Dynamics of Immune Cell Infiltration and Fibroblast-Derived IL-33/ST2 Axis Induction in a Mouse Model of Post-Surgical Lymphedema
Abstract
1. Introduction
2. Results
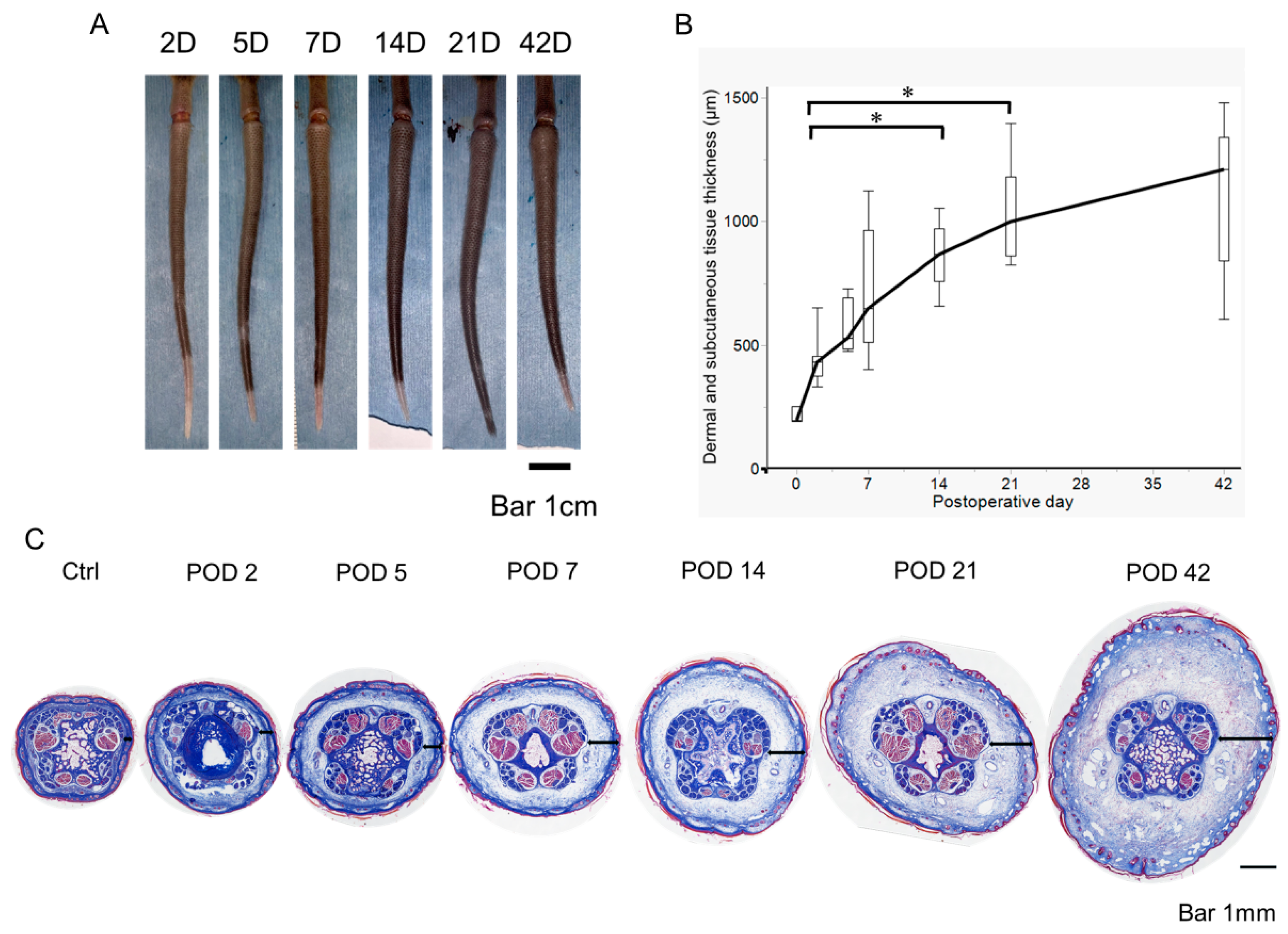
2.1. Early Postoperative Edema Development in a Mouse Tail Lymphedema Model
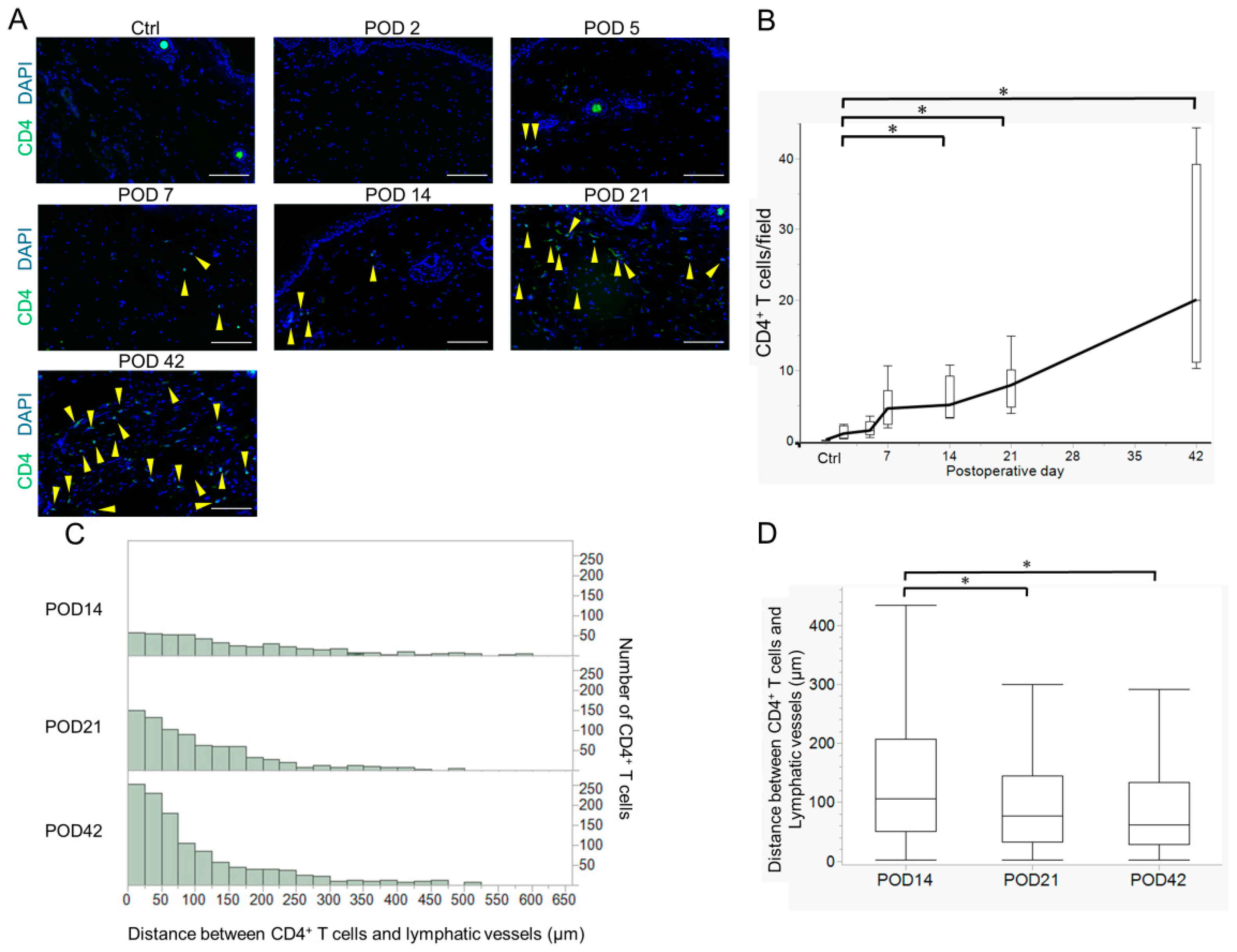
2.2. Variation in Acquired Immune Cells in Lymphedema Development
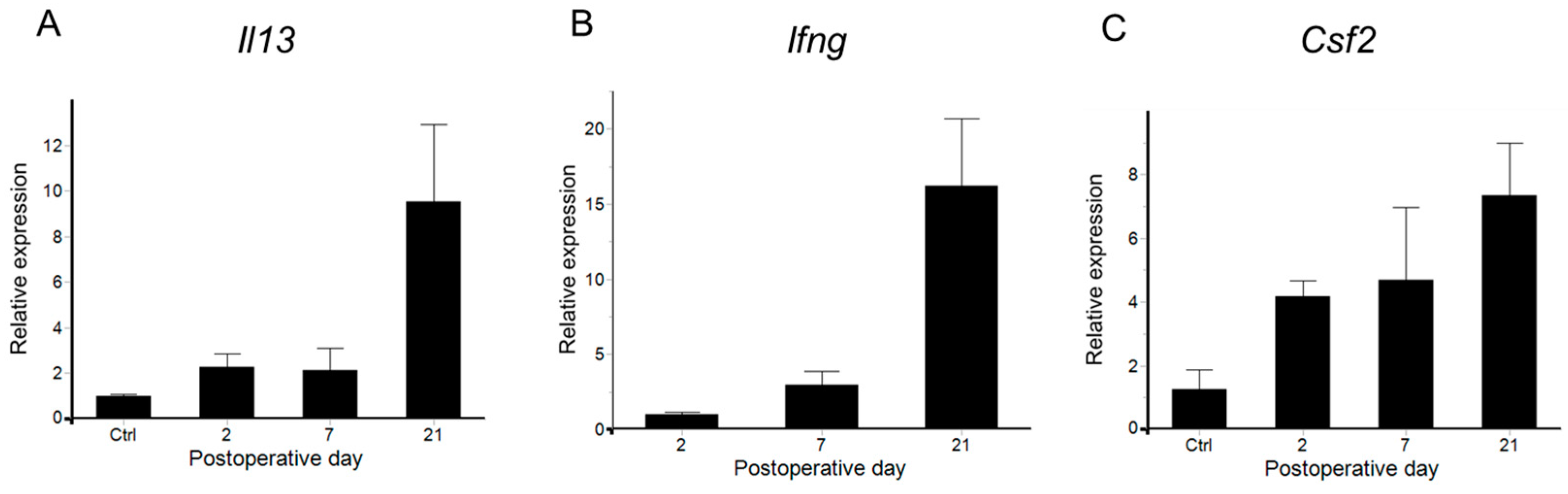
2.3. Cytokine Variation in Lymphedema Development
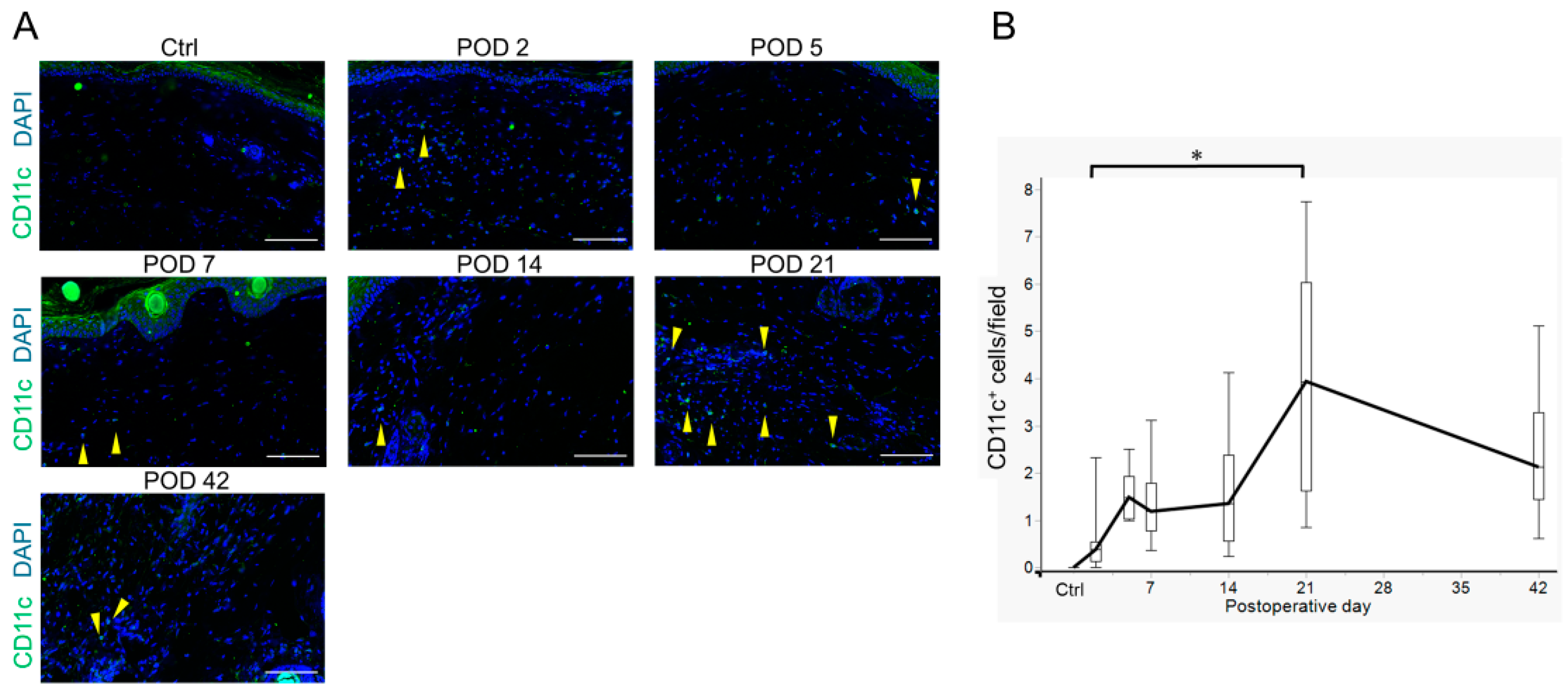
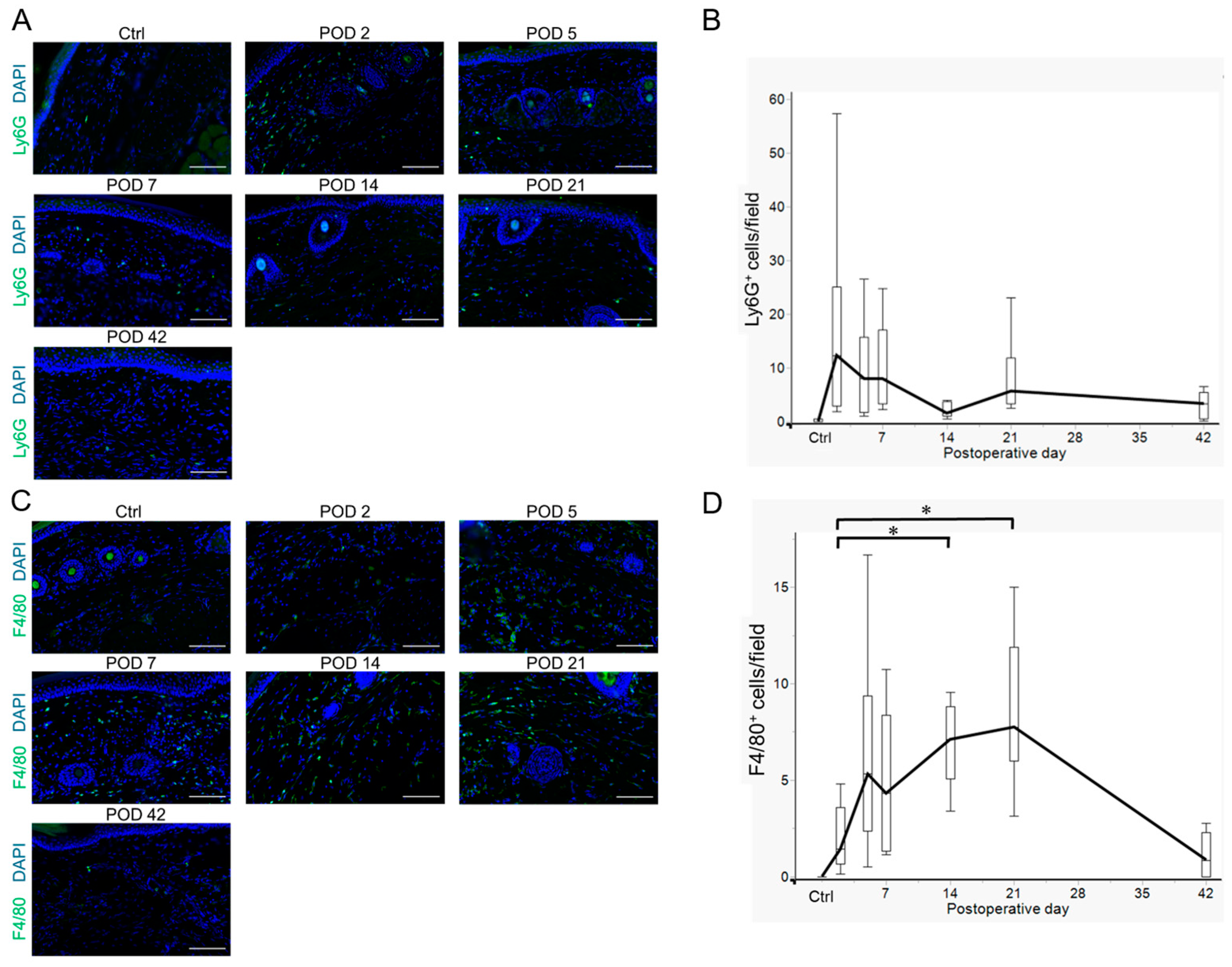
2.4. Innate Immune Cell Variation in Lymphedema Development
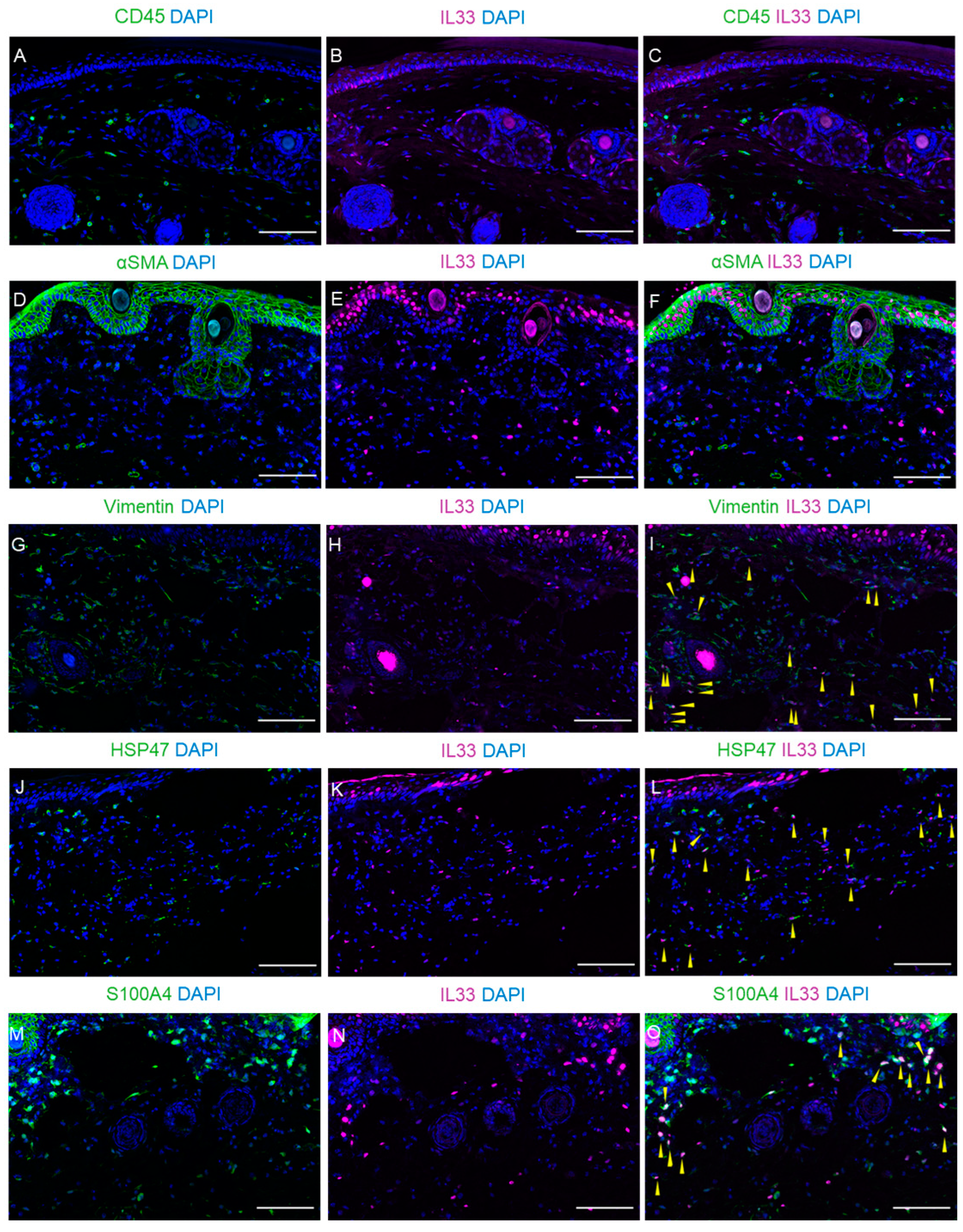
2.5. The IL-33/ST2 Axis in Lymphedema Development
2.6. Expression of IL-33 in Fibroblasts Within Dermal and Subcutaneous Tissues
3. Discussion
4. Materials and Methods
4.1. Surgical Models of Lymphedema
4.2. Measurement of Dermal and Subcutaneous Tissue Thickness
4.3. Real-Time RT-qPCR Analysis
4.4. Histology and Fluorescence IMMUNOHISTOCHEMISTRY
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Th2 | Type 2 T helper |
| IL | interleukin |
| Tregs | regulatory T cells |
| ST2 | suppression of tumorigenicity 2 |
| ILC2 | Group 2 innate lymphoid cells |
| POD | postoperative day |
| RT-qPCR | reverse transcriptase-quantitative polymerase chain reaction |
| Ct | threshold cycle |
| PBS | phosphate-buffered saline |
| EDTA | ethylenediaminetetraacetic acid |
References
- Morfoisse, F.; Zamora, A.; Marchaud, E.; Nougue, M.; Diallo, L.H.; David, F.; Roussel, E.; Lacazette, E.; Prats, A.-C.; Tatin, F.; et al. Sex Hormones in Lymphedema. Cancers 2021, 13, 530. [Google Scholar] [CrossRef]
- Zamora, A.; Nougué, M.; Verdu, L.; Balzan, E.; Draia-Nicolau, T.; Benuzzi, E.; Pujol, F.; Baillif, V.; Lacazette, E.; Morfoisse, F.; et al. 15-Lipoxygenase promotes resolution of inflammation in lymphedema by controlling Treg cell function through IFN-β. Nat. Commun. 2024, 15, 221. [Google Scholar] [CrossRef] [PubMed]
- Herberger, K.; Blome, C.; Heyer, K.; Ellis, F.; Münter, K.C.; Augustin, M. Quality of life in patients with primary and secondary lymphedema in the community. Wound Repair Regen. 2017, 25, 466–473. [Google Scholar] [CrossRef]
- Jonis, Y.M.J.; Wolfs, J.A.G.N.; Hummelink, S.; Tielemans, H.J.P.; Keuter, X.H.A.; van Kuijk, S.; Ulrich, D.J.O.; van der Hulst, R.R.W.J.; Qiu, S.S. The 6 month interim analysis of a randomized controlled trial assessing the quality of life in patients with breast cancer related lymphedema undergoing lymphaticovenous anastomosis vs. conservative therapy. Sci. Rep. 2024, 14, 2238. [Google Scholar] [CrossRef]
- Lau, K.; Patel, S.; Rogers, K.; Smith, S.; Riba, M. Cancer-Related Lymphedema and Psychological Distress. Curr. Psychiatry Rep. 2024, 26, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Warren, A.G.; Brorson, H.; Borud, L.J.; Slavin, S.A. Lymphedema: A Comprehensive Review. Ann. Plast. Surg. 2007, 59, 464–472. [Google Scholar] [CrossRef] [PubMed]
- McGinity, E.N.; Bray, W.F.; Granzow, J.W. The Current State of Lymphedema Surgery. Lymphatics 2024, 2, 212–227. [Google Scholar] [CrossRef]
- Maita, K.C.; Avila, F.R.; Torres-Guzman, R.A.; Garcia, J.P.; Borna, S.; Brown, S.A.; Ho, O.A.; Forte, A.J. Surgery for Lymphedema Prevention and Treatment. Curr. Breast Cancer Rep. 2024, 16, 260–268. [Google Scholar] [CrossRef]
- Eisner, Z.J.; Best, C.S.W.; Lenders, J.; Hespe, G.E.; Myers, P.L.; Kung, T.A. Cross-Sectional Analysis of Patients Referred to a Tertiary Lymphatic Surgery Center. Ann. Surg. Oncol. 2024, 32, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Kataru, R.P.; Mehrara, B.J. Histopathologic Features of Lymphedema: A Molecular Review. Int. J. Mol. Sci. 2020, 21, 2546. [Google Scholar] [CrossRef] [PubMed]
- Michelini, S.; Cestari, M.; Michelini, S.; Camilleri, G.; De Antoni, L.; Sonna, W.N.; Bertelli, M. Study of a supplement and a genetic test for lymphedema management. Acta Biomed. 2020, 91, e2020013. [Google Scholar]
- Ly, C.L.; Kataru, R.P.; Mehrara, B.J. Inflammatory Manifestations of Lymphedema. Int. J. Mol. Sci. 2017, 18, 171. [Google Scholar] [CrossRef]
- Bowman, C.; Rockson, S.G. The Role of Inflammation in Lymphedema: A Narrative Review of Pathogenesis and Opportunities for Therapeutic Intervention. Int. J. Mol. Sci. 2024, 25, 3907. [Google Scholar] [CrossRef]
- Zampell, J.C.; Yan, A.; Elhadad, S.; Avraham, T.; Weitman, E.; Mehrara, B.J. CD4(+) cells regulate fibrosis and lymphangiogenesis in response to lymphatic fluid stasis. PLoS ONE 2012, 7, e49940. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-O.; Kim, I.-K. Molecular pathophysiology of secondary lymphedema. Front. Cell Dev. Biol. 2024, 12, 1363811. [Google Scholar] [CrossRef]
- Avraham, T.; Zampell, J.C.; Yan, A.; Elhadad, S.; Weitman, E.S.; Rockson, S.G.; Bromberg, J.; Mehrara, B.J. Th2 differentiation is necessary for soft tissue fibrosis and lymphatic dysfunction resulting from lymphedema. FASEB J. 2013, 27, 1114–1126. [Google Scholar] [CrossRef] [PubMed]
- Gousopoulos, E.; Proulx, S.T.; Bachmann, S.B.; Scholl, J.; Dionyssiou, D.; Demiri, E.; Halin, C.; Dieterich, L.C.; Detmar, M. Regulatory T cell transfer ameliorates lymphedema and promotes lymphatic vessel function. JCI Insight 2016, 1, e89081. [Google Scholar] [CrossRef]
- Alvarez, F.; Fritz, J.H.; Piccirillo, C.A. Pleiotropic Effects of IL-33 on CD4+ T Cell Differentiation and Effector Functions. Front. Immunol. 2019, 10, 522. [Google Scholar] [CrossRef]
- Griesenauer, B.; Paczesny, S. The ST2/IL-33 Axis in Immune Cells during Inflammatory Diseases. Front. Immunol. 2017, 8, 475. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wu, T.; Hua, F.; Sun, J.; Bai, Y.; Wang, W.; Liu, J.; Zhang, M. IL-33 Promotes ST2-Dependent Fibroblast Maturation via P38 and TGF-β in a Mouse Model of Epidural Fibrosis. J. Tissue Eng. Regen. Med. 2022, 19, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Kotsiou, O.S.; Gourgoulianis, K.I.; Zarogiannis, S.G. IL-33/ST2 Axis in Organ Fibrosis. Front. Immunol. 2018, 9, 2432. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Turnquist, H.R.; Hoffman, R.; Billiar, T.R. Role of the IL-33-ST2 axis in sepsis. Mil. Med. Res. 2017, 4, 3. [Google Scholar] [CrossRef]
- Walker, J.A.; McKenzie, A.N.J. TH2 cell development and function. Nat. Rev. Immunol. 2018, 18, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Oliphant, C.J.; Hwang, Y.Y.; Walker, J.A.; Salimi, M.; Wong, S.H.; Brewer, J.M.; Englezakis, A.; Barlow, J.L.; Hams, E.; Scanlon, S.T. MHCII-mediated dialog between group 2 innate lymphoid cells and CD4+ T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity 2014, 41, 283–295. [Google Scholar] [CrossRef]
- Zhu, J. T helper 2 (Th2) cell differentiation, type 2 innate lymphoid cell (ILC2) development and regulation of interleukin-4 (IL-4) and IL-13 production. Cytokine 2015, 75, 14–24. [Google Scholar] [CrossRef]
- García Nores, G.D.; Ly, C.L.; Cuzzone, D.A.; Kataru, R.P.; Hespe, G.E.; Torrisi, J.S.; Huang, J.J.; Gardenier, J.C.; Savetsky, I.L.; Nitti, M.D.; et al. CD4+ T cells are activated in regional lymph nodes and migrate to skin to initiate lymphedema. Nat. Commun. 2018, 9, 1970. [Google Scholar] [CrossRef]
- Kumegawa, S.; Yamada, G.; Hashimoto, D.; Hirashima, T.; Kajimoto, M.; Isono, K.; Fujimoto, K.; Suzuki, K.; Uemura, K.; Ema, M.; et al. Development of Surgical and Visualization Procedures to Analyze Vasculatures by Mouse Tail Edema Model. Biol. Proced. Online 2021, 23, 21. [Google Scholar] [CrossRef]
- Nishioka, T.; Katayama, K.-I.; Kumegawa, S.; Isono, K.; Baba, T.; Tsujimoto, H.; Yamada, G.; Inoue, N.; Asamura, S. Increased infiltration of CD4+ T cell in the complement deficient lymphedema model. BMC Immunol. 2023, 24, 42. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Liu, C.H.; Roberts, A.I.; Das, J.; Xu, G.; Ren, G.; Zhang, Y.; Zhang, L.; Yuan, Z.R.; Tan, H.S.W.; et al. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and T-cell responses: What we do and don’t know. Cell Res. 2006, 16, 126–133. [Google Scholar] [CrossRef] [PubMed]
- van de Laar, L.; Coffer, P.J.; Woltman, A.M. Regulation of dendritic cell development by GM-CSF: Molecular control and implications for immune homeostasis and therapy. Blood 2012, 119, 3383–3393. [Google Scholar] [CrossRef] [PubMed]
- Mehrara, B.J.; Park, H.J.; Kataru, R.P.; Bromberg, J.; Coriddi, M.; Baik, J.E.; Shin, J.; Li, C.; Cavalli, M.R.; Encarnacion, E.M.; et al. Pilot Study of Anti-Th2 Immunotherapy for the Treatment of Breast Cancer-Related Upper Extremity Lymphedema. Biology 2021, 10, 934. [Google Scholar] [CrossRef]
- Ogata, F.; Fujiu, K.; Matsumoto, S.; Nakayama, Y.; Shibata, M.; Oike, Y.; Koshima, I.; Watabe, T.; Nagai, R.; Manabe, I. Excess Lymphangiogenesis Cooperatively Induced by Macrophages and CD4+ T Cells Drives the Pathogenesis of Lymphedema. J. Investig. Dermatol. 2016, 136, 706–714. [Google Scholar] [CrossRef]
- Ogino, R.; Yokooji, T.; Hayashida, M.; Suda, S.; Yamakawa, S.; Hayashida, K. Emerging Anti-Inflammatory Pharmacotherapy and Cell-Based Therapy for Lymphedema. Int. J. Mol. Sci. 2022, 23, 7614. [Google Scholar] [CrossRef] [PubMed]
- Kataru, R.P.; Baik, J.E.; Park, H.J.; Wiser, I.; Rehal, S.; Shin, J.Y.; Mehrara, B.J. Regulation of Immune Function by the Lymphatic System in Lymphedema. Front. Immunol. 2019, 10, 470. [Google Scholar] [CrossRef]
- Meszaros, A.J.; Reichner, J.S.; Albina, J.E. Macrophage phagocytosis of wound neutrophils. J. Leukoc. Biol. 1999, 65, 35–42. [Google Scholar] [CrossRef]
- Tabibiazar, R.; Cheung, L.; Han, J.; Swanson, J.; Beilhack, A.; An, A.; Dadras, S.S.; Rockson, N.; Joshi, S.; Wagner, R.; et al. Inflammatory manifestations of experimental lymphatic insufficiency. PLoS Med. 2006, 3, e254. [Google Scholar] [CrossRef] [PubMed]
- Cayrol, C.; Girard, J.-P. Interleukin-33 (IL-33): A nuclear cytokine from the IL-1 family. Immunol. Rev. 2018, 281, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Cayrol, C.; Girard, J.-P. IL-33, an alarmin cytokine with crucial roles in innate immunity, inflammation and allergy. Curr. Opin. Immunol. 2014, 31, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, G.K.; D’Cruz, L.M.; Turnquist, H.R. Emerging functions of IL-33 in homeostasis and immunity. Annu. Rev. Immunol. 2022, 40, 15–43. [Google Scholar] [CrossRef]
- Martin, N.T.; Martin, M.U. Interleukin 33 is a guardian of barriers and a local alarmin. Nat. Immunol. 2016, 17, 122–131. [Google Scholar] [CrossRef]
- Kakkar, R.; Hei, H.; Dobner, S.; Lee, R.T. Interleukin 33 as a mechanically responsive cytokine secreted by living cells. J. Biol. Chem. 2012, 287, 6941–6948. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uemura, K.; Katayama, K.-i.; Nishioka, T.; Watanabe, H.; Yamada, G.; Inoue, N.; Asamura, S. Dynamics of Immune Cell Infiltration and Fibroblast-Derived IL-33/ST2 Axis Induction in a Mouse Model of Post-Surgical Lymphedema. Int. J. Mol. Sci. 2025, 26, 1371. https://doi.org/10.3390/ijms26031371
Uemura K, Katayama K-i, Nishioka T, Watanabe H, Yamada G, Inoue N, Asamura S. Dynamics of Immune Cell Infiltration and Fibroblast-Derived IL-33/ST2 Axis Induction in a Mouse Model of Post-Surgical Lymphedema. International Journal of Molecular Sciences. 2025; 26(3):1371. https://doi.org/10.3390/ijms26031371
Chicago/Turabian StyleUemura, Kazuhisa, Kei-ichi Katayama, Toshihiko Nishioka, Hikaru Watanabe, Gen Yamada, Norimitsu Inoue, and Shinichi Asamura. 2025. "Dynamics of Immune Cell Infiltration and Fibroblast-Derived IL-33/ST2 Axis Induction in a Mouse Model of Post-Surgical Lymphedema" International Journal of Molecular Sciences 26, no. 3: 1371. https://doi.org/10.3390/ijms26031371
APA StyleUemura, K., Katayama, K.-i., Nishioka, T., Watanabe, H., Yamada, G., Inoue, N., & Asamura, S. (2025). Dynamics of Immune Cell Infiltration and Fibroblast-Derived IL-33/ST2 Axis Induction in a Mouse Model of Post-Surgical Lymphedema. International Journal of Molecular Sciences, 26(3), 1371. https://doi.org/10.3390/ijms26031371




