Tadalafil Ameliorates Chronic Ischemia-Associated Bladder Overactivity in Fructose-Fed Rats by Exerting Pelvic Angiogenesis and Enhancing p-eNOS Expression
Abstract
1. Introduction
2. Results
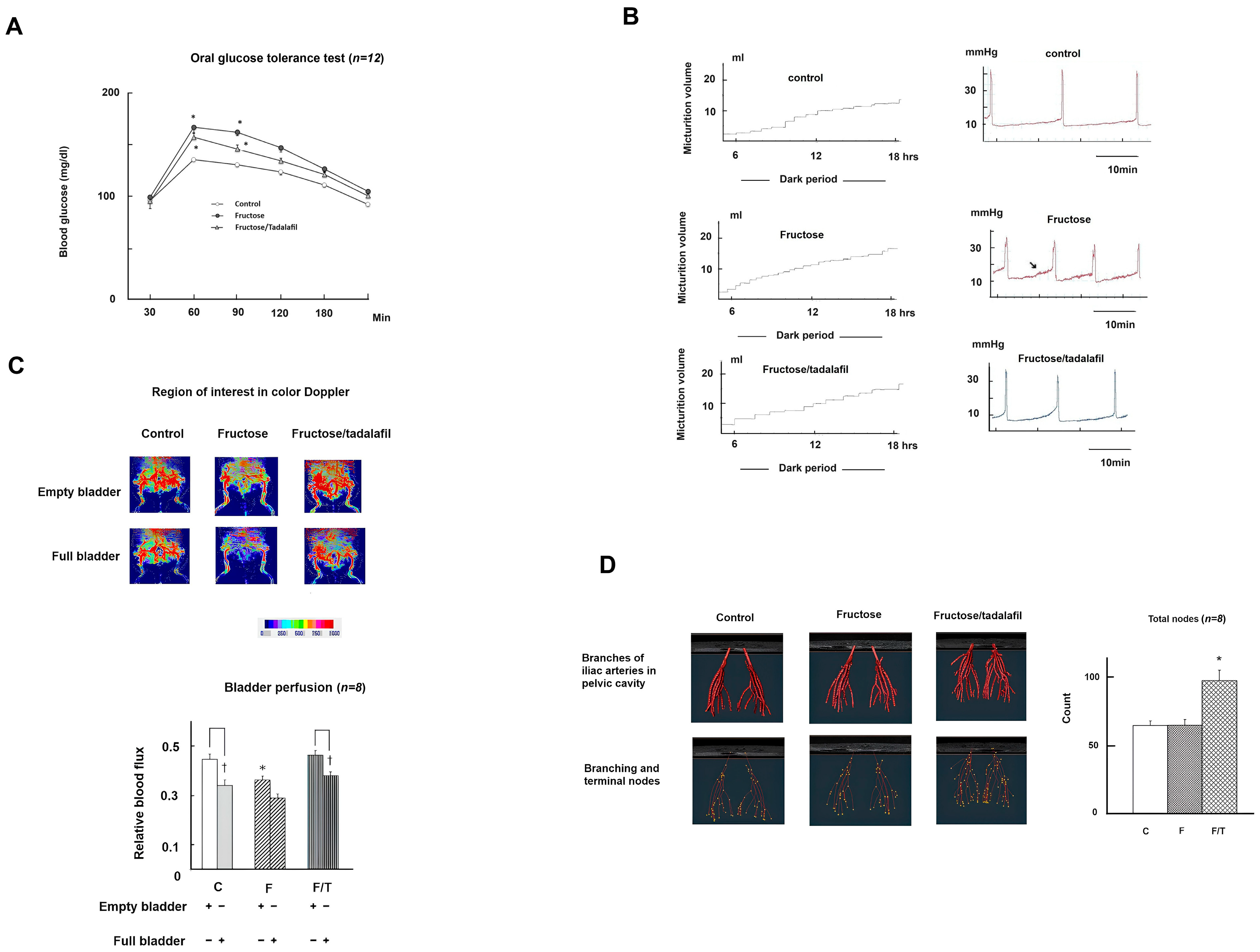
2.1. General Characteristics, Micturition Behavior, Bladder Perfusion, and Pelvis Vasculature Among Groups
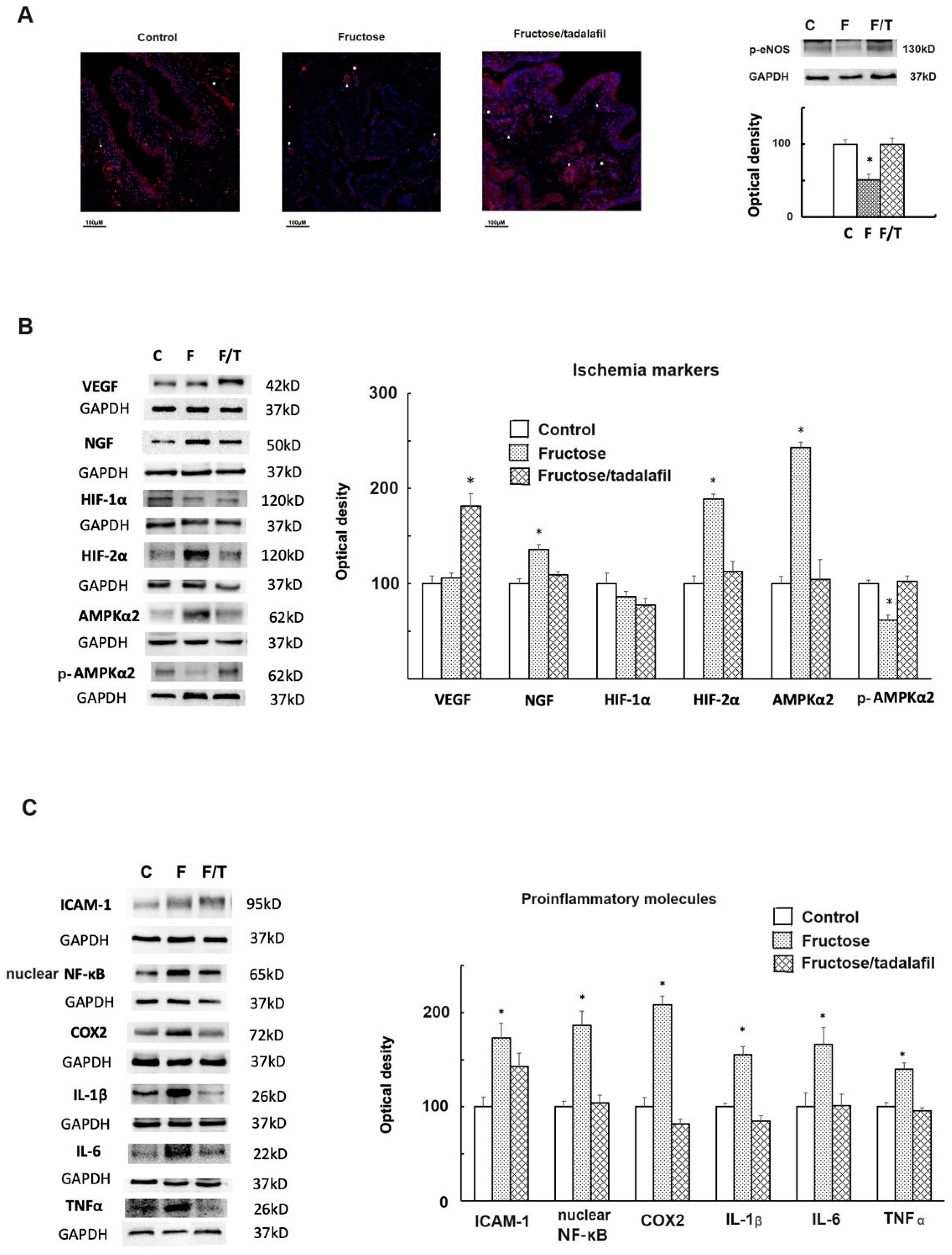
2.2. The Expressions of Phosphorylated-Endothelial Nitric Oxide Synthase (p-eNOS), Ischemia Markers, and Proinflammatory Molecules of the Bladder
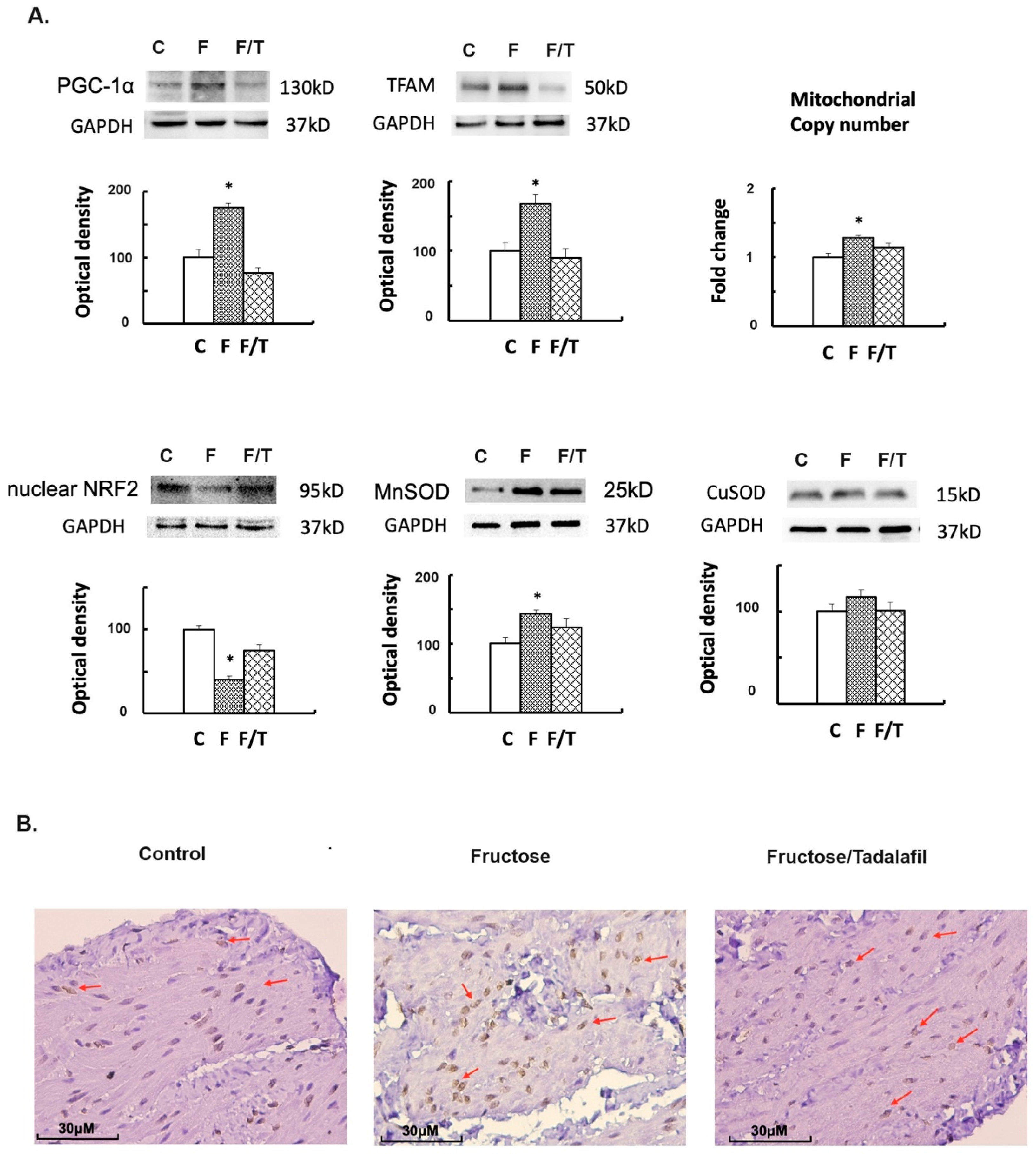
2.3. Alterations in Mitochondrial Biogenesis, Oxidative Stress, and Bladder Fibrosis
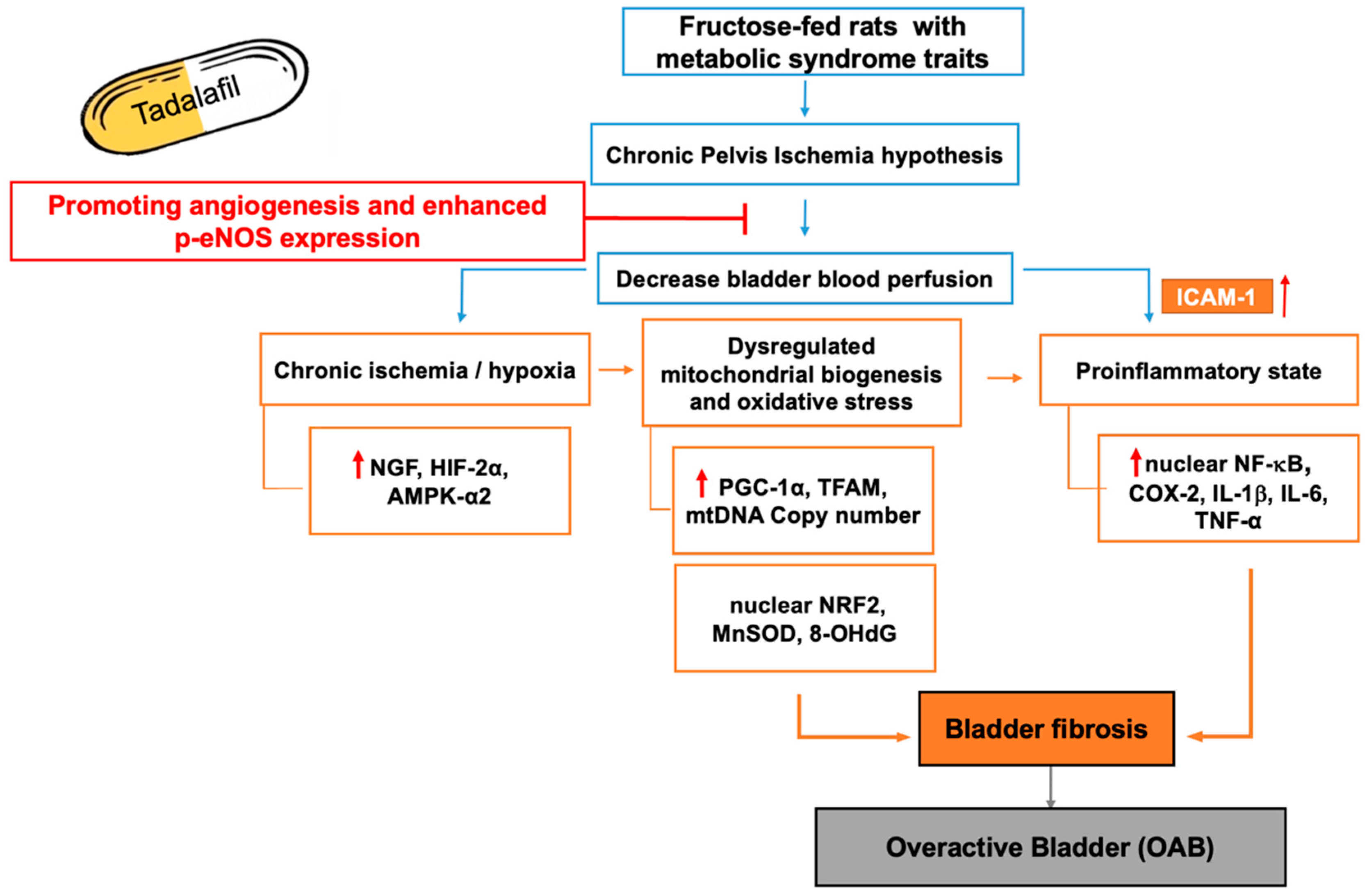
3. Discussion
3.1. Limitations and Future Prospects
3.2. Conclusions
4. Materials and Methods
4.1. Animals
4.2. Metabolic Cage Study and OGTT
4.3. Measurement of Bladder Perfusion by Using Laser Doppler Blood Flow Imaging
4.4. Three-Dimensional Magnetic Resonance Imaging for Pelvis Angiogenesis
4.5. Filling Cystometry
4.6. Urinary Bladder Histological Studies for the Immunofluorescence Staining of p-eNOS, IHC Staining of 8-OHdG, and Masson Trichrome Stain
4.7. mtDNA Copy Number Detection of the Bladder
4.8. Western Blots for Bladder Proteins
5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chuang, Y.C.; Liu, S.P.; Lee, K.S.; Liao, L.; Wang, J.; Yoo, T.K.; Chu, R.; Sumarsono, B. Prevalence of overactive bladder in China, Taiwan and South Korea: Results from a cross-sectional, population-based study. Low. Urin. Tract. Symptoms 2019, 11, 48–55. [Google Scholar] [CrossRef]
- Santander, J.; Plata, M.; Zuluaga, L.; Azuero, J.; Daza, F.; Trujillo, C.G.; Caicedo, J.I.; Rondón, M. What is the real burden of the overactive bladder? Results from a national prevalence study. Neurourol. Urodyn. 2022, 41, 926–934. [Google Scholar] [CrossRef]
- Kinsey, D.; Pretorius, S.; Glover, L.; Alexander, T. The psychological impact of overactive bladder: A systematic review. J. Health Psychol. 2016, 21, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Coyne, K.S.; Sexton, C.C.; Thompson, C.L.; Clemens, J.Q.; Chen, C.I.; Bavendam, T.; Dmochowski, R. Impact of overactive bladder on work productivity. Urology 2012, 80, 97–103. [Google Scholar] [CrossRef]
- Liang, X.; Or, B.; Tsoi, M.F.; Cheung, C.L.; Cheung, B.M.Y. Prevalence of metabolic syndrome in the United States National Health and Nutrition Examination Survey 2011–2018. Postgrad. Med. J. 2023, 99, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, P.; Mathangasinghe, Y.; Jayawardena, R.; Hills, A.P.; Misra, A. Prevalence and trends of metabolic syndrome among adults in the asia-pacific region: A systematic review. BMC Public Health 2017, 17, 101. [Google Scholar] [CrossRef] [PubMed]
- Anis, O.; Cohen, A.D.; Aharony, S.; Kitrey, N.D.; Dotan, I.; Shenhar, C.; Comaneshter, D.; Beckenstein, T.; Yaron, S. Increased prevalence of metabolic syndrome in female patients with overactive bladder: A population-based study. Neurourol. Urodyn. 2024, 43, 1809–1816. [Google Scholar] [CrossRef]
- Hsu, L.N.; Hu, J.C.; Chen, P.Y.; Lee, W.C.; Chyang, Y.C. Metabolic syndrome and overactive bladder syndrome may share common pathophysiologies. Biomedicines 2022, 10, 1957. [Google Scholar] [CrossRef]
- Peyronnet, B.; Mironska, E.; Chapple, C.; Cardozo, L.; Oelke, M.; Dmochowski, R.; Amarenco, G.; Gamé, X.; Kirby, R.; Van Der Aa, F.; et al. A compressive review of overactive bladder pathophysiology: On the way to tailored treatment. Eur. Urol. 2019, 75, 988–1000. [Google Scholar] [CrossRef]
- Andersson, K.E.; Boedtkjer, D.B.; Forman, A. The link between vascular dysfunction, bladder ischemia, and aging bladder dysfunction. Ther. Adv. Urol. 2017, 9, 11–27. [Google Scholar] [CrossRef]
- Yee, C.H.; Yip, J.S.; Cheng, N.M.; Kwan, C.H.; Li, K.M.; Teoh, J.Y.; Chiu, P.K.; Wong, J.H.; Chan, E.S.; Chan, C.K.; et al. The cardiovascular risk factors in men with lower urinary tract symptoms. World J. Urol. 2019, 37, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Azadzoi, K.M.; Tarcan, T.; Siroky, M.B.; Krane, R.J. Atherosclerosis-induced chronic ischemia causes bladder fibrosis and non-compliance in the rabbit. J. Urol. 1999, 161, 1626–1635. [Google Scholar] [CrossRef] [PubMed]
- Azadzoi, K.M.; Yalla, S.V.; Siroky, M.B. Oxidative stress and neurodegeneration in the ischemic overactive bladder. J. Urol. 2007, 178, 710–715. [Google Scholar] [CrossRef]
- Gacci, M.; Corona, G.; Sebastianelli, A.; Serni, S.; De Nunzio, C.; Maggi, M.; Vignozzi, L.; Novara, G.; McVary, K.T.; Kaplan, S.A.; et al. Male lower urinary tract symptoms and cardiovascular events: A systemic review and meta-analysis. Eur. Urol. 2016, 70, 788–796. [Google Scholar] [CrossRef]
- Papaefstathiou, E.; Gatsos, S.; Tigkiropoulos, K.; Apostolidis, I.; Koukourikis, P.; Lazaridis, I.; Apostolidis, A. Role of pelvic ischemia in human lower urinary tract symptoms and sexual function among patients with common iliac artery obstruction undergoing revascularization surgery. Int. Neurourol. J. 2023, 27, 129–138. [Google Scholar] [CrossRef]
- Yokoyama, O.; Igawa, Y.; Takeda, M.; Yamaguchi, T.; Murakami, M.; Viktrup, L. Tadalafil for lower urinary tract symptoms secondary to benign prostatic hyperplasia: A review of clinical data in Asian men and an update on the mechanism of action. Ther. Adv. Urol. 2015, 7, 249–264. [Google Scholar] [CrossRef]
- Gravas, S.; Gacci, M.; Gratzke, C.; Herrmann, T.R.; Karavitakis, M.; Kyriazis, I.; Malde, S.; Mamoulakis, C.; Rieken, M.; Sakalis, V.I.; et al. Summary paper on the 2023 European association of urology guidelines on the management of non-neurogenic male lower urinary tract symptoms. Eur. Urol. 2023, 84, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, F.; Yu, Z.; Zhang, Y.; Liu, C.; Dai, S.; Chen, B.; Lv, J. Efficacy of daily low-dose Tadalafil for treating Overactive Bladder: Results of a randomized, double-blind, placebo-controlled trial. Urology 2017, 100, 59–64. [Google Scholar] [CrossRef]
- Matsuo, T.; Miyata, Y.; Araki, K.; Mukae, Y.; Otsubo, A.; Ohba, K.; Sakai, H. Efficacy of tadalafil therapy and changes in oxidative stress levels in male patients with lower urinary tract symptoms and overactive bladder. Low. Urin. Tract. Symptoms 2020, 12, 47–53. [Google Scholar] [CrossRef]
- Taskinen, M.R.; Packard, C.J.; Borén, J. Dietary fructose and the metabolic syndrome. Nutrients 2019, 11, 1987. [Google Scholar] [CrossRef]
- Wu, L.; Wang, M.; Maher, S.; Fu, P.; Cai, D.; Wang, B.; Gupta, S.; Hijaz, A.; Daneshgari, F.; Liu, G. Effects of different diets used to induce obesity/metabolic syndrome on bladder function in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2023, 324, R70–R81. [Google Scholar] [PubMed]
- Lee, W.C.; Leu, S.; Wu, K.L.H.; Tain, Y.L.; Chuang, Y.C.; Chan, J.Y.H. Tadalafil ameliorates bladder overactivity by restoring insulin-activated detrusor relaxation via the bladder mucosal IRS/PI3K/AKT/eNOS pathway in fructose-fed rats. Sci. Rep. 2021, 11, 8202. [Google Scholar]
- Lee, W.C.; Yu, H.R.; Tain, Y.L.; Wu, K.L.H.; Chuang, Y.C.; Chan, J.Y.H. Vinpocetine ameliorates metabolic syndrome -associated Bladder Overactivity in Fructose-fed Rats by Restoring succinate-modulating cAMP level and Exerting Anti-inflammatory Effects in bladder detrusor muscle. Biomedicines 2022, 10, 2716. [Google Scholar] [CrossRef]
- Christ, G.J.; Bushman, W.; Fraser, M.O. Impact of diabetes and obesity on the prostate and urethra: Implications to improved bladder dysfunction understanding and treatment. J. Urol. 2009, 182, S38–S44. [Google Scholar] [PubMed]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [PubMed]
- Koka, S.; Xi, L.; Kukreja, R.C. Chronic inhibition of phosphodiesterase 5 with tadalafil affords cardioprotection in a mouse model of metabolic syndrome: Role of nitric oxide. Mol. Cell. Biochem. 2020, 468, 47–58. [Google Scholar] [PubMed]
- Tanaka, H.; Mitsui, R.; Oishi, M.; Passlick, S.; Jabs, R.; Steinhäuser, C.; Tanaka, K.F.; Hashitani, H. Nitric oxide-mediated signal transmission in bladder vasculature underlies the therapeutic actions of PDE5 inhibitors in the rat. Br. J. Pharmacol. 2021, 178, 1073–1094. [Google Scholar] [PubMed]
- Loboda, A.; Jozkowicz, A.; Dulak, J. HIF-1 versus HIF-2--is one more important than the other? Vascul. Pharmacol. 2012, 56, 245–251. [Google Scholar]
- Christiaansen, C.E.; Sun, Y.; Hsu, Y.C.; Chai, T.C. Alterations in expression of HIF-1α, HIF-2α, and VEGF by idiopathic overactive bladder urothelial cells during stretch suggest role for hypoxia. Urology 2011, 77, e7–e11. [Google Scholar]
- Yang, J.H.; Choi, H.P.; Niu, W.; Azadzoi, K.M. Cellular Stress and Molecular Responses in Bladder Ischemia. Int. J. Mol. Sci. 2021, 22, 11862. [Google Scholar] [CrossRef]
- Chess-Williams, R.; Sellers, D.J. Pathophysiological mechanisms involved in overactive bladder/detrusor overactivity. Curr. Bladder Dysfunct. Rep. 2023, 18, 79–88. [Google Scholar]
- Yang, J.H.; Niu, W.; Li, Y.; Azadzoi, K.M. Impairment of AMPK-α2 augments detrusor contractions in bladder ischemia. Investig. Clin. Urol. 2021, 62, 600–609. [Google Scholar] [PubMed]
- Jornayvaz, F.R.; Shulman, G.I. Regulation of mitochondrial biogenesis. Essays Biochem. 2010, 47, 69–84. [Google Scholar] [PubMed]
- Yang, J.H.; Siroky, M.B.; Yalla, S.V.; Azadzoi, K.M. Mitochondrial stress and activation of PI3K and Akt survival pathway in bladder ischemia. Res. Rep. Urol. 2017, 9, 93–100. [Google Scholar] [PubMed]
- Rasbach, K.A.; Gupta, R.K.; Ruas, J.L.; Wu, J.; Naseri, E.; Estall, J.L.; Spiegelman, B.M. PGC-1alpha regulates a HIF2alpha-dependent switch in skeletal muscle fiber types. Proc. Natl. Acad. Sci. USA 2010, 107, 21866–21871. [Google Scholar]
- Speich, J.E.; Tarcan, T.; Hashitani, H.; Vahabi, B.; McCloskey, K.D.; Andersson, K.E.; Wein, A.J.; Birder, L.A. Are oxidative stress and ischemia significant causes of bladder damage leading to lower urinary tract dysfunction? Report from the ICI-RS 2019. Neurourol. Urodyn. 2020, 39, S16–S22. [Google Scholar] [CrossRef]
- Lee, W.C.; Chien, C.T.; Yu, H.J.; Lee, S.W. Bladder dysfunction in rats with metabolic syndrome induced by long-term fructose feeding. J. Urol. 2008, 179, 2470–2476. [Google Scholar]
- Khosla, L.; Gong, S.; Weiss, J.P.; Birder, L.A. Oxidative stress biomarkers in age-related lower urinary tract disorders: A systematic review. Int. Neurourol. J. 2022, 26, 3–19. [Google Scholar] [PubMed]
- Dussault, S.; Maingrette, F.; Ménard, C.; Michaud, S.E.; Haddad, P.; Groleau, J.; Turgeon, J.; Perez, G.; Rivard, A. Sildenafil increases endothelial progenitor cell function and improves ischemia-induced neovascularization in hypercholesterolemic apolipoprotein E-deficient mice. Hypertension 2009, 54, 1043–1049. [Google Scholar] [PubMed]
- Glushakova, O.; Kosugi, T.; Roncal, C.; Mu, W.; Heinig, M.; Cirillo, P.; Sánchez-Lozada, L.G.; Johnson, R.J.; Nakagawa, T. Fructose Induces the Inflammatory Molecule ICAM-1 in Endothelial Cells. J. Am. Soc. Nephrol. 2008, 19, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Dehvari, N.; da Silva Junior, E.D.; Bengtsson, T.; Hutchinson, D.S. Mirabegron: Potential off target effects and uses beyond the bladder. Br. J. Pharmacol. 2018, 175, 4072–4082. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Kim, D.Y.; Cho, K.J.; Hashimoto, M.; Matsuoka, K.; Kamijo, T.; Wang, Z.; Karnup, S.; Robertson, A.M.; Tyagi, P.; et al. Pathophysiology of Overactive Bladder and Pharmacologic Treatments Including β3-Adrenoceptor Agonists -Basic Research Perspectives. Int. Neurourol. J. 2024, 28 (Suppl. 1), 12–33. [Google Scholar] [CrossRef] [PubMed]
- Gacci, M.; Corona, G.; Salvi, M.; Vignozzi, L.; McVary, K.T.; Kaplan, S.A.; Roehrborn, C.G.; Serni, S.; Mirone, V.; Carini, M.; et al. A systematic review and meta-analysis on the use of phosphodiesterase 5 inhibitors alone or in combination with α-blockers for lower urinary tract symptoms due to benign prostatic hyperplasia. Eur. Urol. 2012, 61, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Chancellor, M.B.; Lamb, L.E.; Ward, E.P.; Bartolone, S.N.; Carabulea, A.; Sharma, P.; Janicki, J.; Smith, C.; Laudano, M.; Abraham, N.; et al. Comparing Concentration of Urinary Inflammatory Cytokines in Interstitial Cystitis, Overactive Bladder, Urinary Tract Infection, and Bladder Cancer. Uro Sci. 2022, 33, 199–204. [Google Scholar] [CrossRef]
- Lin, C.T.; Chiang, B.J.; Liao, C.H. Perspectives of Medical Treatment for Overactive Bladder. Uro Sci. 2020, 31, 91–98. [Google Scholar] [CrossRef]
- Yamanishi, T.; Kaga, K.; Sakata, K.; Yokoyama, T.; Kageyama, S.; Fuse, M.; Tokunaga, S. A randomized controlled study of the efficacy of tadalafil monotherapy versus combination of tadalafil and mirabegron for the treatment of persistent overactive bladder symptoms in men presenting with lower urinary tract symptoms (CONTACT Study). Neurourol. Urodyn. 2020, 39, 804–812. [Google Scholar] [CrossRef]
- Chiang, B.J.; Mao, S.H.; Chen, T.S.; Chung, S.D.; Chien, C.T. Adipose stem-cell-derived microvesicles ameliorate long-term bladder ischemia-induced bladder underactivity. J. Formos. Med. Assoc. 2024; in press. [Google Scholar] [CrossRef]
- Wu, C.W.; Hung, C.Y.; Hirase, H.; Tain, Y.L.; Lee, W.C.; Chan, J.Y.H.; Fu, M.H.; Chen, L.W.; Liu, W.C.; Liang, C.K.; et al. Pioglitazone reversed the fructose-programmed astrocytic glycolysis and oxidative phosphorylation of female rat offspring. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E622–E634. [Google Scholar] [CrossRef]

| Mean ± SEM | Control | Fructose | Fructose/ Tadalafil |
|---|---|---|---|
| General characteristics: | |||
| Body weight (g) | 290.7 ± 3.4 | 300.3 ± 3.2 | 291.3 ± 3.2 |
| Bladder weight (mg) | 99.5 ± 1.8 | 109.7 ± 4 | 105.8 ± 3.7 |
| Mean arterial pressure (mmHg) | 120.2 ± 3.3 | 148.5 ± 3.4 * | 144.1 ± 3.2 * |
| Fasting biochemistry parameters | |||
| Triglycerides (mg/dL) | 41 ± 2.5 | 88.7 ± 4.2 * | 57.2 ± 2.2 * |
| Cholesterol (mg/dL) | 67.5 ± 3.5 | 85 ± 1.8 * | 79.2 ± 3 * |
| Creatinine (mg/dL) | 0.74 ± 0.06 | 0.67 ± 0.06 | 0.78 ± 0.05 |
| Uric acid (mg/dL) | 1.5 ± 0.06 | 1.9 ± 0.07 * | 1.7 ± 0.08 * |
| Glucose (mM) | 5.2 ± 0.11 | 5.5 ± 0.5 | 5.2 ± 0.12 |
| Insulin (mU/L) | 10.5 ± 1.1 | 27.9 ± 1.6 * | 24.9 ± 1.37 * |
| HOMA-IR | 2.5 ± 0.26 | 6.8 ± 0.45 * | 5.7 ± 0.43 * |
| Nitrite (μM) | 16.6 ± 1.76 | 33.9 ± 3.8 * | 22.3 ± 2.9 |
| Nitrate (μM) | 27.3 ± 2.8 | 21.9 ± 3.6 | 26.4 ± 5 |
| Metabolic cage study/24 h | |||
| Water intake (mL) | 43.7 ± 1.6 | 38.3 ± 2.6 | 41.8 ± 1.2 |
| Urine output (mL) | 22.8 ± 1.9 | 20.9 ± 1.7 | 19.3 ± 0.8 |
| No. voids | 17.4 ± 1.2 | 21.1 ± 0.79 * | 17.1 ± 0.59 |
| Cystometric parameters | |||
| Voiding pressure (mmHg) | 26.4 ± 0.96 | 27.2 ± 0.91 | 25.8 ± 1.1 |
| Intercontractile interval (min) | 12.3 ± 0.5 | 7.9 ± 0.3 * | 11.9 ± 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, W.-C.; Lu, S.; Su, C.-H.; Tain, Y.-L.; Wu, K.L.H.; Hsu, C.-N.; Tzeng, H.-T. Tadalafil Ameliorates Chronic Ischemia-Associated Bladder Overactivity in Fructose-Fed Rats by Exerting Pelvic Angiogenesis and Enhancing p-eNOS Expression. Int. J. Mol. Sci. 2025, 26, 1363. https://doi.org/10.3390/ijms26031363
Lee W-C, Lu S, Su C-H, Tain Y-L, Wu KLH, Hsu C-N, Tzeng H-T. Tadalafil Ameliorates Chronic Ischemia-Associated Bladder Overactivity in Fructose-Fed Rats by Exerting Pelvic Angiogenesis and Enhancing p-eNOS Expression. International Journal of Molecular Sciences. 2025; 26(3):1363. https://doi.org/10.3390/ijms26031363
Chicago/Turabian StyleLee, Wei-Chia, Steve Lu, Chia-Hao Su, You-Lin Tain, Kay L. H. Wu, Chien-Ning Hsu, and Hong-Tai Tzeng. 2025. "Tadalafil Ameliorates Chronic Ischemia-Associated Bladder Overactivity in Fructose-Fed Rats by Exerting Pelvic Angiogenesis and Enhancing p-eNOS Expression" International Journal of Molecular Sciences 26, no. 3: 1363. https://doi.org/10.3390/ijms26031363
APA StyleLee, W.-C., Lu, S., Su, C.-H., Tain, Y.-L., Wu, K. L. H., Hsu, C.-N., & Tzeng, H.-T. (2025). Tadalafil Ameliorates Chronic Ischemia-Associated Bladder Overactivity in Fructose-Fed Rats by Exerting Pelvic Angiogenesis and Enhancing p-eNOS Expression. International Journal of Molecular Sciences, 26(3), 1363. https://doi.org/10.3390/ijms26031363










