Evaluation of Larrea tridentata Extracts and Their Antimicrobial Effects on Strains of Clinical Interest
Abstract
1. Introduction
2. Results
2.1. Phytochemical Characterization
2.2. Antioxidant Characterization
2.3. Hemolysis
2.4. Microbial Inhibition
3. Discussion
4. Materials and Methods
4.1. Plant Collection
4.2. Extraction Procedure
4.3. Qualitative Tests for Chemical Profile
4.4. Determination of pH
4.5. Quantitative Tests for Chemical Profile
4.6. Determination of Hemolysis
4.7. Microbial Strains
4.8. Antimicrobial Evaluation
4.9. Multivariate and Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report. 2022. Available online: https://www.who.int/publications/i/item/9789240062702 (accessed on 15 January 2025).
- Fariña, N. Resistencia bacteriana: Un problema de salud pública mundial de difícil solución. Mem. Del. Inst. De. Investig. En. Cienc. De. La. Salud 2016, 14, 4–5. [Google Scholar] [CrossRef]
- Reygaert, C.W. An Overview of the Antimicrobial Resistance Mechanisms of Bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef] [PubMed]
- Silveira, C.R. Los productos fito-farmacéuticos en la acuicultura. REDVET Rev. Electrónica De Vet. 2006, 7, 1–10. [Google Scholar]
- Plaul, S.E.; Pérez, M.L.; Sansiñena, J.A.; Marino, S.G.P.; Barbeito, C.G. Importancia de las micosis en acuicultura ¿Es la fitoterapia una alternativa superadora para su tratamiento con respecto a los tratamientos convencionales? Rev. De. Investig. Vet. Del. Perú 2022, 33, 1–22. [Google Scholar] [CrossRef]
- World Health Organization. Traditional Medicine Strategy 2002–2005, Ginebra. 2002. Available online: https://www.who.int/publications/i/item/WHO-EDM-TRM-2002.1 (accessed on 15 January 2025).
- Maldonado, C.; Paniagua, Z.N.; Bussmann, R.; Zentero, R.F.; Fuentes, A. La importancia de las plantas medicinales, su taxonomía y la búsqueda de la cura a la enfermedad que causa el coronavirus (COVID-19). Ecol. En. Boliv. 2020, 55, 1–5. [Google Scholar]
- Vélez, G.J.M.; Veloza, L.A.; Sepúlveda, A.J.C. Biotechnology and Its Applications in the Health Sector; Arias, J.C.S., Ed.; Universidad Tecnológica de Pereira: Pereira, Colombia, 2020; pp. 347–362. [Google Scholar]
- Chibane, L.B.; Degraeve, P.; Ferhout, H.; Bouajila, J.; Oulahal, N. Plant Antimicrobial Polyphenols as Potential Natural Food Preservatives. J. Sci. Food Agric. 2019, 99, 1457–1474. [Google Scholar] [CrossRef] [PubMed]
- Lovkova, M.Y.; Buzuk, G.N.; Sokolova, S.M.; Kliment’eva, N.I. Chemical features of medicinal plants. Appl. Biochem. Microbiol. 2001, 37, 229–237. [Google Scholar] [CrossRef]
- Bhowmik, P.; Shohan, F.M.; Baroi, J.A.; Pranto, T.I.; Ullah, M.R.; Rupak, M.A.H.B.; Tashin, R. Evaluation of the Effects of Ethanolic Extract of Ficus benghalensis on the Lipid Profile and Kidney Function in Rat Model. Int. Res. J. Gastroenterol. Hepatol. 2024, 7, 22–28. [Google Scholar]
- Ávalos García, A.; Pérez-Urria Carril, E. Secondary metabolism of plants. Reduca 2009, 2, 119–145. [Google Scholar]
- Hernández, A.J.; Zaragoza, B.A.; López, R.G.; Peláez, A.A.; Olmedo, J.A.; Rivero, P.N. Actividad antibacteriana y sobre nematodos gastrointestinales de metabolitos secundarios vegetales: Enfoque en Medicina Veterinaria. Abanico Vet. 2018, 8, 14–27. [Google Scholar]
- Angelini, P. Plant-Derived Antimicrobials and Their Crucial Role in Combating Antimicrobial Resistance. Antibiotics 2024, 13, 746. [Google Scholar] [CrossRef] [PubMed]
- Laport, R.G.; Minckley, R.L.; Ramsey, J. Phylogeny and cytogeography of the North American creosote bush (Larrea tridentata, Zygophyllaceae). Syst. Bot. 2012, 37, 153–164. [Google Scholar] [CrossRef]
- Morales, U.A.L.; Rivero, P.N.; Valladares, C.B.; Madariaga, N.A.; Higuera, P.R.I.; Delgadillo, R.L.; Bañuelos, V.R.; Zaragoza, B.A. Phytochemical compounds and pharmacological properties of Larrea tridentata. Molecules 2022, 27, 5393. [Google Scholar] [CrossRef] [PubMed]
- Arteaga, S.; Andrade, C.; Cárdenas, R. Larrea tridentata (Creosote bush), an abundant plant of Mexican and US-American deserts and its metabolite nordihydroguaiaretic acid. J. Ethnopharmacol. 2005, 98, 231–239. [Google Scholar] [CrossRef]
- Beltramo, C.; Marinoni, D.T.; Botta, R. Gene expression analysis in female inflorescences of Corylus avellana L. In Proceedings of the 14th International Biotechnology Symposium and Exhibition, Rimini, Italy, 14–18 September 2010; p. 74. [Google Scholar]
- Zhang, L.J.; Wu, J.; Xing, D.M.; Liu, Y.; Jia, F.H.; Li, D.T. Nordihydroguaiaretic acid (NDGA) promotes functional recovery after transient focal cerebral ischemia in rats. Lat. Am. J. Pharm. 2014, 33, 994–1000. [Google Scholar]
- Jitsuno, M.; Mimaki, Y. Triterpene glycosides from the aerial parts of Larrea tridentata. Phytochemistry 2010, 71, 2157–2167. [Google Scholar] [CrossRef] [PubMed]
- Yokosuka, A.; Iguchi, T.; Jitsuno, M.; Mimaki, Y. Structure and cytotoxicity of novel lignans and lignan glycosides from the aerial parts of Larrea tridentata. Molecules 2021, 26, 6186. [Google Scholar] [CrossRef]
- Herrera-Medina, R.E.; Álvarez-Fuentes, G.; Contreras-Servín, C.; García-López, J.C. Creosote Bush (Larrea Tridentata) Phytochemical Traits and Its Different Uses: A Review. J. Appl. Life Sci. Int. 2021, 24, 34–45. [Google Scholar] [CrossRef]
- Vargas-Arispuro, I.; Contreras-Valenzuela, A.; Martínez-Téllez, M.Á. Lignans from Larrea Tridentate (Creosote Bush) as Fungal β-1,3-Glucanase Inhibitors. Pestic. Biochem. Physiol. 2009, 94, 60–63. [Google Scholar] [CrossRef]
- Skouta, R.; Morán, S.K.; Valenzuela, C.A.; Vasquez, A.H.; Fenelon, K. Assessing the antioxidant properties of Larrea tridentata extract as a potential molecular therapy against oxidative stress. Molecules 2018, 23, 1826. [Google Scholar] [CrossRef] [PubMed]
- Favela-Hernández, J.M.J.; García, A.; Garza-Gonzalez, E.; Rivas-Galindo, V.M.; Camacho-Corona, M.D.R. Antibacterial and antimycobacterial lignans and flavonoids from Larrea tridentata. Phytother. Res. 2012, 26, 1957–1960. [Google Scholar] [CrossRef] [PubMed]
- Bañuelos, V.R.; Delgadillo, R.L.; Echavarría, C.F.; Delgadillo, R.O.; Meza, L.C. Chemical composition and FTIR of ethane extracts of Larrea tridentata, Origanum vulgare, Artemisa ludoviciana and Ruta graveolens. Agrociencia 2018, 52, 309–321. [Google Scholar]
- Vélez, T.M.; Gaona, R.C.C.; Sánchez, G.H. Use of plant secondary metabolites to reduce ruminal methanogenesis. Trop. Subtrop. Agroecosystems 2014, 17, 489–499. [Google Scholar]
- Silva, V.M.R.; Bañuelos, V.L.; Delgadilo, R.L.; Gallegos, F.P.; Meza, L.C.; Valladares, C.B.; Ehavarría, C.F. Chemical characterization of guava (Psidium guajava) leaf alcoholic extract and its effect as a motility inhibitor for Escherichia coli O157:H7. Abanico Vet. 2020, 10, 1–13. [Google Scholar]
- López, M.A.; Valbuena, G.E.; Quihui, C.L.; Morales, F.G.G.; Ruiz, C.S.; Campos, G.J.C.; Díaz, M.E.; Pablos, R.D.E. Effect of microemulsions of essential oils on human erythrocyte and pathogens bacteria. Rev. Mex. De Ing. Biomédica 2017, 38, 247–254. [Google Scholar] [CrossRef]
- Mendez, M.; Rodríguez, R.; Ruiz, J.; Morales, A.D.; Castillo, F.; Hernández, C.F.D.; Aguilar, C.N. Antibacterial activity of plant extracts obtained with alternative organics solvents against food-borne pathogen bacteria. Ind. Crops Prod. 2012, 37, 445–450. [Google Scholar] [CrossRef]
- Turner, T.; Ruiz, G.; Gerstel, J.; Langland, J. Characterization of the antibacterial activity from ethanolic extracts of the botanical, Larrea tridentata. BMC Complement. Med. Ther. 2021, 21, 177. [Google Scholar] [CrossRef]
- Montemayor, F.J.A.; Medina, M.D.D.; Cruz, A.Z.; Cisneros, M.d.L.G.; Céspedes, R.I.N.; Velázquez, M.G.N.; Galindo, A.S.; Corona, B.J.J. Minimum inhibitory but maximum non-hemolytic concentration of Larrea tridentata and Origanum vulgare extracts. Afinidad 2023, 80, 164–174. [Google Scholar] [CrossRef]
- Pesewu, G.A.; Cutler, R.R.; Humber, D.P. Antibacterial activity of plants used in traditional medicines of Ghana with particular reference to MRSA. J. Ethnopharmacol. 2008, 116, 102–111. [Google Scholar] [CrossRef]
- Gallegos-Flores, P.; Bañuelos-Valenzuela, R.; Delgadillo-Ruiz, L.; Echavarría-Cháirez, F.; Meza-López, C.; Rodríguez-Tenorio, D. Differential evaluation of oregano extracts in the production of volatile fatty acids and methane during ruminal fermentation in vitro. Abanico Vet. 2019, 9, 1–18. [Google Scholar]
- Domínguez, X.A. Métodos de Investigación Fitoquímica; Editorial Limusa S.A.: Mexico City, Mexico, 1973; p. 281. [Google Scholar]
- Rivero, P.N.; Hernández, A.J.L.; Valladares, C.B.; Delgadillo, R.L.; Ojeda, R.D.; Sosa, G.C.G.; Zaragoza, B.A. Salix babylonica L. as a natural anticoccidial alternative in growing rabbits. Evid. Based Complement. Altern. Med. 2019, 2019, 2107231. [Google Scholar]
- Akrout, A.; Mighri, H.; Krid, M.; Thabet, F.; Turki, H.; El-Jani, H.; Neffati, M. Chemical composition and antioxidant activity of aqueous extracts of some wild medicinal plants in southern Tunisia. Int. J. Life Sci. Med. Sci. 2012, 2, 1–4. [Google Scholar] [CrossRef]
- Georgé, S.; Brat, P.; Pascalino, A.; Amiot, M.J. Rapid determination of polyphenols and vitamin C in plant-derived products. J. Agric. Food Chem. 2005, 53, 1370–1373. [Google Scholar] [CrossRef] [PubMed]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Amarowicz, R.; Pegg, R.B. Legumes as a source of natural antioxidants. Eur. J. Lipid Sci. Technol. 2008, 110, 865–878. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Alvarez-Parrilla, E.; De la Rosa, L.A.; Martínez, N.R.; González, G.A. Total phenols and antioxidant activity of commercial and wild mushrooms from Chihuahua, Mexico. Cienc. Y Tecnol. Aliment. 2007, 5, 329–334. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Lebensm. Wiss. Technol. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Chew, Y.L.; Lim, Y.Y.; Omar, M.; Khoo, K.S. Antioxidant activity of three edible seaweeds from two areas in South East Asia. Lebensm. Wiss. Technol. Food Sci. Technol. 2008, 41, 1067–1072. [Google Scholar] [CrossRef]
- Delgadillo, R.L.; Bañuelos, V.R.; Delgadillo, R.O.; Silva, V.M.; Gallegos, F.P. Chemical composition and antibacterian effect in vitro of extracts of Larrea tridentata, Origanum vulgare, Artemisa ludoviciana and Ruta graveolens. Nova Sci. 2017, 9, 273–290. [Google Scholar] [CrossRef]
- Mith, H.; Dure, R.; Delcenserie, V.; Zhiri, A.; Daube, G.; Clinquart, A. Antimicrobial activities of commercial essential oils and their components against food-borne pathogens and food spoilage bacteria. Food Sci. Nutr. 2014, 2, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.; Barros, L.; Santos, C.; Henriques, M.; Silva, S.; Ferreira, I. Decoction, infusion and hydroalcoholic extract of Origanum vulgare L.: Different performances regarding bioactivity and phenolic compounds. Food Chem. 2014, 158, 73–80. [Google Scholar] [CrossRef]
- Gallegos, F.P.; Bañuelos, V.R.; Delgadillo, R.L.; Meza, L.C.; Echavarría, C.F. Antibacterial activity of five terpenoid compounds: Carvacrol, limonene, linalool, α-terpinene, and thymol. Trop. Subtrop. Agroecosystems 2019, 22, 241–248. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Package ‘vegan’: Community Ecology Package, version 2.5-6; R Fundation for Statical Computing: Vienna, Austria, 2019. [Google Scholar]
- Legendre, P.; Gallagher, E.D. Ecologically meaningful transformations for ordination of species data. Oecologia 2001, 129, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Esparza-Orozco, A.; Lira-Noriega, A.; Martínez-Montoya, J.F.; Pineda-Martínez, L.F.; de Jesus Mendez-Gallegos, S. Influences of environmental heterogeneity on amphibian composition at breeding sites in a semiarid region of Mexico. J. Arid Environ. 2020, 182, 104259. [Google Scholar] [CrossRef]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing, version 4.2.3; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
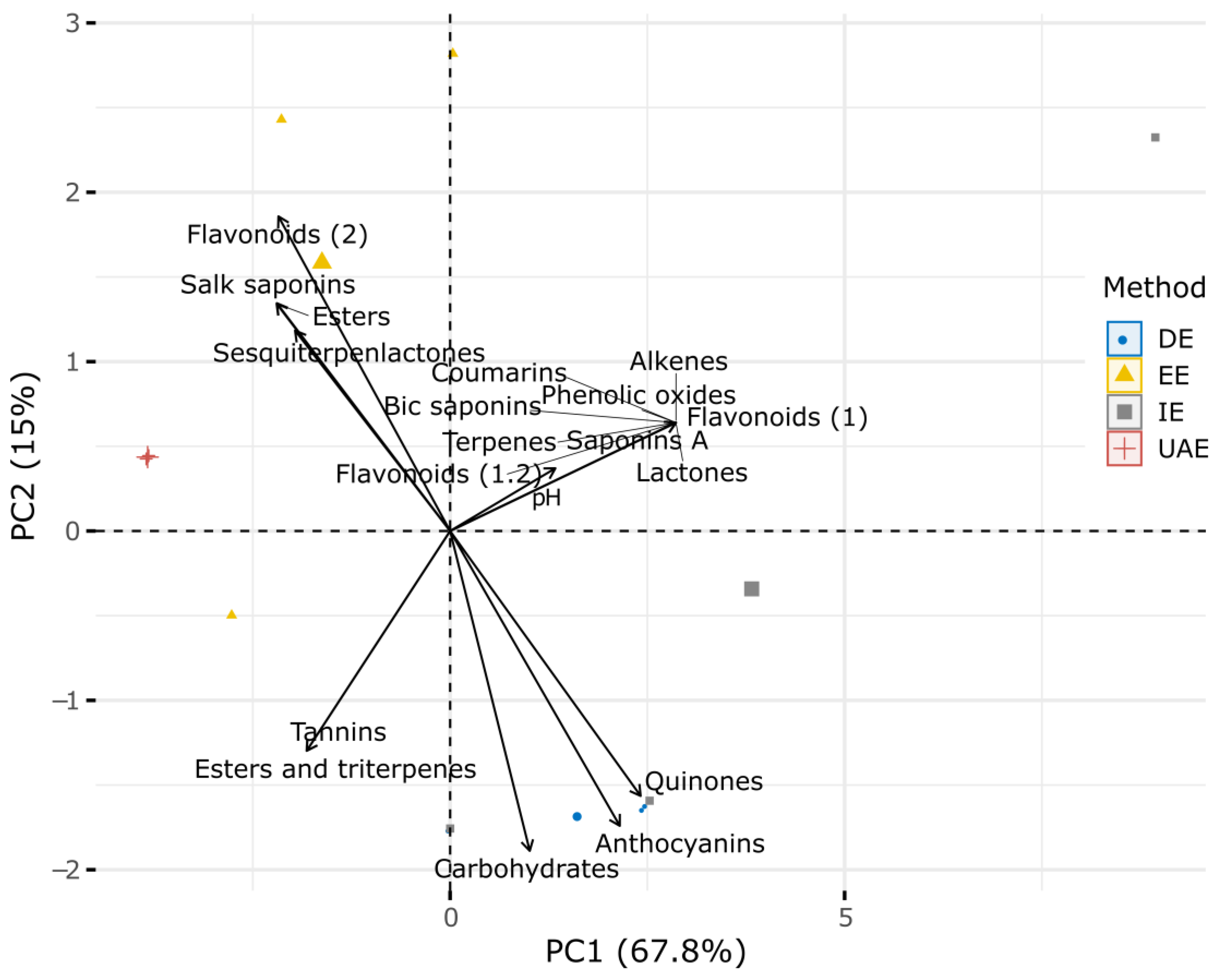

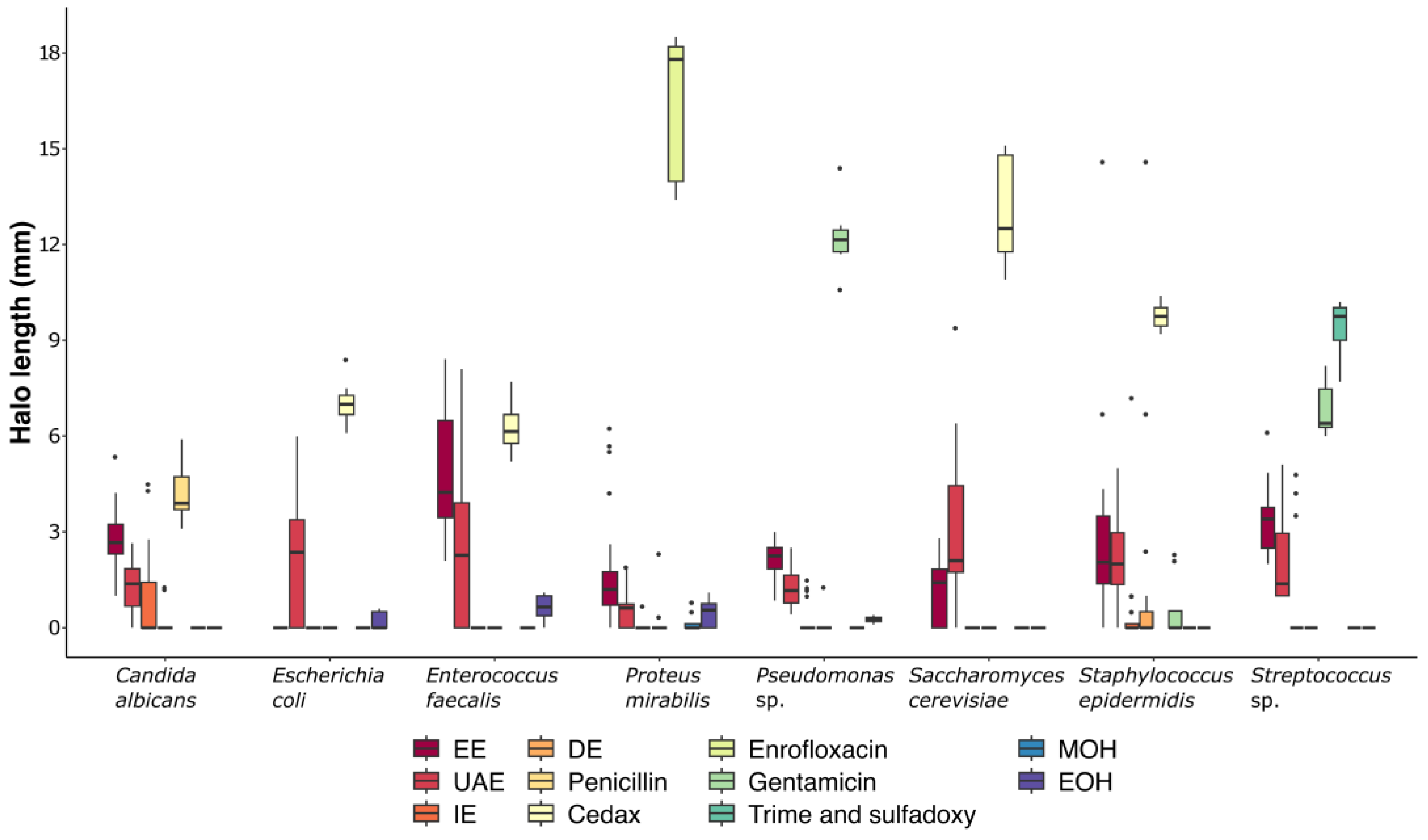
| Metabolites Detected | Extraction Methods | |||
|---|---|---|---|---|
| UAE | EE | DE | IE | |
| Lactones | + | + | + | + |
| Cumarins | + | + | + | + |
| Carbohydrates | + | − | + | + |
| Esters | + | + | + | + |
| Sesquiterpenlactones | + | − | − | − |
| Flavonoids (1) | + | + | + | + |
| Insaturations | + | + | + | + |
| Phenolic oxides (vegetable tannins) | + | + | + | + |
| Saponins A | + | + | + | + |
| Salk saponins | + | + | + | + |
| Bic saponins | + | + | − | − |
| Flavonoids (H2SO4 test) (1.2) | + | + | + | + |
| Salk esters and triterpenes | + | + | + | − |
| Flavonoids (Shinoda test) (2) | + | + | − | − |
| Quinones | − | − | + | + |
| Terpenes | + | + | + | + |
| Anthocyanins | − | − | + | + |
| Tannins | + | + | + | + |
| Extracts | DPPH | FRAP | Flavonoids | Phenols | Tannins |
|---|---|---|---|---|---|
| TE | TE | mg/mL | mg/mL | mg/mL | |
| UAE | 0.36 ± 0.12 | 2.78 ± 0.24 | 4.48 ± 0.68 | 3.69 ± 0.34 | 0.94 ± 0.18 |
| EE | 0.50 ± 0.04 | 2.63 ± 0.55 | 6.88 ± 0.95 | 3.89 ± 0.02 | 0.78 ± 0.18 |
| DE | 0.98 ± 0.42 | 2.91 ± 0.20 | 2.63 ± 0.24 | 2.83 ± 0.55 | 0.20 ± 0.08 |
| IE | 0.67 ± 0.08 | 2.83 ± 0.14 | 1.83 ± 0.14 | 2.68 ± 0.90 | 0.06 ± 0.09 |
| Time (h) | 1:10 | 1:100 | 1:1,000 | 1:10,000 | 1:100,000 | 1:1,000,000 | Total |
|---|---|---|---|---|---|---|---|
| 1 | 6 | 0 | 0 | 0 | 0 | 0 | 6 |
| 2 | 6 | 0 | 0 | 0 | 0 | 0 | 6 |
| 3 | 6 | 6 | 0 | 0 | 0 | 0 | 12 |
| 24 | 6 | 7 | 3 | 1 | 0 | 0 | 5 |
| Total | 6 | 7 | 3 | 1 | 0 | 0 | 29 |
| Compound Chemist | Test | Procedure |
|---|---|---|
| Insaturations | KMnO4 | To 100 µL of the extract, three drops of 2% KMnO4 (Sigma-Aldrich, St. Louis, MO, USA) in water were added dropwise. A positive test was indicated by discoloration or the formation of a brown precipitate (manganese dioxide). |
| Phenolic oxydryls (vegetable tannins) | FeCl3 | To 100 µL of the extract, three drops of 12.5% FeCl3 (Sigma-Aldrich, St. Louis, MO, USA) in water were added. A positive test was indicated by the formation of a red, blue–violet, or green precipitate. |
| Carbonyl groups | 2–4 Dinitxophenylhydrazine | One drop of 2,4-dinitrophenylhydrazine solution in 6N HCl (J.T. Baker®, Radnor, PA, USA) was added to 100 µL of the extract. A positive test was indicated by a yellow or orange precipitate. |
| Triterpenes and sterols | Liebermann–Burchard | The reagent was prepared by mixing 1 mL CH3COOH (J.T. Baker®, Radnor, PA, USA), 1 mL CHCl3 (J.T. Baker®, Radnor, PA, USA), and 1 drop of H2SO4 (J.T. Baker®, Radnor, PA, USA), followed by cooling to 0 °C. To 100 µL of the extract, three drops of the reagent were added. Positive results were observed as blue, green, red, or orange colors over time. |
| Salkowski | To 200 µL of the extract, 500 µL of H2SO4 (J.T. Baker®, Radnor, PA, USA) was added. A positive test was indicated by yellow or red coloration, confirming the presence of sterols or methylsterols. | |
| Carbohydrates | Molish | Two drops of Molisch’s reagent were added to 100 µL of the extract, followed by 500 µL of H2SO4 (J.T. Baker®, Radnor, PA, USA). A positive test was indicated by the presence of a purple ring at the interface. |
| Coumarin | To 100 µL of the extract, 100 µL of 10% NaOH (Sigma-Aldrich, St. Louis, MO, USA) was added. A positive test was indicated by yellow coloration, which disappeared upon acidification with HCl (J.T. Baker®, Radnor, PA, USA). | |
| Lactone | To 100 µL of the extract, 100 µL of a 10% NaOH (Sigma-Aldrich, St. Louis, MO, USA) alcoholic solution was added. A positive test was indicated by yellow or orange coloration that disappeared after the addition of a few drops of HCl. | |
| Sesquitexpenlactones | Baljet | To 100 µL of the extract, 3–4 drops of Baljet solution [10 mL of C6H3N3O7 1% (Sigma-Aldrich, St. Louis, MO, USA), 10 mL NaOH 10% (Sigma-Aldrich, St. Louis, MO, USA)] mixed solution were added. A positive test was indicated by the appearance of orange or dark coloration. |
| Flavonoids | H2SO4 | To 100 µL of the extract, 500 µL of H2SO4 (J.T. Baker®, Radnor, PA, USA) was added. Positive results were indicated by yellow coloration for flavonoids, orange–cherry for flavones, red–blue for chalcones, and red–purple for quinones. |
| Shinoda | 100 µL of the extract was mixed with 100 µL of ethanol, followed by the addition of 0.1 g magnesium filings (J.T. Baker®, Radnor, PA, USA). The sample was boiled, and three drops of concentrated HCl (J.T. Baker®, Radnor, PA, USA) were added. Positive results were indicated by orange, red, pink, blue, or violet coloration. | |
| Alkaloids | Dragendorf | Two to three drops of reagent A (bismuth nitrate (Sigma-Aldrich) and glacial acetic acid) and reagent B (potassium iodide (Sigma-Aldrich)) were added to 100 µL of the extract. A positive test was indicated by orange to reddish coloration. |
| Mayer | To 2 mL of the extract, 4 mL of Mayer’s reagent [Mercury and potassium iodide (Ricca Chemical®, Arlington, TX, USA)] was added. A positive test was indicated by yellow coloration with precipitates. | |
| Saponins | Agitation | 1 mL of the extract was dissolved with 1 mL of water in a test tube and shaken vigorously for 3–5 min. A positive test was indicated by stable foam with a honeycomb appearance for 30 min. |
| NaHCO3 | To 100 µL of the extract, 2–3 drops of H2SO4 (J.T. Baker®, Radnor, PA, USA) were added, and the mixture was lightly shaken. Then, 2–3 drops of 10% NaHCO3 (J.T. Baker®, Radnor, PA, USA) solution were added. A positive test was indicated by bubbles that persisted for over 1 min. | |
| Salkowski | To 100 µL of the extract, 100 µL of CHCl3 (J.T. Baker®, Radnor, PA, USA) was added, followed by 100 µL of H2SO4 (J.T. Baker®, Radnor, PA, USA). A positive test was indicated by the appearance of a red color. | |
| Aromaticity | H2SO4-CH2O | One drop of a mixture of H2SO4 (J.T. Baker®, Radnor, PA, USA) and CH2O (J.T. Baker®, Radnor, PA, USA) was added to 100 µL of the extract dissolved in a non-aromatic solvent. A positive test was indicated by red or violet coloration. |
| Anthocyanins | HCl | To 1 mL of the extract, 5 mL of 10% HCl (J.T. Baker®, Radnor, PA, USA) was added, and the mixture was boiled in a water bath. A positive test was indicated by pale pink coloration. |
| Terpenoids | H2SO4 | To 5 mL of the extract, 4 mL of chloroform and 4 mL of (J.T. Baker®, Radnor, PA, USA) were added. A positive test was indicated by reddish–brown coloration at the interface. |
| Tannins | FeCl3 | 1 mL of the extract was boiled, and 20 mL of water was added, followed by three drops of 0.1% FeCl3 (Sigma-Aldrich, St. Louis, MO, USA). A positive test was indicated by green or blue coloration. |
| Steroids | H2SO4 | To 2 mL of CH3COOH (J.T. Baker®, Radnor, PA, USA), 0.5 mL of the extract was added, followed by 2 mL of H2SO4 (J.T. Baker®, Radnor, PA, USA). A positive test was indicated by violet, blue, or green coloration. |
| Strain | Type | Control Drugs |
|---|---|---|
| Candida albicans | Yeast | Penicillin (Pharmalife®, Veritrade, Mexico City, Mexico) |
| Escherichia coli | Gram-negative | Cedax® (Sanfer®, Laboratorios Sanfer, Mexico City, Mexico) |
| Enterococcus faecalis | Gram-positive | Cedax (Sanfer®,Laboratorios Sanfer, Mexico City, Mexico) |
| Proteus mirabilis | Gram-negative | Enflofloxacin (ALTIA®, PYMES de BME, Spain) |
| Pseudomonas sp. | Gram-negative | Gentamine (Halvet®, Veritrade, Mexico City, Mexico) |
| Saccharomyces cerevisiae | Yeast | Cedax® (Sanfer®, Laboratorios Sanfer, Mexico City, Mexico) |
| Staphylococcus epidermidis | Gram-positive | Cedax® (Sanfer®, Laboratorios Sanfer, Mexico City, Mexico) |
| Streptococcus sp. | Gram-positive | Gentamine and Trimethylsulfadoxy (Novag®, Fressines, France) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-Márquez, R.; Delgadillo-Ruiz, L.; Esparza-Orozco, A.; Delgadillo-Ruiz, E.; Bañuelos-Valenzuela, R.; Valladares-Carranza, B.; Chávez-Ruvalcaba, M.I.; Chávez-Ruvalcaba, F.; Valtierra-Marín, H.E.; Gaytán-Saldaña, N.A.; et al. Evaluation of Larrea tridentata Extracts and Their Antimicrobial Effects on Strains of Clinical Interest. Int. J. Mol. Sci. 2025, 26, 1032. https://doi.org/10.3390/ijms26031032
Morales-Márquez R, Delgadillo-Ruiz L, Esparza-Orozco A, Delgadillo-Ruiz E, Bañuelos-Valenzuela R, Valladares-Carranza B, Chávez-Ruvalcaba MI, Chávez-Ruvalcaba F, Valtierra-Marín HE, Gaytán-Saldaña NA, et al. Evaluation of Larrea tridentata Extracts and Their Antimicrobial Effects on Strains of Clinical Interest. International Journal of Molecular Sciences. 2025; 26(3):1032. https://doi.org/10.3390/ijms26031032
Chicago/Turabian StyleMorales-Márquez, Renata, Lucía Delgadillo-Ruiz, Alfredo Esparza-Orozco, Eladio Delgadillo-Ruiz, Rómulo Bañuelos-Valenzuela, Benjamín Valladares-Carranza, María Isabel Chávez-Ruvalcaba, Francisca Chávez-Ruvalcaba, Héctor Emmanuel Valtierra-Marín, Norma Angélica Gaytán-Saldaña, and et al. 2025. "Evaluation of Larrea tridentata Extracts and Their Antimicrobial Effects on Strains of Clinical Interest" International Journal of Molecular Sciences 26, no. 3: 1032. https://doi.org/10.3390/ijms26031032
APA StyleMorales-Márquez, R., Delgadillo-Ruiz, L., Esparza-Orozco, A., Delgadillo-Ruiz, E., Bañuelos-Valenzuela, R., Valladares-Carranza, B., Chávez-Ruvalcaba, M. I., Chávez-Ruvalcaba, F., Valtierra-Marín, H. E., Gaytán-Saldaña, N. A., Mercado-Reyes, M., & Arias-Hernández, L. A. (2025). Evaluation of Larrea tridentata Extracts and Their Antimicrobial Effects on Strains of Clinical Interest. International Journal of Molecular Sciences, 26(3), 1032. https://doi.org/10.3390/ijms26031032









