Glioblastoma—A Contemporary Overview of Epidemiology, Classification, Pathogenesis, Diagnosis, and Treatment: A Review Article
Abstract
1. Introduction
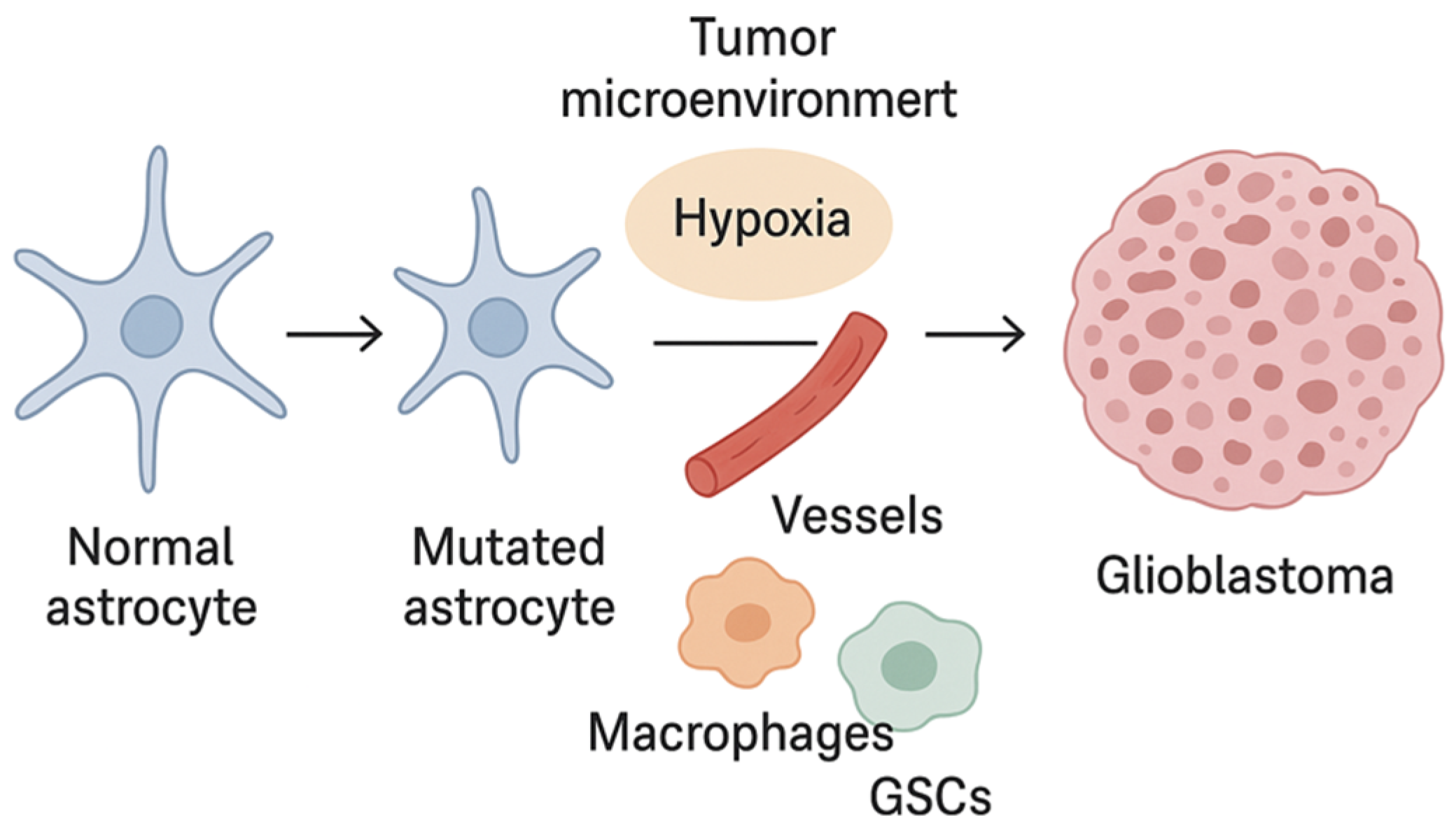
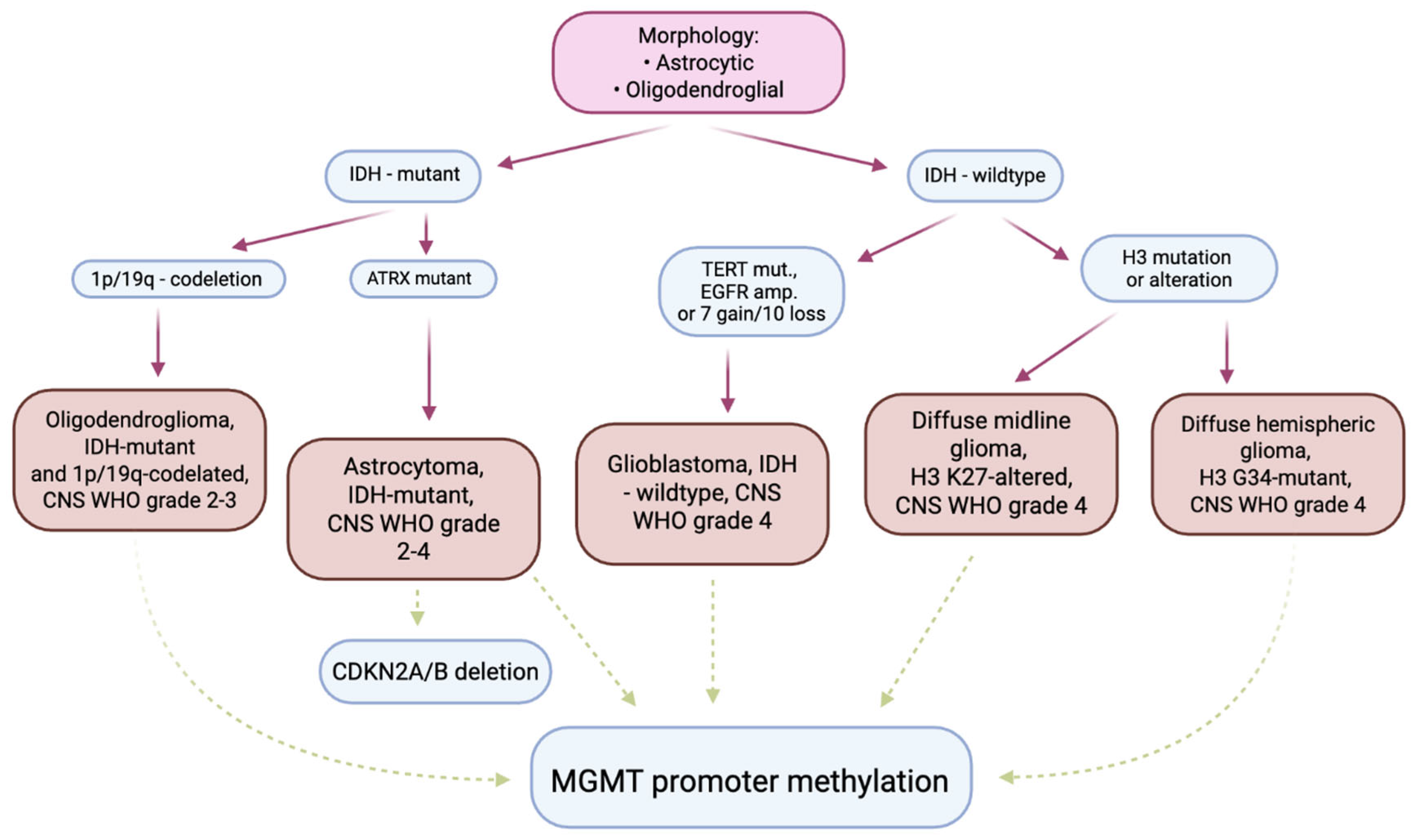
2. Pathogenesis and Molecular Biology
3. Clinical Picture
4. Diagnosis
4.1. Imaging Diagnostics (MRI, fMRI, PET)
4.2. Molecular and Histopathological Diagnostics—The Importance of Biomarkers
4.3. Liquid Biopsy
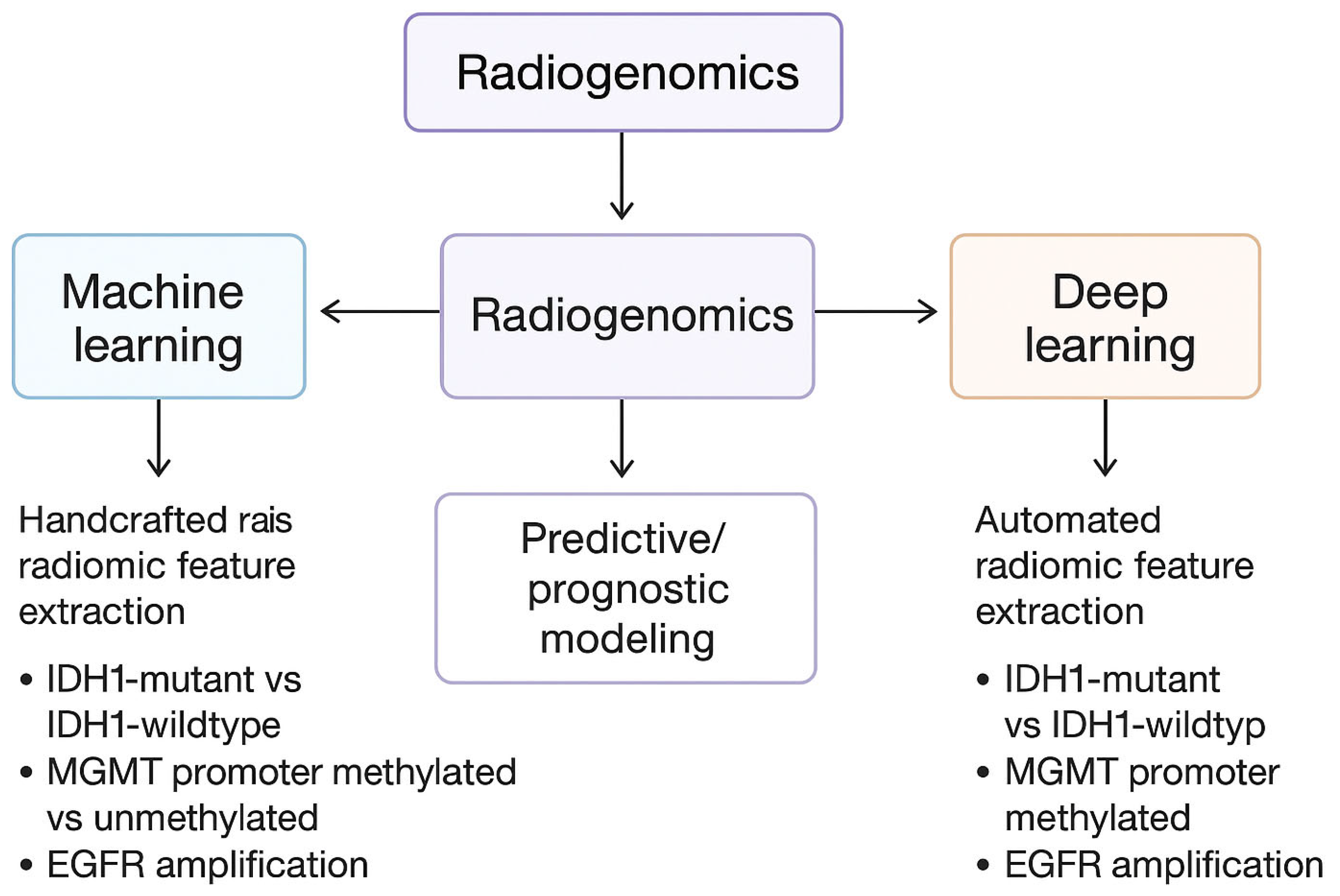
4.4. Diagnostic Innovations
5. Treatment
6. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.M.; Simjee, S.U. Glioblastoma Multiforme: A Review of its Epidemiology and Pathogenesis through Clinical Presentation and Treatment. Asian Pac. J. Cancer Prev. 2017, 18, 3–9. [Google Scholar]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Jacome, M.A.; Wu, Q.; Piña, Y.; Etame, A.B. Evolution of Molecular Biomarkers and Precision Molecular Therapeutic Strategies in Glioblastoma. Cancers 2024, 16, 3635. [Google Scholar] [CrossRef]
- Thakkar, J.P.; Dolecek, T.A.; Horbinski, C.; Ostrom, Q.T.; Lightner, D.D.; Barnholtz-Sloan, J.S.; Villano, J.L. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1985–1996. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Price, M.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2016–2020. Neuro Oncol. 2023, 25, iv1–iv99. [Google Scholar] [CrossRef]
- Crocetti, E.; Trama, A.; Stiller, C.; Caldarella, A.; Soffietti, R.; Jaal, J.; Weber, D.C.; Ricardi, U.; Slowinski, J.; Brandes, A.; et al. Epidemiology of glial and non-glial brain tumours in Europe. Eur. J. Cancer 2012, 48, 1532–1542. [Google Scholar] [CrossRef] [PubMed]
- Monterroso, P.; Moore, K.J.; Sample, J.M.; Sorajja, N.; Domingues, A.; Williams, L.A. Racial/ethnic and sex differences in young adult malignant brain tumor incidence by histologic type. Cancer Epidemiol. 2022, 76, 102078. [Google Scholar] [CrossRef]
- Di Nunno, V.; Gatto, L.; Aprile, M.; Bartolini, S.; Tosoni, A.; Franceschi, E. Economic income and survival in patients affected by glioblastoma: A systematic review and meta-analysis. Neurooncol. Pract. 2024, 11, 546–555. [Google Scholar] [CrossRef]
- Delgado-López, P.D.; Corrales-García, E.M. Survival in glioblastoma: A review on the impact of treatment modalities. Clin. Transl. Oncol. 2016, 18, 1062–1071. [Google Scholar] [CrossRef]
- Sun, T.; Warrington, N.M.; Luo, J.; Brooks, M.D.; Dahiya, S.; Snyder, S.C.; Sengupta, R.; Rubin, J.B. Sexually dimorphic RB inactivation underlies mesenchymal glioblastoma prevalence in males. J. Clin. Investig. 2014, 124, 4123–4133. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.; Perryman, L.; Hargrave, D. Paediatric and adult malignant glioma: Close relatives or distant cousins? Nat. Rev. Clin. Oncol. 2012, 9, 400–413. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board. WHO Classification of Tumours of the Central Nervous System, 5th ed.; CNS5; WHO Classification of Tumours Editorial Board: Lyon, France, 2021. [Google Scholar]
- Aldape, K.; Brindle, K.M.; Chesler, L.; Chopra, R.; Gajjar, A.; Gilbert, M.R.; Gottardo, N.; Gutmann, D.H.; Hargrave, D.; Holland, E.C.; et al. Challenges to curing primary brain tumours. Nat. Rev. Clin. Oncol. 2019, 16, 509–520. [Google Scholar] [CrossRef]
- Ah-Pine, F.; Khettab, M.; Bedoui, Y.; Slama, Y.; Daniel, M.; Doray, B.; Gasque, P. On the origin and development of glioblastoma: Multifaceted role of perivascular mesenchymal stromal cells. Acta Neuropathol. Commun. 2023, 11, 104. [Google Scholar] [CrossRef]
- Gunasegaran, B.; Ashley, C.L.; Marsh-Wakefield, F.; Guillemin, G.J.; Heng, B. Viruses in glioblastoma: An update on evidence and clinical trials. BJC Rep. 2024, 2, 33. [Google Scholar] [CrossRef]
- Testa, U.; Castelli, G.; Pelosi, E. Genetic Abnormalities, Clonal Evolution, and Cancer Stem Cells of Brain Tumors. Med. Sci. 2018, 6, 85. [Google Scholar]
- Fujimoto, K.; Arita, H.; Satomi, K.; Yamasaki, K.; Matsushita, Y.; Nakamura, T.; Miyakita, Y.; Umehara, T.; Kobayashi, K.; Tamura, K.; et al. TERT promoter mutation status is necessary and sufficient to diagnose IDH-wildtype diffuse astrocytic glioma with molecular features of glioblastoma. Acta Neuropathol. 2021, 142, 323–338. [Google Scholar] [CrossRef]
- Olympios, N.; Gilard, V.; Marguet, F.; Clatot, F.; Di Fiore, F.; Fontanilles, M. TERT Promoter Alterations in Glioblastoma: A Systematic Review. Cancers 2021, 13, 1147. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, S.M.B.; Kamel, A.; Ciubotaru, G.V.; Onose, G.; Sevastre, A.S.; Sfredel, V.; Danoiu, S.; Dricu, A.; Tataranu, L.G. An Overview of EGFR Mechanisms and Their Implications in Targeted Therapies for Glioblastoma. Int. J. Mol. Sci. 2023, 24, 11110. [Google Scholar] [CrossRef]
- Fan, X.; Aalto, Y.; Sanko, S.G.; Knuutila, S.; Klatzmann, D.; Castresana, J.S. Genetic profile, PTEN mutation and therapeutic role of PTEN in glioblastomas. Int. J. Oncol. 2002, 21, 1141–1150. [Google Scholar] [CrossRef]
- Zhang, Y.; Dube, C.; Gibert, M., Jr.; Cruickshanks, N.; Wang, B.; Coughlan, M.; Yang, Y.; Setiady, I.; Deveau, C.; Saoud, K.; et al. The p53 Pathway in Glioblastoma. Cancers 2018, 10, 297. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, J.X.; Liu, J.P.; You, C.; Liu, Y.H.; Mao, Q. Gain of function of mutant TP53 in glioblastoma: Prognosis and response to temozolomide. Ann. Surg. Oncol. 2014, 21, 1337–1344. [Google Scholar] [CrossRef]
- Terzi, N.K.; Yilmaz, I.; Oz, A.B. The Place and Prognostic Value of TERT Promoter Mutation in Molecular Classification in Grade II-III Glial Tumors and Primary Glioblastomas. Turk Patoloji Derg. 2022, 38, 90–98. [Google Scholar] [CrossRef]
- Chen, Y.P.; Yin, J.H.; Li, W.F.; Li, H.J.; Chen, D.P.; Zhang, C.J.; Lv, J.W.; Wang, Y.Q.; Li, X.M.; Li, J.Y.; et al. Single-cell transcriptomics reveals regulators underlying immune cell diversity and immune subtypes associated with prognosis in nasopharyngeal carcinoma. Cell Res. 2020, 30, 1024–1042. [Google Scholar] [CrossRef] [PubMed]
- Sottoriva, A.; Spiteri, I.; Piccirillo, S.G.; Touloumis, A.; Collins, V.P.; Marioni, J.C.; Curtis, C.; Watts, C.; Tavaré, S. Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proc. Natl. Acad. Sci. USA 2013, 110, 4009–4014. [Google Scholar] [PubMed]
- Becker, A.P.; Sells, B.E.; Haque, S.J.; Chakravarti, A. Tumor Heterogeneity in Glioblastomas: From Light Microscopy to Molecular Pathology. Cancers 2021, 13, 761. [Google Scholar] [CrossRef]
- Tataranu, L.G.; Turliuc, S.; Kamel, A.; Rizea, R.E.; Dricu, A.; Staicu, G.A.; Baloi, S.C.; Rodriguez, S.M.B.; Manole, A.I.M. Glioblastoma Tumor Microenvironment: An Important Modulator for Tumoral Progression and Therapy Resistance. Curr. Issues Mol. Biol. 2024, 46, 9881–9894. [Google Scholar] [CrossRef]
- Elguindy, M.M.; Young, J.S.; Ho, W.S.; Lu, R.O. Co-evolution of glioma and immune microenvironment. J. Immunother. Cancer 2024, 12, e009175. [Google Scholar] [CrossRef] [PubMed]
- Agosti, E.; Antonietti, S.; Ius, T.; Fontanella, M.M.; Zeppieri, M.; Panciani, P.P. Glioma Stem Cells as Promoter of Glioma Progression: A Systematic Review of Molecular Pathways and Targeted Therapies. Int. J. Mol. Sci. 2024, 25, 7979. [Google Scholar] [CrossRef]
- Lathia, J.D.; Mack, S.C.; Mulkearns-Hubert, E.E.; Valentim, C.L.L.; Rich, J.N. Cancer stem cells in glioblastoma. Genes Dev. 2015, 29, 1203–1217. [Google Scholar] [CrossRef]
- Bush, N.A.O.; Chang, S.M.; Berger, M.S. Current and future strategies for treatment of glioma. Neurosurg. Rev. 2017, 40, 1–14. [Google Scholar]
- Sipos, D.; Raposa, B.L.; Freihat, O.; Simon, M.; Mekis, N.; Cornacchione, P.; Kovács, Á. Glioblastoma: Clinical Presentation, Multidisciplinary Management, and Long-Term Outcomes. Cancers 2025, 17, 146. [Google Scholar] [CrossRef]
- Kanu, O.O.; Mehta, A.; Di, C.; Lin, N.; Bortoff, K.; Bigner, D.D.; Yan, H.; Adamson, D.C. Glioblastoma multiforme: A review of therapeutic targets. Expert Opin. Ther. Targets 2009, 13, 701–718. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar]
- Sipos, D.; Debreczeni-Máté, Z.; Ritter, Z.; Freihat, O.; Simon, M.; Kovács, Á. Complex Diagnostic Challenges in Glioblastoma: The Role of 18F-FDOPA PET Imaging. Pharmaceuticals 2024, 17, 1215. [Google Scholar] [CrossRef]
- Batash, R.; Asna, N.; Schaffer, P.; Francis, N.; Schaffer, M. Glioblastoma Multiforme, Diagnosis and Treatment; Recent Literature Review. Curr. Med. Chem. 2017, 24, 3002–3009. [Google Scholar] [CrossRef]
- Debreczeni-Máté, Z.; Freihat, O.; Törő, I.; Simon, M.; Kovács, Á.; Sipos, D. Value of 11C-Methionine PET Imaging in High-Grade Gliomas: A Narrative Review. Cancers 2024, 16, 3200. [Google Scholar] [CrossRef]
- Capper, D.; Jones, D.T.W.; Sill, M.; Hovestadt, V.; Schrimpf, D.; Sturm, D.; Koelsche, C.; Sahm, F.; Chavez, L.; Reuss, D.E.; et al. DNA methylation-based classification of central nervous system tumours. Nature 2018, 555, 469–474. [Google Scholar] [CrossRef]
- Ginat, D.T.; Schaefer, P.W. Imaging guidelines and findings of extracranial glioblastoma. J. Neurooncol. 2014, 118, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Kokkinos, V.; Chatzisotiriou, A.; Seimenis, I. Functional Magnetic Resonance Imaging and Diffusion Tensor Imaging-Tractography in Resective Brain Surgery: Lesion Coverage Strategies and Patient Outcomes. Brain Sci. 2023, 13, 1574. [Google Scholar] [CrossRef] [PubMed]
- Castellano, A.; Cirillo, S.; Bello, L.; Riva, M.; Falini, A. Functional MRI for Surgery of Gliomas. Curr. Treat. Options Neurol. 2017, 19, 34. [Google Scholar] [CrossRef] [PubMed]
- Law, I.; Albert, N.L.; Arbizu, J.; Boellaard, R.; Drzezga, A.; Galldiks, N.; la Fougère, C.; Langen, K.J.; Lopci, E.; Lowe, V.; et al. Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standards for imaging of gliomas using PET with radiolabelled amino acids and [18F]FDG: Version 1.0. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 540–557. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Katz, S.; Kontos, D.; Roshkovan, L. State of the art: Radiomics and radiomics-related artificial intelligence on the road to clinical translation. BJR Open 2024, 6, tzad004. [Google Scholar]
- Brat, D.J.; Aldape, K.; Colman, H.; Figrarella-Branger, D.; Fuller, G.N.; Giannini, C.; Holland, E.C.; Jenkins, R.B.; Kleinschmidt-DeMasters, B.; Komori, T.; et al. cIMPACT-NOW update 5: Recommended grading criteria and terminologies for IDH-mutant astrocytomas. Acta Neuropathol. 2020, 139, 603–608. [Google Scholar] [PubMed]
- Liu, E.M.; Shi, Z.F.; Li, K.K.; Malta, T.M.; Chung, N.Y.; Chen, H.; Chan, J.Y.; Poon, M.F.; Kwan, J.S.; Chan, D.T.; et al. Molecular landscape of IDH-wild type, pTERT-wild type adult glioblastomas. Brain Pathol. 2022, 32, e13107. [Google Scholar] [CrossRef]
- Lee, D.; Riestenberg, R.A.; Haskell-Mendoza, A.; Bloch, O. Diffuse astrocytic glioma, IDH-Wildtype, with molecular features of glioblastoma, WHO grade IV: A single-institution case series and review. J. Neurooncol. 2021, 152, 89–98. [Google Scholar]
- Hartmann, C.; Hentschel, B.; Wick, W.; Capper, D.; Felsberg, J.; Simon, M.; Westphal, M.; Schackert, G.; Meyermann, R.; Pietsch, T.; et al. Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: Implications for classification of gliomas. Acta Neuropathol. 2010, 120, 707–718. [Google Scholar] [CrossRef]
- Polivka, J.; Polivka, J., Jr.; Rohan, V.; Pesta, M.; Repik, T.; Pitule, P.; Topolcan, O. Isocitrate dehydrogenase-1 mutations as prognostic biomarker in glioblastoma multiforme patients in West Bohemia. BioMed Res. Int. 2014, 2014, 735659. [Google Scholar] [CrossRef]
- Zhang, G.T.; Liu, Q.; Zuo, F.X.; Liu, H.J.; Wang, S.Q.; Yuan, Q.; Liu, A.S.; Hu, K.; Meng, X.L.; Wang, W.J.; et al. Clinical and genomic features in patients with second primary glioblastoma following first primary renal cell carcinoma. BMC Cancer 2023, 23, 104. [Google Scholar] [CrossRef]
- Hegi, M.E.; Diserens, A.C.; Gorlia, T.; Hamou, M.F.; de Tribolet, N.; Weller, M.; Kros, J.M.; Hainfellner, J.A.; Mason, W.; Mariani, L.; et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003. [Google Scholar]
- Horbinski, C.; Nabors, L.B.; Portnow, J.; Baehring, J.; Bhatia, A.; Bloch, O.; Brem, S.; Butowski, N.; Cannon, D.M.; Chao, S.; et al. NCCN Guidelines® Insights: Central Nervous System Cancers, Version 2.2022. J. Natl. Compr. Cancer Netw. 2023, 21, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, C.; Li, K.; Deng, J.; Liu, H.; Lai, G.; Xie, B.; Zhong, X. Identification of Molecular Subtypes and Prognostic Characteristics of Adrenocortical Carcinoma Based on Unsupervised Clustering. Int. J. Mol. Sci. 2023, 24, 15465. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, Y.; Lai, G.; Xie, B. Landscape of infiltrated immune cell characterization in COVID-19. Heliyon 2024, 10, e28174. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Maiti, B.; Konar, K. Histopathological Profile of Central Nervous System Tumors in a Peripheral Tertiary Care Centre of West Bengal. J. Lab. Physicians 2023, 15, 38–44. [Google Scholar]
- Kurdi, M.; Moshref, R.H.; Katib, Y.; Faizo, E.; Najjar, A.A.; Bahakeem, B.; Bamaga, A.K. Simple approach for the histomolecular diagnosis of central nervous system gliomas based on 2021 World Health Organization Classification. World J. Clin. Oncol. 2022, 13, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Lan, Z.; Li, X.; Zhang, X. Glioblastoma: An Update in Pathology, Molecular Mechanisms and Biomarkers. Int. J. Mol. Sci. 2024, 25, 3040. [Google Scholar] [CrossRef]
- Saenz-Antoñanzas, A.; Auzmendi-Iriarte, J.; Carrasco-Garcia, E.; Moreno-Cugnon, L.; Ruiz, I.; Villanua, J.; Egaña, L.; Otaegui, D.; Samprón, N.; Matheu, A. Liquid Biopsy in Glioblastoma: Opportunities, Applications and Challenges. Cancers 2019, 11, 950. [Google Scholar] [CrossRef]
- Heitzer, E.; Haque, I.S.; Roberts, C.E.S.; Speicher, M.R. Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat. Rev. Genet. 2019, 20, 71–88. [Google Scholar]
- Cabezas-Camarero, S.; Pérez-Alfayate, R.; García-Barberán, V.; Gandía-González, M.L.; García-Feijóo, P.; López-Cade, I.; Lorca, V.; Casado-Fariñas, I.; Cerón, M.A.; Paz-Cabezas, M.; et al. ctDNA detection in cerebrospinal fluid and plasma and mutational concordance with the primary tumor in a multicenter prospective study of patients with glioma. Ann. Oncol. 2025, 36, 660–672. [Google Scholar] [CrossRef]
- De Mattos-Arruda, L.; Mayor, R.; Ng, C.K.Y.; Weigelt, B.; Martínez-Ricarte, F.; Torrejon, D.; Oliveira, M.; Arias, A.; Raventos, C.; Tang, J.; et al. Cerebrospinal fluid-derived circulating tumour DNA better represents the genomic alterations of brain tumours than plasma. Nat. Commun. 2015, 6, 8839. [Google Scholar] [CrossRef] [PubMed]
- Galldiks, N.; Zadeh, G.; Lohmann, P. Artificial Intelligence, Radiomics, and Deep Learning in Neuro-Oncology. Neurooncol. Adv. 2020, 2, iv1–iv2. [Google Scholar] [CrossRef] [PubMed]
- Yogananda, C.G.B.; Shah, B.R.; Nalawade, S.S.; Murugesan, G.K.; Yu, F.F.; Pinho, M.C.; Wagner, B.C.; Mickey, B.; Patel, T.R.; Fei, B.; et al. MRI-Based Deep-Learning Method for Determining Glioma MGMT Promoter Methylation Status. AJNR Am. J. Neuroradiol. 2021, 42, 845–852. [Google Scholar] [CrossRef]
- Kickingereder, P.; Bonekamp, D.; Nowosielski, M.; Kratz, A.; Sill, M.; Burth, S.; Wick, A.; Eidel, O.; Schlemmer, H.P.; Radbruch, A.; et al. Radiogenomics of Glioblastoma: Machine Learning-based Classification of Molecular Characteristics by Using Multiparametric and Multiregional MR Imaging Features. Radiology 2016, 281, 907–918. [Google Scholar]
- Bi, W.L.; Hosny, A.; Schabath, M.B.; Giger, M.L.; Birkbak, N.J.; Mehrtash, A.; Allison, T.; Arnaout, O.; Abbosh, C.; Dunn, I.F.; et al. Artificial intelligence in cancer imaging: Clinical challenges and applications. CA Cancer J. Clin. 2019, 69, 127–157. [Google Scholar] [CrossRef]
- Zlochower, A.; Chow, D.S.; Chang, P.; Khatri, D.; Boockvar, J.A.; Filippi, C.G. Deep Learning AI Applications in the Imaging of Glioma. Top. Magn. Reson. Imaging 2020, 29, 115-00. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.K.; Mohan, S.; Agarwal, S.; de Godoy, L.L.; Rajan, A.; Nasrallah, M.P.; Bagley, S.J.; Brem, S.; Loevner, L.A.; Poptani, H.; et al. Distinction of pseudoprogression from true progression in glioblastomas using machine learning based on multiparametric magnetic resonance imaging and O6-methylguanine-methyltransferase promoter methylation status. Neurooncol. Adv. 2024, 6, vdae159. [Google Scholar] [CrossRef]
- Singh, G.; Manjila, S.; Sakla, N.; True, A.; Wardeh, A.H.; Beig, N.; Vaysberg, A.; Matthews, J.; Prasanna, P.; Spektor, V. Radiomics and radiogenomics in gliomas: A contemporary update. Br. J. Cancer 2021, 125, 641–657. [Google Scholar] [CrossRef]
- Debinski, W. (Ed.) Gliomas; Exon Publications: Brisbane, Australia, 2021. [Google Scholar]
- Zhang, C.; Yang, J.; Chen, S.; Sun, L.; Li, K.; Lai, G.; Peng, B.; Zhong, X.; Xie, B. Artificial intelligence in ovarian cancer drug resistance advanced 3PM approach: Subtype classification and prognostic modeling. EPMA J. 2024, 15, 525–544. [Google Scholar] [CrossRef]
- Park, J.E.; Kim, H.S.; Park, S.Y.; Nam, S.J.; Chun, S.M.; Jo, Y.; Kim, J.H. Prediction of Core Signaling Pathway by Using Diffusion- and Perfusion-based MRI Radiomics and Next-generation Sequencing in Isocitrate Dehydrogenase Wild-type Glioblastoma. Radiology 2020, 2, 388–397. [Google Scholar]
- Buzdugan, S.; Mazher, M.; Puig, D. Radiogenomics for Glioblastoma Survival Prediction: Integrating Radiomics, Clinical, and Genomic Features Using Artificial Intelligence. J. Imaging Inform. Med. 2025. [Google Scholar] [CrossRef]
- Jermyn, M.; Mok, K.; Mercier, J.; Desroches, J.; Pichette, J.; Saint-Arnaud, K.; Bernstein, L.; Guiot, M.C.; Petrecca, K.; Leblond, F. Intraoperative brain cancer detection with Raman spectroscopy in humans. Sci. Transl. Med. 2015, 7, 274ra19. [Google Scholar] [CrossRef] [PubMed]
- Elliot, M.; Ségaud, S.; Lavrador, J.P.; Vergani, F.; Bhangoo, R.; Ashkan, K.; Xie, Y.; Stasiuk, G.J.; Vercauteren, T.; Shapey, J. Fluorescence Guidance in Glioma Surgery: A Narrative Review of Current Evidence and the Drive Towards Objective Margin Differentiation. Cancers 2025, 17, 2019. [Google Scholar] [CrossRef] [PubMed]
- Bowden, S.G.; Neira, J.A.; Gill, B.J.A.; Ung, T.H.; Englander, Z.K.; Zanazzi, G.; Chang, P.D.; Samanamud, J.; Grinband, J.; Sheth, S.A.; et al. Sodium Fluorescein Facilitates Guided Sampling of Diagnostic Tumor Tissue in Nonenhancing Gliomas. Neurosurgery 2018, 82, 719–727. [Google Scholar]
- Evers, M.; Brändl, B.; Rohrandt, C.; Kubelt-Kwamin, C.; Müller, F.J.; Danso, D.; Maicher, A.; Wang, G.; Friedrichsen, S.; Kolkenbrock, S. Rapid intraoperative amplicon sequencing of CNS tumor markers. Comput. Struct. Biotechnol. J. 2024, 26, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, S.; Nano, O.; Hana, C.; Bonano-Rios, A.; Hussein, A. Molecular Targeting of the Isocitrate Dehydrogenase Pathway and the Implications for Cancer Therapy. Int. J. Mol. Sci. 2024, 25, 7337. [Google Scholar] [CrossRef]
- Behjati, S.; Tarpey, P.S. What is next generation sequencing? Arch. Dis. Child. Educ. Pract. Ed. 2013, 98, 236–238. [Google Scholar] [PubMed]
- Rolfo, C.; Drilon, A.; Hong, D.; McCoach, C.; Dowlati, A.; Lin, J.J.; Russo, A.; Schram, A.M.; Liu, S.V.; Nieva, J.J.; et al. NTRK1 Fusions identified by non-invasive plasma next-generation sequencing (NGS) across 9 cancer types. Br. J. Cancer 2022, 126, 514–520. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Y.; Chen, X.; Wei, P.; Lin, Y.; Wu, Z.; Lin, Z.; Kang, D.; Ding, C. Single-cell RNA sequencing reveals intra-tumoral heterogeneity of glioblastoma and a pro-tumor subset of tumor-associated macrophages characterized by EZH2 overexpression. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166534. [Google Scholar]
- Patel, A.P.; Tirosh, I.; Trombetta, J.J.; Shalek, A.K.; Gillespie, S.M.; Wakimoto, H.; Cahill, D.P.; Nahed, B.V.; Curry, W.T.; Martuza, R.L.; et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 2014, 344, 1396–1401. [Google Scholar]
- Latzer, P.; Zelba, H.; Battke, F.; Reinhardt, A.; Shao, B.; Bartsch, O.; Rabsteyn, A.; Harter, J.; Schulze, M.; Okech, T.; et al. A real-world observation of patients with glioblastoma treated with a personalized peptide vaccine. Nat. Commun. 2024, 15, 6870. [Google Scholar] [CrossRef]
- Amhis, N. Innovative approaches to glioma treatment: Oncolytic foamy virus and CAR T cell therapy. Mol. Ther. Oncol. 2024, 32, 200876. [Google Scholar] [CrossRef]
- Vandecandelaere, G.; Ramapriyan, R.; Gaffey, M.; Richardson, L.G.; Steuart, S.J.; Tazhibi, M.; Kalaw, A.; Grewal, E.P.; Sun, J.; Curry, W.T.; et al. Pre-Clinical Models for CAR T-Cell Therapy for Glioma. Cells 2024, 13, 1480. [Google Scholar] [CrossRef] [PubMed]
- Shan, K.S.; Musleh Ud Din, S.; Dalal, S.; Gonzalez, T.; Dalal, M.; Ferraro, P.; Hussein, A.; Vulfovich, M. Bispecific Antibodies in Solid Tumors: Advances and Challenges. Int. J. Mol. Sci. 2025, 26, 5838. [Google Scholar] [CrossRef] [PubMed]
- Alomari, O.; Eyvazova, H.; Güney, B.; Al Juhmani, R.; Odabasi, H.; Al-Rawabdeh, L.; Mokresh, M.E.; Erginoglu, U.; Keles, A.; Baskaya, M.K. Oncolytic Therapies for Glioblastoma: Advances, Challenges, and Future Perspectives. Cancers 2025, 17, 2550. [Google Scholar] [CrossRef]
- Rousseau, J.; Lapointe, S.; Roberge, D. Tumor-Treating Fields and Related Treatments in the Management of Pediatric Brain Tumors. Curr Oncol. 2025, 32, 185. [Google Scholar] [CrossRef]
- Liu, S.; Liang, R. Oncolytic Virotherapy for Glioma: A Bibliometric Roadmap for Multidisciplinary Clinical and Research Strategies. J Multidiscip. Healthc. 2025, 18, 6167–6185. [Google Scholar] [CrossRef]
- Manganelli, V.; Misasi, R.; Riitano, G.; Capozzi, A.; Mattei, V.; Caglar, T.R.; Ialongo, D.; Madia, V.N.; Messore, A.; Costi, R.; et al. Role of a Novel Heparanase Inhibitor on the Balance between Apoptosis and Autophagy in U87 Human Glioblastoma Cells. Cells 2023, 12, 1891. [Google Scholar] [CrossRef] [PubMed]
| Symptom/Feature | Clinical Description | References |
|---|---|---|
| Paresis, aphasia | Focal neurological deficits depending on tumor location | [32,33] |
| Seizures | Common, especially with cortical involvement | [32] |
| Headache, nausea | Symptoms of increased intracranial pressure | [32] |
| Cognitive changes | Memory and personality disturbances, disorientation | [32] |
| Rapid progression, recurrence | High mortality and treatment resistance | [32,35,36] |
| Therapy/Agent | Mechanism | Trial Phase | Key Limitations |
|---|---|---|---|
| Standard radiotherapy + temozolomide | DNA damage, cytotoxic effect | Standard of care | Limited efficacy in MGMT unmethylated patients; tumor recurrence common |
| Tumor Treating Fields (TTFields, Optune) | Disrupt mitosis via alternating electric fields | Approved | Availability, compliance, cost; long-term data limited |
| Personalized neoantigen vaccines | Induce patient-specific T-cell responses | Early-phase | Survival benefit unconfirmed; glucocorticoid effect; antigen heterogeneity |
| CAR-T cells (IL13Rα2, EGFRvIII, EGFR) | Target GBM-associated antigens via adoptive transfer | Early clinical | Transient responses; antigen heterogeneity; immunosuppressive TME |
| BiTE molecules (EGFRvIII/EGFR) | Engage T cells with tumor cells | Preclinical/Early-phase | Limited clinical data; effect durability unknown |
| Oncolytic viruses (DNX-2401, PVSRIPO, Delta-24-RGD) | Tumor lysis + immune activation | Phase I/II | Delivery and safety challenges; transient efficacy |
| Nanoparticle/liposome-based delivery | Targeted cytostatic/oligonucleotide delivery | Preclinical/Early-phase | Distribution, elimination, immunogenicity issues |
| Stem cell-based therapies | Tumor-tropic delivery of prodrugs/gene vectors | Preclinical/Early-phase | Safety (transformation potential); function control |
| Heparanase inhibitor (RDS 3337) | Shift autophagy-apoptosis balance toward apoptosis | Preclinical | Clinical translation unproven; pharmacokinetics and safety require study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Królikowska, K.; Błaszczak, K.; Ławicki, S.; Zajkowska, M.; Gudowska-Sawczuk, M. Glioblastoma—A Contemporary Overview of Epidemiology, Classification, Pathogenesis, Diagnosis, and Treatment: A Review Article. Int. J. Mol. Sci. 2025, 26, 12162. https://doi.org/10.3390/ijms262412162
Królikowska K, Błaszczak K, Ławicki S, Zajkowska M, Gudowska-Sawczuk M. Glioblastoma—A Contemporary Overview of Epidemiology, Classification, Pathogenesis, Diagnosis, and Treatment: A Review Article. International Journal of Molecular Sciences. 2025; 26(24):12162. https://doi.org/10.3390/ijms262412162
Chicago/Turabian StyleKrólikowska, Kinga, Katarzyna Błaszczak, Sławomir Ławicki, Monika Zajkowska, and Monika Gudowska-Sawczuk. 2025. "Glioblastoma—A Contemporary Overview of Epidemiology, Classification, Pathogenesis, Diagnosis, and Treatment: A Review Article" International Journal of Molecular Sciences 26, no. 24: 12162. https://doi.org/10.3390/ijms262412162
APA StyleKrólikowska, K., Błaszczak, K., Ławicki, S., Zajkowska, M., & Gudowska-Sawczuk, M. (2025). Glioblastoma—A Contemporary Overview of Epidemiology, Classification, Pathogenesis, Diagnosis, and Treatment: A Review Article. International Journal of Molecular Sciences, 26(24), 12162. https://doi.org/10.3390/ijms262412162








