Circulating hs-CRP, IL-18, Chemerin, Leptin, and Adiponectin Levels Reflect Cardiometabolic Dysfunction in Adults with Excess Weight
Abstract
1. Introduction
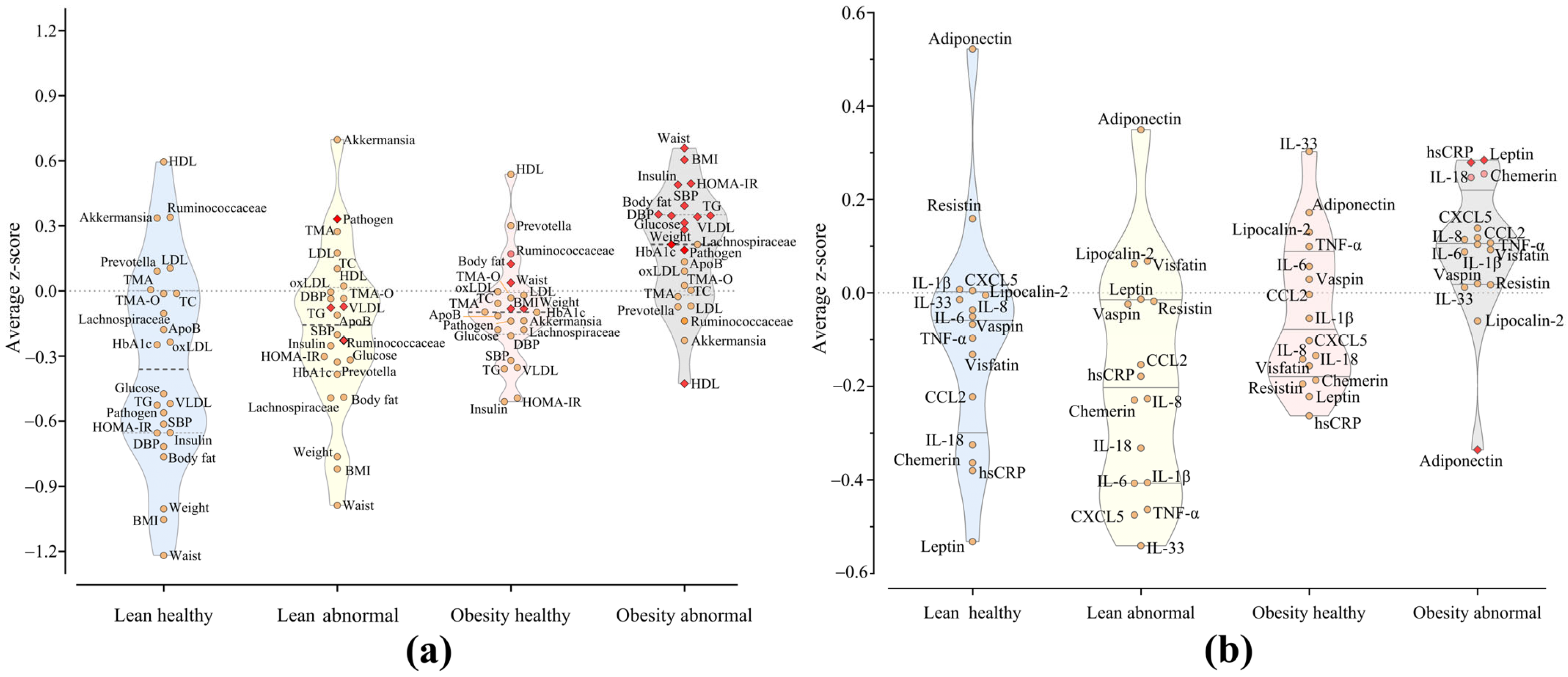
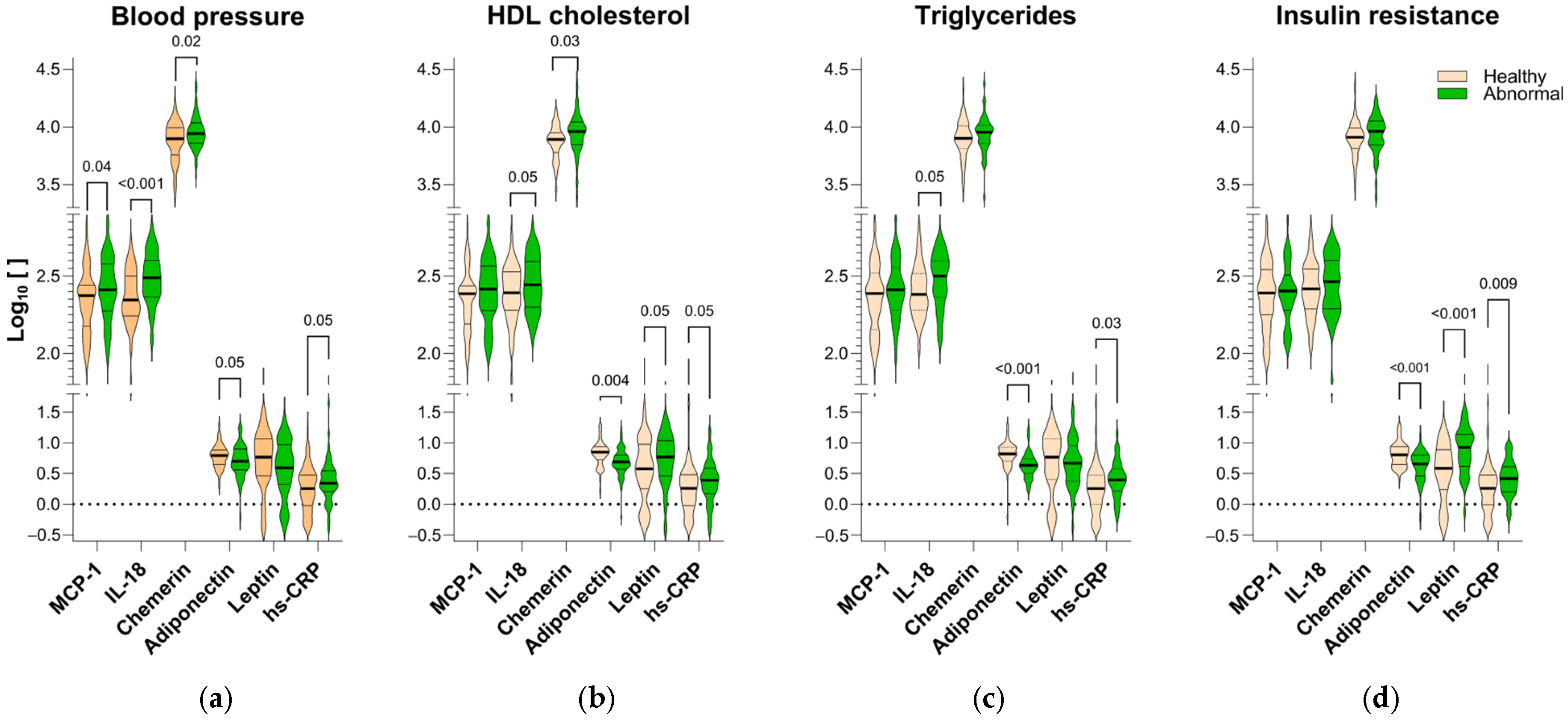
2. Results
3. Discussion
3.1. Heterogeneity Within Obesity
3.2. Gut Microbiota and Metabolic Health
3.3. Adipokines as Biomarkers
4. Materials and Methods
4.1. Study Population
4.2. Subjects
4.3. Adipokines Determination
4.4. TMA and TMA-O Sample Preparation and Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lindstrom, M.; DeCleene, N.; Dorsey, H.; Fuster, V.; Johnson, C.O.; LeGrand, K.E.; Mensah, G.A.; Razo, C.; Stark, B.; Varieur Turco, J.; et al. Global Burden of Cardiovascular Diseases and Risks Collaboration, 1990–2021. J. Am. Coll. Cardiol. 2022, 80, 2372–2425. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Rayner, A.W.; Gregg, E.W.; Sheffer, K.E.; Carrillo-Larco, R.M.; Bennett, J.E.; Shaw, J.E.; Paciorek, C.J.; Singleton, R.K.; Barradas Pires, A.; et al. Worldwide Trends in Diabetes Prevalence and Treatment from 1990 to 2022: A Pooled Analysis of 1108 Population-Representative Studies with 141 Million Participants. Lancet 2024, 404, 2077–2093. [Google Scholar] [CrossRef]
- Blüher, M. Obesity: Global Epidemiology and Pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Gómez-Ambrosi, J.; Silva, C.; Galofré, J.C.; Escalada, J.; Santos, S.; Millán, D.; Vila, N.; Ibañez, P.; Gil, M.J.; Valentí, V.; et al. Body Mass Index Classification Misses Subjects with Increased Cardiometabolic Risk Factors Related to Elevated Adiposity. Int. J. Obes. 2012, 36, 286–294. [Google Scholar] [CrossRef]
- Thomas, F.; Bean, K.; Pannier, B.; Oppert, J.-M.; Guize, L.; Benetos, A. Cardiovascular Mortality in Overweight Subjects: The Key Role of Associated Risk Factors. Hypertension 2005, 46, 654–659. [Google Scholar] [CrossRef]
- Wildman, R.P.; Muntner, P.; Reynolds, K.; McGinn, A.P.; Rajpathak, S.; Wylie-Rosett, J.; Sowers, M.R. The Obese without Cardiometabolic Risk Factor Clustering and the Normal Weight with Cardiometabolic Risk Factor Clustering: Prevalence and Correlates of 2 Phenotypes among the US Population (NHANES 1999–2004). Arch. Intern. Med. 2008, 168, 1617–1624. [Google Scholar] [CrossRef]
- Stefan, N.; Häring, H.-U.; Hu, F.B.; Schulze, M.B. Metabolically Healthy Obesity: Epidemiology, Mechanisms, and Clinical Implications. Lancet Diabetes Endocrinol. 2013, 1, 152–162. [Google Scholar] [CrossRef]
- Primeau, V.; Coderre, L.; Karelis, A.D.; Brochu, M.; Lavoie, M.-E.; Messier, V.; Sladek, R.; Rabasa-Lhoret, R. Characterizing the Profile of Obese Patients Who Are Metabolically Healthy. Int. J. Obes. 2011, 35, 971–981. [Google Scholar] [CrossRef]
- Elías-López, D.; Vargas-Vázquez, A.; Mehta, R.; Cruz Bautista, I.; Del Razo Olvera, F.; Gómez-Velasco, D.; Almeda Valdes, P.; Aguilar-Salinas, C.A. Natural Course of Metabolically Healthy Phenotype and Risk of Developing Cardiometabolic Diseases: A Three Years Follow-up Study. BMC Endocr. Disord. 2021, 21, 85. [Google Scholar] [CrossRef]
- Blüher, M. Metabolically Healthy Obesity. Endocr. Rev. 2020, 41, bnaa004. [Google Scholar] [CrossRef]
- Ler, P.; Ojalehto, E.; Zhan, Y.; Finkel, D.; Dahl Aslan, A.K.; Karlsson, I.K. Conversions between Metabolically Unhealthy and Healthy Obesity from Midlife to Late-Life. Int. J. Obes. 2024, 48, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Choe, S.S.; Huh, J.Y.; Hwang, I.J.; Kim, J.I.; Kim, J.B. Adipose Tissue Remodeling: Its Role in Energy Metabolism and Metabolic Disorders. Front. Endocrinol. 2016, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Emanuela, F.; Grazia, M.; Marco, D.R.; Maria Paola, L.; Giorgio, F.; Marco, B. Inflammation as a Link between Obesity and Metabolic Syndrome. J. Nutr. Metab. 2012, 2012, 476380. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.B.; Reams, G.P.; Spear, R.M.; Freeman, R.H.; Villarreal, D. Leptin: Linking Obesity, the Metabolic Syndrome, and Cardiovascular Disease. Curr. Hypertens. Rep. 2008, 10, 131–137. [Google Scholar] [CrossRef]
- Heiker, J.T. Vaspin (SerpinA12) in Obesity, Insulin Resistance, and Inflammation. J. Pept. Sci. 2014, 20, 299–306. [Google Scholar] [CrossRef]
- Landecho, M.F.; Tuero, C.; Valentí, V.; Bilbao, I.; de la Higuera, M.; Frühbeck, G. Relevance of Leptin and Other Adipokines in Obesity-Associated Cardiovascular Risk. Nutrients 2019, 11, 2664. [Google Scholar] [CrossRef]
- Sethi, J.K. Is PBEF/Visfatin/Nampt an Authentic Adipokine Relevant to the Metabolic Syndrome? Curr. Hypertens. Rep. 2007, 9, 33. [Google Scholar] [CrossRef]
- Moschen, A.R.; Adolph, T.E.; Gerner, R.R.; Wieser, V.; Tilg, H. Lipocalin-2: A Master Mediator of Intestinal and Metabolic Inflammation. Trends Endocrinol. Metab. 2017, 28, 388–397. [Google Scholar] [CrossRef]
- Jacenik, D.; Fichna, J. Chemerin in Immune Response and Gastrointestinal Pathophysiology. Clin. Chim. Acta 2020, 504, 146–153. [Google Scholar] [CrossRef]
- Gómez-Ambrosi, J.; Catalán, V.; Rodríguez, A.; Andrada, P.; Ramírez, B.; Ibáñez, P.; Vila, N.; Romero, S.; Margall, M.A.; Gil, M.J.; et al. Increased Cardiometabolic Risk Factors and Inflammation in Adipose Tissue in Obese Subjects Classified as Metabolically Healthy. Diabetes Care 2014, 37, 2813–2821. [Google Scholar] [CrossRef]
- Netea, M.G.; Joosten, L.A.B. The NLRP1-IL18 Connection: A Stab in the Back of Obesity-Induced Inflammation. Cell Metab. 2016, 23, 6–7. [Google Scholar] [CrossRef] [PubMed]
- Ballak, D.B.; Stienstra, R.; Tack, C.J.; Dinarello, C.A.; van Diepen, J.A. IL-1 Family Members in the Pathogenesis and Treatment of Metabolic Disease: Focus on Adipose Tissue Inflammation and Insulin Resistance. Cytokine 2015, 75, 280–290. [Google Scholar] [CrossRef]
- Jialal, I. Chemerin Levels in Metabolic Syndrome: A Promising Biomarker. Arch. Physiol. Biochem. 2023, 129, 1009–1011. [Google Scholar] [CrossRef]
- Netea, M.G.; Joosten, L.A.B.; Lewis, E.; Jensen, D.R.; Voshol, P.J.; Kullberg, B.J.; Tack, C.J.; van Krieken, H.; Kim, S.-H.; Stalenhoef, A.F.; et al. Deficiency of Interleukin-18 in Mice Leads to Hyperphagia, Obesity and Insulin Resistance. Nat. Med. 2006, 12, 650–656. [Google Scholar] [CrossRef]
- De la Cuesta-Zuluaga, J.; Corrales-Agudelo, V.; Carmona, J.A.; Abad, J.M.; Escobar, J.S. Body Size Phenotypes Comprehensively Assess Cardiometabolic Risk and Refine the Association between Obesity and Gut Microbiota. Int. J. Obes. 2018, 42, 424–432. [Google Scholar] [CrossRef]
- De la Cuesta-Zuluaga, J.; Corrales-Agudelo, V.; Velásquez-Mejía, E.P.; Carmona, J.A.; Abad, J.M.; Escobar, J.S. Gut Microbiota Is Associated with Obesity and Cardiometabolic Disease in a Population in the Midst of Westernization. Sci. Rep. 2018, 8, 11356. [Google Scholar] [CrossRef]
- Dehghan, P.; Farhangi, M.A.; Nikniaz, L.; Nikniaz, Z.; Asghari-Jafarabadi, M. Gut Microbiota-Derived Metabolite Trimethylamine N-Oxide (TMAO) Potentially Increases the Risk of Obesity in Adults: An Exploratory Systematic Review and Dose-Response Meta- Analysis. Obes. Rev. 2020, 21, e12993. [Google Scholar] [CrossRef]
- Nakamura, K.; Fuster, J.J.; Walsh, K. Adipokines: A Link between Obesity and Cardiovascular Disease. J. Cardiol. 2014, 63, 250–259. [Google Scholar] [CrossRef]
- Alfadda, A.A. Circulating Adipokines in Healthy versus Unhealthy Overweight and Obese Subjects. Int. J. Endocrinol. 2014, 2014, 170434. [Google Scholar] [CrossRef]
- Virtue, S.; Vidal-Puig, A. It’s Not How Fat You Are, It’s What You Do with It That Counts. PLoS Biol. 2008, 6, e237. [Google Scholar] [CrossRef]
- Taylor, C.T.; Kent, B.D.; Crinion, S.J.; McNicholas, W.T.; Ryan, S. Human Adipocytes Are Highly Sensitive to Intermittent Hypoxia Induced NF-KappaB Activity and Subsequent Inflammatory Gene Expression. Biochem. Biophys. Res. Commun. 2014, 447, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Trayhurn, P. Hypoxia and Adipose Tissue Function and Dysfunction in Obesity. Physiol. Rev. 2013, 93, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wood, I.S.; Trayhurn, P. Dysregulation of the Expression and Secretion of Inflammation-Related Adipokines by Hypoxia in Human Adipocytes. Pflugers Arch. 2007, 455, 479–492. [Google Scholar] [CrossRef]
- Stenkula, K.G.; Erlanson-Albertsson, C. Adipose Cell Size: Importance in Health and Disease. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R284–R295. [Google Scholar] [CrossRef]
- Nishikawa, T.; Hagihara, K.; Serada, S.; Isobe, T.; Matsumura, A.; Song, J.; Tanaka, T.; Kawase, I.; Naka, T.; Yoshizaki, K. Transcriptional Complex Formation of C-Fos, STAT3, and Hepatocyte NF-1 Alpha Is Essential for Cytokine-Driven C-Reactive Protein Gene Expression. J. Immunol. 2008, 180, 3492–3501. [Google Scholar] [CrossRef]
- D’Alessandris, C.; Lauro, R.; Presta, I.; Sesti, G. C-Reactive Protein Induces Phosphorylation of Insulin Receptor Substrate-1 on Ser307 and Ser 612 in L6 Myocytes, Thereby Impairing the Insulin Signalling Pathway That Promotes Glucose Transport. Diabetologia 2007, 50, 840–849. [Google Scholar] [CrossRef]
- De Jager, J.; Dekker, J.M.; Kooy, A.; Kostense, P.J.; Nijpels, G.; Heine, R.J.; Bouter, L.M.; Stehouwer, C.D.A. Endothelial Dysfunction and Low-Grade Inflammation Explain Much of the Excess Cardiovascular Mortality in Individuals with Type 2 Diabetes: The Hoorn Study. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1086–1093. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, L.; Meng, G.; Zhu, S.; Zhou, R.; Jiang, W. IL-18 Maintains the Homeostasis of Mucosal Immune System via Inflammasome-Independent but Microbiota-Dependent Manner. Sci. Bull. 2021, 66, 2115–2123. [Google Scholar] [CrossRef]
- Levy, M.; Thaiss, C.A.; Zeevi, D.; Dohnalová, L.; Zilberman-Schapira, G.; Mahdi, J.A.; David, E.; Savidor, A.; Korem, T.; Herzig, Y.; et al. Microbiota-Modulated Metabolites Shape the Intestinal Microenvironment by Regulating NLRP6 Inflammasome Signaling. Cell 2015, 163, 1428–1443. [Google Scholar] [CrossRef]
- Qi, H.; Gao, Y.; Li, Y.; Wei, J.; Su, X.; Zhang, C.; Liu, Y.; Zhu, H.; Sui, L.; Xiong, Y.; et al. Induction of Inflammatory Macrophages in the Gut and Extra-Gut Tissues by Colitis-Mediated Escherichia Coli. iScience 2019, 21, 474–489. [Google Scholar] [CrossRef]
- Li, Y.; Lu, H.; Guo, J.; Zhang, M.; Zheng, H.; Liu, Y.; Liu, W. Gut Microbiota-Derived Trimethylamine N-Oxide Is Associated with the Risk of All-Cause and Cardiovascular Mortality in Patients with Chronic Kidney Disease: A Systematic Review and Dose-Response Meta-Analysis. Ann. Med. 2023, 55, 2215542. [Google Scholar] [CrossRef] [PubMed]
- Sikalidis, A.K.; Maykish, A. The Gut Microbiome and Type 2 Diabetes Mellitus: Discussing A Complex Relationship. Biomedicines 2020, 8, 8. [Google Scholar] [CrossRef]
- Arango-González, A.; Lara-Guzmán, O.J.; Rivera, D.A.; Álvarez, R.; Salazar-Serrano, D.; Muñoz-Durango, K.; Escobar, J.S.; Sierra, J.A. Putative Intestinal Permeability Markers Do Not Correlate with Cardiometabolic Health and Gut Microbiota in Humans, except for Peptides Recognized by a Widely Used Zonulin ELISA Kit. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.; Dendale, P.; Beelen, M.; Jonkers, R.A.M.; Mullens, A.; Corluy, L.; Meeusen, R.; van Loon, L.J.C. Plasma Adipokine and Inflammatory Marker Concentrations Are Altered in Obese, as Opposed to Non-Obese, Type 2 Diabetes Patients. Eur. J. Appl. Physiol. 2010, 109, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Al-Hamodi, Z.; Al-Habori, M.; Al-Meeri, A.; Saif-Ali, R. Association of Adipokines, Leptin/Adiponectin Ratio and C-Reactive Protein with Obesity and Type 2 Diabetes Mellitus. Diabetol. Metab. Syndr. 2014, 6, 99. [Google Scholar] [CrossRef]
- Neuparth, M.J.; Proença, J.B.; Santos-Silva, A.; Coimbra, S. Adipokines, Oxidized Low-Density Lipoprotein, and C-Reactive Protein Levels in Lean, Overweight, and Obese Portuguese Patients with Type 2 Diabetes. Int. Sch. Res. Not. 2013, 2013, 142097. [Google Scholar] [CrossRef][Green Version]
- Niklowitz, P.; Rothermel, J.; Lass, N.; Barth, A.; Reinehr, T. Link between Chemerin, Central Obesity, and Parameters of the Metabolic Syndrome: Findings from a Longitudinal Study in Obese Children Participating in a Lifestyle Intervention. Int. J. Obes. 2018, 42, 1743–1752. [Google Scholar] [CrossRef]
- Chu, S.H.; Lee, M.K.; Ahn, K.Y.; Im, J.-A.; Park, M.S.; Lee, D.-C.; Jeon, J.Y.; Lee, J.W. Chemerin and Adiponectin Contribute Reciprocally to Metabolic Syndrome. PLoS ONE 2012, 7, e34710. [Google Scholar] [CrossRef]
- Straczkowski, M.; Kowalska, I.; Nikolajuk, A.; Otziomek, E.; Adamska, A.; Karolczuk-Zarachowicz, M.; Gorska, M. Increased Serum Interleukin-18 Concentration Is Associated with Hypoadiponectinemia in Obesity, Independently of Insulin Resistance. Int. J. Obes. 2007, 31, 221–225. [Google Scholar] [CrossRef]
- Bozaoglu, K.; Bolton, K.; McMillan, J.; Zimmet, P.; Jowett, J.; Collier, G.; Walder, K.; Segal, D. Chemerin Is a Novel Adipokine Associated with Obesity and Metabolic Syndrome. Endocrinology 2007, 148, 4687–4694. [Google Scholar] [CrossRef]
- Bozaoglu, K.; Segal, D.; Shields, K.A.; Cummings, N.; Curran, J.E.; Comuzzie, A.G.; Mahaney, M.C.; Rainwater, D.L.; VandeBerg, J.L.; MacCluer, J.W.; et al. Chemerin Is Associated with Metabolic Syndrome Phenotypes in a Mexican-American Population. J. Clin. Endocrinol. Metab. 2009, 94, 3085–3088. [Google Scholar] [CrossRef] [PubMed]
- Zorrilla, E.P.; Sanchez-Alavez, M.; Sugama, S.; Brennan, M.; Fernandez, R.; Bartfai, T.; Conti, B. Interleukin-18 Controls Energy Homeostasis by Suppressing Appetite and Feed Efficiency. Proc. Natl. Acad. Sci. USA 2007, 104, 11097–11102. [Google Scholar] [CrossRef] [PubMed]
- Lindegaard, B.; Matthews, V.B.; Brandt, C.; Hojman, P.; Allen, T.L.; Estevez, E.; Watt, M.J.; Bruce, C.R.; Mortensen, O.H.; Syberg, S.; et al. Interleukin-18 Activates Skeletal Muscle AMPK and Reduces Weight Gain and Insulin Resistance in Mice. Diabetes 2013, 62, 3064–3074. [Google Scholar] [CrossRef] [PubMed]
- Angelova, P.; Kamenov, Z.; Tsakova, A.; El-Darawish, Y.; Okamura, H. Interleukin-18 and Testosterone Levels in Men with Metabolic Syndrome. Aging Male 2018, 21, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Hu, F.B.; Yu, Z.; Li, H.; Liu, H.; Wang, X.; Yu, D.; Wu, H.; Zhang, G.; Zong, G.; et al. Lean Body Mass, Interleukin 18, and Metabolic Syndrome in Apparently Healthy Chinese. PLoS ONE 2011, 6, e18104. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Li, H.; Wang, J.-J.; Zhang, J.-S.; Shen, J.; An, X.-B.; Zhang, C.-C.; Wu, J.-M.; Song, Y.; Wang, X.-Y.; et al. IL-18 Cleavage Triggers Cardiac Inflammation and Fibrosis upon β-Adrenergic Insult. Eur. Heart J. 2018, 39, 60–69. [Google Scholar] [CrossRef]
- Ahmad, R.; Thomas, R.; Kochumon, S.; Sindhu, S. Increased Adipose Tissue Expression of IL-18R and Its Ligand IL-18 Associates with Inflammation and Insulin Resistance in Obesity. Immunity Inflamm. Dis. 2017, 5, 318–335. [Google Scholar] [CrossRef]
- Orlando, A.; Nava, E.; Giussani, M.; Genovesi, S. Adiponectin and Cardiovascular Risk. From Pathophysiology to Clinic: Focus on Children and Adolescents. Int. J. Mol. Sci. 2019, 20, 3228. [Google Scholar] [CrossRef]
- Oliveira, C.S.V.; Giuffrida, F.M.A.; Crispim, F.; Saddi-Rosa, P.; Reis, A.F. ADIPOQ and Adiponectin: The Common Ground of Hyperglycemia and Coronary Artery Disease? Arq. Bras. Endocrinol. Metabol. 2011, 55, 446–454. [Google Scholar] [CrossRef]
- Zhao, S.; Kusminski, C.M.; Scherer, P.E. Adiponectin, Leptin and Cardiovascular Disorders. Circ. Res. 2021, 128, 136–149. [Google Scholar] [CrossRef]
- Frühbeck, G.; Catalán, V.; Rodríguez, A.; Gómez-Ambrosi, J. Adiponectin-Leptin Ratio: A Promising Index to Estimate Adipose Tissue Dysfunction. Relation with Obesity-Associated Cardiometabolic Risk. Adipocyte 2018, 7, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Lopez, J.P.; Gonzalez, A.M.; Lanza, P.; Martinez-Bello, D.; Gomez-Arbelaez, D.; Otero, J.; Cohen, D.D.; Perez-Mayorga, M.; Garcia-Peña, A.A.; Rangarajan, S.; et al. Waist Circumference Cut-off Points to Identify Major Cardiovascular Events and Incident Diabetes in Latin America: Findings from the Prospective Urban Rural Epidemiology Study Colombia. Front. Cardiovasc. Med. 2023, 10, 1204885. [Google Scholar] [CrossRef]
- Lara-Guzmán, Ó.J.; Rivera, D.A.; Corrales-Agudelo, V.; Salazar-Jaramillo, L.; Gil-Izquierdo, Á.; Medina, S.; Oger, C.; Durand, T.; Galano, J.-M.; Escobar, J.S.; et al. Dietary Antioxidant Intake Is Inversely Associated with 2,3-Dinor Oxylipin Metabolites, the Major Excreted Oxylipins in Overweight and Obese Subjects. Free Radic. Biol. Med. 2022, 190, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Tomiyama, A.J.; Hunger, J.M.; Nguyen-Cuu, J.; Wells, C. Misclassification of Cardiometabolic Health When Using Body Mass Index Categories in NHANES 2005–2012. Int. J. Obes. 2016, 40, 883–886. [Google Scholar] [CrossRef] [PubMed]
| Lean Healthy | Lean Abnormal | Obesity Healthy | Obesity Abnormal | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | CI 95% | Mean ± SD | CI 95% | Mean ± SD | CI 95% | Mean ± SD | CI 95% | Model 1 | Model 2 | |
| N | 26 | - | 15 | - | 18 | - | 57 | - | - | - |
| Age (years) | 36.4 ± 11.6 | 32.0; 41.4 | 38.2 ± 11.0 | 31.0; 45.0 | 43.3 ± 10.9 | 37.9; 48.7 | 42.2 ± 11.8 | 39.1; 45.3 | - | - |
| Female (n) | 11 | - | 9 | - | 11 | 34 | - | - | - | |
| Male (n) | 11 | - | 4 | - | 11 | 25 | - | - | - | |
| Anthropometry | ||||||||||
| BMI (kg/m2) | 23.07 ± 2.02 | 22.25; 23.88 | 24.03 ± 2.45 | 22.66; 25.37 | 28.10 ± 2.91 * | 26.65; 29.55 | 30.84 ± 3.96 * | 29.79; 31.90 | <0.0001 | <0.0001 |
| Weight (kg) | 59.57 ± 6.18 | 57.07; 62.07 | 61.83 ± 9.66 | 56.48; 67.18 | 78.22 ± 12.37 * | 72.07; 84.37 | 84.10 ± 12.72 * | 80.73; 87.48 | <0.0001 | <0.0001 |
| Waist circumference (cm) | 78.81 ± 4.38 | 77.04; 80.58 | 80.74 ± 4.88 | 78.04; 83.45 | 96.33 ± 6.19 * | 93.26; 99.41 | 103.05 ± 9.23 * | 100.60; 105.50 | <0.0001 | <0.0001 |
| Body fat (%) | 33.85 ± 5.27 | 31.72; 35.98 | 35.35 ± 3.80 | 33.25; 37.45 | 38.11 ± 3.00 * | 36.62; 39.61 | 39.11 ± 5.38 * | 37.68; 40.54 | <0.0001 | <0.0001 |
| Blood pressure | ||||||||||
| Systolic (mm Hg) | 114.77 ± 9.95 | 110.75; 118.79 | 120.80 ± 25.33 * | 106.77; 134.83 | 119.22 ± 19.91 | 109.32; 129.12 | 133.77 ± 17.13 *,# | 129.23; 138.32 | <0.0001 | <0.0001 |
| Diastolic (mm Hg) | 70.31 ± 7.54 | 67.26; 73.35 | 77.53 ± 16.63 | 68.33; 86.74 | 78.83 ± 11.83 | 72.95; 84.72 | 84.65 ± 11.01 * | 81.83; 87.57 | <0.0001 | <0.0001 |
| Blood lipids | ||||||||||
| HDL (mg/dL) | 52.15 ± 9.16 | 48.46; 55.85 | 45.07 ± 7.82 | 40.73; 49.40 | 48.56 ± 6.12 | 45.51; 51.60 | 39.47 ± 11.25 *,# | 36.49; 42.46 | <0.0001 | <0.0001 |
| VLDL (mg/dL) | 18.74 ± 6.72 | 16.02; 21.45 | 28.43 ± 11.04 * | 22.31; 34.54 | 22.40 ± 10.13 | 17.36; 27.44 | 37.47 ± 27.62 *,# | 30.14; 44.80 | <0.0001 | <0.0001 |
| LDL (mg/dL) | 122.50 ± 39.11 | 106.70; 138.30 | 127.00 ± 33.65 | 108.36; 145.64 | 114.39 ± 23.63 | 102.64; 126.14 | 113.30 ± 32.84 | 104.51; 122.10 | 0.472 | 0.362 |
| Total cholesterol (mg/dL) | 191.62 ± 44.84 | 173.50; 209.73 | 197.27 ± 36.61 | 176.99; 217.54 | 181.39 ± 29.86 | 166.54; 196.24 | 187.58 ± 39.75 | 177.03; 198.12 | 0.686 | 0.460 |
| Triglycerides (mg/dL) | 93.69 ± 33.48 | 80.17; 107.21 | 142.40 ± 55.57 * | 111.62; 173.18 | 112.06 ± 50.28 | 87.05; 137.06 | 188.65 ± 137.64 *,# | 152.13; 225.17 | <0.0001 | <0.0001 |
| oxLDL (U/L) | 137.29 ± 54.41 | 114.83; 159.75 | 179.96 ± 84.58 | 133.11; 226.80 | 178.26 ± 77.21 | 139.86; 216.66 | 176.34 ± 131.18 | 140.18; 212.49 | 0.465 | 0.157 |
| ApoB (mg/dL) | 90.67 ± 27.49 | 79.56; 101.77 | 94.57 ± 15.84 | 85.79; 103.34 | 91.39 ± 26.33 | 78.30; 104.49 | 108.51 ± 84.26 | 86.15; 130.87 | 0.421 | 0.384 |
| Glycemic profile | ||||||||||
| HbA1c (%) | 5.42 ± 0.30 | 5.30; 5.54 | 5.31 ± 0.27 | 5.16; 5.46 | 5.41 ± 0.34 | 5.30; 5.59 | 5.59 ± 0.50 * | 5.46; 5.72 | 0.075 | 0.018 |
| Glucose (mg/dL) | 83.42 ± 7.06 | 80.57; 86.27 | 85.53 ± 9.46 | 80.29; 90.77 | 85.00 ± 4.10 | 82.96; 87.04 | 92.72 ± 17.24 * | 88.14; 97.29 | 0.005 | 0.050 |
| Insulin (µU/mL) | 7.90 ± 2.41 | 6.93; 8.88 | 12.65 ± 5.91 * | 9.38; 15.93 | 9.15 ± 2.85 | 7.74; 10.57 | 17.67 ± 10.27 *,# | 14.95; 20.40 | <0.0001 | <0.0001 |
| HOMA-B | 158.73 ± 89.12 | 122.74; 194.73 | 228.27 ± 138.21 | 151.73; 304.81 | 153.96 ± 58.47 | 124.89; 183.04 | 259.53 ± 177.39 * | 212.46; 306.60 | <0.0001 | <0.0001 |
| HOMA-S | 68.17 ± 24.15 | 58.42; 77.93 | 49.65 ± 30.20 | 32.92; 66.37 | 58.37 ± 22.37 | 47.25; 69.49 | 33.85 ± 20.30 *,# | 29.05; 40.03 | <0.0001 | <0.0001 |
| HOMA-IR | 1.63 ± 0.49 | 1.43; 1.82 | 2.68 ± 1.29 | 1.96; 3.40 | 1.93 ± 0.63 | 1.61; 2.24 | 4.09 ± 2.63 *,# | 3.39; 4.79 | <0.0001 | <0.0001 |
| Gut microbiota and metabolites | ||||||||||
| CAG-Prevotella | 0.17 ± 0.22 | 0.08; 0.26 | 0.09 ± 0.16 | −0.003; 0.18 | 0.27 ± 0.29 | 0.13; 0.41 | 0.15 ± 0.22 | 0.09; 0.21 | 0.466 | 0.578 |
| CAG-Lachnospiraceae | 0.20 ± 0.22 | 0.11; 0.29 | 0.18 ± 0.17 | 0.08; 0.27 | 0.20 ± 0.22 | 0.09; 0.31 | 0.27 ± 0.25 | 0.21; 0.34 | 0.483 | 0.603 |
| CAG-Pathogen | 0.04 ± 0.06 | 0.02; 0.06 | 0.19 ± 0.28 | 0.04; 0.35 | 0.15 ± 0.21 | 0.05; 0.26 | 0.24 ± 0.30 | 0.16; 0.32 | 0.034 | 0.166 |
| CAG-Akkermansia | 0.25 ± 0.29 | 0.14; 0.37 | 0.34 ± 0.31 | 0.17; 0.51 | 0.14 ± 0.20 | 0.04;0.24 | 0.12 ± 0.19 * | 0.07; 0.17 | 0.004 | 0.043 |
| CAG-Ruminococcaceae | 0.17 ± 0.17 | 0.10; 0.24 | 0.07 ± 0.08 | 0.03; 0.12 | 0.10 ± 0.14 | 0.03; 0.17 | 0.09 ± 0.13 | 0.05; 0.12 | 0.067 | 0.109 |
| TMA (µM) | 1.84 ± 0.63 | 1.59; 2.09 | 2.04 ± 0.59 | 1.71; 2.37 | 1.82 ± 0.68 | 1.48; 2.16 | 1.84 ± 0.67 | 1.66; 2.02 | 0.637 | 0.438 |
| TMA-O (µM) | 3.66 ± 0.55 | 3.46; 3.91 | 3.65 ± 0.57 | 3.34; 3.97 | 3.60 ± 0.64 | 3.28; 3.92 | 3.67 ± 0.52 | 3.53; 3.81 | 0.934 | 0.968 |
| Adipokines and inflammation markers | ||||||||||
| hs-CRP (mg/L) | 1.31 ± 0.90 | 0.94; 1.68 | 2.15 ± 1.21 | 1.48; 2.82 | 1.97 ± 1.11 | 1.41; 2.52 | 4.42 ± 6.15 *,# | 2.78; 6.05 | <0.0001 | <0.0001 |
| TNF-α (pg/mL) | 13.07 ± 5.60 | 10.81; 15.34 | 9.75 ± 4.10 | 7.48; 12.03 | 15.06 ± 7.11 | 11.52; 18.59 | 14.96 ± 9.56 | 12.42; 17.49 | 0.042 | 0.090 |
| IL-6 (pg/mL) | 5.68 ± 4.04 | 4.05; 7.31 | 3.75 ± 2.67 | 2.27; 5.22 | 6.65 ± 4.85 | 4.24; 9.06 | 6.66 ± 5.599 | 5.07; 8.25 | 0.105 | 0.190 |
| IL-33 (pg/mL) | 127.45 ± 15.16 | 121.32; 133.57 | 118.92 ± 19.43 | 108.16; 129.68 | 136.01 ± 23.05 | 124.55; 147.47 | 128.67 ± 17.53 | 124.02; 133.02 | 0.064 | 0.143 |
| IL-8 (pg/mL) | 33.36 ± 45.22 | 15.10; 51.63 | 20.72 ± 25.69 | 6.50; 34.95 | 30.33 ± 32.17 | 14.34; 46.33 | 46.67 ± 86.54 | 23.70; 69.63 | 0.324 | 0.206 |
| MCP-1 (pg/mL) | 229.28 ± 105.71 | 186.59; 271.98 | 245.41 ± 134.01 | 171.28; 319.62 | 286.75 ± 166.51 | 203.94; 369.55 | 284.05 ± 119.69 | 252.30; 315.81 | 0.134 | 0.207 |
| IL-1β (pg/mL) | 11.61 ± 1.69 | 10.92; 12.29 | 10.65 ± 2.05 | 9.51; 11.78 | 11.46 ± 2.25 | 10.34; 12.58 | 11.89 ± 2.52 | 11.22; 12.56 | 0.310 | 0.251 |
| Visfatin (ng/mL) | 2.68 ± 2.41 | 1.71; 3.65 | 3.05 ± 3.36 | 0.89; 4.61 | 2.19 ± 1.89 | 1.25; 3.13 | 3.19 ± 2.82 | 2.45; 3.94 | 0.430 | 0.565 |
| Resistin (ng/mL) | 13.00 ± 5.52 | 10.76; 15.23 | 12.42 ± 2.94 | 10.79; 14.05 | 12.47 ± 4.72 | 10.12; 14.82 | 13.07 ± 6.21 | 11.43; 14.72 | 0.993 | 0.957 |
| Lipocalin-2 (ng/mL) | 28.79 ± 4.61 | 26.92; 30.65 | 29.00 ± 3.53 | 27.04; 30.95 | 29.34 ± 6.17 | 26.27; 32.41 | 28.40 ± 4.86 | 27.16; 29.68 | 0.913 | 0.796 |
| CXCL5 (ng/mL) | 1.40 ± 0.90 | 1.03; 1.76 | 1.32 ± 0.88 | 0.84; 1.81 | 1.31 ± 0.87 | 0.88; 1.74 | 1.47 ± 1.16 | 1.17; 1.78 | 0.916 | 0.711 |
| Chemerin (ng/mL) | 7.34 ± 2.23 | 6.44; 8.24 | 8.06 ± 1.84 | 7.04; 9.08 | 8.47 ± 2.89 | 7.04; 9.91 | 9.59 ± 3.89 | 8.56; 10.62 | 0.056 | 0.058 |
| Vaspin (ng/mL) | 2.16 ± 8.98 | −0.84; 5.16 | 2.54 ± 5.60 | −0.56; 5.63 | 4.07 ± 8.41 | −0.12; 8.25 | 3.37 ± 9.40 | 0.88; 5.87 | 0.331 | 0.460 |
| IL-18 (pg/mL) | 240.66 ± 102.93 | 199.09; 282.23 | 236.95 ± 88.97 | 187.68; 286.22 | 289.18 ± 84.11 | 248.81; 329.54 | 323.17 ± 131.74 | 288.21; 358.12 | 0.008 | 0.102 |
| Leptin (ng/mL) | 4.27 ± 3.46 | 2.87; 5.67 | 7.54 ± 6.79 | 3.79; 11.30 | 5.21 ± 4.36 | 3.04; 7.37 | 8.94 ± 7.61 * | 6.92; 10.96 | 0.004 | <0.001 |
| Adiponectin (µg/mL) | 8.35 ± 3.66 | 6.87; 9.83 | 7.55 ± 4.11 | 5.27; 9.82 | 6.19 ± 2.28 | 5.06; 7.32 | 5.08 ± 2.73 * | 4.35; 5.80 | <0.0001 | <0.001 |
| Adiponectin/leptin | 5.42 ± 5.85 | 3.06; 7.79 | 1.74 ± 1.35 | 0.99; 2.49 | 2.72 ± 3.04 | 1.21; 4.23 | 1.27 ± 1.62 *,# | 0.84; 1.70 | <0.0001 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lara-Guzmán, Ó.J.; Arango-González, Á.M.; Álvarez-Quintero, R.; Escobar, J.S.; Muñoz-Durango, K.; Sierra, J.A. Circulating hs-CRP, IL-18, Chemerin, Leptin, and Adiponectin Levels Reflect Cardiometabolic Dysfunction in Adults with Excess Weight. Int. J. Mol. Sci. 2025, 26, 1176. https://doi.org/10.3390/ijms26031176
Lara-Guzmán ÓJ, Arango-González ÁM, Álvarez-Quintero R, Escobar JS, Muñoz-Durango K, Sierra JA. Circulating hs-CRP, IL-18, Chemerin, Leptin, and Adiponectin Levels Reflect Cardiometabolic Dysfunction in Adults with Excess Weight. International Journal of Molecular Sciences. 2025; 26(3):1176. https://doi.org/10.3390/ijms26031176
Chicago/Turabian StyleLara-Guzmán, Óscar Javier, Ángela María Arango-González, Rafael Álvarez-Quintero, Juan S. Escobar, Katalina Muñoz-Durango, and Jelver Alexander Sierra. 2025. "Circulating hs-CRP, IL-18, Chemerin, Leptin, and Adiponectin Levels Reflect Cardiometabolic Dysfunction in Adults with Excess Weight" International Journal of Molecular Sciences 26, no. 3: 1176. https://doi.org/10.3390/ijms26031176
APA StyleLara-Guzmán, Ó. J., Arango-González, Á. M., Álvarez-Quintero, R., Escobar, J. S., Muñoz-Durango, K., & Sierra, J. A. (2025). Circulating hs-CRP, IL-18, Chemerin, Leptin, and Adiponectin Levels Reflect Cardiometabolic Dysfunction in Adults with Excess Weight. International Journal of Molecular Sciences, 26(3), 1176. https://doi.org/10.3390/ijms26031176







