Colorectal Cancer: Current and Future Therapeutic Approaches and Related Technologies Addressing Multidrug Strategies Against Multiple Level Resistance Mechanisms
Abstract
1. Introduction
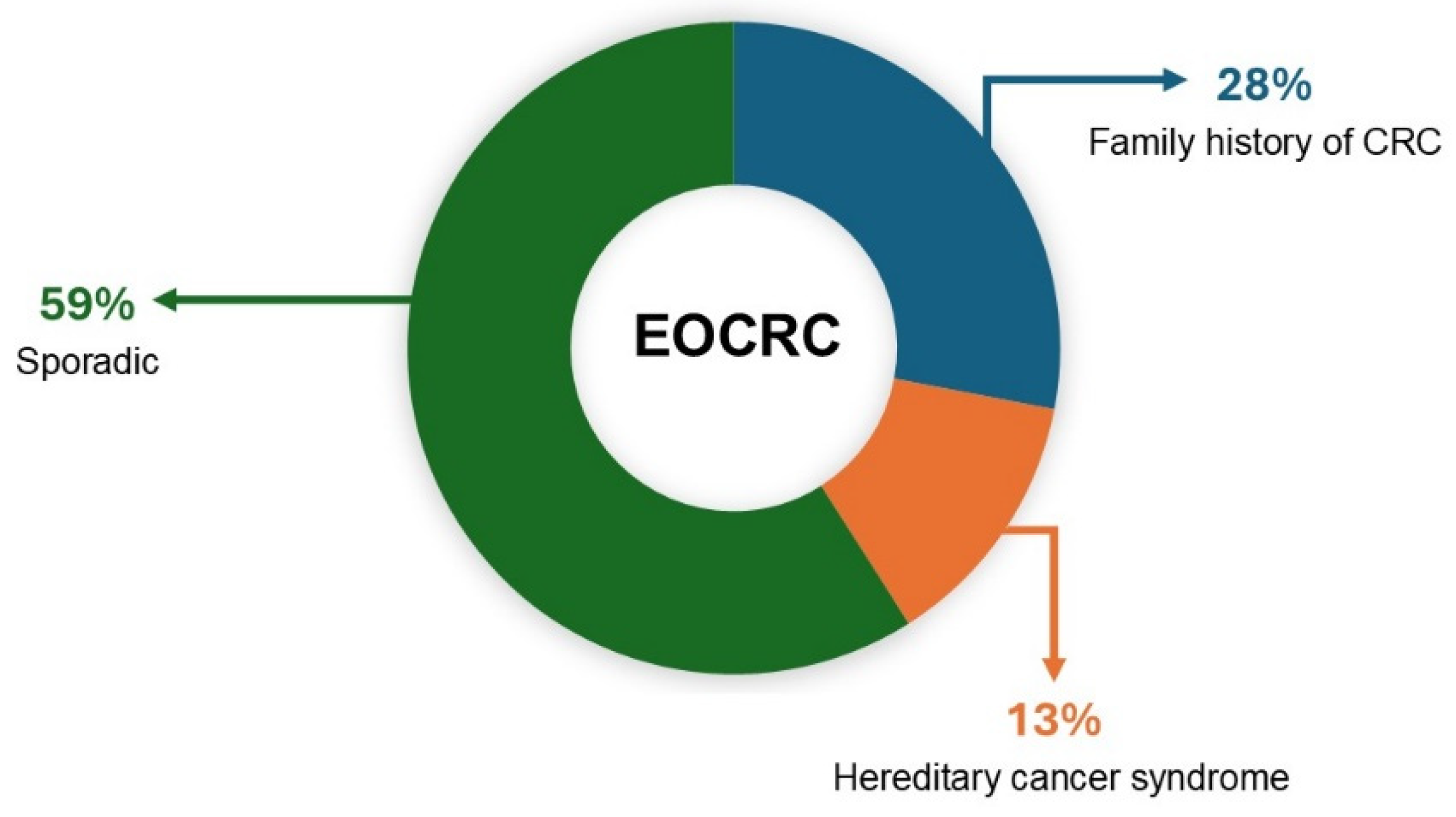
2. Causes of CRC
3. CRC: From Pathogenesis to Molecular Typing
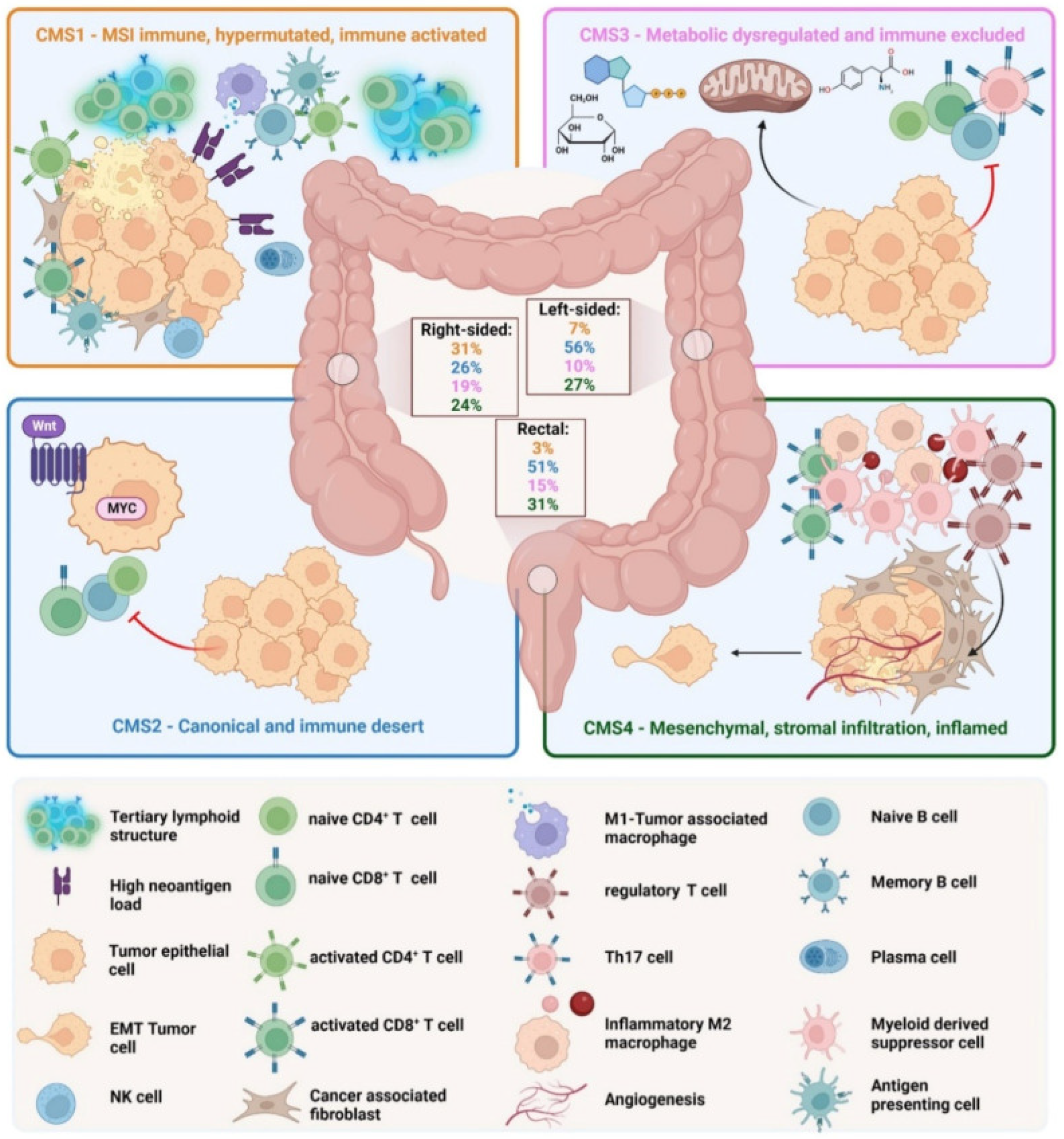
4. Dependence of Progression and Therapeutic Options on Tumor Microenvironment. The Four Consensus Molecular Subtypes
5. CRC: Critical Pathways and Mutation Landscape
5.1. The EGFR Pathway
5.2. The VEGFR Pathway
5.3. The HGF/C-MET Pathway
6. Advanced Therapeutic Approaches
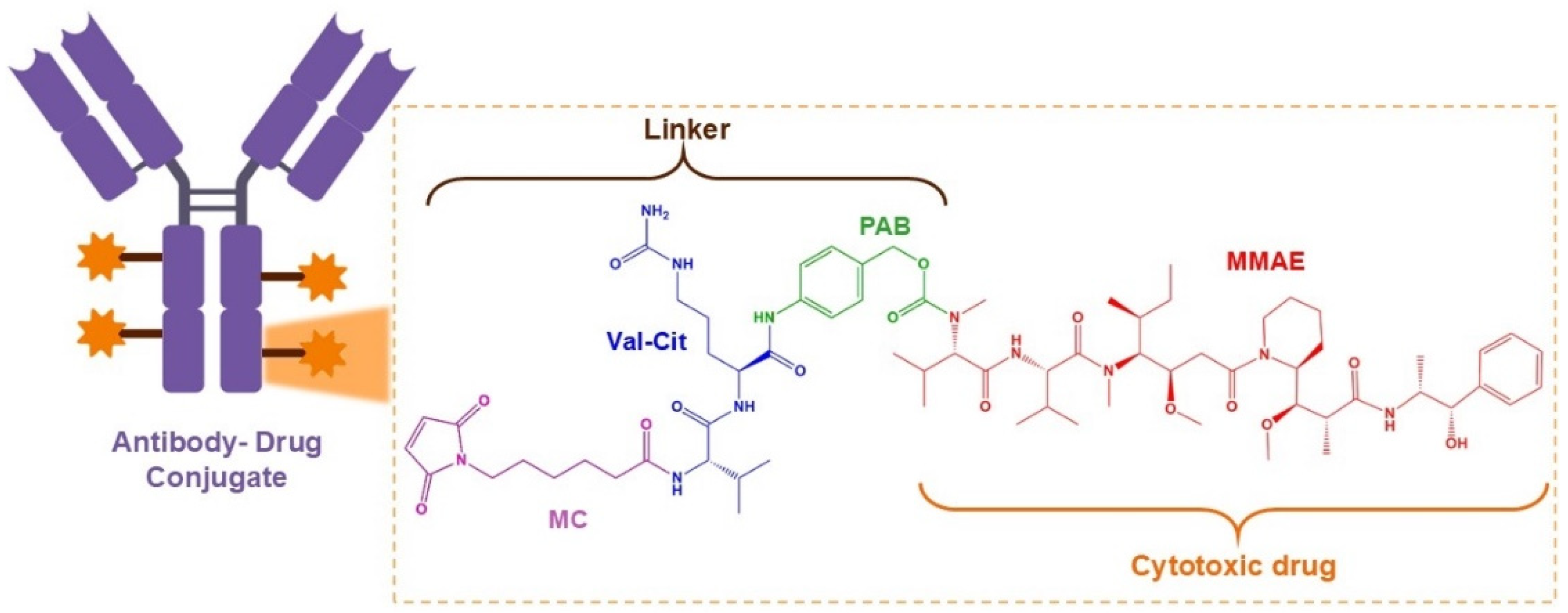
6.1. Antibody–Drug Conjugates
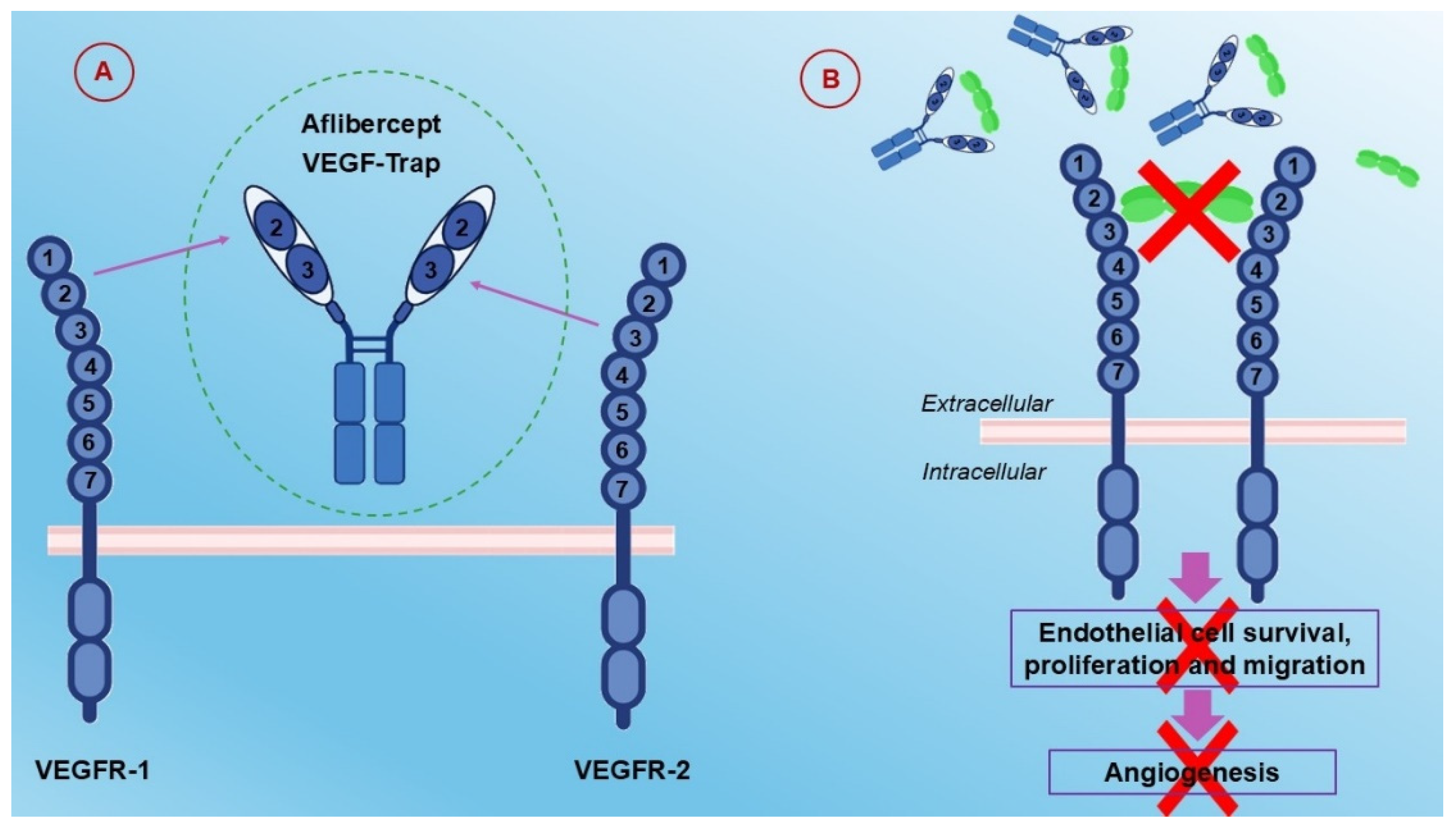
6.2. Aflibercept
7. Opportunities for CRC Through Nanotechnology: Pharmacological Needs and Possible Synergetic Actions to Overcome Multiple Level Resistance Mechanisms
7.1. Nanotechnology Solutions for CRC
7.2. Comparison Between Nanotechnology-Based Therapies and Conventional CRC Treatments
7.3. Nanotechnology Application in Early Colorectal Cancer Detection
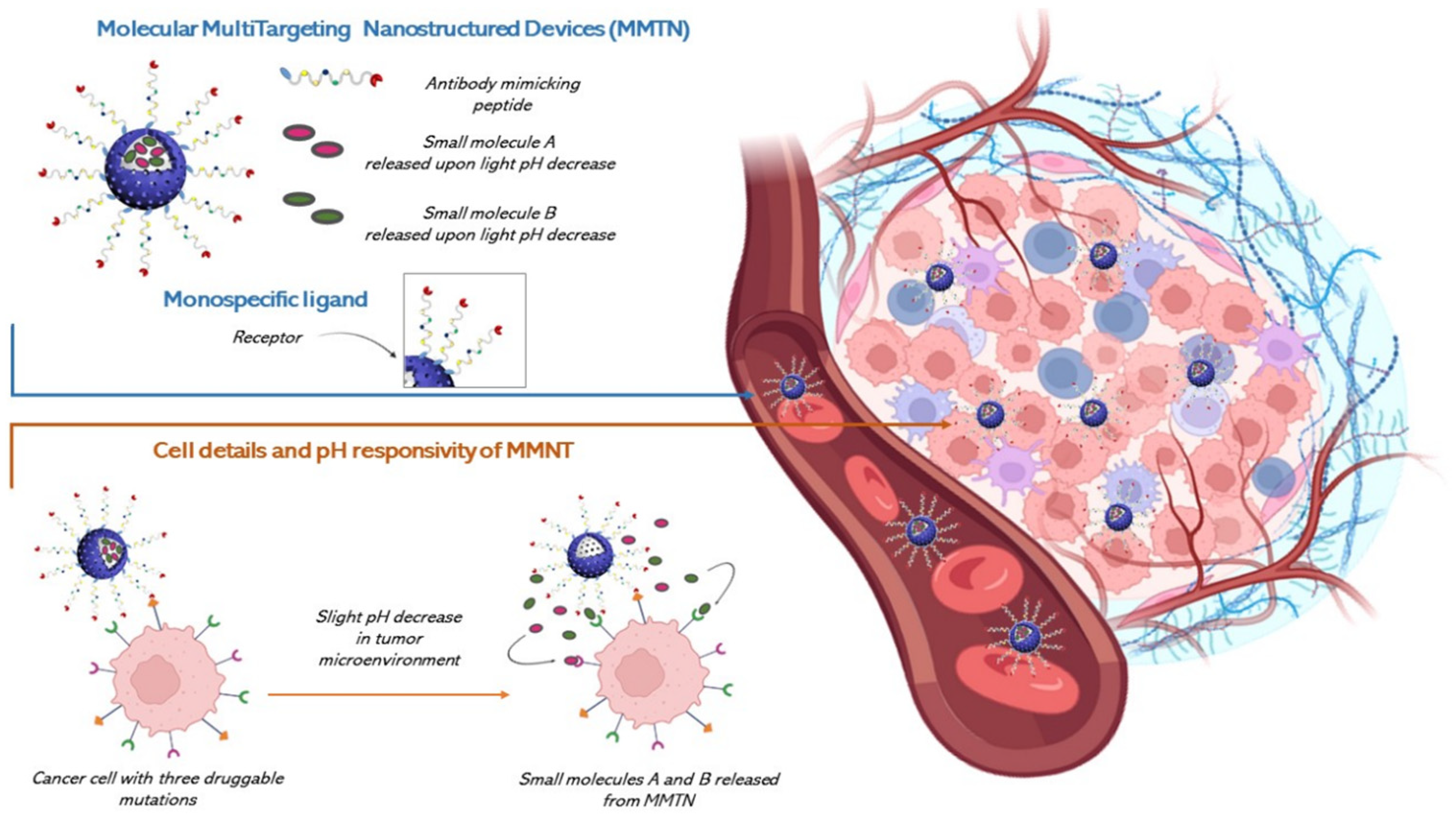
7.4. Possible Synergetic Actions to Overcome Multiple Level Resistance Mechanisms
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Colorectal Cancer in the Top 3 of the Most Diagnosed and Fatal Cancers Both in EU Men and Women. Available online: https://joint-research-centre.ec.europa.eu/jrc-news-and-updates/colorectal-cancer-top-3-most-diagnosed-and-fatal-cancers-both-eu-men-and-women-2021-03-15_en (accessed on 21 November 2024).
- Rawla, P.; Sunkar, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Prz. Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef]
- Ciombor, K.K.; Wu, C.; Goldberg, R.M. Recent therapeutic advances in the treatment of colorectal cancer. Annu. Rev. Med. 2015, 66, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhao, R.; Zhang, G.; Jiao, Y.; Wang, Y.; Wang, D.; Cai, H. Association of Retinol and Carotenoids Content in Diet and Serum With Risk for Colorectal Cancer: A Meta-Analysis. Front. Nutr. 2022, 30, 918777. [Google Scholar] [CrossRef]
- Carotenuto, P.; Pecoraro, A.; Brignola, C.; Barbato, A.; Franco, B.; Longobardi, G.; Conte, C.; Quaglia, F.; Russo, G.; Russo, A. Combining β-Carotene with 5-FU via Polymeric Nanoparticles as a Novel Therapeutic Strategy to Overcome uL3-Mediated Chemoresistance in p53-Deleted Colorectal Cancer Cells. Mol. Pharm. 2023, 1, 2326–2340. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.H.; Chen, Y.X.; Fang, J.Y. Comprehensive review of targeted therapy for colorectal cancer. Signal. Transduct. Target. Ther. 2020, 5, 22. [Google Scholar] [CrossRef]
- Rosales, J.; Leong, L.A. Chemotherapy for metastatic colorectal cancer. J. Natl. Compr. Canc. Netw. 2005, 3, 525–529. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lombardi, L.; Morelli, F.; Cinieri, S.; Santini, D.; Silvestris, N.; Fazio, N.; Orlando, L.; Tonini, G.; Colucci, G.; Maiello, E. Adjuvant colon cancer chemotherapy: Where we are and where we’ll go. Cancer Treat. Rev. 2010, 36, S34–S44. [Google Scholar] [CrossRef] [PubMed]
- Engstrom, P.F.; Arnoletti, J.P.; Benson, A.B., 3rd; Chen, Y.-J.; Choti, M.A.; Cooper, H.S.; Covey, A.; Dilawari, R.A.; Early, D.S.; Enzinger, P.C.; et al. NCCN Clinical Practice Guidelines in Oncology: Colon cancer. J. Natl. Compr. Canc. Netw. 2009, 7, 778–831. [Google Scholar] [CrossRef]
- Wilkinson, N.W.; Yothers, G.; Lopa, S.; Costantino, J.P.; Petrelli, N.J.; Wolmark, N. Long-term survival results of surgery alone versus surgery plus 5-fluorouracil and leucovorin for stage II and stage III colon cancer: Pooled analysis of NSABP C-01 through C-05. A baseline from which to compare modern adjuvant trials. Ann. Surg. Oncol. 2010, 17, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.; Blackstock, A.W.; Goldberg, R.M. Colorectal cancer. Mayo Clin. Proc. 2007, 82, 114–129. [Google Scholar] [CrossRef] [PubMed]
- Scheithauer, W.; Rosen, H.; Kornek, G.V.; Sebesta, C.; Depisch, D. Randomised comparison of combination chemotherapy plus supportive care with supportive care alone in patients with metastatic colorectal cancer. BMJ 1993, 306, 752–755. [Google Scholar] [CrossRef] [PubMed]
- Lucas, A.S.; O’Neil, B.H.; Goldberg, R.M. A decade of advances in cytotoxic chemotherapy for metastatic colorectal cancer. Clin. Color. Cancer 2011, 10, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Guinney, J.; Dienstmann, R.; Wang, X.; de Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Lenz, H.J.; Ou, F.-S.; Venook, A.P.; Hochster, H.S.; Niedzwiecki, D.; Goldberg, R.M.; Mayer, R.J.; Bertagnolli, M.M.; Blanke, C.D.; Zemla, T.; et al. Impact of Consensus Molecular Subtype on Survival in Patients With Metastatic Colorectal Cancer: Results From CALGB/SWOG 80405 (Alliance). J. Clin. Oncol. 2019, 37, 1876–1885. [Google Scholar] [CrossRef] [PubMed]
- Dienstmann, R.; Vermeulen, L.; Guinney, J.; Kopetz, S.; Tejpar, S.; Tabernero, J. Consensus molecular subtypes and the evolution of precision medicine in colorectal cancer. Nat. Rev. Cancer 2017, 17, 79–92, Correction in Nat. Rev. Cancer 2017, 17, 268. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, D.; Liu, D.; Xu, X.; Zhang, K.; Hu, R.; Xiong, P.; Wang, C.; Zeng, X.; Wang, L.; et al. Synergistic antitumor activity between HER2 antibody-drug conjugate and chemotherapy for treating advanced colorectal cancer. Cell Death Dis. 2024, 15, 187. [Google Scholar] [CrossRef] [PubMed]
- Terracciano, R.; Casadonte, F.; Pasqua, L.; Candeloro, P.; Di Fabrizio, E.; Urbani, A.; Savino, R. Enhancing plasma peptide MALDI-TOF-MS profiling by mesoporous silica assisted crystallization. Talanta 2010, 80, 1532–1538. [Google Scholar] [CrossRef] [PubMed]
- Preianò, M.; Pasqua, L.; Gallelli, L.; Galasso, O.; Gasparini, G.; Savino, R.; Terracciano, R. Simultaneous extraction and rapid visualization of peptidomic and lipidomic body fluids fingerprints using mesoporous aluminosilicate and MALDI-TOF MS. Proteomics 2012, 12, 3286–3294. [Google Scholar] [CrossRef]
- Pasqua, L.; Procopio, A.; Oliverio, M.; Paonessa, R.; Prete, R.; Nardi, M.; Casula, M.F.; Testa, F.; Nagy, J.B. Hybrid MCM-41 grafted by a general microwave-assisted procedure: A characterization study. J. Porous Mater. 2013, 20, 865–873. [Google Scholar] [CrossRef]
- Mazzotta, E.; De Santo, M.; Lombardo, D.; Leggio, A.; Pasqua, L. Mesoporous silicas in materials engineering: Nanodevices for bionanotechnologies. Mater. Today Bio. 2022, 17, 100472. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, D.; Calandra, P.; Teresa Caccamo, M.; Magazù, S.; Pasqua, L.; A. Kiselev, M.A. Interdisciplinary Approaches to the Study of Biological Membranes. AIMS Biophys. 2020, 7, 267–290. [Google Scholar] [CrossRef]
- Daca Alvarez, M.; Quintana, I.; Terradas, M.; Mur, P.; Balaguer, F.; Valle, L. The Inherited and Familial Component of Early-Onset Colorectal Cancer. Cells 2021, 10, 710. [Google Scholar] [CrossRef]
- Nardone, O.M.; Zammarchi, I.; Santacroce, G.; Ghosh, S.; Iacucci, M. Inflammation-Driven Colorectal Cancer Associated with Colitis: From Pathogenesis to Changing Therapy. Cancers 2023, 15, 2389. [Google Scholar] [CrossRef]
- Rejali, L.; Seifollahi Asl, R.; Sanjabi, F.; Fatemi, N.; Aghdaei, H.A.; Niasar, M.S.; Moghadam, P.K.; Mojarad, E.N.; Mini, E.; Nobili, S. Principles of Molecular Utility for CMS Classification in Colorectal Cancer Management. Cancers 2023, 15, 2746. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Geng, S.; Luo, H.; Wang, W.; Mo, Y.Q.; Luo, Q.; Wang, L.; Song, G.B.; Sheng, J.; Xu, B. Signaling pathways involved in colorectal cancer: Pathogenesis and targeted therapy. Signal Transduct. Target. Ther. 2024, 9, 266. [Google Scholar]
- Stidham, R.W.; Higgins, P.D.R. Colorectal Cancer in Inflammatory Bowel Disease. Clin. Colon. Rectal Surg. 2018, 31, 168–178. [Google Scholar] [PubMed]
- Fanizza, J.; Bencardino, S.; Allocca, M.; Furfaro, F.; Zilli, A.; Parigi, T.L.; Fiorino, G.; Peyrin-Biroulet, L.; Danese, S.; D’Amico, F. Inflammatory Bowel Disease and Colorectal Cancer. Cancers 2024, 16, 2943. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, B.; Chan, J.J.; Tabatabaeian, H.; Tong, K.J.; Chew, X.H.; Fan, X.; Driguez, P.; Chan, C.; Cheong, F.; et al. An isoform-resolution transcriptomic atlas of colorectal cancer from long-read single-cell sequencing. Cell Genom. 2024, 4. [Google Scholar] [CrossRef]
- Alsolme, E.; Alqahtani, S.; Fageeh, M.; Barakeh, D.; Sharma, N.K.; Mangul, S.; Robinson, H.A.; Fathaddin, A.; Hauser, C.; Abedalthagafi, M. The Genomic Landscape of Colorectal Cancer in the Saudi Arabian Population Using a Comprehensive Genomic Panel. Diagnostics 2023, 13, 2993. [Google Scholar] [CrossRef]
- Perdomo, S.; Abedi-Ardekani, B.; de Carvalho, A.C.; Ferreiro-Iglesias, A.; Gaborieau, V.; Cattiaux, T.; Renard, H.; Chopard, P.; Carreira, C.; Spanu, A.; et al. The Mutographs biorepository: A unique genomic resource to study cancer around the world. Cell Genom. 2024, 3, 100500. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Yang, M. Molecular Network of Colorectal Cancer and Current Therapeutic Options. Front. Oncol. 2022, 12, 852927. [Google Scholar] [CrossRef] [PubMed]
- Krasinskas, A.M. EGFR Signaling in Colorectal Carcinoma. Patholog. Res. Int. 2011, 1, 932932. [Google Scholar] [CrossRef] [PubMed]
- Malki, A.; El Ruz, R.A.; Gupta, I.; Allouch, A.; Vranic, S.; Al Moustafa, A.E. Molecular Mechanisms of Colon Cancer Progression and Metastasis: Recent Insights and Advancements. Int. J. Mol. Sci. 2020, 22, 130. [Google Scholar] [CrossRef] [PubMed]
- Requena, D.O.; Garcia-Buitrago, M. Molecular Insights Into Colorectal Carcinoma. Arch. Med. Res. 2020, 51, 839–844. [Google Scholar] [CrossRef]
- Toyota, M.; Ahuja, N.; Ohe-Toyota, M.; Herman, J.G.; Baylin, S.B.; Issa, J.P. CpG Island methylator phenotype in colorectal cancer. Proc. Natl. Acad. Sci. USA 1999, 96, 8681–8686. [Google Scholar] [CrossRef] [PubMed]
- Braumüller, H.; Mauerer, B.; Andris, J.; Berlin, C.; Wieder, T.; Kesselring, R. The Cytokine Network in Colorectal Cancer: Implications for New Treatment Strategies. Cells 2022, 12, 138. [Google Scholar] [CrossRef] [PubMed]
- Menter, D.G.; Davis, J.S.; Broom, B.M.; Overman, M.J.; Morris, J.; Kopetz, S. Back to the colorectal cancer consensus molecular subtype future. Curr. Gastroenterol. Rep. 2019, 21, 5. [Google Scholar] [CrossRef]
- Martini, G.; Dienstmann, R.; Ros, J.; Baraibar, I.; Cuadra-Urteaga, J.L.; Salva, F.; Ciardiello, D.; Mulet, N.; Argiles, G.; Tabernero, J.; et al. Molecular subtypes and the evolution of treatment management in metastatic colorectal cancer. Ther. Adv. Med. Oncol. 2020, 12, 1758835920936089. [Google Scholar] [CrossRef]
- Su, M.-W.; Chang, C.-K.; Lin, C.-W.; Chu, H.-W.; Tsai, T.-N.; Su, W.-C.; Chen, Y.-W.; Chang, T.-K.; Huang, C.-W.; Tsai, H.-L.; et al. Genomic and metabolomic landscape of right-sided and left-sided colorectal cancer: Potential preventive biomarkers. Cells 2022, 11, 527. [Google Scholar] [CrossRef]
- Tanaka, A.; Ogawa, M.; Zhou, Y.; Namba, K.; Hendrickson, R.C.; Miele, M.M.; Li, Z.; Klimstra, D.S.; Buckley, P.G.; Gulcher, J.; et al. Proteogenomic characterization of primary colorectal cancer and metastatic progression identifies proteome-based subtypes and signatures. Cell Rep. 2024, 43, 113810. [Google Scholar] [CrossRef] [PubMed]
- Cornish, A.J.; Gruber, A.J.; Kinnersley, B.; Chubb, D.; Frangou, A.; Caravagna, G.; Noyvert, B.; Lakatos, E.; Wood, H.M.; Thorn, S.; et al. The genomic landscape of 2023 colorectal cancers. Nature 2024, 633, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Chen, M.; Wei, Y.; Ma, X.; Shi, H. Signaling pathways in colorectal cancer implications for the target therapies. Mol. Biomed. 2024, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Yeoh, Y.; Low, T.Y.; Abu, N.; Lee, P.Y. Regulation of signal transduction pathways in colorectal cancer: Implications for therapeutic resistance. Peer J. 2021, 9, e12338. [Google Scholar] [CrossRef]
- Wang, Q.; Shen, X.; Chen, G.; Du, J. Drug Resistance in Colorectal Cancer: From Mechanism to Clinic. Cancer 2022, 14, 2928. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Shao, X.; Zhang, Y.; Zhu, M.; Wang, F.X.C.; Mu, J.; Li, J.; Yao, H.; Chen, K. Role of tumor microenvironment in cancer progression and therapeutic strategy. Cancer Med. 2023, 12, 11149–11165. [Google Scholar] [CrossRef]
- Koveitypour, Z.; Panahi, F.; Vakilian, M.; Peymani, M.; Seyed Forootan, F.; Nasr Esfahani, M.H.; Ghaedi, K. Signaling pathways involved in colorectal cancer progression. Cell Biosci. 2019, 9, 97. [Google Scholar] [CrossRef] [PubMed]
- Tardito, S.; Matis, S.; Zocchi, M.R.; Benelli, R.; Poggi, A. Epidermal growth factor receptor targeting in colorectal carcinoma: Antibodies and patient-derived organoids as a smart model to study therapy resistance. Int. J. Mol. Sci. 2024, 25, 7131. [Google Scholar] [CrossRef]
- Shin, J.H.; Kim, M.-J.; Kim, J.Y.; Choi, B.; Kang, Y.; Kim, S.H.; Lee, H.-J.; Kwon, D.; Cho, Y.B.; Kim, K.K.; et al. USP21-EGFR signaling axis is functionally implicated in metastatic colorectal cancer. Cell Death Discov. 2024, 10, 492. [Google Scholar] [CrossRef] [PubMed]
- Manzi, J.; Hoff, C.O.; Ferreira, R.; Pimentel, A.; Datta, J.; Livingstone, A.S.; Vianna, R.; Abreu, P. Targeted Therapies in Colorectal Cancer: Recent Advances in Biomarkers, Landmark Trials, and Future Perspectives. Cancers 2023, 15, 3023. [Google Scholar] [CrossRef]
- Leto, S.M.; Trusolino, L. Primary and acquired resistance to EGFR-targeted therapies in colorectal cancer: Impact on future treatment strategies. J. Mol. Med. 2014, 92, 709–722. [Google Scholar] [CrossRef]
- Mendelsohn, J.; Prewett, M.; Rockwell, P.; Goldstein, N.I. CCR 20th anniversary commentary: A chimeric antibody, C225, inhibits EGFR activation and tumor growth. Clin. Cancer Res. 2015, 21, 227–229. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yarom, N.; Jonker, D.J. The role of the epidermal growth factor receptor in the mechanism and treatment of colorectal cancer. Discov. Med. 2011, 11, 95–105. [Google Scholar] [PubMed]
- Ferrara, N.; Gerber, H.P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Olsson, A.K.; Dimberg, A.; Kreuger, J.; Claesson-Welsh, L. VEGF receptor signalling—In control of vascular function. Nat. Rev. Mol. Cell. Biol. 2006, 7, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Shibuya, M. The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin. Sci. 2005, 109, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Goel, H.L.; Mercurio, A.M. VEGF targets the tumour cell. Nat. Rev. Cancer 2013, 13, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Koch, S.; Claesson-Welsh, L. Signal transduction by vascular endothelial growth factor receptors. Cold Spring Harb. Perspect. Med. 2012, 2, a006502. [Google Scholar] [CrossRef]
- Cebe-Suarez, S.; Zehnder-Fjallman, A.; Ballmer-Hofer, K. The role of VEGF receptors in angiogenesis; complex partnerships. Cell Mol. Life Sci. 2006, 63, 601–615. [Google Scholar] [CrossRef]
- Garnier, L.; Gkountidi, A.O.; Hugues, S. Tumor-associated lymphatic vessel features and immunomodulatory functions. Front. Immunol. 2019, 10, 720. [Google Scholar] [CrossRef] [PubMed]
- Secker, G.A.; Harvey, N.L. VEGFR signaling during lymphatic vascular development: From progenitor cells to functional vessels. Dev. Dyn. 2015, 244, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.A.; Cohen, S.J.; Kollmannsberger, C.; Bjarnason, G.; Virik, K.; MacKenzie, M.J.; Lourenco, L.; Wang, L.; Chen, A.; Moor, M.J. Phase II clinical and pharmacokinetic study of aflibercept in patients with previously treated metastatic colorectal cancer. Clin. Cancer Res. 2012, 18, 6023–6031. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, J.; Yoshino, T.; Cohn, A.L.; Obermannova, R.; Bodoky, G.; Garcia-Carbonero, R.; Ciuleanu, T.E.; Portnoy, D.C.; Van Cutsem, E.; Grothey, A.; et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): A randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 2015, 16, 499–508, Erratum in Lancet Oncol. 2015, 16, e262. [Google Scholar]
- Chiron, M.; Bagley, R.G.; Pollard, J.; Mankoo, P.K.; Henry, C.; Vincent, L.; Geslin, C.; Baltes, N.; Bergstrom, D.A. Differential antitumor activity of aflibercept and bevacizumab in patient-derived xenograft models of colorectal cancer. Mol. Cancer Ther. 2014, 13, 1636–1644. [Google Scholar] [CrossRef]
- Goede, V.; Coutelle, O.; Neuneier, J.; Reinacher-Schick, A.; Schnell, R.; Koslowsky, T.C.; Weihrauch, M.R.; Cremer, B.; Kashkar, H.; Odenthal, M.; et al. Identification of serum angiopoietin-2 as a biomarker for clinical outcome of colorectal cancer patients treated with bevacizumab-containing therapy. Br. J. Cancer 2010, 103, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Rigamonti, N.; Kadioglu, E.; Keklikoglou, I.; Wyser Rmili, C.; Leow, C.C.; De Palma, M. Role of angiopoietin-2 in adaptive tumor resistance to VEGF signaling blockade. Cell Rep. 2014, 8, 696–706. [Google Scholar] [CrossRef] [PubMed]
- Scholz, A.; Harter, P.N.; Cremer, S.; Yalcin, B.H.; Gurnik, S.; Yamaji, M.; Di Tacchio, M.; Sommer, K.; Baumgarten, P.; Bähr, O.; et al. Endothelial cell-derived angiopoietin-2 is a therapeutic target in treatment-naive and bevacizumab-resistant glioblastoma. EMBO Mol. Med. 2016, 8, 39–57. [Google Scholar] [CrossRef] [PubMed]
- Kienast, Y.; Klein, C.; Scheuer, W.; Raemsch, R.; Lorenzon, E.; Bernicke, D.; Herting, F.; Yu, S.; The, H.H.; Martarello, L.; et al. Ang-2-VEGF-A CrossMab, a novel bispecific human IgG1 antibody blocking VEGF-A and Ang-2 functions simultaneously, mediates potent antitumor, antiangiogenic, and antimetastatic efficacy. Clin. Cancer Res. 2013, 19, 6730–6740. [Google Scholar] [CrossRef] [PubMed]
- Bradley, C.A.; Salto-Tellez, M.; Laurent-Puig, P.; Bardelli, A.; Rolfo, C.; Tabernero, J.; Khawaja, H.A.; Lawler, M.; Johnston, P.G.; Schaeybroeck, S.V.; et al. Targeting c-MET in gastrointestinal tumours: Rationale, opportunities and challenges. Nat. Rev. Clin. Oncol. 2017, 14, 562–576, Correction in Nat. Rev. Clin. Oncol. 2018, 15, 150. [Google Scholar] [CrossRef] [PubMed]
- Qamsari, E.S.; Ghaderi, S.S.; Zarei, B.; Dorostkar, R.; Bagheri, S.; Jadidi-Niaragh, F.; Hossein Somi, M.; Yousef, M. The c-Met receptor: Implication for targeted therapies in colorectal cancer. Tumour Biol. 2017, 39, 1010428317699118. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Umitsu, M.; De Silva, D.M.; Roy, A.; Bottaro, D.P. Hepatocyte growth factor/MET in cancer progression and biomarker discovery. Cancer Sci. 2017, 108, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, A.; Shahidsales, S.; Khazaei, M.; Ghayour-Mobarhan, M.; Maftouh, M.; Hassanian, S.M.; Avan, A. C-Met as a potential target for the treatment of gastrointestinal cancer: Current status and future perspectives. J. Cell. Physiol. 2017, 232, 2657–2673. [Google Scholar] [CrossRef]
- Mo, H.N.; Liu, P. Targeting MET in cancer therapy. Chronic Dis. Transl. Med. 2017, 3, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Parseghian, C.M.; Napolitano, S.; Loree, J.M.; Kopetz, S. Mechanisms of innate and acquired resistance to anti-EGFR therapy: A review of current knowledge with a focus on rechallenge therapies. Clin. Cancer Res. 2019, 25, 6899–6908. [Google Scholar] [CrossRef]
- Boccaccio, C.; Luraghi, P.; Comoglio, P.M. MET-mediated resistance to EGFR inhibitors: An old liaison rooted in colorectal cancer stem cells. Cancer Res. 2014, 74, 3647–3651. [Google Scholar] [CrossRef]
- Murtuza, A.; Bulbul, A.; Shen, J.P.; Keshavarzian, P.; Woodward, B.D.; Lopez-Diaz, F.J.; Lippman, S.M.; Husain, H. Novel third-generation EGFR tyrosine kinase inhibitors and strategies to overcome therapeutic resistance in lung cancer. Cancer Res. 2019, 79, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Luraghi, P.; Reato, G.; Cipriano, E.; Sassi, F.; Orzan, F.; Bigatto, V.; De Bacco, F.; Menietti, E.; Han, M.; Rideout, W.M., 3rd; et al. MET signaling in colon cancer stem-like cells blunts the therapeutic response to EGFR inhibitors. Cancer Res. 2014, 74, 1857–1869. [Google Scholar] [CrossRef] [PubMed]
- Van Emburgh, B.O.; Sartore-Bianchi, A.; Di Nicolantonio, F.; Siena, S.; Bardelli, A. Acquired resistance to EGFR-targeted therapies in colorectal cancer. Mol. Oncol. 2014, 8, 1084–1094. [Google Scholar] [CrossRef] [PubMed]
- Criscitiello, C.; Morganti, S.; Curigliano, G. Antibody-drug conjugates in solid tumors: A look into novel targets. J. Hematol. Oncol. 2021, 14, 20. [Google Scholar] [CrossRef]
- Cortés, J.; Kim, S.B.; Chung, W.P.; Im, S.A.; Park, Y.H.; Hegg, R.; Kim, M.H.; Tseng, L.M.; Petry, V.; Chung, C.F.; et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. NEJM 2022, 386, 1143–1154. [Google Scholar] [CrossRef]
- Holash, J.; Davis, S.; Papadopoulos, N.; Croll, S.D.; Ho, L.; Russell, M.; Boland, P.; Leidich, R.; Hylton, D.; Burova, E.; et al. VEGF-Trap: A VEGF blocker with potent antitumor effects. Proc. Natl. Acad. Sci. USA 2002, 99, 11393–11398. [Google Scholar] [CrossRef]
- Degenhardt, Y.; Lampkin, T. Targeting Polo-like kinase in cancer therapy. Clin. Cancer Res. 2010, 16, 384–389. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Tabernero, J.; Lakomy, R. Intravenous (IV) aflibercept versus placebo in combination with irinotecan/5-FU (FOLFIRI) for second-line treatment of metastatic colorectal cancer (mCRC): Results of a multinational phase III trial (EFC10262-VELOUR). In Proceedings of the Congress on Gastrointestinal Cancer. European Society for Medical Oncology (ESMO), Barcelona, Spain, 25 June 2011. [Google Scholar]
- Zaltrap [Prescribing Information]; Regeneron Pharmaceuticals, Inc./sanofi-aventis, U.S. LLC: Bridgewater, NJ, USA, 2012.
- Sun, L.; Liu, H.; Ye, Y.; Lei, Y.; Islam, R.; Tan, S.; Tong, R.; Miao, Y.-B.; Cai, L. Smart nanoparticles for cancer therapy. Signal Transduct. Target. Ther. 2023, 8, 418. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, P.; Kaur, G.; Singh, N.K. Nanotechnology for colorectal cancer detection and treatment. World J. Gastroenterol. 2022, 28, 6497–6511. [Google Scholar] [CrossRef] [PubMed]
- Dabaghi, M.; Rasa, S.M.M.; Cirri, E.; Ori, A.; Neri, F.; Quaas, H.I. Iron Oxide Nanoparticles Carrying 5-Fluorouracil in Combination with Magnetic Hyperthermia Induce Thrombogenic Collagen Fibers, Cellular Stress, and Immune Responses in Heterotopic Human Colon Cancer in Mice. Pharmaceutics 2021, 13, 1625. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Zhou, Q.; Zhu, H.; Zhuang, Y.; Bao, J. Enhanced antitumor efficacy in colon cancer using EGF functionalized PLGA nanoparticles loaded with 5-Fluorouracil and perfluorocarbon. BMC Cancer 2020, 20, 354. [Google Scholar] [CrossRef]
- Alibolandi, M.; Taghdisi, S.M.; Ramezani, P.; Hosseini Shamili, F.; Farzad, S.A.; Abnous, K.; Ramezani, M. Smart AS1411-aptamer conjugated pegylated PAMAM dendrimer for the superior delivery of camptothecin to colon adenocarcinoma in vitro and in vivo. Int. J. Pharm. 2017, 519, 352–364. [Google Scholar] [CrossRef]
- England, R.; Hare, J.I.; Barnes, J.; Wilson, J.; Smith, A.; Strittmatter, N.; Kemmitt, P.D.; Waring, M.J.; Barry, S.T.; Alexander, C.; et al. Tumour regression and improved gastrointestinal tolerability from controlled release of SN-38 from novel polyoxazoline-modified dendrimers. J. Control Release 2017, 247, 73–85. [Google Scholar] [CrossRef]
- Narmani, A.; Kamali, M.; Amini, B.; Salimi, A.; Panahi, Y. Targeting delivery of oxaliplatin with smart PEG-modified PAMAM G4 to colorectal cell line: In vitro studies. Process Biochem. 2018, 69, 178–187. [Google Scholar] [CrossRef]
- Meng, J.; Wang, Z.-G.; Zhao, X.; Wang, Y.; Chen, D.-Y.; Liu, D.-L.; Ji, C.-C.; Wang, T.-F.; Zhang, L.-M.; Bai, H.-X.; et al. Silica nanoparticle design for colorectal cancer treatment: Recent progress and clinical potential. World J. Clin. Oncol. 2024, 15, 667–673. [Google Scholar] [CrossRef]
- Linton, S.S.; Sherwood, S.G.; Drews, K.C.; Kester, M. Targeting cancer cells in the tumor microenvironment: Opportunities and challenges in combinatorial nanomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 208–222. [Google Scholar] [CrossRef]
- DeJong, W.H.; Borm, P.J. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef]
- De Santo, M.; Giovinazzo, A.; Fava, M.; Mazzotta, E.; De Napoli, I.E.; Greco, M.; Comande, A.; Nigro, A.; Argurio, P.; Perrotta, I.; et al. Engineered mesoporous silica-based nanoparticles as smart chemotherapy nanodevice for bortezomib administration. Mat. Chem. Front. 2023, 7, 216–229. [Google Scholar] [CrossRef]
- Testa, F.; Pasqua, L.; Crea, F.; Aiello, R.; Lázár, K.; Fejes, P.; Lentz, P.; Nagy, J.B. Synthesis of Fe-MFI zeolites in fluoride-containing media. Microporous Mesoporous Mater. 2003, 57, 57–72. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services Food and Drug Administration, Guidance for Industry on Drug Products, Including Biological Products, That Contain Nanomaterials. Available online: https://www.fda.gov/files/drugs/published/Drug Products-Including-Biological-Products--that-Contain-Nanomaterials---Guidance-for-Industry.pdf (accessed on 27 January 2025).
- Kuipers, E.J.; Grady, W.M.; Lieberman, D.; Seufferlein, T.; Sung, J.J.; Boelens, P.G.; van de Velde, C.J.H.; Watanabe, T. Colorectal cancer. Nat. Rev. Dis. Prim. 2015, 1, 15065. [Google Scholar] [CrossRef]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef]
- Roy, A.; Raza, M.A.; Ajazuddin, V.G. Diagnostic innovations and therapeutic potential of nanoparticulate delivery for colon cancer. Nano-Struct. Nano-Objects 2025, 41, 101426. [Google Scholar] [CrossRef]
- Mostert, B.; Sieuwerts, A.M.; Bolt-deVries, J. mRNA Expression Profiles in Circulating Tumor Cells of Metastatic Colorectal Cancer Patients. Mol. Oncol. 2015, 9, 920–932. [Google Scholar] [CrossRef] [PubMed]
- Nune, S.K.; Gunda, P.; Thallapally, P.K.; Lin, Y.Y.; Forrest, M.L.; Berkland, C.J. Nanoparticles for biomedical imaging. Expert. Opin. Drug Deliv. 2009, 6, 1175–1194. [Google Scholar] [CrossRef]
- Lanone, S.; Boczkowski, J. Biomedical applications and potential health risks of nanomaterials: Molecular mechanisms. Curr. Mol. Med. 2006, 6, 651–663. [Google Scholar] [CrossRef]
- Hiwatari, K.; Sakuma, S.; Iwata, K.; Masaoka, K.; Kataoka, M.; Tachikawa, H.; Shoji, Y.; Yamashita, S. Poly(N-vinylacetamide) chains enhance lectin-induced biorecognition through the reduction of nonspecific interactions with nontargets. Eur. J. Pharm. Biopharm. 2008, 70, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, S.; Yano, T.; Masaoka, Y.; Kataoka, M.; Hiwatari, K.; Tachikawa, H.; Shoji, Y.; Kimura, R.; Ma, H.; Yang, Z.; et al. In vitro/in vivo biorecognition of lectin-immobilized fluorescent nanospheres for human colorectal cancer cells. J. Control. Release 2009, 134, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Feng, S.; Pan, J.; Chen, Y.; Lin, J.; Chen, G.; Xie, S.; Zeng, H.; Chen, R. Colorectal cancer detection by gold nanoparticle based surface-enhanced Raman spectroscopy of blood serum and statistical analysis. Opt. Express 2011, 19, 13565–13577. [Google Scholar] [CrossRef]
- Chen, N.T.; Souris, J.S.; Cheng, S.H.; Chu, C.H.; Wang, Y.C.; Konda, V.; Dougherty, U.; Bissonnette, M.; Mou, C.Y.; Chen, C.T.; et al. Lectin-functionalized mesoporous silica nanoparticles for endoscopic detection of premalignant colonic lesions. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1941–1952. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.; Zhang, B.; Wang, X.; Liu, F.; Cheng, Y. An “imaging-biopsy” strategy for colorectal tumor reconfirmation by multipurpose paramagnetic quantum dots. Biomaterials 2015, 48, 16–25. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Wang, T.; Gu, J.; Zhao, J.; Pan, Z. Detection of AKR1B10 in peripheral blood by anti-AKR1B10-conjugated CdTe/CdS quantum dots. Clin. Lab. 2015, 61, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Liu, F.; Liu, L.; Duan, T.; Zhang, H.; Wang, Z. Lectin-conjugated Fe2O3@Au core@shell nanoparticles as dual mode contrast agents for in vivo detection of tumor. Mol. Pharm. 2014, 11, 738–745. [Google Scholar] [CrossRef]
- Kolitz-Domb, M.; Corem-Salkmon, E.; Grinberg, I.; Margel, S. Synthesis and characterization of bioactive conjugated near-infrared fluorescent proteinoid-poly(L-lactic acid) hollow nanoparticles for optical detection of colon cancer. Int. J. Nanomed. 2014, 31, 5041–5053. [Google Scholar]
- Fakih, M.G.; Kopetz, S.; Kuboki, Y.; Kim, T.W.; Munster, P.N.; Krauss, J.C.; Falchook, G.S.; Han, S.W.; Heinemann, V.; Muro, K.; et al. Sotorasib for previously treated colorectal cancers with KRASG12C mutation (CodeBreaK100): A prespecified analysis of a single-arm, phase 2 trial. Lancet Oncol. 2022, 23, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Fakih, M.G.; Salvatore, L.; Esaki, T.; Modest, D.P.; Lopez-Bravo, D.P.; Taieb, J.; Karamouzis, M.V.; Ruiz-Garcia, E.; Kim, T.W.; Kuboki, Y.; et al. Sotorasib plus panitumumab in refractory colorectal cancer with mutated KRAS G12C. NEJM 2023, 389, 2125–2139. [Google Scholar] [CrossRef]
- Siravegna, G.; Mussolin, B.; Buscarino, M.; Corti, G.; Cassingena, A.; Crisafulli, G.; Ponzetti, A.; Cremolini, C.; Amatu, A.; Lauricella, C.; et al. Clonal evolution and resistance to EGFR blockade in the blood of colorectal cancer patients. Nat. Med. 2015, 21, 795–801. [Google Scholar] [CrossRef]
- Van Emburgh, B.O.; Arena, S.; Siravegna, G.; Lazzari, L.; Crisafulli, G.; Corti, G.; Mussolin, B.; Baldi, F.; Buscarino, M.; Bartolini, A.; et al. Acquired RAS or EGFR mutations and duration of response to EGFR blockade in colorectal cancer. Nature Commun. 2016, 7, 13665. [Google Scholar] [CrossRef] [PubMed]
- Pietrantonio, F.; Petrelli, F.; Coinu, A.; Di Bartolomeo, M.; Borgonovo, K.; Maggi, C.; Cabiddu, M.; Iacovelli, R.; Bossi, I.; Lonati, V.; et al. Predictive role of BRAF mutations in patients with advanced colorectal cancer receiving cetuximab and panitumumab: A meta-analysis. Eur. J. Cancer 2015, 51, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Rowland, A.; Dias, M.M.; Wiese, M.D.; Kichenadasse, G.; McKinnon, R.A.; Karapetis, C.S.; Sorich, M.J. Meta-analysis of BRAF mutation as a predictive biomarker of benefit from anti-EGFR monoclonal antibody therapy for RAS wild-type metastatic colorectal cancer. Br. J. Cancer 2015, 112, 1888–1894. [Google Scholar] [CrossRef] [PubMed]
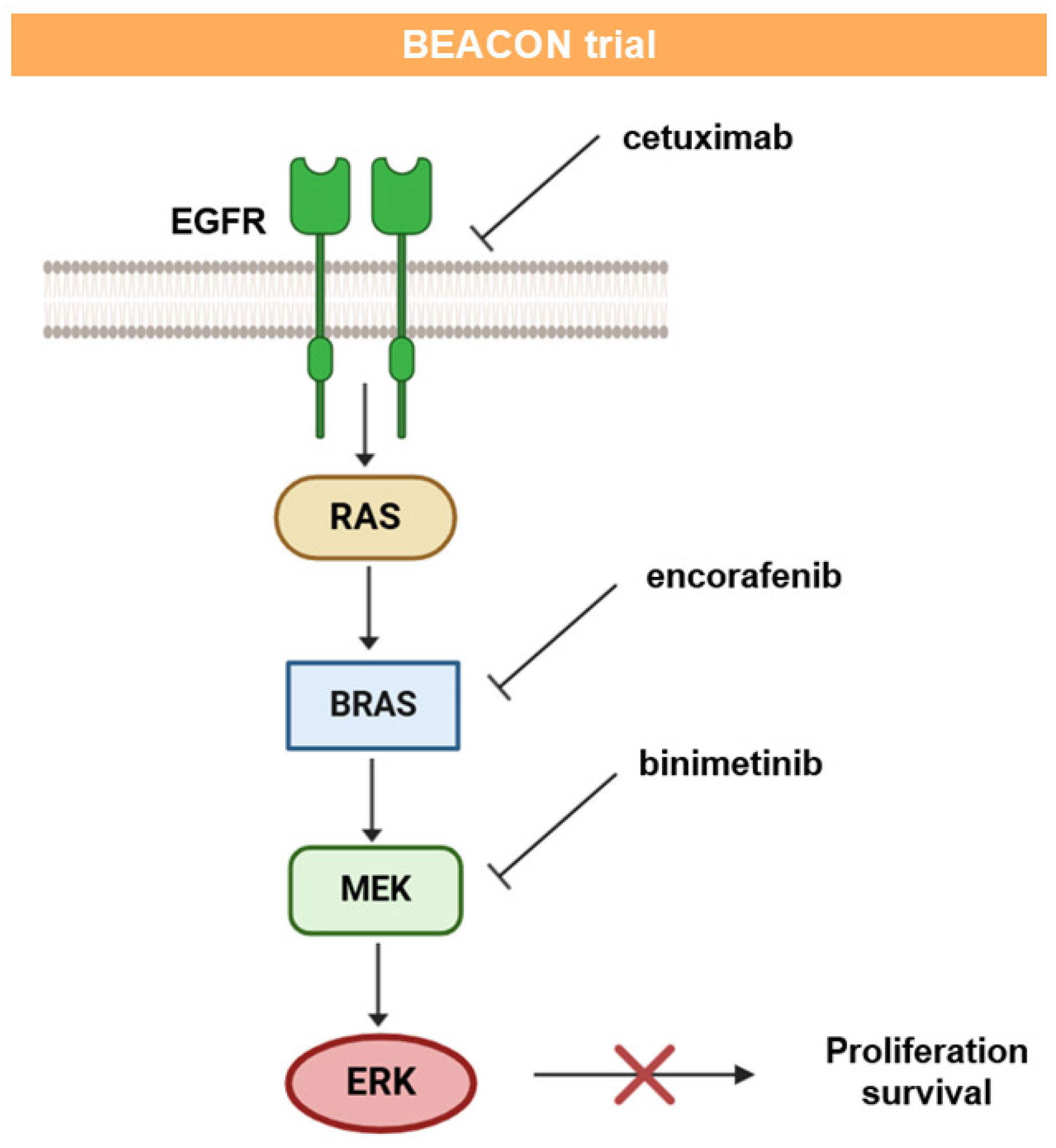
- Van Cutsem, E.; Huijberts, S.; Grothey, A.; Yaeger, R.; Cuyle, P.J.; Elez, E.; Fakih, M.; Montagut, C.; Peeters, M.; Yoshino, T.; et al. Binimetinib, encorafenib, and cetuximab triplet therapy for patients with BRAF V600E–mutant metastatic colorectal cancer: Safety lead-in results from the phase III BEACON colorectal cancer study. J. Clin. Oncol. 2019, 37, 1460–1469. [Google Scholar] [CrossRef]
- Kopetz, S.; Grothey, A.; Yaeger, R.; Cuyle, P.J.; Huijberts, S.; Schellens, J.H.; Elez, E.; Fakih, M.; Viladot, C.M.; Peeters, M.; et al. Updated results of the BEACON CRC safety lead-in: Encorafenib (ENCO)+ binimetinib (BINI)+ cetuximab (CETUX) for BRAFV600E-mutant metastatic colorectal cancer (mCRC). J. Clin. Oncol. 2019, 37, 688. [Google Scholar] [CrossRef]
- Kopetz, S.; Grothey, A.; Van Cutsem, E.; Yaeger, R.; Wasan, H.S.; Yoshino, T.; Desai, J.; Ciardiello, F.; Loupakis, F.; Hong, Y.S.; et al. Encorafenib plus cetuximab with or without binimetinib for BRAF V600E-mutant metastatic colorectal cancer: Quality-of-life results from a randomized, three-arm, phase III study versus the choice of either irinotecan or FOLFIRI plus cetuximab (BEACON CRC). J. Clin. Oncol. 2020, 38, 4039. [Google Scholar] [CrossRef]
- Puzzo, M.; De Santo, M.; Morelli, C.; Leggio, A.; Pasqua, L. The Advent of Molecular Targeted Therapies Against Cancer. Toward Multi-Targeting Drugs Through Materials Engineering: A Possible Future Scenario. Small Sci. 2024, 4, 2400113. [Google Scholar] [CrossRef]
- Ashman, N.; Bargh, J.D.; Spring, D.R. Non-internalising antibody–drug conjugates. Chem. Soc. Rev. 2022, 51, 9182. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Bhattacharya, S.; Joshi, K.; Singh, S. A shift in focus towards precision oncology, driven by revolutionary nanodiagnostics; revealing mysterious pathways in colorectal carcinogenesis. J. Cancer Res. Clin. Oncol. 2023, 149, 16157–16177. [Google Scholar] [CrossRef] [PubMed]
- Ceresa, C.; Nicolini, G.; Rigolio, R.; Bossi, M.; Pasqua, L.; Cavaletti, G. Functionalized Mesoporous Silica Nanoparticles: A Possible Strategy to Target Cancer Cells Reducing Peripheral Nervous System Uptake. Curr. Med. Chem. 2013, 20, 2589–2600. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puzzo, M.; De Santo, M.; Morelli, C.; Leggio, A.; Catalano, S.; Pasqua, L. Colorectal Cancer: Current and Future Therapeutic Approaches and Related Technologies Addressing Multidrug Strategies Against Multiple Level Resistance Mechanisms. Int. J. Mol. Sci. 2025, 26, 1313. https://doi.org/10.3390/ijms26031313
Puzzo M, De Santo M, Morelli C, Leggio A, Catalano S, Pasqua L. Colorectal Cancer: Current and Future Therapeutic Approaches and Related Technologies Addressing Multidrug Strategies Against Multiple Level Resistance Mechanisms. International Journal of Molecular Sciences. 2025; 26(3):1313. https://doi.org/10.3390/ijms26031313
Chicago/Turabian StylePuzzo, Marianna, Marzia De Santo, Catia Morelli, Antonella Leggio, Stefania Catalano, and Luigi Pasqua. 2025. "Colorectal Cancer: Current and Future Therapeutic Approaches and Related Technologies Addressing Multidrug Strategies Against Multiple Level Resistance Mechanisms" International Journal of Molecular Sciences 26, no. 3: 1313. https://doi.org/10.3390/ijms26031313
APA StylePuzzo, M., De Santo, M., Morelli, C., Leggio, A., Catalano, S., & Pasqua, L. (2025). Colorectal Cancer: Current and Future Therapeutic Approaches and Related Technologies Addressing Multidrug Strategies Against Multiple Level Resistance Mechanisms. International Journal of Molecular Sciences, 26(3), 1313. https://doi.org/10.3390/ijms26031313






