Fibrosis and Src Signalling in Glaucoma: From Molecular Pathways to Therapeutic Prospects
Abstract
1. Introduction
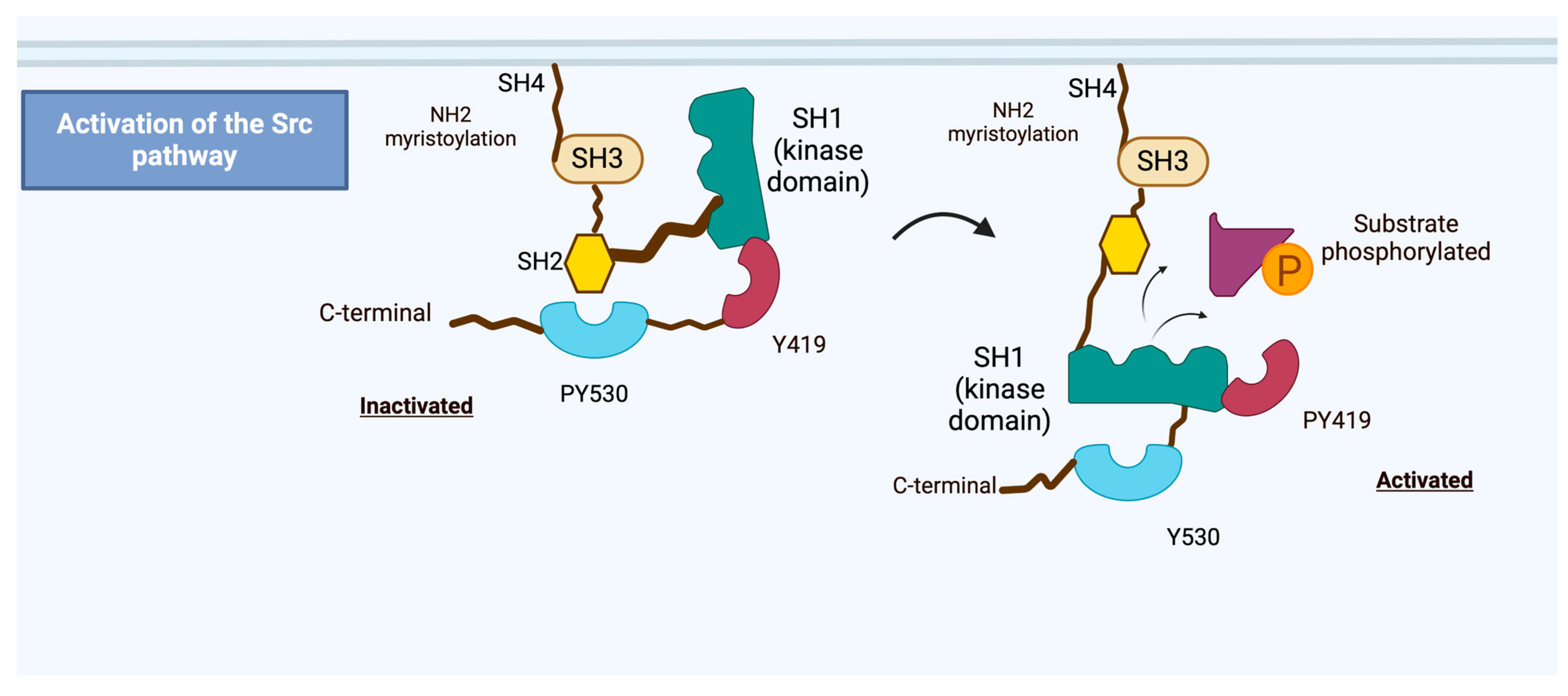
2. Src Proto-Oncogene Overview
3. Role of Src in Fibrosis
4. Role of Src in Ophthalmology
5. Role of Src in Glaucoma
5.1. Src in TM and Aqueous Humour Dynamics
5.2. Src and Cyctoskeletal Dynamics in Glaucoma
5.3. Src in RGC Survival and Neuroprotection
5.4. Src and TGF-β-Mediated Fibrosis in Glaucoma
5.5. Src and YAP/TAZ Pathways in Glaucoma
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jonas, J.B.; Aung, T.; Bourne, R.R.; Bron, A.M.; Ritch, R.; Panda-Jonas, S. Glaucoma. Lancet 2017, 390, 2183–2193. [Google Scholar] [CrossRef] [PubMed]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Sharif, N.A. Glaucomatous optic neuropathy treatment options: The promise of novel therapeutics, techniques and tools to help preserve vision. Neural Regen. Res. 2018, 13, 1145–1150. [Google Scholar] [CrossRef] [PubMed]
- Wilson, G.N.; Inman, D.M.; Dengler Crish, C.M.; Smith, M.A.; Crish, S.D. Early pro-inflammatory cytokine elevations in the DBA/2J mouse model of glaucoma. J. Neuroinflamm. 2015, 12, 176. [Google Scholar] [CrossRef]
- Yang, X.; Luo, C.; Cai, J.; Powell, D.W.; Yu, D.; Kuehn, M.H.; Tezel, G. Neurodegenerative and inflammatory pathway components linked to TNF-α/TNFR1 signaling in the glaucomatous human retina. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8442–8454. [Google Scholar] [CrossRef]
- Thomas, S.M.; Brugge, J.S. Cellular Functions Regulated by Src Family Kinases. Annu. Rev. Cell Dev. Biol. 1997, 13, 513–609. [Google Scholar] [CrossRef]
- Kirihara, T.; Shimazaki, A.; Nakamura, M.; Miyawaki, N. Ocular hypotensive efficacy of Src-family tyrosine kinase inhibitors via different cellular actions from Rock inhibitors. Exp. Eye Res. 2014, 119, 97–105. [Google Scholar] [CrossRef]
- Tsukamoto, T.; Kajiwara, K.; Nada, S.; Okada, M. Src mediates TGF-β-induced intraocular pressure elevation in glaucoma. J. Cell Physiol. 2019, 234, 1730–1744. [Google Scholar] [CrossRef]
- Murphy, R.; Irnaten, M.; Hopkins, A.; O’Callaghan, J.; Stamer, W.D.; Clark, A.F.; Wallace, D.; O’Brien, C.J. Matrix Mechanotransduction via Yes-Associated Protein in Human Lamina Cribrosa Cells in Glaucoma. Investig. Ophthalmol. Vis. Sci. 2022, 63, 16. [Google Scholar] [CrossRef]
- Chitranshi, N.; Dheer, Y.; Mirzaei, M.; Wu, Y.; Salekdeh, G.H.; Abbasi, M.; Gupta, V.; Vander Wall, R.; You, Y.; Graham, S.L.; et al. Loss of Shp2 Rescues BDNF/TrkB Signaling and Contributes to Improved Retinal Ganglion Cell Neuroprotection. Mol. Ther. 2019, 27, 424–441. [Google Scholar] [CrossRef]
- Bugara, K.; Pacwa, A.; Smedowski, A. Molecular pathways in experimental glaucoma models. Front. Neurosci. 2024, 18, 1363170. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.M.; Honeybone, L.M.; Hollings, P.E.; Fitzgerald, P.H. Localization of the SRC Oncogene to Chromosome Band 20ql 1.2 and Loss of This Gene with Deletion (20q) in Two Leukemic Patients. Blood 1989, 74, 1768–1773. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Ouyang, C.; Zhang, L.; Wang, J.; Zhang, J.; Zhang, Y.; Huang, C.; Xiao, Q.; Jiang, B.; Lin, F.; et al. The proto-oncogene tyrosine kinase c-SRC facilitates glioblastoma progression by remodeling fatty acid synthesis. Nat. Commun. 2024, 15, 7455. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yu, D. Targeting Src family kinases in anti-cancer therapies: Turning promise into triumph. Trends Pharmacol. Sci. 2012, 33, 122–128. [Google Scholar] [CrossRef]
- Manning, G.; Whyte, D.B.; Martinez, R.; Hunter, T.; Sudarsanam, S. The protein kinase complement of the human genome. Science 2002, 298, 1912–1934. [Google Scholar] [CrossRef]
- Brown, M.T.; Cooper, J.A. Regulation, substrates and functions of src. Biochim. Biophys. Acta 1996, 1287, 121–149. [Google Scholar] [CrossRef]
- Mitchell, P.J.; Barker, K.T.; Martindale, J.E.; Kamalati, T.; Lowe, P.N.; Page, M.J.; Gusterson, B.A.; Crompton, M.R. Cloning and characterisation of cDNAs encoding a novel non-receptor tyrosine kinase, brk, expressed in human breast tumours. Oncogene 1994, 9, 2383–2390. [Google Scholar]
- Alonso, A.; Sasin, J.; Bottini, N.; Friedberg, I.; Friedberg, I.; Osterman, A.; Godzik, A.; Hunter, T.; Dixon, J.; Mustelin, T. Protein Tyrosine Phosphatases in the Human Genome. Cell 2004, 117, 699–711. [Google Scholar] [CrossRef]
- Mori, S.; Rönnstrand, L.; Yokote, K.; Engström, A.; Courtneidge, S.A.; Claesson-Welsh, L.; Heldin, C.H. Identification of two juxtamembrane autophosphorylation sites in the PDGF beta-receptor; involvement in the interaction with Src family tyrosine kinases. EMBO J. 1993, 12, 2257–2264. [Google Scholar] [CrossRef]
- Roskoski, R., Jr. Src protein-tyrosine kinase structure, mechanism, and small molecule inhibitors. Pharmacol. Res. 2015, 94, 9–25. [Google Scholar] [CrossRef]
- Piwnica-Worms, H.; Saunders, K.B.; Roberts, T.M.; Smith, A.E.; Cheng, S.H. Tyrosine phosphorylation regulates the biochemical and biological properties of pp60c-src. Cell 1987, 49, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Marcotte, R.; Smith, H.W.; Sanguin-Gendreau, V.; McDonough, R.V.; Muller, W.J. Mammary epithelial-specific disruption of c-Src impairs cell cycle progression and tumorigenesis. Proc. Natl. Acad. Sci. USA 2012, 109, 2808–2813. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.C.; Song, L.; Haura, E.B. Src kinases as therapeutic targets for cancer. Nat. Rev. Clin. Oncol. 2009, 6, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Koudelková, L.; Brábek, J.; Rosel, D. Src kinase: Key effector in mechanosignalling. Int. J. Biochem. Cell Biol. 2021, 131, 105908. [Google Scholar] [CrossRef]
- Wang, Z. Regulation of Cell Cycle Progression by Growth Factor-Induced Cell Signaling. Cells 2021, 10, 3327. [Google Scholar] [CrossRef]
- Maat, W.; el Filali, M.; Dirks-Mulder, A.; Luyten, G.P.; Gruis, N.A.; Desjardins, L.; Boender, P.; Jager, M.J.; van der Velden, P.A. Episodic Src activation in uveal melanoma revealed by kinase activity profiling. Br. J. Cancer 2009, 101, 312–319. [Google Scholar] [CrossRef][Green Version]
- Parkin, A.; Man, J.; Timpson, P.; Pajic, M. Targeting the complexity of Src signalling in the tumour microenvironment of pancreatic cancer: From mechanism to therapy. FEBS J. 2019, 286, 3510–3539. [Google Scholar] [CrossRef]
- Barber, T.D.; Vogelstein, B.; Kinzler, K.W.; Velculescu, V.E. Somatic mutations of EGFR in colorectal cancers and glioblastomas. N. Engl. J. Med. 2004, 351, 2883. [Google Scholar] [CrossRef]
- Daub, H.; Weiss, F.U.; Wallasch, C.; Ullrich, A. Role of transactivation of the EGF receptor in signalling by G-protein-coupled receptors. Nature 1996, 379, 557–560. [Google Scholar] [CrossRef]
- Belli, S.; Esposito, D.; Servetto, A.; Pesapane, A.; Formisano, L.; Bianco, R. c-Src and EGFR Inhibition in Molecular Cancer Therapy: What Else Can We Improve? Cancers 2020, 12, 1489. [Google Scholar] [CrossRef]
- Ghalehbandi, S.; Yuzugulen, J.; Pranjol, M.Z.I.; Pourgholami, M.H. The role of VEGF in cancer-induced angiogenesis and research progress of drugs targeting VEGF. Eur. J. Pharmacol. 2023, 949, 175586. [Google Scholar] [CrossRef] [PubMed]
- Parsons, S.J.; Parsons, J.T. Src family kinases, key regulators of signal transduction. Oncogene 2004, 23, 7906–7909. [Google Scholar] [CrossRef] [PubMed]
- Filla, M.S.; Faralli, J.A.; Peotter, J.L.; Peters, D.M. The role of integrins in glaucoma. Exp. Eye Res. 2017, 158, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhang, L.; Song, J.; Nguyen, E.; Lee, R.S.; Rodgers, S.J.; Li, F.; Huang, C.; Schittenhelm, R.B.; Chan, H.; et al. Characterization of the Src-regulated kinome identifies SGK1 as a key mediator of Src-induced transformation. Nat. Commun. 2019, 10, 296. [Google Scholar] [CrossRef]
- Jain, S.; Wang, X.; Chang, C.-C.; Ibarra-Drendall, C.; Wang, H.; Zhang, Q.; Brady, S.W.; Li, P.; Zhao, H.; Dobbs, J.; et al. Src Inhibition Blocks c-Myc Translation and Glucose Metabolism to Prevent the Development of Breast Cancer. Cancer Res. 2015, 75, 4863–4875. [Google Scholar] [CrossRef]
- Gerber, D.E. Targeted therapies: A new generation of cancer treatments. Am. Fam. Physician 2008, 77, 311–319. [Google Scholar]
- Fruman, D.A.; Rommel, C. PI3K and cancer: Lessons, challenges and opportunities. Nat. Rev. Drug Discov. 2014, 13, 140–156. [Google Scholar] [CrossRef]
- Pelaz, S.G.; Tabernero, A. Src: Coordinating metabolism in cancer. Oncogene 2022, 41, 4917–4928. [Google Scholar] [CrossRef]
- Song, L.; Liu, Z.; Hu, H.H.; Yang, Y.; Li, T.Y.; Lin, Z.Z.; Ye, J.; Chen, J.; Huang, X.; Liu, D.T.; et al. Proto-oncogene Src links lipogenesis via lipin-1 to breast cancer malignancy. Nat. Commun. 2020, 11, 5842. [Google Scholar] [CrossRef]
- Hua, T.N.M.; Kim, M.K.; Vo, V.T.A.; Choi, J.W.; Choi, J.H.; Kim, H.W.; Cha, S.K.; Park, K.S.; Jeong, Y. Inhibition of oncogenic Src induces FABP4-mediated lipolysis via PPARγ activation exerting cancer growth suppression. EBioMedicine 2019, 41, 134–145. [Google Scholar] [CrossRef]
- Tian, Q.; Yuan, P.; Quan, C.; Li, M.; Xiao, J.; Zhang, L.; Lu, H.; Ma, T.; Zou, L.; Wang, F.; et al. Phosphorylation of BCKDK of BCAA catabolism at Y246 by Src promotes metastasis of colorectal cancer. Oncogene 2020, 39, 3980–3996. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Palmieri, M.; Chaudhury, A.; Klisch, T.J.; di Ronza, A.; Neilson, J.R.; Rodney, G.G.; Sardiello, M. Src regulates amino acid-mediated mTORC1 activation by disrupting GATOR1-Rag GTPase interaction. Nat. Commun. 2018, 9, 4351. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.H.; Agani, F.; Passaniti, A.; Semenza, G.L. V-SRC induces expression of hypoxia-inducible factor 1 (HIF-1) and transcription of genes encoding vascular endothelial growth factor and enolase 1: Involvement of HIF-1 in tumor progression. Cancer Res. 1997, 57, 5328–5335. [Google Scholar] [PubMed]
- Karni, R.; Dor, Y.; Keshet, E.; Meyuhas, O.; Levitzki, A. Activated pp60c-Src leads to elevated hypoxia-inducible factor (HIF)-1alpha expression under normoxia. J. Biol. Chem. 2002, 277, 42919–42925. [Google Scholar] [CrossRef]
- Courtneidge, S.A. Role of Src in signal transduction pathways. The Jubilee Lecture. Biochem. Soc. Trans. 2002, 30, 11–17. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Q.; Tengholm, A.; Sjöholm, A. Involvement of JAK2 and Src kinase tyrosine phosphorylation in human growth hormone-stimulated increases in cytosolic free Ca2+ and insulin secretion. Am. J. Physiol. Cell Physiol. 2006, 291, C466–C475. [Google Scholar] [CrossRef]
- Sato, H.; Nagashima, K.; Ogura, M.; Sato, Y.; Tahara, Y.; Ogura, K.; Yamano, G.; Sugizaki, K.; Fujita, N.; Tatsuoka, H.; et al. Src regulates insulin secretion and glucose metabolism by influencing subcellular localization of glucokinase in pancreatic β-cells. J. Diabetes Investig. 2016, 7, 171–178. [Google Scholar] [CrossRef]
- Hu, H.; Juvekar, A.; Lyssiotis, C.A.; Lien, E.C.; Albeck, J.G.; Oh, D.; Varma, G.; Hung, Y.P.; Ullas, S.; Lauring, J.; et al. Phosphoinositide 3-Kinase Regulates Glycolysis through Mobilization of Aldolase from the Actin Cytoskeleton. Cell 2016, 164, 433–446. [Google Scholar] [CrossRef]
- Xie, Y.; Shi, X.; Sheng, K.; Han, G.; Li, W.; Zhao, Q.; Jiang, B.; Feng, J.; Li, J.; Gu, Y. PI3K/Akt signaling transduction pathway, erythropoiesis and glycolysis in hypoxia (Review). Mol. Med. Rep. 2019, 19, 783–791. [Google Scholar] [CrossRef]
- Magaway, C.; Kim, E.; Jacinto, E. Targeting mTOR and Metabolism in Cancer: Lessons and Innovations. Cells 2019, 8, 1584. [Google Scholar] [CrossRef]
- Sigismund, S.; Avanzato, D.; Lanzetti, L. Emerging functions of the EGFR in cancer. Mol. Oncol. 2018, 12, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Gupte, R.S.; Floyd, B.C.; Kozicky, M.; George, S.; Ungvari, Z.I.; Neito, V.; Wolin, M.S.; Gupte, S.A. Synergistic activation of glucose-6-phosphate dehydrogenase and NAD(P)H oxidase by Src kinase elevates superoxide in type 2 diabetic, Zucker fa/fa, rat liver. Free Radic. Biol. Med. 2009, 47, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Gupte, R.S.; Vijay, V.; Marks, B.; Levine, R.J.; Sabbah, H.N.; Wolin, M.S.; Recchia, F.A.; Gupte, S.A. Upregulation of glucose-6-phosphate dehydrogenase and NAD(P)H oxidase activity increases oxidative stress in failing human heart. J. Card. Fail. 2007, 13, 497–506. [Google Scholar] [CrossRef]
- Pan, S.; World, C.J.; Kovacs, C.J.; Berk, B.C. Glucose 6-phosphate dehydrogenase is regulated through c-Src-mediated tyrosine phosphorylation in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 895–901. [Google Scholar] [CrossRef]
- Hébert Chatelain, E.; Dupuy, J.W.; Letellier, T.; Dachary-Prigent, J. Functional impact of PTP1B-mediated Src regulation on oxidative phosphorylation in rat brain mitochondria. Cell. Mol. Life Sci. 2011, 68, 2603–2613. [Google Scholar] [CrossRef]
- Palikaras, K.; Lionaki, E.; Tavernarakis, N. Mechanisms of mitophagy in cellular homeostasis, physiology and pathology. Nat. Cell Biol. 2018, 20, 1013–1022. [Google Scholar] [CrossRef]
- Guedouari, H.; Ould Amer, Y.; Pichaud, N.; Hebert-Chatelain, E. Characterization of the interactome of c-Src within the mitochondrial matrix by proximity-dependent biotin identification. Mitochondrion 2021, 57, 257–269. [Google Scholar] [CrossRef]
- Jin, Y.; Cai, Q.; Shenoy, A.K.; Lim, S.; Zhang, Y.; Charles, S.; Tarrash, M.; Fu, X.; Kamarajugadda, S.; Trevino, J.G.; et al. Src drives the Warburg effect and therapy resistance by inactivating pyruvate dehydrogenase through tyrosine-289 phosphorylation. Oncotarget 2016, 7, 25113–25124. [Google Scholar] [CrossRef]
- Pelaz, S.G.; Jaraíz-Rodríguez, M.; Álvarez-Vázquez, A.; Talaverón, R.; García-Vicente, L.; Flores-Hernández, R.; Gómez de Cedrón, M.; Tabernero, M.; Ramírez de Molina, A.; Lillo, C.; et al. Targeting metabolic plasticity in glioma stem cells in vitro and in vivo through specific inhibition of c-Src by TAT-Cx43266–283. EBioMedicine 2020, 62, 103134. [Google Scholar] [CrossRef]
- Liu, L.; Feng, D.; Chen, G.; Chen, M.; Zheng, Q.; Song, P.; Ma, Q.; Zhu, C.; Wang, R.; Qi, W.; et al. Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat. Cell Biol. 2012, 14, 177–185. [Google Scholar] [CrossRef]
- Chen, G.; Han, Z.; Feng, D.; Chen, Y.; Chen, L.; Wu, H.; Huang, L.; Zhou, C.; Cai, X.; Fu, C.; et al. A regulatory signaling loop comprising the PGAM5 phosphatase and CK2 controls receptor-mediated mitophagy. Mol. Cell 2014, 54, 362–377. [Google Scholar] [CrossRef] [PubMed]
- Guedouari, H.; Savoie, M.-C.; Jean, S.; Djeungoue-Petga, M.-A.; Pichaud, N.; Hebert-Chatelain, E. Multi-omics Reveal that c-Src Modulates the Mitochondrial Phosphotyrosine Proteome and Metabolism According to Nutrient Availability. Cell. Physiol. Biochem. 2020, 54, 517–537. [Google Scholar] [PubMed]
- Beyer, C.; Distler, J.H.W. Tyrosine kinase signaling in fibrotic disorders: Translation of basic research to human disease. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2013, 1832, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Smyth, A.; Callaghan, B.; Willoughby, C.E.; O’Brien, C. The Role of miR-29 Family in TGF-β Driven Fibrosis in Glaucomatous Optic Neuropathy. Int. J. Mol. Sci. 2022, 23, 10216. [Google Scholar] [CrossRef]
- Mishra, R.; Zhu, L.; Eckert, R.L.; Simonson, M.S. TGF-β-regulated collagen type I accumulation: Role of Src-based signals. Am. J. Physiol. Cell Physiol. 2007, 292, C1361–C1369. [Google Scholar] [CrossRef]
- Okutani, D.; Lodyga, M.; Han, B.; Liu, M. Src protein tyrosine kinase family and acute inflammatory responses. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2006, 291, L129–L141. [Google Scholar] [CrossRef]
- Lambert, W.; Agarwal, R.; Howe, W.; Clark, A.F.; Wordinger, R.J. Neurotrophin and neurotrophin receptor expression by cells of the human lamina cribrosa. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2315–2323. [Google Scholar]
- Kirwan, R.P.; Fenerty, C.H.; Crean, J.; Wordinger, R.J.; Clark, A.F.; O’Brien, C.J. Influence of cyclical mechanical strain on extracellular matrix gene expression in human lamina cribrosa cells in vitro. Mol. Vis. 2005, 11, 798–810. [Google Scholar]
- Kirwan, R.P.; Leonard, M.O.; Murphy, M.; Clark, A.F.; O’Brien, C.J. Transforming growth factor-beta-regulated gene transcription and protein expression in human GFAP-negative lamina cribrosa cells. Glia 2005, 52, 309–324. [Google Scholar] [CrossRef]
- Kirwan, R.P.; Crean, J.K.; Fenerty, C.H.; Clark, A.F.; O’Brien, C.J. Effect of cyclical mechanical stretch and exogenous transforming growth factor-beta1 on matrix metalloproteinase-2 activity in lamina cribrosa cells from the human optic nerve head. J. Glaucoma 2004, 13, 327–334. [Google Scholar] [CrossRef]
- Quill, B.; Docherty, N.G.; Clark, A.F.; O’Brien, C.J. The effect of graded cyclic stretching on extracellular matrix-related gene expression profiles in cultured primary human lamina cribrosa cells. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1908–1915. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McElnea, E.M.; Quill, B.; Docherty, N.G.; Irnaten, M.; Siah, W.F.; Clark, A.F.; O’Brien, C.J.; Wallace, D.M. Oxidative stress, mitochondrial dysfunction and calcium overload in human lamina cribrosa cells from glaucoma donors. Mol. Vis. 2011, 17, 1182–1191. [Google Scholar] [PubMed]
- Galliher, A.J.; Schiemann, W.P. Src phosphorylates Tyr284 in TGF-beta type II receptor and regulates TGF-beta stimulation of p38 MAPK during breast cancer cell proliferation and invasion. Cancer Res. 2007, 67, 3752–3758. [Google Scholar] [CrossRef] [PubMed]
- Meyn, M.A.; Wilson, M.B.; Abdi, F.A.; Fahey, N.; Schiavone, A.P.; Wu, J.; Hochrein, J.M.; Engen, J.R.; Smithgall, T.E. Src Family Kinases Phosphorylate the Bcr-Abl SH3-SH2 Region and Modulate Bcr-Abl Transforming Activity. J. Biol. Chem. 2006, 281, 30907–30916. [Google Scholar] [CrossRef]
- Liu, B.; Kilpatrick, J.I.; Lukasz, B.; Jarvis, S.P.; McDonnell, F.; Wallace, D.M.; Clark, A.F.; O’Brien, C.J. Increased Substrate Stiffness Elicits a Myofibroblastic Phenotype in Human Lamina Cribrosa Cells. Investig. Ophthalmol. Vis. Sci. 2018, 59, 803–814. [Google Scholar] [CrossRef]
- Li, H.; Zhao, C.; Tian, Y.; Lu, J.; Zhang, G.; Liang, S.; Chen, D.; Liu, X.; Kuang, W.; Zhu, M. Src family kinases and pulmonary fibrosis: A review. Biomed. Pharmacother. 2020, 127, 110183. [Google Scholar] [CrossRef]
- Mitra, S.K.; Schlaepfer, D.D. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr. Opin. Cell Biol. 2006, 18, 516–523. [Google Scholar] [CrossRef]
- Playford, M.P.; Schaller, M.D. The interplay between Src and integrins in normal and tumor biology. Oncogene 2004, 23, 7928–7946. [Google Scholar] [CrossRef]
- Brunton, V.G.; MacPherson, I.R.; Frame, M.C. Cell adhesion receptors, tyrosine kinases and actin modulators: A complex three-way circuitry. Biochim. Biophys. Acta 2004, 1692, 121–144. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Hopkins, A.A.; Murphy, R.; Irnaten, M.; Wallace, D.M.; Quill, B.; O’Brien, C. The role of lamina cribrosa tissue stiffness and fibrosis as fundamental biomechanical drivers of pathological glaucoma cupping. Am. J. Physiol. Cell Physiol. 2020, 319, C611–C623. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Che, P.; Han, X.; Cai, G.-Q.; Liu, G.; Antony, V.; Luckhardt, T.; Siegal, G.P.; Zhou, Y.; Liu, R.-m. Therapeutic targeting of SRC kinase in myofibroblast differentiation and pulmonary fibrosis. J. Pharmacol. Exp. Ther. 2014, 351, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Siesser, P.M.; Meenderink, L.M.; Ryzhova, L.; Michael, K.E.; Dumbauld, D.W.; García, A.J.; Kaverina, I.; Hanks, S.K. A FAK/Src chimera with gain-of-function properties promotes formation of large peripheral adhesions associated with dynamic actin assembly. Cell Motil. Cytoskelet. 2008, 65, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Irnaten, M.; Gaynor, E.; O’Brien, C. The Role of αvβ3 Integrin in Lamina Cribrosa Cell Mechanotransduction in Glaucoma. Cells 2024, 13, 1487. [Google Scholar] [CrossRef] [PubMed]
- Filla, M.S.; Meyer, K.K.; Faralli, J.A.; Peters, D.M. Overexpression and Activation of αvβ3 Integrin Differentially Affects TGFβ2 Signaling in Human Trabecular Meshwork Cells. Cells 2021, 10, 1923. [Google Scholar] [CrossRef]
- Seo, H.Y.; Lee, S.H.; Lee, J.H.; Kang, Y.N.; Hwang, J.S.; Park, K.G.; Kim, M.K.; Jang, B.K. Src Inhibition Attenuates Liver Fibrosis by Preventing Hepatic Stellate Cell Activation and Decreasing Connetive Tissue Growth Factor. Cells 2020, 9, 558. [Google Scholar] [CrossRef]
- Bashir, H.M.; Maeno, H.; Marshall, C.M.; Park, C.; Raju, R.; Seykora, J.T.; Lee, V. Role of Epidermal Growth Factor Receptor and Src-Family Kinase Activation in Human Corneal Epithelial Wound Healing. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1281. [Google Scholar]
- Yeung, V.; Sriram, S.; Tran, J.A.; Guo, X.; Hutcheon, A.E.K.; Zieske, J.D.; Karamichos, D.; Ciolino, J.B. FAK Inhibition Attenuates Corneal Fibroblast Differentiation In Vitro. Biomolecules 2021, 11, 1682. [Google Scholar] [CrossRef]
- Park, G.B.; Kim, D.; Kim, Y.S.; Kim, S.; Lee, H.-K.; Yang, J.W.; Hur, D.Y. The Epstein-Barr Virus Causes Epithelial–Mesenchymal Transition in Human Corneal Epithelial Cells Via Syk/Src and Akt/Erk Signaling Pathways. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1770–1779. [Google Scholar] [CrossRef]
- Xu, K.P.; Yin, J.; Yu, F.S. SRC-family tyrosine kinases in wound- and ligand-induced epidermal growth factor receptor activation in human corneal epithelial cells. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2832–2839. [Google Scholar] [CrossRef]
- Penn, J.S.; Madan, A.; Caldwell, R.B.; Bartoli, M.; Caldwell, R.W.; Hartnett, M.E. Vascular endothelial growth factor in eye disease. Prog. Retin. Eye Res. 2008, 27, 331–371. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Venema, V.J.; Gu, X.; Venema, R.C.; Marrero, M.B.; Caldwell, R.B. Vascular Endothelial Growth Factor Signals Endothelial Cell Production of Nitric Oxide and Prostacyclin through Flk-1/KDR Activation of c-Src. J. Biol. Chem. 1999, 274, 25130–25135. [Google Scholar] [CrossRef] [PubMed]
- Reina-Torres, E.; Wen, J.C.; Liu, K.C.; Li, G.; Sherwood, J.M.; Chang, J.Y.; Challa, P.; Flügel-Koch, C.M.; Stamer, W.D.; Allingham, R.R.; et al. VEGF as a Paracrine Regulator of Conventional Outflow Facility. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1899–1908. [Google Scholar] [CrossRef] [PubMed]
- Kizhatil, K.; Ryan, M.; Marchant, J.K.; Henrich, S.; John, S.W. Schlemm’s canal is a unique vessel with a combination of blood vascular and lymphatic phenotypes that forms by a novel developmental process. PLoS Biol. 2014, 12, e1001912. [Google Scholar] [CrossRef] [PubMed]
- Aspelund, A.; Tammela, T.; Antila, S.; Nurmi, H.; Leppänen, V.M.; Zarkada, G.; Stanczuk, L.; Francois, M.; Mäkinen, T.; Saharinen, P.; et al. The Schlemm’s canal is a VEGF-C/VEGFR-3-responsive lymphatic-like vessel. J. Clin. Investig. 2014, 124, 3975–3986. [Google Scholar] [CrossRef]
- Wen, J.C.; Reina-Torres, E.; Sherwood, J.M.; Challa, P.; Liu, K.C.; Li, G.; Chang, J.Y.; Cousins, S.W.; Schuman, S.G.; Mettu, P.S.; et al. Intravitreal Anti-VEGF Injections Reduce Aqueous Outflow Facility in Patients With Neovascular Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1893–1898. [Google Scholar] [CrossRef]
- Sergeys, J.; Van Hove, I.; Hu, T.-T.; Temps, C.; Carragher, N.O.; Unciti-Broceta, A.; Feyen, J.H.M.; Moons, L.; Porcu, M. The retinal tyrosine kinome of diabetic Akimba mice highlights potential for specific Src family kinase inhibition in retinal vascular disease. Exp. Eye Res. 2020, 197, 108108. [Google Scholar] [CrossRef]
- Liu, D.; Xu, H.; Zhang, C.; Xie, H.; Yang, Q.; Li, W.; Tian, H.; Lu, L.; Xu, J.-Y.; Xu, G.; et al. Erythropoietin maintains VE-cadherin expression and barrier function in experimental diabetic retinopathy via inhibiting VEGF/VEGFR2/Src signaling pathway. Life Sci. 2020, 259, 118273. [Google Scholar] [CrossRef]
- Zhou, J.; Leonard, M.; Van Bockstaele, E.; Menko, A.S. Mechanism of Src kinase induction of cortical cataract following exposure to stress: Destabilization of cell-cell junctions. Mol. Vis. 2007, 13, 1298–1310. [Google Scholar]
- Li, X.; Wang, F.; Ren, M.; Du, M.; Zhou, J. The effects of c-Src kinase on EMT signaling pathway in human lens epithelial cells associated with lens diseases. BMC Ophthalmol. 2019, 19, 219. [Google Scholar] [CrossRef]
- Shahidullah, M.; Mandal, A.; Delamere, N.A. A Role for Calcium-Activated Adenylate Cyclase and Protein Kinase A in the Lens Src Family Kinase and Na,K-ATPase Response to Hyposmotic Stress. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4447–4456. [Google Scholar] [CrossRef] [PubMed]
- Duncan, G.; Bushell, A.R. Ion analyses of human cataractous lenses. Exp. Eye Res. 1975, 20, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Lakk, M.; Križaj, D. TRPV4-Rho signaling drives cytoskeletal and focal adhesion remodeling in trabecular meshwork cells. Am. J. Physiol. Cell Physiol. 2021, 320, C1013–C1030. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Khaw, P.T. Primary open-angle glaucoma. Lancet 2004, 363, 1711–1720. [Google Scholar] [CrossRef]
- Zhang, M.; Maddala, R.; Rao, P.V. Novel molecular insights into RhoA GTPase-induced resistance to aqueous humor outflow through the trabecular meshwork. Am. J. Physiol. Cell Physiol. 2008, 295, C1057–C1070. [Google Scholar] [CrossRef]
- Maddala, R.; Eldawy, C.; Bachman, W.; Soderblom, E.J.; Rao, P.V. Glypican-4 regulated actin cytoskeletal reorganization in glucocorticoid treated trabecular meshwork cells and involvement of Wnt/PCP signaling. J. Cell Physiol. 2023, 238, 631–646. [Google Scholar] [CrossRef]
- Liu, S.T.; Pham, H.; Pandol, S.J.; Ptasznik, A. Src as the link between inflammation and cancer. Front. Physiol. 2013, 4, 416. [Google Scholar] [CrossRef]
- Tezel, G. Immune regulation toward immunomodulation for neuroprotection in glaucoma. Curr. Opin. Pharmacol. 2013, 13, 23–31. [Google Scholar] [CrossRef]
- Howell, G.R.; Soto, I.; Zhu, X.; Ryan, M.; Macalinao, D.G.; Sousa, G.L.; Caddle, L.B.; MacNicoll, K.H.; Barbay, J.M.; Porciatti, V.; et al. Radiation treatment inhibits monocyte entry into the optic nerve head and prevents neuronal damage in a mouse model of glaucoma. J. Clin. Investig. 2012, 122, 1246–1261. [Google Scholar] [CrossRef]
- Nickells, R.W.; Howell, G.R.; Soto, I.; John, S.W. Under pressure: Cellular and molecular responses during glaucoma, a common neurodegeneration with axonopathy. Annu. Rev. Neurosci. 2012, 35, 153–179. [Google Scholar] [CrossRef]
- Yang, H.; Wang, L.; Zang, C.; Wang, Y.; Shang, J.; Zhang, Z.; Liu, H.; Bao, X.; Wang, X.; Zhang, D. Src Inhibition Attenuates Neuroinflammation and Protects Dopaminergic Neurons in Parkinson’s Disease Models. Front. Neurosci. 2020, 14, 45. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shi, C.; He, M.; Xiong, S.; Xia, X. Endoplasmic reticulum stress: Molecular mechanism and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 352. [Google Scholar] [CrossRef] [PubMed]
- Kimura, A.; Namekata, K.; Guo, X.; Harada, C.; Harada, T. Dock3-NMDA receptor interaction as a target for glaucoma therapy. Histol. Histopathol. 2017, 32, 215–221. [Google Scholar]
- Namekata, K.; Harada, C.; Taya, C.; Guo, X.; Kimura, H.; Parada, L.F.; Harada, T. Dock3 induces axonal outgrowth by stimulating membrane recruitment of the WAVE complex. Proc. Natl. Acad. Sci. USA 2010, 107, 7586–7591. [Google Scholar] [CrossRef]
- Namekata, K.; Kimura, A.; Kawamura, K.; Guo, X.; Harada, C.; Tanaka, K.; Harada, T. Dock3 attenuates neural cell death due to NMDA neurotoxicity and oxidative stress in a mouse model of normal tension glaucoma. Cell Death Differ. 2013, 20, 1250–1256. [Google Scholar] [CrossRef]
- Harada, C.; Nakamura, K.; Namekata, K.; Okumura, A.; Mitamura, Y.; Iizuka, Y.; Kashiwagi, K.; Yoshida, K.; Ohno, S.; Matsuzawa, A.; et al. Role of apoptosis signal-regulating kinase 1 in stress-induced neural cell apoptosis in vivo. Am. J. Pathol. 2006, 168, 261–269. [Google Scholar] [CrossRef]
- Harada, C.; Namekata, K.; Guo, X.; Yoshida, H.; Mitamura, Y.; Matsumoto, Y.; Tanaka, K.; Ichijo, H.; Harada, T. ASK1 deficiency attenuates neural cell death in GLAST-deficient mice, a model of normal tension glaucoma. Cell Death Differ. 2010, 17, 1751–1759. [Google Scholar] [CrossRef]
- Dong, L.D.; Gao, F.; Wang, X.H.; Miao, Y.; Wang, S.Y.; Wu, Y.; Li, F.; Wu, J.; Cheng, X.L.; Sun, X.H.; et al. GluA2 trafficking is involved in apoptosis of retinal ganglion cells induced by activation of EphB/EphrinB reverse signaling in a rat chronic ocular hypertension model. J. Neurosci. 2015, 35, 5409–5421. [Google Scholar] [CrossRef]
- Park, I.; Lee, H.S. EphB/ephrinB signaling in cell adhesion and migration. Mol. Cells 2015, 38, 14–19. [Google Scholar] [CrossRef]
- Xu, L.J.; Gao, F.; Cheng, S.; Zhou, Z.X.; Li, F.; Miao, Y.; Niu, W.R.; Yuan, F.; Sun, X.H.; Wang, Z. Activated ephrinA3/EphA4 forward signaling induces retinal ganglion cell apoptosis in experimental glaucoma. Neuropharmacology 2020, 178, 108228. [Google Scholar] [CrossRef]
- Chow, A.; McCrea, L.; Kimball, E.; Schaub, J.; Quigley, H.; Pitha, I. Dasatinib inhibits peripapillary scleral myofibroblast differentiation. Exp. Eye Res. 2020, 194, 107999. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tian, Y.; Cao, Y.; Ma, Q.; Zhao, S. MiR-137 promotes cell growth and inhibits extracellular matrix protein expression in H(2)O(2)-induced human trabecular meshwork cells by targeting Src. Neurosci. Lett. 2021, 755, 135902. [Google Scholar] [CrossRef] [PubMed]
- Halder, G.; Dupont, S.; Piccolo, S. Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat. Rev. Mol. Cell Biol. 2012, 13, 591–600. [Google Scholar] [CrossRef]
- Low, B.C.; Pan, C.Q.; Shivashankar, G.V.; Bershadsky, A.; Sudol, M.; Sheetz, M. YAP/TAZ as mechanosensors and mechanotransducers in regulating organ size and tumor growth. FEBS Lett. 2014, 588, 2663–2670. [Google Scholar] [CrossRef]
- Smoot, R.L.; Werneburg, N.W.; Sugihara, T.; Hernandez, M.C.; Yang, L.; Mehner, C.; Graham, R.P.; Bronk, S.F.; Truty, M.J.; Gores, G.J. Platelet-derived growth factor regulates YAP transcriptional activity via Src family kinase dependent tyrosine phosphorylation. J. Cell Biochem. 2018, 119, 824–836. [Google Scholar] [CrossRef]
- Sugihara, T.; Werneburg, N.W.; Hernandez, M.C.; Yang, L.; Kabashima, A.; Hirsova, P.; Yohanathan, L.; Sosa, C.; Truty, M.J.; Vasmatzis, G.; et al. YAP Tyrosine Phosphorylation and Nuclear Localization in Cholangiocarcinoma Cells Are Regulated by LCK and Independent of LATS Activity. Mol. Cancer Res. 2018, 16, 1556–1567. [Google Scholar] [CrossRef]
- Hsu, P.C.; Yang, C.T.; Jablons, D.M.; You, L. The Crosstalk between Src and Hippo/YAP Signaling Pathways in Non-Small Cell Lung Cancer (NSCLC). Cancers 2020, 12, 1361. [Google Scholar] [CrossRef]
- Noguchi, S.; Saito, A.; Nagase, T. YAP/TAZ Signaling as a Molecular Link between Fibrosis and Cancer. Int. J. Mol. Sci. 2018, 19, 3674. [Google Scholar] [CrossRef]
- Zanconato, F.; Cordenonsi, M.; Piccolo, S. YAP/TAZ at the Roots of Cancer. Cancer Cell 2016, 29, 783–803. [Google Scholar] [CrossRef]
- Li, H.; Raghunathan, V.; Stamer, W.D.; Ganapathy, P.S.; Herberg, S. Extracellular Matrix Stiffness and TGFβ2 Regulate YAP/TAZ Activity in Human Trabecular Meshwork Cells. Front. Cell Dev. Biol. 2022, 10, 844342. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bourke, L.; O’Brien, C. Fibrosis and Src Signalling in Glaucoma: From Molecular Pathways to Therapeutic Prospects. Int. J. Mol. Sci. 2025, 26, 1009. https://doi.org/10.3390/ijms26031009
Bourke L, O’Brien C. Fibrosis and Src Signalling in Glaucoma: From Molecular Pathways to Therapeutic Prospects. International Journal of Molecular Sciences. 2025; 26(3):1009. https://doi.org/10.3390/ijms26031009
Chicago/Turabian StyleBourke, Liam, and Colm O’Brien. 2025. "Fibrosis and Src Signalling in Glaucoma: From Molecular Pathways to Therapeutic Prospects" International Journal of Molecular Sciences 26, no. 3: 1009. https://doi.org/10.3390/ijms26031009
APA StyleBourke, L., & O’Brien, C. (2025). Fibrosis and Src Signalling in Glaucoma: From Molecular Pathways to Therapeutic Prospects. International Journal of Molecular Sciences, 26(3), 1009. https://doi.org/10.3390/ijms26031009




