Doping of Hollow Urchin-like MnO2 Nanoparticles in Beta-Tricalcium Phosphate Scaffold Promotes Stem Cell Osteogenic Differentiation
Abstract
1. Introduction
2. Results
2.1. Morphology of H-MnO2 Nanoparticles
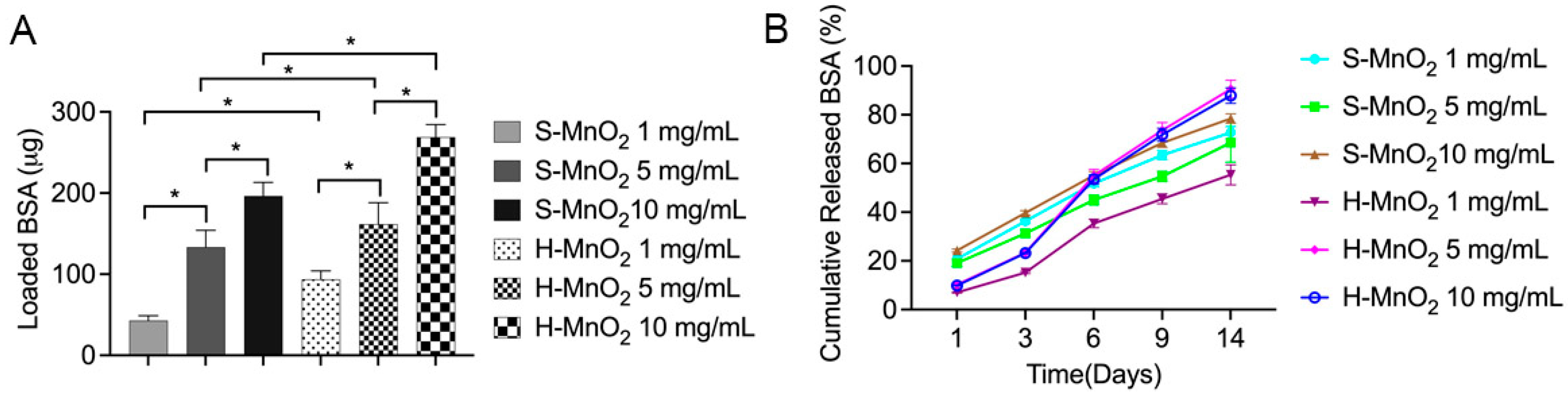
2.2. Loading and Release Profile of the Proteins on H-MnO2 Nanoparticles
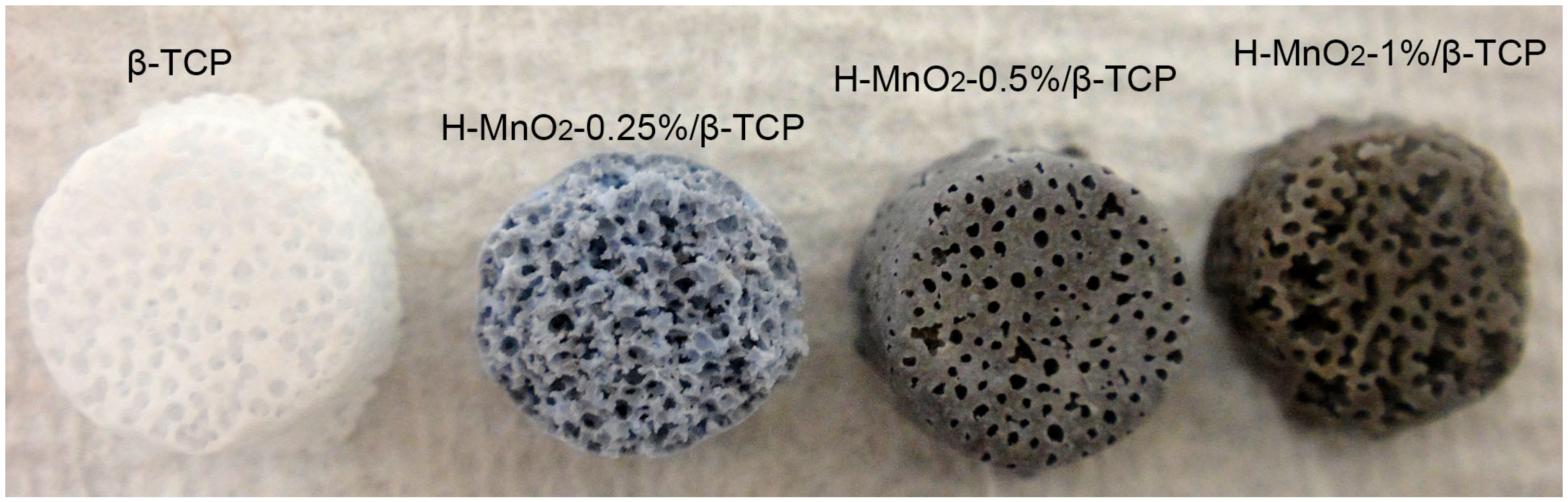
2.3. H-MnO2/β-TCP Scaffold Preparation
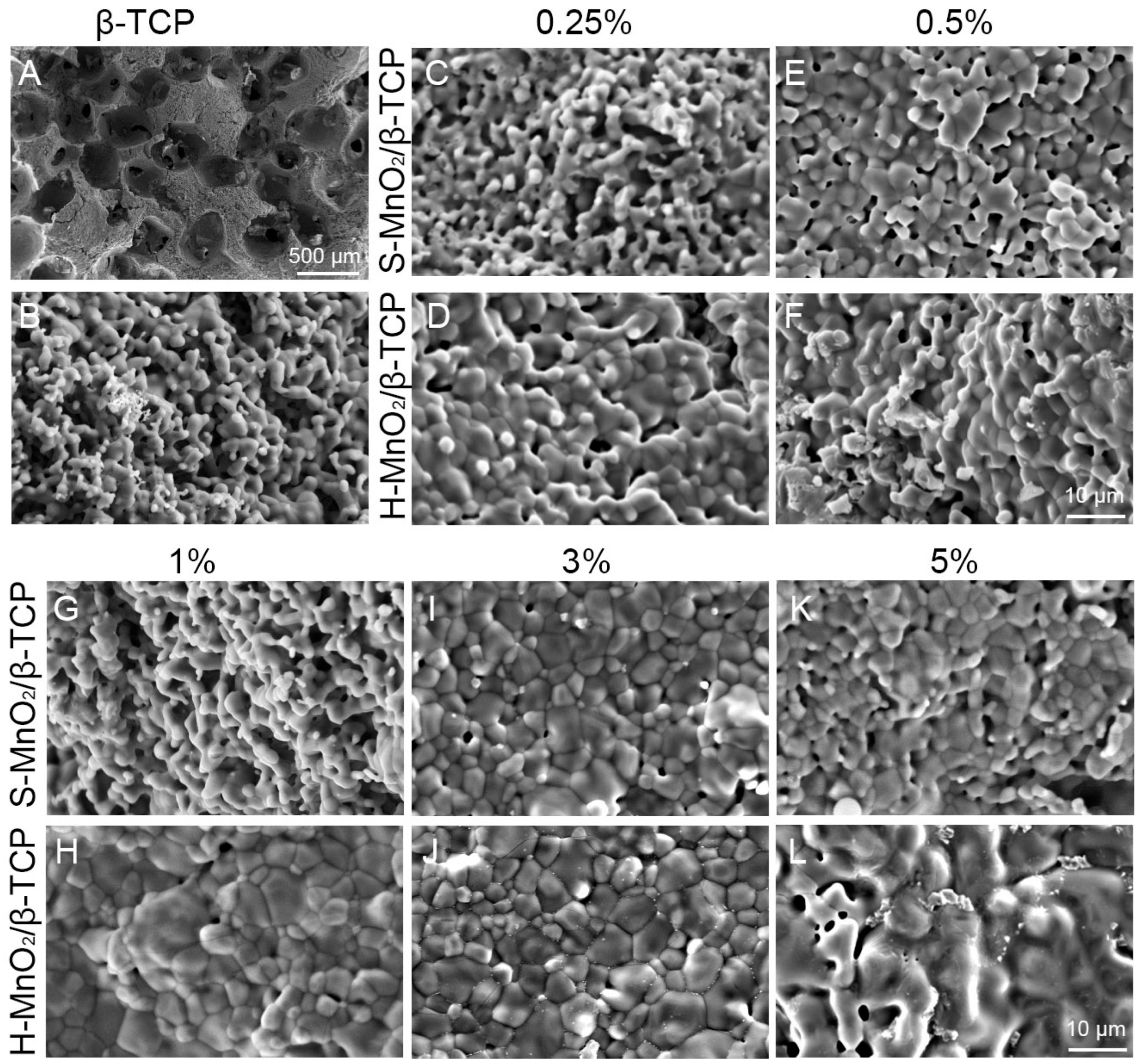
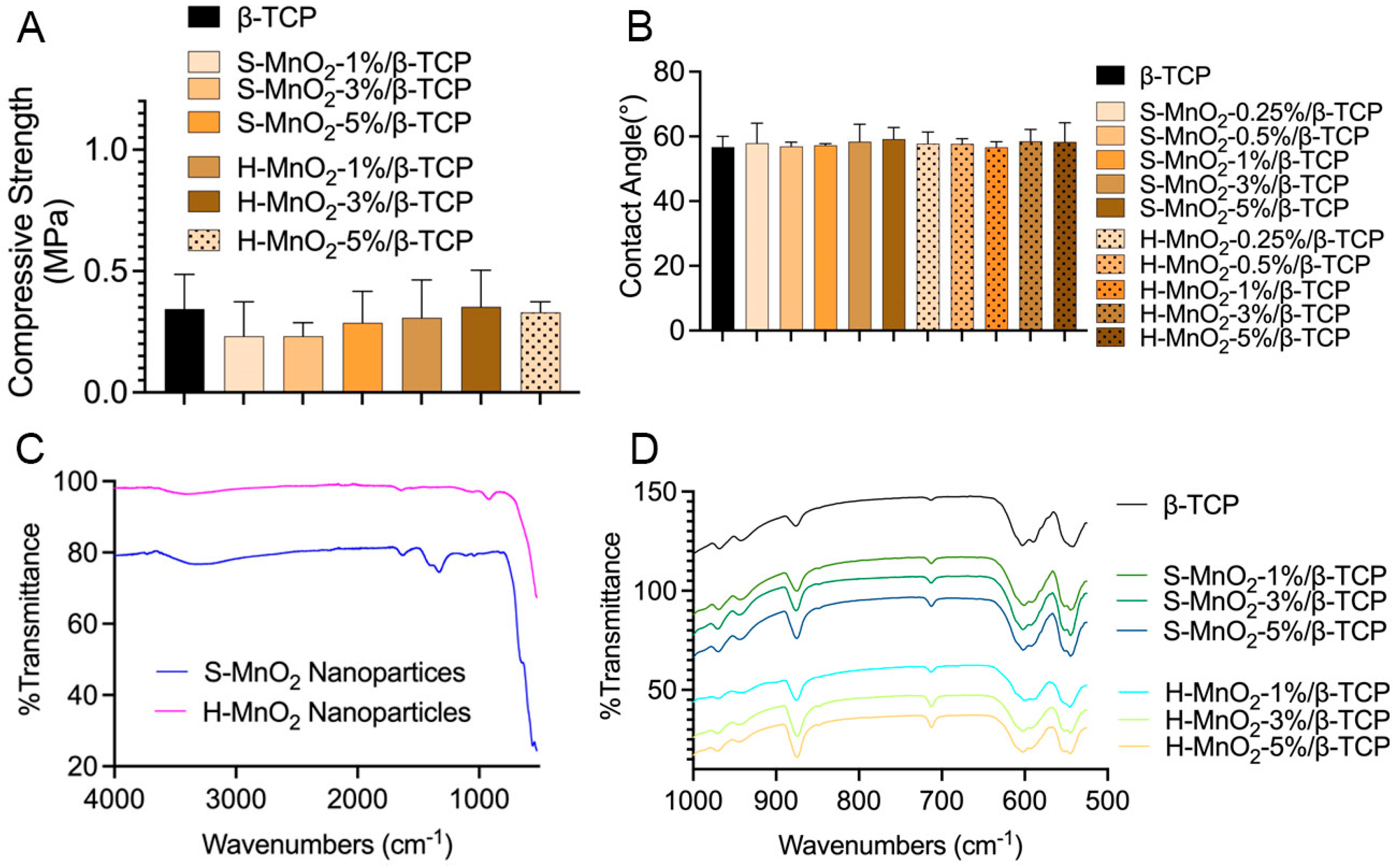
2.4. Characterizations of the Scaffolds
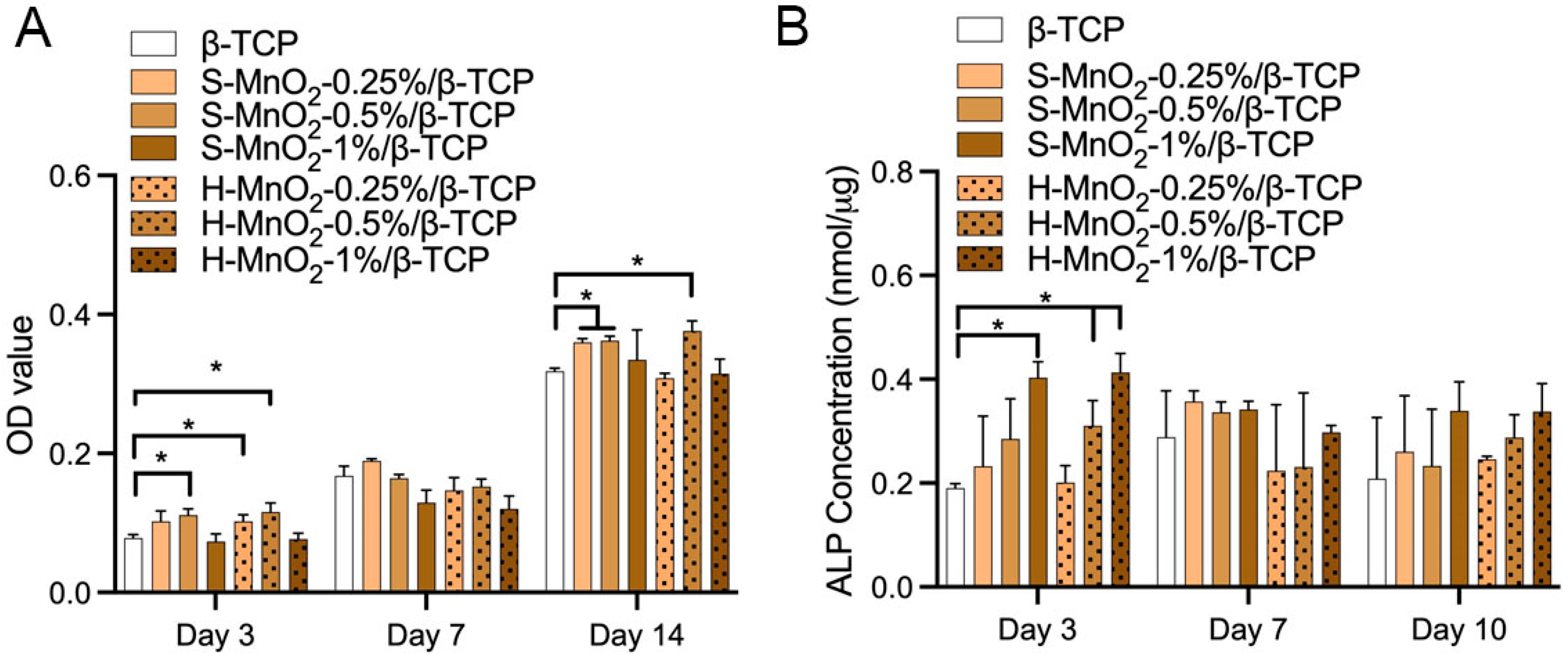
2.5. Cell Viability and Osteogenesis
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation and Characterization of H-MnO2 Nanoparticles
4.3. Preparation of MnO2/β-TCP Scaffolds
4.4. Characterization of the Porous Scaffolds
4.4.1. SEM Observations
4.4.2. Contact Angle Measurement
4.4.3. FTIR Measurement
4.4.4. Compressive Strength of Scaffolds
4.5. hBMSC Cell Behaviors
4.5.1. hBMSC Culture
4.5.2. Cell Proliferation on the Scaffolds
4.5.3. Alkaline Phosphatase (ALP)
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, K.Y.; Jeong, L.; Kang, Y.O.; Lee, S.J.; Park, W.H. Electrospinning of polysaccharides for regenerative medicine. Adv. Drug Deliv. Rev. 2009, 61, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Cen, L.; Liu, W.; Cui, L.; Zhang, W.; Cao, Y. Collagen tissue engineering: Development of novel biomaterials and applications. Pediatr. Res. 2008, 63, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [PubMed]
- Mano, J.F.; Silva, G.A.; Azevedo, H.S.; Malafaya, P.B.; Sousa, R.A.; Silva, S.S.; Boesel, L.F.; Oliveira, J.M.; Santos, T.C.; Marques, A.P.; et al. Natural origin biodegradable systems in tissue engineering and regenerative medicine: Present status and some moving trends. J. R. Soc. Interface/R. Soc. 2007, 4, 999–1030. [Google Scholar] [CrossRef]
- Zhu, J.; Marchant, R.E. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev. Med. Devices 2011, 8, 607–626. [Google Scholar] [CrossRef]
- Fisher, M.B.; Mauck, R.L. Tissue engineering and regenerative medicine: Recent innovations and the transition to translation. Tissue Eng. Part B Rev. 2013, 19, 1–13. [Google Scholar] [CrossRef]
- Tanaka, A.; Woltjen, K.; Miyake, K.; Hotta, A.; Ikeya, M.; Yamamoto, T.; Nishino, T.; Shoji, E.; Sehara-Fujisawa, A.; Manabe, Y.; et al. Efficient and reproducible myogenic differentiation from human iPS cells: Prospects for modeling Miyoshi Myopathy in vitro. PLoS ONE 2013, 8, e61540. [Google Scholar] [CrossRef]
- Hussain, W.; Moens, N.; Veraitch, F.S.; Hernandez, D.; Mason, C.; Lye, G.J. Reproducible culture and differentiation of mouse embryonic stem cells using an automated microwell platform. Biochem. Eng. J. 2013, 77, 246–257. [Google Scholar] [CrossRef]
- Dighe, N.; Khoury, M.; Mattar, C.; Chong, M.; Choolani, M.; Chen, J.; Antoniou, M.N.; Chan, J.K. Long-term reproducible expression in human fetal liver hematopoietic stem cells with a UCOE-based lentiviral vector. PLoS ONE 2014, 9, e104805. [Google Scholar] [CrossRef]
- Peters, A.; Burridge, P.W.; Pryzhkova, M.V.; Levine, M.A.; Park, T.S.; Roxbury, C.; Yuan, X.; Peault, B.; Zambidis, E.T. Challenges and strategies for generating therapeutic patient-specific hemangioblasts and hematopoietic stem cells from human pluripotent stem cells. Int. J. Dev. Biol. 2010, 54, 965–990. [Google Scholar] [CrossRef]
- Das, B.; Bayat-Mokhtari, R.; Tsui, M.; Lotfi, S.; Tsuchida, R.; Felsher, D.W.; Yeger, H. HIF-2alpha suppresses p53 to enhance the stemness and regenerative potential of human embryonic stem cells. Stem Cells 2012, 30, 1685–1695. [Google Scholar] [CrossRef]
- Safwani, W.K.; Makpol, S.; Sathapan, S.; Chua, K. Impact of adipogenic differentiation on stemness and osteogenic gene expression in extensive culture of human adipose-derived stem cells. Arch. Med. Sci. AMS 2014, 10, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Suda, T. Metabolic requirements for the maintenance of self-renewing stem cells. Nat. Rev. Mol. Cell Biol. 2014, 15, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Lovett, M.; Lee, K.; Edwards, A.; Kaplan, D.L. Vascularization strategies for tissue engineering. Tissue Eng. Part B Rev. 2009, 15, 353–370. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Mooney, D.J. Spatiotemporal control over growth factor signaling for therapeutic neovascularization. Adv. Drug Deliv. Rev. 2007, 59, 1340–1350. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Rettig, W.J.; Takaoka, K.; Alderman, E.; Rup, B.; Rosen, V.; Wozney, J.M.; Lane, J.M.; Huvos, A.G.; Garin-Chesa, P. Expression of bone morphogenetic proteins in human osteosarcoma. Immunohistochemical detection with monoclonal antibody. Cancer 1994, 73, 85–91. [Google Scholar] [CrossRef]
- Raval, P.; Hsu, H.H.; Schneider, D.J.; Sarras, M.P., Jr.; Masuhara, K.; Bonewald, L.F.; Anderson, H.C. Expression of bone morphogenetic proteins by osteoinductive and non-osteoinductive human osteosarcoma cells. J. Dent. Res. 1996, 75, 1518–1523. [Google Scholar] [CrossRef]
- Louis-Ugbo, J.; Kim, H.S.; Boden, S.D.; Mayr, M.T.; Li, R.C.; Seeherman, H.; D’Augusta, D.; Blake, C.; Jiao, A.; Peckham, S. Retention of 125I-labeled recombinant human bone morphogenetic protein-2 by biphasic calcium phosphate or a composite sponge in a rabbit posterolateral spine arthrodesis model. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2002, 20, 1050–1059. [Google Scholar] [CrossRef]
- Hollinger, J.O.; Uludag, H.; Winn, S.R. Sustained release emphasizing recombinant human bone morphogenetic protein-2. Adv. Drug Deliv. Rev. 1998, 31, 303–318. [Google Scholar]
- Gu, Y.; Zhang, J.; Zhang, X.; Liang, G.; Xu, T.; Niu, W. Three-dimensional Printed Mg-Doped beta-TCP Bone Tissue Engineering Scaffolds: Effects of Magnesium Ion Concentration on Osteogenesis and Angiogenesis In Vitro. Tissue Eng. Regen. Med. 2019, 16, 415–429. [Google Scholar] [CrossRef]
- Bose, S.; Banerjee, D.; Robertson, S.; Vahabzadeh, S. Enhanced In Vivo Bone and Blood Vessel Formation by Iron Oxide and Silica Doped 3D Printed Tricalcium Phosphate Scaffolds. Ann. Biomed. Eng. 2018, 46, 1241–1253. [Google Scholar] [CrossRef] [PubMed]
- Tohidnezhad, M.; Kubo, Y.; Lichte, P.; Heigl, T.; Roch, D.; Barahmand Pour, N.; Bergmann, C.; Sonmez, T.T.; Hock, J.V.P.; Fragoulis, A.; et al. Effects of Strontium-Doped beta-Tricalcium Scaffold on Longitudinal Nuclear Factor-Kappa Beta and Vascular Endothelial Growth Factor Receptor-2 Promoter Activities during Healing in a Murine Critical-Size Bone Defect Model. Int. J. Mol. Sci. 2020, 21, 3208. [Google Scholar] [CrossRef] [PubMed]
- Buchman, A.R. Manganese. In Modern Nutrition in Health and Disease, 11th ed.; Ross, A.C., Caballero, B., Cousins, R.J., Tucker, K.L., Ziegler, T.R., Eds.; Lippincott Williams Wilkins: Baltim, MD, USA, 2014; p. 7. [Google Scholar]
- Nielsen, F.H. Manganese, Molybdenum, Boron, Chromium, and Other Trace Elements. In Present Knowledge in Nutrition, 10th ed.; Erdman, J.W., Jr., Macdonald, I.A., Zeisel, S.H., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2012; p. 22. [Google Scholar]
- Palacios, C. The role of nutrients in bone health, from A to Z. Crit. Rev. Food Sci. Nutr. 2006, 46, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Saltman, P.D.; Strause, L.G. The role of trace minerals in osteoporosis. J. Am. Coll. Nutr. 1993, 12, 384–389. [Google Scholar] [CrossRef]
- Bae, Y.J.; Kim, M.H. Manganese supplementation improves mineral density of the spine and femur and serum osteocalcin in rats. Biol. Trace Elem. Res. 2008, 124, 28–34. [Google Scholar] [CrossRef]
- Fischer, A.E.; Pettigrew, K.A.; Rolison, D.R.; Stroud, R.M.; Long, J.W. Incorporation of homogeneous, nanoscale MnO2 within ultraporous carbon structures via self-limiting electroless deposition: Implications for electrochemical capacitors. Nano Lett. 2007, 7, 281–286. [Google Scholar] [CrossRef]
- Zhao, Z.; Fan, H.; Zhou, G.; Bai, H.; Liang, H.; Wang, R.; Zhang, X.; Tan, W. Activatable fluorescence/MRI bimodal platform for tumor cell imaging via MnO2 nanosheet-aptamer nanoprobe. J. Am. Chem. Soc. 2014, 136, 11220–11223. [Google Scholar] [CrossRef]
- Chen, Y.; Ye, D.; Wu, M.; Chen, H.; Zhang, L.; Shi, J.; Wang, L. Break-up of two-dimensional MnO2 nanosheets promotes ultrasensitive pH-triggered theranostics of cancer. Adv. Mater. 2014, 26, 7019–7026. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, K.; Zhu, J.; Zhang, L.; Lin, H. A FRET-based carbon dot-MnO2 nanosheet architecture for glutathione sensing in human whole blood samples. Chem. Commun. 2015, 51, 12748–12751. [Google Scholar] [CrossRef]
- Li, P.; Wei, M.; Zhang, F.; Su, J.; Wei, W.; Zhang, Y.; Liu, S. Novel Fluorescence Switch for MicroRNA Imaging in Living Cells Based on DNAzyme Amplification Strategy. ACS Appl. Mater. Interfaces 2018, 10, 43405–43410. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, J.; Zhang, D. Manganese Dioxide-Based Nanomaterials for Medical Applications. ACS Biomater. Sci. Eng. 2024, 10, 2680–2702. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Jia, Z.; Zhang, L.; Liu, G.; Li, J.; Zhao, J.; Xie, Y.; Chen, L.; Jiang, H.; He, W.; et al. A MnO2 nanosheets doping double crosslinked hydrogel for cartilage defect repair through alleviating inflammation and guiding chondrogenic differentiation. Biomaterials 2025, 314, 122875. [Google Scholar] [CrossRef] [PubMed]
- Rathnam, C.; Yang, L.; Castro-Pedrido, S.; Luo, J.; Cai, L.; Lee, K.B. Hybrid SMART spheroids to enhance stem cell therapy for CNS injuries. Sci. Adv. 2021, 7, eabj2281. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chueng, S.D.; Li, Y.; Patel, M.; Rathnam, C.; Dey, G.; Wang, L.; Cai, L.; Lee, K.B. A biodegradable hybrid inorganic nanoscaffold for advanced stem cell therapy. Nat. Commun. 2018, 9, 3147. [Google Scholar] [CrossRef]
- Li, L.; Xiao, B.; Mu, J.; Zhang, Y.; Zhang, C.; Cao, H.; Chen, R.; Patra, H.K.; Yang, B.; Feng, S.; et al. A MnO2 Nanoparticle-Dotted Hydrogel Promotes Spinal Cord Repair via Regulating Reactive Oxygen Species Microenvironment and Synergizing with Mesenchymal Stem Cells. ACS Nano 2019, 13, 14283–14293. [Google Scholar] [CrossRef]
- Greene, A.; Hashemi, J.; Kang, Y. Development of MnO2 hollow nanoparticles for potential drug delivery applications. Nanotechnology 2021, 32, 025713. [Google Scholar] [CrossRef]
- Kang, Y.; Kim, S.; Bishop, J.; Khademhosseini, A.; Yang, Y. The osteogenic differentiation of human bone marrow MSCs on HUVEC-derived ECM and beta-TCP scaffold. Biomaterials 2012, 33, 6998–7007. [Google Scholar] [CrossRef]
- Kang, Y.; Kim, S.; Khademhosseini, A.; Yang, Y. Creation of bony microenvironment with CaP and cell-derived ECM to enhance human bone-marrow MSC behavior and delivery of BMP-2. Biomaterials 2011, 32, 6119–6130. [Google Scholar] [CrossRef]
- Kang, Y.; Scully, A.; Young, D.A.; Kim, S.; Tsao, H.; Sen, M.; Yang, Y. Enhanced mechanical performance and biological evaluation of a PLGA coated beta-TCP composite scaffold for load-bearing applications. Eur. Polym. J. 2011, 47, 1569–1577. [Google Scholar] [CrossRef]
- Qian, F.; Huang, Z.; Liu, W.; Liu, Y.; He, X. Functional β-TCP/MnO/PCL artificial periosteum promoting osteogenic differentiation of BMSCs by reducing locally reactive oxygen species level. J. Biomed. Mater. Res. Part A 2023, 111, 1678–1691. [Google Scholar] [CrossRef]
- Wang, H.; Lu, Z.; Qian, D.; Li, Y.; Zhang, W. Single-crystal α-MnO2 nanorods: Synthesis and electrochemical properties. Nanotechnology 2007, 18, 115616. [Google Scholar] [CrossRef]
- Gangwar, D.; Rath, C. Structural, optical and magnetic properties of α- and β-MnO2 nanorods. Appl. Surf. Sci. 2021, 557, 149693. [Google Scholar] [CrossRef]
- Xie, Y.; Yu, Y.; Gong, X.; Guo, Y.; Guo, Y.; Wang, Y.; Lu, G. Effect of the crystal plane figure on the catalytic performance of MnO2 for the total oxidation of propane. CrystEngComm 2015, 17, 3005–3014. [Google Scholar] [CrossRef]
- Lin, S.; Xu, Z.; Liu, Y.; Yang, G.; Qi, X.; Huang, Y.; Zhou, M.; Jiang, X. Engineered Macrophage Membrane-Camouflaged Nanodecoys Reshape the Infectious Microenvironment for Efficient Periodontitis Treatment. ACS Nano 2025, 19, 15345–15362. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Qing, X.; Liu, S.; Yang, D.; Dong, X.; Zhang, Y. Hollow Mesoporous Manganese Oxides: Application in Cancer Diagnosis and Therapy. Small 2022, 18, e2106511. [Google Scholar] [CrossRef]
- Tamai, Y.; Aratani, K. Experimental study of the relation between contact angle and surface roughness. J. Phys. Chem. 1972, 76, 3267–3271. [Google Scholar] [CrossRef]
- Wenzel, R.N. Surface Roughness and Contact Angle. J. Phys. Colloid Chem. 1949, 53, 1466–1467. [Google Scholar] [CrossRef]
- Bizeau, J.; Tapeinos, C.; Marella, C.; Larranaga, A.; Pandit, A. Synthesis and characterization of hyaluronic acid coated manganese dioxide microparticles that act as ROS scavengers. Colloids Surf. B Biointerfaces 2017, 159, 30–38. [Google Scholar] [CrossRef]
- Tootoonchi, M.H.; Hashempour, M.; Blackwelder, P.L.; Fraker, C.A. Manganese oxide particles as cytoprotective, oxygen generating agents. Acta Biomater. 2017, 59, 327–337. [Google Scholar] [CrossRef]
- Chen, Q.; Li, J.; Han, F.; Meng, Q.; Wang, H.; Wei, Q.; Li, Z.; Li, F.; Xie, E.; Qin, X.; et al. A Multifunctional Composite Hydrogel That Rescues the ROS Microenvironment and Guides the Immune Response for Repair of Osteoporotic Bone Defects. Adv. Funct. Mater. 2022, 32, 2201067. [Google Scholar] [CrossRef]
- Li, J.; Deng, C.; Liang, W.; Kang, F.; Bai, Y.; Ma, B.; Wu, C.; Dong, S. Mn-containing bioceramics inhibit osteoclastogenesis and promote osteoporotic bone regeneration via scavenging ROS. Bioact. Mater. 2021, 6, 3839–3850. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yao, Z.; Sun, Y.; Nie, Y.; Zhang, Y.; Li, Z.; Luo, Z.; Zhang, W.; Wang, X.; Du, Y.; et al. 3D-printed manganese dioxide incorporated scaffold promotes osteogenic-angiogenic coupling for refractory bone defect by remodeling osteo-regenerative microenvironment. Bioact. Mater. 2025, 44, 354–370. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, J.; Jin, X.; Han, Y.; Lin, Y.; Lei, Z.; Wang, S.; Qin, L.; Jiao, S.; Cao, R. A Hollow-Structured Manganese Oxide Cathode for Stable Zn-MnO2 Batteries. Nanomater 2018, 8, 301. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kim, J.H.; Young, D.; Kim, S.; Nishimoto, S.K.; Yang, Y. Novel template-casting technique for fabricating beta-tricalcium phosphate scaffolds with high interconnectivity and mechanical strength and in vitro cell responses. J. Biomed. Mater. Res. A 2010, 92, 997–1006. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, E.; Eltawila, A.; Kang, Y. Doping of Hollow Urchin-like MnO2 Nanoparticles in Beta-Tricalcium Phosphate Scaffold Promotes Stem Cell Osteogenic Differentiation. Int. J. Mol. Sci. 2025, 26, 5092. https://doi.org/10.3390/ijms26115092
Qian E, Eltawila A, Kang Y. Doping of Hollow Urchin-like MnO2 Nanoparticles in Beta-Tricalcium Phosphate Scaffold Promotes Stem Cell Osteogenic Differentiation. International Journal of Molecular Sciences. 2025; 26(11):5092. https://doi.org/10.3390/ijms26115092
Chicago/Turabian StyleQian, Enze, Ahmed Eltawila, and Yunqing Kang. 2025. "Doping of Hollow Urchin-like MnO2 Nanoparticles in Beta-Tricalcium Phosphate Scaffold Promotes Stem Cell Osteogenic Differentiation" International Journal of Molecular Sciences 26, no. 11: 5092. https://doi.org/10.3390/ijms26115092
APA StyleQian, E., Eltawila, A., & Kang, Y. (2025). Doping of Hollow Urchin-like MnO2 Nanoparticles in Beta-Tricalcium Phosphate Scaffold Promotes Stem Cell Osteogenic Differentiation. International Journal of Molecular Sciences, 26(11), 5092. https://doi.org/10.3390/ijms26115092





