; Hantash, B.M. TGF-β1 Regulates differentiation of bone marrow mesenchymal stem cells. Vitam. Horm. 2011, 87, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Lieb, E.; Milz, S.; Vogel, T.; Hacker, M.; Dauner, M.; Schulz, M.B. Effects of transforming growth factor β1 on bonelike tissue formation in three-dimensional cell culture. I. Culture conditions and tissue formation. Tissue Eng. 2004, 10, 1399–1413. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, O.; Yagishita, H.; Yamazaki, M.; Aoba, T. Adsorption of bovine serum albumin onto octacalcium phosphate and its hydrolyzates Cell Mater. 1995, 5, 45–54. 5.
- Furedi-milhofer, H.; Moradian-oldak, J.; Weiner, S.; Veis, A.; Mintz, K.P.; Addadi, L. Interaction of matrix proteins from mineralized tissues with octacalcium phosphate. Connect. Tissue Res. 1994, 30, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Addadi, L.; Weiner, S. Control and design principles in biological mineralization. Angew. Chem. Int. Ed. 1992, 31, 153–169. [Google Scholar] [CrossRef]
- Moradian-Oldak, J.; Iijima, M.; Bouropoulos, N.; Wen, H.B. Assembly of amelogenin proteolytic products and control of octacalcium phosphate crystal morphology. Connect. Tissue Res. 2003, 44, 58–64. [Google Scholar] [CrossRef]
- Hamai, R.; Sakai, S.; Shiwaku, Y.; Anada, T.; Tsuchiya, K.; Ishimoto, T.; Nakano, T.; Suzuki, O. Octacalcium phosphate crystals including a higher density dislocation improve its materials osteogenecity. Appl. Mater. Today 2022, 26, 101279. [Google Scholar] [CrossRef]
- Handa, T.; Anada, T.; Honda, Y.; Yamazaki, H.; Kobayashi, K.; Kanda, N.; Kamakura, S.; Echigo, S.; Suzuki, O. The effect of an octacalcium phosphate co-precipitated gelatin composite on the repair of critical-sized rat calvarial defects. Acta Biomater. 2012, 8, 1190–1200. [Google Scholar] [CrossRef]
- Iijima, M.; Moriwaki, Y.; Kuboki, Y. Oriented growth of octacalcium phosphate on and inside the collagenous matrix in vitro. Connect. Tissue Res. 1995, 32, 519–524. [Google Scholar] [CrossRef]
- Combes, C.; Rey, C.; Freche, M. In vitro crystallization of octacalcium phosphate on type I collagen: Influence of serum albumin. J. Mater. Sci. Mater. Med. 1999, 10, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Moreno, E.C.; Margolis, H.C. Composition of human plaque fluid. J. Dent. Res. 1988, 67, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Eidelman, N.; Chow, L.C.; Brown, W.E. Calcium phosphate saturation levels in ultrafiltered serum. Calcif. Tissue Int. 1987, 40, 71–78. [Google Scholar] [CrossRef]
- Weilbaecher, K.N.; Guise, T.A.; McCauley, L.K. Cancer to bone: A fatal attraction. Nat. Rev. Cancer 2011, 11, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Boskey, A.L.; Posner, A.S. Bone-structure, composition, and mineralization. Orthop. Clin. N. Am. 1984, 15, 597–612. [Google Scholar] [CrossRef]
- Hamai, R.; Tsuchiya, K.; Suzuki, O. Adsorption of serum albumin onto octacalcium phosphate in supersaturated solutions regarding calcium phosphate phases. Materials 2019, 12, 2333. [Google Scholar] [CrossRef]
- Kataoka, T.; Liu, Z.; Yamada, I.; Galindo, T.G.P.; Tagaya, M. Surface functionalization of hydroxyapatite nanoparticles for biomedical applications. J. Mater. Chem. B 2024, 12, 6805–6826. [Google Scholar] [CrossRef]
- Tang, S.; Shen, Y.; Jiang, L.; Zhang, Y. Surface Modification of nano-hydroxyapatite/polymer composite for bone tissue repair applications: A Review. Polymers 2024, 16, 1263. [Google Scholar] [CrossRef]
- Sugiura, Y.; Munar, M.L.; Ishikawa, K. Fabrication of octacalcium phosphate block through a dissolution-precipitation reaction using a calcium sulphate hemihydrate block as a precursor. J. Mater. Sci. Mater. Med. 2018, 29, 151. [Google Scholar] [CrossRef]
- Vashist, S.K.; Schneider, E.M.; Venkatesh, A.G.; Luong, J.H.T. Emerging human fetuin a assays for biomedical diagnostics. Trends Biotechnol. 2017, 35, 407–421. [Google Scholar] [CrossRef]
- Shelton, R.M.; Liu, Y.; Cooper, P.R.; Gbureck, U.; German, M.J.; Barralet, J.E. Bone marrow cell gene expression and tissue construct assembly using octacalcium phosphate microscaffolds. Biomaterials 2006, 27, 2874–2881. [Google Scholar] [CrossRef]
- Moreno, E.C.; Kresak, M.; Hay, D.I. Adsorption of molecules of biological interest onto hydroxyapatite. Calcif. Tissue Int. 1984, 36, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Kandori, K.; Mizumoto, S.; Toshima, S.; Fukusumi, M.; Morisada, Y. Effects of heat treatment of calcium hydroxyapatite particles on the protein adsorption behavior. J. Phys. Chem. B 2009, 113, 11016–11022. [Google Scholar] [CrossRef]
- Swain, S.K.; Sarkar, D. Study of BSA protein adsorption/release on hydroxyapatite nanoparticles. Appl. Surf. Sci. 2013, 286, 99–103. [Google Scholar] [CrossRef]
- Stein, G.S.; Lian, J.B. Molecular mechanisms mediating proliferation/differentiation interrelationships during progressive development of the osteoblast phenotype. Endocr. Rev. 1993, 14, 424–442. [Google Scholar] [CrossRef]
- Komori, T. Regulation of skeletal development and maintenance by Runx2 and Sp7. Int. J. Mol. Sci. 2024, 25, 10102. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, K.; Shiwaku, Y.; Hamai, R.; Mizoguchi, T.; Tsuchiya, K.; Takahashi, T.; Suzuki, O. Differentiation of committed osteoblast progenitors by octacalcium phosphate compared to calcium-deficient hydroxyapatite in Lepr-cre/Tomato mouse tibia. Acta Biomater. 2022, 142, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Anada, T.; Hamai, R.; Shiwaku, Y.; Tsuchiya, K.; Sakai, S.; Baba, K.; Sasaki, K.; Suzuki, O. Culture of hybrid spheroids composed of calcium phosphate materials and mesenchymal stem cells on an oxygen-permeable culture device to predict in vivo bone forming capability. Acta Biomater. 2019, 88, 477–490. [Google Scholar] [CrossRef]
- Hamai, R.; Tsuchiya, K.; Suzuki, O. Enhancement of the cytokine adsorption capacity of octacalcium phosphate by interaction-controlled glycosaminoglycan modification to promote osteoblastic differentiation. Langmuir 2024, 40, 27253–27269. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, O.; Imaizumi, H.; Kamakura, S.; Katagiri, T. Bone regeneration by synthetic octacalcium phosphate and its role in biological mineralization. Curr. Med. Chem. 2008, 15, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Brylka, L.; Jahnen-Dechent, W. The role of fetuin-A in physiological and pathological mineralization. Calcif. Tissue Int. 2013, 93, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Wald, J.; Wiese, S.; Eckert, T.; Jahnen-Dechent, W.; Richtering, W.; Heiss, A. Formation and stability kinetics of calcium phosphate-fetuin-A colloidal particles probed by time-resolved dynamic light scattering. Soft Matter 2011, 7, 2869–2874. [Google Scholar] [CrossRef]
- Combes, C.; Rey, C. Adsorption of proteins and calcium phosphate materials bioactivity. Biomaterials 2002, 23, 2817–2823. [Google Scholar] [CrossRef]
- Ito, N.; Kamitakahara, M.; Yoshimura, M.; Ioku, K. Importance of nucleation in transformation of octacalcium phosphate to hydroxyapatite. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 40, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Aoba, T.E.; Moreno, E.C. Adsorption of phosphoserine onto hydroxyapatite and its inhibitory activity on crystal growth. J. Colloid. Interface Sci. 1985, 106, 110–121. [Google Scholar] [CrossRef]
- McKenna, P. Proteins in the amniotic and allantoic fluids of fetal pigs. Comp. Biochem. Physiol. B 1984, 78, 663–667. [Google Scholar] [CrossRef]
- Moreno, E.C.; Kresak, M.; Zahradni, R.T. Fluoridated hydroxyapatite solubility and caries formation. Nature 1974, 247, 64–65. [Google Scholar] [CrossRef]
- Tung, M.S.; Eidelman, N.; Sieck, B.; Brown, W.E. Octacalcium phosphate solubility product from 4 to 37 °C. J. Res. Natl. Bur. Stand. 1988, 93, 613–624. [Google Scholar] [CrossRef]
- Moreno, E.C.; Brown, W.E.; Osborn, G. Solubility of dicalcium phosphate dihydrate in aqueous systems. Soil. Sci. Soc. Am. J. 1960, 24, 94–98. [Google Scholar] [CrossRef]
- Aoba, T.; Fukae, M.; Tanabe, T.; Shimizu, M.; Moreno, E.C. Selective adsorption of porcine-amelogenins onto hydroxyapatite and their inhibitory activity on hydroxyapatite growth in supersaturated solutions. Calcif. Tissue Int. 1987, 41, 281–289. [Google Scholar] [CrossRef] [PubMed]


| Supernatants | Periods (Day) | Ca (mM) | Pi (mM) | pH | DS at 37 °C | ||
|---|---|---|---|---|---|---|---|
| HA | OCP | DCPD | |||||
| Culture medium | 0 | 2.26 | 1.32 | 7.82 | 1.65 × 1014 | 5.94 × 104 | 1.01 × 100 |
| 0 mg/mL of fetuin | 7 | 2.00 | 6.84 | 7.57 | 4.81 × 1014 | 7.28 × 105 | 3.76 × 100 |
| 0.2 mg/mL of fetuin | 7 | 1.90 | 4.57 | 7.82 | 1.89 × 1015 | 8.74 × 105 | 2.69 × 100 |
| 0.4 mg/mL of fetuin | 7 | 1.89 | 4.08 | 7.76 | 7.42 × 1014 | 4.61 × 105 | 2.40 × 100 |
| 0.8 mg/mL of fetuin | 7 | 1.91 | 3.82 | 7.62 | 4.52 × 1014 | 3.31 × 105 | 2.27 × 100 |
| 0 mg/mL of fetuin + OCP | 7 | 1.96 | 6.79 | 7.51 | 2.37 × 1014 | 4.81 × 105 | 3.61 × 100 |
| 0.2 mg/mL of fetuin + OCP | 7 | 1.72 | 6.38 | 7.58 | 2.20 × 1014 | 3.61 × 105 | 3.09 × 100 |
| 0.4 mg/mL of fetuin + OCP | 7 | 1.84 | 5.99 | 7.63 | 4.37 × 1014 | 5.35 × 105 | 3.16 × 100 |
| 0.8 mg/mL of fetuin +OCP | 7 | 1.97 | 6.72 | 7.59 | 5.24 × 1014 | 7.33 × 105 | 3.67 × 100 |
| 0 mg/mL of fetuin | 14 | 1.18 | 6.51 | 7.45 | 1.00 × 1013 | 4.40 × 104 | 2.11 × 100 |
| 0.2 mg/mL of fetuin | 14 | 1.66 | 6.91 | 7.54 | 1.47 × 1014 | 3.09 × 105 | 3.15 × 100 |
| 0.4 mg/mL of fetuin | 14 | 1.61 | 6.62 | 7.50 | 7.82 × 1013 | 2.01 × 105 | 2.92 × 100 |
| 0.8 mg/mL of fetuin | 14 | 1.54 | 6.97 | 7.33 | 1.26 × 1013 | 7.41 × 104 | 2.76 × 100 |
| 0 mg/mL of fetuin + OCP | 14 | 1.48 | 6.56 | 7.31 | 7.44 × 1012 | 4.93 × 104 | 2.51 × 100 |
| 0.2 mg/mL of fetuin + OCP | 14 | 1.29 | 6.47 | 7.30 | 3.32 × 1012 | 2.64 × 104 | 2.16 × 100 |
| 0.4 mg/mL of fetuin + OCP | 14 | 1.45 | 6.76 | 7.30 | 6.47 × 1012 | 4.60 × 104 | 2.51 × 100 |
| 0.8 mg/mL of fetuin + OCP | 14 | 1.57 | 6.86 | 7.31 | 1.09 × 1013 | 6.87 × 104 | 2.75 × 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsuboi, Y.; Hamai, R.; Okuyama, K.; Tsuchiya, K.; Shiwaku, Y.; Yamauchi, K.; Suzuki, O. Adsorption of Serum Fetuin onto Octacalcium Phosphate and Its Relation to Osteogenic Property. Int. J. Mol. Sci. 2025, 26, 1391. https://doi.org/10.3390/ijms26031391
Tsuboi Y, Hamai R, Okuyama K, Tsuchiya K, Shiwaku Y, Yamauchi K, Suzuki O. Adsorption of Serum Fetuin onto Octacalcium Phosphate and Its Relation to Osteogenic Property. International Journal of Molecular Sciences. 2025; 26(3):1391. https://doi.org/10.3390/ijms26031391
Chicago/Turabian StyleTsuboi, Yuki, Ryo Hamai, Kyosuke Okuyama, Kaori Tsuchiya, Yukari Shiwaku, Kensuke Yamauchi, and Osamu Suzuki. 2025. "Adsorption of Serum Fetuin onto Octacalcium Phosphate and Its Relation to Osteogenic Property" International Journal of Molecular Sciences 26, no. 3: 1391. https://doi.org/10.3390/ijms26031391
APA StyleTsuboi, Y., Hamai, R., Okuyama, K., Tsuchiya, K., Shiwaku, Y., Yamauchi, K., & Suzuki, O. (2025). Adsorption of Serum Fetuin onto Octacalcium Phosphate and Its Relation to Osteogenic Property. International Journal of Molecular Sciences, 26(3), 1391. https://doi.org/10.3390/ijms26031391







