Nanotechnology Meets Phytotherapy: A Cutting-Edge Approach to Treat Bacterial Infections
Abstract
1. Introduction
2. Insights
2.1. Berberine (BRB)
2.1.1. In Vitro Assessment of Antimicrobial Activity of Berberine Nanoparticles (BRB-NPs)
2.1.2. In Vivo Assessment of Antimicrobial Activity of Berberine Nanoparticles (BRB-NPs)
2.1.3. Antimicrobial Activity of Nanoparticles Based on Plant Extracts Containing Berberine
| The Antibacterial Mechanisms | Reference |
|---|---|
| adherence/internalisation to bacteria cell walls; membrane damage and ATP leakage; inactivation of metabolic enzymes | [21,22,23,25,29,34,39] |
| inhibition of bacteria adherence and proliferation; inhibition of bacterial biofilm formation, thereby enhancing antimicrobial, anti-biofilm, anti-quorum sensing, and anti-virulence effects | [20,36] |
| ROS generation | [23] |
| regulation of relative homeostasis; inhibition of the key intracellular mechanism | [41] |
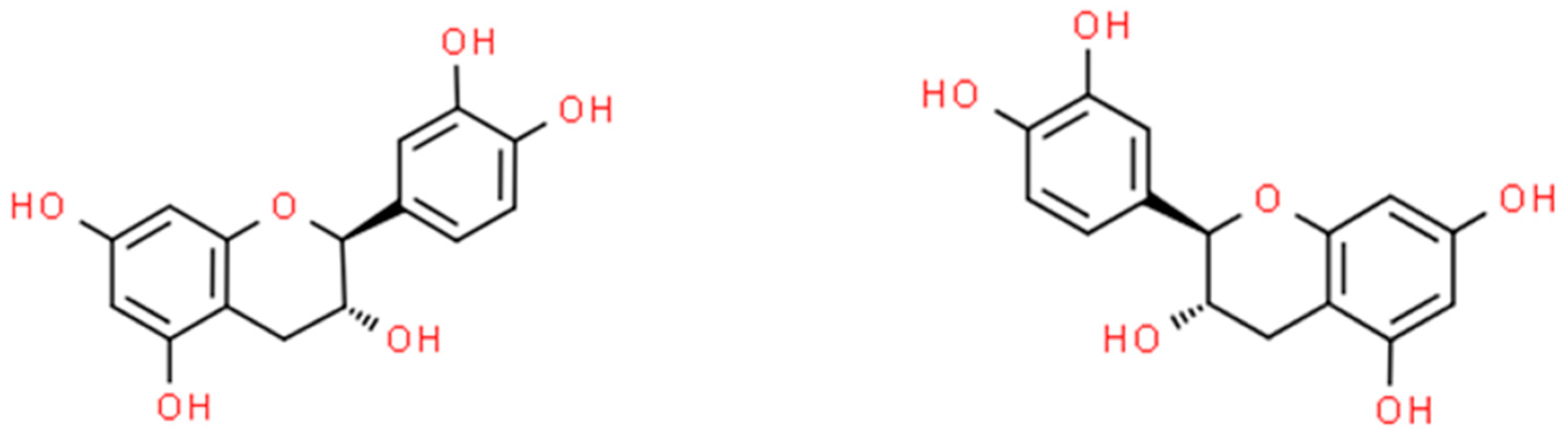
2.2. Catechin (CT)
2.2.1. In Vitro Assessment of Antimicrobial Activity of Catechin Nanoparticles (CT-NPs)
2.2.2. In Vivo Assessment of Antimicrobial Activity of Catechin Nanoparticles (CT-NPs)
2.2.3. Antimicrobial Activity of Nanoparticles Based on Plant Extracts Containing Catechin
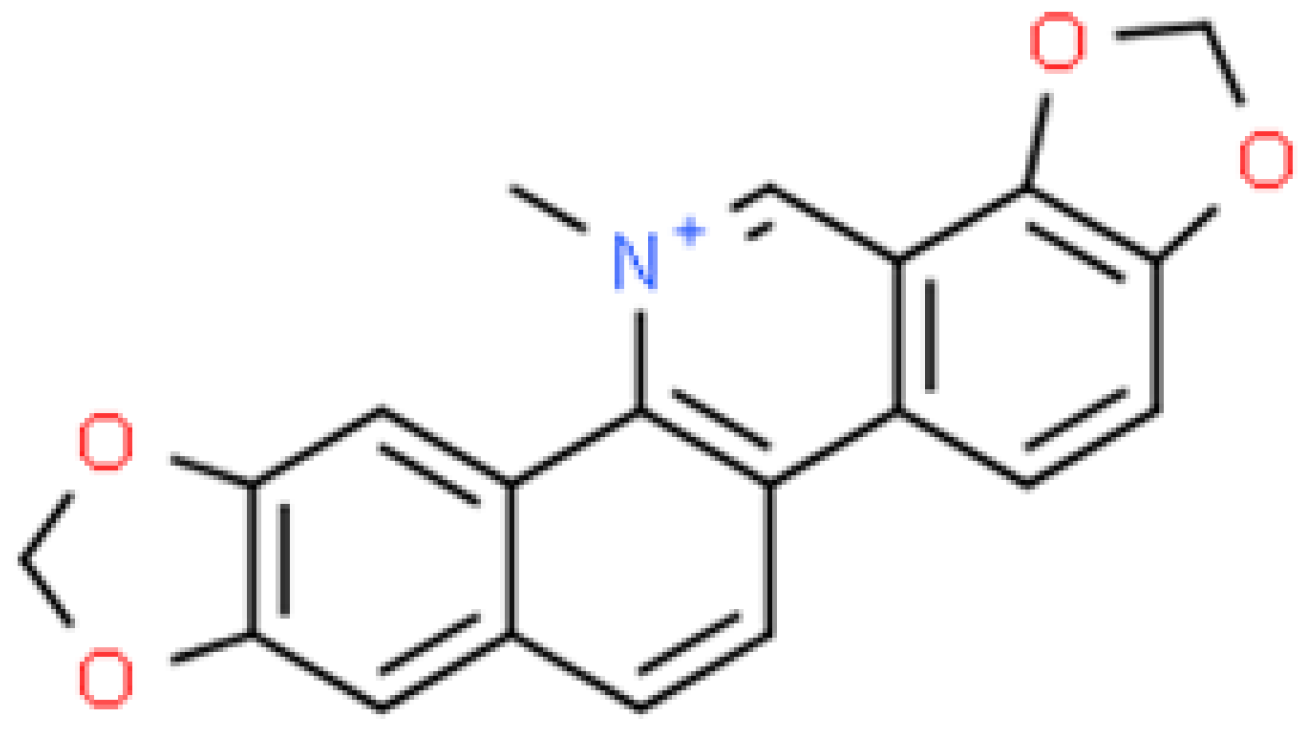
2.3. Chelerythrine (CHE)
2.3.1. In Vitro Assessment of Antimicrobial Activity of Chelerythrine Nanoparticles (CHE-NPs)
2.3.2. In Vivo Assessment of Antimicrobial Activity of Chelerythrine Nanoparticles (CHE-NPs)
2.3.3. Antimicrobial Activity of Nanoparticles Based on Plant Extracts Containing Chelerythrine
2.4. Cinnamaldehyde (CA)
2.4.1. In Vitro Assessment of Antimicrobial Activity of Cinnamaldehyde Nanoparticles (CA-NPs)
2.4.2. In Vivo Assessment of Antimicrobial Activity of Cinnamaldehyde Nanoparticles (CA-NPs)
2.4.3. Antimicrobial Activity of Nanoparticles Based on Plant Extracts Containing Cinnamaldehyde
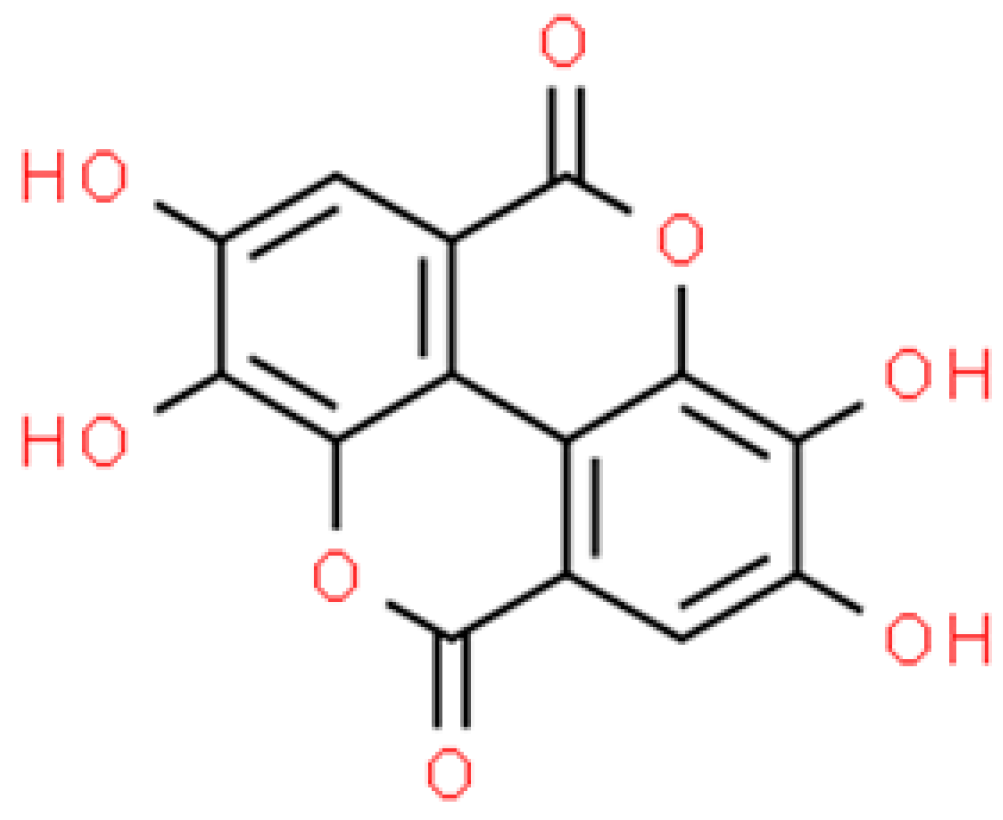
2.5. Ellagic Acid (EA)
2.5.1. In Vitro Assessment of Antimicrobial Activity of Ellagic Acid Nanoparticles (EA-NPs)
2.5.2. In Vivo Assessment of Antimicrobial Activity of Ellagic Acid Nanoparticles (EA-NPs)
2.5.3. Antimicrobial Activity of Nanoparticles Based on Plant Extracts Containing Ellagic Acid
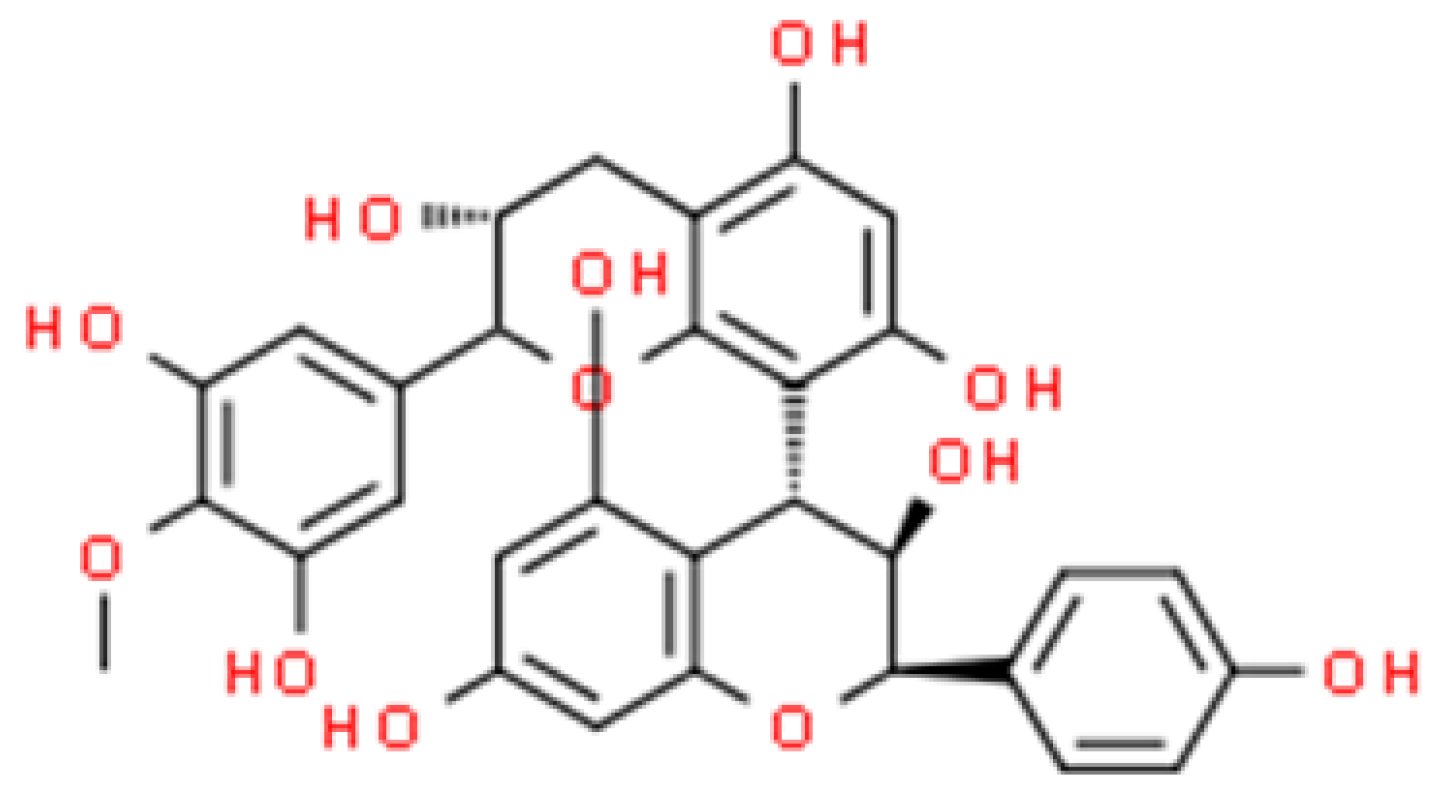
2.6. Proanthocyanidin (PAC)
2.6.1. In Vitro Assessment of Antimicrobial Activity of Proanthocyanidin Nanoparticles (PAC-NPs)
2.6.2. In Vivo Assessment of Antimicrobial Activity of Proanthocyanidin Nanoparticles (PAC-NPs)
2.6.3. Antimicrobial Activity of Nanoparticles Based on Plant Extracts Containing Proanthocyanidin
2.7. Sanguinarine (SG)
2.7.1. In Vitro Assessment of Antimicrobial Activity of Sanguinarine (SG) Nanoparticles (SG-NPs)
2.7.2. In Vivo Assessment of Antimicrobial Activity of Sanguinarine (SG) Nanoparticles (SG-NPs)
2.7.3. Antimicrobial Activity of Nanoparticles Based on Plant Extracts Containing Sanguinarine
2.8. Safety of Using Nanoparticles and Current Regulations in Human and Veterinary Medicine
3. Methodology
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef] [PubMed]
- Muteeb, G.; Rehman, T.; Shahwan, M.; Aatif, M. Origin of antibiotics and antibiotic resistance, and their impacts on drug development: A narrative review. Pharmaceuticals 2023, 16, 1615. [Google Scholar] [CrossRef] [PubMed]
- Salam, A.; Al-amin, Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial resistance: A growing serious threat for global public health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hamid, M.I.; Ibrahim, D.; Elazab, S.T.; Gad, W.M.; Shalaby, M.; El-Neshwy, W.M.; Alshahrani, M.A.; Saif, A.; Algendy, R.M.; AlHarbi, M.; et al. Tackling strong biofilm and multi-virulent vancomycin- resistant Staphylococcus aureus via natural alkaloid-based porous nanoparticles: Perspective towards near future eradication. Front. Cell. Infect. Microbiol. 2024, 13, 1287426. [Google Scholar] [CrossRef] [PubMed]
- Gan, B.H.; Gaynord, J.; Rowe, S.M.; Deingruber, T.; Spring, D.R. The multifaceted nature of antimicrobial peptides: Current synthetic chemistry approaches and future directions. Chem. Soc. Rev. 2021, 50, 7820–7880. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.K.; Chakraborty, S.; Manna, S.; Mandal, S.M. Antimicrobial nanoparticles: Current landscape and future challenges. RSC Pharm. 2024, 1, 388–402. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Ahmed, T.; Wu, W.; Hossain, A.; Hafeez, R.; Masum, M.I.; Wang, Y.; An, Q.; Sun, G.; Li, B. Advancements in plant and microbe-based synthesis of metallic nanoparticles and their antimicrobial activity against plant pathogens. Nanomaterials 2020, 10, 1146. [Google Scholar] [CrossRef] [PubMed]
- Abada, E.; Mashraqi, A.; Modafer, Y.; Al Abboud, M.A.; El-Shabasy, A. Review green synthesis of silver nanoparticles by using plant extracts and their antimicrobial activity. Saudi J. Biol. Sci. 2024, 31, 103877. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.S.; Lins, M.O.; Le, M.; Barros, T.F.; Velozo, E.S. In vitro antibacterial effects of Zanthoxylum tingoassuiba root bark extracts and two of its alkaloids against multiresistant Staphylococcus aureus. Rev. Bras. Farmacogn. 2017, 27, 195–198. [Google Scholar] [CrossRef]
- De Oliveira, E.F.; Yang, X.; Basnayake, N.; Nguyen, C.; Wang, L.; Tikekar, R.; Nitin, N. Screening of antimicrobial synergism between phenolic acids derivatives and UV-A light radiation. J. Photochem. Photobiol. B Biol. 2021, 214, 112081. [Google Scholar] [CrossRef]
- Ivanov, M.; Novovi, K. Polyphenols as inhibitors of antibiotic resistant bacteria—Mechanisms underlying rutin interference with bacterial virulence. Pharmaceuticals 2022, 15, 385. [Google Scholar] [CrossRef]
- Martínez-Ballesta, M.; Gil-Izquierdo, Á.; García-Viguera, C.; Domínguez-Perles, R. Nanoparticles and controlled delivery for bioactive compounds: Outlining challenges for new “Smart-foods” for health. Foods 2018, 7, 72. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, A.; Almotairy, A.R.Z.; Henidi, H.; Alshehri, O.Y.; Aldughaim, M.S. Nanoparticles as drug delivery systems: A review of the implication of nanoparticles’ physicochemical properties on responses in biological systems. Polymers 2023, 15, 1596. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Baek, K.; Perera, C.O. Antibacterial activity and synergistic antibacterial potential of biosynthesized silver nanoparticles against foodborne pathogenic bacteria along with its anticandidal and antioxidant effects. Front. Microbiol. 2017, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomedicine 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed]
- Chehelgerdi, M.; Chehelgerdi, M.; Allela, O.Q.B.; Pecho, R.D.C.; Jayasankar, N.; Rao, D.P.; Thamaraikani, T.; Vasanthan, M.; Viktor, P.; Lakshmaiya, N.; et al. Progressing nanotechnology to improve targeted cancer treatment: Overcoming hurdles in its clinical implementation. Mol. Cancer 2023, 22, 169. [Google Scholar] [CrossRef] [PubMed]
- Foulkes, R.; Man, E.; Thind, J.; Yeung, S.; Joy, A.; Hoskins, C. The regulation of nanomaterials and nanomedicines for clinical application: Current and future perspectives. Biomater. Sci. 2020, 8, 4653–4664. [Google Scholar] [CrossRef]
- Pacyga, K.; Pacyga, P.; Topola, E.; Viscardi, S. Bioactive compounds from plant origin as natural antimicrobial agents for the treatment of wound infections. Int. J. Mol. Sci. 2024, 25, 2100. [Google Scholar] [CrossRef]
- Yu, S.-H.; Wu, S.-J.; Wu, J.-Y.; Wen, D.-Y.; Mi, F.-L. Preparation of fucoidan-shelled and genipin-crosslinked chitosan beads for antibacterial application. Carbohydr. Polym. 2015, 126, 97–107. [Google Scholar] [CrossRef]
- Li, T.; Wang, P.; Guo, W.; Huang, X.; Tian, X.; Wu, G.; Xu, B.; Li, F.; Yan, C.; Liang, X.; et al. Natural berberine-based Chinese herb medicine assembled nanostructures with modified antibacterial application. ACS Nano 2019, 13, 6770–6781. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Wu, L.; Zhou, C.; Sun, H.; Gao, P.; Xu, X.; Zhang, C.; Liang, J.; Fan, Y.; Sun, J.; et al. Berberine/Ag nanoparticle embedded biomimetic calcium phosphate scaffolds for enhancing antibacterial function. Nanotechnol. Rev. 2020, 9, 568–579. [Google Scholar] [CrossRef]
- Dash, S.; Kumar, M.; Pareek, N. Enhanced antibacterial potential of berberine via synergism with chitosan nanoparticles. Mater. Today Proc. 2020, 31, 640–645. [Google Scholar] [CrossRef]
- Li, F.; Zhe, T.; Ma, K.; Li, R.; Li, M.; Liu, Y.; Yuanyuan, C.; Li, W. A naturally derived nanocomposite film with photodynamic antibacterial activity: New prospect for sustainable food packaging. ACS Appl. Mater. Interfaces 2021, 13, 52998–53008. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Pham, T.N.; Le, A.; Thuy, N.T. Antibacterial activity of a berberine nanoformulation. Beilstein J. Nanotechnol. 2022, 13, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Younis, F.A.; Saleh, S.R.; Abd, S.S.; Rahman, E.; Newairy, A.S.A.; El Demellawy, M.A.; Ghareeb, D.A. Preparation, physicochemical characterization, and bioactivity evaluation of berberine—Entrapped albumin nanoparticles. Sci. Rep. 2022, 12, 17431. [Google Scholar] [CrossRef]
- Ma, K.; Zhe, T.; Li, F.; Zhang, Y.; Yu, M.; Li, R.; Wang, L. Sustainable films containing AIE-active berberine-based nanoparticles: A promising antibacterial food packaging. Food Hydrocoll. 2022, 123, 107147. [Google Scholar] [CrossRef]
- Alharthi, S.; Ziora, Z.M.; Janjua, T.; Popat, A.; Moyle, P.M. Formulation and biological evaluation of mesoporous silica nanoparticles loaded with combinations of sortase A inhibitors and antimicrobial peptides. Pharmaceutics 2022, 14, 986. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Chen, H.; Wu, C.; Wang, J.; Cui, M.; Ye, H.; Feng, Y.; Li, Y.; Dong, Z. Fabrication of co-assembly from berberine and tannic acid for multidrug-resistant bacteria infection treatment. Pharmaceutics 2023, 15, 1782. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Cui, M.; Chen, H.; Wang, J.; Ye, H.; Zhang, Q. Co-assembled nanocomplexes comprising epigallocatechin gallate and berberine for enhanced antibacterial activity against multidrug resistant Staphylococcus aureus. Biomed. Pharmacother. 2023, 163, 114856. [Google Scholar] [CrossRef] [PubMed]
- Alharthi, S.; Popat, A.; Ziora, Z.M.; Moyle, P.M. Sortase A inhibitor protein nanoparticle formulations demonstrate antibacterial synergy when combined with antimicrobial peptides. Molecules 2023, 28, 2114. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Yi, X.; Li, Y.; Li, Y.; Qu, X.; Miao, P. Berberine and chlorogenic acid-assembled nanoparticles for highly efficient inhibition of multidrug-resistant Staphylococcus aureus. J. Hazard. Mater. 2024, 473, 134680. [Google Scholar] [CrossRef]
- Andima, M.; Boese, A.; Paul, P.; Koch, M.; Loretz, B.; Lehr, C. Targeting intracellular bacteria with dual drug-loaded lactoferrin nanoparticles. ASC Infect. Dis. 2024, 10, 1696–1710. [Google Scholar] [CrossRef]
- Sadeghi, S.; Agharazi, F.; Hosseinzadeh, S.A.; Mashayekhi, M.; Shafiei, M.; Ebrahimi-rad, M.; Sadeghi, M. Gold nanoparticle conjugation enhances berberine’s antibacterial activity against methicillin-resistant Staphylococcus aureus (MRSA). Talanta 2024, 268, 125358. [Google Scholar] [CrossRef]
- Zuo, F.; Wang, B.; Wang, L.; He, J.; Qiu, X. UV-triggered drug release from mesoporous titanium nanoparticles loaded with berberine hydrochloride: Enhanced antibacterial activity. Molecules 2024, 29, 1607. [Google Scholar] [CrossRef] [PubMed]
- Lenzuni, M.; Fiorentini, F.; Summa, M.; Bertorelli, R.; Suarato, G.; Perotto, G.; Athanassiou, A. Electrosprayed zein nanoparticles as antibacterial and anti-thrombotic coatings for ureteral stents. Int. J. Biol. Macromol. 2024, 257, 128560. [Google Scholar] [CrossRef]
- Zhang, J.; Li, W.; Tao, Z.; Zhou, X.; Chen, X.; Zhou, J.; Sun, H.; Fang, Y.; Liu, Y. Endogenous glucose-driven cascade reaction of nano-drug delivery for boosting multidrug-resistant bacteria-infected diabetic wound healing. J. Colloid Interface Sci. 2024, 672, 63–74. [Google Scholar] [CrossRef]
- Fu, S.; Hu, Y.; Yang, Y.; Yi, X.; Miao, J.; Miao, P. Graphene oxide-supported berberine and aloe-emodin nanocomposites for dual- drug release and antimicrobial therapy. Fundam. Res. 2024. [Google Scholar] [CrossRef]
- Marques, C.; Grenho, L.; Fernandes, M.H.; Lima, S.A.C. Improving the antimicrobial potency of berberine for endodontic canal irrigation using polymeric nanoparticles. Pharmaceutics 2024, 16, 786. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Ao, M.; Zheng, X.; Li, N.; Xia, J.; Li, Y.; Li, D.; Hou, Z.; Qi, Z.; Chen, X.D. PEG-lipid-PLGA hybrid nanoparticles loaded with berberine-phospholipid complex to facilitate the oral delivery efficiency. Drug Deliv. 2017, 24, 825–833. [Google Scholar] [CrossRef]
- Li, H.; Yang, W.; Wu, X.; Tian, L.; Zhang, W.; Tian, H. Cationic fructan-based pH and intestinal flora dual stimulation nanoparticle with berberine for targeted therapy of IBD. Int. J. Biol. Macromol. 2024, 256, 127987. [Google Scholar] [CrossRef]
- Tahan, M.; Zeraatkar, S.; Neshani, A.; Marouzi, P.; Alavi, S.J.; Hamed, S.; Shahri, H.; Hosseini, M. Antibacterial potential of biosynthesized silver nanoparticles using berberine extract against multidrug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa. Indian J. Microbiol. 2023, 64, 125–132. [Google Scholar] [CrossRef]
- Li, H.; Chen, Q.; Zhao, J.; Urmila, K. Enhancing the antimicrobial activity of natural extraction using the synthetic ultrasmall metal nanoparticles. Sci. Rep. 2015, 5, 11033. [Google Scholar] [CrossRef]
- Zhang, H.; Jung, J.; Zhao, Y. Preparation, characterization and evaluation of antibacterial activity of catechins and catechins-Zn complex loaded β-chitosan nanoparticles of different particle sizes. Carbohydr. Polym. 2016, 137, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Jin, H.; Xiao, J.; Yin, X.; Liu, X.; Li, D.; Huang, Q. The simultaneous loading of catechin and quercetin on chitosan-based nanoparticles as effective antioxidant and antibacterial agent. Food Res. Int. 2018, 111, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Oliver, S.; Wagh, H.; Liang, Y.; Yang, S.; Boyer, C. Enhancing the antimicrobial and antibiofilm effectiveness of silver nanoparticles prepared by green synthesis. J. Mater. Chem. B 2018, 6, 4124–4138. [Google Scholar] [CrossRef]
- Liu, L.; Xiao, X.; Li, K.; Li, X.; Yu, K.; Liao, X.; Shi, B. Prevention of bacterial colonization based on self-assembled metal-phenolic nanocoating from rare-earth ions and catechin. ACS Appl. Mater. Interfaces 2020, 12, 22237–22245. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Deng, S.; Li, J.; Sun, C.; Fu, Y.; Liu, Z. Green synthesis, characterization and antibacterial study on the catechin- functionalized ZnO nanoclusters. Mater. Res. Express 2021, 8, 025006. [Google Scholar] [CrossRef]
- Unnikrishnan, B.; Suryani, I.; Tseng, Y.; Chang, H. Controlling morphology evolution of titanium oxide-gold nanourchin for photocatalytic degradation of dyes and photoinactivation of bacteria in the infected wound. J. Colloid Interface Sci. 2021, 598, 260–273. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, N.; Ermenlieva, N.; Simeonova, L.; Kolev, I.; Slavov, I.; Karashanova, D.; Andonova, V. Chlorhexidine-silver nanoparticle conjugation leading to antimicrobial synergism but enhanced cytotoxicity. Pharmaceutics 2023, 15, 2298. [Google Scholar] [CrossRef]
- Hsieh, W.; Fang, C.; Suhail, M.; Lam, Q.; Chuang, C.; Wu, P. Improved skin permeability and whitening effect of catechin-loaded transfersomes through topical delivery. Int. J. Pharm. 2021, 607, 121030. [Google Scholar] [CrossRef]
- Mita, S.R.; Husni, P.; Putriana, N.A.; Maharani, R.; Hendrawan, R.P.; Dewi, D.A. A recent update on the potential use of catechins in cosmeceuticals. Cosmetics 2024, 11, 23. [Google Scholar] [CrossRef]
- Devatha, C.P.; Jagadeesh, K.; Patil, M. Effect of green synthesized iron nanoparticles by Azardirachta indica in different proportions on antibacterial activity. Environ. Nanotechnol. Monit. Manag. 2018, 9, 85–94. [Google Scholar] [CrossRef]
- Rolim, W.R.; Pelegrino, M.T.; De Araújo, B.; Ferraz, L.S. Green tea extract mediated biogenic synthesis of silver nanoparticles: Characterization, cytotoxicity evaluation and antibacterial activity. Appl. Surf. Sci. 2019, 463, 66–74. [Google Scholar] [CrossRef]
- Hashemi, Z.; Ebrahimzadeh, M.A.; Biparva, P.; Mortazavi-Derazkola, S.; Goli, H.R.; Sadeghian, F.; Kardan, M.; Rafiei, A. Biogenic silver and zero-valent iron nanoparticles by Feijoa: Biosynthesis, characterization, cytotoxic, antibacterial and antioxidant activities. Anticancer. Agents Med. Chem. 2020, 20, 1673–1687. [Google Scholar] [CrossRef]
- Das, S.; Langbang, L.; Haque, M.; Kumar, V.B.; Aguan, K.; Roy, A.S. Biocompatible silver nanoparticles: An investigation into their protein binding efficacies, anti-bacterial effects and cell cytotoxicity studies. J. Pharm. Anal. 2021, 11, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Isiksel, E.; Attar, A.; Mutlu, O.; Yapaoz, A.M. Bioinspired fabrication of CuONPs synthesized via cotoneaster and application in dye removal: Antioxidant and antibacterial studies. Environ. Sci. Pollut. Res. 2023, 30, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Alowaiesh, B.F.; Alhaithloul, H.A.S.; Saad, A.M.; Hassanin, A.A. Green biogenic of silver nanoparticles using polyphenolic extract of olive leaf wastes with focus on their anticancer and antimicrobial activities. Plants 2023, 12, 1410. [Google Scholar] [CrossRef] [PubMed]
- Roberto, S.; Avila, R.; Pereira, G.; Schuenck, D.; Pinheiro, L.; Keijok, W.J.; Xavier, L.M.; Endringer, D.C.; Oliveira, J.P.; Schuenck, R.P.; et al. High antibacterial in vitro performance of gold nanoparticles synthesized by epigallocatechin 3-gallate. J. Mater. Res. 2021, 36, 518–532. [Google Scholar]
- Wang, C.; Xiao, R.; Yang, Q.; Pan, J.; Cui, P.; Zhou, S.; Qiu, L.; Zhang, Y.; Wang, J. Green synthesis of epigallocatechin gallate-ferric complex nanoparticles for photothermal enhanced antibacterial and wound healing. Biomed. Pharmacother. 2024, 171, 116175. [Google Scholar] [CrossRef]
- Wei, X.; Guo, J.; Geng, X.; Xue, B.; Huang, S.; Yuan, Z. The combination of membrane disruption and FtsZ targeting by a chemotherapeutic hydrogel synergistically combats pathogens infections. Adv. Healthc. Mater. 2024, 13, e2304600. [Google Scholar] [CrossRef]
- Barbinta-patrascu, M.E.; Badea, N.; Ungureanu, C.; Constantin, M.; Pirvu, C.; Rau, I. Silver-based biohybrids “green” synthesized from Chelidonium majus L. Opt. Mater. 2016, 56, 94–99. [Google Scholar] [CrossRef]
- Dobrucka, R.; Dlugaszewska, J.; Kaczmarek, M. Cytotoxic and antimicrobial effects of biosynthesized ZnO nanoparticles using of Chelidonium majus extract. Biomed. Microdevices 2018, 20, 5. [Google Scholar] [CrossRef]
- Chan, A.C.; Cadena, M.B.; Townley, H.E.; Fricker, M.D.; Thompson, I.P.; Hospital, J.R.; Ox, O.; Thompson, I.P. Effective delivery of volatile biocides employing mesoporous silicates for treating biofilms. J. R. Soc. Interface 2017, 14, 20160650. [Google Scholar] [CrossRef]
- Mohankandhasamy, A.R.; Lee, J.; Lee, J. Direct one-pot synthesis of cinnamaldehyde immobilized on gold nanoparticles and their antibiofilm properties. Colloids Surfaces B Biointerfaces 2017, 160, 639–648. [Google Scholar]
- Ramasamy, M.; Lee, J.-H.; Lee, J. Development of gold nanoparticles coated with silica containing the antibiofilm drug cinnamaldehyde and their effects on pathogenic bacteria. Int. J. Nanomedicine 2017, 12, 2813–2828. [Google Scholar] [CrossRef] [PubMed]
- Salim, A.A.; Bakhtiar, H.; Bidin, N.; Ghoshal, S.K. Antibacterial activity of decahedral cinnamon nanoparticles prepared in honey using PLAL technique. Mater. Lett. 2018, 232, 183–186. [Google Scholar] [CrossRef]
- Subhaswaraj, P.; Barik, S.; Macha, C.; Chiranjeevi, P.V.; Siddhardha, B. Anti quorum sensing and anti biofilm efficacy of cinnamaldehyde encapsulated chitosan nanoparticles against Pseudomonas aeruginosa PAO1. LWT—Food Sci. Technol. 2018, 97, 752–759. [Google Scholar] [CrossRef]
- Gadkari, R.R.; Suwalka, S.; Yogi, M.R.; Ali, W.; Das, A.; Alagirusamy, R. Green synthesis of chitosan-cinnamaldehyde cross-linked nanoparticles: Characterization and antibacterial activity. Carbohydr. Polym. 2019, 226, 115298. [Google Scholar] [CrossRef]
- Pola, C.C.; Moraes, A.R.F.; Medeiros, E.A.A.; Teófilo, R.F.; Soares, N.F.F.; Gomes, C.L. Development and optimization of pH-responsive PLGA-chitosan nanoparticles for triggered release of antimicrobials. Food Chem. 2019, 295, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Gadkari, R.R.; Gupta, A.; Teke, U.; Awadhiya, A.; Shahadat, M.; Ali, W.; Das, A.; Alagirusamy, R. A sustainable way for surface functionalisation of PET nonwoven with novel chitosan-cinnamaldehyde cross-linked nanoparticles. J. Ind. Eng. Chem. 2021, 99, 214–223. [Google Scholar] [CrossRef]
- Chotchindakun, K.; Pekkoh, J.; Ruangsuriya, J.; Zheng, K.; Unalan, I.; Boccaccini, A.R. Fabrication and characterization of cinnamaldehyde-loaded microspheres for preventing bacterial infection and promoting bone tissue regeneration. Polymers 2021, 13, 1794. [Google Scholar]
- Xu, J.; Lin, Q.; Sheng, M.; Ding, T.; Li, B.; Gao, Y.; Tan, Y. Antibiofilm effect of cinnamaldehyde-chitosan nanoparticles against the biofilm of Staphylococcus aureus. Antibiotics 2022, 11, 1403. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Sun, X.; Dong, M.; Zhang, H.; Wang, J.; Bu, T.; Zhao, S.; Wang, L. NIR-regulated dual-functional silica nanoplatform for infected-wound therapy via synergistic sterilization and anti-oxidation. Colloids Surf. B Biointerfaces 2022, 213, 112414. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Ghaderi, J.; Gómez-Guillén, M.C. Tailoring physico-mechanical and antimicrobial/antioxidant properties of biopolymeric films by cinnamaldehyde-loaded chitosan nanoparticles and their application in packaging of fresh rainbow trout fillets. Food Hydrocoll. 2024, 124, 107249. [Google Scholar] [CrossRef]
- Maroufi, L.Y.; Ghorbani, M. Development of a novel antibacterial hydrogel scaffold based on guar gum/poly(methylvinylether-alt-maleic acid containing cinnamaldehyde-loaded chitosan nanoparticles. J. Polym. Environ. 2022, 30, 431–442. [Google Scholar] [CrossRef]
- Batista, A.F.P.; Rosa, L.C.M.; Pizzo, J.S.; da Silva, A.F.; Visentainer, J.V.; de Abreu Filho, B.A.; Kobayashi, R.K.T.; Nakazato, G.; Mikcha, J.M.G. Biogenic silver nanoparticles and cinnamaldehyde as an effective sanitizer for fresh sweet grape tomatoes. J. Food Sci. Technol. 2023, 60, 2477–2485. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, Y.; Piao, Y.; Karimi, M.; Wang, Y.; Wen, F.; Li, H.; Shi, L.; Liu, Y. Two-tailed dynamic covalent amphiphile combats bacterial biofilms. Adv. Mater. 2023, 35, 2301623. [Google Scholar] [CrossRef] [PubMed]
- Morela-Aucejo, A.; Medaglia, S.; Ruiz-Rico, M.; Martínez-Manez, R.; Marcos, M.D.; Bernardos, A. Remarkable enhancement of cinnamaldehyde antimicrobial activity encapsulated in capped mesoporous nanoparticles: A new “nanokiller” approach in the era of antimicrobial resistance. Biomater. Adv. 2024, 160, 213840. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Shan, P.; Lu, Y.; Lian, X.; He, F.; Xiao, J.; Qiu, L.; Liu, Z.; Gan, Z.; Yan, Q.; et al. Heparin mimicking sulfonated polyester synergistically promotes bacterial infected-wound healing with ROS responsive cinnamaldehyde based prodrugs. Chem. Eng. J. 2024, 495, 153513. [Google Scholar] [CrossRef]
- Marcondes, P.; Rosas, G.H.; Elena, M.; González, L.; Antonio, A.; De Queiroz, A.; Marques, P.S. Poly(vinyl alcohol)/poly(glycerol) dendrimer hydrogel mediated green synthesis of silver nanoparticles. Polimeros 2022, 32, e2022034. [Google Scholar] [CrossRef]
- Jose, A.; Mathew, M.; Mathew, A.S.; Aswani, R.; Vimal, J.; Premnath, M.; Parambath, B.; Reshmy, R.; Radhakrishnan, E.K. Cinnamon essential oil induced microbial stress metabolome indicates its active food packaging efficiency when incorporated into poly vinyl alcohol, engineered with zinc oxide nanoparticles and nanocellulose. Int. J. Biol. Macromol. 2024, 278, 134115. [Google Scholar] [CrossRef] [PubMed]
- Alvear, A.G.; Pineda-aguilar, N.; Lozano, P.; Suppan, G.; Galeas, S.; Debut, A.; Vizuete, K.; De Lima, L.; Pablo, J.; Alexis, F.; et al. Synergistic antibacterial properties of silver nanoparticles and its reducing agent from cinnamon bark extract. Bioengineering 2024, 11, 517. [Google Scholar] [CrossRef]
- Tavares, W.D.S.; Barreto, G.A.V.; Pinto, E.P.; Silva, P.G.D.B.; Sousa, F.F.O.D. Influence of gelatin on the functional characteristics and wound healing potential of chitosan/zein films loaded with ellagic acid nanoparticles. J. Drug Deliv. Sci. Technol. 2023, 88, 104942. [Google Scholar] [CrossRef]
- El-Sonbaty, S.M.; Moawed, F.S.M.; Kandil, E.I.; Tamamm, A.M. Antitumor and antibacterial efficacy of gallium nanoparticles coated by ellagic acid. Dose-Response 2022, 20, 15593258211068998. [Google Scholar] [CrossRef]
- Allemailem, K.S. Enhanced activity of ellagic acid in lipid nanoparticles (EA-liposomes) against Acinetobacter baumannii in immunosuppressed mice. Saudi J. Biol. Sci. 2023, 30, 103707. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, F.; Li, Y.; Wang, S.; Ren, Y.; Shi, L.; van der Mei, H.C.; Liu, Y. Ellagic acid-modified gold nanoparticles to combat multi-drug resistant bacterial infections in vitro and in vivo. Mater. Horizons 2024, 11, 4781–4790. [Google Scholar] [CrossRef]
- Norouzalinia, F.; Asadpour, L.; Mokhtary, M. Anti-microbial, anti-biofilm, and efflux pump inhibitory effects of ellagic acid-bonded magnetic nanoparticles against Escherichia coli isolates. Int. Microbiol. 2024, 39105888. [Google Scholar] [CrossRef]
- Appapalam, S.T.; Panchamoorthy, R. Aerva lanata mediated phytofabrication of silver nanoparticles and evaluation of their antibacterial activity against wound associated bacteria. J. Taiwan Inst. Chem. Eng. 2017, 78, 539–551. [Google Scholar] [CrossRef]
- Fernandes, R.A.; Berretta, A.A.; Torres, E.C.; Buszinski, A.F.M.; Fernandes, G.L.; Mendes-Gouvêa, C.C.; Souza-Neto, F.N.; Gorup, L.F.; Camargo, E.R.; Barbosa, D.B. Antimicrobial potential and cytotoxicity of silver nanoparticles phytosynthesized by pomegranate peel extract. Antibiotics 2018, 7, 51. [Google Scholar] [CrossRef]
- Swilam, N.; Nematallah, K.A. Polyphenols profile of pomegranate leaves and their role in green synthesis of silver nanoparticles. Sci. Rep. 2021, 10, 14851. [Google Scholar] [CrossRef]
- Muthusamy, N.; Kanniah, P.; Vijayakumar, P.; Murugan, U.; Raj, D.S.; Sankaran, U. Green-inspired fabrication of silver nanoparticles and examine its potential in-vitro cytotoxic and antibacterial activities. J. Inorg. Organomet. Polym. Mater. 2021, 31, 4693–4709. [Google Scholar] [CrossRef]
- Ekrikaya, S.; Yilmaz, E.; Celik, C.; Demirbuga, S.; Ildiz, N. Investigation of ellagic acid rich-berry extracts directed silver nanoparticles synthesis and their antimicrobial properties with potential mechanisms towards Enterococcus faecalis and Candida albicans. J. Biotechnol. 2021, 341, 155–162. [Google Scholar] [CrossRef]
- Alfaro-viquez, E.; Esquivel-alvarado, D.; Madrigal-carballo, S.; Krueger, C.G.; Reed, J.D. Cranberry proanthocyanidin-chitosan hybrid nanoparticles as a potential inhibitor of extra-intestinal pathogenic Escherichia coli invasion of gut epithelial cells. Int. J. Biol. Macromol. 2018, 111, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Alfaro-viquez, E.; Esquivel-alvarado, D.; Madrigal-carballo, S.; Krueger, C.G.; Reed, J.D. Proanthocyanidin-chitosan composite nanoparticles prevent bacterial invasion and colonization of gut epithelial cells by extra-intestinal pathogenic Escherichia coli. Int. J. Biol. Macromol. 2019, 135, 630–636. [Google Scholar] [CrossRef]
- Shejawal, K.P.; Randive, D.S.; Bhinge, S.D.; Bhutkar, M.A.; Wadkar, G.H.; Jadhav, N.R. Green synthesis of silver and iron nanoparticles of isolated proanthocyanidin: Its characterization, antioxidant, antimicrobial, and cytotoxic activities against COLO320DM and HT29. J. Genet. Eng. Biotechnol. 2020, 18, 43. [Google Scholar] [CrossRef] [PubMed]
- Alfaro-Viquez, E.; Esquivel-Alvarado, D.; Madrigal-Carballo, S.; Krueger, C.G.; Reed, J.D. Antimicrobial proanthocyanidin-chitosan composite nanoparticles loaded with gentamicin. Int. J. Biol. Macromol. 2020, 162, 1500–1508. [Google Scholar] [CrossRef]
- Ding, Z.; Mo, M.; Zhang, K.; Bi, Y.; Kong, F. Preparation, characterization and biological activity of proanthocyanidin-chitosan nanoparticles. Int. J. Biol. Macromol. 2021, 188, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Y.; Ma, S.; Fu, M.; Wu, M.; Li, J.; Wu, K.; Zhuang, X.; Lu, Z.; Guo, J. Injective programmable proanthocyanidin-coordinated zinc-based composite hydrogel for infected bone repair. Adv. Healthc. Mater. 2024, 13, e2302690. [Google Scholar] [CrossRef] [PubMed]
- Araya-Sibaja, A.M.; Wilhelm-Romero, K.; Vargas-Huertas, F.; Alvarado-Corella, D.; Jos, J.; Vega-Baudrit, J.R.; Navarro-Hoyos, M. Hybrid nanoparticles of proanthocyanidins from Uncaria tomentosa leaves: QTOF-ESI MS characterization, antioxidant activity and immune cellular response. Plants 2022, 11, 1737. [Google Scholar] [CrossRef]
- Mehra, C.; Gala, R.; Kakatkar, A.; Kumar, V.; Khurana, R.; Chatterjee, S.; Kumar, N.N.; Barooah, N.; Bhasikuttan, A.C.; Mohanty, J. Cooperative enhancement of antibacterial activity of sanguinarine drug through p-sulfonatocalix[6]arene functionalized silver nanoparticles. Chem. Commun. 2019, 55, 14275–14278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Sun, G.; Zhao, R.; Yang, F.; Jiang, X.; Song, S.; Zhang, J.; Shen, H.; Shen, J. Zwitterion-modified MXene quantum dot as a nanocarrier for traditional chinese medicine sanguinarine delivery and its application for photothermal-chemotherapy synergistic antibacterial and wound healing. Langmuir 2025, 40, 11381–11389. [Google Scholar] [CrossRef]
- Klębowski, B.; Depciuch, J.; Parlińska-Wojtan, M.; Baran, J. Applications of noble metal-based nanoparticles in medicine. Int. J. Mol. Sci. 2018, 19, 4031. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, T.; Li, S.; Chen, X.; Deng, Z.; Huang, Y. Nanoparticles for cancer therapy: A review of influencing factors and evaluation methods for biosafety. Clin. Transl. Oncol. 2023, 25, 2043–2055. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Mitragotri, S. Nanoparticles for topical drug delivery: Potential for skin cancer treatment. Adv. Drug Deliv. Rev. 2020, 153, 87–108. [Google Scholar] [CrossRef]
- Zaiter, T.; Cornu, R.; El Basset, W.; Martin, H.; Diab, M.; Béduneau, A. Toxicity assessment of nanoparticles in contact with the skin. J. Nanoparticle Res. 2022, 24, 149. [Google Scholar] [CrossRef]
- Zhang, N.; Said, A.; Wischke, C.; Kral, V.; Brodwolf, R.; Volz, P.; Boreham, A.; Gerecke, C.; Li, W.; Neffe, A.T.; et al. Poly[acrylonitrile-co-(N-vinyl pyrrolidone)] nanoparticles—Composition-dependent skin penetration enhancement of a dye probe and biocompatibility. Eur. J. Pharm. Biopharm. 2016, 116, 66–75. [Google Scholar] [CrossRef]
- Das, S.; Chaudhury, A. Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. AAPS PharmSciTech 2011, 12, 62–76. [Google Scholar] [CrossRef]
- Chamorro, S.; Gutiérrez, L.; Vaquero, M.P. Safety assessment of chronic oral exposure to iron oxide nanoparticles. Nanotechnology 2015, 26, 205101. [Google Scholar] [CrossRef] [PubMed]
- Sugibayashi, K. Skin Permeation and Disposition of Therapeutic and Cosmeceutical Compounds; Springer: Tokyo, Japan, 2017; ISBN 9784431565246. [Google Scholar]
- Musazzi, U.M.; Marini, V.; Casiraghi, A.; Minghetti, P. Is the European regulatory framework sufficient to assure the safety of citizens using health products containing nanomaterials? Drug Discov. Today 2017, 22, 870–882. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, B.M. Perspectives on FDA’s regulation of nanotechnology: Emerging challenges and potential solutions. Comp. Rev. Food Sci. Food Saf. 2009, 8, 375–393. [Google Scholar] [CrossRef]
- Heo, M.B.; Kwak, M.; An, K.S.; Kim, H.J.; Ryu, H.Y.; Lee, S.M.; Song, K.S.; Kim, I.Y.; Kwon, J.; Lee, T.G. Oral toxicity of titanium dioxide P25 at repeated dose 28-day and 90-day in rats. Part. Fibre Toxicol. 2020, 17, 34. [Google Scholar] [CrossRef]
- Dandekar, P.; Dhumal, R.; Jain, R.; Tiwari, D.; Vanage, G.; Patravale, V. Toxicological evaluation of pH-sensitive nanoparticles of curcumin: Acute, sub-acute and genotoxicity studies. Food Chem. Toxicol. 2010, 48, 2073–2089. [Google Scholar] [CrossRef] [PubMed]



| The Antibacterial Mechanisms | Reference |
|---|---|
| increased attachment and damage to bacterial cell membrane | [58] |
| ROS production | [44,47,48,52,56,57] |
| inhibited bacterial adhesion to bacterial cell walls | [59] |
| binding with proteins and DNA; affecting the respiratory chain; disrupting metabolism | [60] |
| disruption of bacterial biofilm formation | [57] |
| The Antibacterial Mechanisms | Reference |
|---|---|
| DNA fragmentation, caspase accumulation, membrane depolarisation, exposure to phosphatidylserine, and chromosome condensation increased attachment and damage to bacterial cell membrane | [61] |
| ROS production | [63] |
| The Antibacterial Mechanisms | Reference |
|---|---|
| interfere with bacteria by causing aggregation or precipitation, disrupting their normal functions and inhibiting growth | [80] |
| decrease in the mobility of bacterial cells | [68] |
| increased permeability of cell membrane, inhibition of the ATP-synthase | [78] |
| malfunction of critical cellular structures, thickening of bacterial cell walls, premature cell division, disintegration of cytosol and cell lysis | [66] |
| The Antibacterial Mechanisms | Reference |
|---|---|
| decreased expression of genes related to purine, galactose, aminosugars, nucleotides and arginine metabolism; the phosphotransferase system; inhibition of expression of genes associated with QS | [87] |
| decreased expression of key bacterial genes encoding the components of efflux pumps: acrB-1, acrB-2, tolC-1, tolC-2 | [88] |
| The Antibacterial Mechanisms | Reference |
|---|---|
| agglutination of bacterial cells | [97] |
| bacterial cell wall deformation and cell membrane increased permeability | [98] |
| agglutinative interaction with the bacterial fimbriae P structure | [94] |
| The Antibacterial Mechanisms | Reference |
|---|---|
| potentiation of antibiotics activity (β-lactams and glycopeptides) | [33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacyga, K.; Pacyga, P.; Szuba, E.; Viscardi, S.; Topola, E.; Duda-Madej, A. Nanotechnology Meets Phytotherapy: A Cutting-Edge Approach to Treat Bacterial Infections. Int. J. Mol. Sci. 2025, 26, 1254. https://doi.org/10.3390/ijms26031254
Pacyga K, Pacyga P, Szuba E, Viscardi S, Topola E, Duda-Madej A. Nanotechnology Meets Phytotherapy: A Cutting-Edge Approach to Treat Bacterial Infections. International Journal of Molecular Sciences. 2025; 26(3):1254. https://doi.org/10.3390/ijms26031254
Chicago/Turabian StylePacyga, Katarzyna, Paweł Pacyga, Emilia Szuba, Szymon Viscardi, Ewa Topola, and Anna Duda-Madej. 2025. "Nanotechnology Meets Phytotherapy: A Cutting-Edge Approach to Treat Bacterial Infections" International Journal of Molecular Sciences 26, no. 3: 1254. https://doi.org/10.3390/ijms26031254
APA StylePacyga, K., Pacyga, P., Szuba, E., Viscardi, S., Topola, E., & Duda-Madej, A. (2025). Nanotechnology Meets Phytotherapy: A Cutting-Edge Approach to Treat Bacterial Infections. International Journal of Molecular Sciences, 26(3), 1254. https://doi.org/10.3390/ijms26031254







