Anticancer Activity of Enantiomeric Neplanocins A: Exploring the Role of Chirality in Tumor Suppression
Abstract
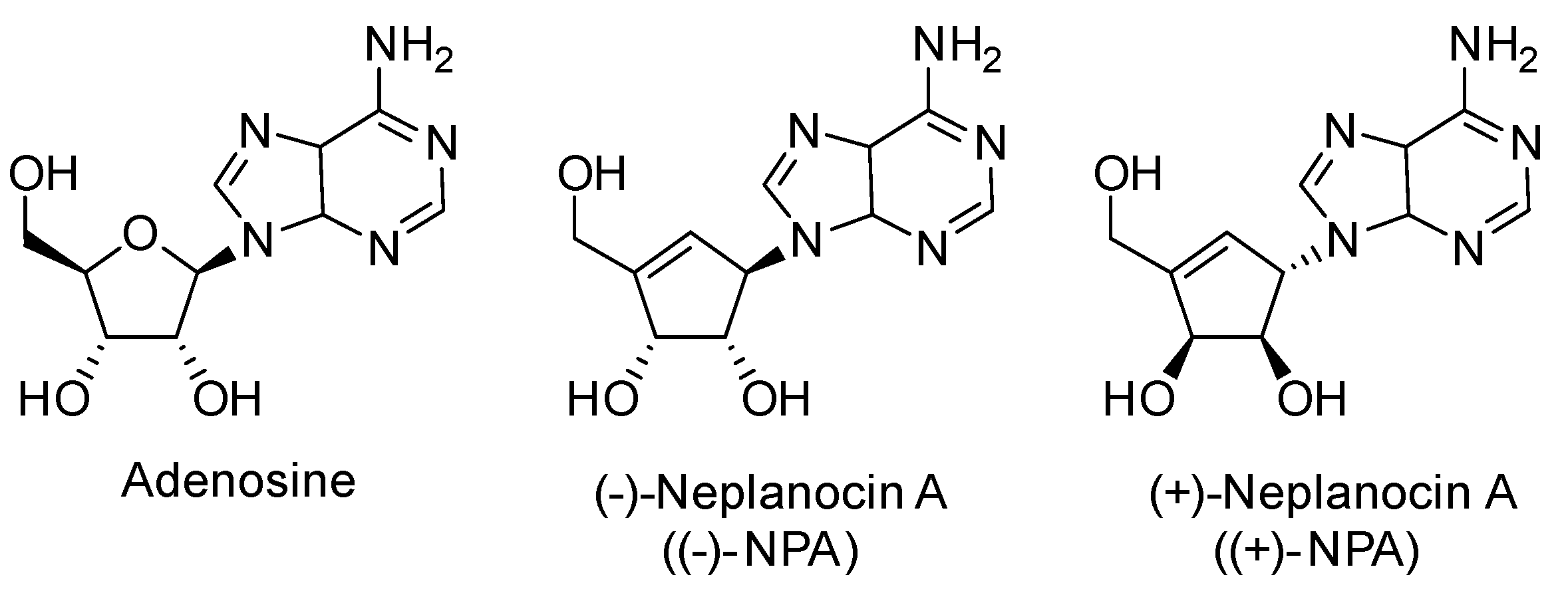
1. Introduction
2. Results
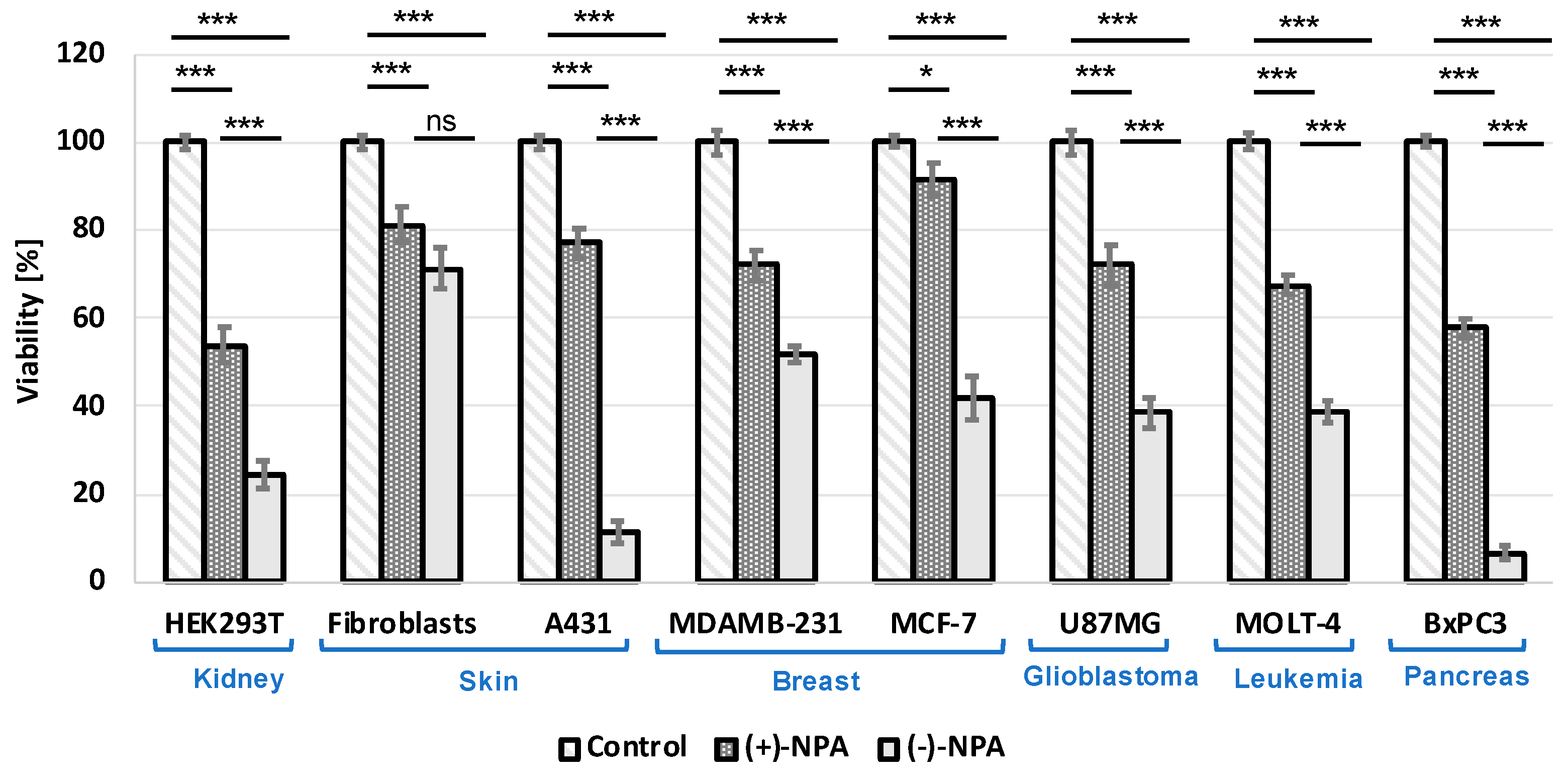
2.1. Cytotoxicity of (-)-Neplanocin A and (+)-Neplanocin A in Various Types of Cancer and Non-Cancerous Cells
2.2. Sensitivity of MDA-MB-231 Cells to Neplanocin A Under Different Conditions
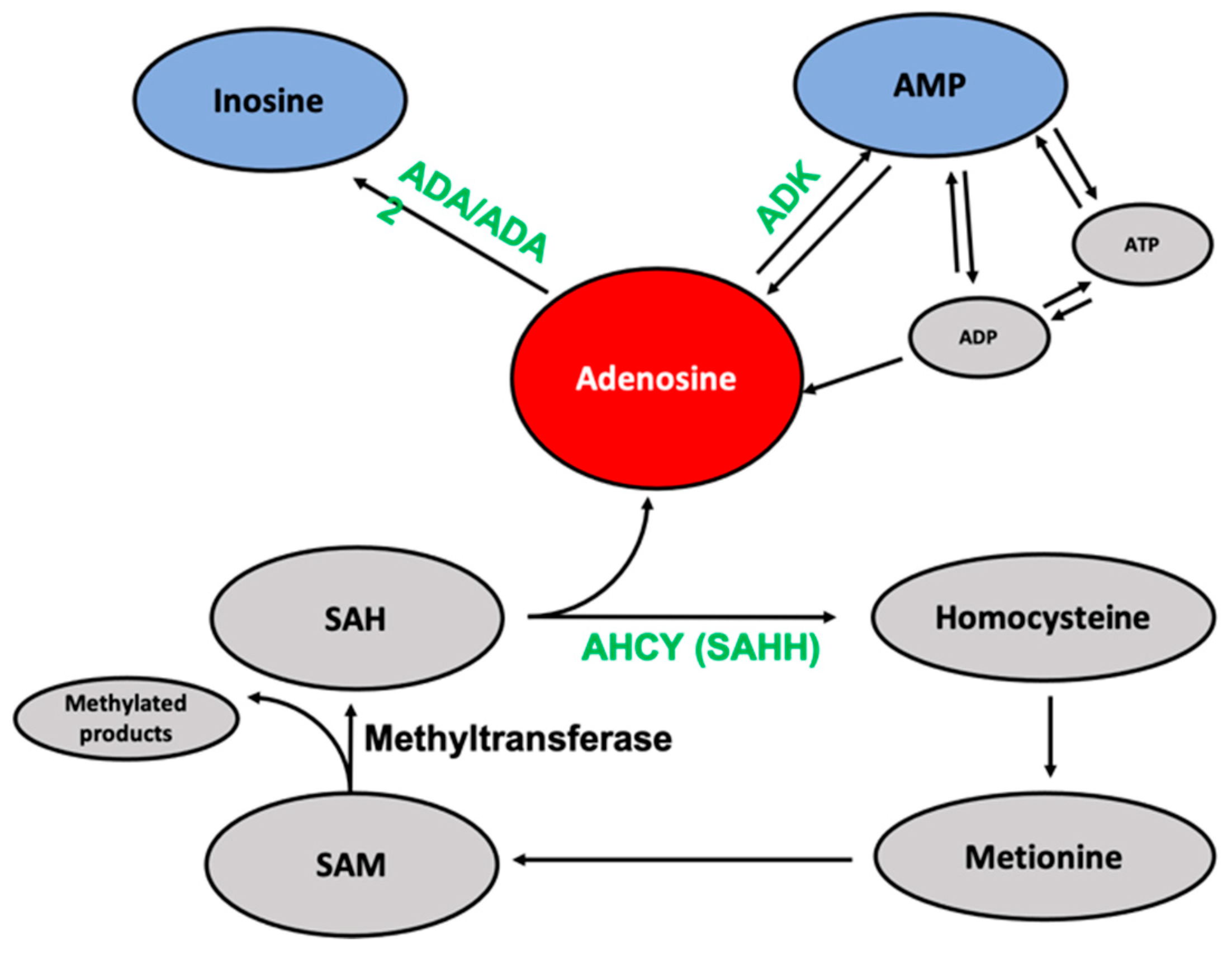
2.3. Analysis of Possible Interaction Partners for Neplanocin A in Cancer Cell Lines
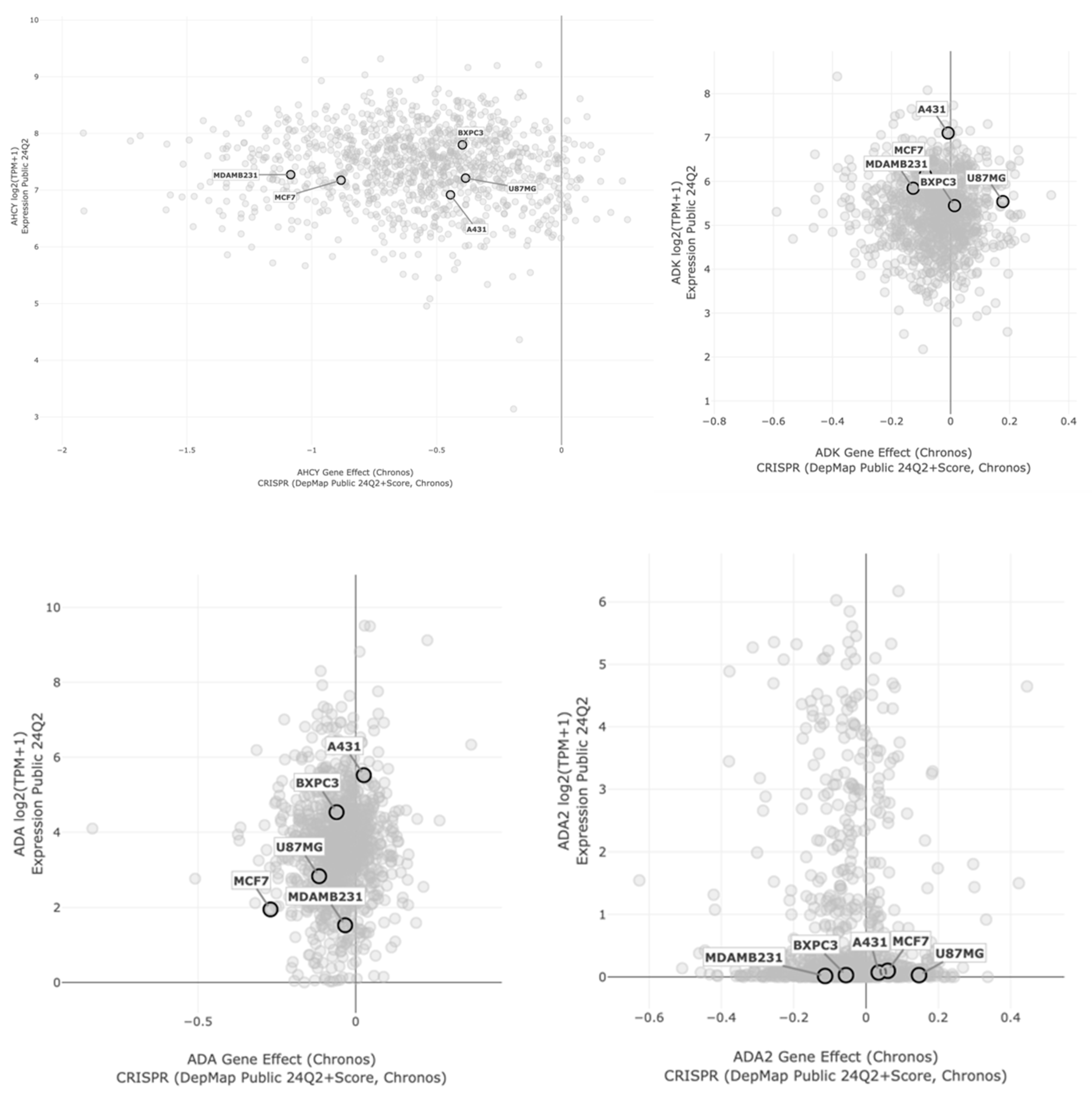
2.4. Bioinformatic Analysis of Neplanocin A Enantiomers with Adenosine-Related Cellular Targets
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Culture
4.3. Cytotoxicity Test
4.4. Anoikis Resistance Assay
4.5. Computational Analysis of Gene Expression and Its Effects on Cancer Cell Lines
4.6. Statistical Analysis of Experimental Studies
4.7. Protein Structures Preparation for Docking Analysis
4.8. Molecular Docking with AutoDock4
4.9. Statistical Analysis of Binding Energy Calculation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- von Frijtag Drabbe Künzel, J.K.; van der Zee, J.; Ijzerman, A.P. Radical scavenging properties of adenosine and derivatives in vitro. Drug Dev. Res. 1996, 37, 48–54. [Google Scholar] [CrossRef]
- Valdes, F.; Brown, N.; Morales-Bayuelo, A.; Prent-Peñaloza, L.; Gutierrez, M. Adenosine Derivates as Antioxidant Agents: Synthesis, Characterization, in Vitro Activity, and Theoretical Insights. Antioxidants 2019, 8, 468. [Google Scholar] [CrossRef] [PubMed]
- Correia, A.S.; Vale, N. Exploring Oxidative Stress in Disease and Its Connection with Adenosine. Oxygen 2024, 4, 325–337. [Google Scholar] [CrossRef]
- Ramkumar, V.; Hallam, D.M.; Nie, Z. Adenosine, Oxidative Stress and Cytoprotection. Jpn. J. Pharmacol. 2001, 86, 265–274. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, A. Adenosine: An endogenous modulator of innate immune system with therapeutic potential. Eur. J. Pharmacol. 2009, 616, 7–15. [Google Scholar] [CrossRef]
- Suwara, J.; Radzikowska-Cieciura, E.; Chworos, A.; Pawlowska, R. The ATP-dependent Pathways and Human Diseases. Curr. Med. Chem. 2023, 30, 1232–1255. [Google Scholar] [CrossRef]
- Graczyk, A.; Radzikowska-Cieciura, E.; Kaczmarek, R.; Pawlowska, R.; Chworos, A. Modified Nucleotides for Chemical and Enzymatic Synthesis of Therapeutic RNA. Curr. Med. Chem. 2023, 30, 1320–1347. [Google Scholar] [CrossRef]
- Samsel, M.; Dzierzbicka, K. Therapeutic potential of adenosine analogues and conjugates. Pharmacol. Rep. 2011, 63, 601–617. [Google Scholar] [CrossRef]
- Pawlowska, R.; Chworos, A. Nucleoside and Nucleotide Analogues as Potential Therapeutics. Curr. Med. Chem. 2023, 30, 1207–1208. [Google Scholar] [CrossRef]
- Valdés Zurita, F.; Brown Vega, N.; Gutiérrez Cabrera, M. Semisynthesis, Characterization and Evaluation of New Adenosine Derivatives as Antiproliferative Agents. Molecules 2018, 23, 1111. [Google Scholar] [CrossRef]
- Converso, A.; Hartingh, T.; Fraley, M.E.; Garbaccio, R.M.; Hartman, G.D.; Huang, S.Y.; Majercak, J.M.; McCampbell, A.; Na, S.J.; Ray, W.J.; et al. Adenosine analogue inhibitors of S-adenosylhomocysteine hydrolase. Bioorganic Med. Chem. Lett. 2014, 24, 2737–2740. [Google Scholar] [CrossRef] [PubMed]
- Fishman, P.; Bar-Yehuda, S.; Synowitz, M.; Powell, J.D.; Klotz, K.N.; Gessi, S.; Borea, P.A. Adenosine Receptors and Cancer. In Adenosine Receptors in Health and Disease; Wilson, C.N., Mustafa, S.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 399–441. [Google Scholar]
- Xia, Y.; Zheng, X.; Wang, E.; Li, D.; Hou, R.; Wang, J. Synthesis of adenosine analogues with indole moiety as human adenosine A3 receptor ligands. R. Soc. Open Sci. 2018, 5, 171596. [Google Scholar] [CrossRef] [PubMed]
- Yaginuma, S.; Muto, N.; Tsujino, M.; Sudate, Y.; Hayashi, M.; Otani, M. Studies on neplanocin A, new antitumor antibiotic. I. Producing organism, isolation and characterization. J. Antibiot. 1981, 34, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Yaginuma, S.; Yoshioka, H.; Nakatsu, K. Studies on neplanocin A, new antitumor antibiotic. II. Structure determination. J. Antibiot. 1981, 34, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Song, G.Y.; Paul, V.; Choo, H.; Morrey, J.; Sidwell, R.W.; Schinazi, R.F.; Chu, C.K. Enantiomeric Synthesis of d- and l-Cyclopentenyl Nucleosides and Their Antiviral Activity Against HIV and West Nile Virus. J. Med. Chem. 2001, 44, 3985–3993. [Google Scholar] [CrossRef]
- De Clercq, E.; Cools, M.; Balzarini, J.; Marquez, V.E.; Borcherding, D.R.; Borchardt, R.T.; Drach, J.C.; Kitaoka, S.; Konno, T. Broad-Spectrum Antiviral Activities of Neplanocin A, 3-Deazaneplanocin A, and their 5′-Nor Derivatives. Antimicrob. Agents Chemother. 1989, 33, 1291–1297. [Google Scholar] [CrossRef]
- De Clercq, E. Antiviral and antimetabolic activities of neplanocins. Antimicrob. Agents Chemother. 1985, 28, 84–89. [Google Scholar] [CrossRef]
- Lukasik, B.; Mikina, M.; Mikolajczyk, M.; Pawlowska, R.; Zurawinski, R. A novel route to a chiral building block for the preparation of cyclopentenyl carbocyclic nucleosides. Synthesis and anticancer activity of enantiomeric neplanocins A. RSC Adv. 2020, 10, 31838–31847. [Google Scholar] [CrossRef]
- Chandra, G.; Moon, Y.W.; Lee, Y.; Jang, J.Y.; Song, J.; Nayak, A.; Oh, K.; Mulamoottil, V.A.; Sahu, P.K.; Kim, G.; et al. Structure-Activity Relationships of Neplanocin A Analogues as S-Adenosylhomocysteine Hydrolase Inhibitors and Their Antiviral and Antitumor Activities. J. Med. Chem. 2015, 58, 5108–5120. [Google Scholar] [CrossRef]
- Zhang, L.; Deng, L.; Chen, F.; Yao, Y.; Wu, B.; Wei, L.; Mo, Q.; Song, Y. Inhibition of histone H3K79 methylation selectively inhibits proliferation, self-renewal and metastatic potential of breast cancer. Oncotarget 2014, 5, 10665–10677. [Google Scholar] [CrossRef]
- Hayden, A.; Johnson, P.W.M.; Packham, G.; Crabb, S.J. S-adenosylhomocysteine hydrolase inhibition by 3-deazaneplanocin A analogues induces anti-cancer effects in breast cancer cell lines and synergy with both histone deacetylase and HER2 inhibition. Breast Cancer Res. Treat. 2011, 127, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Inaba, M.; Nagashima, K.; Tsukagoshi, S.; Sakurai, Y. Biochemical-mode of cytotoxic action of neplanocin A in L1210 leukemic-cells. Cancer Res. 1986, 46, 1063–1067. [Google Scholar] [PubMed]
- Yoneyama, H.; Uemura, K.; Usami, Y.; Harusawa, S. Syntheses of cyclopentyl nucleoside (-)-neplanocin A through tetrazole-fragmentation from cyanophosphates. Tetrahedron 2018, 74, 2143–2150. [Google Scholar] [CrossRef]
- Tan, Y.X.; Santhanakrishnan, S.; Yang, H.Y.; Chai, C.L.L.; Tam, E.K.W. Synthesis of Neplanocin A and Its 3′-Epimer via an Intramolecular Baylis-Hillman Reaction. J. Org. Chem. 2014, 79, 8059–8066. [Google Scholar] [CrossRef]
- Michel, B.Y.; Strazewski, P. Synthesis of (-)-neplanocin A with the highest overall yield via an efficient Mitsunobu coupling. Tetrahedron 2007, 63, 9836–9841. [Google Scholar] [CrossRef]
- Paquette, L.A.; Tian, Z.J.; Seekamp, C.K.; Wang, T. Synthesis of the cyclopentyl nucleoside (-)-neplanocin a from D-glucose via zirconocene-mediated ring contraction. Helv. Chim. Acta 2005, 88, 1185–1198. [Google Scholar] [CrossRef]
- Gallos, J.K.; Stathakis, C.I.; Kotoulas, S.S.; Koumbis, A.E. An improved approach to chiral cyclopentenone building blocks. Total synthesis of pentenomycin I and neplanocin A. J. Org. Chem. 2005, 70, 6884–6890. [Google Scholar] [CrossRef]
- Ono, M.; Nishimura, K.; Tsubouchi, H.; Nagaoka, Y.; Tomioka, K. Total synthesis of (-)-neplanocin A by using lithium thiolate-initiated Michael-Aldol tandem cyclization reaction. J. Org. Chem. 2001, 66, 8199–8203. [Google Scholar] [CrossRef]
- Trost, B.M.; Madsen, R.; Guile, S.D.; Brown, B. Palladium-catalyzed enantioselective synthesis of carbanucleosides. J. Am. Chem. Soc. 2000, 122, 5947–5956. [Google Scholar] [CrossRef]
- Yoshida, N.; Kamikubo, T.; Ogasawara, K. A concise synthesis of (-)-neplanocin A. Tetrahedron Lett. 1998, 39, 4677–4678. [Google Scholar] [CrossRef]
- Trost, B.M.; Madsen, R.; Guile, S.D. An enantio- and diastereo-controlled synthesis of (-)-neplanocin A and its 2,3-di-epi isomer. Tetrahedron Lett. 1997, 38, 1707–1710. [Google Scholar] [CrossRef]
- Niizuma, S.; Shuto, S.; Matsuda, A. New neplanocin analogues.10. The conversion of adenosine to neplanocin A, a carbocyclic nucleoside antibiotic with potent antiviral activity. Tetrahedron 1997, 53, 13621–13632. [Google Scholar] [CrossRef]
- Ohira, S.; Sawamoto, T.; Yamato, M. Synthesis of (-)-Neplanocin A via C-H Insertion of Alkylidenecarbene. Tetrahedron Lett. 1995, 36, 1537–1538. [Google Scholar] [CrossRef]
- Nokami, J.; Matsuura, H.; Takahashi, H.; Yamashita, M. Synthesis of carbocyclic nucleosides, (-)-Neplanocin A and its analogues. Synlett 1994, 491–493. [Google Scholar] [CrossRef]
- Hill, J.M.; Hutchinson, E.J.; Legrand, D.M.; Roberts, S.M.; Thorpe, A.J.; Turner, N.J. Preparation of Neplanocin A from D-Ribose and by a Chemoenzymic Method. J. Chem. Soc. Perkin Trans. 1 1994, 1483–1487. [Google Scholar] [CrossRef]
- Hutchinson, E.J.; Roberts, S.M.; Thorpe, A.J. Enzyme-catalyzed Hydrolysis of Dimethyl 7-Hydroxy-3,3-Dimethyl-2,4-Dioxabicyclo[3.3.0]octane-6,8-dicarboxylate and a Novel Synthesis of Neplanocin, A.J. Chem. Soc. Perkin Trans. 1 1992, 2245–2246. [Google Scholar] [CrossRef]
- Vanhessche, K.; Bello, C.G.; Vandewalle, M. Total Synthesis of (-)-Neplanocin A from L-Ribulose. Synlett 1991, 921–922. [Google Scholar] [CrossRef]
- Wolfe, M.S.; Anderson, B.L.; Borcherding, D.R.; Borchardt, R.T. Enantiospecific Syntheses of Aristeromycin and Neplanocin, A.J. Org. Chem. 1990, 55, 4712–4717. [Google Scholar] [CrossRef]
- Bestmann, H.J.; Roth, D. Synthesis of (-)-Neplanocin A from (R,R)-Tartaric Acid. Angew. Chem. Int. Ed. Engl. 1990, 29, 99–100. [Google Scholar] [CrossRef]
- Marquez, V.E.; Lim, M.I.; Tseng, C.K.H.; Markovac, A.; Priest, M.A.; Khan, M.S.; Kaskar, B. Total synthesis of (-)-neplanocin a. J. Org. Chem. 1988, 53, 5709–5714. [Google Scholar] [CrossRef]
- Medich, J.R.; Kunnen, K.B.; Johnson, C.R. Synthesis of the carbocyclic nucleoside (-)-neplanocin A. Tetrahedron Lett. 1987, 28, 4131–4134. [Google Scholar] [CrossRef]
- Tseng, C.K.H.; Marquez, V.E. An improved method of synthesis of neplanocin and related cyclopentenyl-containing nucleosides. Tetrahedron Lett. 1985, 26, 3669–3672. [Google Scholar] [CrossRef]
- Lim, M.I.; Marquez, V.E. Total Synthesis of (-)-Neplanocin, A. Tetrahedron Lett. 1983, 24, 5559–5562. [Google Scholar] [CrossRef]
- Arita, M.; Adachi, K.; Ito, Y.; Sawai, H.; Ohno, M. Enantioselective synthesis of the carbocyclic nucleosides (-)-aristeromycin and (-)-neplanocin A by a chemicoenzymatic approach. J. Am. Chem. Soc. 1983, 105, 4049–4055. [Google Scholar] [CrossRef]
- Sridhar, P.R.; Reddy, V.D.K.; Suresh, M.; Reddy, N.M.; Kumar, K.S. Synthesis of Carbocyclic Nucleosides (+)-Neplanocin A, (+)-Aristeromycin and 4′-epi-(+)-Aristeromycin from D-Fructose. Lett. Org. Chem. 2019, 16, 750–758. [Google Scholar] [CrossRef]
- Jung, Y.H.; Kim, S.I.; Hong, Y.J.; Park, S.J.; Kang, K.T.; Kim, S.Y.; Kim, I.S. Total synthesis of carbocyclic nucleoside (+)-neplanocin A. Tetrahedron 2015, 71, 1068–1073. [Google Scholar] [CrossRef]
- Hegedus, L.S.; Geisler, L. Synthesis of (+)-neplanocin A from a chromium-carbene complex-derived optically active butenolide. J. Org. Chem. 2000, 65, 4200–4203. [Google Scholar] [CrossRef]
- Keller, B.T.; Borchardt, R.T. Metabolism and Mechanism of Action of Neplanocin A—A Potent Inhibitor of S-Adenosylhomocysteine Hydrolase. In Biological Methylation and Drug Design: Experimental and Clinical Role of S-Adenosylmethionine; Borchardt, R.T., Creveling, C.R., Ueland, P.M., Eds.; Humana Press: Totowa, NJ, USA, 1986; pp. 385–396. [Google Scholar]
- Fisher, E.W.; Decedue, C.J.; Keller, B.T.; Borchardt, R.T. Neplanocin A inhibition of S-adenosylhomocysteine hydrolase in Alcaligenes faecalis has no effect on growth of the microorganism. J. Antibiot. 1987, 40, 873–881. [Google Scholar] [CrossRef]
- Chen, Q.; Davidson, A. Synthesis, conformational study and antiviral activity of l-like neplanocin derivatives. Bioorganic Med. Chem. Lett. 2017, 27, 4436–4439. [Google Scholar] [CrossRef]
- Byun, W.S.; Kim, W.K.; Yoon, J.-s.; Jarhad, D.B.; Jeong, L.S.; Lee, S.K. Antiproliferative and Antimigration Activities of Fluoro-Neplanocin A via Inhibition of Histone H3 Methylation in Triple-Negative Breast Cancer. Biomolecules 2020, 10, 530. [Google Scholar] [CrossRef]
- Zhulai, G.; Oleinik, E.; Shibaev, M.; Ignatev, K. Adenosine-Metabolizing Enzymes, Adenosine Kinase and Adenosine Deaminase, in Cancer. Biomolecules 2022, 12, 418. [Google Scholar] [CrossRef] [PubMed]
- Kloor, D.; Osswald, H. S-Adenosylhomocysteine hydrolase as a target for intracellular adenosine action. Trends Pharmacol. Sci. 2004, 25, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Pawlowska, R.; Korczynski, D.; Nawrot, B.; Stec, W.J.; Chworos, A. The α-thio and/or β-γ-hypophosphate analogs of ATP as cofactors of T4 DNA ligase. Bioorganic Chem. 2016, 67, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Pawlowska, R.; Graczyk, A.; Radzikowska-Cieciura, E.; Wielgus, E.; Madaj, R.; Chworos, A. Substrate Specificity of T7 RNA Polymerase toward Hypophosphoric Analogues of ATP. ACS Omega 2024, 9, 9348–9356. [Google Scholar] [CrossRef]
- Pawlowska, R.; Radzikowska-Cieciura, E.; Jafari, S.; Fastyn, J.; Korkus, E.; Gendaszewska-Darmach, E.; Zhao, G.; Snaar-Jagalska, E.; Chworos, A. Double-modified, thio and methylene ATP analogue facilitates wound healing in vitro and in vivo. Sci. Rep. 2024, 14, 13148. [Google Scholar] [CrossRef]
- Tam, E.K.; Nguyen, T.M.; Lim, C.Z.; Lee, P.L.; Li, Z.; Jiang, X.; Santhanakrishnan, S.; Tan, T.W.; Goh, Y.L.; Wong, S.Y.; et al. 3-Deazaneplanocin A and neplanocin A analogues and their effects on apoptotic cell death. ChemMedChem 2015, 10, 173–182. [Google Scholar] [CrossRef]
- Hermes, M.; Osswald, H.; Riehle, R.; Piesch, C.; Kloor, D. S-Adenosylhomocysteine hydrolase overexpression in HEK-293 cells: Effect on intracellular adenosine levels, cell viability, and DNA methylation. Cell. Physiol. Biochem. 2008, 22, 223–236. [Google Scholar] [CrossRef]
- Tsherniak, A.; Vazquez, F.; Montgomery, P.G.; Weir, B.A.; Kryukov, G.; Cowley, G.S.; Gill, S.; Harrington, W.F.; Pantel, S.; Krill-Burger, J.M.; et al. Defining a Cancer Dependency Map. Cell 2017, 170, 564–576.e16. [Google Scholar] [CrossRef]
- Dempster, J.M.; Boyle, I.; Vazquez, F.; Root, D.E.; Boehm, J.S.; Hahn, W.C.; Tsherniak, A.; McFarland, J.M. Chronos: A cell population dynamics model of CRISPR experiments that improves inference of gene fitness effects. Genome Biol. 2021, 22, 343. [Google Scholar] [CrossRef]

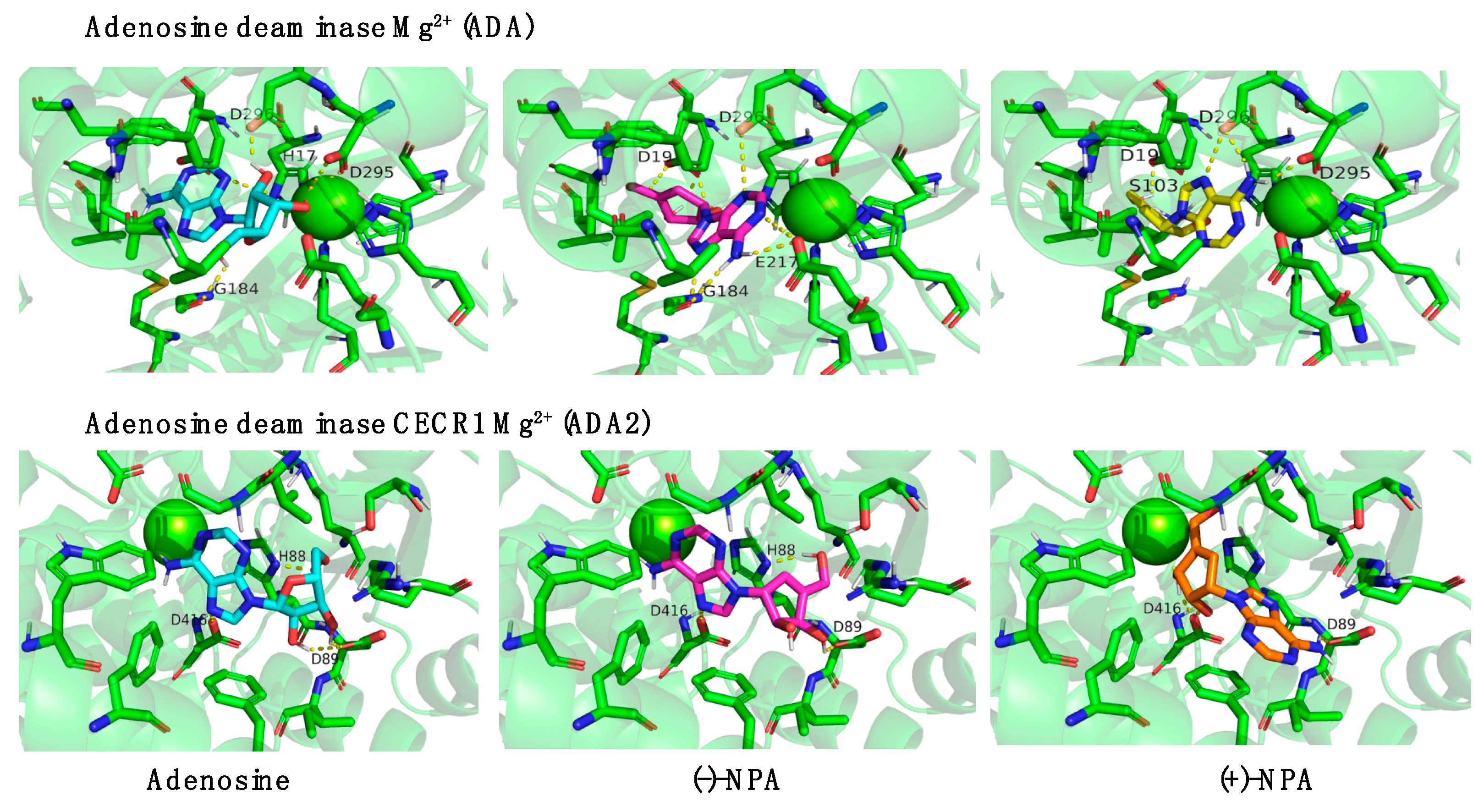
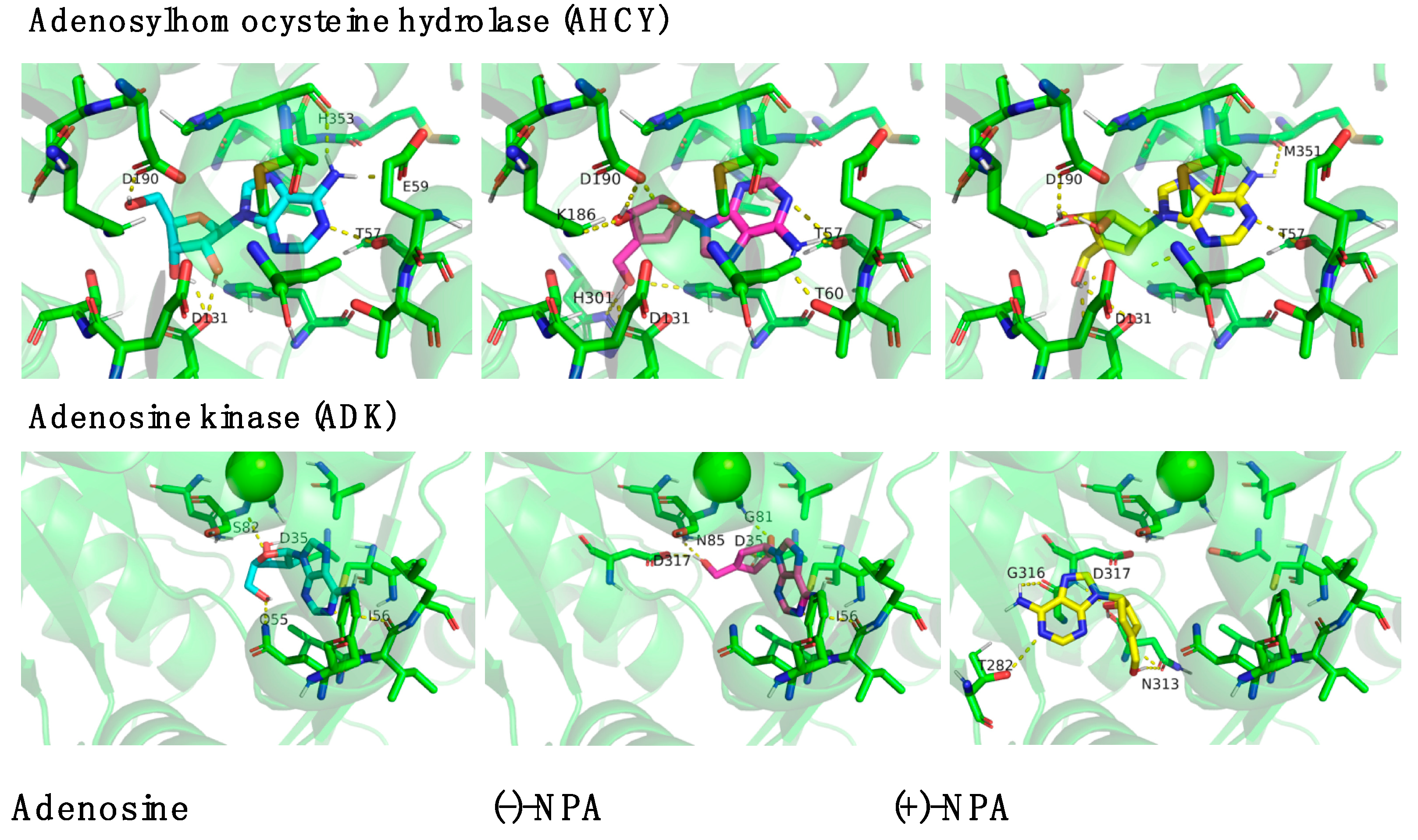
| Av, ΔΔG [kcal/mol] | Adenosine | (-)-NPA | (+)-NPA |
|---|---|---|---|
| AHCY (none) | −12.18 ± 0.03 | −12.29 ± 0.03 | −12.22 ± 0.00 |
| ADA (Zn2+) | −10.45 ± 0.19 | −10.09 ± 0.02 | −11.09 ± 0.03 |
| ADA (Mg2+) | −11.01 ± 0.03 | −11.29 ±0.01 | −11.41 ± 0.01 |
| ADA2 (Zn2+) | −9.61 ± 0.04 | −9.88 ± 0.27 | −10.44 ± 0.08 |
| ADA2 (Mg2+) | −9.65 ± 0.12 | −10.02 ± 0.22 | −10.84 ± 0.07 |
| ADK (Mg2+) | −9.83 ± 0.10 | −10.75 ± 0.10 | −9.71 ± 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawlowska, R.; Banaszkiewicz, H.; Chworos, A.; Żurawiński, R. Anticancer Activity of Enantiomeric Neplanocins A: Exploring the Role of Chirality in Tumor Suppression. Int. J. Mol. Sci. 2025, 26, 1308. https://doi.org/10.3390/ijms26031308
Pawlowska R, Banaszkiewicz H, Chworos A, Żurawiński R. Anticancer Activity of Enantiomeric Neplanocins A: Exploring the Role of Chirality in Tumor Suppression. International Journal of Molecular Sciences. 2025; 26(3):1308. https://doi.org/10.3390/ijms26031308
Chicago/Turabian StylePawlowska, Roza, Hubert Banaszkiewicz, Arkadiusz Chworos, and Remigiusz Żurawiński. 2025. "Anticancer Activity of Enantiomeric Neplanocins A: Exploring the Role of Chirality in Tumor Suppression" International Journal of Molecular Sciences 26, no. 3: 1308. https://doi.org/10.3390/ijms26031308
APA StylePawlowska, R., Banaszkiewicz, H., Chworos, A., & Żurawiński, R. (2025). Anticancer Activity of Enantiomeric Neplanocins A: Exploring the Role of Chirality in Tumor Suppression. International Journal of Molecular Sciences, 26(3), 1308. https://doi.org/10.3390/ijms26031308








