Deciphering the Functions of Raphe–Hippocampal Serotonergic and Glutamatergic Circuits and Their Deficits in Alzheimer’s Disease
Abstract
1. Introduction
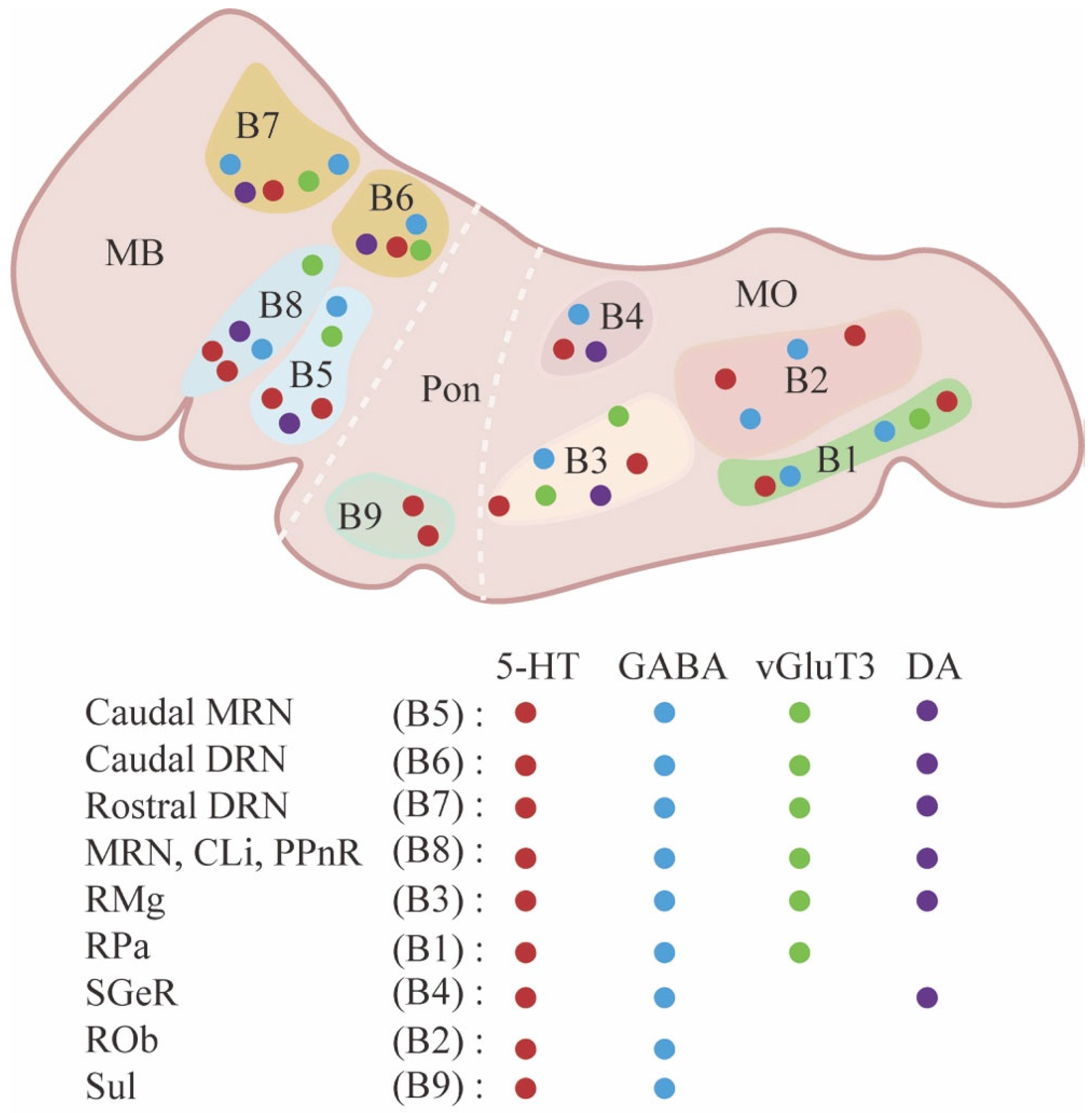
2. Anatomical, Neurochemical, and Electrophysiological Diversity of Raphe Nuclei
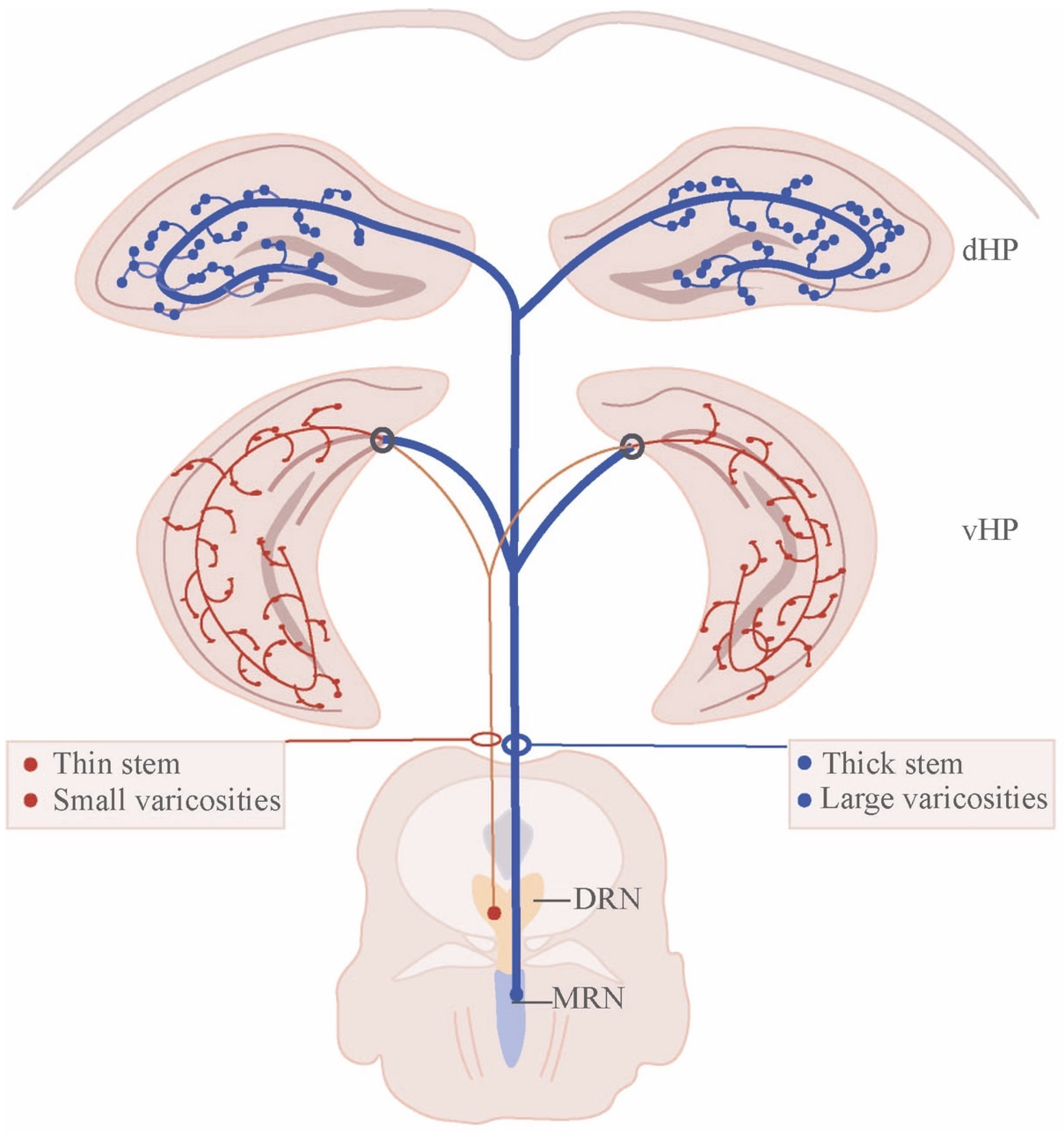
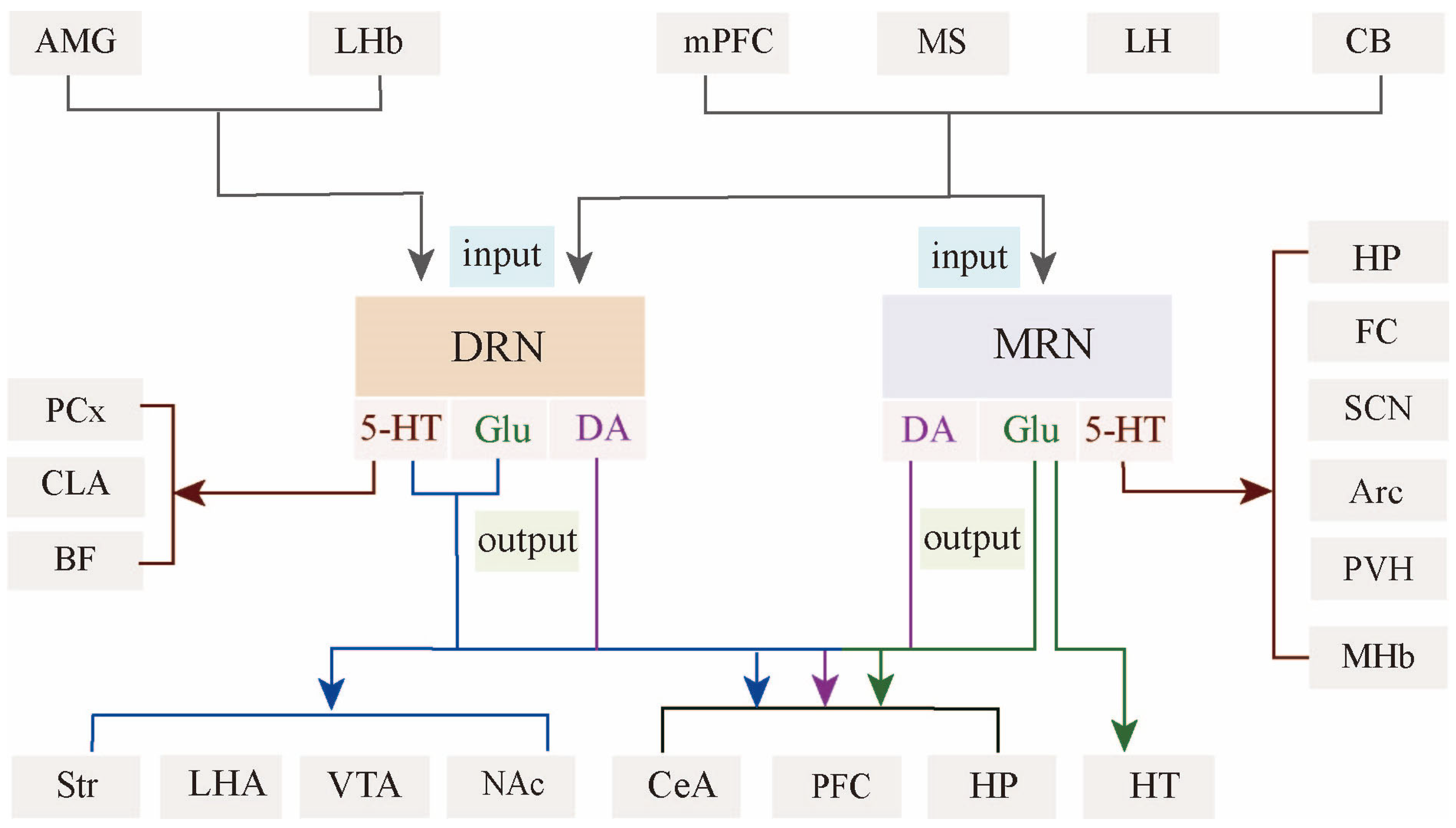
3. Architecture of Raphe Nucleus–Hippocampal Circuits
4. Synaptic Control of Hippocampal Activity by the Raphe Nucleus
4.1. Fast Modulation of Hippocampal Inhibitory Network by Raphe Nucleus
4.2. Subcortical Control of Hippocampal Theta-Rhythm by Raphe Nucleus
4.3. Subcortical Control of Synaptic Plasticity in the Hippocampus
5. Raphe–Hippocampal Serotonergic and Glutamatergic Circuits Regulate Emotional Behavior
5.1. Anxiety
5.2. Depression
5.3. Aggression
5.4. Addiction and Reward
6. Subcortical Modulation of Memory by Raphe–Hippocampal Circuits
6.1. Aversive Memory
6.2. Spatial Memory
6.3. Social Memory
7. Abnormal Raphe–Hippocampal Circuits in AD Patients and Mouse Models
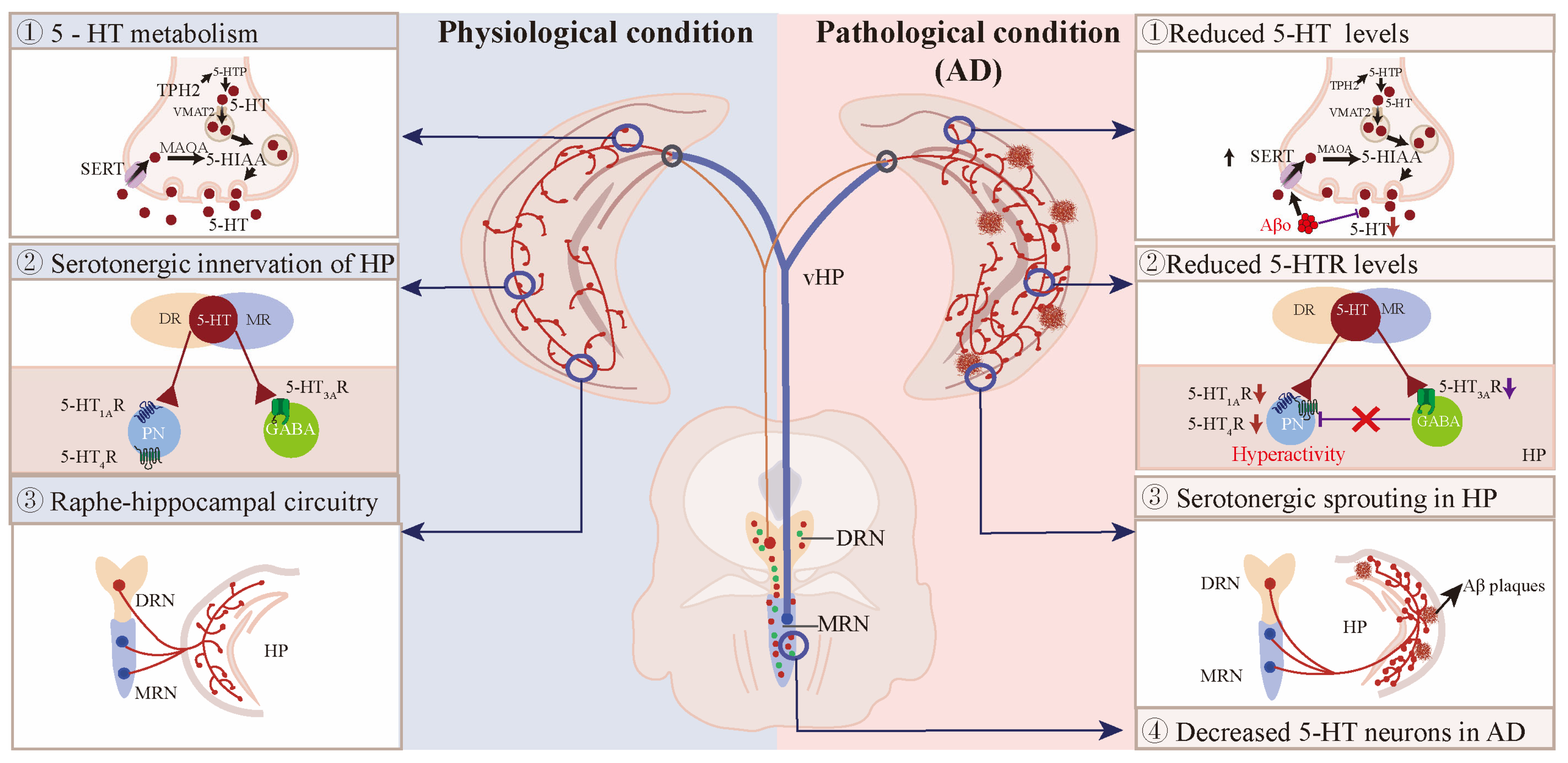
7.1. The 5-HT Neurons in AD Patients and Mouse Models
7.2. Reduced 5-HT and Its Metabolites in the Hippocampus of AD Patients and Mouse Models
| Research Model | Age (Year or Month) | Hippocampal 5-HT/5HIAA | References |
|---|---|---|---|
| MCI patient | 73.2 ± 10.8 y | 5-HT↓ | [185] |
| MCI patient | 83.0 ± 0 y | 5-HIAA↓ | [167] |
| AD patient | 75.0 ± 11.9 y | 5-HT↓, 5-HIAA↓ | [186] |
| AD patient | 70.0 ± 8.7 y | 5-HT↓, 5-HIAA↓ | [187,188] |
| AD patient | 82.0 ± 8.0 y | 5-HT↓, 5-HIAA↓ | [181,189] |
| hTau mice | 4 m | 5-HT↓ | [172] |
| hAPP-J20 mice | 4–5 m | 5-HT↓, 5-HIAA↓ | [49] |
| 5×FAD mice | 9–10 m | 5-HT↓ | [190] |
| APPswe/PS1dE9 mice | 2, 3m 4 m | 5-HIAA (NC), 5-HT↓, 5-HIAA (NC) | [191] |
| 3×Tg-AD mice | 3 m | 5-HT↓, 5-HIAA↑ | [165] |
| THY-Tau22 | 12 m | 5-HT↓, 5-HIAA↑ | [184] |
| APP/PS1 mice | 18 m | 5-HT↓ | [182] |
7.3. The Expression of 5-HTRs and SERT in the Hippocampus of AD Patients and Mouse Models
| Research Model | Age (Year or Month) | Hippocampal 5-HTRs | Reference |
|---|---|---|---|
| MCI patient | 76.8 ± 11.6 y | 5-HT1AR↓ | [197] |
| MCI patient | 83.0 ± 0 y | 5-HT1AR↓ | [208] |
| MCI patient | 73.2 ± 10.8 y | 5-HT1AR↑ | [185] |
| MCI patient | 73.2 ± 10.8 y | 5-HT1AR↑ | [187] |
| AD patient | 81.7 ± 2.0 y | 5-HT1AR↓ | [208] |
| AD patient | 75.0 ± 11.9 y | 5-HT1AR↓ | [197,199] |
| AD patient | 70.0 ± 8.7 y | 5-HT1AR↓ | [185,187] |
| AD patient | 82.0 ± 8.0 y | 5-HT4R↓ | [209] |
| AD patient | 83.1 ± 5.8 y | 5-HT4R↓ | [207] |
| AD patient | 77.0 ± 4.0 y | 5-HT3R (NC) | [206] |
| HTau mice | 4 m | 5-HT1BR↓, 5-HT4R↑, 5-HT1AR↑, 5-HT7R↑ | [172] |
| hAPP-J20 mice | 4, 8 m | 5-HT1AR↓, 5-HT3AR↓, 5-HT4R (NC) | [49] |
| 5×FAD mice | 3, 6 m | 5-HT1BR↓, 5-HT2BR↓, 5-HT3BR↓, 5-HT4R↓, 5-HT6R↓, 5-HT7R↓, 5-HT1FR↓ | [68] |
| APPswe/PS1dE9 mice | 4, 8, 11 m 18 m | 5-HT2AR (NC), 5-HT2BR↑ | [203,205] |
| 3×Tg-AD mice | 24 m | 5-HT1AR↓ | [196] |
| Tg2576 mice | 24 m | 5-HT1BR↓ | [210] |
| APP/PS1 mice | 9 m | 5-HT1BR↓ | [211] |
7.4. Ascending Projections from Raphe to Hippocampus in AD
7.5. Interactions Between Serotonergic and Glutamatergic Circuits in AD
8. Therapeutic Strategies to Rectify Serotonergic System for the Prevention of AD
8.1. Modulating 5-HTRs in the Treatment of AD
| Drugs | Target | Research Model | Results | Reference |
|---|---|---|---|---|
| Erythropoietin | 5-HT4R, 5-HT6R, 5-HT7R, 5-HT1AR | C57BL/6 mice injected with Aβo | Erythropoietin ameliorates cognitive deficits in Aβo-induced AD mouse model by modulating 5-HTRs. | [229] |
| T3 | SERT, 5HT1AR | 3×Tg-AD mice | T3 supplements improve depression- like behavior in 3×Tg-AD mice via activation of 5HT1AR. | [230] |
| Paroxetine | SERT | APP/PS1 mice | Paroxetine treatment ameliorates motional dysfunction in APP/PS1 mice. | [231] |
| Desloratadine | 5-HT2AR | APP/PS1 mice | Desloratadine represses Aβ level via upregulation of 5HT2AR-mediated Sirt1 expression and stimulation of autophagy. | [232] |
| Amisulpride | 5-HT7R | TauP301L-BiFC mice | Amisulpride mitigates Tauopathy via blockade of 5-HT7R activity. | [233] |
| Pimavanserin | 5-HT2AR | APP/PS1 mice | Pimavanserin reduces Aβ levels via suppression of 5HT2A-R activity. | [234] |
| Riluzole | EAAT2/GLT-1 | AβPP/PS1 mice | Riluzole benefits cognition in AβPP/PS1 mice by reducing glutamatergic tone. | [235] |
| Δ9-THC and CBD | GLT-1, EAAT3 | APP/PS1 mice | Chronic combined treatment with Δ9-THC and CBD reduces hippocampal glutamate levels in APP/PS1 mice. | [236] |
| Decanoic acid | AMPAR | 5×FAD mice | Decanoic acid improves cognitive function in 5×FAD mice by normalizing AMPAR-mediated signaling in CA1 hippocampal cells. | [237] |
| Perampanel | AMPAR | C57BL/6 mice injected with Aβ1–42 | Perampanel restores Aβ-impaired hippocampal LTP and blocks Aβ-induced network hyperexcitability. | [238] |
| Troriluzole | vGlut1 | 3×Tg-AD | Troriluzole improves memory in 3×Tg mice by reducing amyloid, tau, vGlut1 levels and restoring glutamate, synaptic functions. | [239] |
8.2. SSRIs in the Treatment of AD
8.3. Stimulation of Raphe Nuclei in the Treatment of AD
9. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frisoni, G.B.; Altomare, D.; Thal, D.R.; Ribaldi, F.; van der Kant, R.; Ossenkoppele, R.; Blennow, K.; Cummings, J.; van Duijn, C.; Nilsson, P.M.; et al. The probabilistic model of Alzheimer disease: The amyloid hypothesis revised. Nat. Rev. Neurosci. 2022, 23, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Giacobini, E.; Cuello, A.C.; Fisher, A. Reimagining cholinergic therapy for Alzheimer’s disease. Brain 2022, 145, 2250–2275. [Google Scholar] [CrossRef] [PubMed]
- Magierski, R.; Sobow, T. Serotonergic drugs for the treatment of neuropsychiatric symptoms in dementia. Expert. Rev. Neurother. 2016, 16, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Amrapala, A.; Sabe, M.; Solmi, M.; Maes, M. Neuropsychiatric disturbances in mild cognitive impairment: A scientometric analysis. Ageing Res. Rev. 2023, 92, 102129. [Google Scholar] [CrossRef]
- Sperling, R.; Salloway, S.; Brooks, D.J.; Tampieri, D.; Barakos, J.; Fox, N.C.; Raskind, M.; Sabbagh, M.; Honig, L.S.; Porsteinsson, A.P.; et al. Amyloid-related imaging abnormalities in patients with Alzheimer’s disease treated with bapineuzumab: A retrospective analysis. Lancet Neurol. 2012, 11, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Haj Ebrahimi, A.; Ali, A.B. Advances in Therapeutics to Alleviate Cognitive Decline and Neuropsychiatric Symptoms of Alzheimer’s Disease. Int. J. Mol. Sci. 2024, 25, 5169. [Google Scholar] [CrossRef] [PubMed]
- Szonyi, A.; Zicho, K.; Barth, A.M.; Gonczi, R.T.; Schlingloff, D.; Torok, B.; Sipos, E.; Major, A.; Bardoczi, Z.; Sos, K.E.; et al. Median raphe controls acquisition of negative experience in the mouse. Science 2019, 366, 6469. [Google Scholar] [CrossRef] [PubMed]
- Teissier, A.; Chemiakine, A.; Inbar, B.; Bagchi, S.; Ray, R.S.; Palmiter, R.D.; Dymecki, S.M.; Moore, H.; Ansorge, M.S. Activity of Raphe Serotonergic Neurons Controls Emotional Behaviors. Cell Rep. 2015, 13, 1965–1976. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, J.; Li, Y.; Hu, F.; Lu, Y.; Ma, M.; Feng, Q.; Zhang, J.E.; Wang, D.; Zeng, J.; et al. Dorsal raphe neurons signal reward through 5-HT and glutamate. Neuron 2014, 81, 1360–1374. [Google Scholar] [CrossRef] [PubMed]
- Okaty, B.W.; Commons, K.G.; Dymecki, S.M. Embracing diversity in the 5-HT neuronal system. Nat. Rev. Neurosci. 2019, 20, 397–424. [Google Scholar] [CrossRef] [PubMed]
- Kosofsky, B.E.; Molliver, M.E. The serotoninergic innervation of cerebral cortex: Different classes of axon terminals arise from dorsal and median raphe nuclei. Synapse 1987, 1, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Beck, S.G.; Pan, Y.Z.; Akanwa, A.C.; Kirby, L.G. Median and dorsal raphe neurons are not electrophysiologically identical. J. Neurophysiol. 2004, 91, 994–1005. [Google Scholar] [CrossRef] [PubMed]
- Slifirski, G.; Krol, M.; Turlo, J. 5-HT Receptors and the Development of New Antidepressants. Int. J. Mol. Sci. 2021, 22, 9015. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.J.; Noristani, H.N.; Verkhratsky, A. The serotonergic system in ageing and Alzheimer’s disease. Prog. Neurobiol. 2012, 99, 15–41. [Google Scholar] [CrossRef] [PubMed]
- Cirrito, J.R.; Disabato, B.M.; Restivo, J.L.; Verges, D.K.; Goebel, W.D.; Sathyan, A.; Hayreh, D.; D’Angelo, G.; Benzinger, T.; Yoon, H.; et al. Serotonin signaling is associated with lower amyloid-beta levels and plaques in transgenic mice and humans. Proc. Natl. Acad. Sci. USA 2011, 108, 14968–14973. [Google Scholar] [CrossRef] [PubMed]
- Cirrito, J.R.; Wallace, C.E.; Yan, P.; Davis, T.A.; Gardiner, W.D.; Doherty, B.M.; King, D.; Yuede, C.M.; Lee, J.M.; Sheline, Y.I. Effect of escitalopram on Abeta levels and plaque load in an Alzheimer mouse model. Neurology 2020, 95, e2666–e2674. [Google Scholar] [CrossRef] [PubMed]
- Bartels, C.; Wagner, M.; Wolfsgruber, S.; Ehrenreich, H.; Schneider, A.; Alzheimer’s Disease Neuroimaging, I. Impact of SSRI Therapy on Risk of Conversion from Mild Cognitive Impairment to Alzheimer’s Dementia in Individuals with Previous Depression. Am. J. Psychiatry 2018, 175, 232–241. [Google Scholar] [CrossRef]
- Qiao, J.; Wang, J.; Wang, H.; Zhang, Y.; Zhu, S.; Adilijiang, A.; Guo, H.; Zhang, R.; Guo, W.; Luo, G.; et al. Regulation of astrocyte pathology by fluoxetine prevents the deterioration of Alzheimer phenotypes in an APP/PS1 mouse model. Glia 2016, 64, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Bougea, A.; Angelopoulou, E.; Vasilopoulos, E.; Gourzis, P.; Papageorgiou, S. Emerging Therapeutic Potential of Fluoxetine on Cognitive Decline in Alzheimer’s Disease: Systematic Review. Int. J. Mol. Sci. 2024, 25, 6542. [Google Scholar] [CrossRef]
- Szonyi, A.; Mayer, M.I.; Cserep, C.; Takacs, V.T.; Watanabe, M.; Freund, T.F.; Nyiri, G. The ascending median raphe projections are mainly glutamatergic in the mouse forebrain. Brain Struct. Funct. 2016, 221, 735–751. [Google Scholar] [CrossRef] [PubMed]
- Amilhon, B.; Lepicard, E.; Renoir, T.; Mongeau, R.; Popa, D.; Poirel, O.; Miot, S.; Gras, C.; Gardier, A.M.; Gallego, J.; et al. VGLUT3 (vesicular glutamate transporter type 3) contribution to the regulation of serotonergic transmission and anxiety. J. Neurosci. 2010, 30, 2198–2210. [Google Scholar] [CrossRef] [PubMed]
- Stinson, H.E.; Ninan, I. Median raphe glutamatergic neuron-mediated enhancement of GABAergic transmission and suppression of long-term potentiation in the hippocampus. Heliyon 2024, 10, e38192. [Google Scholar] [CrossRef] [PubMed]
- He, A.; Zhang, C.; Ke, X.; Yi, Y.; Yu, Q.; Zhang, T.; Yu, H.; Du, H.; Li, H.; Tian, Q.; et al. VGLUT3 neurons in median raphe control the efficacy of spatial memory retrieval via ETV4 regulation of VGLUT3 transcription. Sci. China Life Sci. 2022, 65, 1590–1607. [Google Scholar] [CrossRef] [PubMed]
- Dahlstroem, A.; Fuxe, K. Evidence for the Existence of Monoamine-Containing Neurons in the Central Nervous System. I. Demonstration of Monoamines in the Cell Bodies of Brain Stem Neurons. Acta Physiol. Scand Suppl. 1964, 232, 1–55. [Google Scholar]
- Jacobs, B.L.; Azmitia, E.C. Structure and function of the brain serotonin system. Physiol. Rev. 1992, 72, 165–229. [Google Scholar] [CrossRef]
- Fernandez, S.P.; Cauli, B.; Cabezas, C.; Muzerelle, A.; Poncer, J.C.; Gaspar, P. Multiscale single-cell analysis reveals unique phenotypes of raphe 5-HT neurons projecting to the forebrain. Brain Struct. Funct. 2016, 221, 4007–4025. [Google Scholar] [CrossRef]
- Bjarkam, C.R.; Sorensen, J.C.; Geneser, F.A. Distribution and morphology of serotonin-immunoreactive axons in the retrohippocampal areas of the New Zealand white rabbit. Anat. Embryol. 2005, 210, 199–207. [Google Scholar] [CrossRef]
- Papp, E.C.; Hajos, N.; Acsady, L.; Freund, T.F. Medial septal and median raphe innervation of vasoactive intestinal polypeptide-containing interneurons in the hippocampus. Neuroscience 1999, 90, 369–382. [Google Scholar] [CrossRef]
- McQuade, R.; Sharp, T. Functional mapping of dorsal and median raphe 5-hydroxytryptamine pathways in forebrain of the rat using microdialysis. J. Neurochem. 1997, 69, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Crunelli, V.; Segal, M. An electrophysiological study of neurones in the rat median raphe and their projections to septum and hippocampus. Neuroscience 1985, 15, 47–60. [Google Scholar] [CrossRef]
- Commons, K.G. Ascending serotonin neuron diversity under two umbrellas. Brain Struct. Funct. 2016, 221, 3347–3360. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.D.; Zhu, Y.; Sun, Q.X.; Deng, F.; Wan, J.; Zheng, D.; Gong, W.; Xie, S.Z.; Shen, C.J.; Fu, J.Y.; et al. Distinct serotonergic pathways to the amygdala underlie separate behavioral features of anxiety. Nat. Neurosci. 2022, 25, 1651–1663. [Google Scholar] [CrossRef]
- Wang, X.Y.; Jia, W.B.; Xu, X.; Chen, R.; Wang, L.B.; Su, X.J.; Xu, P.F.; Liu, X.Q.; Wen, J.; Song, X.Y.; et al. A glutamatergic DRN-VTA pathway modulates neuropathic pain and comorbid anhedonia-like behavior in mice. Nat. Commun. 2023, 14, 5124. [Google Scholar] [CrossRef]
- Walsh, J.J.; Christoffel, D.J.; Heifets, B.D.; Ben-Dor, G.A.; Selimbeyoglu, A.; Hung, L.W.; Deisseroth, K.; Malenka, R.C. 5-HT release in nucleus accumbens rescues social deficits in mouse autism model. Nature 2018, 560, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Chien, P.Y.; Su, C.L.; Liu, P.H.; Chang, C.H.; Gean, P.W. The dorsal raphe-to-ventral hippocampal projection modulates reactive aggression through 5-HT(1B) receptors. Eur. J. Pharmacol. 2024, 981, 176918. [Google Scholar] [CrossRef]
- Hensler, J.G. Serotonergic modulation of the limbic system. Neurosci. Biobehav. Rev. 2006, 30, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Dorocic, I.P.; Furth, D.; Xuan, Y.; Johansson, Y.; Pozzi, L.; Silberberg, G.; Carlen, M.; Meletis, K. A Whole-Brain Atlas of Inputs to Serotonergic Neurons of the Dorsal and Median Raphe Nuclei. Neuron 2014, 83, 663–678. [Google Scholar]
- Weissbourd, B.; Ren, J.; DeLoach, K.E.; Guenthner, C.J.; Miyamichi, K.; Luo, L. Presynaptic partners of dorsal raphe serotonergic and GABAergic neurons. Neuron 2014, 83, 645–662. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, M.Z.; Li, Q.; Deng, J.; Mu, D.; Sun, Y.G. Organization of Functional Long-Range Circuits Controlling the Activity of Serotonergic Neurons in the Dorsal Raphe Nucleus. Cell Rep. 2017, 18, 3018–3032. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.K.; Cohen, J.Y.; Hwang, D.; Uchida, N.; Watabe-Uchida, M. Organization of monosynaptic inputs to the serotonin and dopamine neuromodulatory systems. Cell Rep. 2014, 8, 1105–1118. [Google Scholar] [CrossRef]
- Sos, K.E.; Mayer, M.I.; Cserep, C.; Takacs, F.S.; Szonyi, A.; Freund, T.F.; Nyiri, G. Cellular architecture and transmitter phenotypes of neurons of the mouse median raphe region. Brain Struct. Funct. 2017, 222, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Commons, K.G. Dorsal raphe organization. J. Chem. Neuroanat. 2020, 110, 101868. [Google Scholar] [CrossRef]
- Descarries, L.; Watkins, K.C.; Garcia, S.; Beaudet, A. The serotonin neurons in nucleus raphe dorsalis of adult rat: A light and electron microscope radioautographic study. J. Comp. Neurol. 1982, 207, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.W.; Ochandarena, N.E.; Philson, A.C.; Hyun, M.; Birnbaum, J.E.; Cicconet, M.; Sabatini, B.L. Molecular and anatomical organization of the dorsal raphe nucleus. eLife 2019, 8, e46464. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Le Maitre, E.; Fabre, V.; Bernard, J.F.; David Xu, Z.Q.; Hokfelt, T. Chemical neuroanatomy of the dorsal raphe nucleus and adjacent structures of the mouse brain. J. Comp. Neurol. 2010, 518, 3464–3494. [Google Scholar] [CrossRef]
- Fortin-Houde, J.; Henderson, F.; Dumas, S.; Ducharme, G.; Amilhon, B. Parallel streams of raphe VGLUT3-positive inputs target the dorsal and ventral hippocampus in each hemisphere. J. Comp. Neurol. 2023, 531, 702–719. [Google Scholar] [CrossRef] [PubMed]
- Senft, R.A.; Freret, M.E.; Sturrock, N.; Dymecki, S.M. Neurochemically and Hodologically Distinct Ascending VGLUT3 versus Serotonin Subsystems Comprise the r2-Pet1 Median Raphe. J. Neurosci. 2021, 41, 2581–2600. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Maejima, T.; Wyler, S.C.; Casadesus, G.; Herlitze, S.; Deneris, E.S. Pet-1 is required across different stages of life to regulate serotonergic function. Nat. Neurosci. 2010, 13, 1190–1198. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Mei, Y.; Zhang, X.; Wei, X.; Zhang, Y.; Wang, D.; Huang, J.; Zhu, K.; Peng, G.; Sun, B. Aberrant serotonergic signaling contributes to the hyperexcitability of CA1 pyramidal neurons in a mouse model of Alzheimer’s disease. Cell Rep. 2023, 42, 112152. [Google Scholar] [CrossRef] [PubMed]
- Okaty, B.W.; Sturrock, N.; Escobedo Lozoya, Y.; Chang, Y.; Senft, R.A.; Lyon, K.A.; Alekseyenko, O.V.; Dymecki, S.M. A single-cell transcriptomic and anatomic atlas of mouse dorsal raphe Pet1 neurons. eLife 2020, 9, e55523. [Google Scholar] [CrossRef] [PubMed]
- Narboux-Neme, N.; Pavone, L.M.; Avallone, L.; Zhuang, X.; Gaspar, P. Serotonin transporter transgenic (SERTcre) mouse line reveals developmental targets of serotonin specific reuptake inhibitors (SSRIs). Neuropharmacology 2008, 55, 994–1005. [Google Scholar] [CrossRef]
- Sahly, I.; Fabre, V.; Vyas, S.; Milet, A.; Rouzeau, J.D.; Hamon, M.; Lazar, M.; Tronche, F. 5-HT1A-iCre, a new transgenic mouse line for genetic analyses of the serotonergic pathway. Mol. Cell Neurosci. 2007, 36, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Rood, B.D.; Calizo, L.H.; Piel, D.; Spangler, Z.P.; Campbell, K.; Beck, S.G. Dorsal raphe serotonin neurons in mice: Immature hyperexcitability transitions to adult state during first three postnatal weeks suggesting sensitive period for environmental perturbation. J. Neurosci. 2014, 34, 4809–4821. [Google Scholar] [CrossRef] [PubMed]
- Crawford, L.K.; Craige, C.P.; Beck, S.G. Increased intrinsic excitability of lateral wing serotonin neurons of the dorsal raphe: A mechanism for selective activation in stress circuits. J. Neurophysiol. 2010, 103, 2652–2663. [Google Scholar] [CrossRef]
- Okaty, B.W.; Freret, M.E.; Rood, B.D.; Brust, R.D.; Hennessy, M.L.; deBairos, D.; Kim, J.C.; Cook, M.N.; Dymecki, S.M. Multi-Scale Molecular Deconstruction of the Serotonin Neuron System. Neuron 2015, 88, 774–791. [Google Scholar] [CrossRef]
- Domonkos, A.; Nikitidou Ledri, L.; Laszlovszky, T.; Cserep, C.; Borhegyi, Z.; Papp, E.; Nyiri, G.; Freund, T.F.; Varga, V. Divergent in vivo activity of non-serotonergic and serotonergic VGluT3-neurones in the median raphe region. J. Physiol. 2016, 594, 3775–3790. [Google Scholar] [CrossRef]
- Kohler, C.; Steinbusch, H. Identification of serotonin and non-serotonin-containing neurons of the mid-brain raphe projecting to the entorhinal area and the hippocampal formation. A combined immunohistochemical and fluorescent retrograde tracing study in the rat brain. Neuroscience 1982, 7, 951–975. [Google Scholar] [CrossRef] [PubMed]
- Aznar, S.; Qian, Z.X.; Knudsen, G.M. Non-serotonergic dorsal and median raphe projection onto parvalbumin- and calbindin-containing neurons in hippocampus and septum. Neuroscience 2004, 124, 573–581. [Google Scholar] [CrossRef]
- Muzerelle, A.; Scotto-Lomassese, S.; Bernard, J.F.; Soiza-Reilly, M.; Gaspar, P. Conditional anterograde tracing reveals distinct targeting of individual serotonin cell groups (B5-B9) to the forebrain and brainstem. Brain Struct. Funct. 2016, 221, 535–561. [Google Scholar] [CrossRef] [PubMed]
- Vertes, R.P. A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. J. Comp. Neurol. 1991, 313, 643–668. [Google Scholar] [CrossRef] [PubMed]
- Commons, K.G. Two major network domains in the dorsal raphe nucleus. J. Comp. Neurol. 2015, 523, 1488–1504. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.; Farago, A.F.; Awatramani, R.B.; Scott, M.M.; Deneris, E.S.; Dymecki, S.M. Redefining the serotonergic system by genetic lineage. Nat. Neurosci. 2008, 11, 417–419. [Google Scholar] [CrossRef]
- Alonso, A.; Merchan, P.; Sandoval, J.E.; Sanchez-Arrones, L.; Garcia-Cazorla, A.; Artuch, R.; Ferran, J.L.; Martinez-de-la-Torre, M.; Puelles, L. Development of the serotonergic cells in murine raphe nuclei and their relations with rhombomeric domains. Brain Struct. Funct. 2013, 218, 1229–1277. [Google Scholar] [CrossRef]
- Hamada, H.T.; Abe, Y.; Takata, N.; Taira, M.; Tanaka, K.F.; Doya, K. Optogenetic activation of dorsal raphe serotonin neurons induces brain-wide activation. Nat. Commun. 2024, 15, 4152. [Google Scholar] [CrossRef] [PubMed]
- Gradin, K.; Qadri, F.; Nomikos, G.G.; Hillegaart, V.; Svensson, T.H. Substance P injection into the dorsal raphe increases blood pressure and serotonin release in hippocampus of conscious rats. Eur. J. Pharmacol. 1992, 218, 363–367. [Google Scholar] [CrossRef]
- Matos, F.F.; Urban, C.; Yocca, F.D. Serotonin (5-HT) release in the dorsal raphe and ventral hippocampus: Raphe control of somatodendritic and terminal 5-HT release. J. Neural Transm. 1996, 103, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Nagai, Y.; Kisaka, Y.; Nomura, K.; Nishitani, N.; Andoh, C.; Koda, M.; Kawai, H.; Seiriki, K.; Nagayasu, K.; Kasai, A.; et al. Dorsal raphe serotonergic neurons preferentially reactivate dorsal dentate gyrus cell ensembles associated with positive experience. Cell Rep. 2023, 42, 112149. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, C.; Lin, Y.; Chen, Y.; Xie, W.; Huang, X.; Zhang, F.; Fu, C.; Zhuang, K.; Zou, T.; et al. Dorsal raphe nucleus-hippocampus serotonergic circuit underlies the depressive and cognitive impairments in 5×FAD male mice. Transl. Neurodegener. 2024, 13, 34. [Google Scholar] [CrossRef]
- Cheng, J.; Chen, L.; Zheng, Y.N.; Liu, J.; Zhang, L.; Zhang, X.M.; Huang, L.; Yuan, Q.L. Disfunction of dorsal raphe nucleus-hippocampus serotonergic-HTR3 transmission results in anxiety phenotype of Neuroplastin 65-deficient mice. Acta Pharmacol. Sin. 2024, 45, 1393–1405. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.; Bland, B.H.; Antle, M.C. Nonserotonergic projection neurons in the midbrain raphe nuclei contain the vesicular glutamate transporter VGLUT3. Synapse 2009, 63, 31–41. [Google Scholar] [CrossRef]
- Halasy, K.; Miettinen, R.; Szabat, E.; Freund, T.F. GABAergic Interneurons are the Major Postsynaptic Targets of Median Raphe Afferents in the Rat Dentate Gyrus. Eur. J. Neurosci. 1992, 4, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, R.; Freund, T.F. Convergence and segregation of septal and median raphe inputs onto different subsets of hippocampal inhibitory interneurons. Brain Res. 1992, 594, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, R.; Freund, T.F. Neuropeptide Y-containing interneurons in the hippocampus receive synaptic input from median raphe and GABAergic septal afferents. Neuropeptides 1992, 22, 185–193. [Google Scholar] [CrossRef]
- Freund, T.F.; Gulyas, A.I.; Acsady, L.; Gorcs, T.; Toth, K. Serotonergic control of the hippocampus via local inhibitory interneurons. Proc. Natl. Acad. Sci. USA 1990, 87, 8501–8505. [Google Scholar] [CrossRef]
- Bohm, C.; Pangalos, M.; Schmitz, D.; Winterer, J. Serotonin Attenuates Feedback Excitation onto O-LM Interneurons. Cereb. Cortex 2015, 25, 4572–4583. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Waider, J.; Proft, F.; Langlhofer, G.; Asan, E.; Lesch, K.P.; Gutknecht, L. GABA concentration and GABAergic neuron populations in limbic areas are differentially altered by brain serotonin deficiency in Tph2 knockout mice. Histochem. Cell Biol. 2013, 139, 267–281. [Google Scholar] [CrossRef]
- Kao, K.; Sanders, M.J.; Green, E.J. Physiological evidence for hippocampal disinhibition resulting from activation of the median raphe. Brain Res. 1997, 752, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Chittajallu, R.; Craig, M.T.; McFarland, A.; Yuan, X.; Gerfen, S.; Tricoire, L.; Erkkila, B.; Barron, S.C.; Lopez, C.M.; Liang, B.J.; et al. Dual origins of functionally distinct O-LM interneurons revealed by differential 5-HT(3A)R expression. Nat. Neurosci. 2013, 16, 1598–1607. [Google Scholar] [CrossRef] [PubMed]
- Winterer, J.; Stempel, A.V.; Dugladze, T.; Foldy, C.; Maziashvili, N.; Zivkovic, A.R.; Priller, J.; Soltesz, I.; Gloveli, T.; Schmitz, D. Cell-type-specific modulation of feedback inhibition by serotonin in the hippocampus. J. Neurosci. 2011, 31, 8464–8475. [Google Scholar] [CrossRef]
- McMahon, L.L.; Kauer, J.A. Hippocampal interneurons are excited via serotonin-gated ion channels. J. Neurophysiol. 1997, 78, 2493–2502. [Google Scholar] [CrossRef] [PubMed]
- Varga, V.; Losonczy, A.; Zemelman, B.V.; Borhegyi, Z.; Nyiri, G.; Domonkos, A.; Hangya, B.; Holderith, N.; Magee, J.C.; Freund, T.F. Fast synaptic subcortical control of hippocampal circuits. Science 2009, 326, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Wyskiel, D.R.; Andrade, R. Serotonin excites hippocampal CA1 GABAergic interneurons at the stratum radiatum-stratum lacunosum moleculare border. Hippocampus 2016, 26, 1107–1114. [Google Scholar] [CrossRef]
- Luttgen, M.; Ove Ogren, S.; Meister, B. Chemical identity of 5-HT2A receptor immunoreactive neurons of the rat septal complex and dorsal hippocampus. Brain Res. 2004, 1010, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Leung, L.S.; Law, C.S.H. Phasic modulation of hippocampal synaptic plasticity by theta rhythm. Behav. Neurosci. 2020, 134, 595–612. [Google Scholar] [CrossRef] [PubMed]
- Vertes, R.P.; Hoover, W.B.; Viana Di Prisco, G. Theta rhythm of the hippocampus: Subcortical control and functional significance. Behav. Cogn. Neurosci. Rev. 2004, 3, 173–200. [Google Scholar] [CrossRef]
- Bland, B.H.; Bland, C.E.; MacIver, M.B. Median raphe stimulation-induced motor inhibition concurrent with suppression of type 1 and type 2 hippocampal theta. Hippocampus 2016, 26, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Crooks, R.; Jackson, J.; Bland, B.H. Dissociable pathways facilitate theta and non-theta states in the median raphe--septohippocampal circuit. Hippocampus 2012, 22, 1567–1576. [Google Scholar] [CrossRef]
- Kinney, G.G.; Kocsis, B.; Vertes, R.P. Injections of excitatory amino acid antagonists into the median raphe nucleus produce hippocampal theta rhythm in the urethane-anesthetized rat. Brain Res. 1994, 654, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Ikemoto, S.; Wang, D.V. Median Raphe Nonserotonergic Neurons Modulate Hippocampal Theta Oscillations. J. Neurosci. 2022, 42, 1987–1998. [Google Scholar] [CrossRef] [PubMed]
- Kocsis, B.; Varga, V.; Dahan, L.; Sik, A. Serotonergic neuron diversity: Identification of raphe neurons with discharges time-locked to the hippocampal theta rhythm. Proc. Natl. Acad. Sci. USA 2006, 103, 1059–1064. [Google Scholar] [CrossRef]
- Sinnamon, H.M.; Jassen, A.K.; Ilch, C. Hippocampal theta activity and facilitated locomotor stepping produced by GABA injections in the midbrain raphe region. Behav. Brain Res. 2000, 107, 93–103. [Google Scholar] [CrossRef]
- Kinney, G.G.; Kocsis, B.; Vertes, R.P. Injections of muscimol into the median raphe nucleus produce hippocampal theta rhythm in the urethane anesthetized rat. Psychopharmacology 1995, 120, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Varga, V.; Sik, A.; Kocsis, B. GABAergic control of the ascending input from the median raphe nucleus to the limbic system. J. Neurophysiol. 2005, 94, 2561–2574. [Google Scholar] [CrossRef][Green Version]
- Jelitai, M.; Barth, A.M.; Komlosi, F.; Freund, T.F.; Varga, V. Activity and Coupling to Hippocampal Oscillations of Median Raphe GABAergic Cells in Awake Mice. Front. Neural Circuits 2021, 15, 784034. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Vazquez, M.A.; Lopez-Loeza, E.; Lajud Avila, N.; Gutierrez-Guzman, B.E.; Hernandez-Perez, J.J.; Reyes, Y.E.; Olvera-Cortes, M.E. Septal serotonin depletion in rats facilitates working memory in the radial arm maze and increases hippocampal high-frequency theta activity. Eur. J. Pharmacol. 2014, 734, 105–113. [Google Scholar] [CrossRef]
- Hernandez-Perez, J.J.; Gutierrez-Guzman, B.E.; Lopez-Vazquez, M.A.; Olvera-Cortes, M.E. Supramammillary serotonin reduction alters place learning and concomitant hippocampal, septal, and supramammillar theta activity in a Morris water maze. Front. Pharmacol. 2015, 6, 250. [Google Scholar] [CrossRef] [PubMed]
- Matulewicz, P.; Orzel-Gryglewska, J.; Hunt, M.J.; Trojniar, W.; Jurkowlaniec, E. Hippocampal theta rhythm after serotonergic activation of the pedunculopontine tegmental nucleus in anesthetized rats. Brain Res. Bull. 2010, 83, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Law, C.S.H.; Leung, L.S. Long-Term Potentiation and Excitability in the Hippocampus Are Modulated Differently by theta Rhythm. eNeuro 2018, 5, ENEURO.0236-18.2018. [Google Scholar] [CrossRef]
- Teixeira, C.M.; Rosen, Z.B.; Suri, D.; Sun, Q.; Hersh, M.; Sargin, D.; Dincheva, I.; Morgan, A.A.; Spivack, S.; Krok, A.C.; et al. Hippocampal 5-HT Input Regulates Memory Formation and Schaffer Collateral Excitation. Neuron 2018, 98, 992–1004 e4. [Google Scholar] [CrossRef]
- Nozaki, K.; Kubo, R.; Furukawa, Y. Serotonin modulates the excitatory synaptic transmission in the dentate granule cells. J. Neurophysiol. 2016, 115, 2997–3007. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Friedmann, D.; Xiong, J.; Liu, C.D.; Ferguson, B.R.; Weerakkody, T.; DeLoach, K.E.; Ran, C.; Pun, A.; Sun, Y.; et al. Anatomically Defined and Functionally Distinct Dorsal Raphe Serotonin Sub-systems. Cell 2018, 175, 472–487.e20. [Google Scholar] [CrossRef] [PubMed]
- Marcinkiewcz, C.A.; Mazzone, C.M.; D’Agostino, G.; Halladay, L.R.; Hardaway, J.A.; DiBerto, J.F.; Navarro, M.; Burnham, N.; Cristiano, C.; Dorrier, C.E.; et al. Serotonin engages an anxiety and fear-promoting circuit in the extended amygdala. Nature 2016, 537, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Andrade, T.G.; Graeff, F.G. Effect of electrolytic and neurotoxic lesions of the median raphe nucleus on anxiety and stress. Pharmacol. Biochem. Behav. 2001, 70, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Netto, S.M.; Silveira, R.; Coimbra, N.C.; Joca, S.R.; Guimaraes, F.S. Anxiogenic effect of median raphe nucleus lesion in stressed rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2002, 26, 1135–1141. [Google Scholar] [CrossRef]
- Ohmura, Y.; Tanaka, K.F.; Tsunematsu, T.; Yamanaka, A.; Yoshioka, M. Optogenetic activation of serotonergic neurons enhances anxiety-like behaviour in mice. Int. J. Neuropsychopharmacol. 2014, 17, 1777–1783. [Google Scholar] [CrossRef] [PubMed]
- Andrade, T.G.; Zangrossi, H., Jr.; Graeff, F.G. The median raphe nucleus in anxiety revisited. J. Psychopharmacol. 2013, 27, 1107–1115. [Google Scholar] [CrossRef]
- Ohmura, Y.; Tsutsui-Kimura, I.; Sasamori, H.; Nebuka, M.; Nishitani, N.; Tanaka, K.F.; Yamanaka, A.; Yoshioka, M. Different roles of distinct serotonergic pathways in anxiety-like behavior, antidepressant-like, and anti-impulsive effects. Neuropharmacology 2020, 167, 107703. [Google Scholar] [CrossRef] [PubMed]
- Vicente, M.A.; Zangrossi, H., Jr.; dos Santos, L.; de Macedo, C.E.; Andrade, T.G. Involvement of median raphe nucleus 5-HT1A receptors in the regulation of generalized anxiety-related defensive behaviours in rats. Neurosci. Lett. 2008, 445, 204–208. [Google Scholar] [CrossRef]
- Andrews, N.; Hogg, S.; Gonzalez, L.E.; File, S.E. 5-HT1A receptors in the median raphe nucleus and dorsal hippocampus may mediate anxiolytic and anxiogenic behaviours respectively. Eur. J. Pharmacol. 1994, 264, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.; File, S.E.; Fernandes, C.; Gonzalez, L.E.; Barnes, N.M. Evidence that the median raphe nucleus--dorsal hippocampal pathway mediates diazepam withdrawal-induced anxiety. Psychopharmacology 1997, 130, 228–234. [Google Scholar] [CrossRef]
- Abela, A.R.; Browne, C.J.; Sargin, D.; Prevot, T.D.; Ji, X.D.; Li, Z.; Lambe, E.K.; Fletcher, P.J. Median raphe serotonin neurons promote anxiety-like behavior via inputs to the dorsal hippocampus. Neuropharmacology 2020, 168, 107985. [Google Scholar] [CrossRef]
- Fazekas, C.L.; Torok, B.; Correia, P.; Chaves, T.; Bellardie, M.; Sipos, E.; Horvath, H.R.; Gaszner, B.; Dora, F.; Dobolyi, A.; et al. The Role of Vesicular Glutamate Transporter Type 3 in Social Behavior, with a Focus on the Median Raphe Region. eNeuro 2024, 11, ENEURO.0332-23.2024. [Google Scholar] [CrossRef] [PubMed]
- Fazekas, C.L.; Szabo, A.; Torok, B.; Banrevi, K.; Correia, P.; Chaves, T.; Daumas, S.; Zelena, D. A New Player in the Hippocampus: A Review on VGLUT3+ Neurons and Their Role in the Regulation of Hippocampal Activity and Behaviour. Int. J. Mol. Sci. 2022, 23, 790. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.B.; Kim, B.T.; Kim, J.Y.; Ryu, V.; Kang, D.W.; Lee, J.H.; Jahng, J.W. Adolescence fluoxetine increases serotonergic activity in the raphe-hippocampus axis and improves depression-like behaviors in female rats that experienced neonatal maternal separation. Psychoneuroendocrinology 2013, 38, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Johnston, B.A.; Tolomeo, S.; Gradin, V.; Christmas, D.; Matthews, K.; Steele, J.D. Failure of hippocampal deactivation during loss events in treatment-resistant depression. Brain 2015, 138 Pt 9, 2766–2776. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Hu, S.S.; Zhang, Q.L.; Han, X.M.; Chen, Z.G.; Nie, R.Z.; Cao, X.; Yuan, D.H.; Long, Y.; Hong, H.; et al. A circuit from lateral hypothalamic to dorsal hippocampal dentate gyrus modulates behavioral despair in mice. Cereb. Cortex 2024, 34, bhae399. [Google Scholar] [CrossRef]
- Fazekas, C.L.; Bellardie, M.; Torok, B.; Sipos, E.; Toth, B.; Baranyi, M.; Sperlagh, B.; Dobos-Kovacs, M.; Chaillou, E.; Zelena, D. Pharmacogenetic excitation of the median raphe region affects social and depressive-like behavior and core body temperature in male mice. Life Sci. 2021, 286, 120037. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, K.; Chen, H.S.; Gao, S.Q.; Xia, Z.X.; Zhang, J.T.; Wang, F.; Chen, J.G. Dorsal raphe projection inhibits the excitatory inputs on lateral habenula and alleviates depressive behaviors in rats. Brain Struct. Funct. 2018, 223, 2243–2258. [Google Scholar] [CrossRef]
- Meng, X.; Grandjean, J.; Sbrini, G.; Schipper, P.; Hofwijks, N.; Stoop, J.; Calabrese, F.; Homberg, J. Tryptophan Hydroxylase 2 Knockout Male Rats Exhibit a Strengthened Oxytocin System, Are Aggressive, and Are Less Anxious. ACS Chem. Neurosci. 2022, 13, 2974–2981. [Google Scholar] [CrossRef]
- Gorlova, A.; Ortega, G.; Waider, J.; Bazhenova, N.; Veniaminova, E.; Proshin, A.; Kalueff, A.V.; Anthony, D.C.; Lesch, K.P.; Strekalova, T. Stress-induced aggression in heterozygous TPH2 mutant mice is associated with alterations in serotonin turnover and expression of 5-HT6 and AMPA subunit 2A receptors. J. Affect. Disord. 2020, 272, 440–451. [Google Scholar] [CrossRef]
- Koprowska, M.; Romaniuk, A. Behavioral and biochemical alterations in median and dorsal raphe nuclei lesioned cats. Pharmacol. Biochem. Behav. 1997, 56, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fan, Y.; Raza, M.U.; Zhan, Y.; Du, X.D.; Patel, P.D.; Zhu, M.Y. The regulation of corticosteroid receptors in response to chronic social defeat. Neurochem. Int. 2017, 108, 397–409. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, W.; Wang, D.; Feng, Q.; Liu, Z.; Zhou, J.; Jia, C.; Hu, F.; Zeng, J.; Guo, Q.; et al. Serotonin neurons in the dorsal raphe nucleus encode reward signals. Nat. Commun. 2016, 7, 10503. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Zhang, S.; Qi, J.; Wang, H.; Cachope, R.; Mejias-Aponte, C.A.; Gomez, J.A.; Mateo-Semidey, G.E.; Beaudoin, G.M.J.; Paladini, C.A.; et al. Dorsal Raphe Dual Serotonin-Glutamate Neurons Drive Reward by Establishing Excitatory Synapses on VTA Mesoaccumbens Dopamine Neurons. Cell Rep. 2019, 26, 1128–1142.e7. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.Y.; Bromberg-Martin, E.S.; Monosov, I.E. Dorsal raphe neurons integrate the values of reward amount, delay, and uncertainty in multi-attribute decision-making. Cell Rep. 2024, 43, 114341. [Google Scholar] [CrossRef]
- Paquelet, G.E.; Carrion, K.; Lacefield, C.O.; Zhou, P.; Hen, R.; Miller, B.R. Single-cell activity and network properties of dorsal raphe nucleus serotonin neurons during emotionally salient behaviors. Neuron 2022, 110, 2664–2679 e8. [Google Scholar] [CrossRef] [PubMed]
- Luchetti, A.; Bota, A.; Weitemier, A.; Mizuta, K.; Sato, M.; Islam, T.; McHugh, T.J.; Tashiro, A.; Hayashi, Y. Two Functionally Distinct Serotonergic Projections into Hippocampus. J. Neurosci. 2020, 40, 4936–4944. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Drew, M.R.; Mimura, M.; Tanaka, K.F. Serotonin-mediated inhibition of ventral hippocampus is required for sustained goal-directed behavior. Nat. Neurosci. 2019, 22, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Belmer, A.; Depoortere, R.; Beecher, K.; Newman-Tancredi, A.; Bartlett, S.E. Neural serotonergic circuits for controlling long-term voluntary alcohol consumption in mice. Mol. Psychiatry 2022, 27, 4599–4610. [Google Scholar] [CrossRef] [PubMed]
- Avanzi, V.; Castilho, V.M.; de Andrade, T.G.; Brandao, M.L. Regulation of contextual conditioning by the median raphe nucleus. Brain Res. 1998, 790, 178–184. [Google Scholar] [CrossRef]
- Balazsfi, D.G.; Zelena, D.; Farkas, L.; Demeter, K.; Barna, I.; Cserep, C.; Takacs, V.T.; Nyiri, G.; Goloncser, F.; Sperlagh, B.; et al. Median raphe region stimulation alone generates remote, but not recent fear memory traces. PLoS ONE 2017, 12, e0181264. [Google Scholar] [CrossRef]
- Almada, R.C.; Borelli, K.G.; Albrechet-Souza, L.; Brandao, M.L. Serotonergic mechanisms of the median raphe nucleus-dorsal hippocampus in conditioned fear: Output circuit involves the prefrontal cortex and amygdala. Behav. Brain Res. 2009, 203, 279–287. [Google Scholar] [CrossRef]
- Ohmura, Y.; Izumi, T.; Yamaguchi, T.; Tsutsui-Kimura, I.; Yoshida, T.; Yoshioka, M. The serotonergic projection from the median raphe nucleus to the ventral hippocampus is involved in the retrieval of fear memory through the corticotropin-releasing factor type 2 receptor. Neuropsychopharmacology 2010, 35, 1271–1278. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, D.V.; Yau, H.J.; Broker, C.J.; Tsou, J.H.; Bonci, A.; Ikemoto, S. Mesopontine median raphe regulates hippocampal ripple oscillation and memory consolidation. Nat. Neurosci. 2015, 18, 728–735. [Google Scholar] [CrossRef]
- Collins, S.A.; Stinson, H.E.; Himes, A.; Nestor-Kalinoski, A.; Ninan, I. Sex-specific modulation of the medial prefrontal cortex by glutamatergic median raphe neurons. Sci. Adv. 2023, 9, eadg4800. [Google Scholar] [CrossRef]
- Silva, R.C.; Cruz, A.P.; Avanzi, V.; Landeira-Fernandez, J.; Brandao, M.L. Distinct contributions of median raphe nucleus to contextual fear conditioning and fear-potentiated startle. Neural Plast. 2002, 9, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Borelli, K.G.; Gargaro, A.C.; dos Santos, J.M.; Brandao, M.L. Effects of inactivation of serotonergic neurons of the median raphe nucleus on learning and performance of contextual fear conditioning. Neurosci. Lett. 2005, 387, 105–110. [Google Scholar] [CrossRef]
- Riedel, W.J.; Klaassen, T.; Schmitt, J.A. Tryptophan, mood, and cognitive function. Brain Behav. Immun. 2002, 16, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, J.A.; Jorissen, B.L.; Sobczak, S.; van Boxtel, M.P.; Hogervorst, E.; Deutz, N.E.; Riedel, W.J. Tryptophan depletion impairs memory consolidation but improves focussed attention in healthy young volunteers. J. Psychopharmacol. 2000, 14, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Ghaheri, S.; Niapour, A.; Sakhaie, N.; Sadegzadeh, F.; Saadati, H. Postnatal depletion of serotonin affects the morphology of neurons and the function of the hippocampus in male rats. Int. J. Dev. Neurosci. 2022, 82, 222–230. [Google Scholar] [CrossRef]
- Fernandez, S.P.; Muzerelle, A.; Scotto-Lomassese, S.; Barik, J.; Gruart, A.; Delgado-Garcia, J.M.; Gaspar, P. Constitutive and Acquired Serotonin Deficiency Alters Memory and Hippocampal Synaptic Plasticity. Neuropsychopharmacology 2017, 42, 512–523. [Google Scholar] [CrossRef]
- Gutierrez-Guzman, B.E.; Hernandez-Perez, J.J.; Olvera-Cortes, M.E. Serotonergic modulation of septo-hippocampal and septo-mammillary theta activity during spatial learning, in the rat. Behav. Brain Res. 2017, 319, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Richter-Levin, G.; Segal, M. The effects of serotonin depletion and raphe grafts on hippocampal electrophysiology and behavior. J. Neurosci. 1991, 11, 1585–1596. [Google Scholar] [CrossRef] [PubMed]
- Richter-Levin, G.; Segal, M. Raphe cells grafted into the hippocampus can ameliorate spatial memory deficits in rats with combined serotonergic/cholinergic deficiencies. Brain Res. 1989, 478, 184–186. [Google Scholar] [CrossRef] [PubMed]
- Wirtshafter, D.; Asin, K.E. Impaired radial maze performance in rats with electrolytic median raphe lesions. Exp. Neurol. 1983, 79, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Carli, M.; Lazarova, M.; Tatarczynska, E.; Samanin, R. Stimulation of 5-HT1A receptors in the dorsal hippocampus impairs acquisition and performance of a spatial task in a water maze. Brain Res. 1992, 595, 50–56. [Google Scholar] [CrossRef]
- Sarihi, A.; Motamedi, F.; Naghdi, N.; Rashidy-Pour, A. Lidocaine reversible inactivation of the median raphe nucleus has no effect on reference memory but enhances working memory versions of the Morris water maze task. Behav. Brain Res. 2000, 114, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Adams, W.; Kusljic, S.; van den Buuse, M. Serotonin depletion in the dorsal and ventral hippocampus: Effects on locomotor hyperactivity, prepulse inhibition and learning and memory. Neuropharmacology 2008, 55, 1048–1055. [Google Scholar] [CrossRef]
- Carli, M.; Bonalumi, P.; Samanin, R. Stimulation of 5-HT1A receptors in the dorsal raphe reverses the impairment of spatial learning caused by intrahippocampal scopolamine in rats. Eur. J. Neurosci. 1998, 10, 221–230. [Google Scholar] [CrossRef]
- Carli, M.; Balducci, C.; Samanin, R. Low doses of 8-OH-DPAT prevent the impairment of spatial learning caused by intrahippocampal scopolamine through 5-HT(1A) receptors in the dorsal raphe. Br. J. Pharmacol. 2000, 131, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Khodabande, F.; Akbari, E.; Ardeshiri, M.R. The modulation of the spatial reference memory by the orexinergic system of the dorsal raphe nucleus. Life Sci. 2021, 265, 118777. [Google Scholar] [CrossRef] [PubMed]
- Diethorn, E.J.; Gould, E. Development of the hippocampal CA2 region and the emergence of social recognition. Dev. Neurobiol. 2023, 83, 143–156. [Google Scholar] [CrossRef]
- McKittrick, C.R.; Magarinos, A.M.; Blanchard, D.C.; Blanchard, R.J.; McEwen, B.S.; Sakai, R.R. Chronic social stress reduces dendritic arbors in CA3 of hippocampus and decreases binding to serotonin transporter sites. Synapse 2000, 36, 85–94. [Google Scholar] [CrossRef]
- Sargin, D.; Oliver, D.K.; Lambe, E.K. Chronic social isolation reduces 5-HT neuronal activity via upregulated SK3 calcium-activated potassium channels. eLife 2016, 5, e21416. [Google Scholar] [CrossRef]
- Wu, X.; Morishita, W.; Beier, K.T.; Heifets, B.D.; Malenka, R.C. 5-HT modulation of a medial septal circuit tunes social memory stability. Nature 2021, 599, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Chaves, T.; Torok, B.; Fazekas, C.L.; Correia, P.; Sipos, E.; Varkonyi, D.; Hellinger, A.; Erk, D.; Zelena, D. Median raphe region GABAergic neurons contribute to social interest in mouse. Life Sci. 2022, 289, 120223. [Google Scholar] [CrossRef]
- Chaves, T.; Torok, B.; Fazekas, C.L.; Correia, P.; Sipos, E.; Varkonyi, D.; Toth, Z.E.; Dora, F.; Dobolyi, A.; Zelena, D. The Dopaminergic Cells in the Median Raphe Region Regulate Social Behavior in Male Mice. Int. J. Mol. Sci. 2024, 25, 4315. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Yin, C.; Zeng, Y.; Mei, Y. Structural and functional remodeling of neural networks in beta-amyloid driven hippocampal hyperactivity. Ageing Res. Rev. 2024, 101, 102468. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Lennon, J.C.; Malkaram, S.A.; Zeng, Y.; Fisher, D.W.; Dong, H. Serotonergic system, cognition, and BPSD in Alzheimer’s disease. Neurosci. Lett. 2019, 704, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Y.; Gan, Y.H.; Yang, L.; Cheng, W.; Yu, J.T. Depression in Alzheimer’s Disease: Epidemiology, Mechanisms, and Treatment. Biol. Psychiatry 2024, 95, 992–1005. [Google Scholar] [CrossRef] [PubMed]
- Slutsky, I. Linking activity dyshomeostasis and sleep disturbances in Alzheimer disease. Nat. Rev. Neurosci. 2024, 25, 272–284. [Google Scholar] [CrossRef]
- Kovacs, G.G.; Kloppel, S.; Fischer, I.; Dorner, S.; Lindeck-Pozza, E.; Birner, P.; Botefur, I.C.; Pilz, P.; Volk, B.; Budka, H. Nucleus-specific alteration of raphe neurons in human neurodegenerative disorders. Neuroreport 2003, 14, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.P.; Eastwood, S.L.; Hope, T.; McDonald, B.; Francis, P.T.; Esiri, M.M. Immunocytochemical study of the dorsal and median raphe nuclei in patients with Alzheimer’s disease prospectively assessed for behavioural changes. Neuropathol. Appl. Neurobiol. 2000, 26, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Bernedo, V.; Insua, D.; Suarez, M.L.; Santamarina, G.; Sarasa, M.; Pesini, P. Beta-amyloid cortical deposits are accompanied by the loss of serotonergic neurons in the dog. J. Comp. Neurol. 2009, 513, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Noristani, H.N.; Olabarria, M.; Verkhratsky, A.; Rodriguez, J.J. Serotonin fibre sprouting and increase in serotonin transporter immunoreactivity in the CA1 area of hippocampus in a triple transgenic mouse model of Alzheimer’s disease. Eur. J. Neurosci. 2010, 32, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Gottfries, C.G. Alzheimer’s disease and senile dementia: Biochemical characteristics and aspects of treatment. Psychopharmacology 1985, 86, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Cross, A.J.; Crow, T.J.; Johnson, J.A.; Joseph, M.H.; Perry, E.K.; Perry, R.H.; Blessed, G.; Tomlinson, B.E. Monoamine metabolism in senile dementia of Alzheimer type. J. Neurol. Sci. 1983, 60, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Hendricksen, M.; Thomas, A.J.; Ferrier, I.N.; Ince, P.; O’Brien, J.T. Neuropathological study of the dorsal raphe nuclei in late-life depression and Alzheimer’s disease with and without depression. Am. J. Psychiatry 2004, 161, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Tejani-Butt, S.M.; Yang, J.; Pawlyk, A.C. Altered serotonin transporter sites in Alzheimer’s disease raphe and hippocampus. Neuroreport 1995, 6, 1207–1210. [Google Scholar] [CrossRef]
- Vermeiren, Y.; Janssens, J.; Aerts, T.; Martin, J.J.; Sieben, A.; Van Dam, D.; De Deyn, P.P. Brain Serotonergic and Noradrenergic Deficiencies in Behavioral Variant Frontotemporal Dementia Compared to Early-Onset Alzheimer’s Disease. J. Alzheimers Dis. 2016, 53, 1079–1096. [Google Scholar] [CrossRef]
- Vermeiren, Y.; Van Dam, D.; Aerts, T.; Engelborghs, S.; De Deyn, P.P. Monoaminergic neurotransmitter alterations in postmortem brain regions of depressed and aggressive patients with Alzheimer’s disease. Neurobiol. Aging 2014, 35, 2691–2700. [Google Scholar] [CrossRef]
- Khan, K.M.; Balasubramanian, N.; Gaudencio, G.; Wang, R.; Selvakumar, G.P.; Kolling, L.; Pierson, S.; Tadinada, S.M.; Abel, T.; Hefti, M.; et al. Human tau-overexpressing mice recapitulate brainstem involvement and neuropsychiatric features of early Alzheimer’s disease. Acta Neuropathol. Commun. 2023, 11, 57. [Google Scholar] [CrossRef]
- Liu, Y.; Yoo, M.J.; Savonenko, A.; Stirling, W.; Price, D.L.; Borchelt, D.R.; Mamounas, L.; Lyons, W.E.; Blue, M.E.; Lee, M.K. Amyloid pathology is associated with progressive monoaminergic neurodegeneration in a transgenic mouse model of Alzheimer’s disease. J. Neurosci. 2008, 28, 13805–13814. [Google Scholar] [CrossRef] [PubMed]
- Ehrenberg, A.J.; Nguy, A.K.; Theofilas, P.; Dunlop, S.; Suemoto, C.K.; Di Lorenzo Alho, A.T.; Leite, R.P.; Diehl Rodriguez, R.; Mejia, M.B.; Rub, U.; et al. Quantifying the accretion of hyperphosphorylated tau in the locus coeruleus and dorsal raphe nucleus: The pathological building blocks of early Alzheimer’s disease. Neuropathol. Appl. Neurobiol. 2017, 43, 393–408. [Google Scholar] [CrossRef] [PubMed]
- Grinberg, L.T.; Rub, U.; Ferretti, R.E.; Nitrini, R.; Farfel, J.M.; Polichiso, L.; Gierga, K.; Jacob-Filho, W.; Heinsen, H.; Brazilian Brain Bank Study, G. The dorsal raphe nucleus shows phospho-tau neurofibrillary changes before the transentorhinal region in Alzheimer’s disease. A precocious onset? Neuropathol. Appl. Neurobiol. 2009, 35, 406–416. [Google Scholar] [CrossRef]
- Pierson, S.R.; Fiock, K.L.; Wang, R.; Balasubramanian, N.; Reinhardt, J.; Khan, K.M.; James, T.D.; Hunter, M.L.; Cooper, B.J.; Williamsen, H.R.; et al. Tau pathology in the dorsal raphe may be a prodromal indicator of Alzheimer’s disease. Mol. Psychiatry 2024, 30, 532–546. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.S.; Uribe Isaza, J.; Rouhi, N.; Jamani, N.F.; Jabeen, S.; Gill, A.K.; Tsutsui, M.; Visser, F.; Sargin, D. Behavioral and Neurophysiological Implications of Pathological Human Tau Expression in Serotonin Neurons. ACS Chem. Neurosci. 2024, 15, 932–943. [Google Scholar] [CrossRef] [PubMed]
- Arai, H.; Kosaka, K.; Iizuka, R. Changes of biogenic amines and their metabolites in postmortem brains from patients with Alzheimer-type dementia. J. Neurochem. 1984, 43, 388–393. [Google Scholar]
- Reinikainen, K.J.; Paljarvi, L.; Huuskonen, M.; Soininen, H.; Laakso, M.; Riekkinen, P.J. A post-mortem study of noradrenergic, serotonergic and GABAergic neurons in Alzheimer’s disease. J. Neurol. Sci. 1988, 84, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Dekker, A.D.; Vermeiren, Y.; Carmona-Iragui, M.; Benejam, B.; Videla, L.; Gelpi, E.; Aerts, T.; Van Dam, D.; Fernandez, S.; Lleo, A.; et al. Monoaminergic impairment in Down syndrome with Alzheimer’s disease compared to early-onset Alzheimer’s disease. Alzheimers Dement. 2018, 10, 99–111. [Google Scholar] [CrossRef]
- Vermeiren, Y.; Van Dam, D.; Aerts, T.; Engelborghs, S.; De Deyn, P.P. Brain region-specific monoaminergic correlates of neuropsychiatric symptoms in Alzheimer’s disease. J. Alzheimers Dis. 2014, 41, 819–833. [Google Scholar] [CrossRef] [PubMed]
- Szapacs, M.E.; Numis, A.L.; Andrews, A.M. Late onset loss of hippocampal 5-HT and NE is accompanied by increases in BDNF protein expression in mice co-expressing mutant APP and PS1. Neurobiol. Dis. 2004, 16, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hafiz, L.; Muller-Schiffmann, A.; Korth, C.; Fazari, B.; Chao, O.Y.; Nikolaus, S.; Schable, S.; Herring, A.; Keyvani, K.; Lamounier-Zepter, V.; et al. Abeta dimers induce behavioral and neurochemical deficits of relevance to early Alzheimer’s disease. Neurobiol. Aging 2018, 69, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Van der Jeugd, A.; Blum, D.; Raison, S.; Eddarkaoui, S.; Buee, L.; D’Hooge, R. Observations in THY-Tau22 mice that resemble behavioral and psychological signs and symptoms of dementia. Behav. Brain Res. 2013, 242, 34–39. [Google Scholar] [CrossRef]
- Truchot, L.; Costes, S.N.; Zimmer, L.; Laurent, B.; Le Bars, D.; Thomas-Antérion, C.; Croisile, B.; Mercier, B.; Hermier, M.; Vighetto, A.; et al. Up-regulation of hippocampal serotonin metabolism in mild cognitive impairment. Neurology 2007, 69, 1012–1017. [Google Scholar] [CrossRef]
- Gottfries, C.G.; Bartfai, T.; Carlsson, A.; Eckernäs, S.; Svennerholm, L. Multiple biochemical deficits in both gray and white matter of Alzheimer brains. Prog. Progress. Neuro-Psychopharmacol. Biol. Psychiatry 1986, 10, 405–413. [Google Scholar] [CrossRef]
- Truchot, L.; Costes, N.; Zimmer, L.; Laurent, B.; Le Bars, D.; Thomas-Antérion, C.; Mercier, B.; Hermier, M.; Vighetto, A.; Krolak-Salmon, P. A distinct [18F]MPPF PET profile in amnestic mild cognitive impairment compared to mild Alzheimer’s disease. NeuroImage 2008, 40, 1251–1256. [Google Scholar] [CrossRef]
- Gottfries, C.G. Disturbance of the 5-hydroxytryptamine metabolism in brains from patients with Alzheimer’s dementia. J. Neural Transm. Suppl. 1990, 30, 33–43. [Google Scholar] [PubMed]
- Burke, W.J.; Park, D.H.; Chung, H.D.; Marshall, G.L.; Haring, J.H.; Joh, T.H. Evidence for decreased transport of tryptophan hydroxylase in Alzheimer’s disease. Brain Res. 1990, 537, 83–87. [Google Scholar] [CrossRef]
- Tian, J.; Stucky, C.S.; Wang, T.; Muma, N.A.; Johnson, M.; Du, H. Mitochondrial Dysfunction Links to Impaired Hippocampal Serotonin Release in a Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. JAD 2023, 93, 605–619. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.W.; Liu, Y.F.; Shih, Y.H.; Chen, S.J.; Huang, T.Y.; Chang, C.Y.; Lien, C.H.; Yu, L.; Chen, S.H.; Kuo, Y.M. Neurodegeneration in Amygdala Precedes Hippocampus in the APPswe/PS1dE9 Mouse Model of Alzheimer’s Disease. Curr. Alzheimer Res. 2015, 12, 951–963. [Google Scholar] [CrossRef]
- Dengler-Crish, C.M.; Smith, M.A.; Wilson, G.N. Early Evidence of Low Bone Density and Decreased Serotonergic Synthesis in the Dorsal Raphe of a Tauopathy Model of Alzheimer’s Disease. J. Alzheimers Dis. 2017, 55, 1605–1619. [Google Scholar] [CrossRef]
- Aquilani, R.; Cotta Ramusino, M.; Maestri, R.; Iadarola, P.; Boselli, M.; Perini, G.; Boschi, F.; Dossena, M.; Bellini, A.; Buonocore, D.; et al. Several dementia subtypes and mild cognitive impairment share brain reduction of neurotransmitter precursor amino acids, impaired energy metabolism, and lipid hyperoxidation. Front. Aging Neurosci. 2023, 15, 1237469. [Google Scholar] [CrossRef]
- Verdurand, M.; Zimmer, L. Hippocampal 5-HT(1A) receptor expression changes in prodromal stages of Alzheimer’s disease: Beneficial or deleterious? Neuropharmacology 2017, 123, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, H.; Solas, M.; Francis, P.T.; Ramirez, M.J. Serotonin 5-HT(6) Receptor Antagonists in Alzheimer’s Disease: Therapeutic Rationale and Current Development Status. CNS Drugs 2017, 31, 19–32. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, L.Y.; Xu, Y.; Wang, W.Z.; Qiu, N.Z.; Zhang, F.F.; Zhang, F.; Wang, X.D.; Chen, W.; Xu, X.Y.; et al. Imbalance of multiple neurotransmitter pathways leading to depression-like behavior and cognitive dysfunction in the triple transgenic mouse model of Alzheimer disease. Metab. Brain Dis. 2023, 38, 2465–2476. [Google Scholar] [CrossRef]
- Kepe, V.; Barrio, J.R.; Huang, S.C.; Ercoli, L.; Siddarth, P.; Shoghi-Jadid, K.; Cole, G.M.; Satyamurthy, N.; Cummings, J.L.; Small, G.W.; et al. Serotonin 1A receptors in the living brain of Alzheimer’s disease patients. Proc. Natl. Acad. Sci. USA 2006, 103, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Vidal, B.; Sebti, J.; Verdurand, M.; Fieux, S.; Billard, T.; Streichenberger, N.; Troakes, C.; Newman-Tancredi, A.; Zimmer, L. Agonist and antagonist bind differently to 5-HT1A receptors during Alzheimer’s disease: A post-mortem study with PET radiopharmaceuticals. Neuropharmacology 2016, 109, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Mizukami, K.; Ishikawa, M.; Akatsu, H.; Abrahamson, E.E.; Ikonomovic, M.D.; Asada, T. An immunohistochemical study of the serotonin 1A receptor in the hippocampus of subjects with Alzheimer’s disease. Neuropathology 2011, 31, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, P.; Cselenyi, Z.; Andree, B.; Borg, J.; Nag, S.; Halldin, C.; Farde, L. Decreased 5-HT(1A) binding in mild Alzheimer’s disease-A positron emission tomography study. Synapse 2022, 76, e22235. [Google Scholar] [CrossRef] [PubMed]
- Verdurand, M.; Chauveau, F.; Daoust, A.; Morel, A.L.; Bonnefoi, F.; Liger, F.; Berod, A.; Zimmer, L. Differential effects of amyloid-beta 1-40 and 1-42 fibrils on 5-HT1A serotonin receptors in rat brain. Neurobiol. Aging 2016, 40, 11–21. [Google Scholar] [CrossRef]
- Marner, L.; Knudsen, G.M.; Madsen, K.; Holm, S.; Baare, W.; Hasselbalch, S.G. The reduction of baseline serotonin 2A receptors in mild cognitive impairment is stable at two-year follow-up. J. Alzheimers Dis. 2011, 23, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Holm, P.; Ettrup, A.; Klein, A.B.; Santini, M.A.; El-Sayed, M.; Elvang, A.B.; Stensbol, T.B.; Mikkelsen, J.D.; Knudsen, G.M.; Aznar, S. Plaque deposition dependent decrease in 5-HT2A serotonin receptor in AbetaPPswe/PS1dE9 amyloid overexpressing mice. J. Alzheimers Dis. 2010, 20, 1201–1213. [Google Scholar] [CrossRef]
- Christensen, R.; Marcussen, A.B.; Wortwein, G.; Knudsen, G.M.; Aznar, S. Abeta(1-42) injection causes memory impairment, lowered cortical and serum BDNF levels, and decreased hippocampal 5-HT(2A) levels. Exp. Neurol. 2008, 210, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Anzalone, M.; Karam, S.A.; Briting, S.R.R.; Petersen, S.; Thomsen, M.B.; Babcock, A.A.; Landau, A.M.; Finsen, B.; Metaxas, A. Serotonin-2B receptor (5-HT(2B)R) expression and binding in the brain of APP(swe)/PS1(dE9) transgenic mice and in Alzheimer’s disease brain tissue. Neurosci. Lett. 2025, 844, 138013. [Google Scholar] [CrossRef]
- Barnes, N.M.; Costall, B.; Naylor, R.J.; Williams, T.J.; Wischik, C.M. Normal densities of 5-HT3 receptor recognition sites in Alzheimer’s disease. Neuroreport 1990, 1, 253–254. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, G.P.; Mason, S.L.; Meldrum, A.; De Keczer, S.; Parnes, H.; Eglen, R.M.; Wong, E.H. 5-Hydroxytryptamine (5-HT)4 receptors in post mortem human brain tissue: Distribution, pharmacology and effects of neurodegenerative diseases. Br. J. Pharmacol. 1995, 114, 993–998. [Google Scholar] [CrossRef]
- Lai, M.K.; Tsang, S.W.; Esiri, M.M.; Francis, P.T.; Wong, P.T.; Chen, C.P. Differential involvement of hippocampal serotonin1A receptors and re-uptake sites in non-cognitive behaviors of Alzheimer’s disease. Psychopharmacology 2011, 213, 431–439. [Google Scholar] [CrossRef]
- Rebholz, H.; Friedman, E.; Castello, J. Alterations of Expression of the Serotonin 5-HT4 Receptor in Brain Disorders. Int. J. Mol. Sci. 2018, 19, 3581. [Google Scholar] [CrossRef] [PubMed]
- Tajeddinn, W.; Persson, T.; Maioli, S.; Calvo-Garrido, J.; Parrado-Fernandez, C.; Yoshitake, T.; Kehr, J.; Francis, P.; Winblad, B.; Hoglund, K.; et al. 5-HT1B and other related serotonergic proteins are altered in APPswe mutation. Neurosci. Lett. 2015, 594, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.C.; Wang, X.F.; Wang, J.; Li, C.; Xiao, J.; Wang, X.S.; Han, R.; Wang, S.Q.; Lin, Y.F.; Kong, L.H.; et al. Electroacupuncture Alleviates Memory Deficits in APP/PS1 Mice by Targeting Serotonergic Neurons in Dorsal Raphe Nucleus. Curr. Med. Sci. 2024, 44, 987–1000. [Google Scholar] [CrossRef]
- Smith, G.S.; Barrett, F.S.; Joo, J.H.; Nassery, N.; Savonenko, A.; Sodums, D.J.; Marano, C.M.; Munro, C.A.; Brandt, J.; Kraut, M.A.; et al. Molecular imaging of serotonin degeneration in mild cognitive impairment. Neurobiol. Dis. 2017, 105, 33–41. [Google Scholar] [CrossRef] [PubMed]
- van der Zande, J.J.; Joling, M.; Happach, I.G.; Vriend, C.; Scheltens, P.; Booij, J.; Lemstra, A.W. Serotonergic deficits in dementia with Lewy bodies with concomitant Alzheimer’s disease pathology: An (123)I-FP-CIT SPECT study. Neuroimage Clin. 2020, 25, 102062. [Google Scholar] [CrossRef] [PubMed]
- Noristani, H.N.; Meadows, R.S.; Olabarria, M.; Verkhratsky, A.; Rodriguez, J.J. Increased hippocampal CA1 density of serotonergic terminals in a triple transgenic mouse model of Alzheimer’s disease: An ultrastructural study. Cell Death Dis. 2011, 2, e210. [Google Scholar] [CrossRef][Green Version]
- Noristani, H.N.; Verkhratsky, A.; Rodriguez, J.J. High tryptophan diet reduces CA1 intraneuronal beta-amyloid in the triple transgenic mouse model of Alzheimer’s disease. Aging Cell 2012, 11, 810–822. [Google Scholar] [CrossRef]
- Jeon, S.G.; Kim, Y.J.; Kim, K.A.; Mook-Jung, I.; Moon, M. Visualization of Altered Hippocampal Connectivity in an Animal Model of Alzheimer’s Disease. Mol. Neurobiol. 2018, 55, 7886–7899. [Google Scholar] [CrossRef]
- Barrett, F.S.; Workman, C.I.; Sair, H.I.; Savonenko, A.V.; Kraut, M.A.; Sodums, D.J.; Joo, J.J.; Nassery, N.; Marano, C.M.; Munro, C.A.; et al. Association between serotonin denervation and resting-state functional connectivity in mild cognitive impairment. Hum. Brain Mapp. 2017, 38, 3391–3401. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Rodriguez, J.J.; Molina-Gil, S.; Rey-Brea, R.; Berrocoso, E.; Garcia-Alloza, M. Specific serotonergic denervation affects tau pathology and cognition without altering senile plaques deposition in APP/PS1 mice. PLoS ONE 2013, 8, e79947. [Google Scholar] [CrossRef]
- Harkany, T.; Dijkstra, I.M.; Oosterink, B.J.; Horvath, K.M.; Abraham, I.; Keijser, J.; Van der Zee, E.A.; Luiten, P.G. Increased amyloid precursor protein expression and serotonergic sprouting following excitotoxic lesion of the rat magnocellular nucleus basalis: Neuroprotection by Ca(2+) antagonist nimodipine. Neuroscience 2000, 101, 101–114. [Google Scholar] [CrossRef]
- d’Angremont, E.; Begemann, M.J.H.; van Laar, T.; Sommer, I.E.C. Cholinesterase Inhibitors for Treatment of Psychotic Symptoms in Alzheimer Disease and Parkinson Disease: A Meta-analysis. JAMA Neurol. 2023, 80, 813–823. [Google Scholar] [CrossRef]
- Sato, S.; Mizukami, K.; Asada, T. A preliminary open-label study of 5-HT1A partial agonist tandospirone for behavioural and psychological symptoms associated with dementia. Int. J. Neuropsychopharmacol. 2007, 10, 281–283. [Google Scholar] [CrossRef]
- Busceti, C.L.; Di Pietro, P.; Riozzi, B.; Traficante, A.; Biagioni, F.; Nistico, R.; Fornai, F.; Battaglia, G.; Nicoletti, F.; Bruno, V. 5-HT(2C) serotonin receptor blockade prevents tau protein hyperphosphorylation and corrects the defect in hippocampal synaptic plasticity caused by a combination of environmental stressors in mice. Pharmacol. Res. 2015, 99, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Angelucci, F.; Bernardini, S.; Gravina, P.; Bellincampi, L.; Trequattrini, A.; Di Iulio, F.; Vanni, D.; Federici, G.; Caltagirone, C.; Bossu, P.; et al. Delusion symptoms and response to antipsychotic treatment are associated with the 5-HT2A receptor polymorphism (102T/C) in Alzheimer’s disease: A 3-year follow-up longitudinal study. J. Alzheimers Dis. 2009, 17, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Kurhan, F.; Akin, M. A New Hope in Alzheimer’s Disease Psychosis: Pimavanserin. Curr. Alzheimer Res. 2023, 20, 403–408. [Google Scholar] [CrossRef]
- Robert, S.J.; Zugaza, J.L.; Fischmeister, R.; Gardier, A.M.; Lezoualc’h, F. The human serotonin 5-HT4 receptor regulates secretion of non-amyloidogenic precursor protein. J. Biol. Chem. 2001, 276, 44881–44888. [Google Scholar] [CrossRef] [PubMed]
- Tesseur, I.; Pimenova, A.A.; Lo, A.C.; Ciesielska, M.; Lichtenthaler, S.F.; De Maeyer, J.H.; Schuurkes, J.A.; D’Hooge, R.; De Strooper, B. Chronic 5-HT4 receptor activation decreases Abeta production and deposition in hAPP/PS1 mice. Neurobiol. Aging 2013, 34, 1779–1789. [Google Scholar] [CrossRef]
- Hashemi-Firouzi, N.; Shahidi, S.; Soleimani Asl, S. Chronic stimulation of the serotonergic 5-HT4 receptor modulates amyloid-beta-related impairments in synaptic plasticity and memory deficits in male rats. Brain Res. 2021, 1773, 147701. [Google Scholar] [CrossRef]
- Shahidi, S.; Hashemi-Firouzi, N.; Asl, S.S.; Komaki, A. Serotonin type 6 receptor antagonist attenuates the impairment of long-term potentiation and memory induced by Abeta. Behav. Brain Res. 2019, 364, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Shim, K.H.; Ha, S.; Choung, J.S.; Choi, J.I.; Kim, D.Y.; Kim, J.M.; Kim, M. Therapeutic Effect of Erythropoietin on Alzheimer’s Disease by Activating the Serotonin Pathway. Int. J. Mol. Sci. 2022, 23, 8144. [Google Scholar] [CrossRef] [PubMed]
- Maglione, A.V.; do Nascimento, B.P.P.; Ribeiro, M.O.; de Souza, T.J.L.; da Silva, R.E.C.; Sato, M.A.; Penatti, C.A.A.; Britto, L.R.G.; de Souza, J.S.; Maciel, R.M.B.; et al. Triiodothyronine Treatment reverses Depression-Like Behavior in a triple-transgenic animal model of Alzheimer’s Disease. Metab. Brain Dis. 2022, 37, 2735–2750. [Google Scholar] [CrossRef]
- Ai, P.H.; Chen, S.; Liu, X.D.; Zhu, X.N.; Pan, Y.B.; Feng, D.F.; Chen, S.; Xu, N.J.; Sun, S. Paroxetine ameliorates prodromal emotional dysfunction and late-onset memory deficit in Alzheimer’s disease mice. Transl. Neurodegener. 2020, 9, 18. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, C.; Lv, J.; Zhu, X.; Jiang, X.; Lu, W.; Lu, Y.; Tang, Z.; Wang, J.; Shen, X. Antiallergic drug desloratadine as a selective antagonist of 5HT(2A) receptor ameliorates pathology of Alzheimer’s disease model mice by improving microglial dysfunction. Aging Cell 2021, 20, e13286. [Google Scholar] [CrossRef]
- Jahreis, K.; Brüge, A.; Borsdorf, S.; Müller, F.E.; Sun, W.; Jia, S.; Kang, D.M.; Boesen, N.; Shin, S.; Lim, S.; et al. Amisulpride as a potential disease-modifying drug in the treatment of tauopathies. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2023, 19, 5482–5497. [Google Scholar] [CrossRef]
- Yuede, C.M.; Wallace, C.E.; Davis, T.A.; Gardiner, W.D.; Hettinger, J.C.; Edwards, H.M.; Hendrix, R.D.; Doherty, B.M.; Yuede, K.M.; Burstein, E.S.; et al. Pimavanserin, a 5HT(2A) receptor inverse agonist, rapidly suppresses Aβ production and related pathology in a mouse model of Alzheimer’s disease. J. Neurochem. 2021, 156, 658–673. [Google Scholar] [CrossRef] [PubMed]
- Hascup, K.N.; Findley, C.A.; Britz, J.; Esperant-Hilaire, N.; Broderick, S.O.; Delfino, K.; Tischkau, S.; Bartke, A.; Hascup, E.R. Riluzole attenuates glutamatergic tone and cognitive decline in AβPP/PS1 mice. J. Neurochem. 2021, 156, 513–523. [Google Scholar] [CrossRef]
- Sánchez-Fernández, N.; Gómez-Acero, L.; Castañé, A.; Adell, A.; Campa, L.; Bonaventura, J.; Brito, V.; Ginés, S.; Queiróz, F.; Silva, H.; et al. A combination of Δ(9)-tetrahydrocannabinol and cannabidiol modulates glutamate dynamics in the hippocampus of an animal model of Alzheimer’s disease. Neurother. J. Am. Soc. Exp. NeuroTherapeutics 2024, 21, e00439. [Google Scholar] [CrossRef]
- Abghari, M.; Vu, J.; Eckberg, N.; Aldana, B.I.; Kohlmeier, K.A. Decanoic Acid Rescues Differences in AMPA-Mediated Calcium Rises in Hippocampal CA1 Astrocytes and Neurons in the 5×FAD Mouse Model of Alzheimer’s Disease. Biomolecules 2023, 13, 1461. [Google Scholar] [CrossRef] [PubMed]
- Bellingacci, L.; Tallarico, M.; Mancini, A.; Megaro, A.; De Caro, C.; Citraro, R.; De Sarro, G.; Tozzi, A.; Di Filippo, M.; Sciaccaluga, M.; et al. Non-competitive AMPA glutamate receptors antagonism by perampanel as a strategy to counteract hippocampal hyper-excitability and cognitive deficits in cerebral amyloidosis. Neuropharmacology 2023, 225, 109373. [Google Scholar] [CrossRef] [PubMed]
- Pfitzer, J.; Pinky, P.D.; Perman, S.; Redmon, E.; Cmelak, L.; Suppiramaniam, V.; Coric, V.; Qureshi, I.A.; Gramlich, M.W.; Reed, M.N. Troriluzole rescues glutamatergic deficits, amyloid and tau pathology, and synaptic and memory impairments in 3×Tg-AD mice. J. Neurochem. 2025, 169, e16215. [Google Scholar] [CrossRef]
- Nelson, R.L.; Guo, Z.; Halagappa, V.M.; Pearson, M.; Gray, A.J.; Matsuoka, Y.; Brown, M.; Martin, B.; Iyun, T.; Maudsley, S.; et al. Prophylactic treatment with paroxetine ameliorates behavioral deficits and retards the development of amyloid and tau pathologies in 3×TgAD mice. Exp. Neurol. 2007, 205, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Sheline, Y.I.; Snider, B.J.; Beer, J.C.; Seok, D.; Fagan, A.M.; Suckow, R.F.; Lee, J.M.; Waligorska, T.; Korecka, M.; Aselcioglu, I.; et al. Effect of escitalopram dose and treatment duration on CSF Abeta levels in healthy older adults: A controlled clinical trial. Neurology 2020, 95, e2658–e2665. [Google Scholar] [CrossRef]
- Sivasaravanaparan, M.; Olesen, L.O.; Severino, M.; von Linstow, C.U.; Lambertsen, K.L.; Gramsbergen, J.B.; Hasselstrom, J.; Metaxas, A.; Wiborg, O.; Finsen, B. Efficacy of Chronic Paroxetine Treatment in Mitigating Amyloid Pathology and Microgliosis in APPSWE/PS1DeltaE9 Transgenic Mice. J. Alzheimers Dis. 2022, 87, 685–699. [Google Scholar] [CrossRef]
- von Linstow, C.U.; Waider, J.; Grebing, M.; Metaxas, A.; Lesch, K.P.; Finsen, B. Serotonin augmentation therapy by escitalopram has minimal effects on amyloid-beta levels in early-stage Alzheimer’s-like disease in mice. Alzheimers Res. Ther. 2017, 9, 74. [Google Scholar] [CrossRef]
- Severino, M.; Sivasaravanaparan, M.; Olesen, L.O.; von Linstow, C.U.; Metaxas, A.; Bouzinova, E.V.; Khan, A.M.; Lambertsen, K.L.; Babcock, A.A.; Gramsbergen, J.B.; et al. Established amyloid-beta pathology is unaffected by chronic treatment with the selective serotonin reuptake inhibitor paroxetine. Alzheimers Dement. 2018, 4, 215–223. [Google Scholar]
- von Linstow, C.U.; Waider, J.; Bergh, M.S.; Anzalone, M.; Madsen, C.; Nicolau, A.B.; Wirenfeldt, M.; Lesch, K.P.; Finsen, B. The Combined Effects of Amyloidosis and Serotonin Deficiency by Tryptophan Hydroxylase-2 Knockout Impacts Viability of the APP/PS1 Mouse Model of Alzheimer’s Disease. J. Alzheimers Dis. 2022, 85, 1283–1300. [Google Scholar] [CrossRef] [PubMed]
- Carballosa Gonzalez, M.M.; Blaya, M.O.; Alonso, O.F.; Bramlett, H.M.; Hentall, I.D. Midbrain raphe stimulation improves behavioral and anatomical recovery from fluid-percussion brain injury. J. Neurotrauma 2013, 30, 119–130. [Google Scholar] [CrossRef]
- Madhav, T.R.; Pei, Q.; Grahame-Smith, D.G.; Zetterstrom, T.S. Repeated electroconvulsive shock promotes the sprouting of serotonergic axons in the lesioned rat hippocampus. Neuroscience 2000, 97, 677–683. [Google Scholar] [CrossRef]
- Lesch, K.P.; Waider, J. Serotonin in the modulation of neural plasticity and networks: Implications for neurodevelopmental disorders. Neuron 2012, 76, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Li, H.H.; Yao, X.Y.; Tao, S.; Sun, X.; Li, P.P.; Li, X.X.; Liu, Z.L.; Ren, C. Serotonin 2 Receptors, Agomelatine, and Behavioral and Psychological Symptoms of Dementia in Alzheimer’s Disease. Behav. Neurol. 2021, 2021, 5533827. [Google Scholar] [CrossRef]
| Sample Type | Age (Year or Month) | Measurement Method | Raphe Neurons | Reference |
|---|---|---|---|---|
| MCI patient | 73.2 ± 10.8 y | Biochemical Detection | DRN5-HT↓ | [166] |
| MCI patient | 83.0 ± 0 y | HPLC | DRN5-HT↓, MRN5-HT↓ | [167] |
| AD patient | 70.0 ± 8.7 y | Biochemical Detection | DRN5-HT↓ | [166] |
| AD patient | 83.1 ± 5.8 y | ICC, 2DIA | MRN5-HT↓ | [168] |
| AD patient | 79.3 ± 8.7 y | QAR | DRN5-HT↓ | [169] |
| AD patient | 82.0 ± 1.0 y | ICC | DRN5-HT↓ | [163] |
| AD patient | 76.5 ± 10.2 y | RP-HPLC | DRN5-HT↓, MRN5-HT↓ | [170] |
| AD patient | 75.9 ± 7.3 y | RP-HPLC | DRN5-HT↑ | [171] |
| hTau mice | 4 m | IF | DRN5-HT↓ | [172] |
| hAPP-J20 mice | 4 m | IF, IHC | NC | [49] |
| 3×Tg-AD mice | 3–18 m | IHC | NC | [165] |
| 5×FAD mice | 3 m | IF | DRN5-HT↓ | [68] |
| APPswe/PS1dE9 mice | 24 m | IHC | DRN5-HT↓ | [173] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, W.; Zhang, R.; Zhang, A.; Mei, Y. Deciphering the Functions of Raphe–Hippocampal Serotonergic and Glutamatergic Circuits and Their Deficits in Alzheimer’s Disease. Int. J. Mol. Sci. 2025, 26, 1234. https://doi.org/10.3390/ijms26031234
Yu W, Zhang R, Zhang A, Mei Y. Deciphering the Functions of Raphe–Hippocampal Serotonergic and Glutamatergic Circuits and Their Deficits in Alzheimer’s Disease. International Journal of Molecular Sciences. 2025; 26(3):1234. https://doi.org/10.3390/ijms26031234
Chicago/Turabian StyleYu, Wanting, Ruonan Zhang, Aohan Zhang, and Yufei Mei. 2025. "Deciphering the Functions of Raphe–Hippocampal Serotonergic and Glutamatergic Circuits and Their Deficits in Alzheimer’s Disease" International Journal of Molecular Sciences 26, no. 3: 1234. https://doi.org/10.3390/ijms26031234
APA StyleYu, W., Zhang, R., Zhang, A., & Mei, Y. (2025). Deciphering the Functions of Raphe–Hippocampal Serotonergic and Glutamatergic Circuits and Their Deficits in Alzheimer’s Disease. International Journal of Molecular Sciences, 26(3), 1234. https://doi.org/10.3390/ijms26031234







