Discovery of TRPV4-Targeting Small Molecules with Anti-Influenza Effects Through Machine Learning and Experimental Validation
Abstract
1. Introduction
2. Result
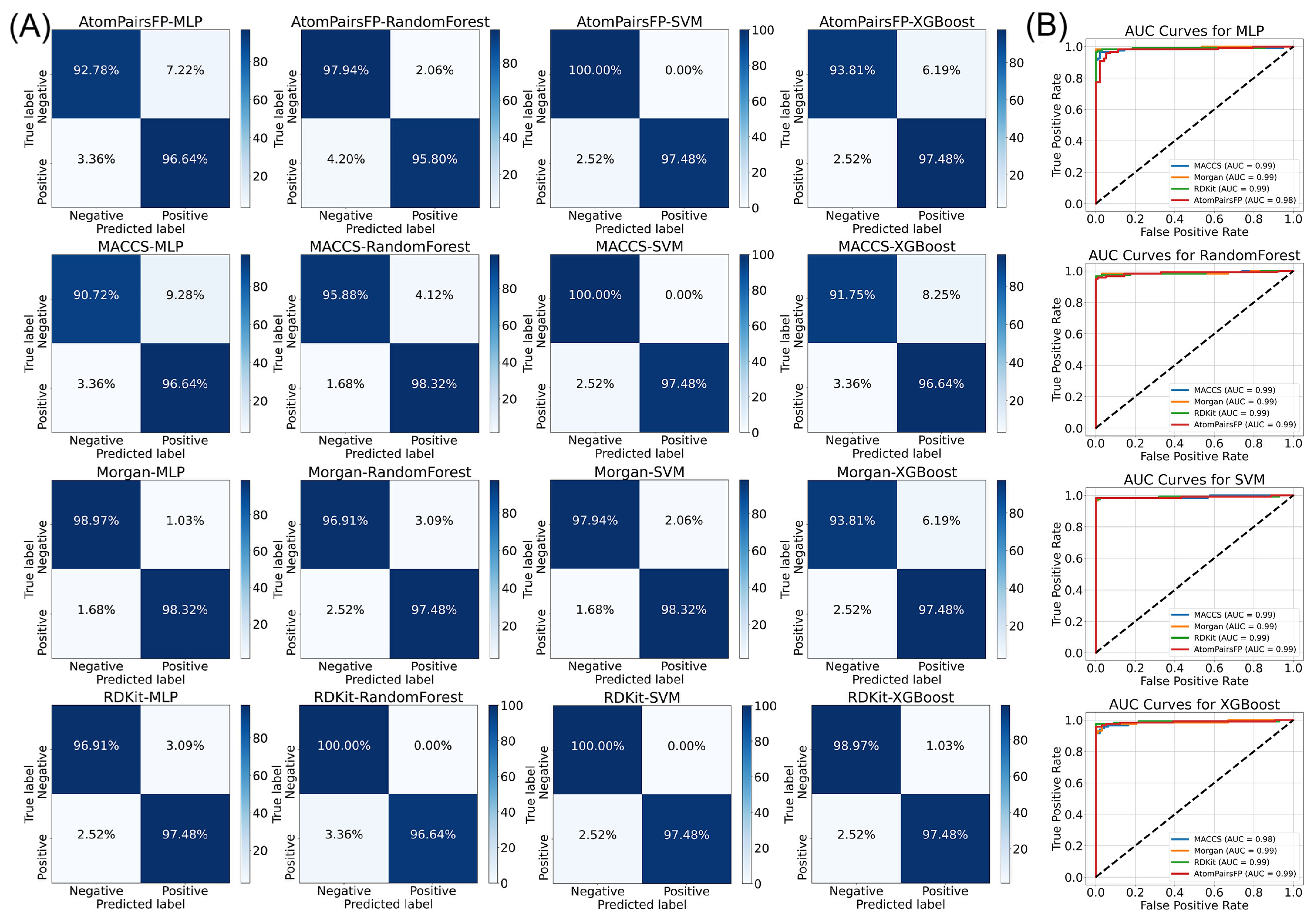
2.1. Machine Learning Integration
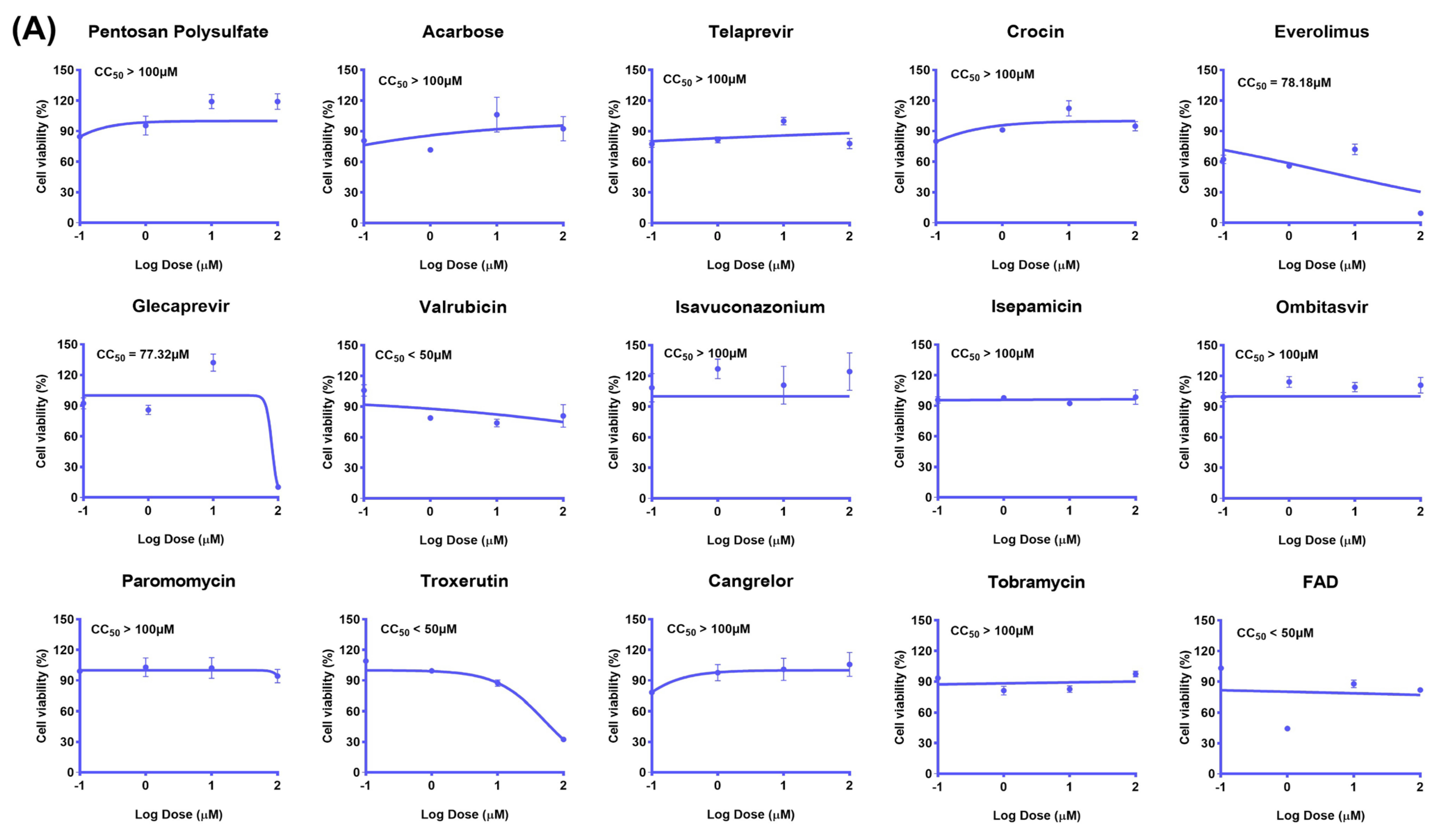
2.2. The Inhibitory Effect of the Drug Candidate on the Virus In Vitro
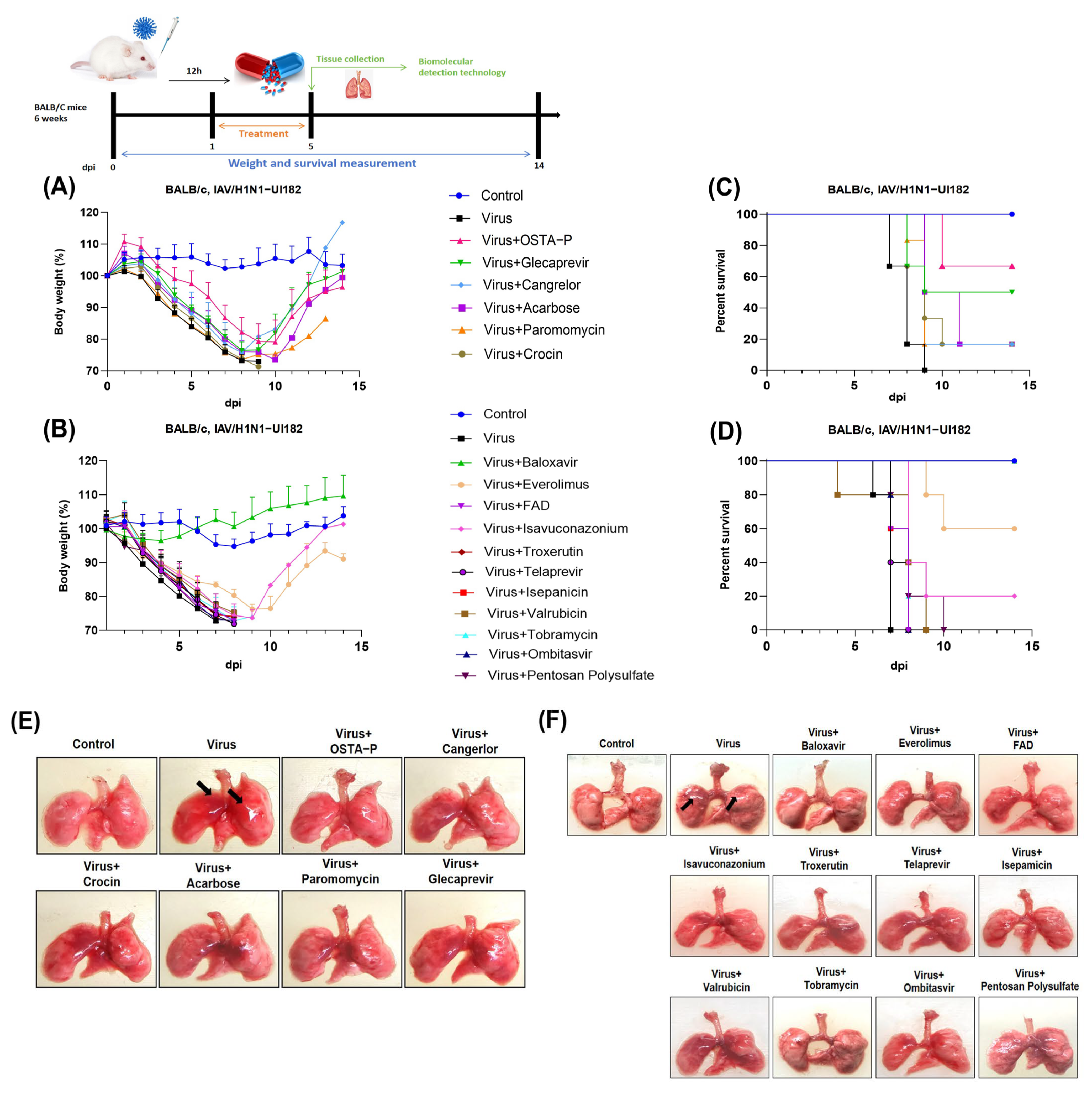
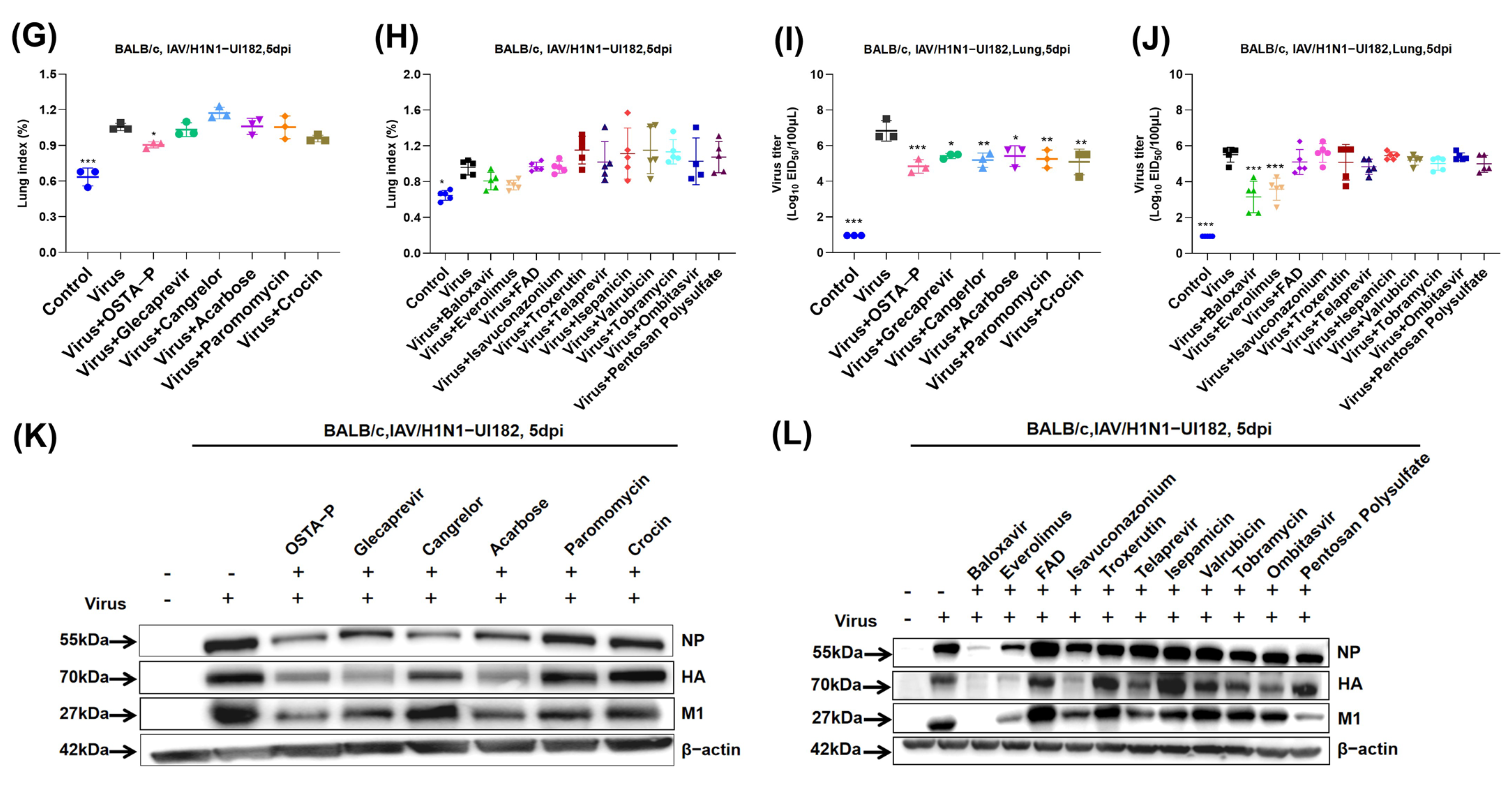
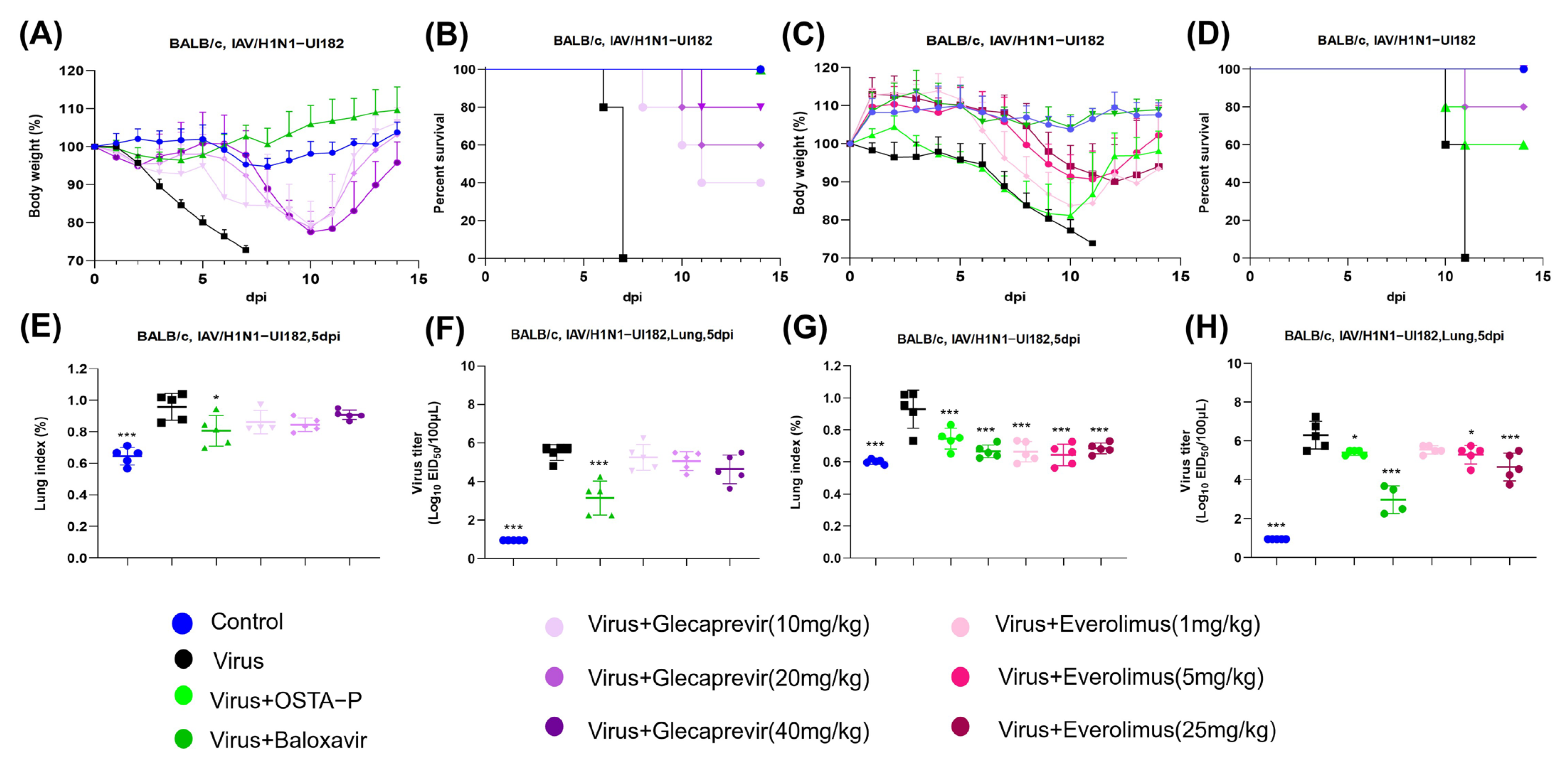
2.3. Evaluation of Candidate Drugs on Mice Infected with H1N1 Lethal Virus
2.4. Protection of Glecaprevir and Everolimus at Different Concentrations in Mice Infected with H1N1 Lethal Virus
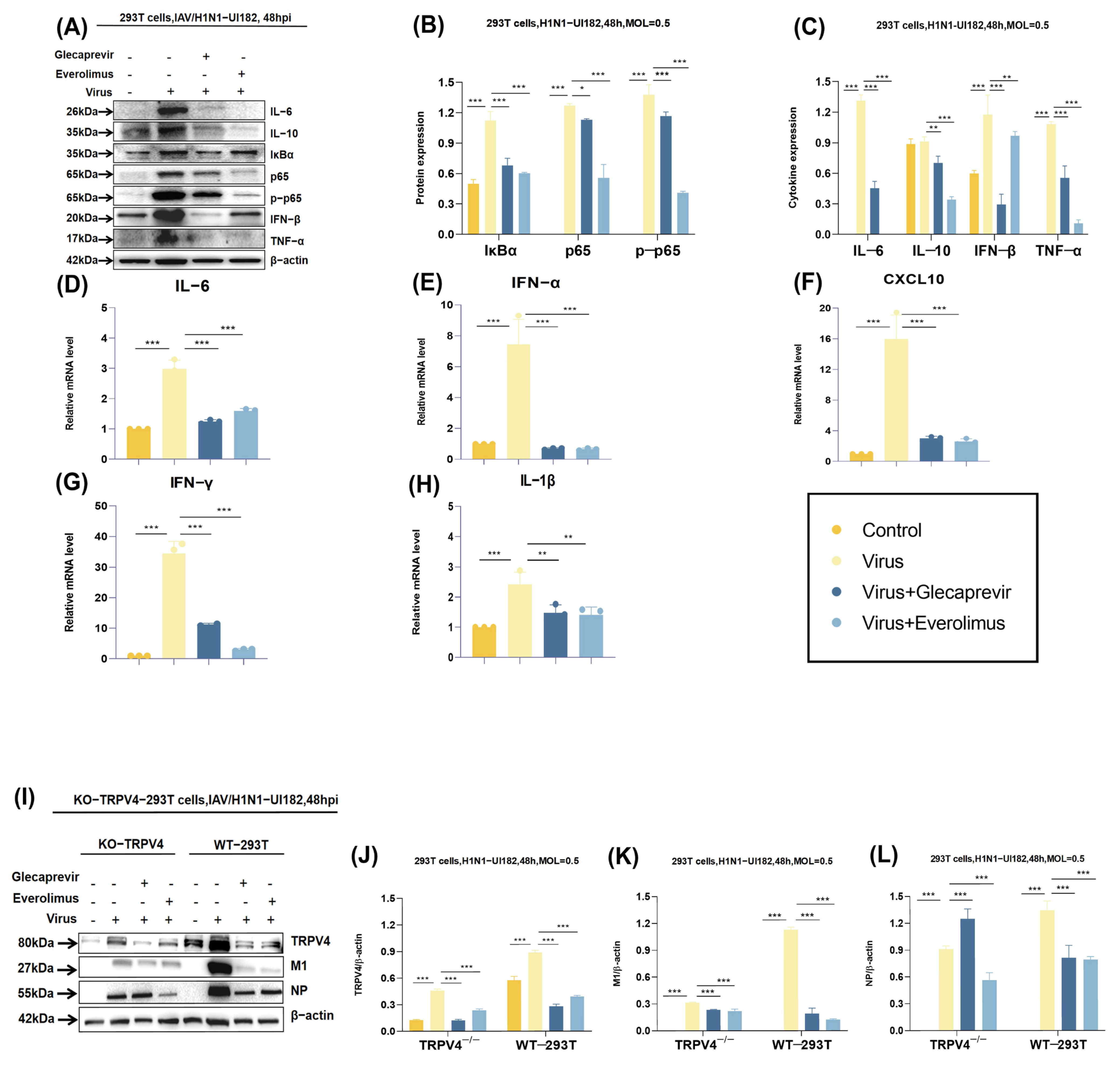
2.5. Molecular Mechanisms of Drug Candidates
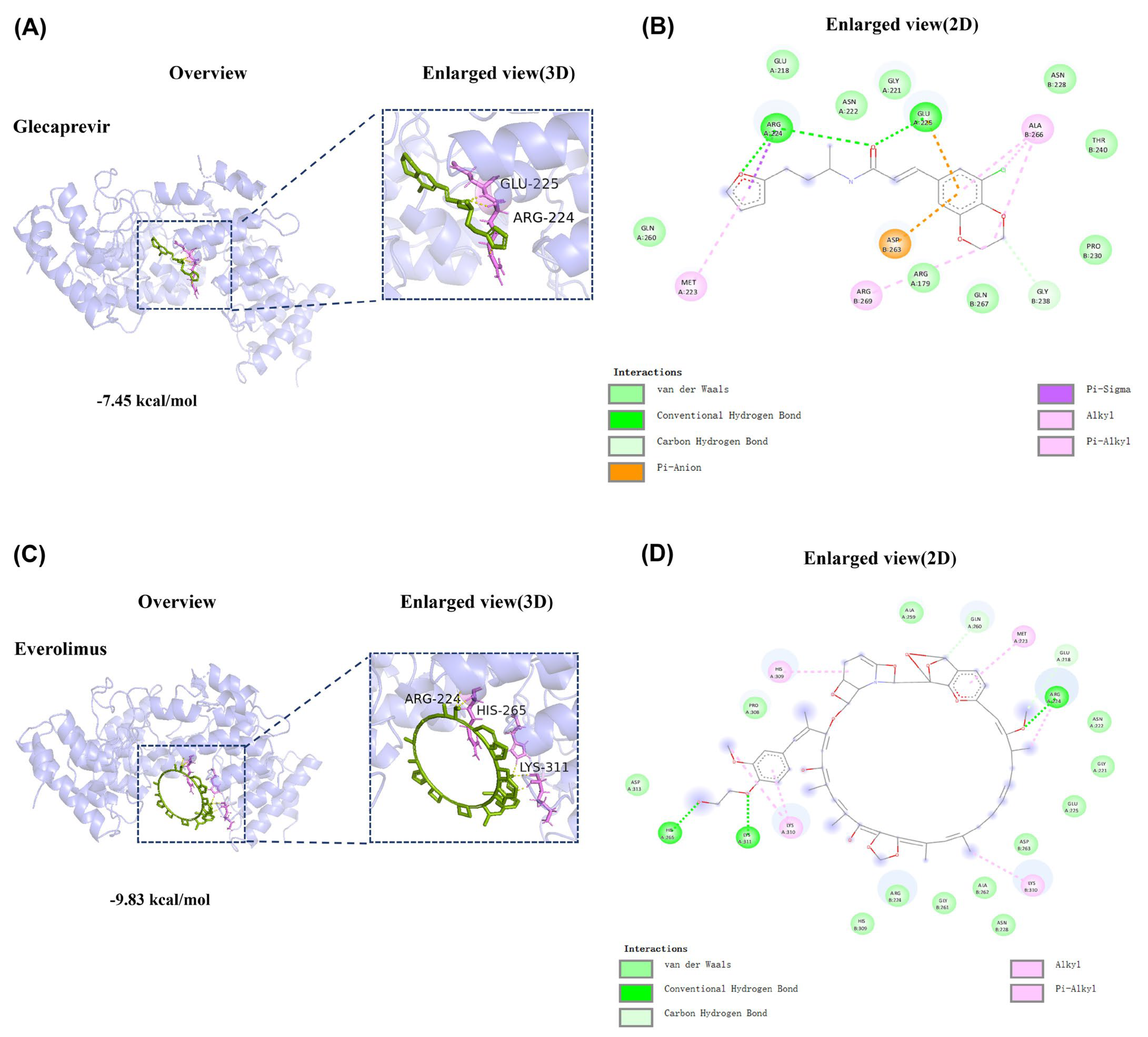
2.6. Binding Mode of the Compounds with TRPV4 Receptor Protein
3. Discussion
4. Materials and Methods
4.1. Machine Learning Models
4.2. Molecular Docking for Compounds and Target
4.3. Cell Culture, Reagents, Virus
4.4. Cytotoxicity Test and In Vitro Antiviral Activity Determination
4.5. Immunofluorescence Staining (IF)
4.6. Infection in Mice and Determination of Lung Index
4.7. RNA Isolation and Quantitative RT-qPCR
4.8. EID50 Detection
4.9. Pathological Analysis
4.10. Western Blot (WB)
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 293T | Human embryonic kidney 293T cells |
| AUC | Area under the curve |
| BA | Balanced accuracy |
| BSA | Bovine serum albumin |
| CADD | Computer-aided drug design |
| CC50 | Maximum cytotoxic concentration |
| CCK-8 | Cell counting kit-8 |
| DPI | Days post-infection |
| DMSO | Dimethyl sulfoxide |
| DMEM | Dulbecco modified Eagle culture medium |
| EID50 | 50% egg infectious dose |
| FBS | Fetal bovine serum |
| H&E | Hematoxylin and eosin |
| HRP | Horseradish peroxidase |
| HSV-2 | Herpes simplex virus type 2 |
| IV | Influenza virus |
| IAV | Influenza A virus |
| IL-6 | Interleukin 6 |
| IL-10 | Interleukin 10 |
| IFN-β | Interferon β |
| MDCK | Madin–Darby canine kidney |
| M1 | Matrix protein 1 |
| mTOR1 | Mammalian target of rapamycin complex 1 |
| MLP | Multi-layer perceptron |
| MCC | Matthews correlation coefficient |
| MACCS | Molecular access system |
| NP | Nucleoprotein |
| PA | Polymerase acidic |
| PDB | Protein data bank |
| PFA | Paraformaldehyde |
| RBC | Red blood cells |
| RT-qPCR | Real-time quantitative fluorescent PCR |
| RF | Random forest |
| SPF-grade | Specific pathogen free-grade |
| SMILES | Simplified molecular input line entry system |
| SVM | Support vector machine |
| TRPV4 | Transient receptor potential vanilloid 4 |
| TNF-α | Tumor necrosis factor α |
| TPSA | Topological polar surface areas |
| WB | Western blot |
| XGBoost | Extreme gradient boosting |
References
- Dadonaite, B.; Gilbertson, B.; Knight, M.L.; Trifkovic, S.; Rockman, S.; Laederach, A.; Brown, L.E.; Fodor, E.; Bauer, D.L.V. The structure of the influenza A virus genome. Nat. Microbiol. 2019, 4, 1781–1789. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Wang, L.F.; Sun, B.W.; Wan, W.B.; Ji, X.; Baele, G.; Bi, Y.H.; Suchard, M.A.; Lai, A.; Zhang, M.; et al. Zoonotic infections by avian influenza virus: Changing global epidemiology, investigation, and control. Lancet Infect. Dis. 2024, 24, e522–e531. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Sharma, S.D.; Kumar, A.; Ende, Z.; Mishina, M.; Wang, Y.; Falls, Z.; Samudrala, R.; Pohl, J.; Knight, P.R.; et al. Antiviral Approaches against Influenza Virus. Clin. Microbiol. Rev. 2023, 36, e0004022. [Google Scholar] [CrossRef] [PubMed]
- Kosik, I.; Angeletti, D.; Gibbs, J.S.; Angel, M.; Takeda, K.; Kosikova, M.; Nair, V.; Hickman, H.D.; Xie, H.; Brooke, C.B.; et al. Neuraminidase inhibition contributes to influenza A virus neutralization by anti-hemagglutinin stem antibodies. J. Exp. Med. 2019, 216, 304–316. [Google Scholar] [CrossRef]
- Upadhayay, A.; Ling, J.; Pal, D.; Xie, Y.; Ping, F.F.; Kumar, A. Resistance-proof antimicrobial drug discovery to combat global antimicrobial resistance threat. Drug Resist. Updat. 2023, 66, 100890. [Google Scholar] [CrossRef]
- Shen, Z.; Lou, K.; Wang, W. New small-molecule drug design strategies for fighting resistant influenza A. Acta Pharm. Sin. B 2015, 5, 419–430. [Google Scholar] [CrossRef][Green Version]
- Masuyama, R.; Mizuno, A.; Komori, H.; Kajiya, H.; Uekawa, A.; Kitaura, H.; Okabe, K.; Ohyama, K.; Komori, T. Calcium/calmodulin-signaling supports TRPV4 activation in osteoclasts and regulates bone mass. J. Bone Min. Res. 2012, 27, 1708–1721. [Google Scholar] [CrossRef]
- Grace, M.S.; Bonvini, S.J.; Belvisi, M.G.; McIntyre, P. Modulation of the TRPV4 ion channel as a therapeutic target for disease. Pharmacol. Ther. 2017, 177, 9–22. [Google Scholar] [CrossRef]
- Weber, M.; Sediri, H.; Felgenhauer, U.; Binzen, I.; Bänfer, S.; Jacob, R.; Brunotte, L.; García-Sastre, A.; Schmid-Burgk, J.L.; Schmidt, T.; et al. Influenza virus adaptation PB2-627K modulates nucleocapsid inhibition by the pathogen sensor RIG-I. Cell Host Microbe 2015, 17, 309–319. [Google Scholar] [CrossRef]
- Doñate-Macián, P.; Jungfleisch, J.; Pérez-Vilaró, G.; Rubio-Moscardo, F.; Perálvarez-Marín, A.; Diez, J.; Valverde, M.A. The TRPV4 channel links calcium influx to DDX3X activity and viral infectivity. Nat. Commun. 2018, 9, 2307. [Google Scholar] [CrossRef]
- Luo, J.; Feng, J.; Yu, G.; Yang, P.; Mack, M.R.; Du, J.; Yu, W.; Qian, A.; Zhang, Y.; Liu, S.; et al. Transient receptor potential vanilloid 4-expressing macrophages and keratinocytes contribute differentially to allergic and nonallergic chronic itch. J. Allergy Clin. Immunol. 2018, 141, 608–619.e7. [Google Scholar] [CrossRef] [PubMed]
- Doñate-Macian, P.; Duarte, Y.; Rubio-Moscardo, F.; Pérez-Vilaró, G.; Canan, J.; Díez, J.; González-Nilo, F.; Valverde, M.A. Structural determinants of TRPV4 inhibition and identification of new antagonists with antiviral activity. Br. J. Pharmacol. 2022, 179, 3576–3591. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Li, S.S.; Xu, X.F.; Yang, C.; Cheng, C.; Wang, J.S.; Zhou, P.Z.; Liu, S.W. TRPV4 channel is involved in HSV-2 infection in human vaginal epithelial cells through triggering Ca(2+) oscillation. Acta Pharmacol. Sin. 2023, 44, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Beroza, P.; Crawford, J.J.; Ganichkin, O.; Gendelev, L.; Harris, S.F.; Klein, R.; Miu, A.; Steinbacher, S.; Klingler, F.M.; Lemmen, C. Chemical space docking enables large-scale structure-based virtual screening to discover ROCK1 kinase inhibitors. Nat. Commun. 2022, 13, 6447. [Google Scholar] [CrossRef]
- Lyu, J.; Wang, S.; Balius, T.E.; Singh, I.; Levit, A.; Moroz, Y.S.; O’Meara, M.J.; Che, T.; Algaa, E.; Tolmachova, K.; et al. Ultra-large library docking for discovering new chemotypes. Nature 2019, 566, 224–229. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Wang, Y.; Zou, J.; Yang, R.; Luo, X.; Wu, C.; Yang, W.; Tian, C.; Xu, H.; et al. Generative deep learning enables the discovery of a potent and selective RIPK1 inhibitor. Nat. Commun. 2022, 13, 6891. [Google Scholar] [CrossRef]
- Zacharioudakis, E.; Agianian, B.; Mv, V.K.; Biris, N.; Garner, T.P.; Rabinovich-Nikitin, I.; Ouchida, A.T.; Margulets, V.; Nordstrøm, L.U.; Riley, J.S.; et al. Modulating mitofusins to control mitochondrial function and signaling. Nat. Commun. 2022, 13, 3775. [Google Scholar] [CrossRef]
- Clyde, A.; Galanie, S.; Kneller, D.W.; Ma, H.; Babuji, Y.; Blaiszik, B.; Brace, A.; Brettin, T.; Chard, K.; Chard, R.; et al. High-Throughput Virtual Screening and Validation of a SARS-CoV-2 Main Protease Noncovalent Inhibitor. J. Chem. Inf. Model. 2022, 62, 116–128. [Google Scholar] [CrossRef]
- Kumar, N.; Xin, Z.T.; Liang, Y.; Ly, H.; Liang, Y. NF-kappaB signaling differentially regulates influenza virus RNA synthesis. J. Virol. 2008, 82, 9880–9889. [Google Scholar] [CrossRef]
- Bai, H.; Si, L.; Jiang, A.; Belgur, C.; Zhai, Y.; Plebani, R.; Oh, C.Y.; Rodas, M.; Patil, A.; Nurani, A.; et al. Mechanical control of innate immune responses against viral infection revealed in a human lung alveolus chip. Nat. Commun. 2022, 13, 1928. [Google Scholar] [CrossRef]
- Zhang, X.; Lee, M.D.; Buckley, C.; Wilson, C.; McCarron, J.G. Mitochondria regulate TRPV4-mediated release of ATP. Br. J. Pharmacol. 2022, 179, 1017–1032. [Google Scholar] [CrossRef] [PubMed]
- Saurav, S.; Tanwar, J.; Ahuja, K.; Motiani, R.K. Dysregulation of host cell calcium signaling during viral infections: Emerging paradigm with high clinical relevance. Mol. Aspects Med. 2021, 81, 101004. [Google Scholar] [CrossRef] [PubMed]
- Acharya, D.; Reis, R.; Volcic, M.; Liu, G.; Wang, M.K.; Chia, B.S.; Nchioua, R.; Groß, R.; Münch, J.; Kirchhoff, F.; et al. Actin cytoskeleton remodeling primes RIG-I-like receptor activation. Cell 2022, 185, 3588–3602.e21. [Google Scholar] [CrossRef] [PubMed]
- Madreiter-Sokolowski, C.T.; Waldeck-Weiermair, M.; Bourguignon, M.P.; Villeneuve, N.; Gottschalk, B.; Klec, C.; Stryeck, S.; Radulovic, S.; Parichatikanond, W.; Frank, S.; et al. Enhanced inter-compartmental Ca(2+) flux modulates mitochondrial metabolism and apoptotic threshold during aging. Redox Biol. 2019, 20, 458–466. [Google Scholar] [CrossRef]
- Bihari, S.; Dixon, D.L.; Bersten, A.D. Ruthenium Red Inhibits Intravenous Fluid Induced Permeability Lung Odema Suggesting A Trpv4 Mechanism. Am. J. Respir. Crit. Care Med. 2014, 189, A3275. [Google Scholar]
- Thorneloe, K.S.; Cheung, M.; Bao, W.; Alsaid, H.; Lenhard, S.; Jian, M.Y.; Costell, M.; Maniscalco-Hauk, K.; Krawiec, J.A.; Olzinski, A.; et al. An orally active TRPV4 channel blocker prevents and resolves pulmonary edema induced by heart failure. Sci. Transl. Med. 2012, 4, 159ra148. [Google Scholar] [CrossRef]
- Lamb, Y.N. Glecaprevir/Pibrentasvir: First Global Approval. Drugs 2017, 77, 1797–1804. [Google Scholar] [CrossRef]
- Kandeel, M.; Al-Nazawi, M. Virtual screening and repurposing of FDA approved drugs against COVID-19 main protease. Life Sci. 2020, 251, 117627. [Google Scholar] [CrossRef]
- Dore, G.J.; Feld, J.J.; Thompson, A.; Martinello, M.; Muir, A.J.; Agarwal, K.; Müllhaupt, B.; Wedemeyer, H.; Lacombe, K.; Matthews, G.V.; et al. Simplified monitoring for hepatitis C virus treatment with glecaprevir plus pibrentasvir, a randomised non-inferiority trial. J. Hepatol. 2020, 72, 431–440. [Google Scholar] [CrossRef]
- Forns, X.; Lee, S.S.; Valdes, J.; Lens, S.; Ghalib, R.; Aguilar, H.; Felizarta, F.; Hassanein, T.; Hinrichsen, H.; Rincon, D.; et al. Glecaprevir plus pibrentasvir for chronic hepatitis C virus genotype 1, 2, 4, 5, or 6 infection in adults with compensated cirrhosis (EXPEDITION-1): A single-arm, open-label, multicentre phase 3 trial. Lancet Infect. Dis. 2017, 17, 1062–1068. [Google Scholar] [CrossRef]
- Nnani, D.U.; Campbell, A.; Ajaimy, M.; Saeed, O.; Patel, S.R.; Ahmed, S.; Graham, J.A.; Jorde, U.P. Effect of glecaprevir/pibrentasvir on weight-adjusted tacrolimus trough/dose ratios in heart and kidney transplant recipients. Transpl. Infect. Dis. 2021, 23, e13716. [Google Scholar] [CrossRef] [PubMed]
- Strueber, M.; Warnecke, G.; Fuge, J.; Simon, A.R.; Zhang, R.; Welte, T.; Haverich, A.; Gottlieb, J. Everolimus Versus Mycophenolate Mofetil De Novo After Lung Transplantation: A Prospective, Randomized. Open-Label Trial. Am. J. Transpl. 2016, 16, 3171–3180. [Google Scholar] [CrossRef] [PubMed]
- Kloker, L.D.; Berchtold, S.; Smirnow, I.; Beil, J.; Krieg, A.; Sipos, B.; Lauer, U.M. Oncolytic vaccinia virus GLV-1h68 exhibits profound antitumoral activities in cell lines originating from neuroendocrine neoplasms. BMC Cancer 2020, 20, 628. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, T.; Asthana, S.; Kneteman, N.M. m-TOR inhibitors: What role in liver transplantation? J. Hepatol. 2011, 55, 1441–1451. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Wu, J.; Shen, B.; Yang, H.; Cui, H.; Han, W.; Luo, R.; Zhang, S.; Li, H.; Qian, B.; et al. Discovery of TRPV4-Targeting Small Molecules with Anti-Influenza Effects Through Machine Learning and Experimental Validation. Int. J. Mol. Sci. 2025, 26, 1381. https://doi.org/10.3390/ijms26031381
Sun Y, Wu J, Shen B, Yang H, Cui H, Han W, Luo R, Zhang S, Li H, Qian B, et al. Discovery of TRPV4-Targeting Small Molecules with Anti-Influenza Effects Through Machine Learning and Experimental Validation. International Journal of Molecular Sciences. 2025; 26(3):1381. https://doi.org/10.3390/ijms26031381
Chicago/Turabian StyleSun, Yan, Jiajing Wu, Beilei Shen, Hengzheng Yang, Huizi Cui, Weiwei Han, Rongbo Luo, Shijun Zhang, He Li, Bingshuo Qian, and et al. 2025. "Discovery of TRPV4-Targeting Small Molecules with Anti-Influenza Effects Through Machine Learning and Experimental Validation" International Journal of Molecular Sciences 26, no. 3: 1381. https://doi.org/10.3390/ijms26031381
APA StyleSun, Y., Wu, J., Shen, B., Yang, H., Cui, H., Han, W., Luo, R., Zhang, S., Li, H., Qian, B., Fan, L., Zhang, J., Wang, T., Xia, X., Yan, F., & Gao, Y. (2025). Discovery of TRPV4-Targeting Small Molecules with Anti-Influenza Effects Through Machine Learning and Experimental Validation. International Journal of Molecular Sciences, 26(3), 1381. https://doi.org/10.3390/ijms26031381






