Role of 11β-Hydroxysteroid Dehydrogenase and Mineralocorticoid Receptor on Alzheimer’s Disease Onset: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Identification of the Research Question
2.2. Study Selection Process
- 11β-HSD and Alzheimer disease;
- 11beta-HSD and Alzheimer disease;
- 11beta-hydroxysteroid dehydrogenase and Alzheimer disease.
3. Results and Discussion
- Section 1 focuses on the biology and genetics of 11β-HSD1.
- Section 2 presents the preclinical groundwork that supports therapeutic inhibition.
- Section 3 addresses clinical trials directly, separating successes (Xanamem) from challenges (ABT-384).
- Section 4 highlights innovations in inhibitor design and their effects.
- From basic biology → preclinical studies → clinical applications → novel designs and disease-modifying effects.
3.1. Role of 11β-HSD1 and HSD11B1 Polymorphisms in AD Pathophysiology
3.1.1. Basic Research on 11β-HSD1 Function
- Herbert and Lucassen [14] reported that 11β-HSD1 inhibition protects neurons and improves cognition in aging models, suggesting that excessive glucocorticoid activity is neurotoxic. Conversely, they found that 11β-HSD2 induction plays a protective role in cerebellar neurons. These findings highlight the dual roles of 11β-HSD enzymes in regulating glucocorticoid metabolism and their potential as therapeutic targets.
- Surprisingly, the authors later pointed out a study by de Quervain Dominique [15], which suggested that a rare variant of the HSD11B1 gene associated with reduced 11β-HSD1 activity increases AD risk sixfold. This finding contrasts with the prevailing understanding of 11β-HSD1 as harmful in AD. Upon further review of this study, they clarified that the observed effects were context-dependent: in cell homogenates, 11β-HSD1 favored dehydrogenase activity (converting active cortisol to inactive cortisone), whereas in cell cultures, the enzyme primarily acted as a reductase, regenerating active cortisol. This discrepancy underscores the complexity of 11β-HSD1’s role in AD and the need for further study.
3.1.2. Polymorphisms of the HSD11B1 Gene
- rs846911 polymorphism: A rare A allele of this polymorphism was found in 2.9% of AD patients compared to 0.5% of controls. This suggests that certain genetic variants of 11β-HSD1 might predispose individuals to AD [15].
- 83,557 insA polymorphism: In a study by Smit et al., the percentage of this variant was high (34.6% heterozygous and 4.8% homozygous) in the study groups consisting in 6105 subjects, but no significant correlation was found with dementia incidence. This suggests that not all genetic variations of 11β-HSD1 are equally impactful in AD [16,17].
- rs12086634-G/T, rs846910-A/G: Deary et al. tested the hypothesis that these polymorphisms were associated with lifetime cognitive change in humans. They evaluated the presence of polymorphisms in 194 participants at age 11 and age 79. They found no association between HSD11B1 SNPs and cognitive variation with aging in a Scottish cohort, concluding that further studies in Alzheimer’s disease in different populations were warranted [18].
3.2. Preclinical Evidence Supporting 11β-HSD1 Inhibition
- This section synthesizes evidence from animal and cellular preclinical studies, underlining the neuroprotective potential of 11β-HSD1 inhibition in AD and suggesting that modulating glucocorticoid metabolism may alleviate AD-related damage. These studies offer valuable insights into the role of 11β-HSD1 in regulating local glucocorticoid levels and its impact on neurodegeneration. Li et al. [19] demonstrated that in animal models of AD, pharmacological inhibition or genetic knockdown of 11β-HSD1 prevented cognitive impairment caused by excessive local steroid action. This study linked excessive glucocorticoid activity to hippocampal damage and validated 11β-HSD1 inhibition as a neuroprotective strategy.
- Chowdhury et al. [20] examined the overexpression of 11β-HSD1 in cell models, which resulted in reduced cell proliferation and increased apoptosis, suggesting that hyperactivity of this enzyme directly contributes to neuronal loss.
- Wang et al. [21] investigated the effects of Akebia saponin D (ASD) in an amyloid-beta rat model of AD. ASD reversed corticosterone increases via HPA axis regulation, though it did not directly inhibit 11β-HSD1. These findings indicate that reducing corticosterone levels may have therapeutic benefits in AD.
3.3. Clinical Trials of 11β-HSD1 Inhibitors
3.3.1. ABT-384
- Phase II Trial: ABT-384, a selective 11β-HSD1 inhibitor, was tested in AD patients to assess its ability to reduce intracellular cortisol and improve cognition. While the compound successfully inhibited 11β-HSD1 in the brain, no significant cognitive improvements were observed, as measured by the Alzheimer’s Disease Assessment Scale—Cognitive (ADAS-Cog). The trial was terminated early for futility, highlighting the challenges of targeting glucocorticoid metabolism in AD [22].
- Katz et al. [23]: This study confirmed that ABT-384 effectively inhibited both peripheral and central 11β-HSD1 at low doses (1–2 mg). However, the lack of clinical efficacy despite strong enzymatic inhibition contrasts with the hypothesis that cortisol is deregulated.
3.3.2. Xanamem (UE2343)
- Webster et al. [24]: This study demonstrated that Xanamem, another selective 11β-HSD1 inhibitor, effectively penetrated the brain, showing favorable safety and pharmacokinetics in early trials.
- Taylor et al. [25]: In a Phase II trial, Xanamem improved cognition in patients with high levels of pTau181, a biomarker of tauopathy. This suggests that Xanamem may be particularly effective in tau-driven subtypes of AD.
- Dodd et al.: In a Phase II, Xanamem did not achieve statistically significant results at a dose of 10 mg/day and new results about 20 mg daily dose to reach cognitive protection have to be published [12].
3.4. Novel and Optimized 11β-HSD1 Inhibitors
- Leiva et al. [26]: The authors of this study developed inhibitors featuring unexplored pyrrolidine-based polycyclic substituents, demonstrating a high affinity for 11β-HSD1. This compound improved memory and learning in animal models of age-related cognitive decline, reduced neuroinflammation, and decreased oxidative stress.
- Puigoriol-Illamola et al. [27,28]: In this study, researchers developed RL-118, a pyrrolidine-based inhibitor, which alleviated cognitive impairments in aging mice by promoting autophagy and improving mitochondrial function. RL-118 boosted Beclin1 and LC3B levels, facilitating the clearance of tau. It also reduced glucocorticoid receptor expression and systemic glucocorticoid levels, demonstrating broad neuroprotective effects. Moreover, the study found that inhibiting 11β-HSD1 with RL-118 mitigated the harmful effects caused by chronic mild stress, including epigenetic and cognitive disruptions. This suggests that reducing glucocorticoid excess could be a promising therapeutic approach for age-related cognitive decline and Alzheimer’s disease.
- Sooy et al. [29]: In this study, the authors evaluated UE2316 (more details on Table 2), which reduced amyloid plaque burden in Tg2576 mice and improved memory. This compound lowered glucocorticoid levels in the brain, restoring the balance between glucocorticoid and mineralocorticoid receptor activity. In addition, in [29], the chronic administration of this inhibitor significantly reduced amyloid plaques in the cortex and amygdala of Tg2576 mice. This was associated with improvements in memory and synaptic integrity. Mohler et al. [30] characterized two novel and selective HSD1 inhibitors, A-918446 and A-801195, and learning, memory consolidation, and recall were evaluated as well as CREB, a transcription factor involved in cognition. Treatments inhibited cortisol production in the ex vivo assay by ∼35–90%, significantly improving short-term memory in rats.
- Canet et al. [7] focused on the role of early dysregulation of the hypothalamic–pituitary–adrenal axis (HPA axis or stress axis) observed in AD patients and the inhibition of 11β-HSD1 was able to partially restore it. The authors summarized all the different 11β-HSD1 inhibitors, demonstrating that all of them, according to the different animal models, lead to memory improvement and Aβ plaques decrease. Other effects of 11β-HSD1 on the HPA axis were reported by Seckl [31]. In mice, the inhibition of 11β-HSD1 disrupted glucocorticoid feedback regulation in the HPA axis, leading to reduced glucocorticoid levels and the compensatory activation of the axis. This resulted in elevated ACTH and adrenal activity. In humans, 11β-HSD1 inhibitors typically do not alter cortisol but cause modest increases in ACTH and adrenal products like DHEA, reflecting the compensatory activation of the HPA axis. Indeed, elevated ACTH and DHEA are useful biomarkers of effective 11β-HSD1 inhibition in humans.
4. Limitations of the Study and the Selection of Articles
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Preziuso, A.; Piccirillo, S.; Cerqueni, G.; Serfilippi, T.; Terenzi, V.; Vinciguerra, A.; Orciani, M.; Amoroso, S.; Magi, S.; Lariccia, V. Exploring the Role of NCX1 and NCX3 in an In Vitro Model of Metabolism Impairment: Potential Neuroprotective Targets for Alzheimer’s Disease. Biology 2023, 12, 1005. [Google Scholar] [CrossRef] [PubMed]
- de la Monte, S.M. Insulin Resistance and Alzheimer’s Disease. BMB Rep. 2009, 42, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T. The Role of Orexin in Motivated Behaviours. Nat. Rev. Neurosci. 2014, 15, 719–731. [Google Scholar] [CrossRef]
- Alster, P.; Madetko-Alster, N.; Otto-Ślusarczyk, D.; Migda, A.; Migda, B.; Struga, M.; Friedman, A. Role of Orexin in Pathogenesis of Neurodegenerative Parkinsonisms. Neurol. Neurochir. Pol. 2023, 57, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Almeida, V.N. Somatostatin and the Pathophysiology of Alzheimer’s Disease. Ageing Res. Rev. 2024, 96, 102270. [Google Scholar] [CrossRef]
- Lezoualc’h, F.; Engert, S.; Berning, B.; Behl, C. Corticotropin-Releasing Hormone-Mediated Neuroprotection against Oxidative Stress Is Associated with the Increased Release of Non-Amyloidogenic Amyloid Beta Precursor Protein and with the Suppression of Nuclear Factor-KappaB. Mol. Endocrinol. 2000, 14, 147–159. [Google Scholar] [CrossRef]
- Canet, G.; Hernandez, C.; Zussy, C.; Chevallier, N.; Desrumaux, C.; Givalois, L. Is AD a Stress-Related Disorder? Focus on the HPA Axis and Its Promising Therapeutic Targets. Front. Aging Neurosci. 2019, 11, 269. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.J.; Chakraborty, S.; Rao, B.S.S. Remediation of Chronic Immobilization Stress-Induced Negative Affective Behaviors and Altered Metabolism of Monoamines in the Prefrontal Cortex by Inactivation of Basolateral Amygdala. Neurochem. Int. 2020, 141, 104858. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.J.; Chakraborty, S.; Srikumar, B.N.; Raju, T.R.; Shankaranarayana Rao, B.S. Prevention of Chronic Immobilization Stress-Induced Enhanced Expression of Glucocorticoid Receptors in the Prefrontal Cortex by Inactivation of Basolateral Amygdala. J. Chem. Neuroanat. 2019, 95, 134–145. [Google Scholar] [CrossRef]
- Caffarini, M.; Armeni, T.; Pellegrino, P.; Cianfruglia, L.; Martino, M.; Offidani, A.; Di Benedetto, G.; Arnaldi, G.; Campanati, A.; Orciani, M. Cushing Syndrome: The Role of MSCs in Wound Healing, Immunosuppression, Comorbidities, and Antioxidant Imbalance. Front. Cell Dev. Biol. 2019, 7, 227. [Google Scholar] [CrossRef] [PubMed]
- Diederich, S.; Eigendorff, E.; Burkhardt, P.; Quinkler, M.; Bumke-Vogt, C.; Rochel, M.; Seidelmann, D.; Esperling, P.; Oelkers, W.; Bähr, V. 11β-Hydroxysteroid Dehydrogenase Types 1 and 2: An Important Pharmacokinetic Determinant for the Activity of Synthetic Mineralo- and Glucocorticoids. J. Clin. Endocrinol. Metab. 2002, 87, 5695–5701. [Google Scholar] [CrossRef] [PubMed]
- Dodd, S.; Skvarc, D.R.; Dean, O.M.; Anderson, A.; Kotowicz, M.; Berk, M. Effect of Glucocorticoid and 11β-Hydroxysteroid-Dehydrogenase Type 1 (11β-HSD1) in Neurological and Psychiatric Disorders. Int. J. Neuropsychopharmacol. 2022, 25, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Arksey, H.; O’Malley, L. Scoping Studies: Towards a Methodological Framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Herbert, J.; Lucassen, P.J. Depression as a Risk Factor for Alzheimer’s Disease: Genes, Steroids, Cytokines and Neurogenesis—What Do We Need to Know? Front. Neuroendocrinol. 2016, 41, 153–171. [Google Scholar] [CrossRef] [PubMed]
- de Quervain, D.J.-F. Glucocorticoid-Related Genetic Susceptibility for Alzheimer’s Disease. Hum. Mol. Genet. 2003, 13, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Gregory, S.; Hill, D.; Grey, B.; Ketelbey, W.; Miller, T.; Muniz-Terrera, G.; Ritchie, C.W. 11β-Hydroxysteroid Dehydrogenase Type 1 Inhibitor Use in Human Disease-a Systematic Review and Narrative Synthesis. Metabolism 2020, 108, 154246. [Google Scholar] [CrossRef]
- Smit, P.; Dekker, M.J.H.J.; de Jong, F.J.; van den Beld, A.W.; Koper, J.W.; Pols, H.A.P.; Brinkmann, A.O.; de Jong, F.H.; Breteler, M.M.B.; Lamberts, S.W.J. Lack of Association of the 11β-Hydroxysteroid Dehydrogenase Type 1 Gene 83,557insA and Hexose-6-Phosphate Dehydrogenase Gene R453Q Polymorphisms with Body Composition, Adrenal Androgen Production, Blood Pressure, Glucose Metabolism, and Dementia. J. Clin. Endocrinol. Metab. 2007, 92, 359–362. [Google Scholar] [CrossRef][Green Version]
- Deary, I.J.; Hayward, C.; Permana, P.A.; Nair, S.; Whalley, L.J.; Starr, J.M.; Chapman, K.E.; Walker, B.R.; Seckl, J.R. Polymorphisms in the Gene Encoding 11B-Hydroxysteroid Dehydrogenase Type 1 (HSD11B1) and Lifetime Cognitive Change. Neurosci. Lett. 2006, 393, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qiu, W.; Deng, L.; Lin, J.; Huang, W.; Xu, Y.; Zhang, M.; Jones, N.C.; Lin, R.; Xu, H.; et al. 11β-HSD1 Participates in Epileptogenesis and the Associated Cognitive Impairment by Inhibiting Apoptosis in Mice. J. Transl. Med. 2022, 20, 406. [Google Scholar] [CrossRef]
- Chowdhury, S.; Grimm, L.; Gong, Y.J.K.; Wang, B.; Li, B.; Srikant, C.B.; Gao, Z.; Liu, J.-L. Decreased 11β-Hydroxysteroid Dehydrogenase 1 Level and Activity in Murine Pancreatic Islets Caused by Insulin-Like Growth Factor I Overexpression. PLoS ONE 2015, 10, e0136656. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shen, J.; Yang, X.; Jin, Y.; Yang, Z.; Wang, R.; Zhang, F.; Linhardt, R.J. Akebia Saponin D Reverses Corticosterone Hypersecretion in an Alzheimer’s Disease Rat Model. Biomed. Pharmacother. 2018, 107, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Marek, G.J.; Katz, D.A.; Meier, A.; Greco, N.; Zhang, W.; Liu, W.; Lenz, R.A. Efficacy and Safety Evaluation of HSD-1 Inhibitor ABT-384 in Alzheimer’s Disease. Alzheimer’s Dement. 2014, 10, S364–S373. [Google Scholar] [CrossRef] [PubMed]
- Katz, D.A.; Liu, W.; Locke, C.; Jacobson, P.; Barnes, D.M.; Basu, R.; An, G.; Rieser, M.J.; Daszkowski, D.; Groves, F.; et al. Peripheral and Central Nervous System Inhibition of 11β-Hydroxysteroid Dehydrogenase Type 1 in Man by the Novel Inhibitor ABT-384. Transl. Psychiatry 2013, 3, e295. [Google Scholar] [CrossRef] [PubMed]
- Webster, S.P.; McBride, A.; Binnie, M.; Sooy, K.; Seckl, J.R.; Andrew, R.; Pallin, T.D.; Hunt, H.J.; Perrior, T.R.; Ruffles, V.S.; et al. Selection and Early Clinical Evaluation of the Brain-penetrant 11β-hydroxysteroid Dehydrogenase Type 1 (11β-HSD1) Inhibitor UE2343 (XanamemTM). Br. J. Pharmacol. 2017, 174, 396–408. [Google Scholar] [CrossRef]
- Taylor, J.; Jaros, M.; Chen, C.; Harrison, J.; Hilt, D. Plasma PTau181 Predicts Clinical Progression in a Phase 2 Randomized Controlled Trial of the 11β-HSD1 Inhibitor Xanamem® for Mild Alzheimer’s Disease. J. Alzheimer’s Dis. 2024, 100, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Leiva, R.; Griñan-Ferré, C.; Seira, C.; Valverde, E.; McBride, A.; Binnie, M.; Pérez, B.; Luque, F.J.; Pallàs, M.; Bidon-Chanal, A.; et al. Design, Synthesis and in Vivo Study of Novel Pyrrolidine-Based 11β-HSD1 Inhibitors for Age-Related Cognitive Dysfunction. Eur. J. Med. Chem. 2017, 139, 412–428. [Google Scholar] [CrossRef] [PubMed]
- Puigoriol-Illamola, D.; Leiva, R.; Vázquez-Carrera, M.; Vázquez, S.; Griñán-Ferré, C.; Pallàs, M. 11β-HSD1 Inhibition Rescues SAMP8 Cognitive Impairment Induced by Metabolic Stress. Mol. Neurobiol. 2020, 57, 551–565. [Google Scholar] [CrossRef]
- Puigoriol-Illamola, D.; Companys-Alemany, J.; McGuire, K.; Homer, N.Z.M.; Leiva, R.; Vázquez, S.; Mole, D.J.; Griñán-Ferré, C.; Pallàs, M. Inhibition of 11β-HSD1 Ameliorates Cognition and Molecular Detrimental Changes after Chronic Mild Stress in SAMP8 Mice. Pharmaceuticals 2021, 14, 1040. [Google Scholar] [CrossRef] [PubMed]
- Sooy, K.; Noble, J.; McBride, A.; Binnie, M.; Yau, J.L.W.; Seckl, J.R.; Walker, B.R.; Webster, S.P. Cognitive and Disease-Modifying Effects of 11β-Hydroxysteroid Dehydrogenase Type 1 Inhibition in Male Tg2576 Mice, a Model of Alzheimer’s Disease. Endocrinology 2015, 156, 4592–4603. [Google Scholar] [CrossRef] [PubMed]
- Mohler, E.G.; Browman, K.E.; Roderwald, V.A.; Cronin, E.A.; Markosyan, S.; Scott Bitner, R.; Strakhova, M.I.; Drescher, K.U.; Hornberger, W.; Rohde, J.J.; et al. Acute Inhibition of 11β-Hydroxysteroid Dehydrogenase Type-1 Improves Memory in Rodent Models of Cognition. J. Neurosci. 2011, 31, 5406–5413. [Google Scholar] [CrossRef] [PubMed]
- Seckl, J. 11β-Hydroxysteroid Dehydrogenase and the Brain: Not (yet) Lost in Translation. J. Intern. Med. 2024, 295, 20–37. [Google Scholar] [CrossRef] [PubMed]


| N. Records | |||
|---|---|---|---|
| PubMed | Web of Science | Scopus | |
| 11β-HSD and Alzheimer disease | 22 | 8 | 7 |
| 11beta-HSD and Alzheimer disease | 22 | 9 | 7 |
| 11beta-hydroxysteroid dehydrogenase and Alzheimer disease | 27 | 54 | 7 |
| IUPAC Name | Molecular Formula | Chemical Structure Depiction | URL | |
| ABT-384 | 4-[[2-methyl-2-[4-[5-(trifluoromethyl)pyridin-2-yl]piperazin-1-yl]propanoyl]amino]adamantane-1-carboxamide | C25H34F3N5O2 | 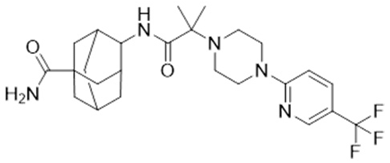 | https://pubchem.ncbi.nlm.nih.gov/compound/Abt-384 (21 January 2025) |
| Xanamem UE2343 | [(1R,5S)-3-hydroxy-3-pyrimidin-2-yl-8-azabicyclo [3.2.1]octan-8-yl]-[5-(1H-pyrazol-4-yl)thiophen-3-yl]methanone | C19H19N5O2S | 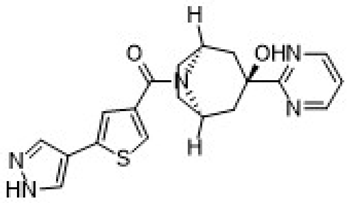 | https://pubchem.ncbi.nlm.nih.gov/compound/137530063 (21 January 2025) |
| UE2316 | (5-(1H-pyrazol-4-yl)thiophen-3-yl)(4-(2-chlorophenyl)-4-fluoropiperidin-1-yl)methanone | C19H17ClFN3OS | 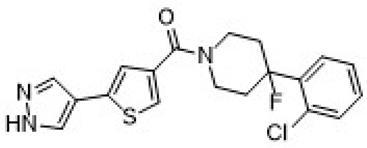 | https://www.medkoo.com/products/50127 (21 January 2025) |
| Category | Study | Key Findings |
|---|---|---|
| Basic Research on 11β-HSD1 Function | Herbert and Lucassen [13] | 11β-HSD1 inhibition protects neurons and improves cognition in aging models. 11β-HSD2 induction protects cerebellar neurons, showing dual roles in glucocorticoid metabolism. |
| de Quervain Dominique [14] | Rare HSD11B1 variant with reduced 11β-HSD1 activity increases AD risk sixfold. | |
| Polymorphisms of HSD11B1 Gene | de Quervain Dominique [14] | A rare rs846911 allele found in 2.9% of AD patients vs. 0.5% of controls, suggesting that genetic variants may be predisposed to AD. |
| Gregory et al. [15]; Smit et al. [16] | High prevalence of 83,557 insA polymorphism in 6105 subjects, but no significant correlation with dementia incidence. | |
| Deary et al. [17] | No association between rs12086634-G/T, rs846910-A/G, and cognitive variation in a cohort at ages 11 and 79. | |
| Preclinical Evidence Supporting 11β-HSD1 Inhibition | Li et al. [18] | Inhibition or knockdown of 11β-HSD1 in AD animal models prevents cognitive impairment and hippocampal damage. |
| Chowdhury et al. [19] | 11β-HSD1 overexpression in cell models results in reduced cell proliferation and increased apoptosis. | |
| Wang et al. [20] | Akebia saponin D (ASD) reduces corticosterone levels but does not directly inhibit 11β-HSD1. | |
| Clinical Trials of 11β-HSD1 Inhibitors | Marek et al. [21]; Katz et al. [22] | No cognitive improvement in AD patients despite the effective inhibition of 11β-HSD1 by ABT-384. |
| Webster et al. [23]; Taylor et al. [24] | Xanamem shows favorable brain penetration and safety. In a Phase II trial, it improves cognition in patients with tauopathy biomarkers (pTau181). | |
| Dodd et al. [12] | In Phase II, 10 mg/day Xanamem did not achieve statistically significant results, but new data on 20 mg/day dose may yield cognitive protection. | |
| Novel and Optimized 11β-HSD1 Inhibitors | Leiva et al. [25] | Developed pyrrolidine-based inhibitors improving memory and learning in animal models. |
| Puigoriol-Illamola et al. [26,27] | RL-118 alleviates cognitive impairments in aging mice, reducing neuroinflammation and glucocorticoid excess. | |
| Sooy et al. [28] | UE2316 reduces amyloid plaque burden and improved memory in Tg2576 mice by restoring glucocorticoid and mineralocorticoid receptor balance. | |
| Mohler et al. [29] | Two novel inhibitors (A-918446, A-801195) significantly improve short-term memory in rats, inhibiting cortisol production and enhancing cognition. | |
| Canet et al. [30] | Various 11β-HSD1 inhibitors show consistent memory improvements and reductions in amyloid plaques across animal models. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Vincenzo, M.; Pellegrino, P.; Schiappa, G.; Campanati, A.; Del Vescovo, V.; Piccirillo, S.; Ambrogini, P.; Arnaldi, G.; Orciani, M. Role of 11β-Hydroxysteroid Dehydrogenase and Mineralocorticoid Receptor on Alzheimer’s Disease Onset: A Systematic Review. Int. J. Mol. Sci. 2025, 26, 1357. https://doi.org/10.3390/ijms26031357
Di Vincenzo M, Pellegrino P, Schiappa G, Campanati A, Del Vescovo V, Piccirillo S, Ambrogini P, Arnaldi G, Orciani M. Role of 11β-Hydroxysteroid Dehydrogenase and Mineralocorticoid Receptor on Alzheimer’s Disease Onset: A Systematic Review. International Journal of Molecular Sciences. 2025; 26(3):1357. https://doi.org/10.3390/ijms26031357
Chicago/Turabian StyleDi Vincenzo, Mariangela, Pamela Pellegrino, Genny Schiappa, Anna Campanati, Valerio Del Vescovo, Silvia Piccirillo, Patrizia Ambrogini, Giorgio Arnaldi, and Monia Orciani. 2025. "Role of 11β-Hydroxysteroid Dehydrogenase and Mineralocorticoid Receptor on Alzheimer’s Disease Onset: A Systematic Review" International Journal of Molecular Sciences 26, no. 3: 1357. https://doi.org/10.3390/ijms26031357
APA StyleDi Vincenzo, M., Pellegrino, P., Schiappa, G., Campanati, A., Del Vescovo, V., Piccirillo, S., Ambrogini, P., Arnaldi, G., & Orciani, M. (2025). Role of 11β-Hydroxysteroid Dehydrogenase and Mineralocorticoid Receptor on Alzheimer’s Disease Onset: A Systematic Review. International Journal of Molecular Sciences, 26(3), 1357. https://doi.org/10.3390/ijms26031357







