Exploring the Role of Lower Genital Tract Microbiota and Cervical–Endometrial Immune Metabolome in Unknown Genesis of Recurrent Pregnancy Loss
Abstract
1. Introduction
2. Results
3. Methods
3.1. Study Design and Population
3.2. Inclusion and Exclusion Criteria
3.3. Immunocytochemical Examination
3.4. Sample Collection and Clinical Assessment
3.5. Cytological Examination
3.6. HPV Detection and Typing
3.7. Immunocytochemical Testing
3.8. Colposcopic Examination
3.9. RNA Isolation and Analysis
3.10. Evaluation of miRNA Expression: RT-PCR
3.11. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, C.; Bai, S.; Zhang, J.; Sun, X.; Meng, A.; Chen, H. Understanding recurrent pregnancy loss: Recent advances on its etiology, clinical diagnosis, and management. Med. Rev. 2022, 2, 570–589. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Kwak-Kim, J.; Bao, S. Unexplained recurrent pregnancy loss: Novel causes and advanced treatment. J. Reprod. Immunol. 2023, 155, 103785. [Google Scholar] [CrossRef] [PubMed]
- Franasiak, J.M.; Alecsandru, D.; Forman, E.J.; Gemmell, L.C.; Goldberg, J.M.; Llarena, N.; Margolis, C.; Laven, J.; Schoenmakers, S.; Seli, E. A review of the pathophysiology of recurrent implantation failure. Fertil. Steril. 2021, 116, 1436–1448. [Google Scholar] [CrossRef]
- Dimitriadis, E.; Menkhorst, E.; Saito, S.; Kutteh, W.H.; Brosens, J.J. Recurrent pregnancy loss. Nat. Rev. Dis. Primers 2020, 6, 98. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Diaz, D.J.; Tyssen, D.; Hayward, J.A.; Gugasyan, R.; Hearps, A.C.; Tachedjian, G. Distinct immune responses elicited from cervicovaginal epithelial cells by lactic acid and short chain fatty acids associated with optimal and non-optimal vaginal microbiota. Front. Cell. Infect. Microbiol. 2019, 9, 446. [Google Scholar] [CrossRef] [PubMed]
- Nunn, K.L.; Witkin, S.S.; Schneider, G.M.; Boester, A.; Nasioudis, D.; Minis, E.; Gliniewicz, K.; Forney, L.J. Changes in the Vaginal Microbiome during the Pregnancy to Postpartum Transition. Reprod. Sci. 2021, 28, 1996–2005. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sohn, K.; Underwood, M.A. Prenatal and postnatal administration of prebiotics and probiotics. Semin. Fetal Neonatal Med. 2017, 22, 284–289. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G. Gestational shaping of the maternal vaginal microbiome. Nat. Med. 2019, 25, 882–883. [Google Scholar] [CrossRef]
- Onyango, S.; Mi, J.D.; Koech, A.; Okiro, P.; Temmerman, M.; von Dadelszen, P.; Tribe, R.M.; Omuse, G.; PRECISE Network. Microbiota dynamics, metabolic and immune interactions in the cervicovaginal environment and their role in spontaneous preterm birth. Front. Immunol. 2023, 14, 1306473. [Google Scholar] [CrossRef]
- Gudnadottir, U.; Debelius, J.W.; Du, J.; Hugerth, L.W.; Danielsson, H.; Schuppe-Koistinen, I.; Fransson, E.; Brusselaers, N. The vaginal microbiome and the risk of preterm birth: A systematic review and network meta-analysis. Sci. Rep. 2022, 12, 7926. [Google Scholar] [CrossRef] [PubMed Central]
- Fettweis, J.M.; Serrano, M.G.; Brooks, J.P.; Edwards, D.J.; Girerd, P.H.; Parikh, H.I.; Huang, B.; Arodz, T.J.; Edupuganti, L.; Glascock, A.L.; et al. The vaginal microbiome and preterm birth. Nat. Med. 2019, 25, 1012–1021. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, H.; Ma, Y.; Li, R.; Chen, X.; Wan, L.; Zhao, W. Associations of Cervicovaginal Lactobacilli With High-Risk Human Papillomavirus Infection, Cervical Intraepithelial Neoplasia, and Cancer: A Systematic Review and Meta-Analysis. J. Infect. Dis. 2019, 220, 1243–1254. [Google Scholar] [CrossRef] [PubMed]
- Norenhag, J.; Du, J.; Olovsson, M.; Verstraelen, H.; Engstrand, L.; Brusselaers, N. The vaginal microbiota, human papillomavirus and cervical dysplasia: A systematic review and network meta-analysis. BJOG 2020, 127, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Basonidis, A.; Liberis, A.; Daniilidis, A.; Petousis, S.; Dinas, K. Human papilloma virus infection and miscarriage: Is there an association? Taiwan. J. Obstet. Gynecol. 2020, 59, 656–659. [Google Scholar] [CrossRef] [PubMed]
- Aldhous, M.C.; Bhatia, R.; Pollock, R.; Vragkos, D.; Cuschieri, K.; Cubie, H.A.; Norman, J.E.; Stock, S.J. HPV infection and pre-term birth: A data-linkage study using Scottish Health Data. Wellcome Open Res. 2019, 4, 48. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xiong, Y.Q.; Mo, Y.; Luo, Q.M.; Huo, S.T.; He, W.Q.; Chen, Q. The Risk of Human Papillomavirus Infection for Spontaneous Abortion, Spontaneous Preterm Birth, and Pregnancy Rate of Assisted Reproductive Technologies: A Systematic Review and Meta-Analysis. Gynecol. Obstet. Investig. 2018, 83, 417–427. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, L.B.; Pereira, C.C.; Merçon-de-Vargas, P.R.; Spano, L.C. Human papillomavirus in foetal and maternal tissues from miscarriage cases. J. Obstet. Gynaecol. 2018, 38, 1083–1087. [Google Scholar] [CrossRef] [PubMed]
- Niyibizi, J.; Mayrand, M.H.; Audibert, F.; Monnier, P.; Brassard, P.; Laporte, L.; Lacaille, J.; Zahreddine, M.; Bédard, M.J.; Girard, I.; et al. Association Between Human Papillomavirus Infection Among Pregnant Women and Preterm Birth. JAMA Netw. Open 2021, 4, e2125308. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chan, D.; Bennett, P.R.; Lee, Y.S.; Kundu, S.; Teoh, T.G.; Adan, M.; Ahmed, S.; Brown, R.G.; David, A.L.; Lewis, H.V.; et al. Microbial-driven preterm labour involves crosstalk between the innate and adaptive immune response. Nat. Commun. 2022, 13, 975. [Google Scholar] [CrossRef]
- Abu-Raya, B.; Michalski, C.; Sadarangani, M.; Lavoie, P.M. Maternal Immunological Adaptation During Normal Pregnancy. Front. Immunol. 2020, 11, 575197. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Guo, H.; Geng, L.; Wang, Y. p16/Ki-67 dual-stained cytology used for triage in cervical cancer opportunistic screening. Chin. J. Cancer Res. 2020, 32, 208–217. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, L.; Fei, L.; Liu, X.; Pi, X.; Wang, L.; Chen, S. Application of p16/Ki-67 dual-staining cytology in cervical cancers. J. Cancer 2019, 10, 2654–2660. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Deleye, Y.; Cotte, A.K.; Hannou, S.A.; Hennuyer, N.; Bernard, L.; Derudas, B.; Caron, S.; Legry, V.; Vallez, E.; Dorchies, E.; et al. CDKN2A/p16INK4a suppresses hepatic fatty acid oxidation through the AMPKα2-SIRT1-PPARα signaling pathway. J. Biol. Chem. 2020, 295, 17310–17322. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Burd, E.M. Human papillomavirus laboratory testing: The changing paradigm. Clin. Microbiol. Rev. 2016, 29, 291–319. [Google Scholar] [CrossRef]
- Yellon, S.M. Immunobiology of Cervix Ripening. Front. Immunol. 2020, 10, 3156. [Google Scholar] [CrossRef]
- Shah, N.M.; Lai, P.F.; Imami, N.; Johnson, M.R. Progesterone-Related Immune Modulation of Pregnancy and Labor. Front. Endocrinol. 2019, 10, 198. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Motomura, K.; Miller, D.; Galaz, J.; Liu, T.N.; Romero, R.; Gomez-Lopez, N. The effects of progesterone on immune cellular function at the maternal-fetal interface and in maternal circulation. J. Steroid Biochem. Mol. Biol. 2023, 229, 106254. [Google Scholar] [CrossRef]
- Babaei, K.; Aziminezhad, M.; Mirzajani, E.; Mozdarani, H.; Sharami, S.H.; Norollahi, S.E.; Samadani, A.A. A critical review of the recent concept of regulatory performance of DNA Methylations, and DNA methyltransferase enzymes alongside the induction of immune microenvironment elements in recurrent pregnancy loss. Toxicol. Rep. 2024, 12, 546–563. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, R.; Yu, Y.; Jaafar, S.O.; Baghchi, B.; Farsimadan, M.; Arabipour, I.; Vaziri, H. Genetic Variants miR-126, miR-146a, miR-196a2, and miR-499 in Polycystic Ovary Syndrome. Br. J. Biomed. Sci. 2022, 79, 10209. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gomari, M.M.; Farsimadan, M.; Rostami, N.; Mahmoudi, Z.; Fadaie, M.; Farhani, I.; Tarighi, P. CD44 polymorphisms and its variants, as an inconsistent marker in cancer investigations. Mutat. Res. Rev. Mutat. Res. 2021, 787, 108374. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, G.K.; Khullar, N.; Sidhu, I.S.; Navik, U.S.; Reddy, A.P.; Reddy, P.H.; Bhatti, J.S. Emerging role of non-coding RNA in health and disease. Metab. Brain Dis. 2021, 36, 1119–1134. [Google Scholar] [CrossRef] [PubMed]
- Kamalidehghan, B.; Habibi, M.; Afjeh, S.S.; Shoai, M.; Alidoost, S.; Almasi Ghale, R.; Eshghifar, N.; Pouresmaeili, F. The Importance of Small Non-Coding RNAs in Human Reproduction: A Review Article. Appl. Clin. Genet. 2020, 13, 1–11. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jairajpuri, D.S.; Malalla, Z.H.; Mahmood, N.; Khan, F.; Almawi, W.Y. Differentially expressed circulating microRNAs associated with idiopathic recurrent pregnancy loss. Gene 2021, 768, 145334. [Google Scholar] [CrossRef] [PubMed]
- Ezat, S.A.; Haji, A.I. Study of association between different microRNA variants and the risk of idiopathic recurrent pregnancy loss. Arch. Gynecol. Obstet. 2022, 306, 1281–1286. [Google Scholar] [CrossRef]
- Nevalainen, J.; Skarp, S.; Savolainen, E.R.; Ryynänen, M.; Järvenpää, J. Intrauterine growth restriction and placental gene expression in severe preeclampsia, comparing early-onset and late-onset forms. J. Perinat. Med. 2017, 45, 869–877. [Google Scholar] [CrossRef]
- Tian, Q.X.; Xia, S.H.; Wu, Y.H.; Zhang, J.H.; Wang, L.Y.; Zhu, W.P. Comprehensive analysis of the differential expression profile of microRNAs in missed abortion. Kaohsiung J. Med. Sci. 2020, 36, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, M.; Nazari, F.; Ghanbarian, H.; Taheripanah, R.; Hajivalili, M.; Amani, D. miR-146b-5p and miR-520h Expressions Are Upregulated in Serum of Women with Recurrent Spontaneous Abortion. Biochem. Genet. 2022, 60, 1716–1732. [Google Scholar] [CrossRef]
- Farrell, A.; Alahari, S.; Ermini, L.; Tagliaferro, A.; Litvack, M.; Post, M.; Caniggia, I. Faulty oxygen sensing disrupts angiomotin function in trophoblast cell migration and predisposes to preeclampsia. JCI Insight 2019, 4, e127009. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kramer, M.F. Stem-loop RT-qPCR for miRNAs. Curr. Protoc. Mol. Biol. 2011, 95, 15.10. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lange, T.; Stracke, S.; Rettig, R.; Lendeckel, U.; Kuhn, J.; Schlüter, R.; Rippe, V.; Endlich, K.; Endlich, N. Identification of miR-16 as an endogenous reference gene for the normalization of urinary exosomal miRNA expression data from CKD patients. PLoS ONE 2017, 12, e0183435. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Feng, F.; Hou, Y.M.; Zhang, Y.; Wang, L.Y.; Li, P.P.; Guo, Y.; An, R.F. Correlation analysis of vaginal microecology and different types of human papillomavirus infection: A study conducted at a hospital in northwest China. Front. Med. 2023, 10, 1138507. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ma, Q.; Zhang, L.; Ma, L.; Wang, D.; Yang, Y.; Jia, P.; Wu, Y.; Wang, F. Human papillomavirus and cervical cancer in the microbial world: Exploring the vaginal microecology. Front. Cell. Infect. Microbiol. 2024, 14, 1325500. [Google Scholar] [CrossRef] [PubMed]
- Gomez Cherey, J.F.; Payalef, S.N.; Fleider, L.; Reyes, A.P.; Maldonado, V.A.; Losada, M.O.; Chen, X.; Cardinal, L.H.; Wang, Y.; Tatti, S.A.; et al. Microbiota unbalance in relation to high-risk human papillomavirus cervical infection. Int. J. Gynecol. Cancer 2023, 33, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Aromseree, S.; Wongjumpa, W.; Ekalaksananan, T.; Temtanakitpaisan, A.; Kleebkaow, P.; Srisathaporn, S.; Tongchai, P.; Pientong, C. P16/Ki-67 Dual Staining in Positive Human Papillomavirus DNA Testing for Predictive Diagnosis of Abnormal Cervical Lesions in Northeastern Thai Women. Asian Pac. J. Cancer Prev. 2022, 23, 3405–3411. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, X.-H.; Song, T.-Z.; Zheng, H.-Y.; Li, Y.-H.; Zheng, Y.-T. Jejunal epithelial barrier disruption triggered by reactive oxygen species in early SIV infected rhesus macaques. Free Radic. Biol. Med. 2021, 177, 143–155. [Google Scholar] [CrossRef]
- Sahu, U.; Khare, P. Role of interleukin-17 in human papillomavirus infection and associated malignancies. Microb. Pathog. 2021, 161, 105294. [Google Scholar] [CrossRef]
- Arenas-Hernandez, M.; Romero, R.; Xu, Y.; Panaitescu, B.; Garcia-Flores, V.; Miller, D.; Ahn, H.; Done, B.; Hassan, S.S.; Hsu, C.D.; et al. Effector and Activated T Cells Induce Preterm Labor and Birth That Is Prevented by Treatment with Progesterone. J. Immunol. 2019, 202, 2585–2608. [Google Scholar] [CrossRef]
- Wang, W.J.; Zhang, H.; Chen, Z.Q.; Zhang, W.; Liu, X.M.; Fang, J.Y.; Liu, F.J.; Kwak-Kim, J. Endometrial TGF-β, IL-10, IL-17 and autophagy are dysregulated in women with recurrent implantation failure with chronic endometritis. Reprod. Biol. Endocrinol. 2019, 17, 2. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ng, S.W.; Norwitz, G.A.; Pavlicev, M.; Tilburgs, T.; Simón, C.; Norwitz, E.R. Endometrial Decidualization: The Primary Driver of Pregnancy Health. Int. J. Mol. Sci. 2020, 21, 4092. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jang, S.-E.; Jeong, J.-J.; Choi, S.-Y.; Kim, H.; Han, M.J.; Kim, D.-H. Lactobacillus rhamnosus HN001 and lactobacillus acidophilus la-14 attenuate gardnerella vaginalis-infected bacterial vaginosis in mice. Nutrients 2017, 9, 531. [Google Scholar] [CrossRef]
- Jara, D.; Carvajal, P.; Castro, I.; Barrera, M.J.; Aguilera, S.; González, S.; Molina, C.; Hermoso, M.; González, M.J. Type I Interferon Dependent hsa-miR-145-5p Downregulation Modulates MUC1 and TLR4 Overexpression in Salivary Glands From Sjögren’s Syndrome Patients. Front. Immunol. 2021, 12, 685837. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, J.; Li, X.; Wu, L.; Pei, M.; Li, H.; Jiang, Y. miR-145 inhibits glutamine metabolism through c-myc/GLS1 pathways in ovarian cancer cells. Cell Biol. Int. 2019, 43, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Lan, S. Regulation of IRS-1, insulin signaling and glucose uptake by miR-143/145 in vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 2020, 529, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Kassem, N.M.; Makar, W.S.; Kassem, H.A.; Talima, S.; Tarek, M.; Hesham, H.; El-Desouky, M.A. Circulating miR-34a and miR-125b as Promising non Invasive Biomarkers in Egyptian Locally Advanced Breast Cancer Patients. Asian Pac. J. Cancer Prev. 2019, 20, 2749–2755. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cavallari, I.; Grassi, A.; Del Bianco, P.; Aceti, A.; Zaborra, C.; Sharova, E.; Bertazzolo, I.; D’Agostino, D.M.; Iafrate, M.; Ciminale, V. Prognostic Stratification of Bladder Cancer Patients with a MicroRNA-based Approach. Cancers 2020, 12, 3133. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, M.; Wang, W.; Chen, H.; Lu, Y.; Yuan, D.; Deng, Y.; Ran, D. miR-9, miR-21, miR-27b, and miR-34a Expression in HPV16/58/52-Infected Cervical Cancer. BioMed Res. Int. 2020, 2020, 2474235. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jahanbakhsh, J.; Farid Mojtahedi, M.; Moradi, N.; Fadaei, R.; Tehranian, A.; Rostami, R.; Kashani, L.; Moeini, A.; Alizadeh-Fanalou, S.; Barzin Tond, S.; et al. Circulating and Endometrial Profiles of miR-145, miR-155-5p, miR-224, MPP-5, and PECAM-1 Expression in Patients with Repeated Implantation Failure: A Case Control Study. Cell J. 2023, 25, 427–436. [Google Scholar]
- Jin, M.; Xu, Q.; Li, J.; Xu, S.; Tang, C. Micro-RNAs in human placenta: Tiny molecules, immense power. Molecules. 2022, 27, 5943. [Google Scholar] [CrossRef]
- Sirohi, V.K.; Gupta, K.; Kapoor, R.; Dwivedi, A. MicroRNA-145 targets Smad1 in endometrial stromal cells and regulates decidualization in rat. J. Mol. Med. 2019, 97, 509–522. [Google Scholar] [CrossRef]
- Luo, D.; Zou, W.; He, Y.; Xian, H.; Wang, L.; Shen, J.; Lian, K.; Lin, D. Modified dynamic hip screw loaded with autologous bone graft for treating Pauwels type-3 vertical femoral neck fractures. Injury 2017, 48, 1579–1583. [Google Scholar] [CrossRef]
- Włodarczyk, M.; Nowicka, G.; Ciebiera, M.; Ali, M.; Yang, Q.; Al-Hendy, A. Epigenetic regulation in uterine fibroids—The role of ten-eleven translocation enzymes and their potential therapeutic application. Int. J. Mol. Sci. 2022, 23, 2720. [Google Scholar] [CrossRef]
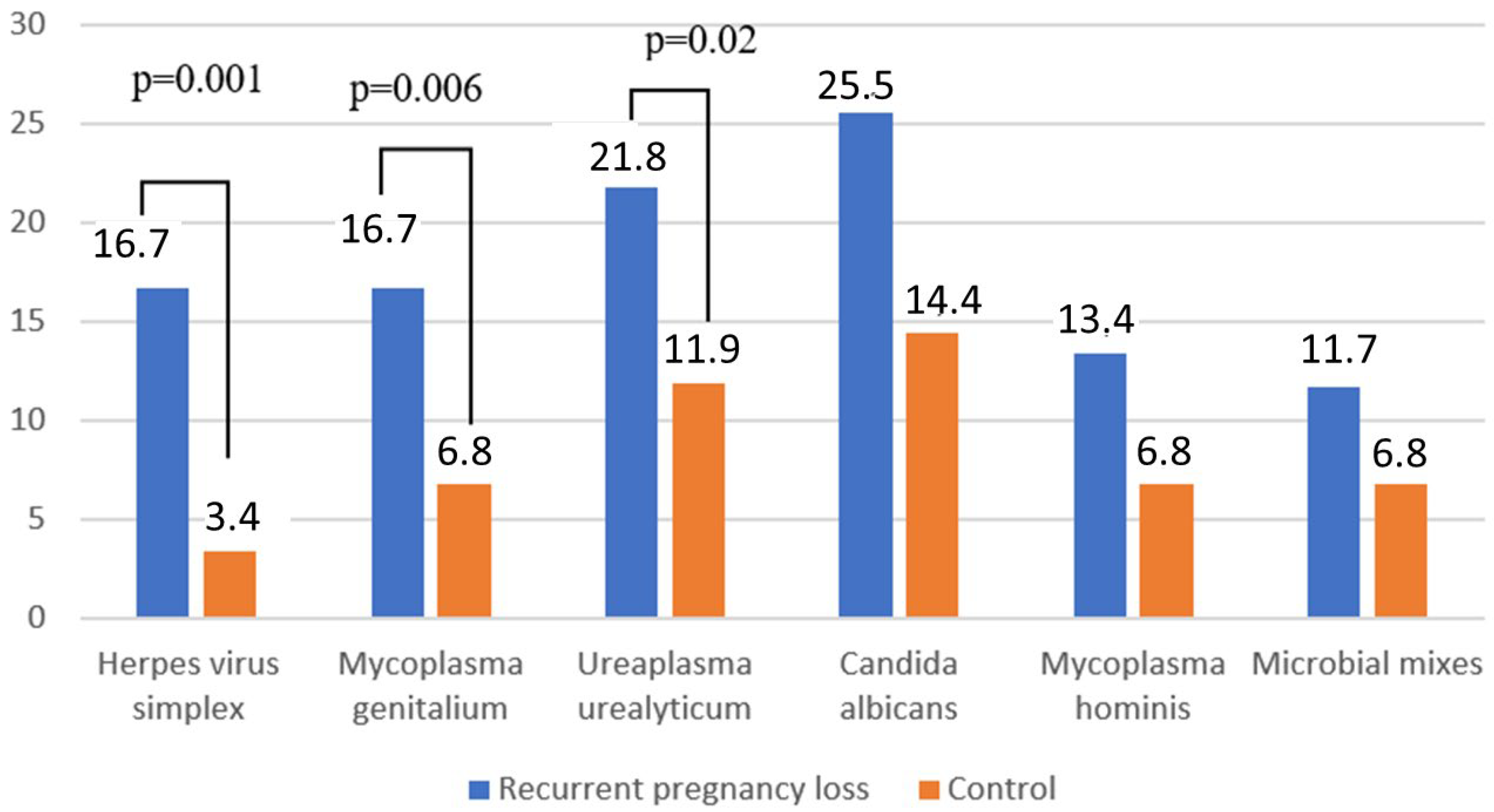
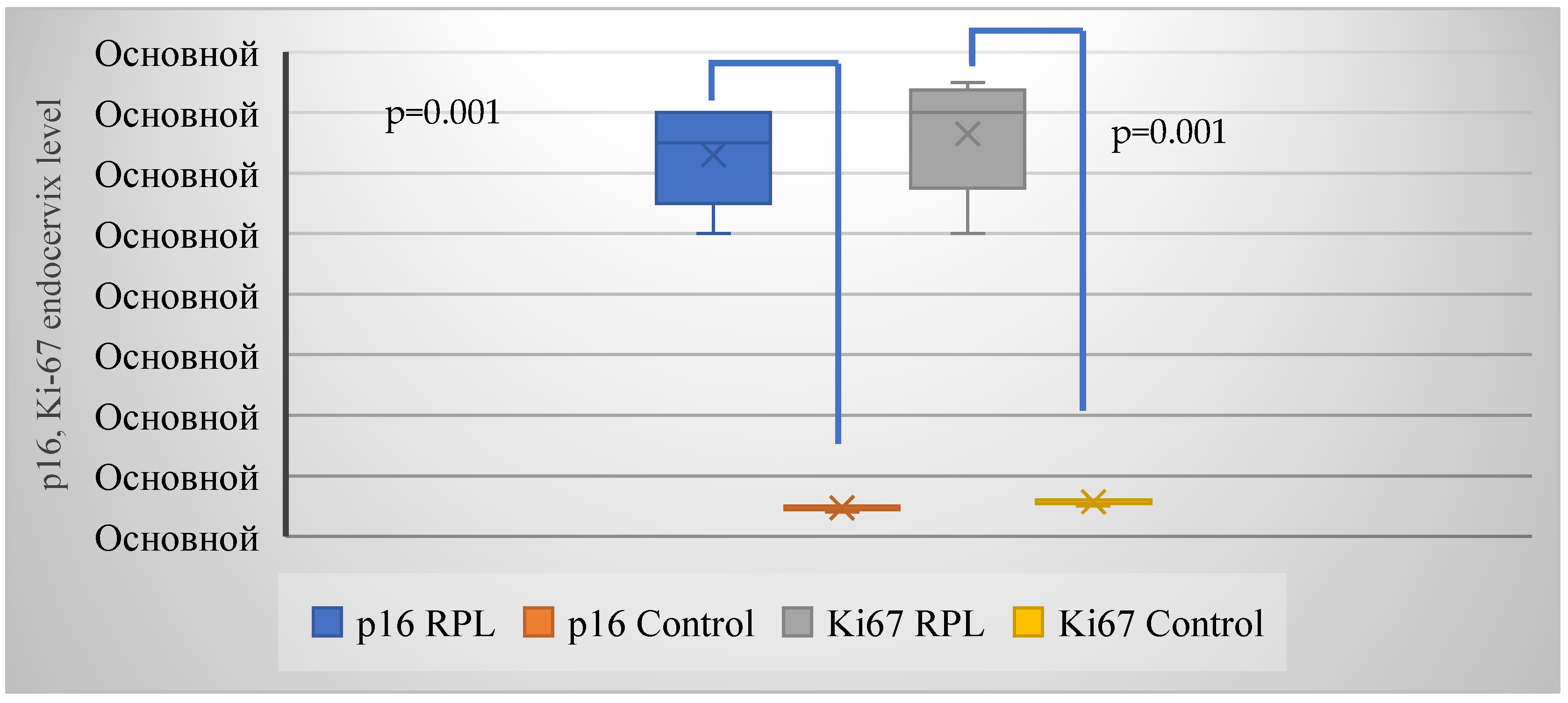
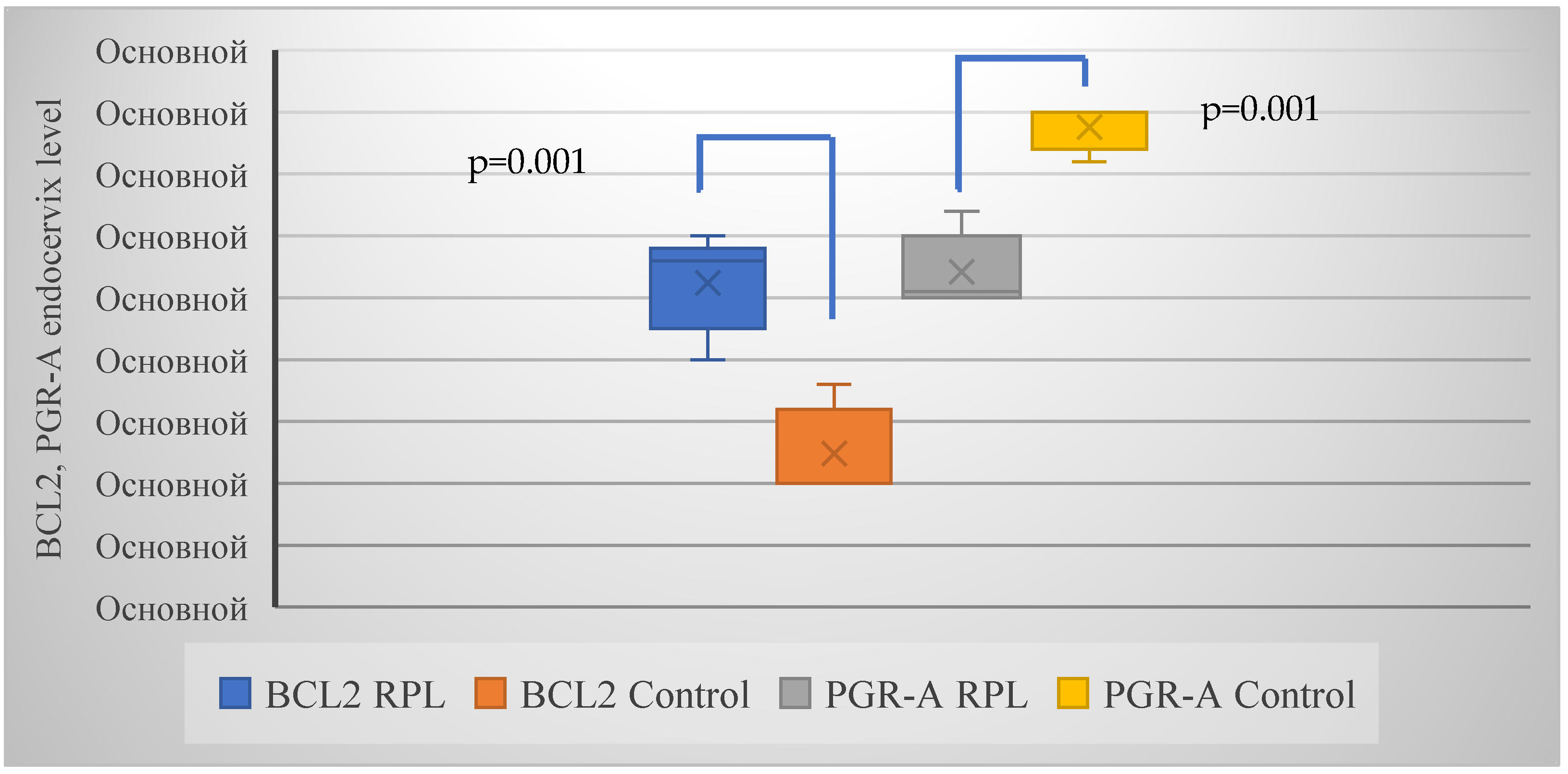
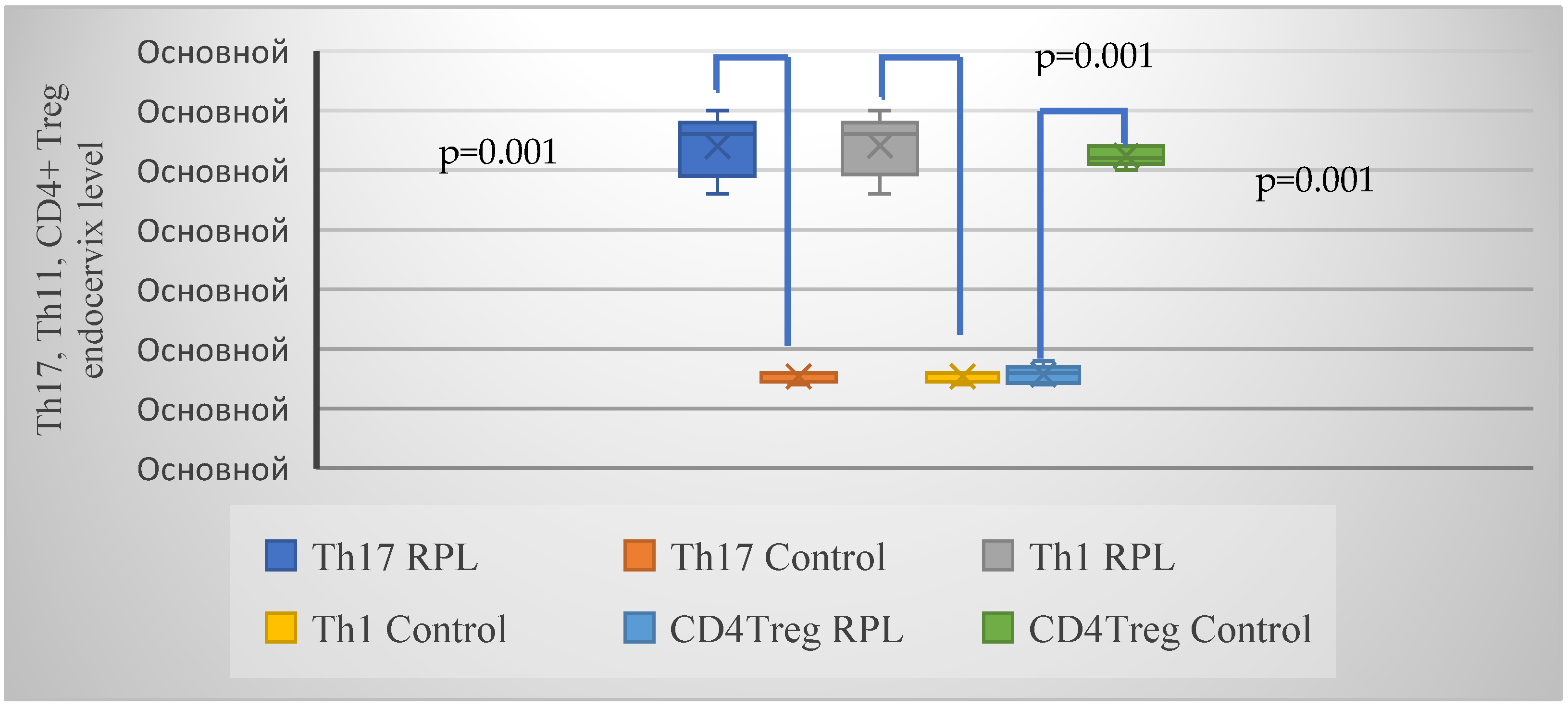
| Variables | RPL (n = 239) | Healthy Women (n = 118) | p |
|---|---|---|---|
| Age (average value ± SD) | 32.7 ± 3.2 | 33.9 ± 4.4 | >0.05 |
| Body mass index (BMI) | 26.8 ± 5.4 | 28.4 ± 3.5 | >0.05 |
| BMI ≥ 30 kg/m2 | 46 (19.2%) | 17 (14.4%) | >0.05 |
| Parity | |||
| First-time mothers | 142 (59.4%) | 75 (63.6%) | >0.05 |
| History of diseases | |||
| Hypertension | 43 (18.0%) | 11 (9.3%) | >0.05 |
| Anemia | 54 (22.6%) | 20 (16.9%) | >0.05 |
| Diabetes mellitus | 15 (6.3%) | 6 (5.1%) | >0.05 |
| Urinary tract infections | 21 (8.8%) | 14 (11.9%) | >0.05 |
| Parameters | RPL (n = 239) | Control (n = 118) | p (χ2) |
|---|---|---|---|
| Normocenosis | 44 (18.4%) | 68 (57.6%) | 0.001 (56.1) |
| Non-specific vaginitis | 65 (27.2%) | 25 (21.2%) | >0.05 |
| Bacterial vaginosis (BV) | 81 (33.9%) | 15 (12.7%) | 0.001 (18.0) |
| Candida vaginitis | 49 (20.5%) | 10 (8.5%) | 0.04 (8.3) |
| RPL | Control Group | p | RPL | Control Group | p | |
|---|---|---|---|---|---|---|
| Overall | Opportunistic Microorganisms in High Diagnostic Titer (>104) | |||||
| Enterococcus spp. | 90 (43.9%) | 10(8.5%) | <0.001 45.5 | 82 (34.3%) | 6 (5.1%) | <0.001 36.3 |
| Streptococcus agalactiae | 90 (37.7%) | 8 (6.8%) | <0.001 37.8 | 75 (31.4%) | 6 (5.1%) | <0.001 31.1 |
| Streptococcus spp. | 15 (6.3%) | 9 (7.6%) | >0.05 | 7 (2.9%) | 5 (4.2%) | >0.05 |
| Corynebacterium spp. | 15 (6.3%) | 5 (4.2%) | >0.05 | 15 (6.3%) | 2 (1.7%) | >0.05 |
| Klebsiella spp. | 13 (5.4%) | 0 (0.0%) | >0.05 | 7 (2.9%) | 0 (0.0%) | >0.05 |
| Escherichia coli | 52 (21.8%) | 9 (7.6%) | <0.001 11.1 | 45 (18.8%) | 3 (2.5%) | <0.001 18.0 |
| Staphylococcus spp. | 30 (12.6%) | 12(10.2%) | >0.05 | 7 (2.9%) | 5 (4.2%) | >0.05 |
| Staphylococcus epidermidis | 22 (9.2%) | 6 (5.1%) | >0.05 | 15 (6.3%) | 4 (3.4%) | >0.05 |
| Actinomyces spp. | 22 (9.2%) | 15 (12.7%) | >0.05 | 7 (2.9%) | 5 (4.2%) | >0.05 |
| Corynebacterium spp. | 19 (7.9%) | 6 (5.1%) | >0.05 | 3 (1.3%) | 3 (2.5%) | >0.05 |
| Bacillus spp. | 8 (3.3%) | 4 (3.4%) | >0.05 | 0 (0.0%) | 4 (3.4%) | >0.05 |
| Parameters | RPL (n = 113) | Control (n = 9) | p (χ2) |
|---|---|---|---|
| HPV HR | 68 (60.2%) | 2 (22.2%) | 0.001 (16.8) |
| HPV 16 type | 43 (31.1%) | 1 (11.1%) | 0.005 (8.1) |
| HPV + bacterial vaginosis | 85 (75.2%) | 1 (11.1%) | 0.001 (35.1) |
| Two or more HPV types | 68 (60.2%) | 0 (0.0%) | - |
| Parameters | RPL (n = 239) | Control (n = 118) | p (χ2) |
|---|---|---|---|
| NILM (negative for intraepithelial lesion or malignancy) | 150 (62.8%) | 95 (80.5%) | 0.001 (11.6) |
| ASC-US (atypical squamous cells of undetermined significance) | 47 (19.7%) | 12 (10.2%) | 0.02 (5.2) |
| LSIL (low-grade intraepitelial lesion) | 42 (17.6%) | 11(9.3%) | 0.04 (4.2) |
| Markers | RPL (n = 44) | Control (n = 222) | p (χ2) |
|---|---|---|---|
| p16 | 23 (52.3%) | 4 (18.2%) | 0.005 (7.9) |
| Ki-67 | 23 (52.3%) | 4 (18.2%) | 0.005 (7.9) |
| BCL-2 | 24 (54.5%) | 3 (13.6%) | 0.002 (10.1) |
| PGR-A | 22 (50.0%) | 3 (13.6%) | 0.005 (8.2) |
| Parameters | RPL (n = 44) | Control (n = 22) | p (χ2) |
|---|---|---|---|
| Th17/Th1 | 22 (50.0%) | 3 (13.6%) | 0.005 (8.2) |
| CD4+ Treg | 10 (22.7%) | 16 (72.7%) | 0.001 (15.3) |
| Parameters | RPL (n = 44) | Control (n = 22) | p (χ2) |
|---|---|---|---|
| miR-145 | 24 (54.5%) | 3 (13.6%) | 0.002 (10.1) |
| miR-34a | 27 (61.4%) | 3 (13.6%) | 0.001 (13.5) |
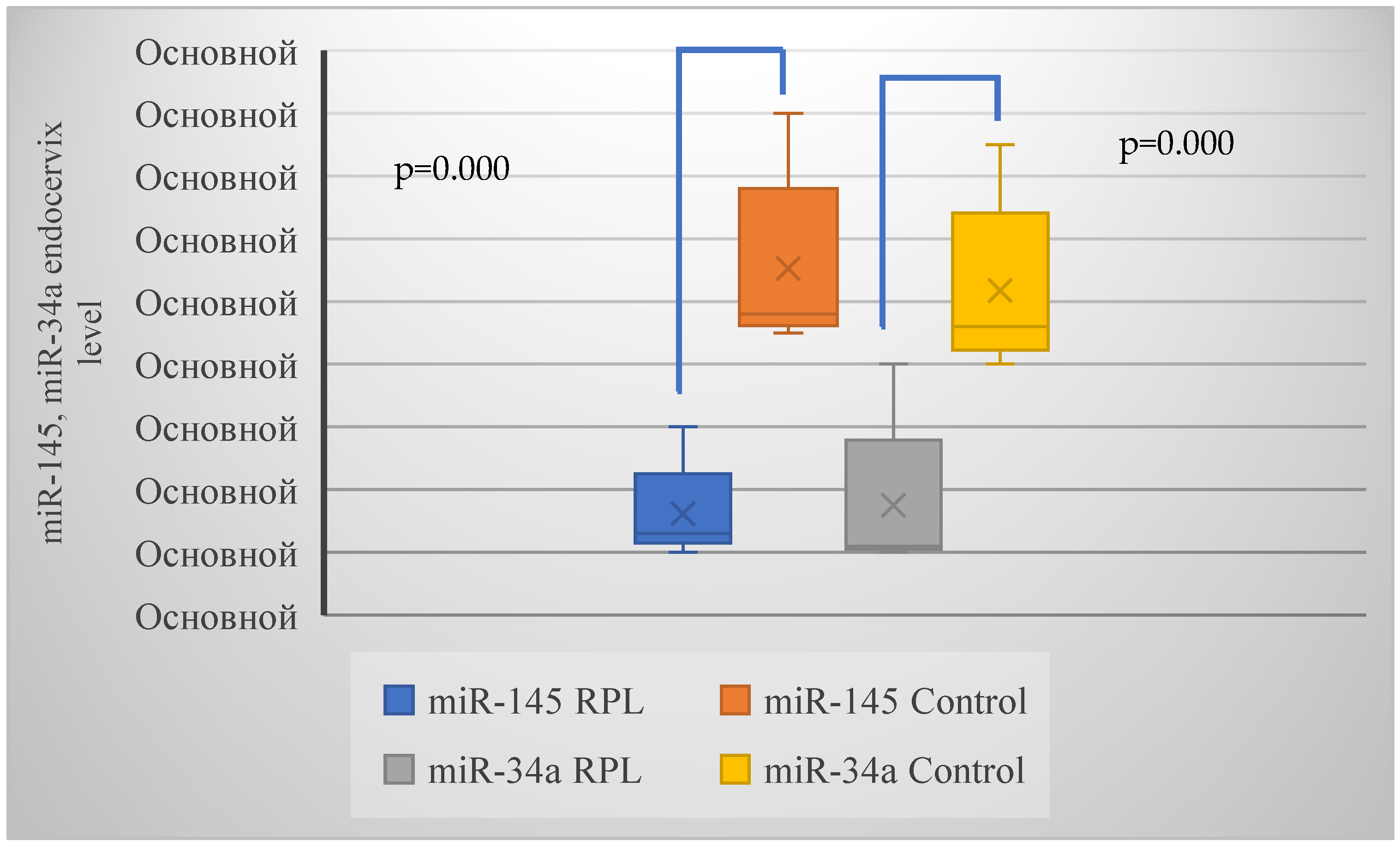
| Parameters | RPL (n = 24/27) | Control (n = 4) | p |
|---|---|---|---|
| miR-145 (HPV HR +) (n = 16) | 0.8 (0.7; 0.9) | 1.1 (1.0;1.2) | <0.05 |
| miR-145(HPV HR −) (n = 8) | 1.6 (1.5; 1.7) | <0.05 | |
| miR-34a (HPV HR +) (n = 16) | 0.6 (0.5; 0.7) | 1.1 (1.0;1.2) | <0.05 |
| miR-34a (HPV HR −) (n = 11) | 1.85 (1.7; 2.0) | <0.05 |
| miR | Primer for Reverse Transcription | Direct PCR Primer | Reverse PCR Primer |
|---|---|---|---|
| hsa-miR-145-5p | 5′GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAGGGA3′3′ | 5′AACAAGGTCCAGTTTTCCCAG3′ | 5′GTCGTATCCAGTGCAGGGT3′ |
| hsa-miR-34a-5p | 5′GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACACAACC3′ | 5′AACAGTGTGGCAGTGTCTTAG 3′ | 5′GTCGTATCCAGTGCAGGGT3′ |
| hsa-miR-16-5p | 5′GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCGCCAA3′ | 5′AACAGTGTAGCAGCACGTAAA3′ | 5′GTCGTATCCAGTGCAGGGT3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikhalev, S.A.; Kurtser, M.A.; Radzinsky, V.E.; Orazov, M.R.; Beeraka, N.M.; Mikhaleva, L.M. Exploring the Role of Lower Genital Tract Microbiota and Cervical–Endometrial Immune Metabolome in Unknown Genesis of Recurrent Pregnancy Loss. Int. J. Mol. Sci. 2025, 26, 1326. https://doi.org/10.3390/ijms26031326
Mikhalev SA, Kurtser MA, Radzinsky VE, Orazov MR, Beeraka NM, Mikhaleva LM. Exploring the Role of Lower Genital Tract Microbiota and Cervical–Endometrial Immune Metabolome in Unknown Genesis of Recurrent Pregnancy Loss. International Journal of Molecular Sciences. 2025; 26(3):1326. https://doi.org/10.3390/ijms26031326
Chicago/Turabian StyleMikhalev, Sergey A., Mark A. Kurtser, Victor E. Radzinsky, Mekan R. Orazov, Narasimha M. Beeraka, and Lyudmila M. Mikhaleva. 2025. "Exploring the Role of Lower Genital Tract Microbiota and Cervical–Endometrial Immune Metabolome in Unknown Genesis of Recurrent Pregnancy Loss" International Journal of Molecular Sciences 26, no. 3: 1326. https://doi.org/10.3390/ijms26031326
APA StyleMikhalev, S. A., Kurtser, M. A., Radzinsky, V. E., Orazov, M. R., Beeraka, N. M., & Mikhaleva, L. M. (2025). Exploring the Role of Lower Genital Tract Microbiota and Cervical–Endometrial Immune Metabolome in Unknown Genesis of Recurrent Pregnancy Loss. International Journal of Molecular Sciences, 26(3), 1326. https://doi.org/10.3390/ijms26031326






