Special Issue: Emerging Topics in Metal Complexes: Pharmacological Activity, 2nd Edition
1. Introduction and Scope
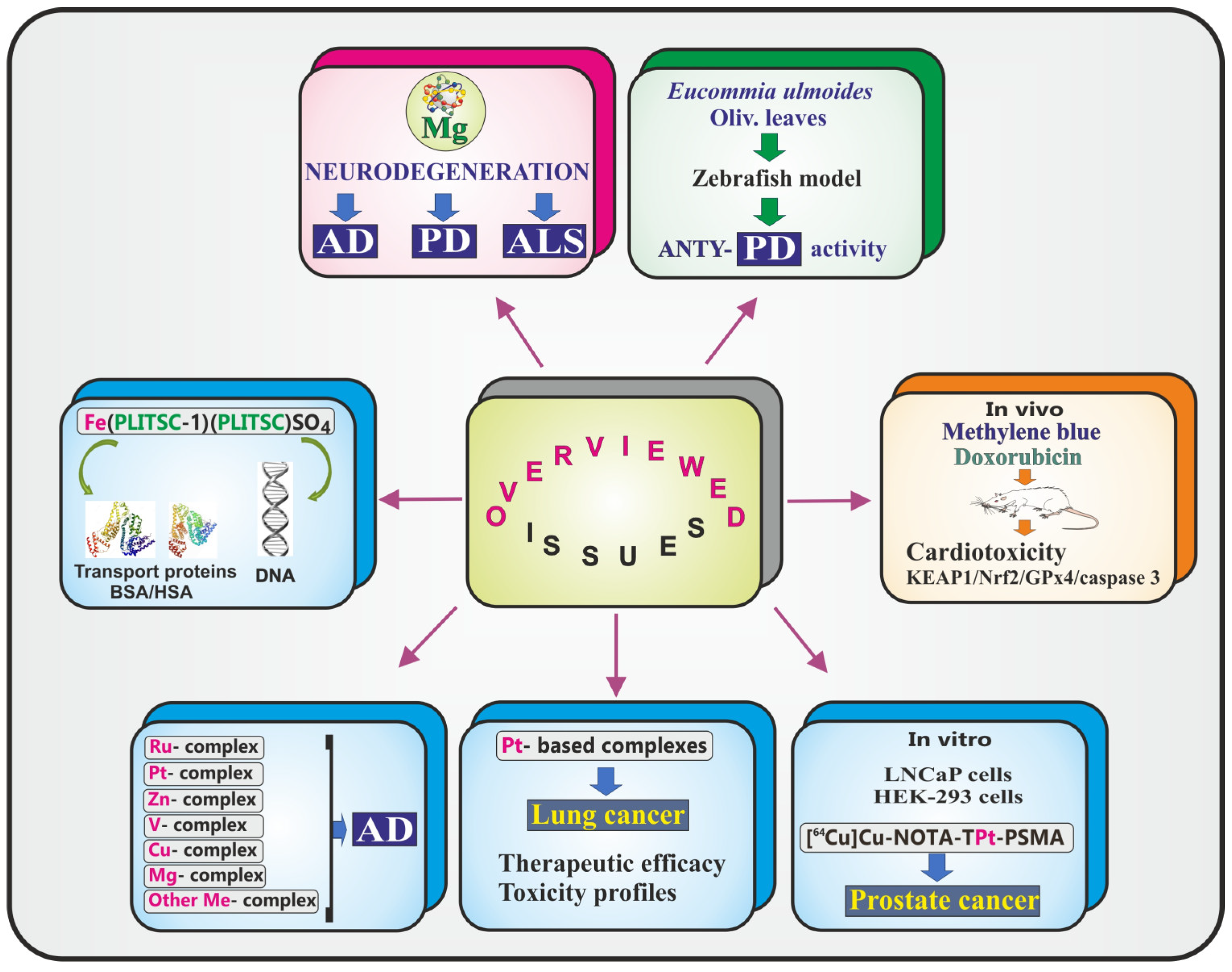
2. Contributions
2.1. Metals
2.2. Metal Complexes
2.3. Drugs
2.4. Extracts of Plant Origin
3. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
List of Contributions
- Ścibior, A.; Llopis, J.; Dobrakowski, P.P.; Męcik-Kronenberg, T. Magnesium (Mg) and neurodegeneration: A comprehensive overview of studies on Mg levels in biological specimens in humans affected some neurodegenerative disorders with an update on therapy and clinical trials supplemented with selected animal studies. Int. J. Mol. Sci. 2024, 25, 12595. https://doi.org/10.3390/ijms252312595.
- Liu, Y.; Ma, J.; Zhang, Q.; Wang, Y.; Sun, Q. Mechanism of metal complexes in Alzheimer’s disease. Int. J. Mol. Sci. 2024, 25, 11873. https://doi.org/10.3390/ijms252211873.
- Liang, W.; Huang, Y.; Wang, Y.; Lu, D.; Sun, Q. Research progress of platinum-based complexes in lung cancer treatment: Mechanisms, applications, and challenges. Int. J. Mol. Sci. 2025, 26, 7958. https://doi.org/10.3390/ijms26167958.
- Talebian, H.; Ait-Mohand, S.; Doss, P.M.I.A.; Sanche, L.; Guérin, B. Development and in vitro evaluation of [64Cu]-NOTA-TP-PSMA, a novel radiotheranostic agent against prostate cancer. Int. J. Mol. Sci. 2025, 26, 11651.
- Jevtovic, V.; Golubović, L.; Alshammari, B.; Alshammari, M.R.; Rajeh, S.Y.; Alreshidi, M.A.; Alshammari, O.A.O.; Rakić, A.; Dimić, D. Crystallographic structure, theoretical analysis, and protein/DNA binding activity of iron(III) complex containing differently protonated pyridoxal-S-methyl-isothiosemicarbazone ligands. Int. J. Mol. Sci. 2024, 25, 7058. https://doi.org/10.3390/ijms25137058.
- Ibrahim, S.G.; Abu-Dief, A.M.; Gad, A.M.; Gad, E.S.; Alzahrani, A.Y.A.; Alraih, A.M.; Barnawi, I.O.; Mansour, M.; Gadelmawla, M.H.A.; Khames, A. Methylene blue mitigates doxorubicin-induced cardiotoxicity via KEAP1/Nrf2/GPx-4/Caspase 3 modulation. Int. J. Mol. Sci. 2025, 26, 7680. https://doi.org/10.3390/ijms26167680.
- Li, Y.; Shi, R.; Xia, L.; Zhang, X.; Zhang, P.; Liu, S.; Liu, K.; Sik, A.; Stoika, R.; Jin, M. Identification of key active constituents in Eucommia ulmoides oliv. leaves against Parkinson’s disease and the alleviate effects via 4E-BP1 up-regulation. Int. J. Mol. Sci. 2025, 26, 2762. https://doi.org/10.3390/ijms26062762.
References
- Ścibior, A.; Pietrzyk, Ł.; Plewa, Z.; Skiba, A. Vanadium: Risks and possible benefits in the light of a comprehensive overview of its pharmacotoxicological mechanisms and multi-applications with a summary of further research trends. J. Trace Elem. Med. Biol. 2020, 61, 126508. [Google Scholar] [CrossRef] [PubMed]
- Safiri, S.; Ghaffari Jolfayi, A.; Fazlollahi, A.; Morsali, S.; Sarkesh, A.; Daei Sorkhabi, A.; Golabi, B.; Aletaha, R.; Motlagh Asghari, K.; Hamidi, S.; et al. Alzheimer’s disease: A comprehensive review of epidemiology, risk factors, symptoms diagnosis, management, caregiving, advanced treatments and associated challenges. Front. Med. 2024, 11, 1474043. [Google Scholar] [CrossRef] [PubMed]
- Okafor, M.; Faller, P.; Vitale, N. Cell-specific copper dyshomeostasis mechanism in Alzheimer’s disease. Transl. Neurodegener. 2025, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Díaz, A.; Vázquez-Roque, R.; Carreto-Meneses, K.; Moroni-González, D.; Moreno-Rodríguez, J.A.; Treviño, S. Polyoxidovanadates as a pharmacological option against brain aging. J. Chem. Neuroanat. 2023, 129, 102256. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Liu, Z.; Zhao, H.; Zhang, H.; Ren, J.; Qu, X. Polyoxometalates: Metallodrug agents for combating amyloid aggregation. Natl. Sci. Rev. 2024, 11, nwae226. [Google Scholar] [CrossRef] [PubMed]
- Aureliano, M.; Mateus, J.; Rijo, D.M. Polyoxometalates’ progress for the treatment of Alzheimer’s disease. BioChem 2025, 5, 41. [Google Scholar] [CrossRef]
- Chu, F.; Chen, L.; Guan, Q.; Chen, Z.; Ji, Q.; Ma, Y.; Ji, J.; Sun, M.; Huang, T.; Song, H.; et al. Global burden of prostate cancer: Age-period-cohort analysis from 1990 to 2021 and projections until 2040. World J. Surg. Onc. 2025, 23, 98. [Google Scholar] [CrossRef] [PubMed]
- Jevtovic, V.; Alabbosh, K.F.S.; Pržić, Z.; Nikolić, J.; Ali Alyami, R.; Alshammari, M.R.; Alshammari, B.; Rakic, V.; Alshammari, O.A.O.; Mitić, M. Targeted recovery of phenolic antioxidants from grape stems: A sequential approach. Molecules 2025, 30, 3546. [Google Scholar] [CrossRef] [PubMed]
- Kciuk, M.; Gielecińska, A.; Mujwar, S.; Kołat, D.; Kałuzińska-Kołat, Ż.; Celik, I.; Kontek, R. Doxorubicin—An agent with multiple mechanisms of anticancer activity. Cells 2023, 12, 659. [Google Scholar] [CrossRef] [PubMed]
- Bhutani, V.; Varzideh, F.; Wilson, S.; Kansakar, U.; Jankauskas, S.S.; Santulli, G. Doxorubicin-induced cardiotoxicity: A comprehensive update. J. Cardiovasc. Dev. Dis. 2025, 12, 207, Erratum in J. Cardiovasc. Dev. Dis. 2025, 12, 242. https://doi.org/10.3390/jcdd12070242. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.J.; Zhang, J.; Xiao, M.; Wang, S.; Wang, B.J.; Guo, Y.; Tang, Y.; Gu, J. Molecular mechanisms of doxorubicin-induced cardiotoxicity: Novel roles of sirtuin 1-mediated signaling pathways. Cell. Mol. Life Sci. 2021, 78, 3105–3125. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Sharma, P.; Patel, A.; Kumar Gaurav, A. Mechanisms, clinical manifestations, and management of doxorubicin-induced cardiotoxicity: A comprehensive review. Int. J. Pharm. Sci. 2025, 3, 1516–1530. [Google Scholar] [CrossRef]
- Xue, H.; Thaivalappil, A.; Cao, K. The potentials of methylene blue as an anti-aging drug. Cells 2021, 10, 3379. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zheng, W.; Li, Y.; Zhong, J.; Ji, J.; Shen, P. Apoptosis induced by methylene-blue-mediated photodynamic therapy in melanomas and the involvement of mitochondrial dysfunction revealed by proteomics. Cancer Sci. 2008, 99, 2019–2027. [Google Scholar] [CrossRef] [PubMed]
- Abdelall, A.M.A.; Khames, A.; Bekhit, A.A.; Fathy, M. Potential effect of etoricoxib in reducing inflammation in methotrexate-induced pulmonary injury in rats: Role of oxidative stress and the TLR4/p38-MAPK/NF-κB signaling pathway. Inflammation 2025, 48, 2407–2416. [Google Scholar] [CrossRef] [PubMed]
- Mhyre, T.R.; Boyd, J.T.; Hamill, R.W.; Maguire-Zeiss, K.A. Parkinson’s disease. In Protein Aggregation and Fibrillogenesis in Cerebral and Systemic Amyloid Disease; Springer: Berlin/Heidelberg, Germany, 2012; Volume 65, pp. 389–455. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ścibior, A.; Aureliano, M.; Llopis, J. Special Issue: Emerging Topics in Metal Complexes: Pharmacological Activity, 2nd Edition. Int. J. Mol. Sci. 2025, 26, 12165. https://doi.org/10.3390/ijms262412165
Ścibior A, Aureliano M, Llopis J. Special Issue: Emerging Topics in Metal Complexes: Pharmacological Activity, 2nd Edition. International Journal of Molecular Sciences. 2025; 26(24):12165. https://doi.org/10.3390/ijms262412165
Chicago/Turabian StyleŚcibior, Agnieszka, Manuel Aureliano, and Juan Llopis. 2025. "Special Issue: Emerging Topics in Metal Complexes: Pharmacological Activity, 2nd Edition" International Journal of Molecular Sciences 26, no. 24: 12165. https://doi.org/10.3390/ijms262412165
APA StyleŚcibior, A., Aureliano, M., & Llopis, J. (2025). Special Issue: Emerging Topics in Metal Complexes: Pharmacological Activity, 2nd Edition. International Journal of Molecular Sciences, 26(24), 12165. https://doi.org/10.3390/ijms262412165






