The Loop-In Binding Mode of Dihydroorotase: Implications for Ligand Binding and Therapeutic Targeting
Abstract
1. Introduction
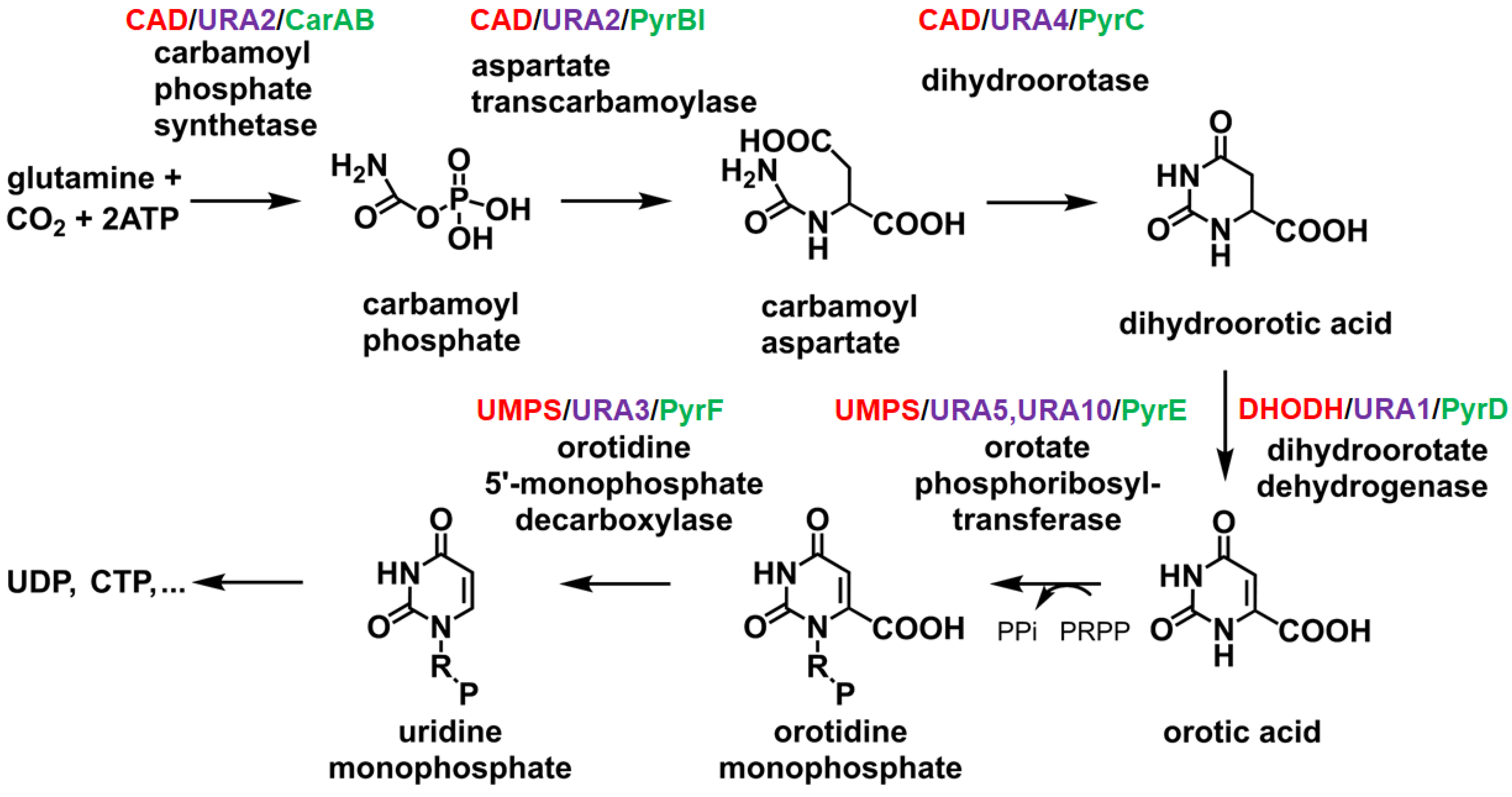
2. DHOase Enzymes
2.1. Physiological Roles and Disease Implications of DHOase
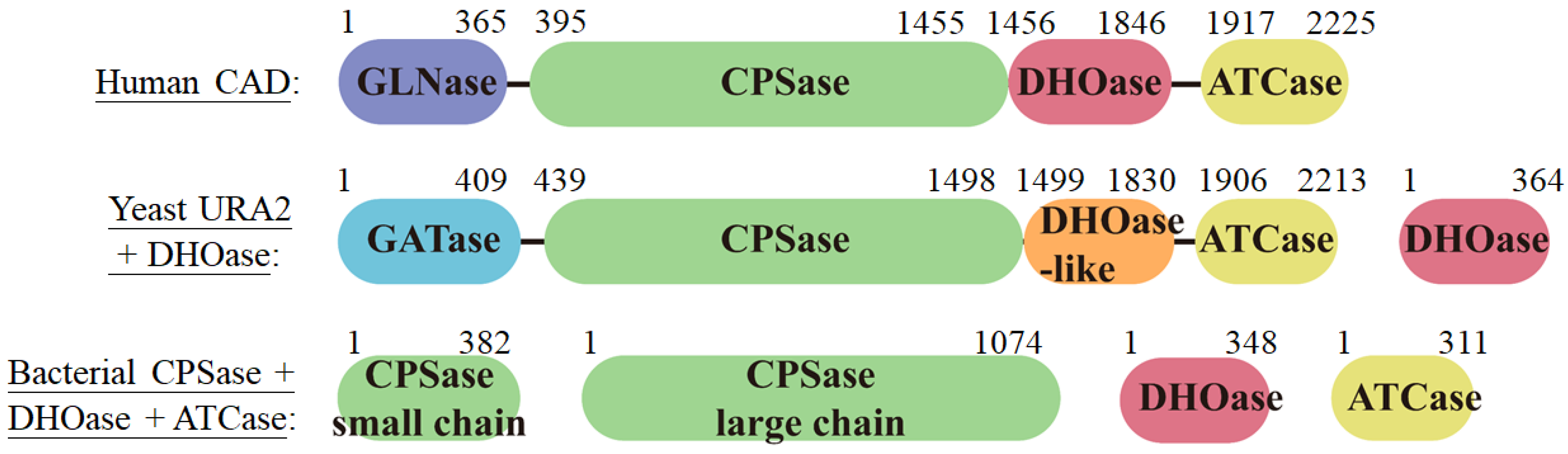
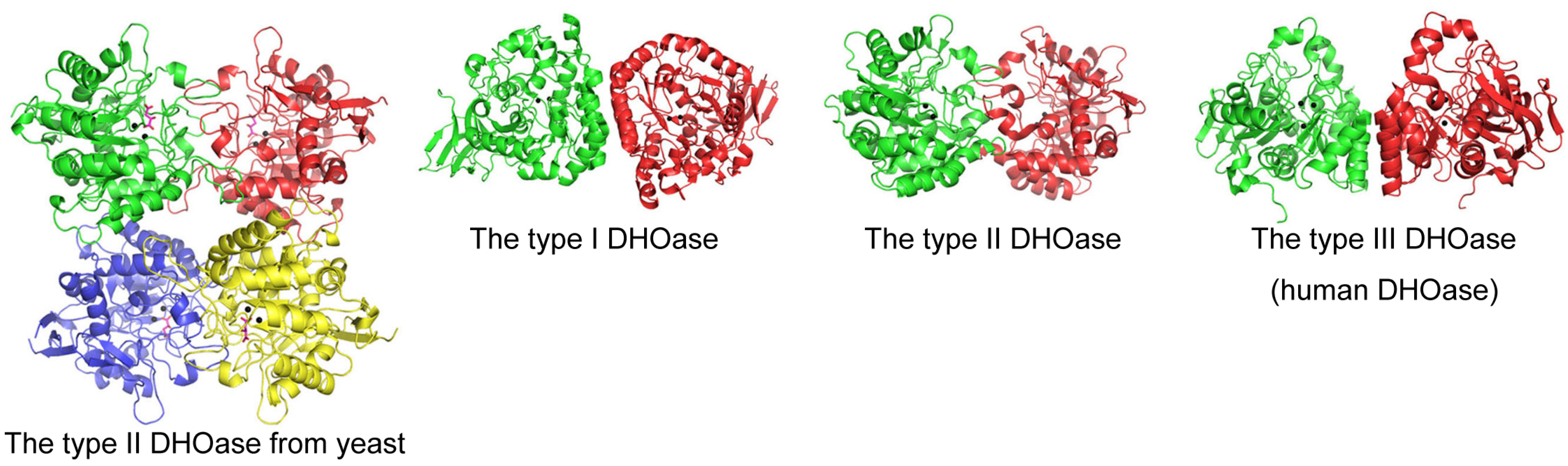
2.2. Diversity and Classification of DHOase
2.3. Biochemistry and Enzymatic Functions of DHOase
2.4. Structural Features and Active Site Dynamics of DHOase
2.5. DHOase Belongs to the Cyclic Amidohydrolase Family with a Similar Active Site for Catalysis
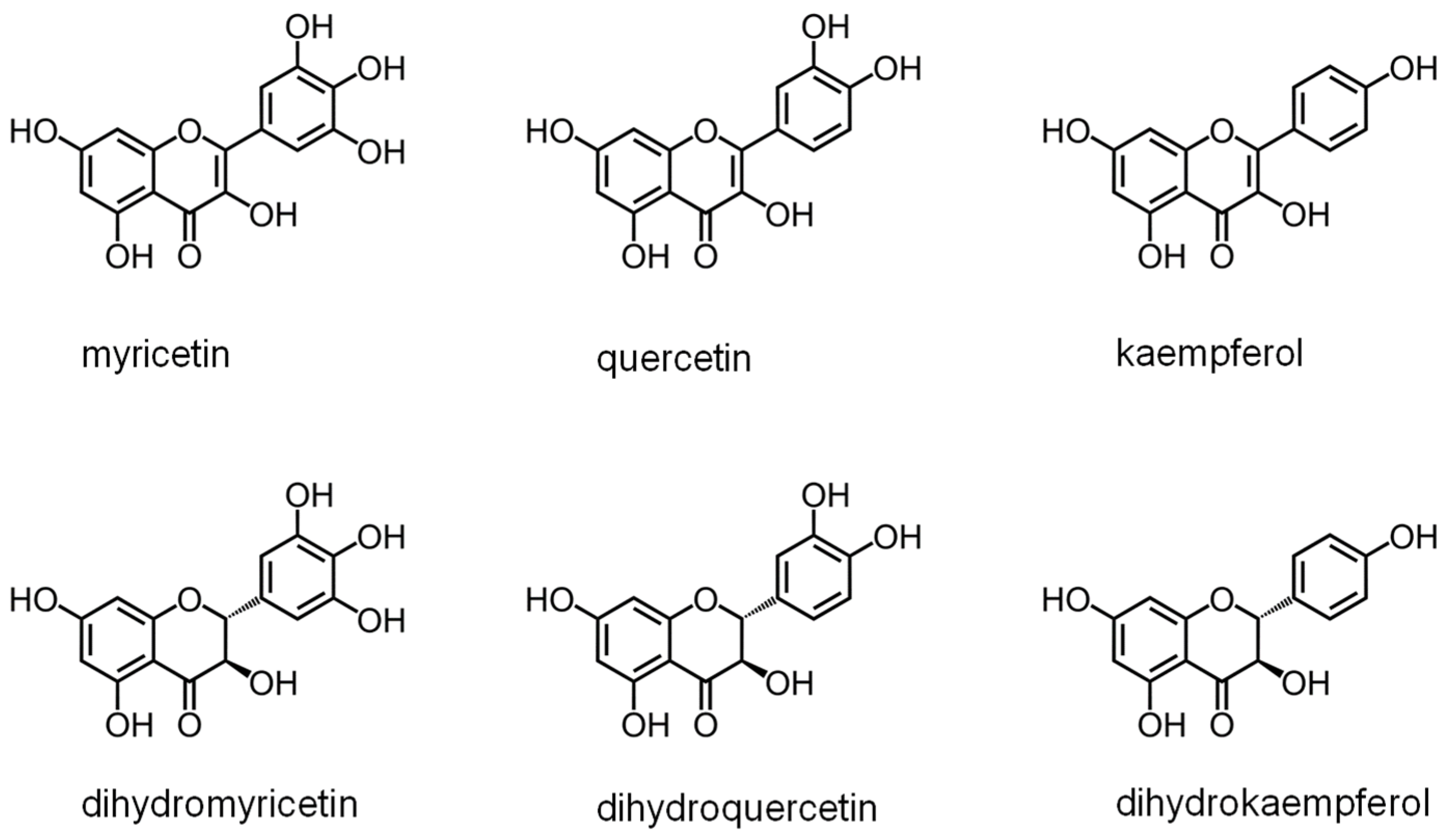
2.6. DHOase as a Drug Target
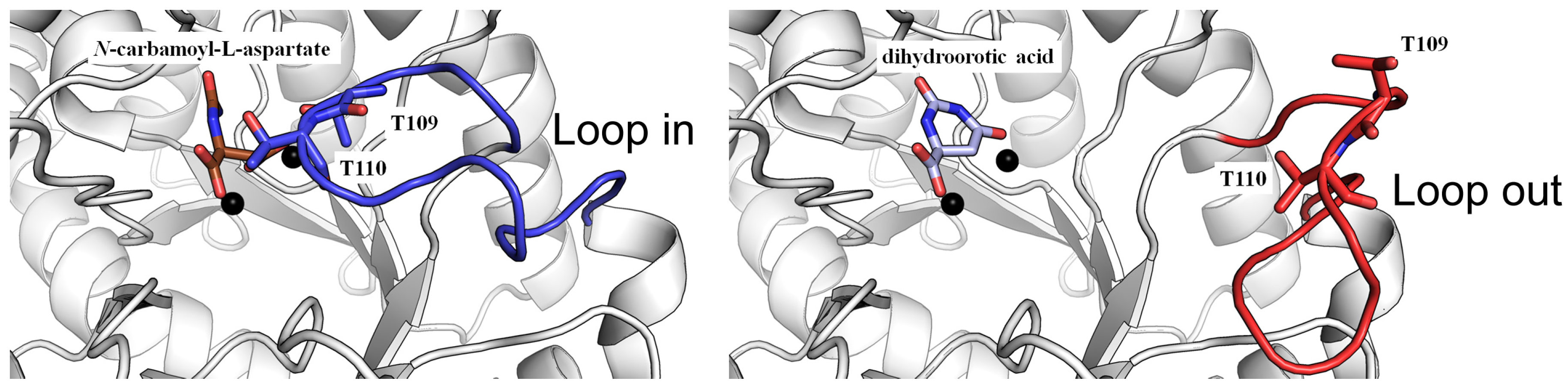
3. The Loop-In Binding Mechanism for Ligand Binding and Therapeutic Targeting
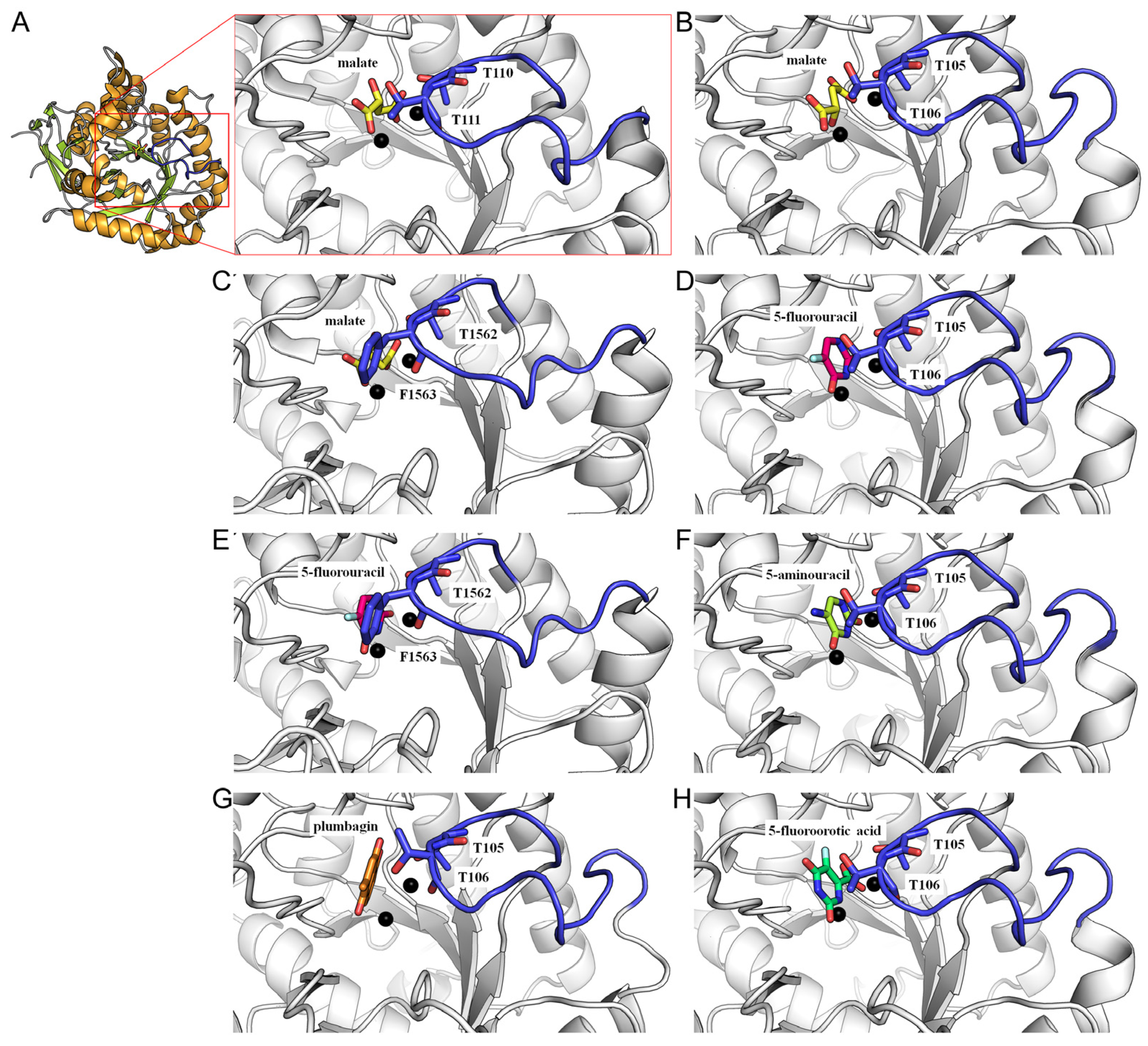
3.1. Revisiting the Loop-In Binding Mode
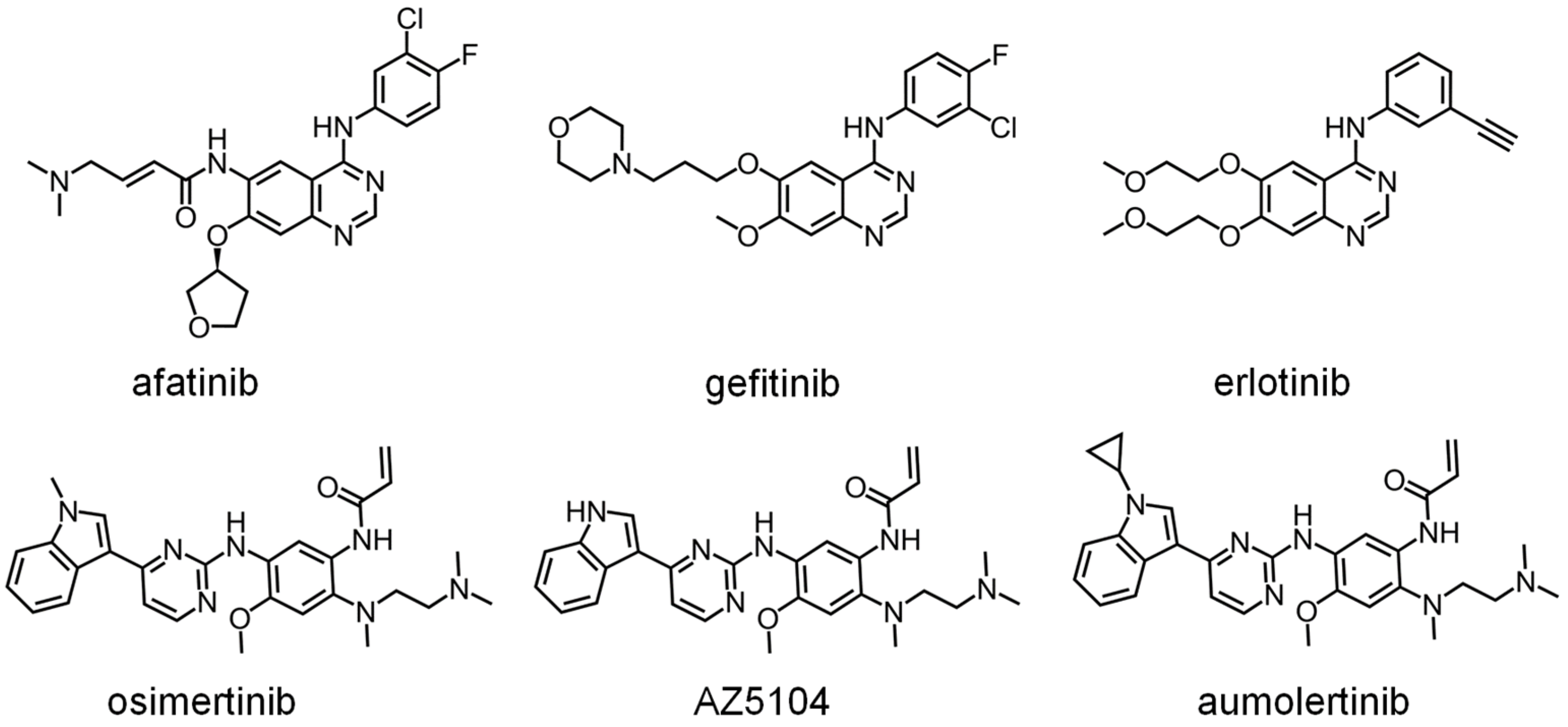
3.2. Therapeutic Implications of the Loop-In Binding Mode
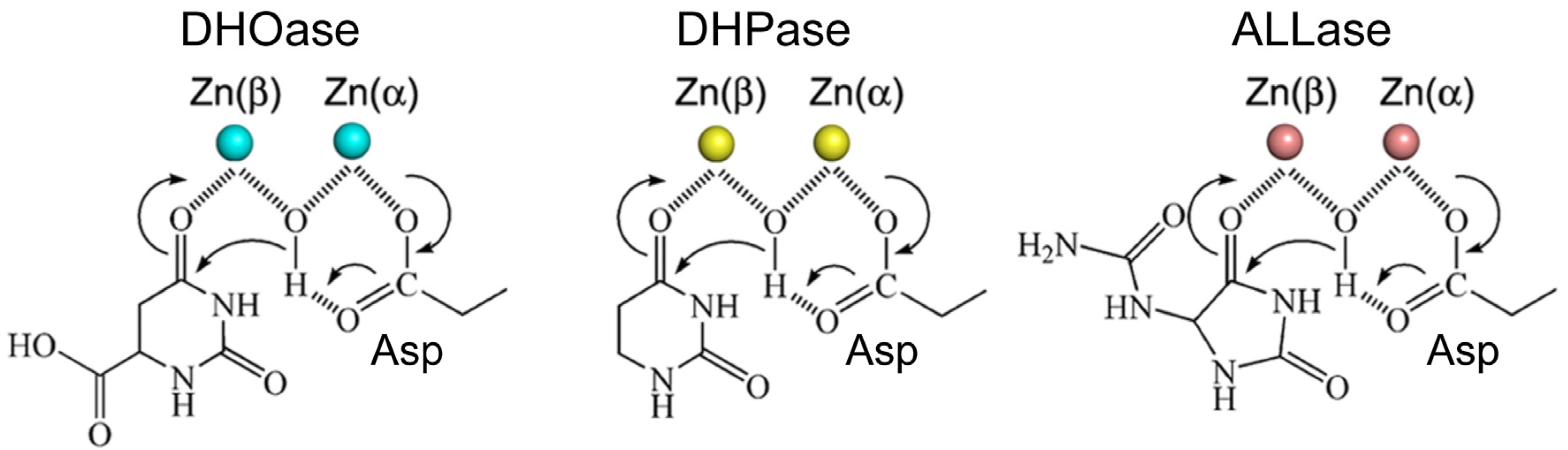
3.3. The Loop-In Binding Mode Observed in DHPase and ALLase
4. Conclusions
Funding
Conflicts of Interest
References
- Del Caño-Ochoa, F.; Ramón-Maiques, S. Deciphering CAD: Structure and function of a mega-enzymatic pyrimidine factory in health and disease. Protein Sci. 2021, 30, 1995–2008. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.R.; Guy, H.I. Mammalian pyrimidine biosynthesis: Fresh insights into an ancient pathway. J. Biol. Chem. 2004, 279, 33035–33038. [Google Scholar] [CrossRef]
- Pardee, A.B.; Yates, R.A. Pyrimidine biosynthesis in Escherichia coli. J. Biol. Chem. 1956, 221, 743–756. [Google Scholar] [PubMed]
- Yang, C.; Zhao, Y.; Wang, L.; Guo, Z.; Ma, L.; Yang, R.; Wu, Y.; Li, X.; Niu, J.; Chu, Q.; et al. De novo pyrimidine biosynthetic complexes support cancer cell proliferation and ferroptosis defence. Nat. Cell Biol. 2023, 25, 836–847. [Google Scholar] [CrossRef]
- Wan, Q.; Tavakoli, L.; Wang, T.Y.; Tucker, A.J.; Zhou, R.; Liu, Q.; Feng, S.; Choi, D.; He, Z.; Gack, M.U.; et al. Hijacking of nucleotide biosynthesis and deamidation-mediated glycolysis by an oncogenic herpesvirus. Nat. Commun. 2024, 15, 1442. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Mir, H.; Khurram, M.A.; Fujihara, K.M.; Dynlacht, B.D.; Cardozo, T.J.; Possemato, R. Allosteric regulation of CAD modulates de novo pyrimidine synthesis during the cell cycle. Nat. Metab. 2023, 5, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Gai, X.; Wu, Y.; Zhang, B.; Wu, X.; Cheng, R.; Tang, B.; Shang, K.; Zhao, N.; Deng, W.; et al. Oncogenic β-catenin stimulation of AKT2-CAD-mediated pyrimidine synthesis is targetable vulnerability in liver cancer. Proc. Natl. Acad. Sci. USA 2022, 119, e2202157119. [Google Scholar] [CrossRef]
- Claiborne, M.D.; Sengupta, S.; Zhao, L.; Arwood, M.L.; Sun, I.M.; Wen, J.; Thompson, E.A.; Mitchell-Flack, M.; Laiho, M.; Powell, J.D. Persistent CAD activity in memory CD8(+) T cells supports rRNA synthesis and ribosomal biogenesis required at rechallenge. Sci. Immunol. 2022, 7, eabh4271. [Google Scholar] [CrossRef]
- O’Donovan, G.A.; Neuhard, J. Pyrimidine metabolism in microorganisms. Bacteriol. Rev. 1970, 34, 278–343. [Google Scholar] [CrossRef]
- Jones, M.E. Pyrimidine nucleotide biosynthesis in animals: Genes, enzymes, and regulation of UMP biosynthesis. Annu. Rev. Biochem. 1980, 49, 253–279. [Google Scholar] [CrossRef]
- Del Cano-Ochoa, F.; Moreno-Morcillo, M.; Ramon-Maiques, S. CAD, A Multienzymatic Protein at the Head of de Novo Pyrimidine Biosynthesis. Subcell. Biochem. 2019, 93, 505–538. [Google Scholar] [PubMed]
- D’Alessio, C.; Caramelo, J.J.; Parodi, A.J. UDP-GlC:glycoprotein glucosyltransferase-glucosidase II, the ying-yang of the ER quality control. Semin. Cell Dev. Biol. 2010, 21, 491–499. [Google Scholar] [CrossRef]
- Huang, M.; Graves, L.M. De novo synthesis of pyrimidine nucleotides; emerging interfaces with signal transduction pathways. Cell Mol. Life Sci. 2003, 60, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Srere, P.A. Complexes of sequential metabolic enzymes. Annu. Rev. Biochem. 1987, 56, 89–124. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, A.L.; Zhou, Q.; Toska, E.; Galeas, J.; Ku, A.A.; Koche, R.P.; Bandyopadhyay, S.; Scaltriti, M.; Lebrilla, C.B.; McCormick, F.; et al. UDP-glucose pyrophosphorylase 2, a regulator of glycogen synthesis and glycosylation, is critical for pancreatic cancer growth. Proc. Natl. Acad. Sci. USA 2021, 118, e2103592118. [Google Scholar] [CrossRef]
- Wang, X.; Feng, J.K.; Mao, F.F.; Hou, Y.C.; Zhang, Y.Q.; Liu, L.H.; Wei, Q.; Sun, J.X.; Liu, C.; Shi, J.; et al. Prognostic and Immunotherapeutic Predictive Value of CAD Gene in Hepatocellular Carcinoma: Integrated Bioinformatics and Experimental Analysis. Mol. Biotechnol. 2024. online ahead of print. [Google Scholar]
- Li, G.; Xiao, K.; Li, Y.; Gao, J.; He, S.; Li, T. CHIP promotes CAD ubiquitination and degradation to suppress the proliferation and colony formation of glioblastoma cells. Cell. Oncol. 2024, 47, 851–865. [Google Scholar] [CrossRef]
- Song, X.; Wang, L.; Wang, T.; Hu, J.; Wang, J.; Tu, R.; Su, H.; Jiang, J.; Qing, G.; Liu, H. Synergistic targeting of CHK1 and mTOR in MYC-driven tumors. Carcinogenesis 2021, 42, 448–460. [Google Scholar] [CrossRef]
- Ridder, D.A.; Schindeldecker, M.; Weinmann, A.; Berndt, K.; Urbansky, L.; Witzel, H.R.; Heinrich, S.; Roth, W.; Straub, B.K. Key Enzymes in Pyrimidine Synthesis, CAD and CPS1, Predict Prognosis in Hepatocellular Carcinoma. Cancers 2021, 13, 744. [Google Scholar] [CrossRef] [PubMed]
- Chernova, O.B.; Chernov, M.V.; Ishizaka, Y.; Agarwal, M.L.; Stark, G.R. MYC abrogates p53-mediated cell cycle arrest in N-(phosphonacetyl)-L-aspartate-treated cells, permitting CAD gene amplification. Mol. Cell Biol. 1998, 18, 536–545. [Google Scholar] [CrossRef]
- Pan, M.; Ge, C.C.; Niu, S.Z.; Duan, Y.Y.; Fan, Y.M.; Jin, Q.W.; Chen, X.; Tao, J.P.; Huang, S.Y. Functional analyses of Toxoplasma gondii dihydroorotase reveal a promising anti-parasitic target. FASEB J. 2024, 38, e23397. [Google Scholar] [CrossRef]
- Yang, P.; Yuan, Y.; Sun, Y.; Lv, B.; Du, H.; Zhou, Z.; Yang, Z.; Liu, X.; Duan, H.; Shen, C. The Host Protein CAD Regulates the Replication of FMDV through the Function of Pyrimidines’ De Novo Synthesis. J. Virol. 2023, 97, e0036923. [Google Scholar] [CrossRef]
- Qin, C.; Rao, Y.; Yuan, H.; Wang, T.Y.; Zhao, J.; Espinosa, B.; Liu, Y.; Zhang, S.; Savas, A.C.; Liu, Q.; et al. SARS-CoV-2 couples evasion of inflammatory response to activated nucleotide synthesis. Proc. Natl. Acad. Sci. USA 2022, 119, e2122897119. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, K.; Wu, Q.; Kim, L.J.Y.; Morton, A.R.; Gimple, R.C.; Prager, B.C.; Shi, Y.; Zhou, W.; Bhargava, S.; et al. Targeting pyrimidine synthesis accentuates molecular therapy response in glioblastoma stem cells. Sci. Transl. Med. 2019, 11, eaau4972. [Google Scholar] [CrossRef] [PubMed]
- Zi, L.; Ma, W.; Zhang, L.; Qiao, B.; Qiu, Z.; Xu, J.; Zhang, J.; Ye, Y.; Yang, Y.; Dong, K.; et al. Uridine Inhibits Hepatocellular Carcinoma Cell Development by Inducing Ferroptosis. J. Clin. Med. 2023, 12, 3552. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Liu, S.; Rodrigues, R.M.; Han, Y.; Guo, C.; Zhu, Z.; He, Y.; Mackowiak, B.; Feng, D.; Gao, B.; et al. Activation of VIPR1 suppresses hepatocellular carcinoma progression by regulating arginine and pyrimidine metabolism. Int. J. Biol. Sci. 2022, 18, 4341–4356. [Google Scholar] [CrossRef] [PubMed]
- Dumenci, O.E.; U, A.M.; Khan, S.A.; Holmes, E.; Taylor-Robinson, S.D. Exploring Metabolic Consequences of CPS1 and CAD Dysregulation in Hepatocellular Carcinoma by Network Reconstruction. J. Hepatocell. Carcinoma 2020, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Adler, L.; Karathia, H.; Carmel, N.; Rabinovich, S.; Auslander, N.; Keshet, R.; Stettner, N.; Silberman, A.; Agemy, L.; et al. Urea Cycle Dysregulation Generates Clinically Relevant Genomic and Biochemical Signatures. Cell 2018, 174, 1559–1570.e22. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, S.; Adler, L.; Yizhak, K.; Sarver, A.; Silberman, A.; Agron, S.; Stettner, N.; Sun, Q.; Brandis, A.; Helbling, D.; et al. Diversion of aspartate in ASS1-deficient tumours fosters de novo pyrimidine synthesis. Nature 2015, 527, 379–383. [Google Scholar] [CrossRef]
- Tu, H.F.; Ko, C.J.; Lee, C.T.; Lee, C.F.; Lan, S.W.; Lin, H.H.; Lin, H.Y.; Ku, C.C.; Lee, D.Y.; Chen, I.C.; et al. Afatinib Exerts Immunomodulatory Effects by Targeting the Pyrimidine Biosynthesis Enzyme CAD. Cancer Res. 2021, 81, 3270–3282. [Google Scholar] [CrossRef]
- Silva, S.; Rosas, M.; Guerra, B.; Muñoz, M.; Fujita, A.; Sakamoto, M.; Matsumoto, N. Adolescent-onset epilepsy and deterioration associated with CAD deficiency: A case report. Brain Dev. 2024, 46, 250–253. [Google Scholar] [CrossRef]
- Del Caño-Ochoa, F.; Ng, B.G.; Rubio-Del-Campo, A.; Mahajan, S.; Wilson, M.P.; Vilar, M.; Rymen, D.; Sánchez-Pintos, P.; Kenny, J.; Ley Martos, M.; et al. Beyond genetics: Deciphering the impact of missense variants in CAD deficiency. J. Inherit. Metab. Dis. 2023, 46, 1170–1185. [Google Scholar] [CrossRef] [PubMed]
- Yarahmadi, S.G.; Morovvati, S. CAD gene and early infantile epileptic encephalopathy-50; three Iranian deceased patients and a novel mutation: Case report. BMC Pediatr. 2022, 22, 125. [Google Scholar] [CrossRef] [PubMed]
- McGraw, C.M.; Mahida, S.; Jayakar, P.; Koh, H.Y.; Taylor, A.; Resnick, T.; Rodan, L.; Schwartz, M.A.; Ejaz, A.; Sankaran, V.G.; et al. Uridine-responsive epileptic encephalopathy due to inherited variants in CAD: A Tale of Two Siblings. Ann. Clin. Transl. Neurol. 2021, 8, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Frederick, A.; Sherer, K.; Nguyen, L.; Ali, S.; Garg, A.; Haas, R.; Sahagian, M.; Bui, J. Triacetyluridine treats epileptic encephalopathy from CAD mutations: A case report and review. Ann. Clin. Transl. Neurol. 2021, 8, 284–287. [Google Scholar] [CrossRef]
- Kamate, M.; Patil, S. CAD Deficiency-Another Treatable Early Infantile Epileptic Encephalopathy. Pediatr. Neurol. 2020, 110, 97–98. [Google Scholar] [CrossRef]
- Koch, J.; Mayr, J.A.; Alhaddad, B.; Rauscher, C.; Bierau, J.; Kovacs-Nagy, R.; Coene, K.L.; Bader, I.; Holzhacker, M.; Prokisch, H.; et al. CAD mutations and uridine-responsive epileptic encephalopathy. Brain 2017, 140, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Nyhan, W.L. Disorders of purine and pyrimidine metabolism. Mol. Genet. Metab. 2005, 86, 25–33. [Google Scholar] [CrossRef]
- Hoelz, L.V.; Calil, F.A.; Nonato, M.C.; Pinheiro, L.C.; Boechat, N. Plasmodium falciparum dihydroorotate dehydrogenase: A drug target against malaria. Future Med. Chem. 2018, 10, 1853–1874. [Google Scholar] [CrossRef] [PubMed]
- Krungkrai, S.R.; Wutipraditkul, N.; Krungkrai, J. Dihydroorotase of human malarial parasite Plasmodium falciparum differs from host enzyme. Biochem. Biophys. Res. Commun. 2008, 366, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Seymour, K.K.; Lyons, S.D.; Phillips, L.; Rieckmann, K.H.; Christopherson, R.I. Cytotoxic effects of inhibitors of de novo pyrimidine biosynthesis upon Plasmodium falciparum. Biochemistry 1994, 33, 5268–5274. [Google Scholar] [CrossRef] [PubMed]
- Krungkrai, J.; Krungkrai, S.R.; Phakanont, K. Antimalarial activity of orotate analogs that inhibit dihydroorotase and dihydroorotate dehydrogenase. Biochem. Pharmacol. 1992, 43, 1295–1301. [Google Scholar] [CrossRef]
- Rathod, P.K.; Khatri, A.; Hubbert, T.; Milhous, W.K. Selective activity of 5-fluoroorotic acid against Plasmodium falciparum in vitro. Antimicrob. Agents Chemother. 1989, 33, 1090–1094. [Google Scholar] [CrossRef] [PubMed]
- Grande-Garcia, A.; Lallous, N.; Diaz-Tejada, C.; Ramon-Maiques, S. Structure, functional characterization, and evolution of the dihydroorotase domain of human CAD. Structure 2014, 22, 185–198. [Google Scholar] [CrossRef]
- Rice, A.J.; Pesavento, R.P.; Ren, J.; Youn, I.; Kwon, Y.; Ellepola, K.; Che, C.T.; Johnson, M.E.; Lee, H. Identification of Small Molecule Inhibitors against Staphylococcus aureus Dihydroorotase via HTS. Int. J. Mol. Sci. 2021, 22, 9984. [Google Scholar] [CrossRef] [PubMed]
- Rice, A.J.; Lei, H.; Santarsiero, B.D.; Lee, H.; Johnson, M.E. Ca-asp bound X-ray structure and inhibition of Bacillus anthracis dihydroorotase (DHOase). Bioorg. Med. Chem. 2016, 24, 4536–4543. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, A.; Purcarea, C.; Ebert, R.; Sadecki, S.; Guy, H.I.; Evans, D.R. Aquifex aeolicus dihydroorotase: Association with aspartate transcarbamoylase switches on catalytic activity. J. Biol. Chem. 2004, 279, 53136–53144. [Google Scholar] [CrossRef] [PubMed]
- Lipowska, J.; Miks, C.D.; Kwon, K.; Shuvalova, L.; Zheng, H.; Lewinski, K.; Cooper, D.R.; Shabalin, I.G.; Minor, W. Pyrimidine biosynthesis in pathogens—Structures and analysis of dihydroorotases from Yersinia pestis and Vibrio cholerae. Int. J. Biol. Macromol. 2019, 136, 1176–1187. [Google Scholar] [CrossRef]
- Souciet, J.L.; Nagy, M.; Le Gouar, M.; Lacroute, F.; Potier, S. Organization of the yeast URA2 gene: Identification of a defective dihydroorotase-like domain in the multifunctional carbamoylphosphate synthetase-aspartate transcarbamylase complex. Gene 1989, 79, 59–70. [Google Scholar] [CrossRef]
- Washabaugh, M.W.; Collins, K.D. Dihydroorotase from Escherichia coli. Purification and characterization. J. Biol. Chem. 1984, 259, 3293–3298. [Google Scholar] [CrossRef] [PubMed]
- Simmer, J.P.; Kelly, R.E.; Rinker, A.G., Jr.; Zimmermann, B.H.; Scully, J.L.; Kim, H.; Evans, D.R. Mammalian dihydroorotase: Nucleotide sequence, peptide sequences, and evolution of the dihydroorotase domain of the multifunctional protein CAD. Proc. Natl. Acad. Sci. USA 1990, 87, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.E.; Mally, M.I.; Evans, D.R. The dihydroorotase domain of the multifunctional protein CAD. Subunit structure, zinc content, and kinetics. J. Biol. Chem. 1986, 261, 6073–6083. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.; Kelly, R.E.; Pastra-Landis, S.C.; Evans, D.R. Oligomeric structure of the multifunctional protein CAD that initiates pyrimidine biosynthesis in mammalian cells. Proc. Natl. Acad. Sci. USA 1985, 82, 6802–6806. [Google Scholar] [CrossRef] [PubMed]
- Nagy, M.; Le Gouar, M.; Potier, S.; Souciet, J.L.; Hervé, G. The primary structure of the aspartate transcarbamylase region of the URA2 gene product in Saccharomyces cerevisiae. Features involved in activity and nuclear localization. J. Biol. Chem. 1989, 264, 8366–8374. [Google Scholar] [CrossRef] [PubMed]
- Guyonvarch, A.; Nguyen-Juilleret, M.; Hubert, J.C.; Lacroute, F. Structure of the Saccharomyces cerevisiae URA4 gene encoding dihydroorotase. Mol. Gen. Genet. 1988, 212, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y. Structure, catalytic mechanism, posttranslational lysine carbamylation, and inhibition of dihydropyrimidinases. Adv. Protein Chem. Struct. Biol. 2020, 122, 63–96. [Google Scholar]
- Thoden, J.B.; Phillips, G.N., Jr.; Neal, T.M.; Raushel, F.M.; Holden, H.M. Molecular structure of dihydroorotase: A paradigm for catalysis through the use of a binuclear metal center. Biochemistry 2001, 40, 6989–6997. [Google Scholar] [CrossRef]
- Lee, M.; Chan, C.W.; Graham, S.C.; Christopherson, R.I.; Guss, J.M.; Maher, M.J. Structures of ligand-free and inhibitor complexes of dihydroorotase from Escherichia coli: Implications for loop movement in inhibitor design. J. Mol. Biol. 2007, 370, 812–825. [Google Scholar] [CrossRef]
- Del Caño-Ochoa, F.; Ramadane-Morchadi, L.; Eixerés, L.; Moreno-Morcillo, M.; Fernández-Leiro, R.; Ramón-Maiques, S. Disruption of CAD Oligomerization by Pathogenic Variants. J. Mol. Biol. 2024, 436, 168832. [Google Scholar] [CrossRef]
- Hervé, G. Structural Insight into the Core of CAD. Structure 2017, 25, 819–820. [Google Scholar] [CrossRef]
- Evans, H.G.; Fernando, R.; Vaishnav, A.; Kotichukkala, M.; Heyl, D.; Hachem, F.; Brunzelle, J.S.; Edwards, B.F.; Evans, D.R. Intersubunit communication in the dihydroorotase-aspartate transcarbamoylase complex of Aquifex aeolicus. Protein Sci. 2014, 23, 100–109. [Google Scholar] [CrossRef]
- Zhang, P.; Martin, P.D.; Purcarea, C.; Vaishnav, A.; Brunzelle, J.S.; Fernando, R.; Guy-Evans, H.I.; Evans, D.R.; Edwards, B.F. Dihydroorotase from the hyperthermophile Aquifex aeolicus is activated by stoichiometric association with aspartate transcarbamoylase and forms a one-pot reactor for pyrimidine biosynthesis. Biochemistry 2009, 48, 766–778. [Google Scholar] [CrossRef] [PubMed]
- Serre, V.; Guy, H.; Liu, X.; Penverne, B.; Hervé, G.; Evans, D. Allosteric regulation and substrate channeling in multifunctional pyrimidine biosynthetic complexes: Analysis of isolated domains and yeast-mammalian chimeric proteins. J. Mol. Biol. 1998, 281, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Guy, H.I.; Evans, D.R. Trapping an activated conformation of mammalian carbamyl-phosphate synthetase. J. Biol. Chem. 1997, 272, 19906–19912. [Google Scholar] [CrossRef] [PubMed]
- Serre, V.; Guy, H.; Penverne, B.; Lux, M.; Rotgeri, A.; Evans, D.; Hervé, G. Half of Saccharomyces cerevisiae carbamoyl phosphate synthetase produces and channels carbamoyl phosphate to the fused aspartate transcarbamoylase domain. J. Biol. Chem. 1999, 274, 23794–23801. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sigoillot, F.D.; Berkowski, J.A.; Sigoillot, S.M.; Kotsis, D.H.; Guy, H.I. Cell cycle-dependent regulation of pyrimidine biosynthesis. J. Biol. Chem. 2003, 278, 3403–3409. [Google Scholar] [CrossRef] [PubMed]
- Porter, T.N.; Li, Y.; Raushel, F.M. Mechanism of the dihydroorotase reaction. Biochemistry 2004, 43, 16285–16292. [Google Scholar] [CrossRef]
- Guan, H.H.; Huang, Y.H.; Lin, E.S.; Chen, C.J.; Huang, C.Y. Structural Analysis of Saccharomyces cerevisiae Dihydroorotase Reveals Molecular Insights into the Tetramerization Mechanism. Molecules 2021, 26, 7249. [Google Scholar] [CrossRef] [PubMed]
- Del Cano-Ochoa, F.; Grande-Garcia, A.; Reverte-Lopez, M.; D’Abramo, M.; Ramon-Maiques, S. Characterization of the catalytic flexible loop in the dihydroorotase domain of the human multi-enzymatic protein CAD. J. Biol. Chem. 2018, 293, 18903–18913. [Google Scholar] [CrossRef]
- Lin, E.S.; Huang, C.Y. Binding Pattern and Structural Interactome of the Anticancer Drug 5-Fluorouracil: A Critical Review. Int. J. Mol. Sci. 2024, 25, 3404. [Google Scholar] [CrossRef]
- Lin, E.S.; Huang, Y.H.; Yang, P.C.; Peng, W.F.; Huang, C.Y. Complexed Crystal Structure of the Dihydroorotase Domain of Human CAD Protein with the Anticancer Drug 5-Fluorouracil. Biomolecules 2023, 13, 149. [Google Scholar] [CrossRef]
- Guan, H.H.; Huang, Y.H.; Lin, E.S.; Chen, C.J.; Huang, C.Y. Complexed Crystal Structure of Saccharomyces cerevisiae Dihydroorotase with Inhibitor 5-Fluoroorotate Reveals a New Binding Mode. Bioinorg. Chem. Appl. 2021, 2021, 2572844. [Google Scholar] [CrossRef]
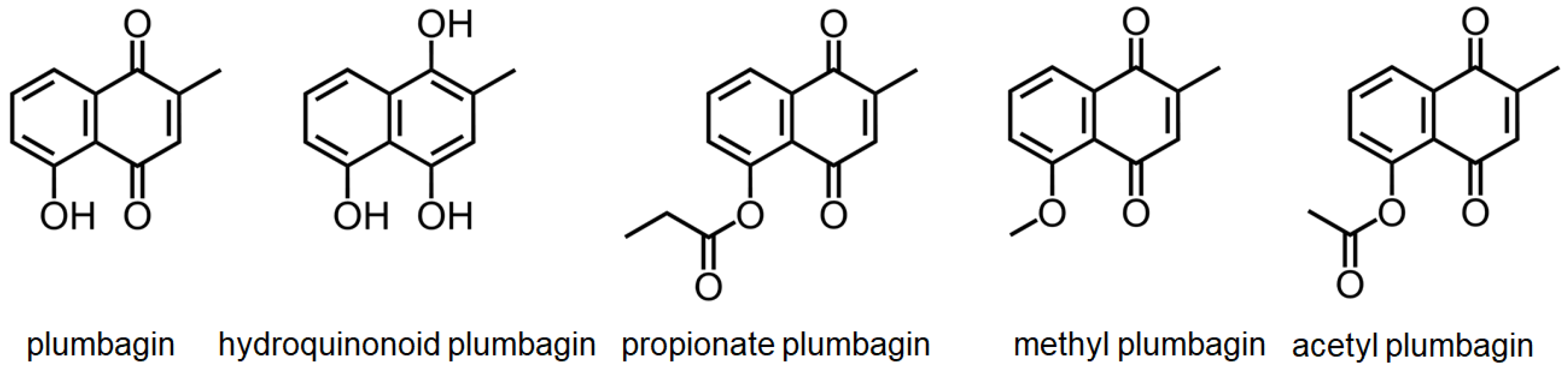
- Guan, H.H.; Huang, Y.H.; Lin, E.S.; Chen, C.J.; Huang, C.Y. Plumbagin, a Natural Product with Potent Anticancer Activities, Binds to and Inhibits Dihydroorotase, a Key Enzyme in Pyrimidine Biosynthesis. Int. J. Mol. Sci. 2021, 22, 6861. [Google Scholar] [CrossRef]
- Guan, H.H.; Huang, Y.H.; Lin, E.S.; Chen, C.J.; Huang, C.Y. Structural basis for the interaction modes of dihydroorotase with the anticancer drugs 5-fluorouracil and 5-aminouracil. Biochem. Biophys. Res. Commun. 2021, 551, 33–37. [Google Scholar] [CrossRef]
- Peng, W.F.; Huang, C.Y. Allantoinase and dihydroorotase binding and inhibition by flavonols and the substrates of cyclic amidohydrolases. Biochimie 2014, 101, 113–122. [Google Scholar] [CrossRef]
- Carrey, E.A. Phosphorylation, allosteric effectors and inter-domain contacts in CAD; their role in regulation of early steps of pyrimidine biosynthesis. Biochem. Soc. Trans. 1993, 21, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Gerlt, J.A.; Babbitt, P.C. Divergent evolution of enzymatic function: Mechanistically diverse superfamilies and functionally distinct suprafamilies. Annu. Rev. Biochem. 2001, 70, 209–246. [Google Scholar] [CrossRef]
- Kim, G.J.; Kim, H.S. Identification of the structural similarity in the functionally related amidohydrolases acting on the cyclic amide ring. Biochem. J. 1998, 330 Pt 1, 295–302. [Google Scholar] [CrossRef]
- Huang, Y.H.; Huang, C.Y. The complexed crystal structure of dihydropyrimidinase reveals a potential interactive link with the neurotransmitter γ-aminobutyric acid (GABA). Biochem. Biophys. Res. Commun. 2024, 692, 149351. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Lien, Y.; Chen, J.H.; Lin, E.S.; Huang, C.Y. Identification and characterization of dihydropyrimidinase inhibited by plumbagin isolated from Nepenthes miranda extract. Biochimie 2020, 171–172, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, C.T.; Huang, Y.H.; Huang, C.Y. Crystal structure of dihydropyrimidinase from Pseudomonas aeruginosa PAO1: Insights into the molecular basis of formation of a dimer. Biochem. Biophys. Res. Commun. 2016, 478, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y. Inhibition of a putative dihydropyrimidinase from Pseudomonas aeruginosa PAO1 by flavonoids and substrates of cyclic amidohydrolases. PLoS ONE 2015, 10, e0127634. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hsieh, Y.C.; Chen, M.C.; Hsu, C.C.; Chan, S.I.; Yang, Y.S.; Chen, C.J. Crystal structures of vertebrate dihydropyrimidinase and complexes from Tetraodon nigroviridis with lysine carbamylation: Metal and structural requirements for post-translational modification and function. J. Biol. Chem. 2013, 288, 30645–30658. [Google Scholar] [CrossRef] [PubMed]
- Abendroth, J.; Niefind, K.; Schomburg, D. X-ray structure of a dihydropyrimidinase from Thermus sp. at 1.3 A resolution. J. Mol. Biol. 2002, 320, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Yang, P.C.; Lin, E.S.; Ho, Y.Y.; Peng, W.F.; Lu, H.P.; Huang, C.C.; Huang, C.Y. Crystal Structure of Allantoinase from Escherichia coli BL21: A Molecular Insight into a Role of the Active Site Loops in Catalysis. Molecules 2023, 28, 827. [Google Scholar] [CrossRef]
- Lin, E.S.; Huang, C.Y. Cytotoxic Activities and the Allantoinase Inhibitory Effect of the Leaf Extract of the Carnivorous Pitcher Plant Nepenthes miranda. Plants 2022, 11, 2265. [Google Scholar] [CrossRef]
- Ho, Y.Y.; Hsieh, H.C.; Huang, C.Y. Biochemical characterization of allantoinase from Escherichia coli BL21. Protein J. 2011, 30, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, M.I.; Chung, J.; Ahn, J.H.; Rhee, S. Crystal structure of metal-dependent allantoinase from Escherichia coli. J. Mol. Biol. 2009, 387, 1067–1074. [Google Scholar] [CrossRef]
- Ho, Y.Y.; Huang, Y.H.; Huang, C.Y. Chemical rescue of the post-translationally carboxylated lysine mutant of allantoinase and dihydroorotase by metal ions and short-chain carboxylic acids. Amino Acids 2013, 44, 1181–1191. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Hsu, C.C.; Chen, M.C.; Yang, Y.S. Effect of metal binding and posttranslational lysine carboxylation on the activity of recombinant hydantoinase. J. Biol. Inorg. Chem. 2009, 14, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.H.; Huang, Y.H.; Lin, J.J.; Huang, C.Y. Crystal structures of monometallic dihydropyrimidinase and the human dihydroorotase domain K1556A mutant reveal no lysine carbamylation within the active site. Biochem. Biophys. Res. Commun. 2018, 505, 439–444. [Google Scholar] [CrossRef]
- Martin, P.D.; Purcarea, C.; Zhang, P.; Vaishnav, A.; Sadecki, S.; Guy-Evans, H.I.; Evans, D.R.; Edwards, B.F. The crystal structure of a novel, latent dihydroorotase from Aquifex aeolicus at 1.7A resolution. J. Mol. Biol. 2005, 348, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Hermoso, J.A. Getting CAD in shape: The atomic structure of human dihydroorotase domain. Structure 2014, 22, 179–181. [Google Scholar] [CrossRef]
- Bowers, E.C.; Cavalcante, A.M.; Nguyen, K.; Li, C.; Wang, Y.; El-Zein, R.; Chen, S.H.; Kim, M.P.; McKay, B.S.; Ramos, K.S. Long Interspersed Nuclear Element-1 Analytes in Extracellular Vesicles as Tools for Molecular Diagnostics of Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2024, 25, 1169. [Google Scholar] [CrossRef] [PubMed]
- Fung, S.K.; Lok, A.S. Drug insight: Nucleoside and nucleotide analog inhibitors for hepatitis B. Nat. Clin. Pract. Gastroenterol. Hepatol. 2004, 1, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Rice, A.J.; Truong, L.; Johnson, M.E.; Lee, H. A colorimetric assay optimization for high-throughput screening of dihydroorotase by detecting ureido groups. Anal. Biochem. 2013, 441, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Maher, M.J.; Christopherson, R.I.; Guss, J.M. Kinetic and structural analysis of mutant Escherichia coli dihydroorotases: A flexible loop stabilizes the transition state. Biochemistry 2007, 46, 10538–10550. [Google Scholar] [CrossRef]
- Huang, Y.H.; Ning, Z.J.; Huang, C.Y. Crystal structure of dihydropyrimidinase in complex with anticancer drug 5-fluorouracil. Biochem. Biophys. Res. Commun. 2019, 519, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Barba, M.; Glansdorff, N.; Labedan, B. Evolution of cyclic amidohydrolases: A highly diversified superfamily. J. Mol. Evol. 2013, 77, 70–80. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-Y. The Loop-In Binding Mode of Dihydroorotase: Implications for Ligand Binding and Therapeutic Targeting. Int. J. Mol. Sci. 2025, 26, 1359. https://doi.org/10.3390/ijms26031359
Huang C-Y. The Loop-In Binding Mode of Dihydroorotase: Implications for Ligand Binding and Therapeutic Targeting. International Journal of Molecular Sciences. 2025; 26(3):1359. https://doi.org/10.3390/ijms26031359
Chicago/Turabian StyleHuang, Cheng-Yang. 2025. "The Loop-In Binding Mode of Dihydroorotase: Implications for Ligand Binding and Therapeutic Targeting" International Journal of Molecular Sciences 26, no. 3: 1359. https://doi.org/10.3390/ijms26031359
APA StyleHuang, C.-Y. (2025). The Loop-In Binding Mode of Dihydroorotase: Implications for Ligand Binding and Therapeutic Targeting. International Journal of Molecular Sciences, 26(3), 1359. https://doi.org/10.3390/ijms26031359




