Altered Network Function in Hippocampus After Sub-Chronic Activation of Cannabinoid Receptors in Early Adolescence
Abstract
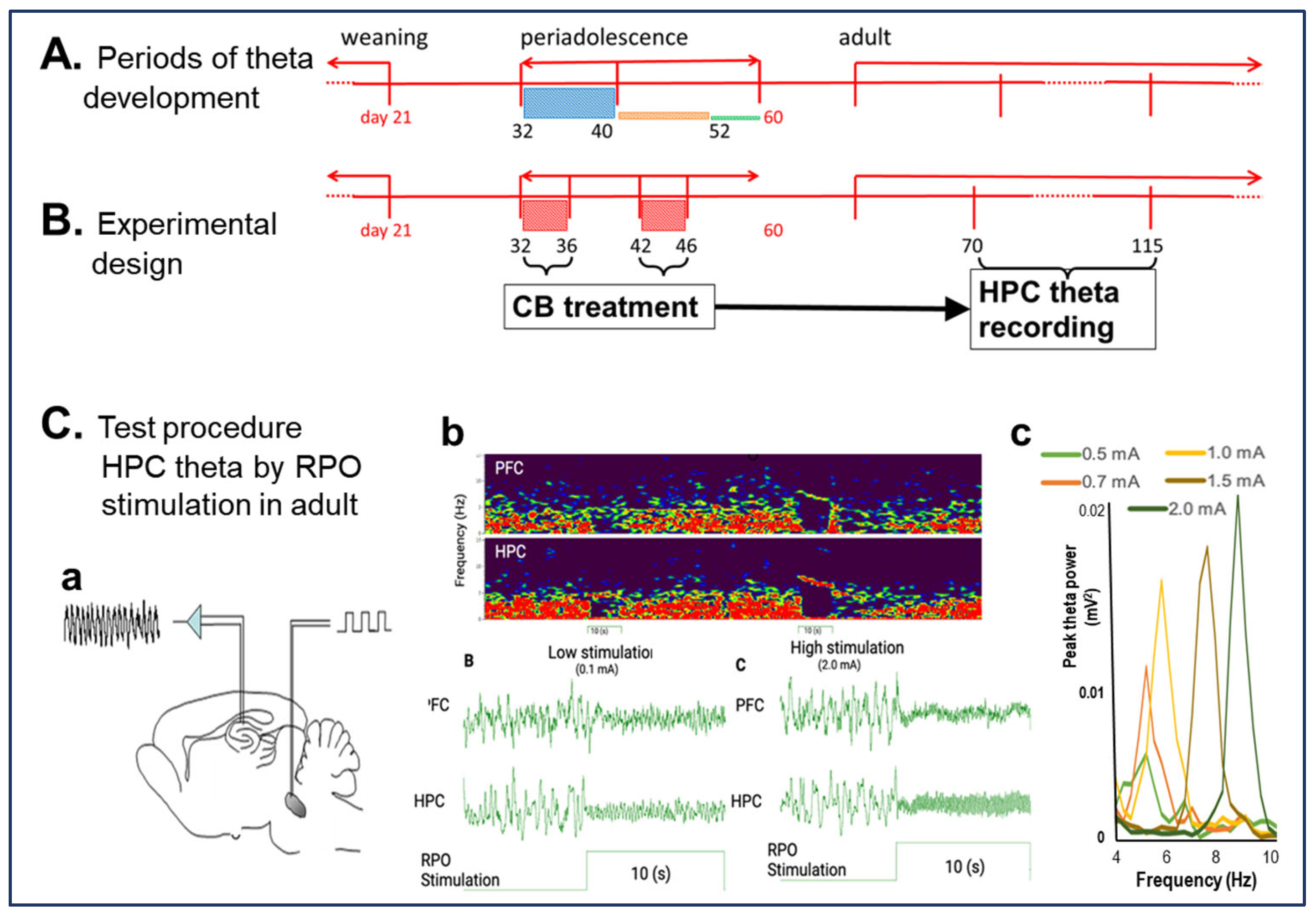
1. Introduction
2. Results
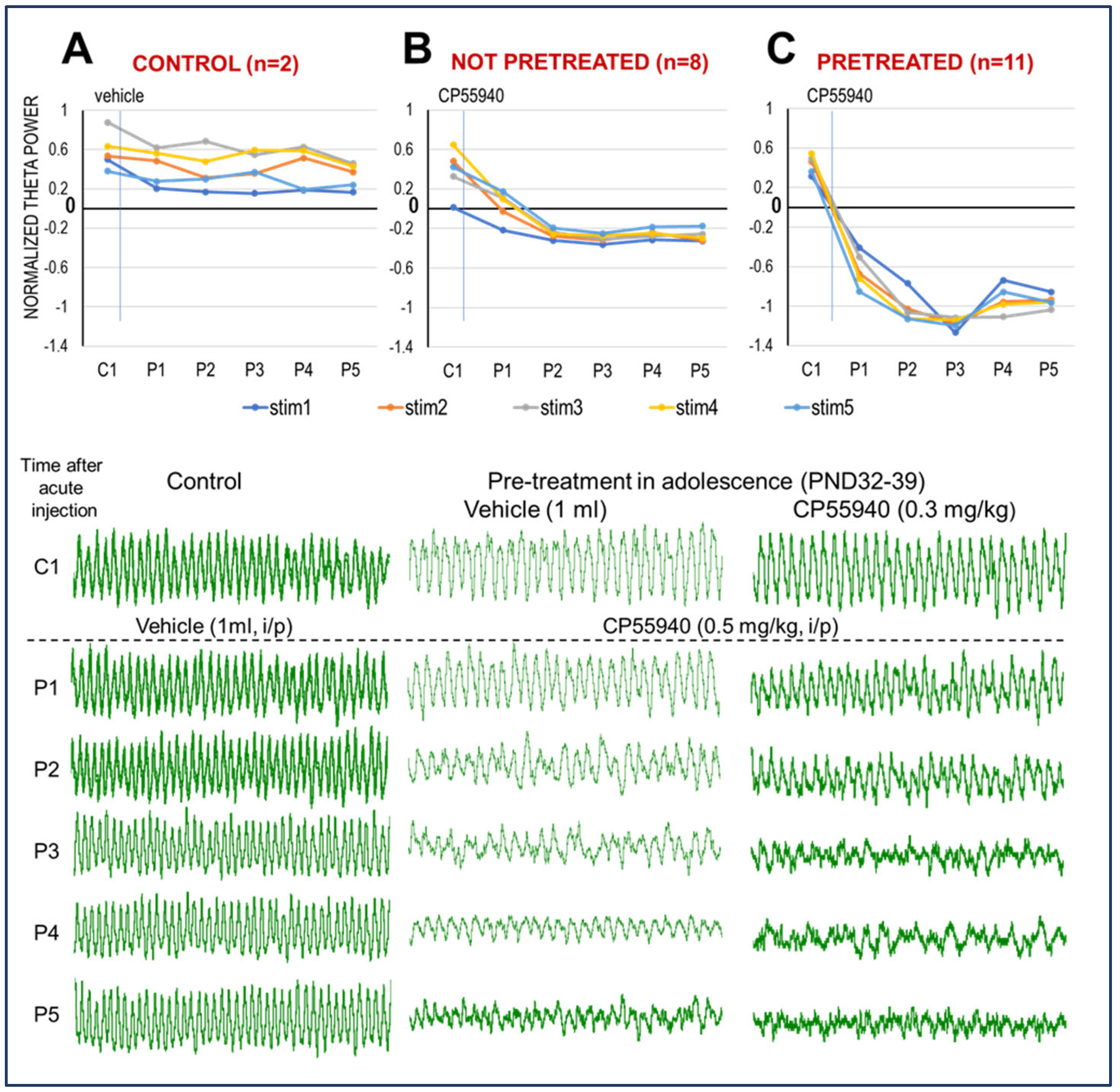
2.1. CB1R Activation Suppresses HPC Theta Oscillation in Adult Rats
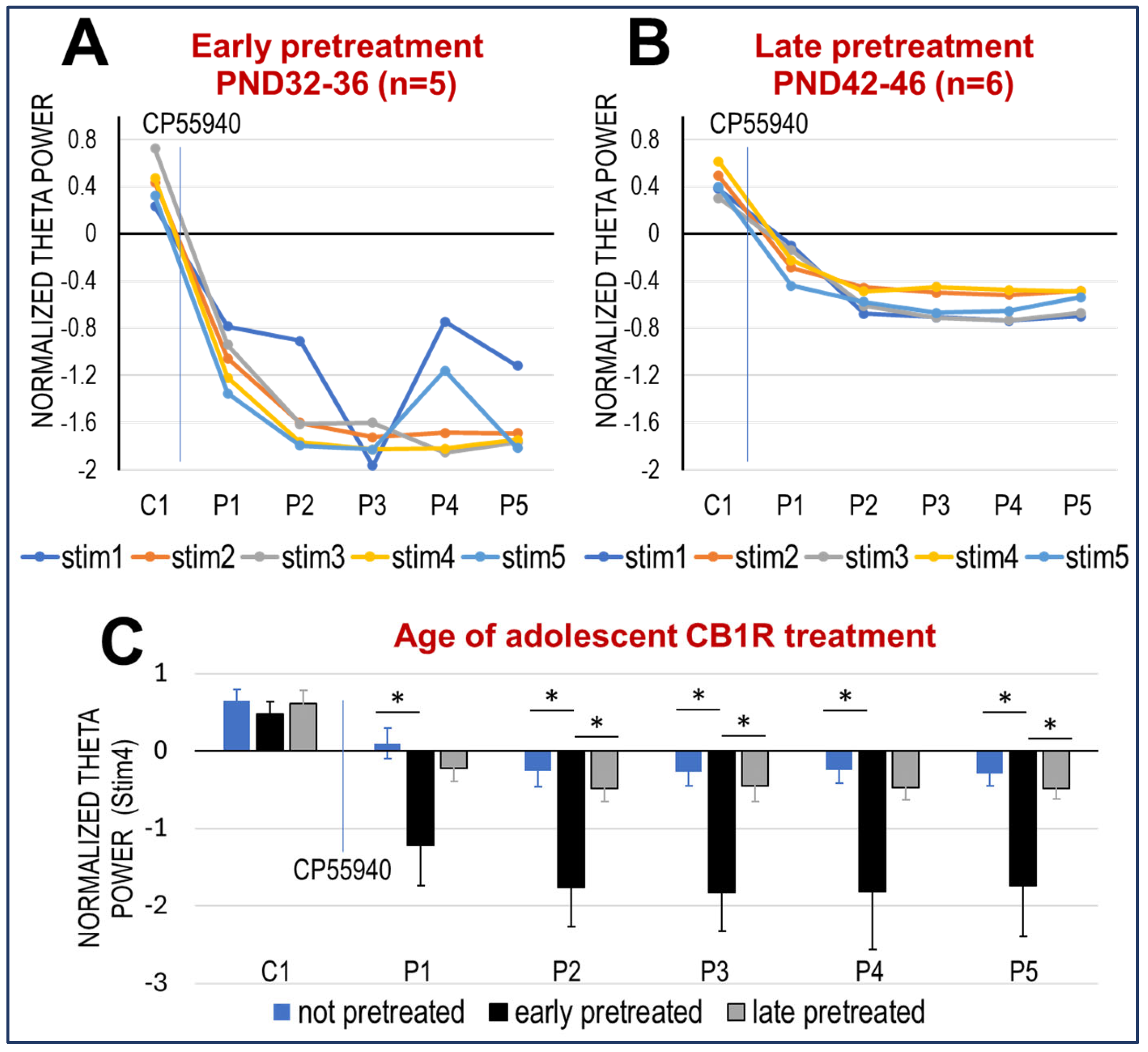
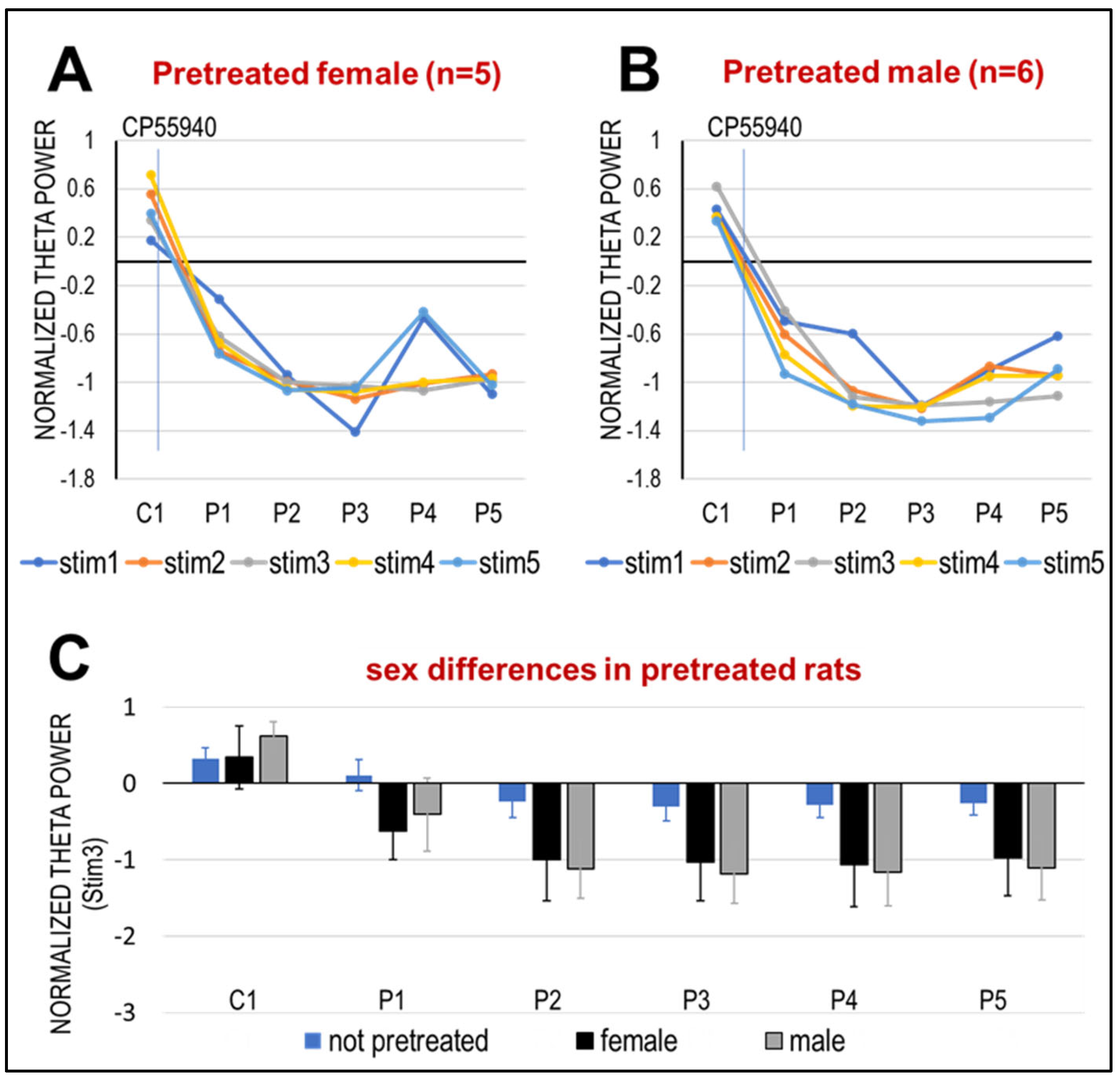
2.2. Effect of Pre-Treatment with CP-55940 in Adolescence on HPC Network Oscillations
3. Discussion
3.1. CB1R Activation Suppresses HPC Theta Oscillation in Adult Urethane-Anesthetized Rats
3.2. The Effect of CB1R Activation in Adult Rats Is Enhanced After Subchronic Periadolescent Treatment with CP-55940, More in Early (PND 32–36) than Late (PND 42–46) Adolescence
3.3. Potential Implications for Schizophrenia
4. Methods
4.1. Drug Preparation and Administration
4.2. Anesthesia and Surgical Procedures
4.3. Electrophysiological Recordings
4.4. RPO Stimulation to Elicit HPC Theta Rhythm
4.5. Statistical Modeling
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kendall, D.A.; Yudowski, G.A. Cannabinoid Receptors in the Central Nervous System: Their Signaling and Roles in Disease. Front. Cell Neurosci. 2016, 10, 294. [Google Scholar] [CrossRef] [PubMed]
- D'Addario, C.; Micale, V.; Di Bartolomeo, M.; Stark, T.; Pucci, M.; Sulcova, A.; Palazzo, M.; Babinska, Z.; Cremaschi, L.; Drago, F.; et al. A preliminary study of endocannabinoid system regulation in psychosis: Distinct alterations of CNR1 promoter DNA methylation in patients with schizophrenia. Schizophr. Res. 2017, 188, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Ceccarini, J.; De Hert, M.; Van Winkel, R.; Peuskens, J.; Bormans, G.; Kranaster, L.; Enning, F.; Koethe, D.; Leweke, F.M.; Van Laere, K. Increased ventral striatal CB1 receptor binding is related to negative symptoms in drug-free patients with schizophrenia. Neuroimage 2013, 79, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.F.; Kuwabara, H.; Horti, A.G.; Raymont, V.; Brasic, J.; Guevara, M.; Ye, W.; Dannals, R.F.; Ravert, H.T.; Nandi, A.; et al. Quantification of cerebral cannabinoid receptors subtype 1 (CB1) in healthy subjects and schizophrenia by the novel PET radioligand [11C]OMAR. Neuroimage 2010, 52, 1505–1513. [Google Scholar] [CrossRef]
- Newell, K.A.; Deng, C.; Huang, X.F. Increased cannabinoid receptor density in the posterior cingulate cortex in schizophrenia. Exp. Brain Res. 2006, 172, 556–560. [Google Scholar] [CrossRef]
- D'Souza, D.C.; Sewell, R.A.; Ranganathan, M. Cannabis and psychosis/schizophrenia: Human studies. Eur. Arch. Psychiatry Clin. Neurosci. 2009, 259, 413–431. [Google Scholar] [CrossRef]
- Hall, W.; Degenhardt, L. Cannabis use and the risk of developing a psychotic disorder. World Psychiatry 2008, 7, 68–71. [Google Scholar] [CrossRef]
- Schneider, M.; Koch, M. Chronic pubertal, but not adult chronic cannabinoid treatment impairs sensorimotor gating, recognition memory, and the performance in a progressive ratio task in adult rats. Neuropsychopharmacology 2003, 28, 1760–1769. [Google Scholar] [CrossRef]
- Heng, L.; Beverley, J.A.; Steiner, H.; Tseng, K.Y. Differential developmental trajectories for CB1 cannabinoid receptor expression in limbic/associative and sensorimotor cortical areas. Synapse 2011, 65, 278–286. [Google Scholar] [CrossRef]
- Long, L.E.; Lind, J.; Webster, M.; Weickert, C.S. Developmental trajectory of the endocannabinoid system in human dorsolateral prefrontal cortex. BMC Neurosci. 2012, 13, 87. [Google Scholar] [CrossRef]
- Hajos, M.; Hoffmann, W.E.; Kocsis, B. Activation of cannabinoid-1 receptors disrupts sensory gating and neuronal oscillation: Relevance to schizophrenia. Biol. Psychiatry 2008, 63, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Robbe, D.; Buzsaki, G. Alteration of theta timescale dynamics of hippocampal place cells by a cannabinoid is associated with memory impairment. J. Neurosci. 2009, 29, 12597–12605. [Google Scholar] [CrossRef] [PubMed]
- Robbe, D.; Montgomery, S.M.; Thome, A.; Rueda-Orozco, P.E.; McNaughton, B.L.; Buzsaki, G. Cannabinoids reveal importance of spike timing coordination in hippocampal function. Nat. Neurosci. 2006, 9, 1526–1533. [Google Scholar] [CrossRef] [PubMed]
- Freund, T.F.; Katona, I.; Piomelli, D. Role of endogenous cannabinoids in synaptic signaling. Physiol. Rev. 2003, 83, 1017–1066. [Google Scholar] [CrossRef]
- Skosnik, P.D.; Hajos, M.; Cortes-Briones, J.A.; Edwards, C.R.; Pittman, B.P.; Hoffmann, W.E.; Sewell, A.R.; D’Souza, D.C.; Ranganathan, M. Cannabinoid receptor-mediated disruption of sensory gating and neural oscillations: A translational study in rats and humans. Neuropharmacology 2018, 135, 412–423. [Google Scholar] [CrossRef]
- Sibilska, S.; Mofleh, R.; Kocsis, B. Development of network oscillations through adolescence in male and female rats. Front. Cell Neurosci. 2023, 17, 1135154. [Google Scholar] [CrossRef]
- Thorn, C.W.; Kafetzopoulos, V.; Kocsis, B. Differential Effect of Dopamine D4 Receptor Activation on Low-Frequency Oscillations in the Prefrontal Cortex and Hippocampus May Bias the Bidirectional Prefrontal-Hippocampal Coupling. Int. J. Mol. Sci. 2022, 23, 1705. [Google Scholar] [CrossRef]
- Mofleh, R.; Kocsis, B. Respiratory coupling between prefrontal cortex and hippocampus of rats anaesthetized with urethane in theta and non-theta states. Eur. J. Neurosci. 2021, 54, 5507–5517. [Google Scholar] [CrossRef]
- Li, S.; Topchiy, I.; Kocsis, B. The effect of atropine administered in the medial septum or hippocampus on high- and low-frequency theta rhythms in the hippocampus of urethane anesthetized rats. Synapse 2007, 61, 412–419. [Google Scholar] [CrossRef]
- Hajos, M.; Siok, C.J.; Hoffmann, W.E.; Li, S.; Kocsis, B. Modulation of hippocampal theta oscillation by histamine H3 receptors. J. Pharmacol. Exp. Ther. 2008, 324, 391–398. [Google Scholar] [CrossRef]
- Kramis, R.; Vanderwolf, C.H.; Bland, B.H. Two types of hippocampal rhythmical slow activity in both the rabbit and the rat: Relations to behavior and effects of atropine, diethyl ether, urethane, and pentobarbital. Exp. Neurol. 1975, 49, 58–85. [Google Scholar] [CrossRef] [PubMed]
- Laviola, G.; Macri, S.; Morley-Fletcher, S.; Adriani, W. Risk-taking behavior in adolescent mice: Psychobiological determinants and early epigenetic influence. Neurosci. Biobehav. Rev. 2003, 27, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Renard, J.; Vitalis, T.; Rame, M.; Krebs, M.O.; Lenkei, Z.; Le Pen, G.; Jay, T.M. Chronic cannabinoid exposure during adolescence leads to long-term structural and functional changes in the prefrontal cortex. Eur. Neuropsychopharmacol. 2016, 26, 55–64. [Google Scholar] [CrossRef] [PubMed]
- O'Shea, M.; Singh, M.E.; McGregor, I.S.; Mallet, P.E. Chronic cannabinoid exposure produces lasting memory impairment and increased anxiety in adolescent but not adult rats. J. Psychopharmacol. 2004, 18, 502–508. [Google Scholar] [CrossRef]
- Biscaia, M.; Marin, S.; Fernandez, B.; Marco, E.M.; Rubio, M.; Guaza, C.; Ambrosio, E.; Viveros, M.P. Chronic treatment with CP 55,940 during the peri-adolescent period differentially affects the behavioural responses of male and female rats in adulthood. Psychopharmacology 2003, 170, 301–308. [Google Scholar] [CrossRef]
- Minney, S.M.; Lopez, H.H. Adolescent cannabinoid treatment negatively affects reproductive behavior in female rats. Pharmacol. Biochem. Behav. 2013, 112, 82–88. [Google Scholar] [CrossRef]
- Burston, J.J.; Wiley, J.L.; Craig, A.A.; Selley, D.E.; Sim-Selley, L.J. Regional enhancement of cannabinoid CB1 receptor desensitization in female adolescent rats following repeated Delta-tetrahydrocannabinol exposure. Br. J. Pharmacol. 2010, 161, 103–112. [Google Scholar] [CrossRef]
- Higuera-Matas, A.; Botreau, F.; Miguens, M.; Del Olmo, N.; Borcel, E.; Perez-Alvarez, L.; Garcia-Lecumberri, C.; Ambrosio, E. Chronic periadolescent cannabinoid treatment enhances adult hippocampal PSA-NCAM expression in male Wistar rats but only has marginal effects on anxiety, learning and memory. Pharmacol. Biochem. Behav. 2009, 93, 482–490. [Google Scholar] [CrossRef]
- Viveros, M.P.; Llorente, R.; Moreno, E.; Marco, E.M. Behavioural and neuroendocrine effects of cannabinoids in critical developmental periods. Behav. Pharmacol. 2005, 16, 353–362. [Google Scholar] [CrossRef]
- Chadwick, B.; Saylor, A.J.; Lopez, H.H. Adolescent cannabinoid exposure attenuates adult female sexual motivation but does not alter adulthood CB1R expression or estrous cyclicity. Pharmacol. Biochem. Behav. 2011, 100, 157–164. [Google Scholar] [CrossRef]
- Rinaldi-Carmona, M.; Pialot, F.; Congy, C.; Redon, E.; Barth, F.; Bachy, A.; Breliere, J.C.; Soubrie, P.; Le Fur, G. Characterization and distribution of binding sites for [3H]-SR 141716A, a selective brain (CB1) cannabinoid receptor antagonist, in rodent brain. Life Sci. 1996, 58, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.; Harte-Hargrove, L.; Izenwasser, S.; Frank, A.; Wade, D.; Dow-Edwards, D. Sex-specific alterations in hippocampal cannabinoid 1 receptor expression following adolescent delta-9-tetrahydrocannabinol treatment in the rat. Neurosci. Lett. 2015, 602, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.R. Comparing Postnatal Development of Gonadal Hormones and Associated Social Behaviors in Rats, Mice, and Humans. Endocrinology 2018, 159, 2596–2613. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Ma, X.; Wang, G.; Yang, J.; Wang, C. Why sex differences in schizophrenia? J. Transl. Neurosci. 2016, 1, 37–42. [Google Scholar]
- Cuttler, C.; Mischley, L.K.; Sexton, M. Sex Differences in Cannabis Use and Effects: A Cross-Sectional Survey of Cannabis Users. Cannabis Cannabinoid Res. 2016, 1, 166–175. [Google Scholar] [CrossRef]
- Buzsaki, G.; Logothetis, N.; Singer, W. Scaling brain size, keeping timing: Evolutionary preservation of brain rhythms. Neuron 2013, 80, 751–764. [Google Scholar] [CrossRef]
- Pittman-Polletta, B.R.; Kocsis, B.; Vijayan, S.; Whittington, M.A.; Kopell, N.J. Brain rhythms connect impaired inhibition to altered cognition in schizophrenia. Biol. Psychiatry 2015, 77, 1020–1030. [Google Scholar] [CrossRef]
- Moran, L.V.; Hong, L.E. High vs low frequency neural oscillations in schizophrenia. Schizophr. Bull. 2011, 37, 659–663. [Google Scholar] [CrossRef]
- Sohal, V.S. Transforming Discoveries About Cortical Microcircuits and Gamma Oscillations Into New Treatments for Cognitive Deficits in Schizophrenia. Am. J. Psychiatry 2022, 179, 267–276. [Google Scholar] [CrossRef]
- Jadi, M.P.; Behrens, M.M.; Sejnowski, T.J. Abnormal Gamma Oscillations in N-Methyl-D-Aspartate Receptor Hypofunction Models of Schizophrenia. Biol. Psychiatry 2016, 79, 716–726. [Google Scholar] [CrossRef]
- Topchiy, I.; Kocsis, B. CB-1 receptor agonist drastically changes oscillatory activity, defining active sleep. Proc. Natl. Acad. Sci. USA 2025, 122, e2411063122. [Google Scholar] [CrossRef]
- Ferrarelli, F.; Tononi, G. The thalamic reticular nucleus and schizophrenia. Schizophr. Bull. 2011, 37, 306–315. [Google Scholar] [CrossRef]
- Manoach, D.S.; Pan, J.Q.; Purcell, S.M.; Stickgold, R. Reduced Sleep Spindles in Schizophrenia: A Treatable Endophenotype That Links Risk Genes to Impaired Cognition? Biol. Psychiatry 2016, 80, 599–608. [Google Scholar] [CrossRef]
- Ferrarelli, F. Sleep spindles as neurophysiological biomarkers of schizophrenia. Eur. J. Neurosci. 2024, 59, 1907–1917, Correction in Eur. J. Neurosci. 2024, 59, 3147–3148. [Google Scholar] [CrossRef]
- Solis-Vivanco, R.; Mondragon-Maya, A.; Reyes-Madrigal, F.; de la Fuente-Sandoval, C. Impairment of novelty-related theta oscillations and P3a in never medicated first-episode psychosis patients. npj Schizophr. 2021, 7, 15. [Google Scholar] [CrossRef]
- Cousijn, J.; Wiers, R.W.; Ridderinkhof, K.R.; van den Brink, W.; Veltman, D.J.; Goudriaan, A.E. Grey matter alterations associated with cannabis use: Results of a VBM study in heavy cannabis users and healthy controls. Neuroimage 2012, 59, 3845–3851. [Google Scholar] [CrossRef]
- Sorman, E.; Wang, D.; Hajos, M.; Kocsis, B. Control of hippocampal theta rhythm by serotonin: role of 5-HT2c receptors. Neuropharmacology 2011, 61, 489–494. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rehn, J.; Admeus, L.; Kocsis, B. Altered Network Function in Hippocampus After Sub-Chronic Activation of Cannabinoid Receptors in Early Adolescence. Int. J. Mol. Sci. 2025, 26, 12182. https://doi.org/10.3390/ijms262412182
Rehn J, Admeus L, Kocsis B. Altered Network Function in Hippocampus After Sub-Chronic Activation of Cannabinoid Receptors in Early Adolescence. International Journal of Molecular Sciences. 2025; 26(24):12182. https://doi.org/10.3390/ijms262412182
Chicago/Turabian StyleRehn, Johanna, Lucas Admeus, and Bernat Kocsis. 2025. "Altered Network Function in Hippocampus After Sub-Chronic Activation of Cannabinoid Receptors in Early Adolescence" International Journal of Molecular Sciences 26, no. 24: 12182. https://doi.org/10.3390/ijms262412182
APA StyleRehn, J., Admeus, L., & Kocsis, B. (2025). Altered Network Function in Hippocampus After Sub-Chronic Activation of Cannabinoid Receptors in Early Adolescence. International Journal of Molecular Sciences, 26(24), 12182. https://doi.org/10.3390/ijms262412182





