Demyelination and Cognitive Performance in Long COVID Patients with Insomnia and/or Depression
Abstract
1. Introduction
2. Results
2.1. COVID-19-Related Parameters
2.2. Neuropsychological Results
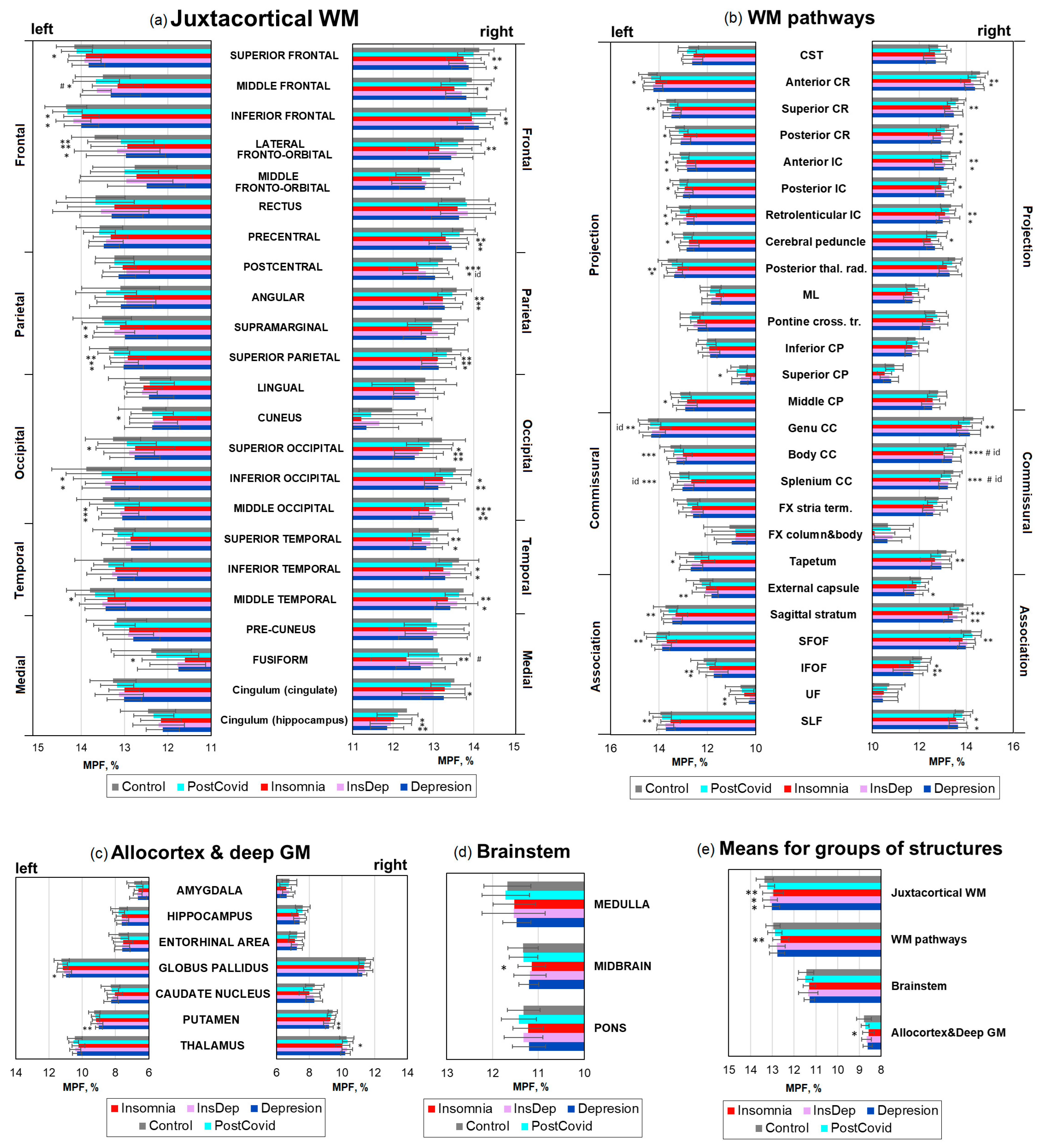
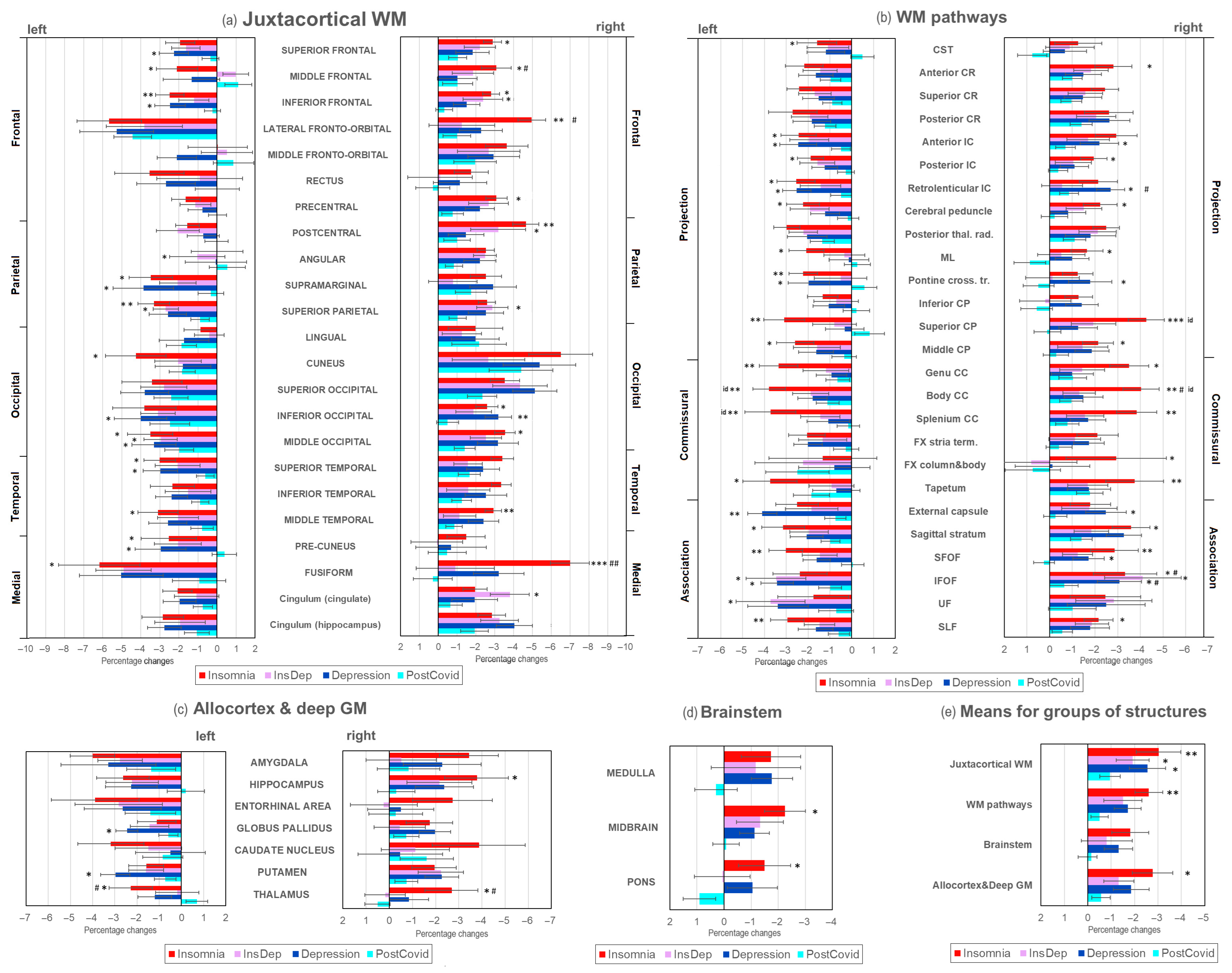
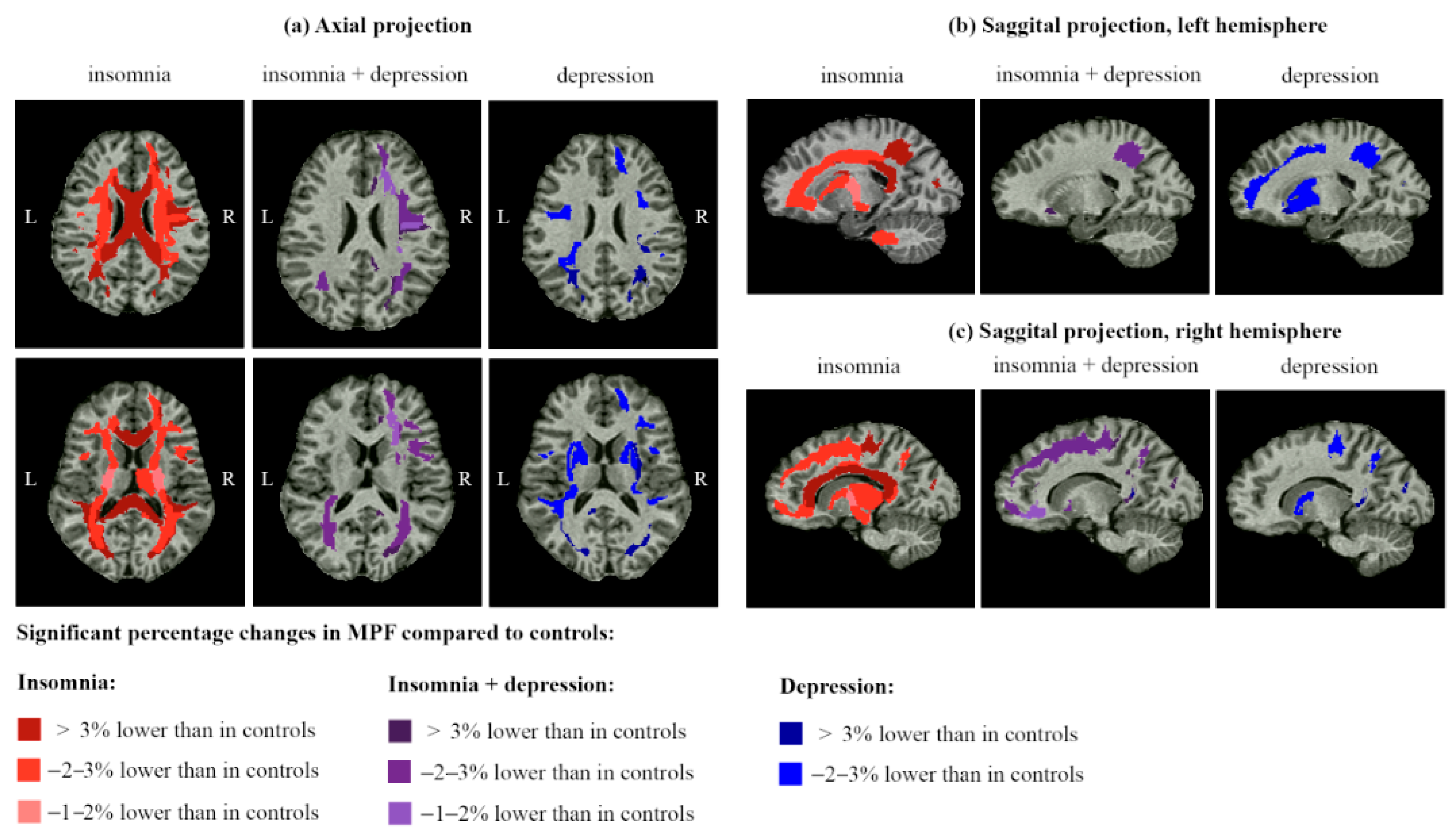
2.3. Brain Demyelination in Post-COVID Patients with Insomnia and Depression
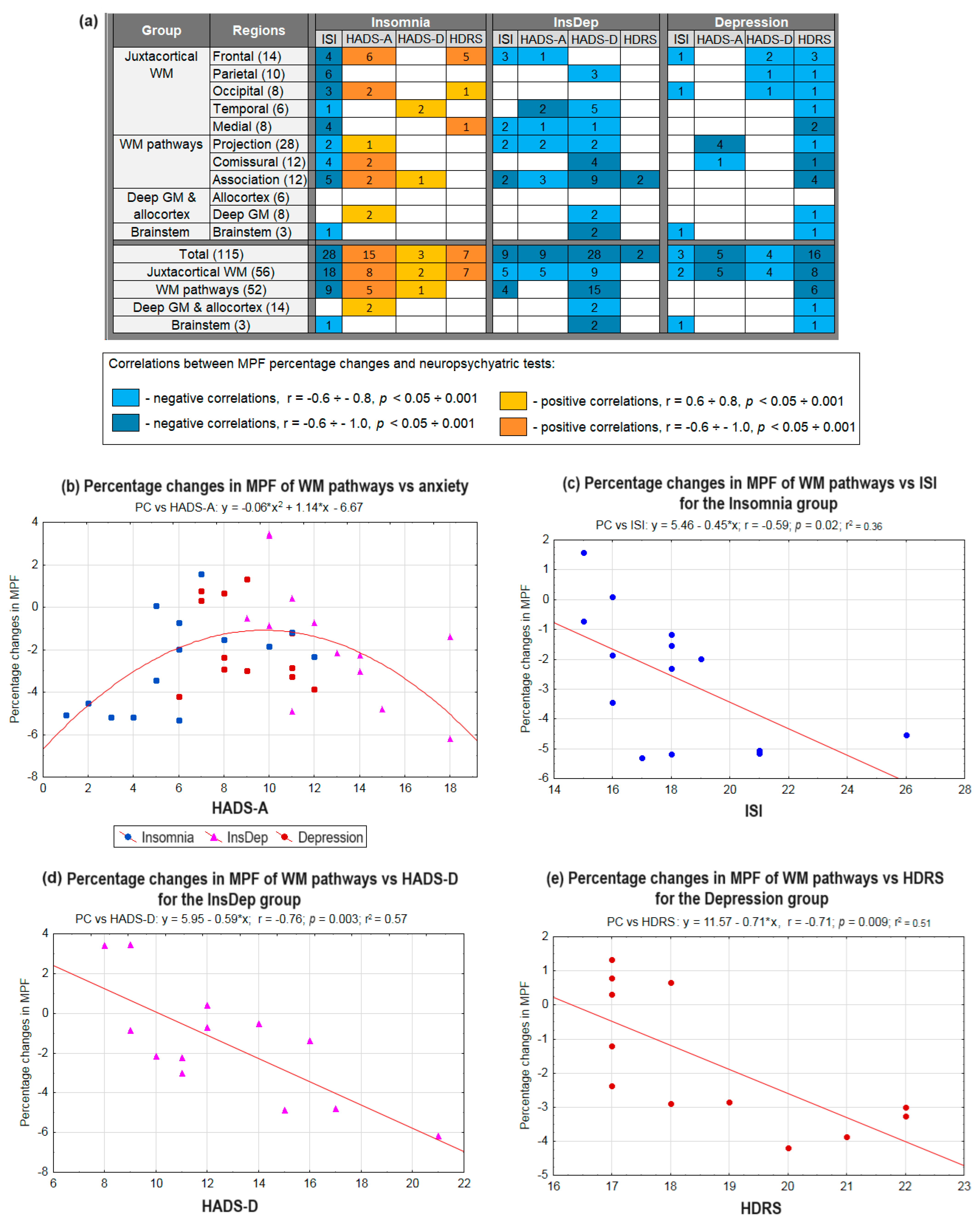
2.4. Associations Between Neuropsychiatric Parameters and Brain Myelination in Post-COVID Patients with Insomnia and Depression
2.5. Associations of the Levels of BDNF, Anti-S100, Anti-SARS-CoV-2, and Myelin-Specific Autoantibodies in Blood Plasma with Neuropsychiatric Parameters and Demyelination
3. Discussion
3.1. Summary of Results
3.2. Demyelination and Cognitive Performance in Long COVID Patients with Insomnia
3.3. Demyelination and Cognitive Performance in Long COVID Patients with Depression
3.4. Demyelination and Cognitive Performance in Long COVID Patients with Comorbid Insomnia–Depression
3.5. Specificity of WM Microstructure Changes for Insomnia and Depression After COVID-19
3.6. Autoimmunity as a Possible Mechanism of Demyelination in Long COVID
3.7. Study Limitations
4. Materials and Methods
4.1. Study Participants
4.2. Neurocognitive Testing
4.3. MRI Data Acquisition
- Magnetization Transfer (MT)-Weighted: TR = 20 ms; echo time (TE) = 4.76 ms; flip angle (FA) = 8°; scan time: 5 min 40 s;
- T1-Weighted: TR = 16 ms; TE = 4.76 ms; FA = 18°; scan time: 4 min 32 s;
- Proton Density (PD)-Weighted: TR = 16 ms; TE = 4.76 ms; FA = 3°; scan time: 4 min 32 s.
- 3D Fluid Attenuated Inversion Recovery (T2/FLAIR): TR = 5000 ms; TE = 390 ms; TI = 1800 ms;
- 3D T1-Weighted: TR = 16 ms; TE = 4.76 ms;
- 3D T2-Weighted: TR = 3000 ms; TE = 335 ms.
4.4. Image Processing
- WM Pathways and Fasciculi: (1) projection tracts—corticospinal tract (CST); anterior, superior, and posterior corona radiata (CR); anterior, posterior limb, and retrolenticular part of internal capsule (IC); cerebral peduncles; posterior thalamic radiation; medial lemniscus (ML); pontine crossing tract; inferior, superior, and middle cerebellar peduncles (CP). (2) Commissural tracts—genu, body, and splenium of corpus callosum (CC); fornix (FX) (stria terminalis, column, and body); tapetum. (3) Association tracts—superior longitudinal (SL) fasciculus; superior (SFOF) and inferior fronto-occipital (IFOF) fasciculi; uncinate fasciculus (UF); sagittal stratum; external capsule.
- Subcortical and Allocortical GM Structures: (1) allocortex—amygdala; hippocampus; entorhinal area. (2) Deep GM—caudate nucleus; putamen; globus pallidus; thalamus.
- Brainstem Structures: medulla; midbrain; pons.
4.5. ELISA
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sansone, V.; Angelillo, S.; Paduano, G.; Pileggi, C.; Nobile, C.G.A.; Di Giuseppe, G. Quality of Sleep after COVID-19 Infection: A Cross-Sectional Study in the Southern Italy. Front. Psychiatry 2024, 15, 1428423. [Google Scholar] [CrossRef]
- Shin, M.; Crouse, J.J.; Byrne, E.M.; Mitchell, B.L.; Lind, P.; Parker, R.; Tonini, E.; Carpenter, J.S.; Wray, N.R.; Colodro-Conde, L.; et al. Changes in Sleep Patterns in People with a History of Depression during the COVID-19 Pandemic: A Natural Experiment. BMJ Ment. Health 2024, 27, e301067. [Google Scholar] [CrossRef]
- Sarkanen, T.; Partinen, M.; Bjorvatn, B.; Merikanto, I.; Benedict, C.; Nadorff, M.R.; Bolstad, C.J.; Espie, C.; Matsui, K.; Chung, F.; et al. Association between Hypersomnolence and the COVID-19 Pandemic: The International COVID-19 Sleep Study (ICOSS). Sleep Med. 2023, 107, 108–115. [Google Scholar] [CrossRef]
- Chen, S.-J.; Charles, M.M.; Ivers, H.; Wing, Y.K.; Partinen, M.; Merikanto, I.; Holzinger, B.; Espie, C.A.; De Gennaro, L.; Dauvilliers, Y.; et al. The Association of Insomnia with Long COVID: An International Collaborative Study (ICOSS-II). Sleep Med. 2023, 112, 216–222. [Google Scholar] [CrossRef]
- Rahmati, M.; Udeh, R.; Kang, J.; Dolja-Gore, X.; McEvoy, M.; Kazemi, A.; Soysal, P.; Smith, L.; Kenna, T.; Fond, G.; et al. Long-Term Sequelae of COVID-19: A Systematic Review and Meta-Analysis of Symptoms 3 Years Post-SARS-CoV-2 Infection. J. Med. Virol. 2025, 97, e70429. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Qin, X.; Lu, Y.; Yu, Y.; Liu, R.; Zhu, R.; Su, K.P.; Wang, F. The Impact of Acute COVID-19 Symptoms on Insomnia: A Longitudinal Study among Medical Students. Brain Behav. Immun.-Health 2025, 48, 101067. [Google Scholar] [CrossRef] [PubMed]
- Levenson, J.C.; Kay, D.B.; Buysse, D.J. The Pathophysiology of Insomnia. Chest 2015, 147, 1179–1192. [Google Scholar] [CrossRef]
- Kronholm, E.; Puusniekka, R.; Jokela, J.; Villberg, J.; Urrila, A.S.; Paunio, T.; Välimaa, R.; Tynjälä, J. Trends in Self-Reported Sleep Problems, Tiredness and Related School Performance among Finnish Adolescents from 1984 to 2011. J. Sleep Res. 2015, 24, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Lian, Y.; Xiao, J.; Liu, Y.; Ning, L.; Guan, S.; Ge, H.; Li, F.; Liu, J. Associations between Insomnia, Sleep Duration and Poor Work Ability. J. Psychosom. Res. 2015, 78, 45–51. [Google Scholar] [CrossRef]
- Hensch, T.; Wozniak, D.; Spada, J.; Sander, C.; Ulke, C.; Wittekind, D.A.; Thiery, J.; Löffler, M.; Jawinski, P.; Hegerl, U. Vulnerability to Bipolar Disorder Is Linked to Sleep and Sleepiness. Transl. Psychiatry 2019, 9, 294. [Google Scholar] [CrossRef]
- Gao, T.; Xiang, H.; Wu, Q.N.; Zhu, L.S.; Pei, W.J.; Fu, W.J.; Chou, T.S. Advances in the Research of Comorbid Insomnia and Depression: Mechanisms, Impacts, and Interventions. Front. Psychiatry 2025, 16, 1468212. [Google Scholar] [CrossRef]
- Fang, H.; Tu, S.; Sheng, J.; Shao, A. Depression in Sleep Disturbance: A Review on a Bidirectional Relationship, Mechanisms and Treatment. J. Cell. Mol. Med. 2019, 23, 2324–2332. [Google Scholar] [CrossRef]
- Oakes, D.J.; Pearce, H.A.; Roberts, C.; Gehrman, P.G.; Lewis, C.; Jones, I.; Lewis, K.J.S. Associations between Comorbid Anxiety and Sleep Disturbance in People with Bipolar Disorder: Findings from Actigraphy and Subjective Sleep Measures. J. Affect. Disord. 2022, 309, 165–171. [Google Scholar] [CrossRef]
- Kay, D.B.; Buysse, D.J. Hyperarousal and beyond: New Insights to the Pathophysiology of Insomnia Disorder through Functional Neuroimaging Studies. Brain Sci. 2017, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Van Someren, E.J.W. Brain Mechanisms of Insomnia: New Perspectives on Causes and Consequences. Physiol. Rev. 2021, 101, 995–1046. [Google Scholar] [CrossRef]
- de Vivo, L.; Bellesi, M. The Role of Sleep and Wakefulness in Myelin Plasticity. Glia 2019, 67, 2142–2152. [Google Scholar] [CrossRef]
- Kurth, S.; Riedner, B.A.; Dean, D.C.; O’Muircheartaigh, J.; Huber, R.; Jenni, O.G.; Deoni, S.C.L.; LeBourgeois, M.K. Traveling Slow Oscillations during Sleep: A Marker of Brain Connectivity in Childhood. Sleep 2017, 40, 10–12. [Google Scholar] [CrossRef]
- Andica, C.; Kamagata, K.; Takabayashi, K.; Mahemuti, Z. Myelin Changes in Poor Sleepers: Insights into Glymphatic Clearance Function and Regional Circadian Clock Gene Expression. Aging Dis. 2024, 16, 2453–2467. [Google Scholar] [CrossRef] [PubMed]
- Altendahl, M.; Cotter, D.L.; Staffaroni, A.M.; Wolf, A.; Mumford, P.; Cobigo, Y.; Casaletto, K.; Elahi, F.; Ruoff, L.; Javed, S.; et al. REM Sleep Is Associated with White Matter Integrity in Cognitively Healthy, Older Adults. PLoS ONE 2020, 15, e0235395. [Google Scholar] [CrossRef]
- Stolicyn, A.; Lyall, L.M.; Lyall, D.M.; Høier, N.K.; Adams, M.J.; Shen, X.; Cole, J.H.; McIntosh, A.M.; Whalley, H.C.; Smith, D.J. Comprehensive Assessment of Sleep Duration, Insomnia, and Brain Structure within the UK Biobank Cohort. Sleep 2024, 47, zsad274. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tian, J.; Bauer, A.; Huang, R.; Wen, H.; Li, M.; Wang, T.; Xia, L.; Jiang, G. Reduced Integrity of Right Lateralized White Matter in Patients with Primary Insomnia: A Diffusion-Tensor Imaging Study. Radiology 2016, 280, 520–528. [Google Scholar] [CrossRef]
- Sexton, C.E.; Zsoldos, E.; Filippini, N.; Griffanti, L.; Winkler, A.; Mahmood, A.; Allan, C.L.; Topiwala, A.; Kyle, S.D.; Spiegelhalder, K.; et al. Associations between Self-Reported Sleep Quality and White Matter in Community-Dwelling Older Adults: A Prospective Cohort Study. Hum. Brain Mapp. 2017, 38, 5465–5473. [Google Scholar] [CrossRef]
- Spiegelhalder, K.; Regen, W.; Prem, M.; Baglioni, C.; Nissen, C.; Feige, B.; Schnell, S.; Kiselev, V.G.; Hennig, J.; Riemann, D. Reduced Anterior Internal Capsule White Matter Integrity in Primary Insomnia. Hum. Brain Mapp. 2014, 35, 3431–3438. [Google Scholar] [CrossRef]
- Kim, M.; Lee, J.; Kim, N.; Hwang, Y.; Lee, K.H.; Lee, J.; Lee, Y.J.; Kim, S.J. The Influence of Life Stress and Sleep Disturbance on White Matter Integrity. Psychiatry Investig. 2023, 20, 439–444. [Google Scholar] [CrossRef]
- Bruce, H.A.; Kochunov, P.; Kvarta, M.D.; Goldwaser, E.L.; Chiappelli, J.; Schwartz, A.; Lightner, S.; Endres, J.; Yuen, A.; Ma, Y.; et al. Frontal White Matter Association with Sleep Quality and the Role of Stress. J. Sleep Res. 2023, 32, e13669. [Google Scholar] [CrossRef]
- Lu, F.M.; Dai, J.; Couto, T.A.; Liu, C.H.; Chen, H.; Lu, S.L.; Tang, L.R.; Le Tie, C.; Chen, H.F.; He, M.X.; et al. Diffusion Tensor Imaging Tractography Reveals Disrupted White Matter Structural Connectivity Network in Healthy Adults with Insomnia Symptoms. Front. Hum. Neurosci. 2017, 11, 583. [Google Scholar] [CrossRef]
- Jamieson, D.; Kannis-dymand, L.; Beaudequin, D.A.; Schwenn, P.; Shan, Z.; Mcloughlin, L.T.; Lagopoulos, J.; Hermens, D.F. Can Measures of Sleep Quality or White Matter Structural Integrity Predict Level of Worry or Rumination in Adolescents Facing Stressful Situations? Lessons from the COVID-19 Pandemic. J. Adolesc. 2021, 91, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Rostampour, M.; Gharaylou, Z.; Rostampour, N.; Kaveh, D.; Noori, K.; Fadaei, R.; Tahmasian, M.; Khazaie, H.; Zarei, M. Asymmetric Alterations of White Matter Integrity in Patients with Insomnia Disorder. Brain Imaging Behav. 2022, 16, 389–396. [Google Scholar] [CrossRef]
- Bresser, T.; Foster-Dingley, J.C.; Wassing, R.; Leerssen, J.; Ramautar, J.R.; Stoffers, D.; Lakbila-Kamal, O.; van de Heuvel, M.P.; Van Someren, E.J.W.; van den Heuvel, M.; et al. Consistent Altered Internal Capsule White Matter Microstructure in Insomnia Disorder. Sleep 2020, 43, zsaa031. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, L.; Xi, Y.; Dai, T.; Fei, N.; Liu, L.; Xu, Z.; Yang, X.; Fu, C.; Sun, J.; et al. White Matter Microstructural Properties Are Related to Inter-Individual Differences in Cognitive Instability after Sleep Deprivation. Neuroscience 2017, 365, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.M.; Joo, S.W.; Son, Y.D.; Kim, H.; Ko, K.P.; Lee, J.S.; Kang, S.G. Low White-Matter Integrity between the Left Thalamus and Inferior Frontal Gyrus in Patients with Insomnia Disorder. J. Psychiatry Neurosci. 2018, 43, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, K.; Nasrallah, I.; Hoang, T.D.; Lauderdale, D.S.; Knutson, K.L.; Carnethon, M.R.; Launer, L.J.; Lewis, C.E.; Sidney, S. Sleep Duration and White Matter Quality in Middle-Aged Adults. Sleep 2016, 39, 1743–1747. [Google Scholar] [CrossRef]
- Ning, J.; Zhang, W.; Chen, S.F.; Li, H.Q.; Wang, R.Z.; Feng, J.; Cheng, W.; Dong, Q.; Yu, J.T. Association of Sleep Behaviors with White Matter Hyperintensities and Microstructural Injury: A Cross-Sectional and Longitudinal Analysis of 26 354 Participants. Sleep 2023, 46, zsad020. [Google Scholar] [CrossRef]
- Sanjari Moghaddam, H.; Mohammadi, E.; Dolatshahi, M.; Mohebi, F.; Ashrafi, A.; Khazaie, H.; Aarabi, M.H. White Matter Microstructural Abnormalities in Primary Insomnia: A Systematic Review of Diffusion Tensor Imaging Studies. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 105, 110132. [Google Scholar] [CrossRef]
- Carvalho, D.Z.; Kolla, B.P.; McCarter, S.J.; St. Louis, E.K.; Machulda, M.M.; Przybelski, S.A.; Fought, A.J.; Lowe, V.J.; Somers, V.K.; Boeve, B.F.; et al. Associations of Chronic Insomnia, Longitudinal Cognitive Outcomes, Amyloid-PET, and White Matter Changes in Cognitively Normal Older Adults. Neurology 2025, 105, e214155. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Schreiber, J.; Bittner, N.; Li, S.; Huang, R.; Moebus, S.; Bauer, A.; Caspers, S.; Elmenhorst, D. White Matter Microstructure Underlies the Effects of Sleep Quality and Life Stress on Depression Symptomatology in Older Adults. Front. Aging Neurosci. 2020, 12, 578037. [Google Scholar] [CrossRef]
- Reyes, S.; Rimkus, C.d.M.; Lozoff, B.; Algarin, C.; Peirano, P. Nighttime Sleep Characteristics and White Matter Integrity in Young Adults. Nat. Sci. Sleep 2022, 14, 1363–1373. [Google Scholar] [CrossRef]
- Choea, A.S.; Stepniewska, I.; Colvin, D.C.; Ding, Z.; Anderson, A.W. Validation of Diffusion Tensor MRI in the Central Nervous System Using Light Microscopy: Quantitative Comparison of Fiber Properties. NMR Biomed 2012, 25, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Khodanovich, M.Y.Y.; Sorokina, I.V.V.; Glazacheva, V.Y.Y.; Akulov, A.E.E.; Nemirovich-Danchenko, N.M.M.; Romashchenko, A.V.V.; Tolstikova, T.G.G.; Mustafina, L.R.R.; Yarnykh, V.L.L. Histological Validation of Fast Macromolecular Proton Fraction Mapping as a Quantitative Myelin Imaging Method in the Cuprizone Demyelination Model. Sci. Rep. 2017, 7, 46686. [Google Scholar] [CrossRef]
- Khodanovich, M.Y.; Pishchelko, A.O.; Glazacheva, V.Y.; Pan, E.S.; Akulov, A.E.; Svetlik, M.V.; Tyumentseva, Y.A.; Anan’ina, T.V.; Yarnykh, V.L. Quantitative Imaging of White and Gray Matter Remyelination in the Cuprizone Demyelination Model Using the Macromolecular Proton Fraction. Cells 2019, 8, 1204. [Google Scholar] [CrossRef]
- Khodanovich, M.Y.; Kisel, A.A.; Akulov, A.E.; Atochin, D.N.; Kudabaeva, M.S.; Glazacheva, V.Y.; Svetlik, M.V.; Medvednikova, Y.A.; Mustafina, L.R.; Yarnykh, V.L. Quantitative Assessment of Demyelination in Ischemic Stroke In Vivo Using Macromolecular Proton Fraction Mapping. J. Cereb. Blood Flow Metab. 2018, 38, 919–931, Erratum in J. Cereb. Blood Flow Metab. 2018, 38, 932. [Google Scholar] [CrossRef]
- Khodanovich, M.Y.; Gubskiy, I.L.; Kudabaeva, M.S.; Namestnikova, D.D.; Kisel, A.A.; Anan’ina, T.V.; Tumentceva, Y.A.; Mustafina, L.R.; Yarnykh, V.L. Long-Term Monitoring of Chronic Demyelination and Remyelination in a Rat Ischemic Stroke Model Using Macromolecular Proton Fraction Mapping. J. Cereb. Blood Flow Metab. 2021, 41, 2856–2869. [Google Scholar] [CrossRef] [PubMed]
- Drobyshevsky, A.; Synowiec, S.; Goussakov, I.; Lu, J.; Gascoigne, D.; Aksenov, D.P.; Yarnykh, V. NeuroImage Temporal Trajectories of Normal Myelination and Axonal Development Assessed by Quantitative Macromolecular and Diffusion MRI: Ultrastructural and Immunochemical Validation in a Rabbit Model. Neuroimage 2023, 270, 119974. [Google Scholar] [CrossRef]
- Yarnykh, V.L. Time-Efficient, High-Resolution, Whole Brain Three-Dimensional Macromolecular Proton Fraction Mapping. Magn. Reson. Med. 2016, 75, 2100–2106. [Google Scholar] [CrossRef] [PubMed]
- Naumova, A.V.; Akulov, A.E.; Khodanovich, M.Y.; Yarnykh, V.L. High-Resolution Three-Dimensional Quantitative Map of the Macromolecular Proton Fraction Distribution in the Normal Rat Brain. Data Br. 2017, 10, 381–384. [Google Scholar] [CrossRef]
- Underhill, H.R.; Yuan, C.; Yarnykh, V.L. Direct Quantitative Comparison between Cross-Relaxation Imaging and Diffusion Tensor Imaging of the Human Brain at 3.0 T. Neuroimage 2009, 47, 1568–1578. [Google Scholar] [CrossRef]
- Yarnykh, V.L.; Krutenkova, E.P.; Aitmagambetova, G.; Henson, L.K.J.; Piedmont, H.; Repovic, P.; Mayadev, A.; Qian, P.; Gangadharan, B. Iron-Insensitive Quantitative Assessment of Subcortical Gray Matter Demyelination in Multiple Sclerosis Using Macromolecular Proton Fraction. Am. J. Neuroradiol. 2018, 39, 618–625. [Google Scholar] [CrossRef]
- Yarnykh, V.L.; Kisel, A.A.; Khodanovich, M.Y. Scan–Rescan Repeatability and Impact of B0 and B1 Field Nonuniformity Corrections in Single-Point Whole-Brain Macromolecular Proton Fraction Mapping. J. Magn. Reson. Imaging 2020, 51, 1789–1798. [Google Scholar] [CrossRef]
- Smirnova, L.P.; Yarnykh, V.L.; Parshukova, D.A.; Kornetova, E.G.; Semke, A.V.; Usova, A.V.; Pishchelko, A.O.; Khodanovich, M.Y.; Ivanova, S.A. Global Hypomyelination of the Brain White and Gray Matter in Schizophrenia: Quantitative Imaging Using Macromolecular Proton Fraction. Transl. Psychiatry 2021, 11, 365. [Google Scholar] [CrossRef] [PubMed]
- Khodanovich, M.; Svetlik, M.; Kamaeva, D.; Usova, A.; Kudabaeva, M.; Anan’ina, T.; Vasserlauf, I.; Pashkevich, V.; Moshkina, M.; Obukhovskaya, V.; et al. Demyelination in Patients with POST-COVID Depression. Biomedicines 2024, 13, 4692. [Google Scholar] [CrossRef]
- Khodanovich, M.; Svetlik, M.; Naumova, A.; Kamaeva, D.; Usova, A.; Kudabaeva, M.; Anan’ina, T.; Wasserlauf, I.; Pashkevich, V.; Moshkina, M.; et al. Age-Related Decline in Brain Myelination: Quantitative Macromolecular Proton Fraction Mapping, T2-FLAIR Hyperintensity Volume, and Anti-Myelin Antibodies Seven Years Apart. Biomedicines 2024, 12, 61. [Google Scholar] [CrossRef]
- Lu, J.; Synowiec, S.; Lu, L.; Yu, Y.; Bretherick, T.; Takada, S.; Yarnykh, V.; Caplan, J.; Caplan, M.; Claud, E.C.; et al. Microbiota Influence the Development of the Brain and Behaviors in C57BL/6J Mice. PLoS ONE 2018, 13, e0201829. [Google Scholar] [CrossRef]
- Khodanovich, M.Y.; Svetlik, M.V.; Naumova, A.V.; Usova, A.V.; Pashkevich, V.Y.; Moshkina, M.V.; Shadrina, M.M.; Kamaeva, D.A.; Obukhovskaya, V.B.; Kataeva, N.G.; et al. Global and Regional Sex-Related Differences, Asymmetry, and Peak Age of Brain Myelination in Healthy Adults. J. Clin. Med. 2024, 13, 7065. [Google Scholar] [CrossRef]
- Khodanovich, M.; Naumova, A.; Kamaeva, D.; Obukhovskaya, V.; Vasilieva, S.; Schastnyy, E.; Kataeva, N.; Levina, A.; Kudabaeva, M.; Pashkevich, V.; et al. Neurocognitive Changes in Patients with Post-COVID Depression. J. Clin. Med. 2024, 13, 1442. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.K.; Farah, L.N.; Saint, S.A.; Song, C.; Field, T.S.; Sossi, V.; Stoessl, A.J.; Wellington, C.; Honer, W.G.; Lang, D.; et al. Diffusion Tensor Imaging after COVID-19 Infection: A Systematic Review. Neuroimage 2025, 310, 121150. [Google Scholar] [CrossRef]
- Yuan, M.; Lu, R.; Liu, Y.; Zhu, H.; Wang, H.; Wang, J.; Song, Y.; Yang, L.; Xiao, M. White Matter Changes in Recovered COVID-19 Patients: Insights from DTI, DKI, and NODDI Metrics. Front. Neurol. 2025, 16, 1580262. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Wang, Y.; Lu, L.; Zhang, Y.; Yu, Q.; Dong, S.; Wang, Y.; Liu, Y.; Dong, G.; Dai, J.; et al. Long-Term White Matter Changes in Patients Who Develop Severe Depression after Multiple COVID-19 Infections: A 3–6-Month Study. Quant. Imaging Med. Surg. 2025, 15, 4364–4374. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Zhou, X.; Zhao, W.; Du, Y.; Yang, D.; Huang, Y.; Chen, Y.; Zhang, H.; Yang, G.; Liu, J.; et al. Dynamic White Matter Changes in Recovered COVID-19 Patients: A Two-Year Follow-up Study. Theranostics 2023, 13, 724–735. [Google Scholar] [CrossRef]
- Huang, S.; Zhou, Z.; Yang, D.; Zhao, W.; Zeng, M.; Xie, X.; Du, Y.; Jiang, Y.; Zhou, X.; Yang, W.; et al. Persistent White Matter Changes in Recovered COVID-19 Patients at the 1-Year Follow-Up. Brain 2022, 145, 1830–1838. [Google Scholar] [CrossRef]
- Qin, Y.; Wu, J.; Chen, T.; Li, J.; Zhang, G.; Wu, D.; Zhou, Y.; Zheng, N.; Cai, A.; Ning, Q.; et al. Long-Term Microstructure and Cerebral Blood Flow Changes in Patients Recovered from COVID-19 without Neurological Manifestations. J. Clin. Investig. 2021, 131, e147329. [Google Scholar] [CrossRef]
- Bispo, D.D.d.C.; Brandão, P.R.d.P.; Pereira, D.A.; Maluf, F.B.; Dias, B.A.; Paranhos, H.R.; von Glehn, F.; de Oliveira, A.C.P.; Regattieri, N.A.T.; Silva, L.S.; et al. Brain Microstructural Changes and Fatigue after COVID-19. Front. Neurol. 2022, 13, 1029302. [Google Scholar] [CrossRef]
- Nelson, B.K.; Farah, L.N.; Grier, A.; Su, W.; Chen, J.; Sossi, V.; Sekhon, M.S.; Stoessl, A.J.; Wellington, C.; Honer, W.G.; et al. Differences in Brain Structure and Cognitive Performance between Patients with Long-COVID and Those with Normal Recovery. Neuroimage 2024, 300, 120859. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Duan, G.; Zhou, K.; Qin, L.; Lai, Y.; Liu, Y.; Lu, Y.; Peng, B.; Zhang, Y.; Zhou, X.; et al. Alteration of White Matter Microstructure in Patients with Sleep Disorders after COVID-19 Infection. Sleep Med. 2024, 114, 109–118. [Google Scholar] [CrossRef]
- Benedetti, F.; Palladini, M.; Paolini, M.; Melloni, E.; Vai, B.; De Lorenzo, R.; Furlan, R.; Rovere-Querini, P.; Falini, A.; Mazza, M.G. Brain Correlates of Depression, Post-Traumatic Distress, and Inflammatory Biomarkers in COVID-19 Survivors: A Multimodal Magnetic Resonance Imaging Study. Brain Behav. Immun.-Health 2021, 18, 100387. [Google Scholar] [CrossRef]
- Khodanovich, M.Y.; Kamaeva, D.A.; Naumova, A.V. Role of Demyelination in the Persistence of Neurological and Mental Impairments after COVID-19. Int. J. Mol. Sci. 2022, 23, 11291. [Google Scholar] [CrossRef]
- Forkel, S.J.; Thiebaut de Schotten, M.; Kawadler, J.M.; Dell’Acqua, F.; Danek, A.; Catani, M. The Anatomy of Fronto-Occipital Connections from Early Blunt Dissections to Contemporary Tractography. Cortex 2014, 56, 73–84. [Google Scholar] [CrossRef]
- Kwon, A.; Gu, P.K.; Zhang, C.; Davidson Ward, S.L.; Perez, I.A. Sleep Disorders in Pediatric Patients with Agenesis of the Corpus Callosum. J. Clin. Sleep Med. 2024, 20, 1663–1667. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.; Montplaisir, J.; Lassonde, M. Sleep Architecture in Agenesis of the Corpus Callosum: Laboratory Assessment of Four Cases. J. Sleep Res. 1992, 1, 197–200. [Google Scholar] [CrossRef]
- Avvenuti, G.; Handjaras, G.; Betta, M.; Cataldi, J.; Imperatori, L.S.; Lattanzi, S.; Riedner, B.A.; Pietrini, P.; Ricciardi, E.; Tononi, G.; et al. Integrity of Corpus Callosum Is Essential for the Cross-Hemispheric Propagation of Sleep Slow Waves: A High-Density Eeg Study in Split-Brain Patients. J. Neurosci. 2020, 40, 5589–5603. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yin, J.; Sun, X.; Qu, Z.; Zhang, J.; Zhang, H. The Association between Insomnia and Cognitive Decline: A Scoping Review. Sleep Med. 2024, 124, 540–550. [Google Scholar] [CrossRef]
- Caverzasi, E.; Papinutto, N.; Amirbekian, B.; Berger, M.S.; Henry, R.G. Q-Ball of Inferior Fronto-Occipital Fasciculus and Beyond. PLoS ONE 2014, 9, e100274. [Google Scholar] [CrossRef]
- Conner, A.K.; Briggs, R.G.; Sali, G.; Rahimi, M.; Baker, C.M.; Burks, J.D.; Glenn, C.A.; Battiste, J.D.; Sughrue, M.E. A Connectomic Atlas of the Human Cerebrum-Chapter 13: Tractographic Description of the Inferior Fronto-Occipital Fasciculus. Oper. Neurosurg. 2018, 15, 5436–5443. [Google Scholar] [CrossRef] [PubMed]
- Hausman, H.K.; Hardcastle, C.; Albizu, A.; Kraft, J.N.; Evangelista, N.D.; Boutzoukas, E.M.; Langer, K.; O’Shea, A.; Van Etten, E.J.; Bharadwaj, P.K.; et al. Cingulo-Opercular and Frontoparietal Control Network Connectivity and Executive Functioning in Older Adults. GeroScience 2022, 44, 847–866. [Google Scholar] [CrossRef]
- Panesar, S.S.; Yeh, F.C.; Deibert, C.P.; Fernandes-Cabral, D.; Rowthu, V.; Celtikci, P.; Celtikci, E.; Hula, W.D.; Pathak, S.; Fernández-Miranda, J.C. A Diffusion Spectrum Imaging-Based Tractographic Study into the Anatomical Subdivision and Cortical Connectivity of the Ventral External Capsule: Uncinate and Inferior Fronto-Occipital Fascicles. Neuroradiology 2017, 59, 971–987. [Google Scholar] [CrossRef]
- Von Der Heide, R.J.; Skipper, L.M.; Klobusicky, E.; Olson, I.R. Dissecting the Uncinate Fasciculus: Disorders, Controversies and a Hypothesis. Brain 2013, 136, 1692–1707. [Google Scholar] [CrossRef]
- Nogueira, P.A.; Neiva, J.F.; Couto, M.P.; Giglio, M.V.; Maldaun, M.V.C.; Joaquim, A.F.; Ghizoni, E.; Formentin, C. From Classic Models to New Pathways: Unraveling the Anatomy and Function of the Inferior Fronto-Occipital Fasciculus in Language Processing. Front. Psychol. 2025, 16, 1561482. [Google Scholar] [CrossRef]
- Dadario, N.; Tariq, R.; Tang, S.; Valdivia, D.; Brenner, D.; Tanglay, O.; Sughrue, M. The Structural and Functional Connectivity of the Orbitofrontal Cortex: Deconvoluting Brodmann Areas 11, 13, 14, and 47. J. Clin. Neurophysiol. 2025, 42, 571–582. [Google Scholar] [CrossRef]
- Lai, C.H.; Wu, Y. Te The White Matter Microintegrity Alterations of Neocortical and Limbic Association Fibers in Major Depressive Disorder and Panic Disorder: The Comparison. Medicine 2016, 95, e2982. [Google Scholar] [CrossRef]
- Coloigner, J.; Batail, J.M.; Commowick, O.; Corouge, I.; Robert, G.; Barillot, C.; Drapier, D. White Matter Abnormalities in Depression: A Categorical and Phenotypic Diffusion MRI Study. NeuroImage Clin. 2019, 22, 101710. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Xu, J.; Yu, H.; Nie, B.; Li, N.; Luo, C.; Li, H.; Liu, F.; Bai, Y.; Shan, B.; et al. Delineation of Early and Later Adult Onset Depression by Diffusion Tensor Imaging. PLoS ONE 2014, 9, e112307. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Wang, Q.; Kong, X.; Deng, W.; Yang, X.; Li, X.; Zhang, Z.; Zhang, J.; Zhang, C.; Li, X.; et al. White Matter Abnormalities in Major Depression Biotypes Identified by Diffusion Tensor Imaging. Neurosci. Bull. 2019, 35, 867–876. [Google Scholar] [CrossRef]
- Wu, J.Y.; Zhang, Y.; Wu, W.B.; Hu, G.; Xu, Y. Impaired Long Contact White Matter Fibers Integrity Is Related to Depression in Parkinson’s Disease. CNS Neurosci. Ther. 2018, 24, 108–114. [Google Scholar] [CrossRef] [PubMed]
- van Velzen, L.S.; Kelly, S.; Isaev, D.; Aleman, A.; Aftanas, L.I.; Bauer, J.; Baune, B.T.; Brak, I.V.; Carballedo, A.; Connolly, C.G.; et al. White Matter Disturbances in Major Depressive Disorder: A Coordinated Analysis across 20 International Cohorts in the ENIGMA MDD Working Group. Mol. Psychiatry 2020, 25, 1511–1525. [Google Scholar] [CrossRef]
- Ota, M.; Noda, T.; Sato, N.; Hattori, K.; Hori, H.; Sasayama, D.; Teraishi, T.; Nagashima, A.; Obu, S.; Higuchi, T.; et al. White Matter Abnormalities in Major Depressive Disorder with Melancholic and Atypical Features: A Diffusion Tensor Imaging Study. Psychiatry Clin. Neurosci. 2015, 69, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Radoeva, P.D.; Milev, V.T.; Hunt, J.I.; Legere, C.H.; Deoni, S.C.L.; Sheinkopf, S.J.; Mazefsky, C.A.; Philip, N.S.; Dickstein, D.P. Systematic Review: White Matter Microstructural Organization in Adolescents With Depression. JAACAP Open 2023, 1, 233–245. [Google Scholar] [CrossRef] [PubMed]
- LeWinn, K.Z.; Connolly, C.G.; Wu, J.; Drahos, M.; Hoeft, F.; Ho, T.C.; Simmons, A.N.; Yang, T.T. White Matter Correlates of Adolescent Depression: Structural Evidence for Frontolimbic Disconnectivity. J. Am. Acad. Child Adolesc. Psychiatry 2014, 53, 899–909. [Google Scholar] [CrossRef]
- Bubb, E.J.; Metzler-Baddeley, C.; Aggleton, J.P. The Cingulum Bundle: Anatomy, Function, and Dysfunction. Neurosci. Biobehav. Rev. 2018, 92, 104–127. [Google Scholar] [CrossRef]
- Bracht, T.; Linden, D.; Keedwell, P. A Review of White Matter Microstructure Alterations of Pathways of the Reward Circuit in Depression. J. Affect. Disord. 2015, 187, 45–53. [Google Scholar] [CrossRef]
- Roos, A.L.; Goetz, T.; Voracek, M.; Krannich, M.; Bieg, M.; Jarrell, A.; Pekrun, R. Test Anxiety and Physiological Arousal: A Systematic Review and Meta-Analysis. Educ. Psychol. Rev. 2021, 33, 579–618. [Google Scholar] [CrossRef]
- Hou, J.; Liu, S.; van Wingen, G. Increased Subcortical Brain Activity in Anxious but Not Depressed Individuals. J. Psychiatr. Res. 2023, 160, 38–46. [Google Scholar] [CrossRef]
- Mitew, S.; Gobius, I.; Fenlon, L.R.; McDougall, S.J.; Hawkes, D.; Xing, Y.L.; Bujalka, H.; Gundlach, A.L.; Richards, L.J.; Kilpatrick, T.J.; et al. Pharmacogenetic Stimulation of Neuronal Activity Increases Myelination in an Axon-Specific Manner. Nat. Commun. 2018, 9, 306. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, J. Neuronal Activity and Remyelination: New Insights into the Molecular Mechanisms and Therapeutic Advancements. Front. Cell Dev. Biol. 2023, 11, 1221890. [Google Scholar] [CrossRef]
- Pan, S.; Mayoral, S.R.; Choi, H.S.; Chan, J.R.; Mazen, A.; Francisco, S.; Francisco, S.; Francisco, S.; Francisco, S.; Francisco, S.; et al. Preservation of a Remote Fear Memory Requires New Myelin Formation. Nat. Neurosci. 2020, 23, 487–499. [Google Scholar] [CrossRef]
- Kenwood, M.M.; Kalin, N.H.; Barbas, H. The Prefrontal Cortex, Pathological Anxiety, and Anxiety Disorders. Neuropsychopharmacology 2022, 47, 260–275, Erratum in Neuropsychopharmacology 2022, 47, 1141. [Google Scholar] [CrossRef]
- Khodanovich, M.Y.; Pishchelko, A.O.; Glazacheva, V.Y.; Pan, E.S.; Krutenkova, E.P.; Trusov, V.B.; Yarnykh, V.L. Plant Polyprenols Reduce Demyelination and Recover Impaired Oligodendrogenesis and Neurogenesis in the Cuprizone Murine Model of Multiple Sclerosis. Phyther. Res. 2019, 33, 1363–1373. [Google Scholar] [CrossRef]
- Pishchelko, A.; Khodanovich, M.; Pan, E.; Glazacheva, V.; Akulov, A.; Yarnykh, V. Oligodendrogenesis and Neurogenesis in Remyelination in the Cuprizone Model of Multiple Sclerosis: Correlation with the Degree of Lesion. J. Phys. Conf. Ser. 2017, 886, 012013. [Google Scholar] [CrossRef]
- Krutenkova, E.P.; Khodanovich, M.; Bowen, J.; Gangadharan, B.; Henson, L.K.J.; Mayadev, A.; Repovic, P.; Qian, P.Q.; Yarnykh, V.L. Demyelination and Iron Accumulation in Subcortical Gray Matter (GM) in Multiple Sclerosis (MS). Ann. Neurol. 2015, 78, S65. [Google Scholar]
- Rouen, A.; Taïeb, J.; Caetano, G.; Pitron, V.; Elbaz, M.; Salmon, D.; Leger, D. Polysomnographic Parameters in Long-COVID Chronic Insomnia Patients. Dialogues Clin. Neurosci. 2023, 25, 43–49. [Google Scholar] [CrossRef]
- Chang, S.E.E.; Feng, A.; Meng, W.; Apostolidis, S.A.A.; Mack, E.; Artandi, M.; Barman, L.; Bennett, K.; Chakraborty, S.; Chang, I.; et al. New-Onset IgG Autoantibodies in Hospitalized Patients with COVID-19. Nat. Commun. 2021, 12, 5417. [Google Scholar] [CrossRef]
- Rojas, M.; Rodríguez, Y.; Acosta-Ampudia, Y.; Monsalve, D.M.; Zhu, C.; Li, Q.Z.; Ramírez-Santana, C.; Anaya, J.M. Autoimmunity Is a Hallmark of Post-COVID Syndrome. J. Transl. Med. 2022, 20, 129. [Google Scholar] [CrossRef] [PubMed]
- Weissert, R. Nervous System-Related Tropism of SARS-CoV-2 and Autoimmunity in COVID-19 Infection. Eur. J. Immunol. 2024, 54, 2250230. [Google Scholar] [CrossRef] [PubMed]
- Lake, C.M.; Breen, J.J. Sequence Similarity between SARS-CoV-2 Nucleocapsid and Multiple Sclerosis-Associated Proteins Provides Insight into Viral Neuropathogenesis Following Infection. Sci. Rep. 2023, 13, 389. [Google Scholar] [CrossRef] [PubMed]
- Almulla, A.F.; Maes, M.; Zhou, B.; Al-Hakeim, H.K.; Vojdani, A. Brain-Targeted Autoimmunity Is Strongly Associated with Long COVID and Its Chronic Fatigue Syndrome as Well as Its Affective Symptoms. J. Adv. Res. 2025, 75, 621–633. [Google Scholar] [CrossRef]
- Rzymski, P.; Niedziela, J.; Poniedziałek, B.; Rosińska, J.; Zarębska-Michaluk, D.; Sobala-Szczygieł, B.; Flisiak, R.; Gąsior, M.; Jaroszewicz, J. Humoral Anti-SARS-CoV-2 Response in Patients with Different Long COVID Phenotypes. Virology 2024, 596, 110118. [Google Scholar] [CrossRef]
- Yukari Magawa, J.; Jacintho, L.C.; Alves Ferreira, M.; Ramos Oliveira, J.; Rahal Guaragna Machado, R.; Kuramoto Takara, A.; Moreno Lima de Oliveira, R.; Cesario Lima, A.; Sasahara, G.L.; Lopes Adami, F.; et al. Protective Role of Anti-SARS-CoV-2 Antibody Responses against Vital Organ Related Long COVID Symptoms. Sci. Rep. 2025, 15, 23705. [Google Scholar] [CrossRef]
- Porter, G.A.; O’Connor, J.C. Brain-Derived Neurotrophic Factor and Inflammation in Depression: Pathogenic Partners in Crime? World J. Psychiatry 2022, 12, 77–97. [Google Scholar] [CrossRef]
- Shafiee, A.; Seighali, N.; Teymouri Athar, M.; Abdollahi, A.K.; Jafarabady, K.; Bakhtiyari, M. Levels of Brain-Derived Neurotrophic Factor (BDNF) among Patients with COVID-19: A Systematic Review and Meta-Analysis. Eur. Arch. Psychiatry Clin. Neurosci. 2024, 274, 1137–1152. [Google Scholar] [CrossRef] [PubMed]
- Demir, B.; Beyazyüz, E.; Beyazyüz, M.; Çelikkol, A.; Albayrak, Y. Long-Lasting Cognitive Effects of COVID-19: Is There a Role of BDNF? Eur. Arch. Psychiatry Clin. Neurosci. 2023, 273, 1339–1347. [Google Scholar] [CrossRef]
- Petrella, C.; Nenna, R.; Petrarca, L.; Tarani, F.; Paparella, R.; Mancino, E.; Di Mattia, G.; Conti, M.G.; Matera, L.; Bonci, E.; et al. Serum NGF and BDNF in Long-COVID-19 Adolescents: A Pilot Study. Diagnostics 2022, 12, 1162. [Google Scholar] [CrossRef]
- Tang, N.; Kido, T.; Shi, J.; McCafferty, E.; Ford, J.M.; Dal Bon, K.; Pulliam, L. Blood Markers Show Neural Consequences of LongCOVID-19. Cells 2024, 13, 478. [Google Scholar] [CrossRef]
- Ditmer, M.; Gabryelska, A.; Turkiewicz, S.; Sochal, M. Investigating the Role of BDNF in Insomnia: Current Insights. Nat. Sci. Sleep 2023, 15, 1045–1060. [Google Scholar] [CrossRef]
- Lee, B.H.; Kim, Y.K. The Roles of BDNF in the Pathophysiology of Major Depression and in Antidepressant Treatment. Psychiatry Investig. 2010, 7, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Bastien, C.H.; Vallières, A.; Morin, C.M. Validation of the Insomnia Severity Index as an Outcome Measure for Insomnia Research. Sleep Med. 2001, 2, 297–307. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snalth, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, M. Hamilton Depression Rating Scale (HDRS). Arch. Gen. Psychiatry 1960, 23, 56–62. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bedirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool For Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699, Erratum in J. Am. Geriatr. Soc. 2019, 67, 1991. [Google Scholar] [CrossRef]
- Green, P.; Montijo, J.; Brockhaus, R. High Specificity of the Word Memory Test and Medical Symptom Validity Test in Groups with Severe Verbal Memory Impairment. Appl. Neuropsychol. 2011, 18, 86–94. [Google Scholar] [CrossRef]
- Allen, M.; Bigler, E.; Larsen, J.; Goodrich-Hunsaker, N.; Hopkins, R. Functional Neuroimaging Evidence for High Cognitive Effort on the Word Memory Test in the Absence of External Incentives. Brain Inj. 2007, 21, 1425–1428. [Google Scholar] [CrossRef]
- Reitan, R.M. Validity of the Trail Making Test as an Indicator of Organic Brain Damage. Percept. Mot. Ski. 1958, 8, 271–276. [Google Scholar] [CrossRef]
- Stroop, J.R. Studies of Interference in Serial Verbal Reactions. J. Exp. Psychol. 1935, 18, 643–662. [Google Scholar] [CrossRef]
- Huang, C.L.-C. The Value of Patient-Administered Depression Rating Scale in Detecting Cognitive Deficits in Depressed Patients. J. Clin. Med. Res. 2010, 2, 27–33. [Google Scholar] [CrossRef]
- Freud, T.; Vostrikov, A.; Dwolatzky, T.; Punchik, B.; Press, Y. Validation of the Russian Version of the MoCA Test as a Cognitive Screening Instrument in Cognitively Asymptomatic Older Individuals and Those With Mild Cognitive Impairment. Front. Med. 2020, 7, 447. [Google Scholar] [CrossRef] [PubMed]
- Mahendran, R.; Chua, J.; Feng, L.; Kua, E.H.; Preedy, V.R. The Mini-Mental State Examination and Other Neuropsychological Assessment Tools for Detecting Cognitive Decline; Elsevier Inc.: Amsterdam, The Netherlands, 2015; ISBN 9780124079397. [Google Scholar]
- Cousijn, J.; van Benthem, P.; van der Schee, E.; Spijkerman, R. Motivational and Control Mechanisms Underlying Adolescent Cannabis Use Disorders: A Prospective Study. Dev. Cogn. Neurosci. 2015, 16, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Tydecks, S.; Merten, T.; Gubbay, J. The Word Memory Test and the One-in-Five-Test in an Analogue Study with Russian Speaking Participants. Int. J. Forensic Psychol. 2006, 1, 29–37. [Google Scholar]
- Yarnykh, V.L.; Tartaglione, E.V.; Ioannou, G.N. Fast Macromolecular Proton Fraction Mapping of the Human Liver In Vivo for Quantitative Assessment of Hepatic Fibrosis. NMR Biomed. 2015, 28, 1716–1725. [Google Scholar] [CrossRef] [PubMed]
- Avants, B.B.; Tustison, N.J.; Song, G.; Cook, P.A.; Klein, A.; Gee, J.C. NeuroImage A Reproducible Evaluation of ANTs Similarity Metric Performance in Brain Image Registration. Neuroimage 2011, 54, 2033–2044. [Google Scholar] [CrossRef]
- Avants, B.B.; Yushkevich, P.; Pluta, J.; Minkoff, D.; Korczykowski, M.; Detre, J.; Gee, J.C. NeuroImage The Optimal Template Effect in Hippocampus Studies of Diseased Populations. Neuroimage 2010, 49, 2457–2466. [Google Scholar] [CrossRef]
- Oishi, K.; Faria, A.; Jiang, H.; Li, X.; Akhter, K.; Zhang, J.; Hsu, J.T.; Miller, M.I.; Van Zijl, P.C.M.; Albert, M.; et al. NeuroImage Atlas-Based Whole Brain White Matter Analysis Using Large Deformation Diffeomorphic Metric Mapping: Application to Normal Elderly and Alzheimer’s Disease Participants. Neuroimage 2009, 46, 486–499. [Google Scholar] [CrossRef]


| Parameter | Insomnia | Ins-Dep | Depression | PostCovid | Control |
|---|---|---|---|---|---|
| Severity, mild/moderate/severe/critical (%) | 57/29/14/0 | 84/8/8/0 | 92/8/0/0 | 77/7/11/5 | - |
| Number of COVID-19 episodes, mean ± SD | 1.64 ± 0.63 | 1.54 ± 0.66 | 1.75 ± 0.873 | 1.63 ± 0.79 | - |
| Time after the first COVID-19, months ± SD | 21.5 ± 8.9 | 22.2 ± 7.2 | 18.3 ± 9.1 | 22.1 ± 10.5 | - |
| Time after the last COVID-19, months ± SD | 14.9 ± 8.5 | 15.7 ± 10.5 | 10.3 ± 9.8 | 15.1 ± 11.5 | - |
| Number of acute symptoms | 6.8 ± 1.5 | 7.4 ± 1.9 | 7.1 ± 1.8 | 6.1 ± 2.4 | - |
| Number of post-COVID symptoms | 6.9 ± 2.7 | 8.2 ± 2.1 * | 7.8 ± 2.4 | 6.0 ± 3.0 | - |
| Vaccinated at the time of study (%) | 42.9 | 53.9 | 100 | 50.0 | 68.2 |
| Test | Parameter | Insomnia | InsDep | Depression | PostCovid | Control |
|---|---|---|---|---|---|---|
| ISI | Total score | 17.1 ± 2.9 D, PC, C | 20.1 ± 4.9 D, PC, C | 8.6 ± 2.5 I, ID, PC | 5.7 ± 3.6 I, ID, D | 6.1 ± 4.9 I, ID |
| HADS | Total score | 12.6 ± 7.0 ID, C | 25.1 ± 6.3 A | 16.7 ± 4.9 I, PC, C | 10.4 ± 4.9 I, ID | 7.4 ± 3.8 A |
| Anxiety | 6.9 ± 3.7 D, ID, C | 12.7 ± 3.0 A | 8.8 ± 2.2 ID, PC, C | 6.0 ± 3.7 D, ID, C | 4.05 ± 2.44 A | |
| Depression | 6.3 ± 3.9 ID, C | 12.7 ± 3.7 A | 7.8 ± 4.6 I, PC, C | 4.6 ± 2.9 D, ID | 3.4 ± 2.4 I, ID, D | |
| HDRS | Total score | 11.4 ± 4.7 A | 21.3 ± 4.0 I, PC, C | 18.3 ± 2.4 I, PC, C | 8.4 ± 4.4 A | 3.5 ± 3.4 A |
| Test | Parameter | Insomnia | InsDep | Depression | PostCovid | Control |
|---|---|---|---|---|---|---|
| MoCA | Total score | 25.9 ± 2.8 C | 26.6 ± 1.8 | 26.3 ± 2.5 | 26.8 ± 1.9 | 27.6 ± 1.5 |
| WMT | Total score | 18.6 ± 2.2 | 17.7 ± 3.1 | 19.0 ± 1.0 | 18.9 ± 1.5 | 19.1 ± 1.3 |
| Immediate recall | 7.5 ± 1.0 | 6.6 ± 1.3 PC, C | 7.6 ± 1.7 | 7.7 ± 1.3 ID | 8.4 ± 1.4 ID | |
| Immediate recall with assistance | 9.7 ± 0.5 ID | 8.8 ± 1.5 I, PC, C | 9.3 ± 1.3 | 9.7 ± 0.5 ID | 9.8 ± 0.5 ID, D | |
| Delayed recall | 6.3 ± 2.1 | 6.5 ± 2.4 | 7.3 ± 1.6 | 7.0 ± 1.8 | 7.2 ± 2.1 | |
| Delayed recall with assistance | 9.3 ± 0.9 | 8.8 ± 2.0 | 9.3 ± 1.2 | 9.3 ± 1.1 | 9.2 ± 1.2 | |
| SCWT | W, time (s) | 51.9 ± 6.5 | 53.3 ± 10.9 | 57.7 ± 13.6 PC, C | 51.8 ± 7.4 | 51.0 ± 9.1 |
| C, time (s) | 74.0 ± 17.5 | 65.1 ± 24.2 | 72.8 ± 19.0 | 66.9 ± 10.1 | 65.5 ± 18.7 | |
| CW, time (s) | 132.1 ± 27.6 C | 117.3 ± 40.4 | 121.1 ± 30.9 | 118.8 ± 22.0 | 114.0 ± 42.7 | |
| TMT | Processing time, s | 37.0 ± 11.1 D | 37.5 ± 14.9 | 46.0 ± 21.1 I, PC, C | 32.5 ± 7.9 D | 34.1 ± 7.9 D |
| Errors, mean ± SD | 0.6 ± 0.6 ID | 0.1 ± 0.3 I | 0.2 ± 0.4 | 0.4 ± 0.6 | 0.5 ± 0.7 |
| Antibodies | Control | PostCovid | Depression | InsDep | Insomnia |
|---|---|---|---|---|---|
| BDNF ng/m | 69.73 ± 11.20 | 64.01 ± 11.64 | 66.44 ± 11.01 | 68.54 ± 10.60 | 71.50 ± 8.48 |
| Anti-S100, ng/mL | 7.12 ± 5.15 | 6.99 ± 3.80 | 5.93 ± 6.74 | 8.44 ± 7.37 | 9.33 ± 8.07 |
| Anti-MBP ab ng/mL | 2.41 ± 3.04 | 10.28 ± 18.24 | 17.46 ± 40.38 | 0.97 ± 1.61 | 25.73 ± 45.10 |
| Anti-PLP, ng/mL | 1.33 ± 0.22 | 0.99 ± 0.54 ** | 1.04 ± 0.85 # | 1.44 ± 0.69 | 1.77 ± 1.16 * |
| IgG, ng/mL | 1.63 ± 3.84 | 2.60 ± 3.81 | 4.78 ± 5.58 | 4.49 ± 5.65 | 1.76 ± 3.94 |
| Parameters | All Patients | Insomnia | InsDep | Depression | PostCovid | |
|---|---|---|---|---|---|---|
| Neuropsychiatric scales | ISI | Anti-PLP (0.30 *) | S-IgG (−0.63 *) | BDNF (0.59 *) | BDNF (0.61 *) | |
| HADS-A | BDNF (0.71 *) | BDNF (−0.43 *) | ||||
| HADS-D | BDNF (−0.74 *) | |||||
| HDRS | Anti-PLP (0.31 *) | BDNF (0.58 *) S-IgG (−0.68 *) | BDNF (−0.53 *) | |||
| MFP PC | Juxtacortical WM | Anti-PLP (−0.34 *) | S-IgG (0.85 **) | Anti-PLP (−0.42 *) | ||
| WM pathways | Anti-PLP (−0.30 *) | S-IgG (0.86 ***) | Anti-PLP (−0.46 *) | |||
| Allocortex and deep GM | Anti-PLP (−0.30 *) BDNF (−0.29 *) S-IgG (0.26 *) | Anti-MBP (−0.63 *) | Anti-PLP (−0.58 **) | |||
| Brainstem | S-IgG (0.27 *) | Anti-MBP (−0.64 *) | Anti-MBP (−0.68 *) | BDNF (−0.44 *) | ||
| Parameter | Insomnia | InsDep | Depression | PostCovid | Control |
|---|---|---|---|---|---|
| Sample size | 14 | 13 | 12 | 32 | 22 |
| Male (%)/Female (%) | 3(21)/11(79) | 2(15)/11(85) * | 2(17)/10(83) | 17(39)/27(61) | 11(50)/11(50) |
| Age, years ± SD | 45.2 ± 8.9 | 38.5 ± 12.6 | 35.3 ± 15.2 | 41.6 ± 10.9 | 40.6 ± 11.2 |
| Age, median (min-max) | 46(22–55) | 42(19–59) | 35(20–58) | 42(20–60) | 40(20–58) |
| Education, years ± SD | 15.1 ± 2.2 | 16.1 ± 1.1 | 15.1 ± 2.4 | 15.2 ± 2.4 | 16.4 ± 1.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khodanovich, M.; Kamaeva, D.; Usova, A.; Pashkevich, V.; Moshkina, M.; Obukhovskaya, V.; Kataeva, N.; Levina, A.; Tumentceva, Y.; Shadrina, M.; et al. Demyelination and Cognitive Performance in Long COVID Patients with Insomnia and/or Depression. Int. J. Mol. Sci. 2025, 26, 12141. https://doi.org/10.3390/ijms262412141
Khodanovich M, Kamaeva D, Usova A, Pashkevich V, Moshkina M, Obukhovskaya V, Kataeva N, Levina A, Tumentceva Y, Shadrina M, et al. Demyelination and Cognitive Performance in Long COVID Patients with Insomnia and/or Depression. International Journal of Molecular Sciences. 2025; 26(24):12141. https://doi.org/10.3390/ijms262412141
Chicago/Turabian StyleKhodanovich, Marina, Daria Kamaeva, Anna Usova, Valentina Pashkevich, Marina Moshkina, Victoria Obukhovskaya, Nadezhda Kataeva, Anastasia Levina, Yana Tumentceva, Maria Shadrina, and et al. 2025. "Demyelination and Cognitive Performance in Long COVID Patients with Insomnia and/or Depression" International Journal of Molecular Sciences 26, no. 24: 12141. https://doi.org/10.3390/ijms262412141
APA StyleKhodanovich, M., Kamaeva, D., Usova, A., Pashkevich, V., Moshkina, M., Obukhovskaya, V., Kataeva, N., Levina, A., Tumentceva, Y., Shadrina, M., Ranzaeva, A., Vasilieva, S., Schastnyy, E., Naumova, A., & Svetlik, M. (2025). Demyelination and Cognitive Performance in Long COVID Patients with Insomnia and/or Depression. International Journal of Molecular Sciences, 26(24), 12141. https://doi.org/10.3390/ijms262412141






