Selenoproteins: Zoom-In to Their Metal-Binding Properties in Neurodegenerative Diseases
Abstract
1. Introduction
2. Metal-Binding Properties of Selenoproteins
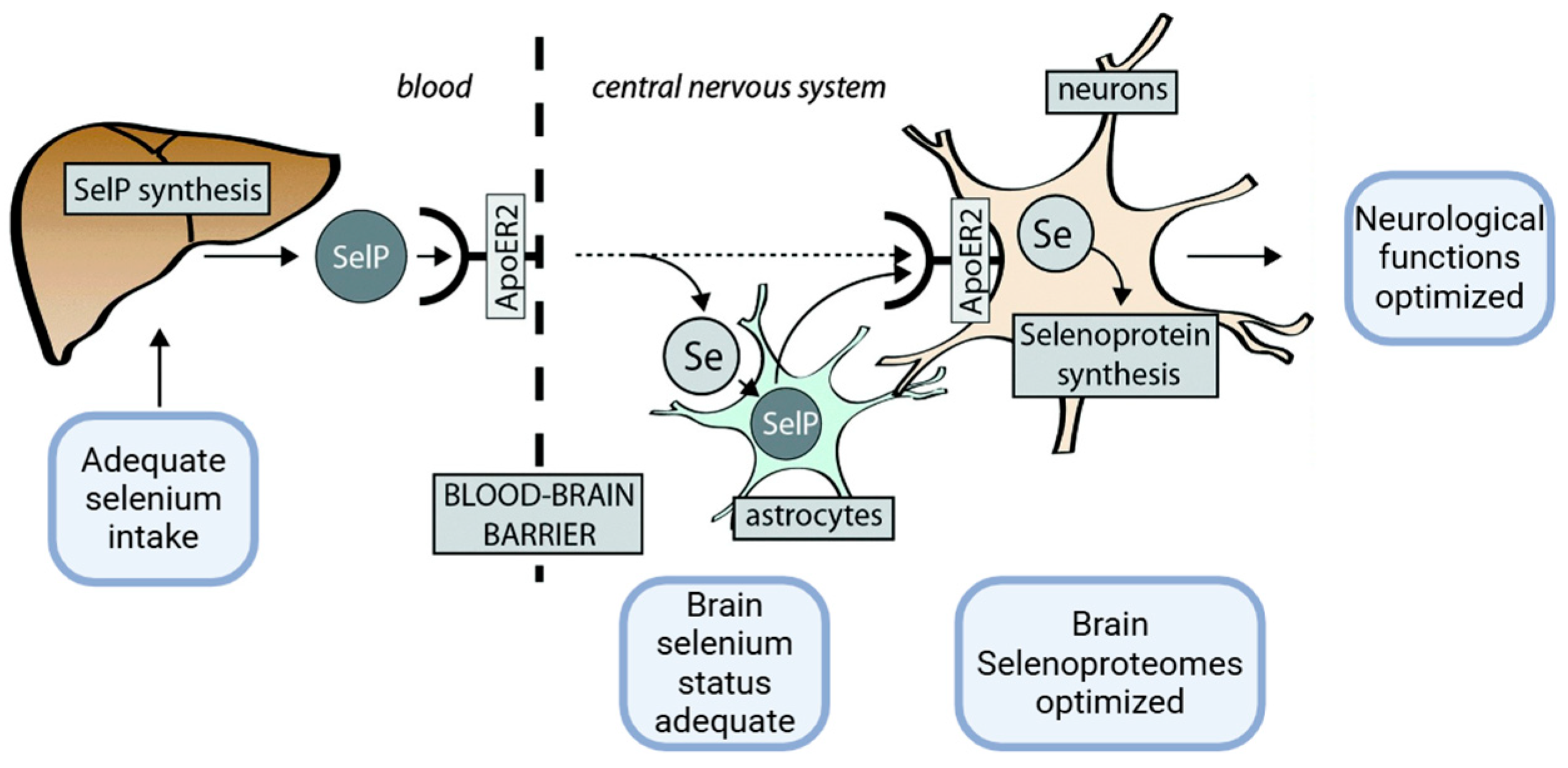
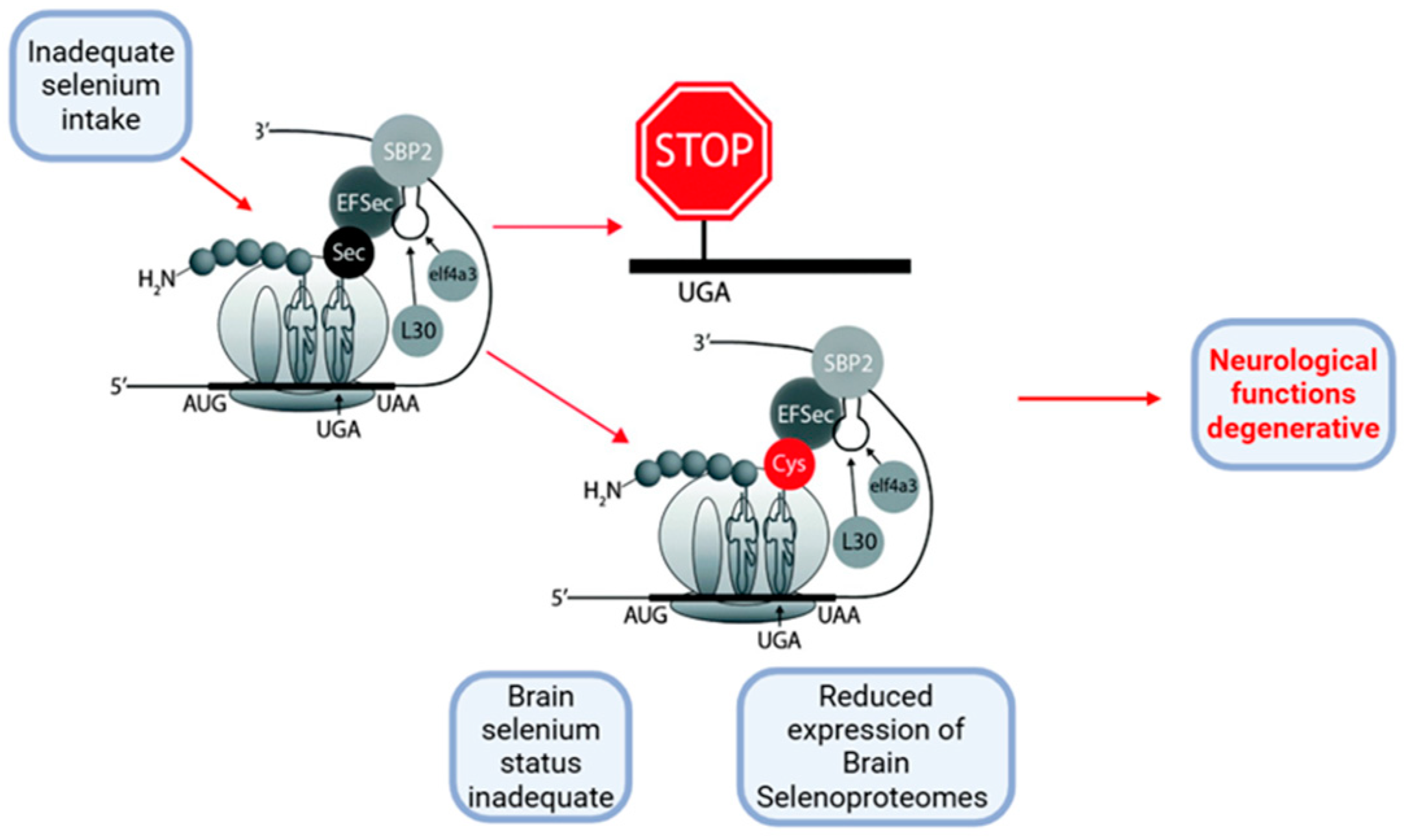
2.1. Metal-Binding Properties of Selenoprotein P (SELENOP)
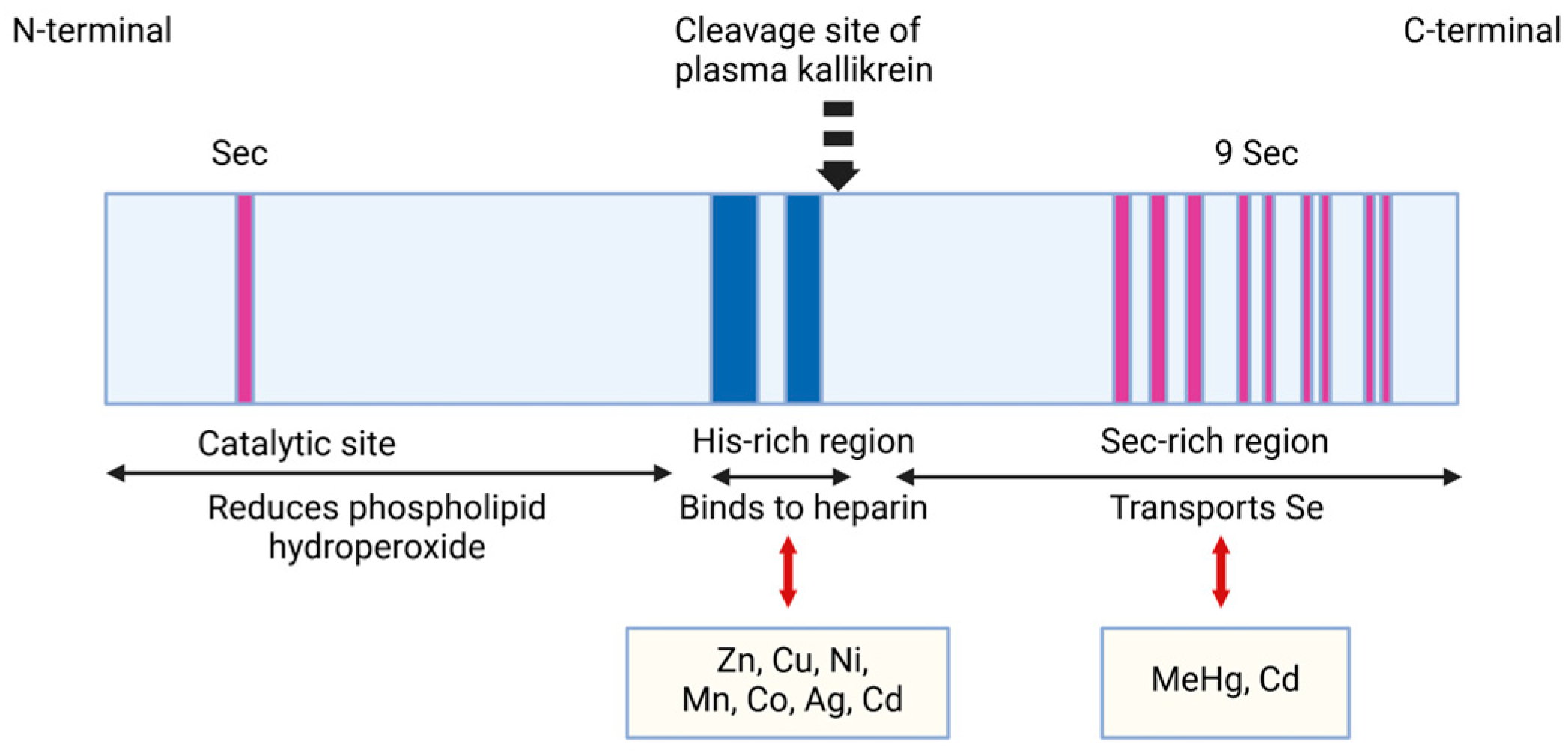
2.1.1. Structural Characteristics of SELENOP
2.1.2. Metal-Binding Properties and Effects of SELENOP in Physiological Versus Pathological Neurodegenerative Conditions
2.1.3. The Properties and Roles of SELENOP in Binding Toxic HEAVY Metals
2.2. Metal-Binding Properties of Selenoprotein W (SELENOW)
2.3. Metal-Binding Properties of Selenoprotein M (SELENOM)
2.4. Metal-Binding Properties of Selenoprotein S (SELENOS)
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Minich, W.B. Selenium Metabolism and Biosynthesis of Selenoproteins in the Human Body. Biochemistry 2022, 87, S168–S177. [Google Scholar] [CrossRef] [PubMed]
- Roman, M.; Jitaru, P.; Barbante, C. Selenium biochemistry and its role for human health. Metallomics 2014, 6, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Maier, K.J.; Knight, A.W. Ecotoxicology of selenium in freshwater systems. Rev. Environ. Contam. Toxicol. 1994, 134, 31–48. [Google Scholar] [PubMed]
- Vinceti, M.; Mandrioli, J.; Borella, P.; Michalke, B.; Tsatsakis, A.; Finkelstein, Y. Selenium neurotoxicity in humans: Bridging laboratory and epidemiologic studies. Toxicol. Lett. 2014, 230, 295–303. [Google Scholar] [CrossRef]
- Solovyev, N.; Drobyshev, E.; Bjørklund, G.; Dubrovskii, Y.; Lysiuk, R.; Rayman, M.P. Selenium, selenoprotein P, and Alzheimer’s disease: Is there a link? Free Radic. Biol. Med. 2018, 127, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Berry, M.J. Selenium and selenoproteins in the brain and brain diseases. J. Neurochem. 2003, 86, 1–12. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Lobanov, A.V.; Hatfield, D.L.; Gladyshev, V.N. Eukaryotic selenoproteins and selenoproteomes. Biochim. Biophys. Acta 2009, 1790, 1424–1428. [Google Scholar] [CrossRef]
- Turanov, A.A.; Everley, R.A.; Hybsier, S.; Renko, K.; Schomburg, L.; Gygi, S.P.; Hatfield, D.L.; Gladyshev, V.N. Regulation of selenocysteine content of human selenoprotein p by dietary selenium and insertion of cysteine in place of selenocysteine. PLoS ONE 2015, 10, e10140353. [Google Scholar] [CrossRef]
- Hill, K.E.; Wu, S.; Motley, A.K.; Stevenson, T.D.; Winfrey, V.P.; Capecchi, M.R.; Atkins, J.F.; Burk, R.F. Production of selenoprotein P (Sepp1) by hepatocytes is central to selenium homeostasis. J. Biol. Chem. 2012, 287, 40414–40424. [Google Scholar] [CrossRef]
- Burk, R.F.; Hill, K.E. Selenoprotein P—Expression, functions, and roles in mammals. Biochim. Biophys. Acta 2009, 1790, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Pillai, R.; Uyehara-Lock, J.H.; Bellinger, F.P. Selenium and selenoprotein function in brain disorders. IUBMB Life 2014, 66, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, U.; Fabiano, M. Selenoproteins in brain development and function. Free Radic. Biol. Med. 2022, 190, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Olson, G.E.; Winfrey, V.P.; NagDas, S.K.; Hill, K.E.; Burk, R.F. Apolipoprotein E Receptor-2 (ApoER2) Mediates Selenium Uptake from Selenoprotein P by the Mouse Testis. J. Biol. Chem. 2007, 282, 12290–12297. [Google Scholar] [CrossRef]
- Abo El-Magd, N.F.; Barbosa, P.O.; Nick, J.; Covalero, V.; Grignetti, G.; Bermano, G. Selenium, as selenite, prevents adipogenesis by modulating selenoproteins gene expression and oxidative stress-related genes. Nutrition 2022, 93, 111424. [Google Scholar] [CrossRef]
- Steinbrenner, H.; Hotze, A.L.; Speckmann, B.; Pinto, A.; Sies, H.; Schott, M.; Ehlers, M.; Scherbaum, W.A.; Schinner, S. Localization and regulation of pancreatic selenoprotein P. J. Mol. Endocrinol. 2013, 50, 31–42. [Google Scholar] [CrossRef]
- Solovyev, N.; Berthele, A.; Michalke, B. Selenium speciation in paired serum and cerebrospinal fluid samples. Anal. Bioanal. Chem. 2013, 405, 1875–1884. [Google Scholar] [CrossRef]
- Saito, Y. Selenium Transport Mechanism via Selenoprotein P—Its Physiological Role and Related Diseases. Front. Nutr. 2021, 8, 685517. [Google Scholar] [CrossRef]
- Saito, Y.; Sato, N.; Hirashima, M.; Takebe, G.; Nagasawa, S.; Takahashi, K. Domain structure of bi-functional selenoprotein P. Biochem. J. 2004, 381 Pt 3, 841–846. [Google Scholar] [CrossRef]
- Hill, K.E.; Zhou, J.; McMahan, W.J.; Motley, A.K.; Atkins, J.F.; Gesteland, R.F.; Burk, R.F. Deletion of Selenoprotein P Alters Distribution of Selenium in the Mouse. J. Biol. Chem. 2003, 278, 13640–13646. [Google Scholar] [CrossRef]
- Cardoso, B.R.; Roberts, B.R.; Bush, A.I.; Hare, D.J. Selenium, selenoproteins and neurodegenerative diseases. Metallomics 2015, 7, 1213–1228. [Google Scholar] [CrossRef]
- Miller, J.A.; Oldham, M.C.; Geschwind, D.H. A systems level analysis of transcriptional changes in Alzheimer’s disease and normal aging. J. Neurosci. 2008, 28, 1410–1420. [Google Scholar] [CrossRef] [PubMed]
- Rueli, R.H.L.H.; Parubrub, A.C.; Dewing, A.S.T.; Hashimoto, A.C.; Bellinger, M.T.; Weeber, E.J.; Uyehara-Lock, J.H.; White, L.R.; Berry, M.J.; Bellinger, F.P. Increased selenoprotein P in choroid plexus and cerebrospinal fluid in Alzheimer’s disease brain. J. Alzheimers Dis. 2015, 44, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.H.; Song, G.L. Roles of Selenoproteins in Brain Function and the Potential Mechanism of Selenium in Alzheimer’s Disease. Front. Neurosci. 2021, 15, 646518. [Google Scholar] [CrossRef] [PubMed]
- Toyama, T.; Kaneko, T.; Arisawa, K.; Saito, Y. Metal-binding properties of selenoprotein P—Its relation to structure and function. Met. Res. 2022, 2, rev18–rev27. [Google Scholar] [CrossRef]
- Dogaru, C.B.; Muscurel, C.; Duță, C.; Stoian, I. “Alphabet” Selenoproteins: Their Characteristics and Physiological Roles. Int. J. Mol. Sci. 2023, 24, 15992. [Google Scholar] [CrossRef]
- Solovyev, N.; Vinceti, M.; Grill, P.; Mandrioli, J.; Michalke, B. Redox speciation of iron, manganese, and copper in cerebrospinal fluid by strong cation exchange chromatography—Sector field inductively coupled plasma mass spectrometry. Anal. Chim. Acta 2017, 973, 25–33. [Google Scholar] [CrossRef]
- Kozlowski, H.; Luczkowski, M.; Remelli, M.; Valensin, D. Copper, zinc and iron in neurodegenerative diseases (Alzheimer’s, Parkinson’s and prion diseases). Coord. Chem. Rev. 2012, 256, 2129–2141. [Google Scholar] [CrossRef]
- Du, X.; Li, H.; Wang, Z.; Qiu, S.; Liu, Q.; Ni, J. Selenoprotein P and selenoprotein M block Zn2+-mediated Aβ42 aggregation and toxicity. Metallomics 2013, 5, 861–870. [Google Scholar] [CrossRef]
- Hondal, R.J.; Ma, S.; Caprioli, R.M.; Hill, K.E.; Burk, R.F. Heparin-binding Histidine and Lysine Residues of Rat Selenoprotein P. J. Biol. Chem. 2001, 276, 15823–15831. [Google Scholar] [CrossRef]
- Sidenius, U.; Farver, O.; Jøns, O.; Gammelgaard, B. Comparison of different transition metal ions for immobilized metal affinity chromatography of selenoprotein P from human plasma. J. Chromatogr. B Biomed. Sci. Appl. 1999, 735, 85–91. [Google Scholar] [CrossRef]
- Outten, C.E.; O’Halloran, T.V. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science 2001, 292, 2488–2492. [Google Scholar] [CrossRef]
- Budimir, A. Metal ions, Alzheimer’s disease and chelation therapy. Acta Pharm. 2011, 61, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Crichton, R.R.; Dexter, D.T.; Ward, R.J. Brain iron metabolism and its perturbation in neurological diseases. J. Neural Transm. 2011, 118, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Duce, J.A.; Bush, A.I. Biological metals and Alzheimer’s disease: Implications for therapeutics and diagnostics. Prog. Neurobiol. 2010, 92, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Santner, A.; Uversky, V.N. Metalloproteomics and metal toxicology of α-synuclein. Metallomics 2010, 2, 378–392. [Google Scholar] [CrossRef] [PubMed]
- Kenche, V.B.; Barnham, K.J. Alzheimer’s disease & metals: Therapeutic opportunities. Br. J. Pharmacol. 2011, 163, 211–219. [Google Scholar] [CrossRef]
- Stankiewicz, J.; Panter, S.S.; Neema, M.; Arora, A.; Batt, C.E.; Bakshi, R. Iron in chronic brain disorders: Imaging and neurotherapeutic implications. Neurotherapeutics 2007, 4, 371–386. [Google Scholar] [CrossRef]
- Friedman, A.; Galazka-Friedman, J.; Koziorowski, D. Iron as a cause of Parkinson disease—A myth or a well established hypothesis? Park. Relat. Disord. 2009, 15 (Suppl. S3), S212–S214. [Google Scholar] [CrossRef]
- Uitti, R.J.; Rajput, A.H.; Rozdilsky, B.; Bickis, M.; Wollin, T.; Yuen, W.K. Regional Metal Concentrations in Parkinson’s Disease, Other Chronic Neurological Diseases, and Control Brains. Can. J. Neurol. Sci./J. Can. Des Sci. Neurol. 1989, 16, 310–314. [Google Scholar] [CrossRef]
- Doroszkiewicz, J.; Farhan, J.A.; Mroczko, J.; Winkel, I.; Perkowski, M.; Mroczko, B. Common and Trace Metals in Alzheimer’s and Parkinson’s Diseases. Int. J. Mol. Sci. 2023, 24, 15721. [Google Scholar] [CrossRef] [PubMed]
- Walter, E.D.; Stevens, D.J.; Spevacek, A.R.; Visconte, M.P.; Dei Rossi, A.; Millhauser, G.L. Copper binding extrinsic to the octarepeat region in the prion protein. Curr. Protein Pept. Sci. 2009, 10, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Jin, Y.; Hall, K.S.; Liang, C.; Unverzagt, F.W.; Ji, R.; Murrell, J.R.; Cao, J.; Shen, J.; Ma, F.; et al. Selenium level and cognitive function in rural elderly Chinese. Am. J. Epidemiol. 2007, 165, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Akbaraly, N.T.; Hininger-Favier, I.; Carrière, I.; Arnaud, J.; Gourlet, V.; Roussel, A.-M.; Berr, C. Plasma Selenium Over Time and Cognitive Decline in the Elderly. Epidemiology 2007, 18, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Maret, W.; Sandstead, H.H. Zinc requirements and the risks and benefits of zinc supplementation. J. Trace Elem. Med. Biol. 2006, 20, 3–18. [Google Scholar] [CrossRef]
- Adlard, P.A.; Bush, A.I. Metals and Alzheimer’s Disease: How Far Have We Come in the Clinic? J. Alzheimers Dis. 2018, 62, 1369–1379. [Google Scholar] [CrossRef]
- Xiong, Y.; Jing, X.P.; Zhou, X.W.; Wang, X.L.; Yang, Y.; Sun, X.Y.; Qiu, M.; Cao, F.Y.; Lu, Y.M.; Liu, R.; et al. Zinc induces protein phosphatase 2A inactivation and tau hyperphosphorylation through Src dependent PP2A (tyrosine 307) phosphorylation. Neurobiol. Aging 2013, 34, 745–756. [Google Scholar] [CrossRef]
- Sun, X.Y.; Wei, Y.P.; Xiong, Y.; Wang, X.C.; Xie, A.J.; Wang, X.L.; Yang, Y.; Wang, Q.; Lu, Y.M.; Liu, R.; et al. Synaptic released zinc promotes tau hyperphosphorylation by inhibition of Protein Phosphatase 2A (PP2A). J. Biol. Chem. 2012, 287, 11174–11182. [Google Scholar] [CrossRef]
- Brion, J.P.; Anderton, B.H.; Authelet, M.; Dayanandan, R.; Leroy, K.; Lovestone, S.; Octave, J.N.; Pradier, L.; Touchet, N.; Tremp, G. Neurofibrillary tangles and tau phosphorylation. Biochem. Soc. Symp. 2001, 67, 81–88. [Google Scholar] [CrossRef]
- Hoffmann, P.R.; Höge, S.C.; Li, P.A.; Hoffmann, F.W.; Hashimoto, A.C.; Berry, M.J. The selenoproteome exhibits widely varying, tissue-specific dependence on selenoprotein P for selenium supply. Nucleic Acids Res. 2007, 35, 3963–3973. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Miura, C.; Kikuchi, K.; Celino, F.T.; Agusa, T.; Tanabe, S.; Miura, T. Zinc is an essential trace element for spermatogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 10859–10864. [Google Scholar] [CrossRef] [PubMed]
- Sensi, S.L.; Paoletti, P.; Koh, J.Y.; Aizenman, E.; Bush, A.I.; Hershfinkel, M. The neurophysiology and pathology of brain zinc. J. Neurosci. 2011, 31, 16076–16085. [Google Scholar] [CrossRef] [PubMed]
- Danscher, G.; Jensen, K.B.; Frederickson, C.J.; Kemp, K.; Andreasen, A.; Juhl, S.; Stoltenberg, M.; David, R. Increased amount of zinc in the hippocampus and amygdala of Alzheimer’s diseased brains: A proton-induced X-ray emission spectroscopic analysis of cryostat sections from autopsy material. J. Neurosci. Methods. 1997, 76, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Frederickson, C.J.; Danscher, G. Zinc-containing neurons in hippocampus and related CNS structures. Prog. Brain Res. 1990, 83, 71–84. [Google Scholar] [CrossRef]
- Slomianka, L.; Danscher, G.; Frederickson, C.J. Labeling of the neurons of origin of zinc-containing pathways by intraperitoneal injections of sodium selenite. Neuroscience 1990, 38, 843–854. [Google Scholar] [CrossRef]
- Tian, Y.; Yang, Z.; Zhang, T. Zinc ion as modulator effects on excitability and synaptic transmission in hippocampal CA1 neurons in Wistar rats. Neurosci. Res. 2010, 68, 167–175. [Google Scholar] [CrossRef]
- Frederickson, C.J.; Koh, J.Y.; Bush, A.I. The neurobiology of zinc in health and disease. Nat. Rev. Neurosci. 2005, 6, 449–462. [Google Scholar] [CrossRef]
- Mandelkow, E.M.; Mandelkow, E. Biochemistry and cell biology of Tau protein in neurofibrillary degeneration. Cold Spring Harb. Perspect. Med. 2012, 2, a006247. [Google Scholar] [CrossRef]
- Small, S.A.; Duff, K. Linking Aβ and Tau in Late-Onset Alzheimer’s Disease: A Dual Pathway Hypothesis. Neuron 2008, 60, 534–542. [Google Scholar] [CrossRef]
- Bancher, C.; Braak, H.; Fischer, P.; Jellinger, K.A. Neuropathological staging of Alzheimer lesions and intellectual status in Alzheimer’s and Parkinson’s disease patients. Neurosci. Lett. 1993, 162, 179–182. [Google Scholar] [CrossRef]
- Arriagada, P.V.; Growdon, J.H.; Hedley-Whyte, E.T.; Hyman, B.T. Neurofibrillary tangles but not senile plaques parallel duration and severiti of azheimer’s disease. Neurology 1992, 42 (3 Pt 1), 631–639. [Google Scholar] [CrossRef]
- Bolognin, S.; Messori, L.; Drago, D.; Gabbiani, C.; Cendron, L.; Zatta, P. Aluminum, copper, iron and zinc differentially alter amyloid-Aβ 1-42 aggregation and toxicity. Int. J. Biochem. Cell Biol. 2011, 43, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Bramblett, G.T.; Goedert, M.; Jakes, R.; Merrick, S.E.; Trojanowski, J.Q.; Lee, V.M.Y. Abnormal tau phosphorylation at Ser396 in alzheimer’s disease recapitulates development and contributes to reduced microtubule binding. Neuron 1993, 10, 1089–1099. [Google Scholar] [CrossRef] [PubMed]
- Dogaru, C.B.; Duță, C.; Muscurel, C.; Stoian, I. “Alphabet” Selenoproteins: Implications in Pathology. Int. J. Mol. Sci. 2023, 24, 15344. [Google Scholar] [CrossRef] [PubMed]
- Sayre, L.M.; Perry, G.; Harris, P.L.; Liu, Y.; Schubert, K.A.; Smith, M.A. In situ oxidative catalysis by neurofibrillary tangles and senile plaques in Alzheimer’s disease: A central role for bound transition metals. J. Neurochem. 2000, 74, 270–279. [Google Scholar] [CrossRef]
- Du, X.; Zheng, Y.; Wang, Z.; Chen, Y.; Zhou, R.; Song, G.; Ni, J.; Liu, Q. Inhibitory act of selenoprotein P on Cu+/Cu2+-induced tau aggregation and neurotoxicity. Inorg. Chem. 2014, 53, 11221–11230. [Google Scholar] [CrossRef]
- Wille, H.; Drewes, G.; Biernat, J.; Mandelkow, E.M.; Mandelkow, E. Alzheimer-like paired helical filaments and antiparallel dimers formed from microtubule-associated protein tau in vitro. J. Cell Biol. 1992, 118, 573–584. [Google Scholar] [CrossRef]
- Berriman, J.; Serpell, C.L.; Oberg, A.K.; Fink, L.A.; Goedert, M.; Crowther, R.A. Tau filaments from human brain and from in vitro assembly of recombinant protein show cross-beta structure. Proc. Natl. Acad. Sci. USA 2003, 100, 9034–9038. [Google Scholar] [CrossRef]
- Okuyama, K.; Nishiura, C.; Mizushima, F.; Minoura, K.; Sumida, M.; Taniguchi, T.; Tomoo, K.; Ishida, T. Linkage-dependent contribution of repeat peptides to self-aggregation of three- or four-repeat microtubule-binding domains in tau protein. FEBS J. 2008, 275, 1529–1539. [Google Scholar] [CrossRef]
- Flach, K.; Hilbrich, I.; Schiffmann, A.; Gärtner, U.; Krüger, M.; Leonhardt, M.; Waschipky, H.; Wick, L.; Arendt, T.; Holzer, M. Tau oligomers impair artificial membrane integrity and cellular viability. J. Biol. Chem. 2012, 287, 43223–43233. [Google Scholar] [CrossRef]
- Du, X.; Wang, Z.; Tian, J.; Qiu, S.; Wang, R.; Wang, C.; Tian, J.; Liu, Q. Direct interaction between selenoprotein P and tubulin. Int. J. Mol. Sci. 2014, 15, 10199–10214. [Google Scholar] [CrossRef] [PubMed]
- Bellinger, F.P.; He, Q.-P.; Bellinger, M.T.; Lin, Y.; Raman, A.V.; White, L.R.; Berry, M.J. Association of Selenoprotein P with Alzheimer’s Pathology in Human Cortex. J. Alzheimers Dis. 2008, 15, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Myhre, O.; Utkilen, H.; Duale, N.; Brunborg, G.; Hofer, T. Metal dyshomeostasis and inflammation in Alzheimer’s and Parkinson’s diseases: Possible impact of environmental exposures. Oxid. Med. Cell. Longev. 2013, 2013, 726954. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, J.L.; Wang, Y.R.; Fa, X.Z. Apigenin attenuates copper-mediated β-amyloid neurotoxicity through antioxidation, mitochondrion protection and MAPK signal inactivation in an AD cell model. Brain Res. 2013, 1492, 33–45. [Google Scholar] [CrossRef]
- Syme, C.D.; Nadal, R.C.; Rigby, S.E.J.; Viles, J.H. Copper Binding to the Amyloid-β (Aβ) Peptide Associated with Alzheimer’s Disease: Folding, coordination geometry, pH dependence, stoichiometry, and affinity of Abeta-(1-28): Insights from a range of complementary spectroscopic techniques. J. Biol. Chem. 2004, 279, 18169–18177. [Google Scholar] [CrossRef]
- Furlan, S.; La Penna, G. Modeling of the Zn2+ binding in the 1-16 region of the amyloid β peptide involved in Alzheimer’s disease. Phys. Chem. Chem. Phys. 2009, 11, 6468–6481. [Google Scholar] [CrossRef]
- Syme, C.D.; Viles, J.H. Solution 1H NMR investigation of Zn2+ and Cd2+ binding to amyloid-beta peptide (Aβ) of Alzheimer’s disease. Biochim. Biophys. Acta 2006, 1764, 246–256. [Google Scholar] [CrossRef]
- Mutter, J.; Curth, A.; Naumann, J.; Deth, R.; Walach, H. Does inorganic mercury play a role in Alzheimer’s disease? A systematic review and an integrated molecular mechanism. J. Alzheimers Dis. 2010, 22, 357–374. [Google Scholar] [CrossRef]
- Cuajungco, M.P.; Lees, G.J. Zinc and Alzheimer’s disease: Is there a direct link? Brain Res. Rev. 1997, 23, 219–236. [Google Scholar] [CrossRef]
- Urbano, T.; Vinceti, M.; Mandrioli, J.; Chiari, A.; Filippini, T.; Bedin, R.; Tondelli, M.; Simonini, C.; Zamboni, G.; Shimizu, M.; et al. Selenoprotein P Concentrations in the Cerebrospinal Fluid and Serum of Individuals Affected by Amyotrophic Lateral Sclerosis, Mild Cognitive Impairment and Alzheimer’s Dementia. Int. J. Mol. Sci. 2022, 23, 9865. [Google Scholar] [CrossRef]
- Kiyohara, A.C.P.; Torres, D.J.; Hagiwara, A.; Pak, J.; Rueli, R.H.L.H.; Shuttleworth, C.W.R.; Bellinger, F.P. Selenoprotein P Regulates Synaptic Zinc and Reduces Tau Phosphorylation. Front. Nutr. 2021, 8, 683154. [Google Scholar] [CrossRef]
- Linert, W.; Kozlowski, H. Metals in the brain. Monatshefte Chem. 2011, 142, 321. [Google Scholar] [CrossRef][Green Version]
- Burk, R.F.; Hill, K.E.; Motley, A.K.; Winfrey, V.P.; Kurokawa, S.; Mitchell, S.L.; Zhang, W. Selenoprotein P and apolipoprotein e receptor-2 interact at the blood-brain barrier and also within the brain to maintain an essential selenium pool that protects against neurodegeneration. FASEB J. 2014, 28, 3579–3588. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Zhao, N.; Caulfield, T.R.; Liu, C.C.; Bu, G. Apolipoprotein E and Alzheimer disease: Pathobiology and targeting strategies. Nat. Rev. Neurol. 2019, 15, 501–518. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Gupta, V.B.; Martins, I.J.; Martins, R.N.; Fowler, C.J.; Bush, A.I.; Finkelstein, D.I.; Adlard, P.A. Zinc affects the proteolytic stability of Apolipoprotein E in an isoform-dependent way. Neurobiol. Dis. 2015, 81, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, B.R.; Hare, D.J.; Bush, A.I.; Roberts, B.R. Glutathione peroxidase 4: A new player in neurodegeneration? Mol. Psychiatry 2017, 22, 328–335. [Google Scholar] [CrossRef]
- Marshall, K.R.; Gong, M.; Wodke, L.; Lamb, J.H.; Jones, D.J.L.; Farmer, P.B.; Scrutton, N.S.; Munro, A.W. The human apoptosis-inducing protein AMID is an oxidoreductase with a modified flavin cofactor and DNA binding activity. J. Biol. Chem. 2005, 280, 30735–30740. [Google Scholar] [CrossRef]
- Duță, C.; Muscurel, C.; Dogaru, C.B.; Stoian, I. Ferroptosis-A Shared Mechanism for Parkinson’s Disease and Type 2 Diabetes. Int. J. Mol. Sci. 2024, 25, 8838. [Google Scholar] [CrossRef]
- Maret, W. Cellular zinc and redox states converge in the metallothionein/thionein pair. J. Nutr. 2003, 133 (Suppl. S1), 1460S–1462S. [Google Scholar] [CrossRef]
- Frederickson, C.J.; Bush, A.I. Synaptically released zinc: Physiological functions and pathological effects. Biometals 2001, 14, 353–366. [Google Scholar] [CrossRef]
- Shu, N.; Zhou, T.; Hovmöller, S. Prediction of zinc-binding sites in proteins from sequence. Bioinformatics 2008, 24, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Zwolak, I. The Role of Selenium in Arsenic and Cadmium Toxicity: An Updated Review of Scientific Literature. Biol. Trace Elem. Res. 2019, 1, 44–63. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.A.; Balal, R.M.; Khan, N.; Zotarelli, L.; Liu, G.D.; Sarkhosh, A.; Fernández-Zapata, J.C.; Nicolás, J.J.M.; Garcia-Sanchez, F. Selenium impedes cadmium and arsenic toxicity in potato by modulating carbohydrate and nitrogen metabolism. Ecotoxicol. Environ. Saf. 2019, 180, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Klaassen, C.D.; Liu, J.; Diwan, B.A. Metallothionein protection of cadmium toxicity. Toxicol. Appl. Pharmacol. 2009, 238, 215–220. [Google Scholar] [CrossRef]
- Sasakura, C.; Suzuki, K.T. Biological interaction between transition metals (Ag, Cd and Hg), selenide/sulfide and selenoprotein P. J. Inorg. Biochem. 1998, 71, 159–162. [Google Scholar] [CrossRef]
- Liu, X.; Hu, Q.; Yang, J.; Huang, S.; Wei, T.; Chen, W.; He, Y.; Wang, D.; Liu, Z.; Wang, K.; et al. Selective cadmium regulation mediated by a cooperative binding mechanism in CadR. Proc. Natl. Acad. Sci. USA 2019, 116, 20398–20403. [Google Scholar] [CrossRef]
- Satish Nair, P.; Robinson, W.E. Histidine-rich glycoprotein in the blood of the bivalve Mytilus edulis: Role in cadmium speciation and cadmium transfer to the kidney. Aquat. Toxicol. 2001, 52, 133–142. [Google Scholar] [CrossRef]
- Xiong, L.; Zhou, B.; Young, J.L.; Wintergerst, K.; Cai, L. Exposure to low-dose cadmium induces testicular ferroptosis. Ecotoxicol. Environ. Saf. 2022, 234, 113373. [Google Scholar] [CrossRef]
- Ramírez-Acosta, S.; Uhlírová, R.; Navarro, F.; Gómez-Ariza, J.L.; García-Barrera, T. Antagonistic Interaction of Selenium and Cadmium in Human Hepatic Cells Through Selenoproteins. Front. Chem. 2022, 10, 891933. [Google Scholar] [CrossRef]
- Achouba, A.; Dumas, P.; Ouellet, N.; Lemire, M.; Ayotte, P. Plasma levels of selenium-containing proteins in Inuit adults from Nunavik. Environ. Int. 2016, 96, 8–15. [Google Scholar] [CrossRef]
- Chen, C.; Yu, H.; Zhao, J.; Li, B.; Qu, L.; Liu, S.; Zhang, P.; Chai, Z. The roles of serum selenium and selenoproteins on mercury toxicity in environmental and occupational exposure. Environ. Health Perspect. 2006, 114, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, W.; Zhao, J.; Lin, X.; Liu, J.; Cui, L.; Gao, Y.; Zhang, T.L.; Li, B.; Li, Y.F. Selenoprotein P as the major transporter for mercury in serum from methylmercury-poisoned rats. J. Trace Elem. Med. Biol. 2018, 50, 589–595. [Google Scholar] [CrossRef]
- Fujimura, M.; Usuki, F.; Unoki, T. Decreased plasma thiol antioxidant capacity precedes neurological signs in a rat methylmercury intoxication model. Food Chem. Toxicol. 2020, 146, 111810. [Google Scholar] [CrossRef] [PubMed]
- Naganuma, A.; Ishii, Y.; Imura, N. Effect of administration sequence of mercuric chloride and sodium selenite on their fates and toxicities in mice. Ecotoxicol. Environ. Saf. 1984, 8, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.T.; Sasakura, C.; Yoneda, S. Binding sites for the (Hg-Se) complex on selenoprotein P. Biochim. Biophys. Acta 1998, 1429, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Hossain, K.F.B.; Banik, S.; Sikder, M.T.; Akter, M.; Bondad, S.E.C.; Rahaman, M.S.; Hosokawa, T.; Saito, T.; Kurasaki, M. Selenium and zinc protections against metal-(loids)-induced toxicity and disease manifestations: A review. Ecotoxicol. Environ. Saf. 2019, 168, 146–163. [Google Scholar] [CrossRef]
- Battin, E.E.; Zimmerman, M.T.; Ramoutar, R.R.; Quarles, C.E.; Brumaghim, J.L. Preventing metal-mediated oxidative DNA damage with selenium compounds. Metallomics 2011, 3, 503–512. [Google Scholar] [CrossRef]
- Zwolak, I.; Zaporowska, H. Selenium interactions and toxicity: A review. Cell Biol. Toxicol. 2012, 28, 31–46. [Google Scholar] [CrossRef]
- Burns, F.J.; Rossman, T.; Vega, K.; Uddin, A.; Vogt, S.; Lai, B.; Reeder, R.J. Mechanism of selenium-induced inhibition of arsenic-enhanced UVR carcinogenesis in mice. Environ. Health Perspect. 2008, 116, 703–708. [Google Scholar] [CrossRef][Green Version]
- Naderi, M.; Puar, P.; Zonouzi-Marand, M.; Chivers, D.P.; Niyogi, S.; Kwong, R.W.M. A comprehensive review on the neuropathophysiology of selenium. Sci. Total Environ. 2021, 767, 144329. [Google Scholar] [CrossRef]
- Yue, C.; Shan, Z.; Tan, Y.; Yao, C.; Liu, Y.; Liu, Q.; Tan, X.; Du, X. His-Rich Domain of Selenoprotein P Ameliorates Neuropathology and Cognitive Deficits by Regulating TrkB Pathway and Zinc Homeostasis in an Alzheimer Model of Mice. ACS Chem. Neurosci. 2020, 11, 4098–4110. [Google Scholar] [CrossRef] [PubMed]
- Solovyev, N. Selenoprotein P and its potential role in Alzheimer’s disease. Hormones 2020, 19, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Vinceti, M.; Urbano, T.; Chiari, A.; Filippini, T.; Wise, L.A.; Tondelli, M.; Michalke, B.; Shimizu, M.; Saito, Y. Selenoprotein P concentrations and risk of progression from mild cognitive impairment to dementia. Sci. Rep. 2023, 13, 8792. [Google Scholar] [CrossRef]
- Sun, Y.; Butler, J.A.; Whanger, P.D. Glutathione peroxidase activity and selenoprotein W levels in different brain regions of selenium-depleted rats. J. Nutr. Biochem. 2001, 12, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.W.; Jeong, D.; Noh, O.J.; Park, Y.H.; Kang, S.I.; Lee, M.G.; Lee, T.H.; Yim, M.B.; Kim, I.Y. Antioxidative Role of Selenoprotein W in Oxidant-Induced Mouse Embryonic Neuronal Cell Death. Mol. Cells. 2009, 27, 609–614. [Google Scholar] [CrossRef]
- Jeong, D.W.; Kim, E.H.; Kim, T.S.; Chung, Y.W.; Kim, H.; Kim, I.Y. Different Distributions of Selenoprotein W and Thioredoxin during Postnatal Brain Development and Embryogenesis. Mol. Cells. 2004, 17, 156–159. [Google Scholar] [CrossRef]
- Raman, A.V.; Pitts, M.W.; Seyedali, A.; Hashimoto, A.C.; Bellinger, F.P.; Berry, M.J. Selenoprotein W expression and regulation in mouse brain and neurons. Brain Behav. 2013, 3, 562–574. [Google Scholar] [CrossRef]
- Chen, H.; Liu, S.; Li, S.; Chen, J.; Ni, J.; Liu, Q. Blocking the Thiol at Cysteine-322 Destabilizes Tau Protein and Prevents Its Oligomer Formation. ACS Chem. Neurosci. 2018, 9, 1560–1565. [Google Scholar] [CrossRef]
- Reeves, M.A.; Hoffmann, P.R. The human selenoproteome: Recent insights into functions and regulation. Cell Mol. Life Sci. 2009, 66, 2457–2478. [Google Scholar] [CrossRef]
- Reeves, M.A.; Bellinger, F.P.; Berry, M.J. The neuroprotective functions of selenoprotein M and its role in cytosolic calcium regulation. Antioxid. Redox Signal. 2010, 12, 809–818. [Google Scholar] [CrossRef]
- Chen, P.; Wang, R.R.; Ma, X.J.; Liu, Q.; Ni, J.Z. Different forms of selenoprotein M differentially affect Aβ aggregation and ROS generation. Int. J. Mol. Sci. 2013, 14, 4385–4399. [Google Scholar] [CrossRef] [PubMed]
- Dikiy, A.; Novoselov, S.V.; Fomenko, D.E.; Sengupta, A.; Carlson, B.A.; Cerny, R.L.; Ginalski, K.; Grishin, N.V.; Hatfield, D.L.; Gladyshev, V.N. SelT, SelW, SelH, and Rdx 12: Genomics and molecular insights into the functions of selenoproteins of a novel thioredoxin-like family. Biochemistry 2007, 46, 6871–6882. [Google Scholar] [CrossRef]
- Bennett, L.E.; Bharadwaj, P.; Waddington, L.; Sudharmarajan, S.; Xu, X.; Szoeke, C.; Ralph, M.; Richard, H. P4-003: Modulation of Amyloid Beta (Aβ) Structure and Toxicity by a Dairy-derived Peptide Product. Alzheimers Dement. 2010, 6, e21. [Google Scholar] [CrossRef]
- Galoyan, A.A.; Sarkissian, J.S.; Chavushyan, V.A.; Meliksetyan, I.B.; Avagyan, Z.E.; Poghosyan, M.V.; Vahradyan, H.G.; Mkrtchian, H.H.; Abrahamyan, D.O. Neuroprotection by hypothalamic peptide proline-rich peptide-1 in Aβ25–35 model of Alzheimer’s disease. Alzheimers Dement. 2008, 4, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Yenkoyan, K.; Safaryan, K.; Chavushyan, V.; Meliksetyan, I.; Navasardyan, G.; Sarkissian, J.; Galoyan, A.; Aghajanov, M. Neuroprotective action of proline-rich polypeptide-1 in β-amyloid induced neurodegeneration in rats. Brain Res. Bull. 2011, 86, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Molina-Holgado, F.; Hider, R.C.; Gaeta, A.; Williams, R.; Francis, P. Metals ions and neurodegeneration. Biometals 2007, 20, 639–654. [Google Scholar] [CrossRef]
- Faller, P.; Hureau, C. Bioinorganic chemistry of copper and zinc ions coordinated to amyloid-β peptide. Dalton Trans. 2009, 7, 1080–1094. [Google Scholar] [CrossRef]
- Park, L.; Anrather, J.; Zhou, P.; Frys, K.; Pitstick, R.; Younkin, S.; Carlson, G.A.; Iadecola, C. NADPH oxidase-derived reactive oxygen species mediate the cerebrovascular dysfunction induced by the amyloid β peptide. J. Neurosci. 2005, 25, 1769–1777. [Google Scholar] [CrossRef]
- Park, L.; Zhou, P.; Pitstick, R.; Capone, C.; Anrather, J.; Norris, E.H.; Younkin, L.; Younkin, S.; Carlson, G.; McEwen, B.S.; et al. Nox2-derived radicals contribute to neurovascular and behavioral dysfunction in mice overexpressing the amyloid precursor protein. Proc. Natl. Acad. Sci. USA 2008, 105, 1347–1352. [Google Scholar] [CrossRef]
- Chaney, M.O.; Stine, W.B.; Kokjohn, T.A.; Kuo, Y.M.; Esh, C.; Rahman, A.; Luehrs, D.C.; Schmidt, A.M.; Stern, D.; Yan, S.D.; et al. RAGE and amyloid beta interactions: Atomic force microscopy and molecular modeling. Biochim. Biophys. Acta 2005, 1741, 199–205. [Google Scholar] [CrossRef]
- Hwang, D.Y.; Cho, J.S.; Oh, J.H.; Shim, S.B.; Jee, S.W.; Lee, S.H.; Seo, S.J.; Lee, S.K.; Lee, S.H.; Kim, Y.K. Differentially Expressed Genes in Transgenic Mice Carrying Human Mutant Presenilin-2 (N141I): Correlation of Selenoprotein M with Alzheimer’s Disease. Neurochem. Res. 2005, 30, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Rueli, R.H.L.H.; Torres, D.J.; Dewing, A.S.T.; Kiyohara, A.C.; Barayuga, S.M.; Bellinger, M.T.; Uyehara-Lock, J.H.; White, L.R.; Moreira, P.I.; Berry, M.J.; et al. Selenoprotein S Reduces Endoplasmic Reticulum Stress-Induced Phosphorylation of Tau: Potential Role in Selenate Mitigation of Tau Pathology. J. Alzheimers Dis. 2017, 55, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.K.; Park, K.J.; Lee, J.H.; Ko, K.Y.; Kang, S.; Kim, I.Y. Selenoprotein S is required for clearance of C99 through endoplasmic reticulum-associated degradation. Biochem. Biophys. Res. Commun. 2017, 486, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Rizek, P.; Kumar, N.; Jog, M.S. An update on the diagnosis and treatment of Parkinson disease. CMAJ 2016, 188, 1157–1165. [Google Scholar] [CrossRef]
- Lev, N.; Melamed, E.; Offen, D. Apoptosis and Parkinson’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2003, 27, 245–250. [Google Scholar] [CrossRef]
- Jomova, K.; Vondrakova, D.; Lawson, M.; Valko, M. Metals, oxidative stress and neurodegenerative disorders. Mol. Cell Biochem. 2010, 345, 91–104. [Google Scholar] [CrossRef]
- Maass, F.; Michalke, B.; Willkommen, D.; Schulte, C.; Tönges, L.; Boerger, M.; Zerr, I.; Bähr, M.; Lingor, P. Selenium speciation analysis in the cerebrospinal fluid of patients with Parkinson’s disease. J. Trace Elem. Med. Biolol. 2020, 57, 126412. [Google Scholar] [CrossRef]
- Salaramoli, S.; Joshaghani, H.; Hashemy, S.I. Selenium Effects on Oxidative Stress-Induced Calcium Signaling Pathways in Parkinson’s Disease. Indian. J. Clin. Biochem. 2022, 37, 257–266. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, K.J.; Jang, J.K.; Jeon, Y.H.; Ko, K.Y.; Kwon, J.H.; Lee, S.R.; Kin, I.Y. Selenoprotein S-dependent Selenoprotein K Binding to p97(VCP) Protein Is Essential for Endoplasmic Reticulum-associated Degradation. J. Biol. Chem. 2015, 290, 29941–29952. [Google Scholar] [CrossRef]
- Salaramoli, S.; Joshaghani, H.R.; Shoeibi, A.; Hashemy, S.I. Selenium and selenoproteins role in Parkinson’s disease: Is there a link between selenoproteins and accumulated alpha-synuclein? J. Trace Elem. Med. Biol. 2024, 81, 127344. [Google Scholar] [CrossRef]
- Xin, Q.; Ji, B.; Cheng, B.; Wang, C.; Liu, H.; Chen, X.; Chen, J.; Bai, B. Endoplasmic reticulum stress in cerebral ischemia. Neurochem. Int. 2014, 68, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Bellinger, F.P.; Raman, A.V.; Rueli, R.H.; Bellinger, M.T.; Dewing, A.S.; Seale, L.A.; Andres, M.A.; Uyehara-Lock, J.H.; White, L.R.; Ross, G.W.; et al. Changes in selenoprotein P in substantia nigra and putamen in Parkinson’s disease. J. Parkinsons Dis. 2012, 2, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Pia Rigobello, M.; Messori, L.; Marcon, G.; Agostina Cinellu, M.; Bragadin, M.; Folda, A.; Scutari, G.; Bindoli, A. Gold complexes inhibit mitochondrial thioredoxin reductase: Consequences on mitochondrial functions. J. Inorg. Biochem. 2004, 98, 1634–1641. [Google Scholar] [CrossRef] [PubMed]
- Prast-Nielsen, S.; Cebula, M.; Pader, I.; Arnér, E.S.J. Noble metal targeting of thioredoxin reductase—covalent complexes with thioredoxin and thioredoxin-related protein of 14 kDa triggered by cisplatin. Free Radic. Biol. Med. 2010, 49, 1765–1778. [Google Scholar] [CrossRef] [PubMed]
- Chaudiere, J.; Tappel, A.L. Interaction of Gold(I) with the Active Site of Selenium-Glutathione Peroxidase. J. Inorg. Biochem. 1984, 20, 313–325. [Google Scholar] [CrossRef]
- Pereira, M.E.; Souza, J.V.; Galiciolli, M.E.A.; Sare, F.; Vieira, G.S.; Kruk, I.L.; Oliveira, C.S. Effects of Selenium Supplementation in Patients with Mild Cognitive Impairment or Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 3205. [Google Scholar] [CrossRef]

| Binding Site | Metal | References | ||
|---|---|---|---|---|
| Selenoprotein P | His-rich domain | Zn, Cu, Ni, Mn, Co, Ag, Cd | [29,31,66,95] | |
| Sec | MeHg, Cd | [99,102] | ||
| Other selenoproteins | TrxR | Sec | Au, Pt, Pd | [143,144] |
| GPx | Sec | Au | [143,145] | |
| Selenium | Hg, Ag, Pb, As, Mn, Cu, Fe | [106,107,109] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duță, C.; Muscurel, C.; Dogaru, C.B.; Stoian, I. Selenoproteins: Zoom-In to Their Metal-Binding Properties in Neurodegenerative Diseases. Int. J. Mol. Sci. 2025, 26, 1305. https://doi.org/10.3390/ijms26031305
Duță C, Muscurel C, Dogaru CB, Stoian I. Selenoproteins: Zoom-In to Their Metal-Binding Properties in Neurodegenerative Diseases. International Journal of Molecular Sciences. 2025; 26(3):1305. https://doi.org/10.3390/ijms26031305
Chicago/Turabian StyleDuță, Carmen, Corina Muscurel, Carmen Beatrice Dogaru, and Irina Stoian. 2025. "Selenoproteins: Zoom-In to Their Metal-Binding Properties in Neurodegenerative Diseases" International Journal of Molecular Sciences 26, no. 3: 1305. https://doi.org/10.3390/ijms26031305
APA StyleDuță, C., Muscurel, C., Dogaru, C. B., & Stoian, I. (2025). Selenoproteins: Zoom-In to Their Metal-Binding Properties in Neurodegenerative Diseases. International Journal of Molecular Sciences, 26(3), 1305. https://doi.org/10.3390/ijms26031305





