Correlations Between Gut Microbiota Composition, Medical Nutrition Therapy, and Insulin Resistance in Pregnancy—A Narrative Review
Abstract
1. Introduction
2. Methodology
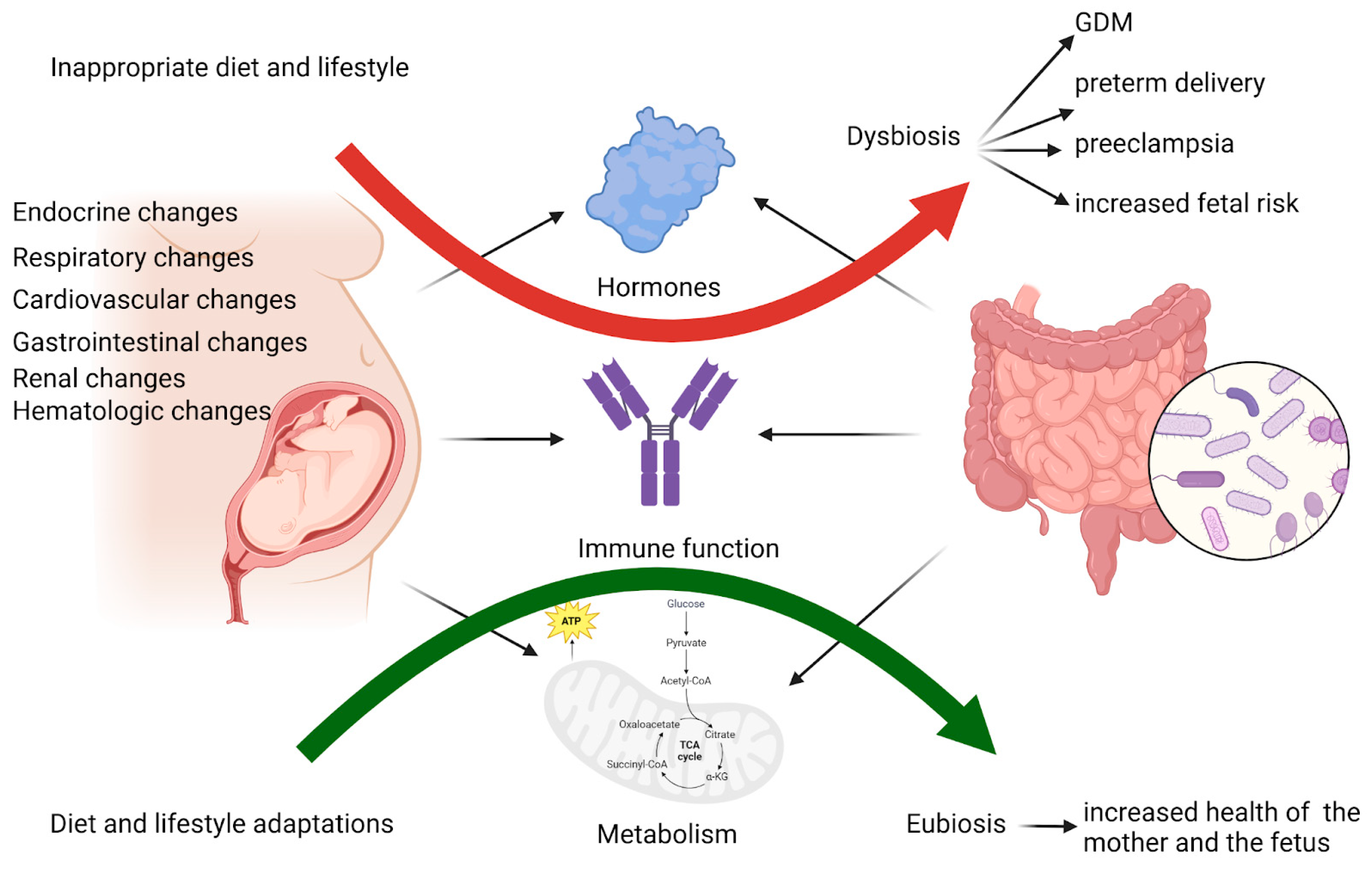
3. Intestinal Microbiota and Host Metabolic Interactions During Pregnancy
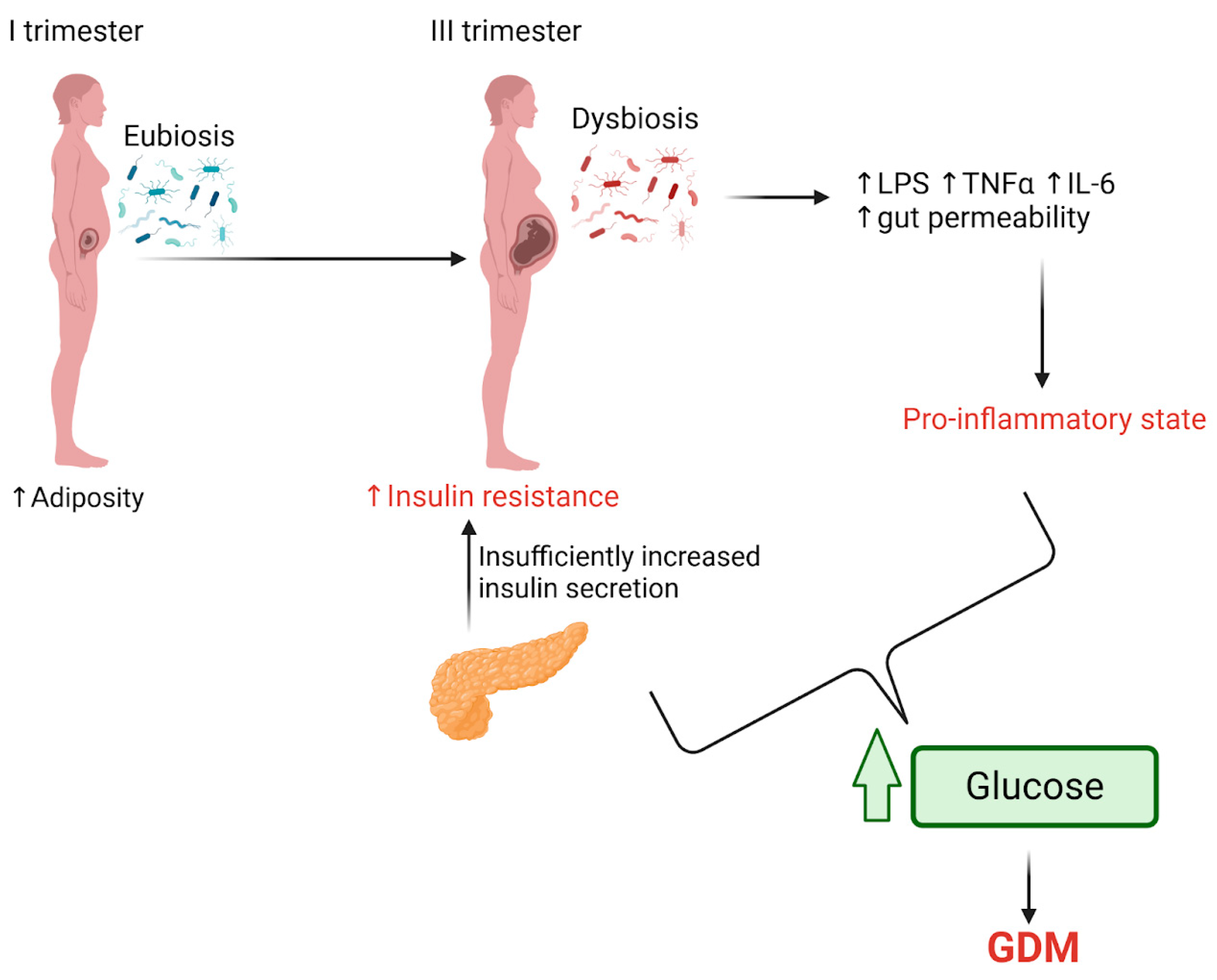
4. Changes in Gut Microbiota in Pregnancies Complicated with Insulin Resistance and GDM
5. The Role of Microbial Metabolites Resulting from an Imbalanced Microbiota
- BAs are small molecules synthesized from cholesterol in hepatocytes, chenodeoxycholic acid and cholic acid (primary BAs). These are conjugated with glycine and taurine to facilitate lipid and vitamin digestion and absorption. Gut microbiota deconjugate these into secondary BAs. In patients with obesity and metabolic syndrome, BA metabolism is disrupted, particularly affecting primary BA metabolism, which contributes to hepatic steatosis and altered glucose and lipid metabolism. BAs play a crucial role in metabolic regulation by influencing serum triglyceride synthesis through the FGF19/FGF15 pathways and interacting with nuclear receptors such as through Farnesoid-X receptor (FXR) and Takeda G-protein-coupled receptor 5. Activation of these pathways increases hepatic glycogen synthesis, insulin sensitivity, pancreatic insulin secretion, energy expenditure (in the liver, brown adipose tissue, and muscles), and thermogenesis, resulting in weight loss and increased satiety in the brain. Gut microbiota dysbiosis impairs ileal absorption of BAs, normally mediated by the apical sodium-dependent bile acid transporter. This impairment reduces FXR and FGF19 expression, leading to an imbalance in BAs, particularly an increase in colonic primary conjugated Bas, which have pro-inflammatory effects on intestinal epithelial cells. These effects weaken intestinal barrier function and increase permeability through the phosphorylation occludin in intestinal Caco-2 cells. Research has shown that a high intake of animal fats increases taurocholic acid levels, stimulating the growth of sulfite-reducing bacteria like Bilophila wadsworthia, which raises susceptibility to colitis, exacerbates liver steatosis, impairs intestinal barrier function, and disrupts glucose metabolism. Other studies have demonstrated that secondary BA can regulate metabolic homeostasis in mice. Antibiotic supplementation, which reduces secondary BA-producing bacteria, has been found to lower hepatic concentrations of deoxycholic acid and lithocholic acid and decrease serum triglyceride levels [75,78,79,80,81].
- SCFAs (butyrate, propionate, and acetate) are end-products of microbial fermentation with numerous physiological roles, mainly mediated through specific G protein-coupled receptors and epigenetic mechanisms. These roles include maintaining intestinal mucosal integrity, enhancing glucose and lipid metabolism, regulating energy expenditure, and modulating immune responses and inflammation. Research has shown that SCFA-producing bacteria and SCFAs are reduced in the fecal samples of obese or diabetic patients with gut dysbiosis. Supplementation with SCFAs (inulin-propionate ester, acetate, or propionate) has increased energy expenditure, improved glucose tolerance and metabolic homeostasis, and enhanced the production of GLP-1 and PYY, leading to reduced weight gain. Interestingly, some studies have shown that maternal gut microbiota, through the production of SCFAs, can activate embryonic GPR41 and GPR43 receptors, influencing the prenatal development of the neural, enteroendocrine, and pancreatic systems in offspring. This process helps maintain postnatal energy homeostasis and may prevent the growth of metabolic disorders [75,82,83,84,85,86].
- BCAAs (valine, isoleucine, and leucine) are essential amino acids that plants, fungi, and bacteria synthesize, especially the gut microbiota. They are key in regulating protein synthesis, glucose and lipid metabolism, insulin resistance, hepatocyte proliferation, immunity, and thermogenesis in brown adipose tissue. Some studies have shown that increased calorie consumption, which can lead to gut dysbiosis, raises systemic BCAA levels and is associated with obesity and diabetes by promoting insulin resistance. Insulin resistance has been linked to elevated levels of Prevotella copri and Bacteroides vulgatus (which produce BCAAs) and reduced levels of Butyrivibrio crossotus and Eubacterium siraeum (which can utilize BCAAs) [23,75,87,88,89].
- The gut microbiota produces TMAO through dietary choline and L-carnitine metabolism, leading to trimethylamine formation. This compound is absorbed and transported to the liver, where it is converted into TMAO by hepatic flavin monooxygenase 3. Studies have shown that elevated TMAO levels from dietary sources are directly involved in the development of metabolic diseases such as diabetes and obesity, as well as increasing the risk of cardiovascular disease and kidney failure. Research has also demonstrated that in antibiotic-treated mice with secondary dysbiosis, dietary supplementation with TMAO increases the risk of atherosclerosis [90,91,92].
- Tryptophan is an essential aromatic amino acid obtained from the diet, involved in protein synthesis and metabolite production through three main pathways: the kynurenine pathway, the serotonin pathway, and a gut microbiota-mediated pathway that converts tryptophan into indole and its derivatives. Studies have shown that metabolic disorders and gut dysbiosis reduce the microbiota’s ability to metabolize tryptophan, leading to decreased production of GLP-1 and IL-22, increased intestinal permeability, and LPS translocation, which contribute to inflammation, insulin resistance, obesity, and liver steatosis. Other studies have found that Lactobacillus reuteri administration can produce aryl hydrocarbon receptor ligands that help reverse metabolic dysfunction, while indole supplementation in mice can prevent LPS-induced disruptions in cholesterol metabolism and reduce liver inflammation [75,93,94,95].
- Imidazole propionate is a metabolite produced from the gut microbiota’s metabolism of histidine, which has been linked to insulin resistance and T2DM by disrupting the insulin signaling pathway through activation of the mammalian target of rapamycin complex 1 (mTORC1) in the liver. Some studies have also identified a connection between imidazole propionate and low-grade inflammation in individuals with an unhealthy diet and secondary dysbiosis [75,96].
6. Diet–Microbiota Interactions in Normal and Complicated Pregnancies
7. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Bas | bile acids |
| BCAAs | branched-chain amino acids |
| FXR | Farnesoid-X receptor |
| GDM | gestational diabetes mellitus |
| hCG | human chorionic gonadotropin |
| HOMA-IR | Homeostasis Model Assessment of Insulin Resistance |
| LPS | lipopolysaccharide |
| MNT | medical nutritional therapy |
| SCFAs | short-chain fatty acids |
| T2DM | type 2 diabetes mellitus |
| TMAO | trimethylamine N-oxide |
References
- Ferrara, A. Increasing prevalence of gestational diabetes mellitus: A public health perspective. Diabetes Care 2007, 30 (Suppl. S2), S141–S146. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, C. Prevalence of Gestational Diabetes and Risk of Progression to Type 2 Diabetes: A Global Perspective. Curr. Diabetes Rep. 2016, 16, 7. [Google Scholar] [CrossRef] [PubMed]
- Metzger, B.E.; Lowe, L.P.; Dyer, A.R.; Trimble, E.R.; Chaovarindr, U.; Coustan, D.R.; Hadden, D.R.; McCance, D.R.; Hod, M.; McIntyre, H.D.; et al. Hyperglycemia and adverse pregnancy outcomes. N. Engl. J. Med. 2008, 358, 1991–2002. [Google Scholar] [CrossRef] [PubMed]
- Kampmann, U.; Madsen, L.R.; Skajaa, G.O.; Iversen, D.S.; Moeller, N.; Ovesen, P. Gestational diabetes: A clinical update. World J. Diabetes 2015, 6, 1065–1072. [Google Scholar] [CrossRef]
- Kim, C.; Newton, K.M.; Knopp, R.H. Gestational diabetes and the incidence of type 2 diabetes: A systematic review. Diabetes Care 2002, 25, 1862–1868. [Google Scholar] [CrossRef]
- Bellamy, L.; Casas, J.P.; Hingorani, A.D.; Williams, D. Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta-analysis. Lancet 2009, 373, 1773–1779. [Google Scholar] [CrossRef]
- Sheiner, E. Gestational Diabetes Mellitus: Long-Term Consequences for the Mother and Child Grand Challenge: How to Move on Towards Secondary Prevention? Front. Clin. Diabetes Healthc. 2020, 1, 546256. [Google Scholar] [CrossRef]
- Fuchs, O.; Sheiner, E.; Meirovitz, M.; Davidson, E.; Sergienko, R.; Kessous, R. The association between a history of gestational diabetes mellitus and future risk for female malignancies. Arch. Gynecol. Obstet. 2017, 295, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Bernea, E.G.; Uyy, E.; Mihai, D.A.; Ceausu, I.; Ionescu-Tirgoviste, C.; Suica, V.I.; Ivan, L.; Antohe, F. New born macrosomia in gestational diabetes mellitus. Exp. Ther. Med. 2022, 24, 710. [Google Scholar] [CrossRef] [PubMed]
- Mitanchez, D. Foetal and neonatal complications in gestational diabetes: Perinatal mortality, congenital malformations, macrosomia, shoulder dystocia, birth injuries, neonatal complications. Diabetes Metab. 2010, 36, 617–627. [Google Scholar] [CrossRef]
- Dabelea, D.; Pettitt, D.J. Intrauterine diabetic environment confers risks for type 2 diabetes mellitus and obesity in the offspring, in addition to genetic susceptibility. J. Pediatr. Endocrinol. Metab. 2001, 14, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Reece, E.A.; Leguizamón, G.; Wiznitzer, A. Gestational diabetes: The need for a common ground. Lancet 2009, 373, 1789–1797. [Google Scholar] [CrossRef]
- McIntyre, H.D.; Catalano, P.; Zhang, C.; Desoye, G.; Mathiesen, E.R.; Damm, P. Gestational diabetes mellitus. Nat. Rev. Dis. Primers 2019, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Canfora, E.E.; Meex, R.C.R.; Venema, K.; Blaak, E.E. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat. Rev. Endocrinol. 2019, 15, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Letchumanan, G.; Abdullah, N.; Marlini, M.; Baharom, N.; Lawley, B.; Omar, M.R.; Mohideen, F.B.S.; Addnan, F.H.; Nur Fariha, M.M.; Ismail, Z.; et al. Gut Microbiota Composition in Prediabetes and Newly Diagnosed Type 2 Diabetes: A Systematic Review of Observational Studies. Front. Cell. Infect. Microbiol. 2022, 12, 943427. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Ren, H.; Lu, Y.; Fang, C.; Hou, G.; Yang, Z.; Chen, B.; Yang, F.; Zhao, Y.; Shi, Z.; et al. Distinct gut metagenomics and metaproteomics signatures in prediabetics and treatment-naïve type 2 diabetics. EBioMedicine 2019, 47, 373–383. [Google Scholar] [CrossRef]
- Karlsson, F.H.; Tremaroli, V.; Nookaew, I.; Bergström, G.; Behre, C.J.; Fagerberg, B.; Nielsen, J.; Bäckhed, F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 2013, 498, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Tremaroli, V.; Schmidt, C.; Lundqvist, A.; Olsson, L.M.; Krämer, M.; Gummesson, A.; Perkins, R.; Bergström, G.; Bäckhed, F. The Gut Microbiota in Prediabetes and Diabetes: A Population-Based Cross-Sectional Study. Cell Metab. 2020, 32, 379–390.e373. [Google Scholar] [CrossRef]
- Wu, H.; Esteve, E.; Tremaroli, V.; Khan, M.T.; Caesar, R.; Mannerås-Holm, L.; Ståhlman, M.; Olsson, L.M.; Serino, M.; Planas-Fèlix, M.; et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat. Med. 2017, 23, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Ben-Haroush, A.; Yogev, Y.; Hod, M. Epidemiology of gestational diabetes mellitus and its association with Type 2 diabetes. Diabet. Med. 2004, 21, 103–113. [Google Scholar] [CrossRef]
- Krautkramer, K.A.; Fan, J.; Bäckhed, F. Gut microbial metabolites as multi-kingdom intermediates. Nat. Rev. Microbiol. 2021, 19, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G.; et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 2016, 535, 376–381. [Google Scholar] [CrossRef]
- Pedersen, H.K.; Forslund, S.K.; Gudmundsdottir, V.; Petersen, A.; Hildebrand, F.; Hyötyläinen, T.; Nielsen, T.; Hansen, T.; Bork, P.; Ehrlich, S.D.; et al. A computational framework to integrate high-throughput ‘-omics’ datasets for the identification of potential mechanistic links. Nat. Protoc. 2018, 13, 2781–2800. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, Z.Z.; Al-Rumaihi, S.; Al-Absi, R.S.; Farah, H.; Elamin, M.; Nader, R.; Bouabidi, S.; Suleiman, S.E.; Nasr, S.; Al-Asmakh, M. Physiological Changes and Interactions Between Microbiome and the Host During Pregnancy. Front. Cell. Infect. Microbiol. 2022, 12, 824925. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, R.F.; Enache, R.M.; Cretoiu, S.M.; Gaspar, B.S. Gut Microbiome Changes in Gestational Diabetes. Int. J. Mol. Sci. 2022, 23, 12839. [Google Scholar] [CrossRef]
- Giannella, L.; Grelloni, C.; Quintili, D.; Fiorelli, A.; Montironi, R.; Alia, S.; Delli Carpini, G.; Di Giuseppe, J.; Vignini, A.; Ciavattini, A. Microbiome Changes in Pregnancy Disorders. Antioxidants 2023, 12, 463. [Google Scholar] [CrossRef] [PubMed]
- Gorczyca, K.; Obuchowska, A.; Kimber-Trojnar, Ż.; Wierzchowska-Opoka, M.; Leszczyńska-Gorzelak, B. Changes in the Gut Microbiome and Pathologies in Pregnancy. Int. J. Environ. Res. Public Health 2022, 19, 9961. [Google Scholar] [CrossRef]
- Koren, O.; Goodrich, J.K.; Cullender, T.C.; Spor, A.; Laitinen, K.; Bäckhed, H.K.; Gonzalez, A.; Werner, J.J.; Angenent, L.T.; Knight, R.; et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 2012, 150, 470–480. [Google Scholar] [CrossRef]
- Conlon, M.A.; Bird, A.R. The impact of diet and lifestyle on gut microbiota and human health. Nutrients 2014, 7, 17–44. [Google Scholar] [CrossRef]
- Kunasegaran, T.; Balasubramaniam, V.; Arasoo, V.J.T.; Palanisamy, U.D.; Ramadas, A. Diet Gut Microbiota Axis in Pregnancy: A Systematic Review of Recent Evidence. Curr. Nutr. Rep. 2023, 12, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Rosendo-Silva, D.; Viana, S.; Carvalho, E.; Reis, F.; Matafome, P. Are gut dysbiosis, barrier disruption, and endotoxemia related to adipose tissue dysfunction in metabolic disorders? Overview of the mechanisms involved. Intern. Emerg. Med. 2023, 18, 1287–1302. [Google Scholar] [CrossRef] [PubMed]
- Aziz, T.; Hussain, N.; Hameed, Z.; Lin, L. Elucidating the role of diet in maintaining gut health to reduce the risk of obesity, cardiovascular and other age-related inflammatory diseases: Recent challenges and future recommendations. Gut Microbes 2024, 16, 2297864. [Google Scholar] [CrossRef] [PubMed]
- Pheiffer, C.; Riedel, S.; Dias, S.; Adam, S. Gestational Diabetes and the Gut Microbiota: Fibre and Polyphenol Supplementation as a Therapeutic Strategy. Microorganisms 2024, 12, 633. [Google Scholar] [CrossRef] [PubMed]
- Ziętek, M.; Celewicz, Z.; Szczuko, M. Short-Chain Fatty Acids, Maternal Microbiota and Metabolism in Pregnancy. Nutrients 2021, 13, 1244. [Google Scholar] [CrossRef]
- Miller, C.; McQuade, M.; MacCallum, J.; Zhang, Y.V. Persistently elevated hCG in a patient with no clear evidence of pregnancy. Clin. Chim. Acta 2020, 510, 703–706. [Google Scholar] [CrossRef] [PubMed]
- Sykes, L.; Bennett, P.R. Efficacy of progesterone for prevention of preterm birth. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 52, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Barjaktarovic, M.; Korevaar, T.I.; Jaddoe, V.W.; de Rijke, Y.B.; Visser, T.J.; Peeters, R.P.; Steegers, E.A. Human chorionic gonadotropin (hCG) concentrations during the late first trimester are associated with fetal growth in a fetal sex-specific manner. Eur. J. Epidemiol. 2017, 32, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Mockridge, A.; Maclennan, K. Physiology of pregnancy. Anaesth. Intensive Care Med. 2019, 20, 397–401. [Google Scholar] [CrossRef]
- Morton, A. Physiological Changes and Cardiovascular Investigations in Pregnancy. Heart Lung Circ. 2021, 30, e6–e15. [Google Scholar] [CrossRef] [PubMed]
- Martell Claros, N.; Asenjo de la Fuente, J.E.; Abad Cardiel, M.; García Donaire, J.A.; Herráiz, M.A. [Role of the renin-angiotensin system in pregnancy and preeclampsia]. Hipertens. Riesgo Vasc. 2020, 37, 72–77. [Google Scholar] [CrossRef]
- Bernstein, I.M.; Ziegler, W.; Badger, G.J. Plasma volume expansion in early pregnancy. Obstet. Gynecol. 2001, 97, 669–672. [Google Scholar] [CrossRef] [PubMed]
- Lemos, A.; de Souza, A.I.; Figueiroa, J.N.; Cabral-Filho, J.E.; de Andrade, A.D. Respiratory muscle strength in pregnancy. Respir. Med. 2010, 104, 1638–1644. [Google Scholar] [CrossRef][Green Version]
- Lee, S.Y.; Chien, D.K.; Huang, C.H.; Shih, S.C.; Lee, W.C.; Chang, W.H. Dyspnea in pregnancy. Taiwan J. Obstet. Gynecol. 2017, 56, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, S.M.; Park, J.S.; Hong, J.S.; Chin, H.J.; Na, K.Y.; Kim, D.K.; Oh, K.H.; Joo, K.W.; Kim, Y.S.; et al. Midterm eGFR and Adverse Pregnancy Outcomes: The Clinical Significance of Gestational Hyperfiltration. Clin. J. Am. Soc. Nephrol. 2017, 12, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Abbassi-Ghanavati, M.; Greer, L.G.; Cunningham, F.G. Pregnancy and laboratory studies: A reference table for clinicians. Obstet. Gynecol. 2009, 114, 1326–1331. [Google Scholar] [CrossRef] [PubMed]
- Kattah, A.; Milic, N.; White, W.; Garovic, V. Spot urine protein measurements in normotensive pregnancies, pregnancies with isolated proteinuria and preeclampsia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 313, R418–R424. [Google Scholar] [CrossRef] [PubMed]
- Alto, W.A. No need for glycosuria/proteinuria screen in pregnant women. J. Fam. Pract. 2005, 54, 978–983. [Google Scholar] [PubMed]
- Edwards, S.M.; Cunningham, S.A.; Dunlop, A.L.; Corwin, E.J. The Maternal Gut Microbiome During Pregnancy. MCN Am. J. Matern. Child Nurs. 2017, 42, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Gewirtz, A.T. Gut microbiota, low-grade inflammation, and metabolic syndrome. Toxicol. Pathol. 2014, 42, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Guo, R.; Li, S.; Liang, F.; Tian, C.; Zhao, X.; Long, Y.; Liu, F.; Jiang, M.; Zhang, Y.; et al. Systematic analysis of gut microbiota in pregnant women and its correlations with individual heterogeneity. NPJ Biofilms Microbiomes 2020, 6, 32. [Google Scholar] [CrossRef]
- Paul, H.A.; Collins, K.H.; Bomhof, M.R.; Vogel, H.J.; Reimer, R.A. Potential Impact of Metabolic and Gut Microbial Response to Pregnancy and Lactation in Lean and Diet-Induced Obese Rats on Offspring Obesity Risk. Mol. Nutr. Food Res. 2018, 62, 1700820. [Google Scholar] [CrossRef] [PubMed]
- Hiltunen, H.; Collado, M.C.; Ollila, H.; Kolari, T.; Tölkkö, S.; Isolauri, E.; Salminen, S.; Rautava, S. Spontaneous preterm delivery is reflected in both early neonatal and maternal gut microbiota. Pediatr. Res. 2022, 91, 1804–1811. [Google Scholar] [CrossRef] [PubMed]
- Dahl, C.; Stanislawski, M.; Iszatt, N.; Mandal, S.; Lozupone, C.; Clemente, J.C.; Knight, R.; Stigum, H.; Eggesbø, M. Gut microbiome of mothers delivering prematurely shows reduced diversity and lower relative abundance of Bifidobacterium and Streptococcus. PLoS ONE 2017, 12, e0184336. [Google Scholar] [CrossRef]
- Nuriel-Ohayon, M.; Neuman, H.; Koren, O. Microbial Changes during Pregnancy, Birth, and Infancy. Front. Microbiol. 2016, 7, 1031. [Google Scholar] [CrossRef]
- DiGiulio, D.B.; Romero, R.; Kusanovic, J.P.; Gómez, R.; Kim, C.J.; Seok, K.S.; Gotsch, F.; Mazaki-Tovi, S.; Vaisbuch, E.; Sanders, K.; et al. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am. J. Reprod. Immunol. 2010, 64, 38–57. [Google Scholar] [CrossRef]
- Morrison, J.L.; Regnault, T.R. Nutrition in Pregnancy: Optimising Maternal Diet and Fetal Adaptations to Altered Nutrient Supply. Nutrients 2016, 8, 342. [Google Scholar] [CrossRef]
- Gomez-Arango, L.F.; Barrett, H.L.; McIntyre, H.D.; Callaway, L.K.; Morrison, M.; Dekker Nitert, M. Connections Between the Gut Microbiome and Metabolic Hormones in Early Pregnancy in Overweight and Obese Women. Diabetes 2016, 65, 2214–2223. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Knight, R.; Mazmanian, S.K.; Cryan, J.F.; Tillisch, K. Gut microbes and the brain: Paradigm shift in neuroscience. J. Neurosci. 2014, 34, 15490–15496. [Google Scholar] [CrossRef] [PubMed]
- Vela, G.; Stark, P.; Socha, M.; Sauer, A.K.; Hagmeyer, S.; Grabrucker, A.M. Zinc in gut-brain interaction in autism and neurological disorders. Neural Plast. 2015, 2015, 972791. [Google Scholar] [CrossRef] [PubMed]
- Josefson, J.L.; Hoffmann, J.A.; Metzger, B.E. Excessive weight gain in women with a normal pre-pregnancy BMI is associated with increased neonatal adiposity. Pediatr. Obes. 2013, 8, e33–e36. [Google Scholar] [CrossRef] [PubMed]
- Brantsæter, A.L.; Myhre, R.; Haugen, M.; Myking, S.; Sengpiel, V.; Magnus, P.; Jacobsson, B.; Meltzer, H.M. Intake of Probiotic Food and Risk of Preeclampsia in Primiparous Women: The Norwegian Mother and Child Cohort Study. Am. J. Epidemiol. 2011, 174, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Medici Dualib, P.; Ogassavara, J.; Mattar, R.; Mariko Koga da Silva, E.; Atala Dib, S.; de Almeida Pititto, B. Gut microbiota and gestational Diabetes Mellitus: A systematic review. Diabetes Res. Clin. Pract. 2021, 180, 109078. [Google Scholar] [CrossRef]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef]
- DiGiulio, D.B.; Callahan, B.J.; McMurdie, P.J.; Costello, E.K.; Lyell, D.J.; Robaczewska, A.; Sun, C.L.; Goltsman, D.S.A.; Wong, R.J.; Shaw, G.; et al. Temporal and spatial variation of the human microbiota during pregnancy. Proc. Natl. Acad. Sci. USA 2015, 112, 11060–11065. [Google Scholar] [CrossRef] [PubMed]
- Ferrocino, I.; Ponzo, V.; Gambino, R.; Zarovska, A.; Leone, F.; Monzeglio, C.; Goitre, I.; Rosato, R.; Romano, A.; Grassi, G.; et al. Changes in the gut microbiota composition during pregnancy in patients with gestational diabetes mellitus (GDM). Sci. Rep. 2018, 8, 12216. [Google Scholar] [CrossRef]
- Pavelescu, L.A.; Profir, M.; Enache, R.M.; Roşu, O.A.; Creţoiu, S.M.; Gaspar, B.S. A Proteogenomic Approach to Unveiling the Complex Biology of the Microbiome. Int. J. Mol. Sci. 2024, 25, 10467. [Google Scholar] [CrossRef]
- Kuang, Y.-S.; Lu, J.-H.; Li, S.-H.; Li, J.-H.; Yuan, M.-Y.; He, J.-R.; Chen, N.-N.; Xiao, W.-Q.; Shen, S.-Y.; Qiu, L.; et al. Connections between the human gut microbiome and gestational diabetes mellitus. GigaScience 2017, 6, gix058. [Google Scholar] [CrossRef]
- Cortez, R.V.; Taddei, C.R.; Sparvoli, L.G.; Ângelo, A.G.S.; Padilha, M.; Mattar, R.; Daher, S. Microbiome and its relation to gestational diabetes. Endocrine 2019, 64, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Crusell, M.K.W.; Hansen, T.H.; Nielsen, T.; Allin, K.H.; Rühlemann, M.C.; Damm, P.; Vestergaard, H.; Rørbye, C.; Jørgensen, N.R.; Christiansen, O.B.; et al. Gestational diabetes is associated with change in the gut microbiota composition in third trimester of pregnancy and postpartum. Microbiome 2018, 6, 89. [Google Scholar] [CrossRef]
- Festa, C.; Drago, L.; Martorelli, M.; Di Marino, V.P.; Bitterman, O.; Corleto, C.C.; Corleto, V.D.; Napoli, A. Flash on gut microbiome in gestational diabetes: A pilot study. New Microbiol. 2020, 43, 195–197. [Google Scholar]
- Hrncir, T. Gut Microbiota Dysbiosis: Triggers, Consequences, Diagnostic and Therapeutic Options. Microorganisms 2022, 10, 578. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.-M.; Kennedy, S.; et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef]
- Agus, A.; Clément, K.; Sokol, H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut 2021, 70, 1174–1182. [Google Scholar] [CrossRef]
- Wu, J.; Wang, K.; Wang, X.; Pang, Y.; Jiang, C. The role of the gut microbiome and its metabolites in metabolic diseases. Protein Cell 2021, 12, 360–373. [Google Scholar] [CrossRef]
- Monnerie, S.; Comte, B.; Ziegler, D.; Morais, J.A.; Pujos-Guillot, E.; Gaudreau, P. Metabolomic and Lipidomic Signatures of Metabolic Syndrome and its Physiological Components in Adults: A Systematic Review. Sci. Rep. 2020, 10, 669. [Google Scholar] [CrossRef]
- Ðanić, M.; Stanimirov, B.; Pavlović, N.; Goločorbin-Kon, S.; Al-Salami, H.; Stankov, K.; Mikov, M. Pharmacological Applications of Bile Acids and Their Derivatives in the Treatment of Metabolic Syndrome. Front. Pharmacol. 2018, 9, 1382. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Hara, H. Dietary fat and bile juice, but not obesity, are responsible for the increase in small intestinal permeability induced through the suppression of tight junction protein expression in LETO and OLETF rats. Nutr. Metab. 2010, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Devkota, S.; Wang, Y.; Musch, M.W.; Leone, V.; Fehlner-Peach, H.; Nadimpalli, A.; Antonopoulos, D.A.; Jabri, B.; Chang, E.B. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature 2012, 487, 104–108. [Google Scholar] [CrossRef]
- Kuno, T.; Hirayama-Kurogi, M.; Ito, S.; Ohtsuki, S. Reduction in hepatic secondary bile acids caused by short-term antibiotic-induced dysbiosis decreases mouse serum glucose and triglyceride levels. Sci. Rep. 2018, 8, 1253. [Google Scholar] [CrossRef] [PubMed]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [PubMed]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-Generated Metabolites Promote Metabolic Benefits via Gut-Brain Neural Circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Lin, S.; Zheng, B.; Cheung, P.C.K. Short-chain fatty acids in control of energy metabolism. Crit. Rev. Food Sci. Nutr. 2018, 58, 1243–1249. [Google Scholar] [CrossRef]
- Priyadarshini, M.; Kotlo, K.U.; Dudeja, P.K.; Layden, B.T. Role of Short Chain Fatty Acid Receptors in Intestinal Physiology and Pathophysiology. Compr. Physiol. 2018, 8, 1091–1115. [Google Scholar] [PubMed]
- Kimura, I.; Miyamoto, J.; Ohue-Kitano, R.; Watanabe, K.; Yamada, T.; Onuki, M.; Aoki, R.; Isobe, Y.; Kashihara, D.; Inoue, D.; et al. Maternal gut microbiota in pregnancy influences offspring metabolic phenotype in mice. Science 2020, 367, eaaw8429. [Google Scholar] [CrossRef]
- Tajiri, K.; Shimizu, Y. Branched-chain amino acids in liver diseases. Transl. Gastroenterol. Hepatol. 2018, 3, 47. [Google Scholar] [CrossRef] [PubMed]
- Arany, Z.; Neinast, M. Branched Chain Amino Acids in Metabolic Disease. Curr. Diabetes Rep. 2018, 18, 76. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Shao, J.; Wu, C.-Y.; Shu, L.; Dong, W.; Liu, Y.; Chen, M.; Wynn, R.M.; Wang, J.; Wang, J.; et al. Targeting BCAA Catabolism to Treat Obesity-Associated Insulin Resistance. Diabetes 2019, 68, 1730–1746. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, P.; Farhangi, M.A.; Nikniaz, L.; Nikniaz, Z.; Asghari-Jafarabadi, M. Gut microbiota-derived metabolite trimethylamine N-oxide (TMAO) potentially increases the risk of obesity in adults: An exploratory systematic review and dose-response meta- analysis. Obes. Rev. 2020, 21, e12993. [Google Scholar] [CrossRef]
- Shan, Z.; Sun, T.; Huang, H.; Chen, S.; Chen, L.; Luo, C.; Yang, W.; Yang, X.; Yao, P.; Cheng, J.; et al. Association between microbiota-dependent metabolite trimethylamine-N-oxide and type 2 diabetes. Am. J. Clin. Nutr. 2017, 106, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef]
- Taleb, S. Tryptophan Dietary Impacts Gut Barrier and Metabolic Diseases. Front. Immunol. 2019, 10, 2113. [Google Scholar] [CrossRef] [PubMed]
- Natividad, J.M.; Agus, A.; Planchais, J.; Lamas, B.; Jarry, A.C.; Martin, R.; Michel, M.-L.; Chong-Nguyen, C.; Roussel, R.; Straube, M.; et al. Impaired Aryl Hydrocarbon Receptor Ligand Production by the Gut Microbiota Is a Key Factor in Metabolic Syndrome. Cell Metab. 2018, 28, 737–749.e734. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, M.; Neyrinck, A.M.; Olivares, M.; Rodriguez, J.; de Rocca Serra, A.; Roumain, M.; Bindels, L.B.; Cani, P.D.; Evenepoel, P.; Muccioli, G.G.; et al. The gut microbiota metabolite indole alleviates liver inflammation in mice. FASEB J. 2018, 32, 6681–6693. [Google Scholar] [CrossRef]
- Koh, A.; Molinaro, A.; Ståhlman, M.; Khan, M.T.; Schmidt, C.; Mannerås-Holm, L.; Wu, H.; Carreras, A.; Jeong, H.; Olofsson, L.E.; et al. Microbially Produced Imidazole Propionate Impairs Insulin Signaling through mTORC1. Cell 2018, 175, 947–961.e917. [Google Scholar] [CrossRef]
- Mostafavi Abdolmaleky, H.; Zhou, J.R. Gut Microbiota Dysbiosis, Oxidative Stress, Inflammation, and Epigenetic Alterations in Metabolic Diseases. Antioxidants 2024, 13, 985. [Google Scholar] [CrossRef]
- Kunst, C.; Schmid, S.; Michalski, M.; Tümen, D.; Buttenschön, J.; Müller, M.; Gülow, K. The Influence of Gut Microbiota on Oxidative Stress and the Immune System. Biomedicines 2023, 11, 1388. [Google Scholar] [CrossRef] [PubMed]
- Yardeni, T.; Tanes, C.E.; Bittinger, K.; Mattei, L.M.; Schaefer, P.M.; Singh, L.N.; Wu, G.D.; Murdock, D.G.; Wallace, D.C. Host mitochondria influence gut microbiome diversity: A role for ROS. Sci. Signal. 2019, 12, eaaw3159. [Google Scholar] [CrossRef] [PubMed]
- The International Diabetes Federation. IDF Diabetes Atlas 9th Edition. 2019. Available online: https://diabetesatlas.org/atlas/ninth-edition/ (accessed on 20 November 2024).
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef]
- Hoet, J.P.; Lukens, F.D. Carbohydrate metabolism during pregnancy. Diabetes 1954, 3, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Metzger, B.E.; Gabbe, S.G.; Persson, B.; Buchanan, T.A.; Catalano, P.A.; Damm, P.; Dyer, A.R.; Leiva, A.; Hod, M.; Kitzmiler, J.L.; et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Hod, M.; Kapur, A.; Sacks, D.A.; Hadar, E.; Agarwal, M.; Di Renzo, G.C.; Roura, L.C.; McIntyre, H.D.; Morris, J.L.; Divakar, H. The International Federation of Gynecology and Obstetrics (FIGO) Initiative on gestational diabetes mellitus: A pragmatic guide for diagnosis, management, and care. Int. J. Gynaecol. Obstet. 2015, 131 (Suppl. S3), S173–S211. [Google Scholar] [CrossRef]
- Marchi, J.; Berg, M.; Dencker, A.; Olander, E.K.; Begley, C. Risks associated with obesity in pregnancy, for the mother and baby: A systematic review of reviews. Obes. Rev. 2015, 16, 621–638. [Google Scholar] [CrossRef] [PubMed]
- Spaight, C.; Gross, J.; Horsch, A.; Puder, J.J. Gestational Diabetes Mellitus. Endocr. Dev. 2016, 31, 163–178. [Google Scholar] [CrossRef]
- Stamilio, D.M.; Scifres, C.M. Extreme obesity and postcesarean maternal complications. Obstet. Gynecol. 2014, 124, 227–232. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 13. Management of Diabetes in Pregnancy: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018, 41, S137–S143. [Google Scholar] [CrossRef]
- Hummel, S.; Much, D.; Rossbauer, M.; Ziegler, A.G.; Beyerlein, A. Postpartum outcomes in women with gestational diabetes and their offspring: POGO study design and first-year results. Rev. Diabet. Stud. 2013, 10, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Berger, D.K.; Chamany, S. Recurrence of gestational diabetes mellitus: A systematic review. Diabetes Care 2007, 30, 1314–1319. [Google Scholar] [CrossRef]
- Gunderson, E.P.; Jacobs, D.R., Jr.; Chiang, V.; Lewis, C.E.; Feng, J.; Quesenberry, C.P., Jr.; Sidney, S. Duration of lactation and incidence of the metabolic syndrome in women of reproductive age according to gestational diabetes mellitus status: A 20-Year prospective study in CARDIA (Coronary Artery Risk Development in Young Adults). Diabetes 2010, 59, 495–504. [Google Scholar] [CrossRef]
- Lauenborg, J.; Mathiesen, E.; Hansen, T.; Glümer, C.; Jørgensen, T.; Borch-Johnsen, K.; Hornnes, P.; Pedersen, O.; Damm, P. The prevalence of the metabolic syndrome in a danish population of women with previous gestational diabetes mellitus is three-fold higher than in the general population. J. Clin. Endocrinol. Metab. 2005, 90, 4004–4010. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.J.; Hiscock, R.J.; Wein, P.; Walker, S.P.; Permezel, M. Gestational diabetes mellitus: Clinical predictors and long-term risk of developing type 2 diabetes: A retrospective cohort study using survival analysis. Diabetes Care 2007, 30, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Ponzo, V.; Fedele, D.; Goitre, I.; Leone, F.; Lezo, A.; Monzeglio, C.; Finocchiaro, C.; Ghigo, E.; Bo, S. Diet-Gut Microbiota Interactions and Gestational Diabetes Mellitus (GDM). Nutrients 2019, 11, 330. [Google Scholar] [CrossRef]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Wang, X.; Li, J.; Zhang, Y.; Zhong, H.; Liu, R.; Zhang, D.; Feng, Q.; Xie, X.; Hong, J.; et al. Analyses of gut microbiota and plasma bile acids enable stratification of patients for antidiabetic treatment. Nat. Commun. 2017, 8, 1785. [Google Scholar] [CrossRef] [PubMed]
- Pilmis, B.; Le Monnier, A.; Zahar, J.R. Gut Microbiota, Antibiotic Therapy and Antimicrobial Resistance: A Narrative Review. Microorganisms 2020, 8, 269. [Google Scholar] [CrossRef]
- Griffin, C. Probiotics in obstetrics and gynaecology. Aust. N. Z. J. Obstet. Gynaecol. 2015, 55, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Chang, H.W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.P.; Gratz, S.W.; Sheridan, P.O.; Flint, H.J.; Duncan, S.H. The influence of diet on the gut microbiota. Pharmacol. Res. 2013, 69, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Selma-Royo, M.; García-Mantrana, I.; Calatayud, M.; Parra-Llorca, A.; Martínez-Costa, C.; Collado, M.C. Maternal diet during pregnancy and intestinal markers are associated with early gut microbiota. Eur. J. Nutr. 2021, 60, 1429–1442. [Google Scholar] [CrossRef]
- Barrett, H.L.; Gomez-Arango, L.F.; Wilkinson, S.A.; McIntyre, H.D.; Callaway, L.K.; Morrison, M.; Dekker Nitert, M. A Vegetarian Diet Is a Major Determinant of Gut Microbiota Composition in Early Pregnancy. Nutrients 2018, 10, 890. [Google Scholar] [CrossRef]
- Mandal, S.; Godfrey, K.M.; McDonald, D.; Treuren, W.V.; Bjørnholt, J.V.; Midtvedt, T.; Moen, B.; Rudi, K.; Knight, R.; Brantsæter, A.L.; et al. Fat and vitamin intakes during pregnancy have stronger relations with a pro-inflammatory maternal microbiota than does carbohydrate intake. Microbiome 2016, 4, 55. [Google Scholar] [CrossRef]
- Gershuni, V.; Li, Y.; Elovitz, M.; Li, H.; Wu, G.D.; Compher, C.W. Maternal gut microbiota reflecting poor diet quality is associated with spontaneous preterm birth in a prospective cohort study. Am. J. Clin. Nutr. 2021, 113, 602–611. [Google Scholar] [CrossRef]
- Ramsden, C.E.; Makrides, M.; Yuan, Z.X.; Horowitz, M.S.; Zamora, D.; Yelland, L.N.; Best, K.; Jensen, J.; Taha, A.Y.; Gibson, R.A. Plasma oxylipins and unesterified precursor fatty acids are altered by DHA supplementation in pregnancy: Can they help predict risk of preterm birth? Prostaglandins Leukot. Essent. Fat. Acids 2020, 153, 102041. [Google Scholar] [CrossRef]
- Englund-Ögge, L.; Brantsæter, A.L.; Sengpiel, V.; Haugen, M.; Birgisdottir, B.E.; Myhre, R.; Meltzer, H.M.; Jacobsson, B. Maternal dietary patterns and preterm delivery: Results from large prospective cohort study. BMJ 2014, 348, g1446. [Google Scholar] [CrossRef]
- Grieger, J.A.; Grzeskowiak, L.E.; Clifton, V.L. Preconception dietary patterns in human pregnancies are associated with preterm delivery. J. Nutr. 2014, 144, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Hillesund, E.R.; Øverby, N.C.; Engel, S.M.; Klungsøyr, K.; Harmon, Q.E.; Haugen, M.; Bere, E. Associations of adherence to the New Nordic Diet with risk of preeclampsia and preterm delivery in the Norwegian Mother and Child Cohort Study (MoBa). Eur. J. Epidemiol. 2014, 29, 753–765. [Google Scholar] [CrossRef] [PubMed]
- Vasile, F.C.; Preda, A.; Ștefan, A.G.; Vladu, M.I.; Forțofoiu, M.C.; Clenciu, D.; Gheorghe, I.O.; Forțofoiu, M.; Moța, M. An Update of Medical Nutrition Therapy in Gestational Diabetes Mellitus. J. Diabetes Res. 2021, 2021, 5266919. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Liu, Z.-L.; Steinmann, P.; Chen, J.; Chen, C.; Ma, X.-T.; Han, S.-H. Medical nutrition therapy for pregnant women with gestational diabetes mellitus—A retrospective cohort study. Taiwan J. Obstet. Gynecol. 2016, 55, 666–671. [Google Scholar] [CrossRef]
- Gómez-Ruiz, R.P.; Cabello-Hernández, A.I.; Gómez-Pérez, F.J.; Gómez-Sámano, M. Meal frequency strategies for the management of type 2 diabetes subjects: A systematic review. PLoS ONE 2024, 19, e0298531. [Google Scholar] [CrossRef]
- Fu, J.; Zheng, Y.; Gao, Y.; Xu, W. Dietary Fiber Intake and Gut Microbiota in Human Health. Microorganisms 2022, 10, 2507. [Google Scholar] [CrossRef]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of Action of Probiotics. Adv. Nutr. 2019, 10, S49–S66. [Google Scholar] [CrossRef]
- Seo, Y.S.; Lee, H.B.; Kim, Y.; Park, H.Y. Dietary Carbohydrate Constituents Related to Gut Dysbiosis and Health. Microorganisms 2020, 8, 427. [Google Scholar] [CrossRef] [PubMed]
- Evert, A.B.; Dennison, M.; Gardner, C.D.; Garvey, W.T.; Lau, K.H.K.; MacLeod, J.; Mitri, J.; Pereira, R.F.; Rawlings, K.; Robinson, S.; et al. Nutrition Therapy for Adults With Diabetes or Prediabetes: A Consensus Report. Diabetes Care 2019, 42, 731–754. [Google Scholar] [CrossRef]
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2022, 65, 1925–1966. [Google Scholar] [CrossRef]
- Murillo, S.; Mallol, A.; Adot, A.; Juárez, F.; Coll, A.; Gastaldo, I.; Roura, E. Culinary strategies to manage glycemic response in people with type 2 diabetes: A narrative review. Front. Nutr. 2022, 9, 1025993. [Google Scholar] [CrossRef]
- Gerontiti, E.; Shalit, A.; Stefanaki, K.; Kazakou, P.; Karagiannakis, D.S.; Peppa, M.; Psaltopoulou, T.; Paschou, S.A. The role of low glycemic index and load diets in medical nutrition therapy for type 2 diabetes: An update. Hormones 2024, 23, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Zou, H.; Zhang, T.; Huo, Y.; Yang, J.; Wang, Z.; Li, Y.; Zhao, J. Gestational Diabetes Mellitus: What Can Medical Nutrition Therapy Do? Nutrients 2024, 16, 1217. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.; Poulsen, C.W.; Kampmann, U.; Smedegaard, S.B.; Ovesen, P.G.; Fuglsang, J. Diet and Healthy Lifestyle in the Management of Gestational Diabetes Mellitus. Nutrients 2020, 12, 3050. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.; Jalaludin, B. Gestational diabetes mellitus: Who requires insulin therapy? Aust. N. Z. J. Obstet. Gynaecol. 2011, 51, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Lepretti, M.; Martucciello, S.; Burgos Aceves, M.A.; Putti, R.; Lionetti, L. Omega-3 Fatty Acids and Insulin Resistance: Focus on the Regulation of Mitochondria and Endoplasmic Reticulum Stress. Nutrients 2018, 10, 350. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, L.; Leng, J.; Liu, H.; Li, W.; Zhang, T.; Li, N.; Li, W.; Tian, H.; Baccarelli, A.A.; et al. Hypertensive disorders of pregnancy in women with gestational diabetes mellitus on overweight status of their children. J. Hum. Hypertens. 2017, 31, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wu, N. Gestational Diabetes Mellitus and Preeclampsia: Correlation and Influencing Factors. Front. Cardiovasc. Med. 2022, 9, 831297. [Google Scholar] [CrossRef] [PubMed]
- Stephansson, O.; Sandström, A. Can short- and long-term maternal and infant risks linked to hypertension and diabetes during pregnancy be reduced by therapy? J. Intern. Med. 2024, 296, 216–233. [Google Scholar] [CrossRef]
- Sousa, K.S.; Leite, H.V.; Corrêa, M.D.; Sousa, M.S.; Queiroz, A.L.R. Prevalence of macrosomic newborn and maternal and neonatal complications in a high-risk maternity. Rev. Bras. Ginecol. Obstet. 2024, 46, e-rbgo48. [Google Scholar] [CrossRef] [PubMed]
- Vally, F.; Presneill, J.; Cade, T. Macrosomia Rates in Women with Diet-Controlled Gestational Diabetes: A Retrospective Study. J. Pregnancy 2017, 2017, 4935397. [Google Scholar] [CrossRef]
- Barrett, H.L.; Callaway, L.K.; Nitert, M.D. Probiotics: A potential role in the prevention of gestational diabetes? Acta Diabetol. 2012, 49 (Suppl. S1), 1–13. [Google Scholar] [CrossRef]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
| Condition | Gut Microbiota Composition | References | |
|---|---|---|---|
| Increased | Decreased | ||
| Healthy Pregnancy | ↑ Beta diversity ↑ Bifidobacteria ↑ Actinobacteria ↑ Proteobacteria ↑ Bacillota/Bacteroides ratio ↑ Blautia ↑ Collinsela ↑ Bifidobacterium | ↓ Alpha diversity ↓ Acinetobacter ↓ Bacteroides ↓ Parabacteroides | [51,52] |
| Preeclampsia | ↑ Fusobacterium ↑ Veillonella ↑ Bulleidia moorei ↑ Clostridium perfringens | ↓ Faecalibacterium ↓ Akkermansia ↓ Coproccocus catus | [25,51] |
| GDM | ↑ Ruminococcaceae ↑ Enterobacteriaceae ↑ Prevotella ↑ Collinsella ↑ Parabacteroides distasonis ↑ Bacillota/Bacteroides ratio ↑ Lachnospiraceae ↑ Phascolarctobacterium ↑ Christensenellaceae | ↓ Bifidobacterium ↓ Faecalibacterium ↓ Akkermansia ↓ Bacteroides vulgatus ↓ Eubacterium eligens ↓ Lactobacillus rogoase ↓ Prevotella copri | [25,51,52] |
| Obesity | ↑ Bacillota ↑ Inflammatory markers ↑ Actinobacteria | ↓ Bifidobacterium ↓ Alpha diversity | [25,51] |
| Study | Increased Species | Decreased Species | Healthy/Pathological Pregnancy |
|---|---|---|---|
| Ferrocino et al. [66] | α-diversity Bacillota Blautia Butyricicoccus Clostridium Coprococcus Dorea Faecalibacterium L-Ruminococcus Lachnospiraceae Sutterella Phascolarctobacterium | Bacteroidetes Actinobacteria Bacteroides Collinsella Rikenellaceae | Healthy pregnancy |
| Koren et al. [29] | α-diversity—I trimester β-diversity—III trimester Proteobacteria Actinobacteria | Faecalibacterium | Pathological pregnancy |
| DiGiulio et al. [65] | No significant changes in gut microbiota diversity and composition | Healthy pregnancy | |
| Kuang et al. [68] | Bacteroides spp. Parabacteroides distasonis Klebsiella variicola Megamonas Phascolarctobacterium Catenibacterium mitsuokai Coprococcus comes Enterobacteriaceae Citrobacter spp. | Bifidobacterium spp. (B. pseudocatenulatum, B. animalis, one unclassified) Eubacterium spp. (E. siraeum, E. eligens, two unclassified Eubacterium species) Roseburia spp. | Pathological pregnancy |
| Cortez et al. [69] | Bacillota/Bacteroides ratio Lachnospiraceae Phascolarctobacterium Christensenellaceae | NA | Pathological pregnancy |
| Crusell et al. [70] | Actinobacteria Collinsella Rothia Actinomyces Desulfovibrio Leuconostoc Granulicatella Mogibacterium | NA | Pathological pregnancy |
| Festa et al. [71] | Bacteroides caccae Bacteroides massiliensis Bacteroides thetaiotaomicron | Bacteroides vulgatus Eubacterium eligens Lactobacillus rogosae Prevotella copri | Pathological pregnancy |
| Metabolites | Metabolic Effects | References |
|---|---|---|
| BAs | hepatic steatosis alter glucose and lipid metabolism pro-inflammatory effects | [75,78,79,80,81] |
| SCFAs | alter intestinal mucosal integrity alter glucose and lipid metabolism pro-inflammatory effects immune effects | [69,76,77,78,79,80] |
| BCAAs | alter protein, glucose, and lipid metabolism increase insulin resistance immune effects | [69,81,82,83,84] |
| TMAO | increased risk of diabetes, obesity, cardiovascular disease, and kidney failure | [85,86,87] |
| Tryptophan and indole derivatives | alter protein metabolism pro-inflammatory effects increase insulin resistance liver steatosis | [75,93,94,95] |
| Imidazole propionate | increase insulin resistance low-grade inflammation | [75,96] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enache, R.-M.; Roşu, O.A.; Profir, M.; Pavelescu, L.A.; Creţoiu, S.M.; Gaspar, B.S. Correlations Between Gut Microbiota Composition, Medical Nutrition Therapy, and Insulin Resistance in Pregnancy—A Narrative Review. Int. J. Mol. Sci. 2025, 26, 1372. https://doi.org/10.3390/ijms26031372
Enache R-M, Roşu OA, Profir M, Pavelescu LA, Creţoiu SM, Gaspar BS. Correlations Between Gut Microbiota Composition, Medical Nutrition Therapy, and Insulin Resistance in Pregnancy—A Narrative Review. International Journal of Molecular Sciences. 2025; 26(3):1372. https://doi.org/10.3390/ijms26031372
Chicago/Turabian StyleEnache, Robert-Mihai, Oana Alexandra Roşu, Monica Profir, Luciana Alexandra Pavelescu, Sanda Maria Creţoiu, and Bogdan Severus Gaspar. 2025. "Correlations Between Gut Microbiota Composition, Medical Nutrition Therapy, and Insulin Resistance in Pregnancy—A Narrative Review" International Journal of Molecular Sciences 26, no. 3: 1372. https://doi.org/10.3390/ijms26031372
APA StyleEnache, R.-M., Roşu, O. A., Profir, M., Pavelescu, L. A., Creţoiu, S. M., & Gaspar, B. S. (2025). Correlations Between Gut Microbiota Composition, Medical Nutrition Therapy, and Insulin Resistance in Pregnancy—A Narrative Review. International Journal of Molecular Sciences, 26(3), 1372. https://doi.org/10.3390/ijms26031372








