Diagnostic and Prognostic Significance of a Four-miRNA Signature in Colorectal Cancer
Abstract
1. Introduction
2. Results
2.1. Clinical Validation of the Diagnostic Role of a Four-miRNA Signature in Colorectal Cancer Samples
2.2. Bioinformatics Evaluation of the Prognostic Value of the Four-miRNA Signature
3. Discussion
4. Materials and Methods
4.1. Patient Cohorts and Specimens
4.2. RNA Extraction and microRNA Reverse Transcription
4.3. ddPCR for Absolute Quantification of microRNA Expression Levels
4.4. Bioinformatics Analyses
4.5. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CRC | Colorectal cancer |
| FOBT | Fecal occult blood test |
| FIT | Fecal immunochemical test |
| miRNA | microRNA |
| EMT | Epithelial-to-mesenchymal transition |
| FFPE | Formalin-fixed paraffin-embedded |
| ddPCR | droplet digital PCR |
| STRING | Search Tool Retrieval of Interacting Genes/Proteins |
| TCGA | The Cancer Genome Atlas |
| GEPIA | Gene Expression Profiling Interactive Analysis |
| ROC | Receiver Operating Characteristics |
References
- Filho, A.M.; Laversanne, M.; Ferlay, J.; Colombet, M.; Piñeros, M.; Znaor, A.; Parkin, D.M.; Soerjomataram, I.; Bray, F. The GLOBOCAN 2022 cancer estimates: Data sources, methods, and a snapshot of the cancer burden worldwide. Int. J. Cancer 2024. Early View. [Google Scholar] [CrossRef]
- Blom, J.; Saraste, D.; Törnberg, S.; Jonsson, H. Routine Fecal Occult Blood Screening and Colorectal Cancer Mortality in Sweden. JAMA Netw. Open 2024, 7, e240516. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, X.; Hu, Y.; Chen, K.; Zheng, S.; Ding, K.; Colorectal Cancer Screening Cohort Research Group. Nonadherence to Referral Colonoscopy After Positive Fecal Immunochemical Test Results Increases the Risk of Distal Colorectal Cancer Mortality. Gastroenterology 2023, 165, 1558–1560.e4. [Google Scholar] [CrossRef]
- Metaxas, G.; Papachristou, A.; Stathaki, M. Colorectal cancer screening: Modalities and adherence. World J. Gastroenterol. 2024, 30, 3048–3051. [Google Scholar] [CrossRef] [PubMed]
- Kildusiene, I.; Dulskas, A.; Smailyte, G. Value of combined serum CEA, CA72-4, and CA19-9 marker detection in diagnosis of colorectal cancer. Tech. Coloproctol. 2024, 28, 33. [Google Scholar] [CrossRef]
- Lakemeyer, L.; Sander, S.; Wittau, M.; Henne-Bruns, D.; Kornmann, M.; Lemke, J. Diagnostic and Prognostic Value of CEA and CA19-9 in Colorectal Cancer. Diseases 2021, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Tian, Y.; Deng, Z.; Yang, F.; Chen, E. Epigenetic Alteration in Colorectal Cancer: Potential Diagnostic and Prognostic Implications. Int. J. Mol. Sci. 2024, 25, 3358. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Zhong, R.; Wang, L.; Sun, L. Research progress of DNA methylation in colorectal cancer. Mol. Med. Rep. 2024, 30, 154. [Google Scholar] [CrossRef]
- Qiu, W.; Akanyibah, F.A.; Xia, Y.; Ocansey, D.K.W.; Mao, F.; Liang, Y. Emerging role of small RNAs in inflammatory bowel disease and associated colorectal cancer. Int. J. Mol. Med. 2025, 55, 33. [Google Scholar] [CrossRef]
- Candido, S.; Lupo, G.; Pennisi, M.; Basile, M.S.; Anfuso, C.D.; Petralia, M.C.; Gattuso, G.; Vivarelli, S.; Spandidos, D.A.; Libra, M.; et al. The analysis of miRNA expression profiling datasets reveals inverse microRNA patterns in glioblastoma and Alzheimer’s disease. Oncol. Rep. 2019, 42, 911–922. [Google Scholar] [CrossRef]
- Gao, X.; Yang, X.; He, F.; Liu, X.; Liu, D.; Yuan, X. Downregulation of microRNA 494 inhibits cell proliferation in lung squamous cell carcinoma via the induction of PUMA α mediated apoptosis. Exp. Ther. Med. 2023, 25, 242. [Google Scholar] [CrossRef]
- Falzone, L.; Grimaldi, M.; Celentano, E.; Augustin, L.S.A.; Libra, M. Identification of Modulated MicroRNAs Associated with Breast Cancer, Diet, and Physical Activity. Cancers 2020, 12, 2555. [Google Scholar] [CrossRef]
- Falzone, L.; Lupo, G.; La Rosa, G.R.M.; Crimi, S.; Anfuso, C.D.; Salemi, R.; Rapisarda, E.; Libra, M.; Candido, S. Identification of Novel MicroRNAs and Their Diagnostic and Prognostic Significance in Oral Cancer. Cancers 2019, 11, 610. [Google Scholar] [CrossRef]
- Stella, M.; Russo, G.I.; Leonardi, R.; Carcò, D.; Gattuso, G.; Falzone, L.; Ferrara, C.; Caponnetto, A.; Battaglia, R.; Libra, M.; et al. Extracellular RNAs from Whole Urine to Distinguish Prostate Cancer from Benign Prostatic Hyperplasia. Int. J. Mol. Sci. 2024, 25, 10079. [Google Scholar] [CrossRef] [PubMed]
- Pekarek, L.; Sánchez Cedra, A.; Jaudenes, Y.D.Y.; Ospino, L.R.; Iglesias Pedrejón, B.; Bernier, L.; Roberts Cervantes, E.D.; Sánchez Cendra, C.; Cassinello, J.; Trasobares, L.; et al. Paradigm of biomarkers in metastatic melanoma. Oncol. Lett. 2024, 29, 78. [Google Scholar] [CrossRef] [PubMed]
- Ždralević, M.; Raonić, J.; Popovic, N.; Vučković, L.; Rovčanin Dragović, I.; Vukčević, B.; Todorović, V.; Vukmirović, F.; Marzano, F.; Tullo, A.; et al. The role of miRNA in colorectal cancer diagnosis: A pilot study. Oncol. Lett. 2023, 25, 267. [Google Scholar] [CrossRef]
- Gattuso, G.; Crimi, S.; Lavoro, A.; Rizzo, R.; Musumarra, G.; Gallo, S.; Facciponte, F.; Paratore, S.; Russo, A.; Bordonaro, R.; et al. Liquid Biopsy and Circulating Biomarkers for the Diagnosis of Precancerous and Cancerous Oral Lesions. Noncoding RNA 2022, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Wang, H.; Yao, X.; Zhang, D.; Xie, Y.; Cui, R.; Zhang, X. Circulating MicroRNAs in Cancer: Potential and Challenge. Front. Genet. 2019, 10, 626. [Google Scholar] [CrossRef] [PubMed]
- Pekarek, L.; Torres-Carranza, D.; Fraile-Martinez, O.; García-Montero, C.; Pekarek, T.; Saez, M.A.; Rueda-Correa, F.; Pimentel-Martinez, C.; Guijarro, L.G.; Diaz-Pedrero, R.; et al. An Overview of the Role of MicroRNAs on Carcinogenesis: A Focus on Cell Cycle, Angiogenesis and Metastasis. Int. J. Mol. Sci. 2023, 24, 7268. [Google Scholar] [CrossRef]
- Bader El Din, N.G.; El Shenawy, R.; Moustafa, R.I.; Khairy, A.; Farouk, S. Association between the expression level of miRNA 374a and TGF-beta;1 in patients with colorectal cancer. World Acad. Sci. J. 2024, 6, 68. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, Y.; Liu, X.; Cao, L.; Han, M.; Wang, C.; Chen, J.; Zhang, X. miR-151a-5p promotes the proliferation and metastasis of colorectal carcinoma cells by targeting AGMAT. Oncol. Rep. 2023, 49, 50. [Google Scholar] [CrossRef] [PubMed]
- Pizzini, S.; Bisognin, A.; Mandruzzato, S.; Biasiolo, M.; Facciolli, A.; Perilli, L.; Rossi, E.; Esposito, G.; Rugge, M.; Pilati, P.; et al. Impact of microRNAs on regulatory networks and pathways in human colorectal carcinogenesis and development of metastasis. BMC Genom. 2013, 14, 589. [Google Scholar] [CrossRef] [PubMed]
- Gaedcke, J.; Grade, M.; Camps, J.; Søkilde, R.; Kaczkowski, B.; Schetter, A.J.; Difilippantonio, M.J.; Harris, C.C.; Ghadimi, B.M.; Møller, S.; et al. The rectal cancer microRNAome--microRNA expression in rectal cancer and matched normal mucosa. Clin. Cancer Res. 2012, 18, 4919–4930. [Google Scholar] [CrossRef] [PubMed]
- Hedayat, S.; Cascione, L.; Cunningham, D.; Schirripa, M.; Lampis, A.; Hahne, J.C.; Tunariu, N.; Hong, S.P.; Marchetti, S.; Khan, K.; et al. Circulating microRNA Analysis in a Prospective Co-clinical Trial Identifies MIR652-3p as a Response Biomarker and Driver of Regorafenib Resistance Mechanisms in Colorectal Cancer. Clin. Cancer Res. 2024, 30, 2140–2159. [Google Scholar]
- AlZaabi, A.; Shalaby, A. A Systematic Review of Diagnostic Performance of Circulating MicroRNAs in Colorectal Cancer Detection with a Focus on Early-Onset Colorectal Cancer. Int. J. Mol. Sci. 2024, 25, 9565. [Google Scholar] [CrossRef] [PubMed]
- Falzone, L.; Scola, L.; Zanghì, A.; Biondi, A.; Di Cataldo, A.; Libra, M.; Candido, S. Integrated analysis of colorectal cancer microRNA datasets: Identification of microRNAs associated with tumor development. Aging 2018, 10, 1000–1014. [Google Scholar] [CrossRef] [PubMed]
- Crimi, S.; Falzone, L.; Gattuso, G.; Grillo, C.M.; Candido, S.; Bianchi, A.; Libra, M. Droplet Digital PCR Analysis of Liquid Biopsy Samples Unveils the Diagnostic Role of hsa-miR-133a-3p and hsa-miR-375-3p in Oral Cancer. Biology 2020, 9, 379. [Google Scholar] [CrossRef]
- Miotto, E.; Saccenti, E.; Lupini, L.; Callegari, E.; Negrini, M.; Ferracin, M. Quantification of circulating miRNAs by droplet digital PCR: Comparison of EvaGreen- and TaqMan-based chemistries. Cancer Epidemiol. Biomarkers Prev. 2014, 23, 2638–2642. [Google Scholar] [CrossRef] [PubMed]
- Gahlawat, A.W.; Witte, T.; Sinn, P.; Schott, S. Circulating cf-miRNA as a more appropriate surrogate liquid biopsy marker than cfDNA for ovarian cancer. Sci. Rep. 2023, 13, 5503. [Google Scholar] [CrossRef]
- Falzone, L.; Bordonaro, R.; Libra, M. SnapShot: Cancer Chemotherapy. Cell 2023, 186, 1816–1816.e1. [Google Scholar] [CrossRef]
- Li, J.; Chen, H.; Sun, G.; Zhang, X.; Ye, H.; Wang, P. Role of miR-21 in the diagnosis of colorectal cancer: Meta-analysis and bioinformatics. Pathol. Res. Pract. 2023, 248, 154670. [Google Scholar] [CrossRef]
- Liu, T.; Liu, D.; Guan, S.; Dong, M. Diagnostic role of circulating MiR-21 in colorectal cancer: A update meta-analysis. Ann. Med. 2017, 53, 87–102. [Google Scholar] [CrossRef]
- Rattan Negi, R.; Rana, S.V.; Gupta, V.; Gupta, R.; Dhawan, D.K. Evaluation of the Plasma Expression Levels of miR-21 and miR-145 as Potential Non-Invasive Biomarkers for Early Detection of Colorectal Cancer. Asian Pac. J. Cancer Prev. 2024, 25, 2797–2804. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, J.; Gao, C.; Zhu, D.; Xu, X.; Wu, C.; Jiang, J. MicroRNA-497 inhibits tumor growth through targeting insulin receptor substrate 1 in colorectal cancer. Oncol. Lett. 2017, 14, 6379–6386. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, L.; Jiang, C.F.; Li, D.M.; Ge, X.; Shi, Z.M.; Li, C.Y.; Liu, X.; Yin, Y.; Zhen, L.; Liu, L.Z.; et al. MicroRNA-497 inhibits tumor growth and increases chemosensitivity to 5-fluorouracil treatment by targeting KSR1. Oncotarget 2016, 7, 2660–2671. [Google Scholar] [CrossRef]
- Fonseca, A.; Ramalhete, S.V.; Mestre, A.; Pires das Neves, R.; Marreiros, A.; Castelo-Branco, P.; Roberto, V.P. Identification of colorectal cancer associated biomarkers: An integrated analysis of miRNA expression. Aging 2021, 13, 21991–22029. [Google Scholar] [CrossRef]
- Xu, L.; Li, M.; Wang, M.; Yan, D.; Feng, G.; An, G. The expression of microRNA-375 in plasma and tissue is matched in human colorectal cancer. BMC Cancer 2014, 14, 714. [Google Scholar] [CrossRef]
- Yan, L.X.; Liu, Y.H.; Xiang, J.W.; Wu, Q.N.; Xu, L.B.; Luo, X.L.; Zhu, X.L.; Liu, C.; Xu, F.P.; Luo, D.L.; et al. PIK3R1 targeting by miR-21 suppresses tumor cell migration and invasion by reducing PI3K/AKT signaling and reversing EMT, and predicts clinical outcome of breast cancer. Int. J. Oncol. 2016, 48, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Li, X.; Cheng, T.; Wu, J. miR-21-5p/SMAD7 axis promotes the progress of lung cancer. Thorac. Cancer 2021, 12, 2307–2313. [Google Scholar] [CrossRef]
- Qiu, Y.; Yu, H.; Shi, X.; Xu, K.; Tang, Q.; Liang, B.; Hu, S.; Bao, Y.; Xu, J.; Cai, J.; et al. microRNA-497 inhibits invasion and metastasis of colorectal cancer cells by targeting vascular endothelial growth factor-A. Cell Prolif. 2016, 49, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Zaharie, F.; Muresan, M.S.; Petrushev, B.; Berce, C.; Gafencu, G.A.; Selicean, S.; Jurj, A.; Cojocneanu-Petric, R.; Lisencu, C.I.; Pop, L.A.; et al. Exosome-Carried microRNA-375 Inhibits Cell Progression and Dissemination via Bcl-2 Blocking in Colon Cancer. J. Gastrointestin. Liver Dis. 2015, 24, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Zou, G.; Wang, R.; Wang, M. Clinical response and prognostic significance of serum miR-497 expression in colorectal cancer. Cancer Biomark. 2019, 25, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhou, Y.; Liang, S. Correlation Between miR-497-5p Expression with Clinicopathological Characteristics and Prognosis in Patients with Breast Cancer. Appl. Immunohistochem. Mol. Morphol. 2024, 32, 200–205. [Google Scholar] [CrossRef]
- Gan, J.; Zhang, Y.; Liu, S.; Mu, G.; Zhao, J.; Jiang, W.; Li, J.; Li, Q.; Wu, Y.; Wang, X.; et al. MicroRNA-375 restrains the progression of lung squamous cell carcinoma by modulating the ERK pathway via UBE3A-mediated DUSP1 degradation. Cell Death Discov. 2023, 9, 199. [Google Scholar] [CrossRef]
- De Nunzio, V.; Donghia, R.; Pesole, P.L.; Coletta, S.; Calò, N.; Notarnicola, M. Serum Cytokine and miRNA Levels Are Differently Expressed in Right- and Left-Sided Colon Cancer. J. Clin. Med. 2023, 12, 5986. [Google Scholar] [CrossRef] [PubMed]
- Slattery, M.L.; Herrick, J.S.; Pellatt, D.F.; Mullany, L.E.; Stevens, J.R.; Wolff, E.; Hoffman, M.D.; Wolff, R.K.; Samowitz, W. Site-specific associations between miRNA expression and survival in colorectal cancer cases. Oncotarget 2016, 7, 60193–60205. [Google Scholar] [CrossRef]
- Slattery, M.L.; Wolff, E.; Hoffman, M.D.; Pellatt, D.F.; Milash, B.; Wolff, R.K. MicroRNAs and colon and rectal cancer: Differential expression by tumor location and subtype. Genes Chromosomes Cancer 2011, 50, 196–206. [Google Scholar] [CrossRef]
- Min, L.; Zhu, S.; Chen, L.; Liu, X.; Wei, R.; Zhao, L.; Yang, Y.; Zhang, Z.; Kong, G.; Li, P.; et al. Evaluation of circulating small extracellular vesicles derived miRNAs as biomarkers of early colon cancer: A comparison with plasma total miRNAs. J. Extracell. Vesicles 2019, 8, 1643670. [Google Scholar] [CrossRef] [PubMed]
- Marletta, S.; Rizzo, A.; Spoto, G.; Falzone, L. Predictive and prognostic biomarkers in cancer: Towards the precision medicine era. Explor. Target Antitumor. Ther. 2024, 5, 1321–1325. [Google Scholar] [CrossRef] [PubMed]
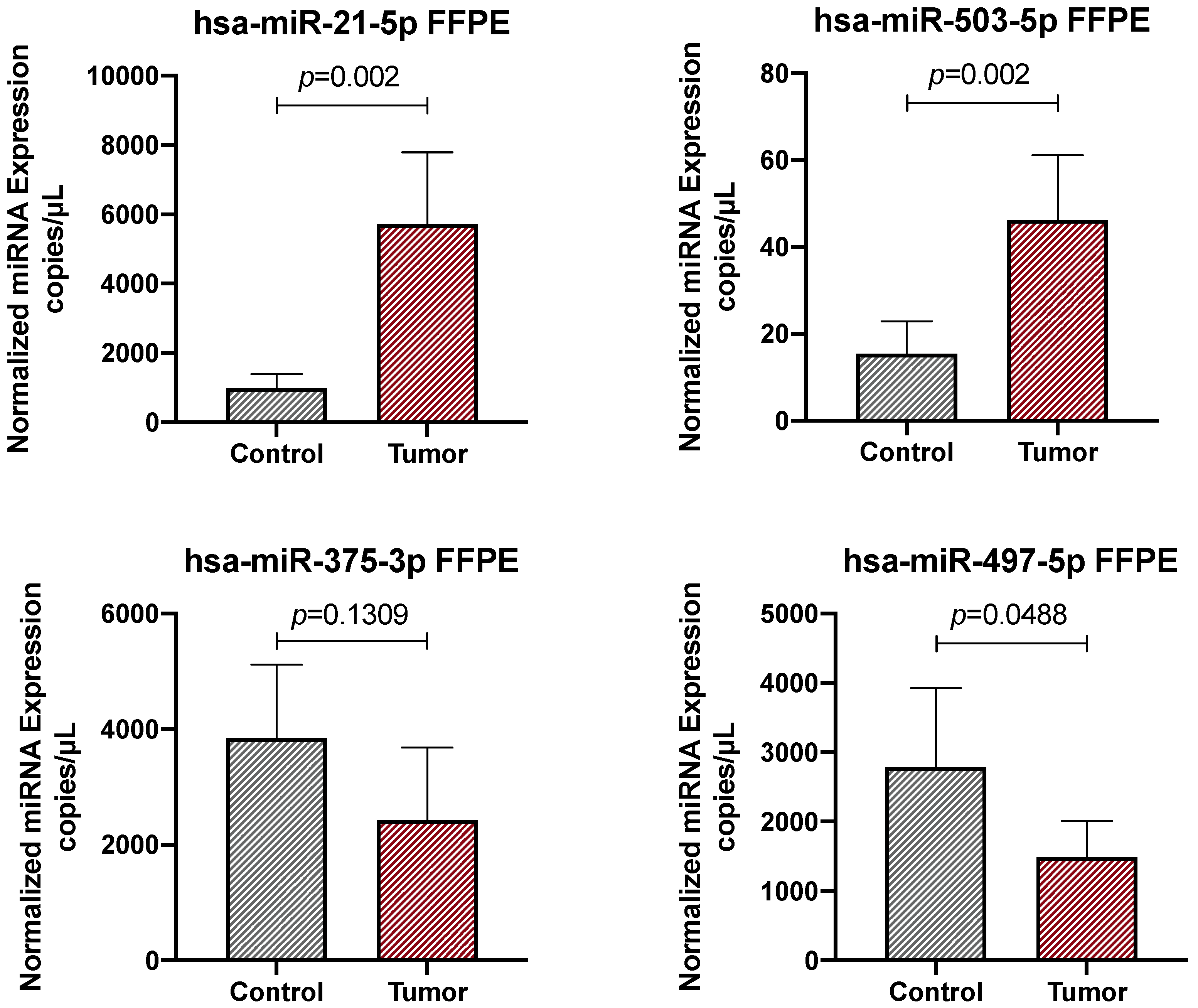

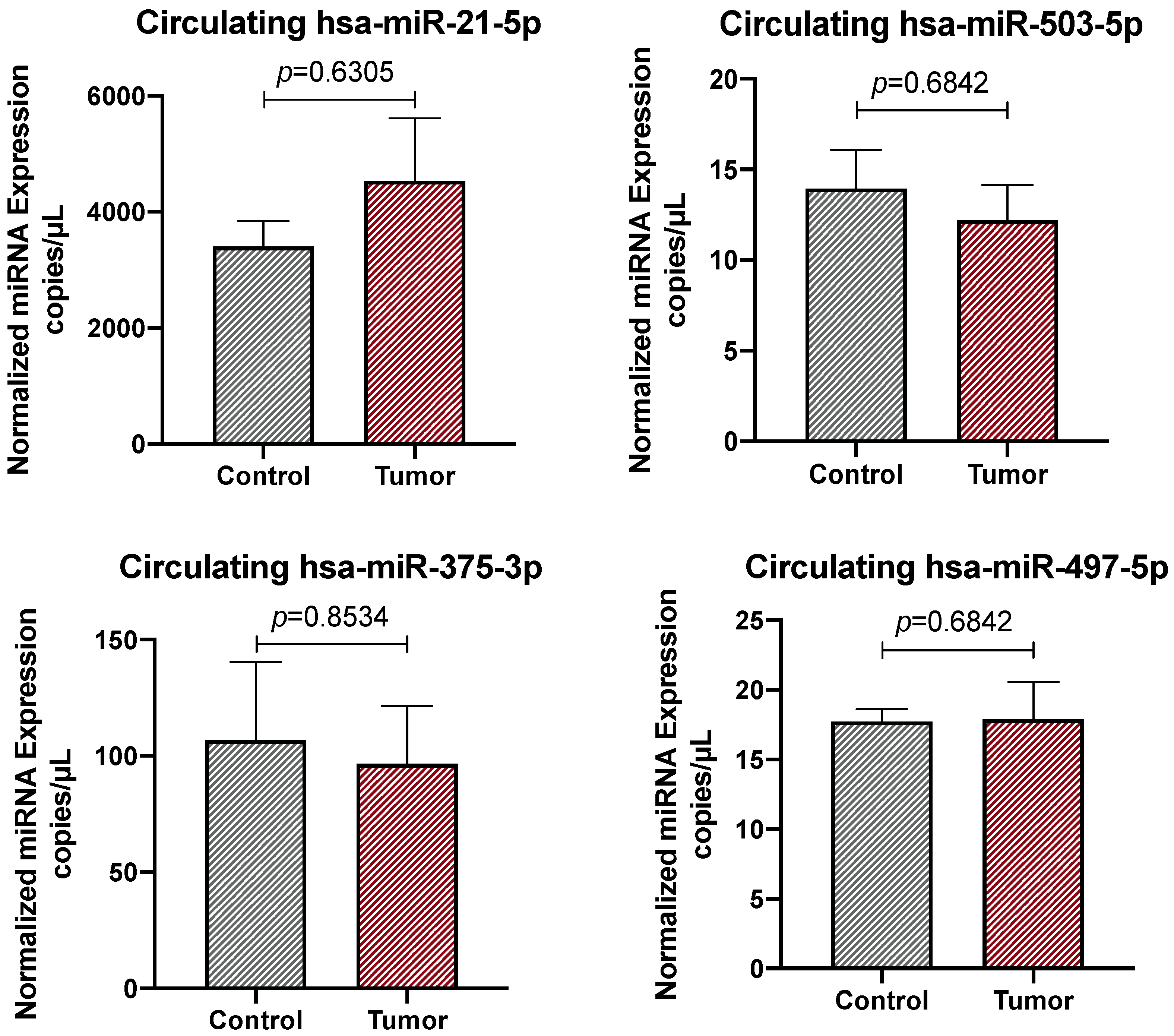

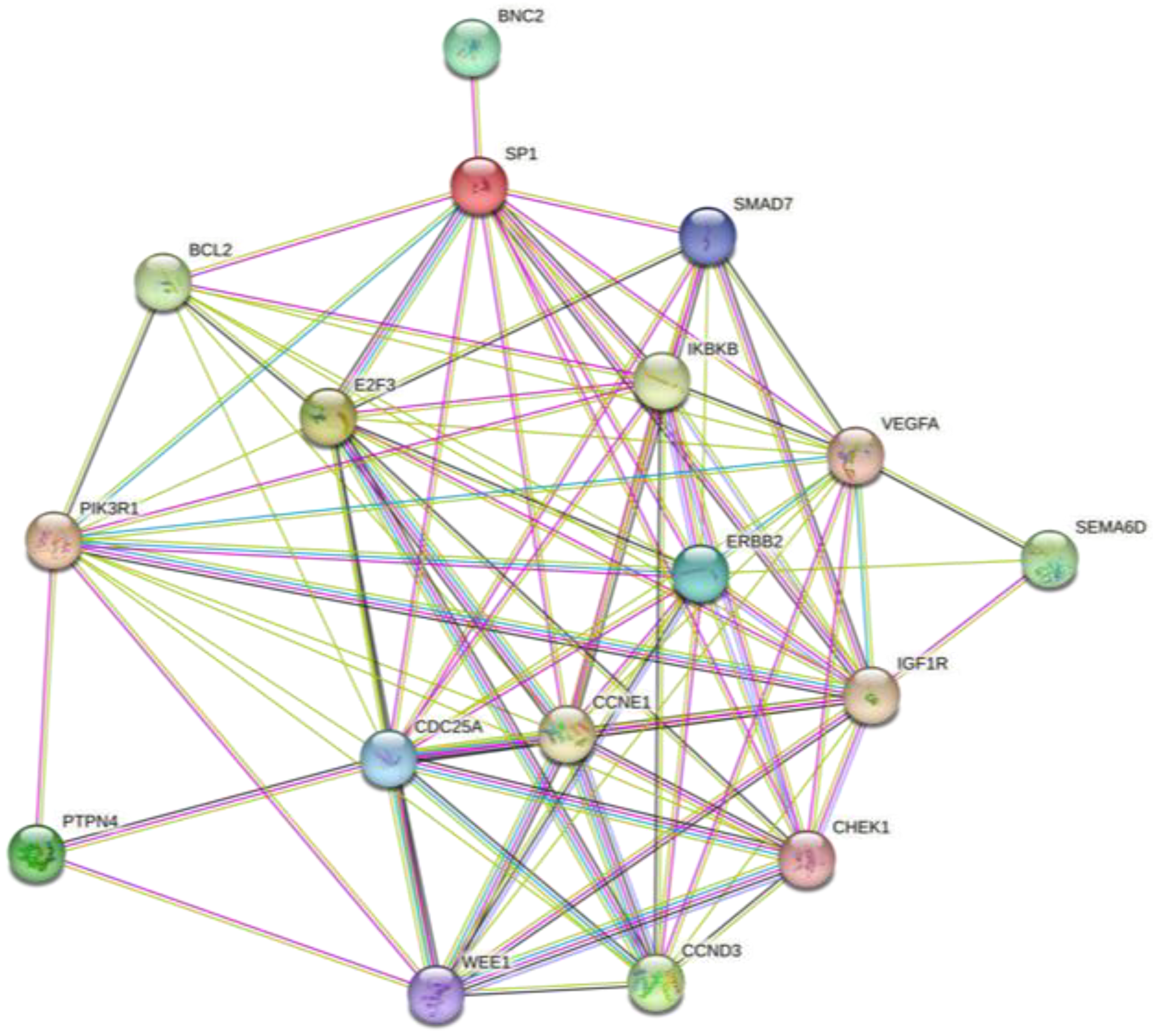
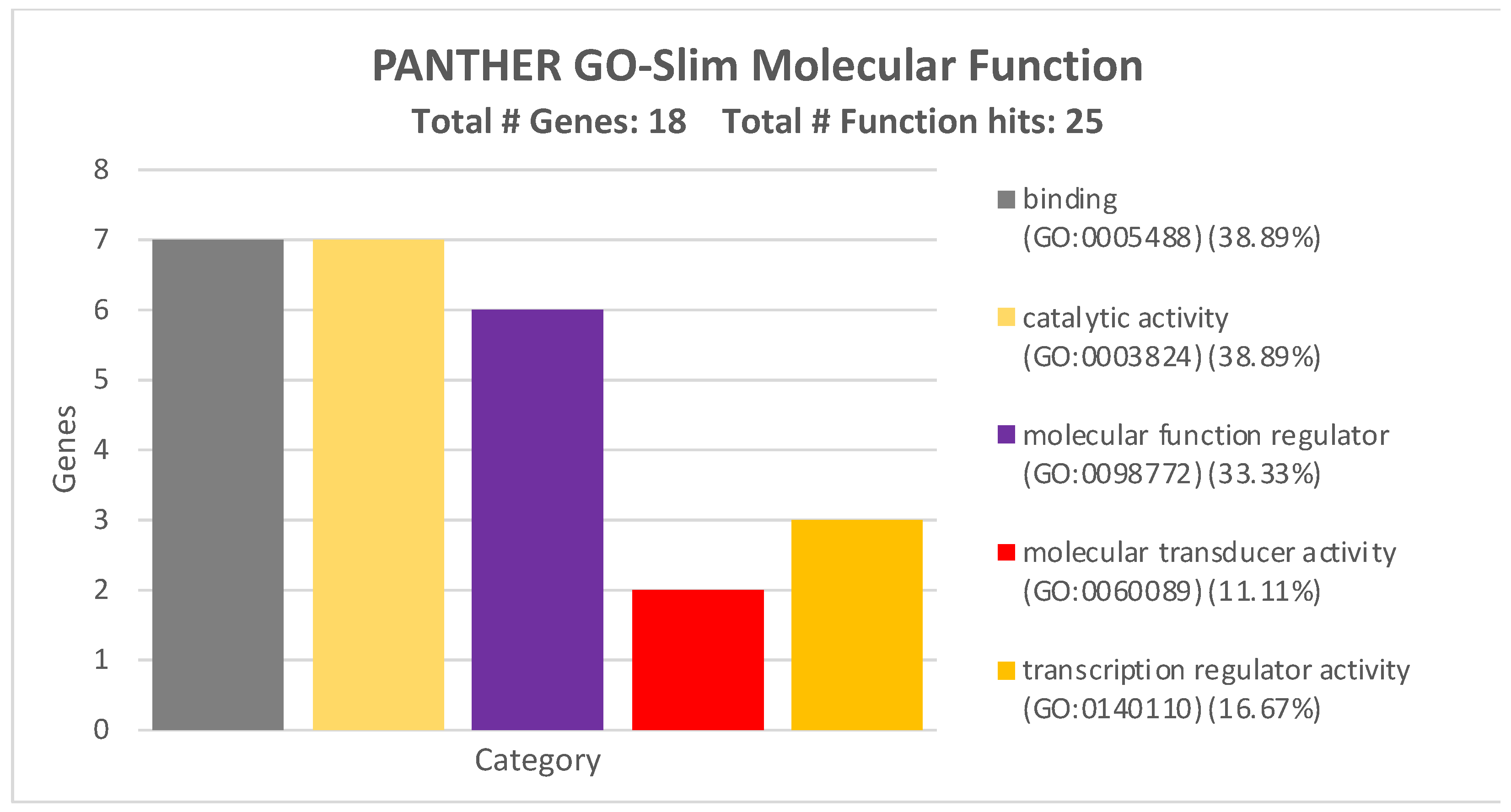
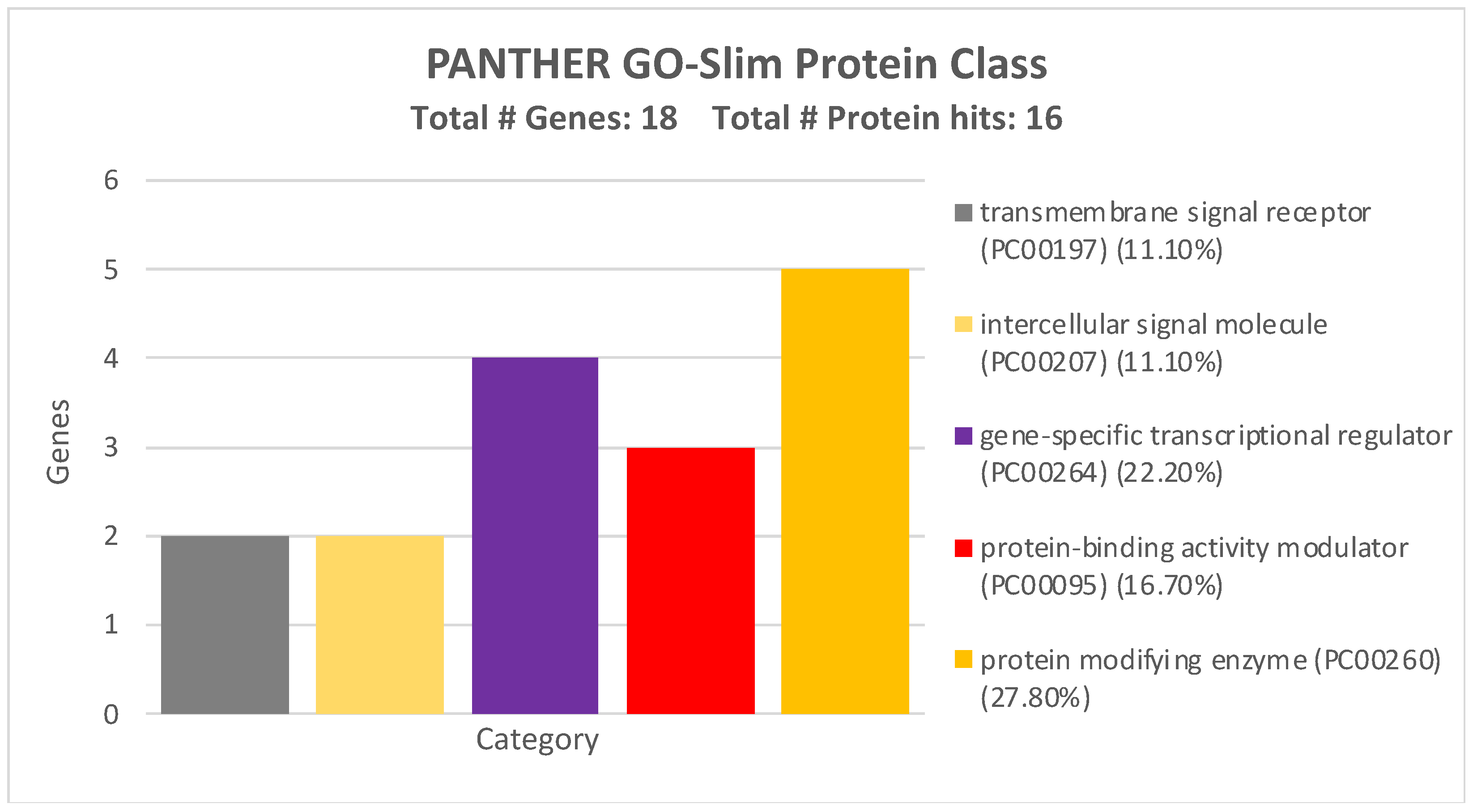

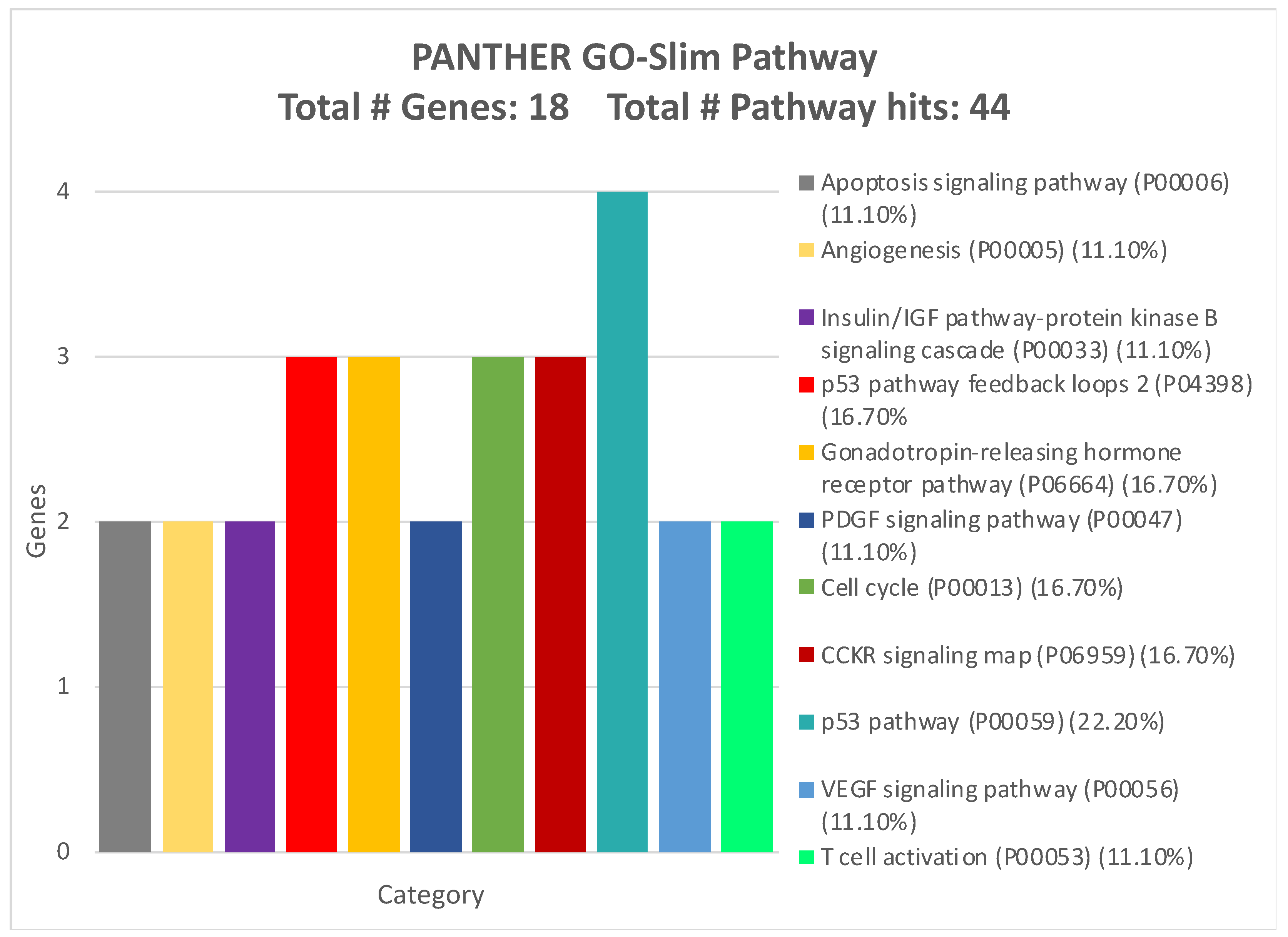

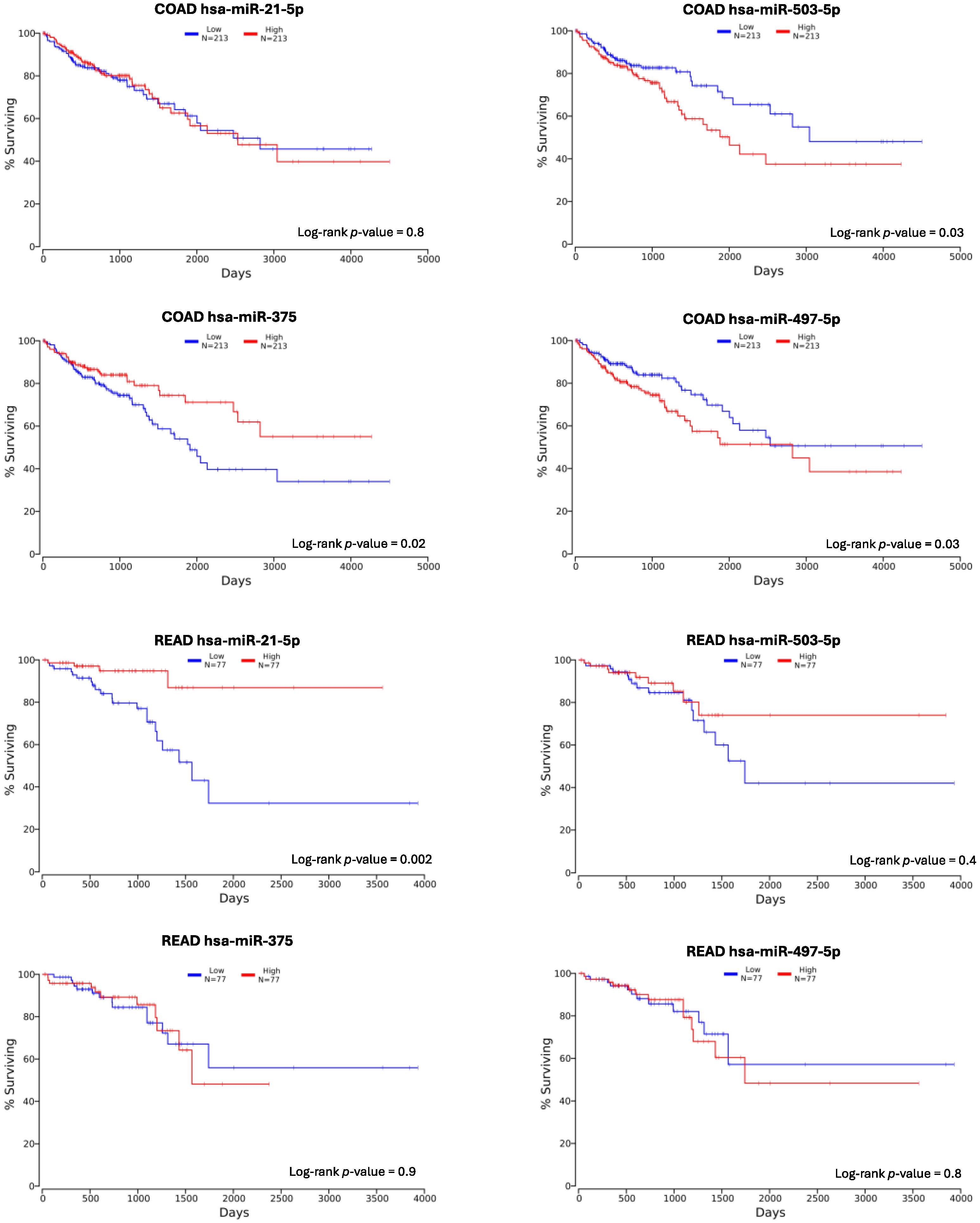
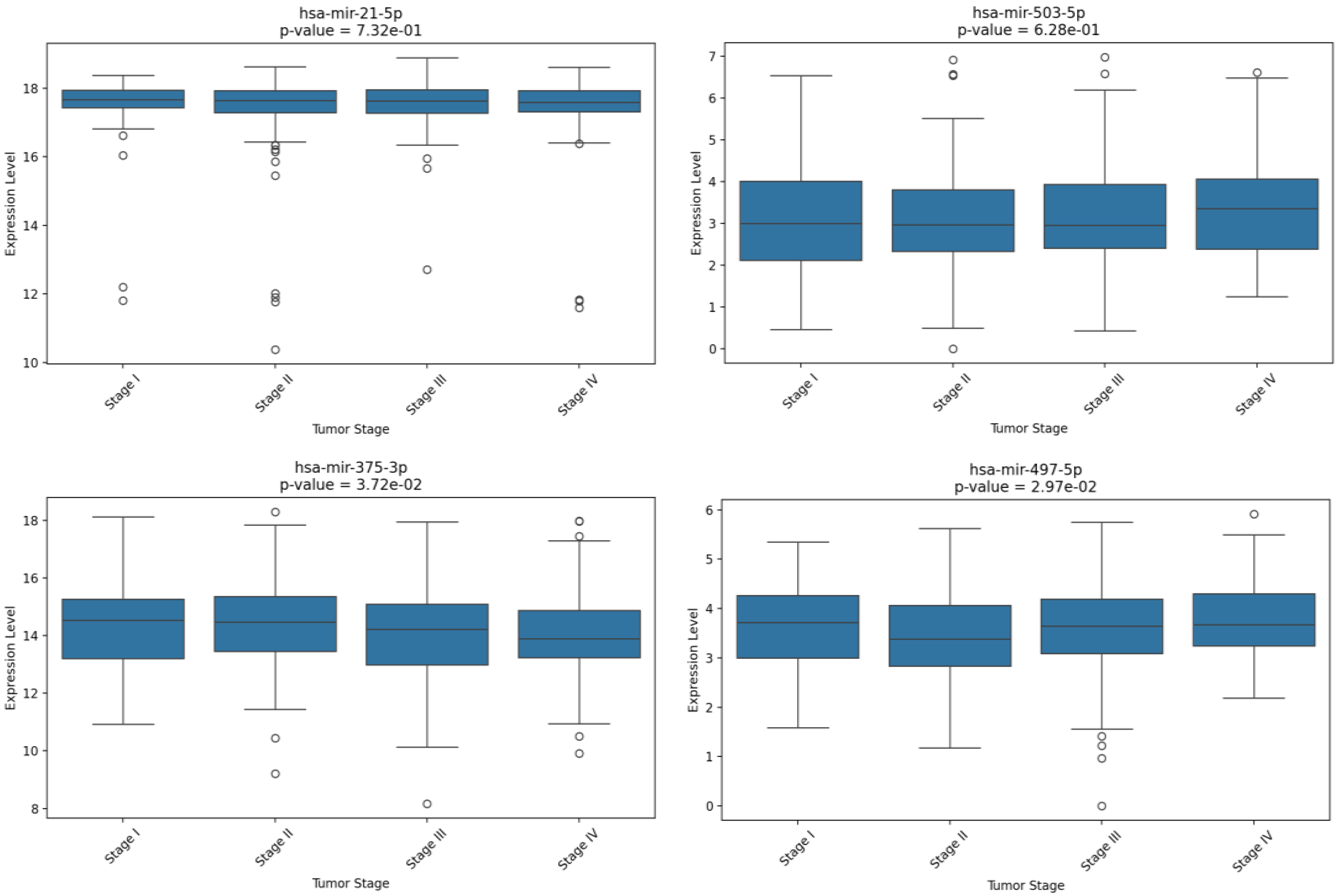
| miRNA | Sensitivity | Specificity |
|---|---|---|
| hsa-miR-21-5p | 100% | 80% |
| hsa-miR-503-5p | 90% | 70% |
| hsa-miR-375-3p | - | - |
| hsa-miR-497-5p | - | - |
| FFPE | Liquid Biopsy | Fisher’s Test Chi-Square Test | |||||
|---|---|---|---|---|---|---|---|
| Cancer Patients (N. 10) | Cancer Patients (N. 10) | Normal Controls (N. 10) | |||||
| N. | % | N. | % | N. | % | ||
| Sex | |||||||
| Male | 5 | 50 | 7 | 70 | 9 | 90 | 0.5820 |
| Female | 5 | 50 | 3 | 30 | 1 | 10 | |
| Age | |||||||
| <60 | 0 | 0 | 2 | 20 | 6 | 60 | 0.1698 |
| ≥60 | 10 | 100 | 8 | 80 | 4 | 40 | |
| Smoke | |||||||
| Yes | NA | 2 | 20 | 3 | 30 | 0.8646 | |
| No | 6 | 60 | 5 | 50 | |||
| Ex-smoker | 2 | 20 | 2 | 20 | |||
| Tumor Stage | |||||||
| T1 | 0 | 0 | 1 | 10 | NA | ||
| T2 | 1 | 10 | 2 | 20 | |||
| T3 | 6 | 60 | 5 | 50 | |||
| T4 | 3 | 30 | 1 | 10 | |||
| Lymph Nodes | |||||||
| Positive | 5 | 50 | 5 | 50 | NA | ||
| Negative | 4 | 40 | 5 | 50 | |||
| Nx | 1 | 10 | 0 | 0 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gattuso, G.; Longo, F.; Spoto, G.; Ricci, D.; Lavoro, A.; Candido, S.; Di Cataldo, A.; Broggi, G.; Salvatorelli, L.; Magro, G.; et al. Diagnostic and Prognostic Significance of a Four-miRNA Signature in Colorectal Cancer. Int. J. Mol. Sci. 2025, 26, 1219. https://doi.org/10.3390/ijms26031219
Gattuso G, Longo F, Spoto G, Ricci D, Lavoro A, Candido S, Di Cataldo A, Broggi G, Salvatorelli L, Magro G, et al. Diagnostic and Prognostic Significance of a Four-miRNA Signature in Colorectal Cancer. International Journal of Molecular Sciences. 2025; 26(3):1219. https://doi.org/10.3390/ijms26031219
Chicago/Turabian StyleGattuso, Giuseppe, Federica Longo, Graziana Spoto, Daria Ricci, Alessandro Lavoro, Saverio Candido, Antonio Di Cataldo, Giuseppe Broggi, Lucia Salvatorelli, Gaetano Magro, and et al. 2025. "Diagnostic and Prognostic Significance of a Four-miRNA Signature in Colorectal Cancer" International Journal of Molecular Sciences 26, no. 3: 1219. https://doi.org/10.3390/ijms26031219
APA StyleGattuso, G., Longo, F., Spoto, G., Ricci, D., Lavoro, A., Candido, S., Di Cataldo, A., Broggi, G., Salvatorelli, L., Magro, G., Libra, M., & Falzone, L. (2025). Diagnostic and Prognostic Significance of a Four-miRNA Signature in Colorectal Cancer. International Journal of Molecular Sciences, 26(3), 1219. https://doi.org/10.3390/ijms26031219











