The Determinant of Tau Spreading in Alzheimer’s Disease: Dependent on Senile Plaque, Neural Circuits, or Spatial Proximity?
Abstract
1. Introduction
- Introduction
- Aβ and Tau Pathology in AD
- 2.2
- Neuropathology of AD
- 2.3
- Is Tau a Bystander of Aβ in AD Pathology?
- Controversies of Tau Pathology in AD
- 3.1
- Spatiotemporal Discrepancy Between Senile Plaque and NFTs in the Human Brain, from the Viewpoint of the ‘Amyloid Cascade’
- 3.2
- Is AD a System Degeneration of Memory Circuits? From the Viewpoint of ‘Tauopathy’
- Conclusions
2. Aβ and Tau Pathology in AD
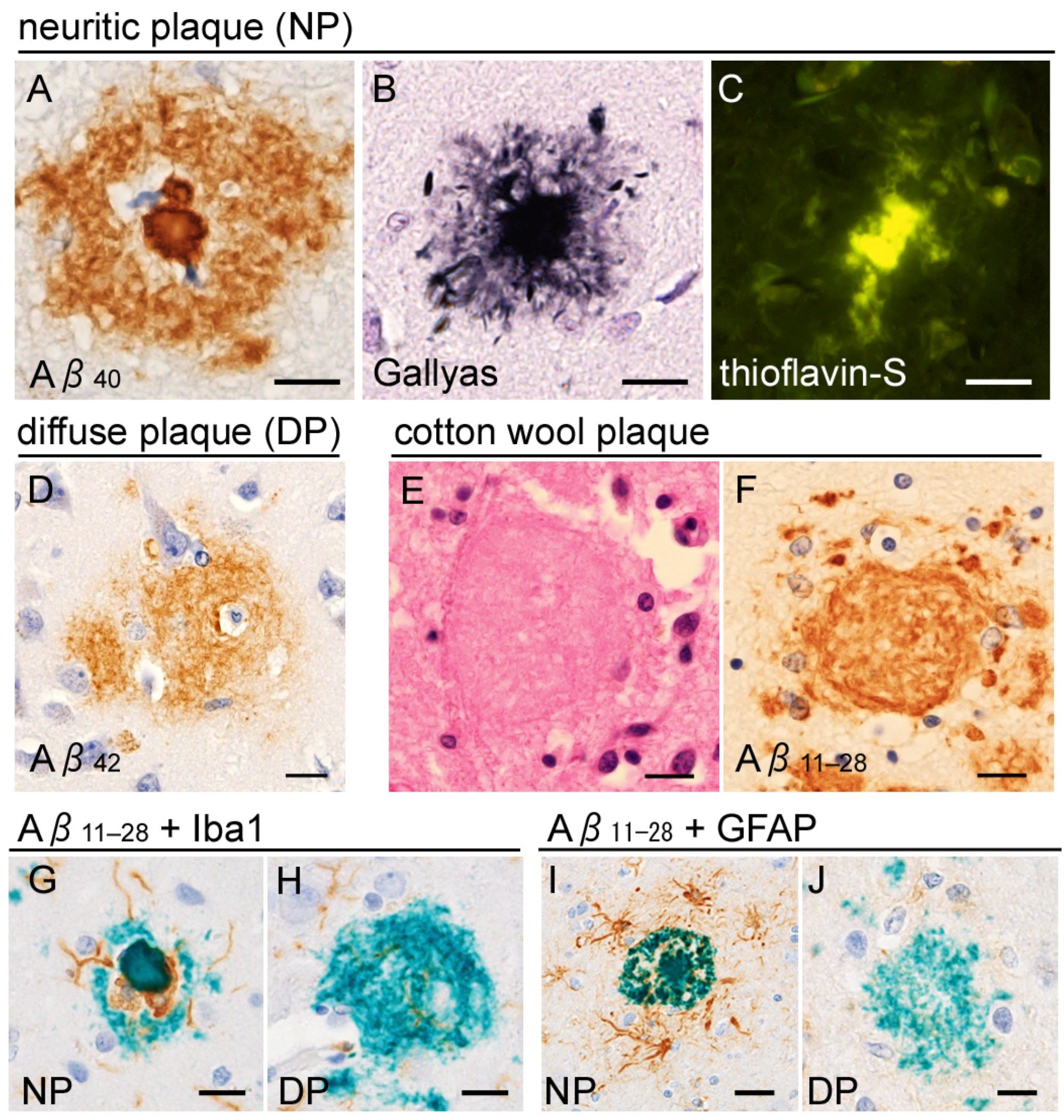
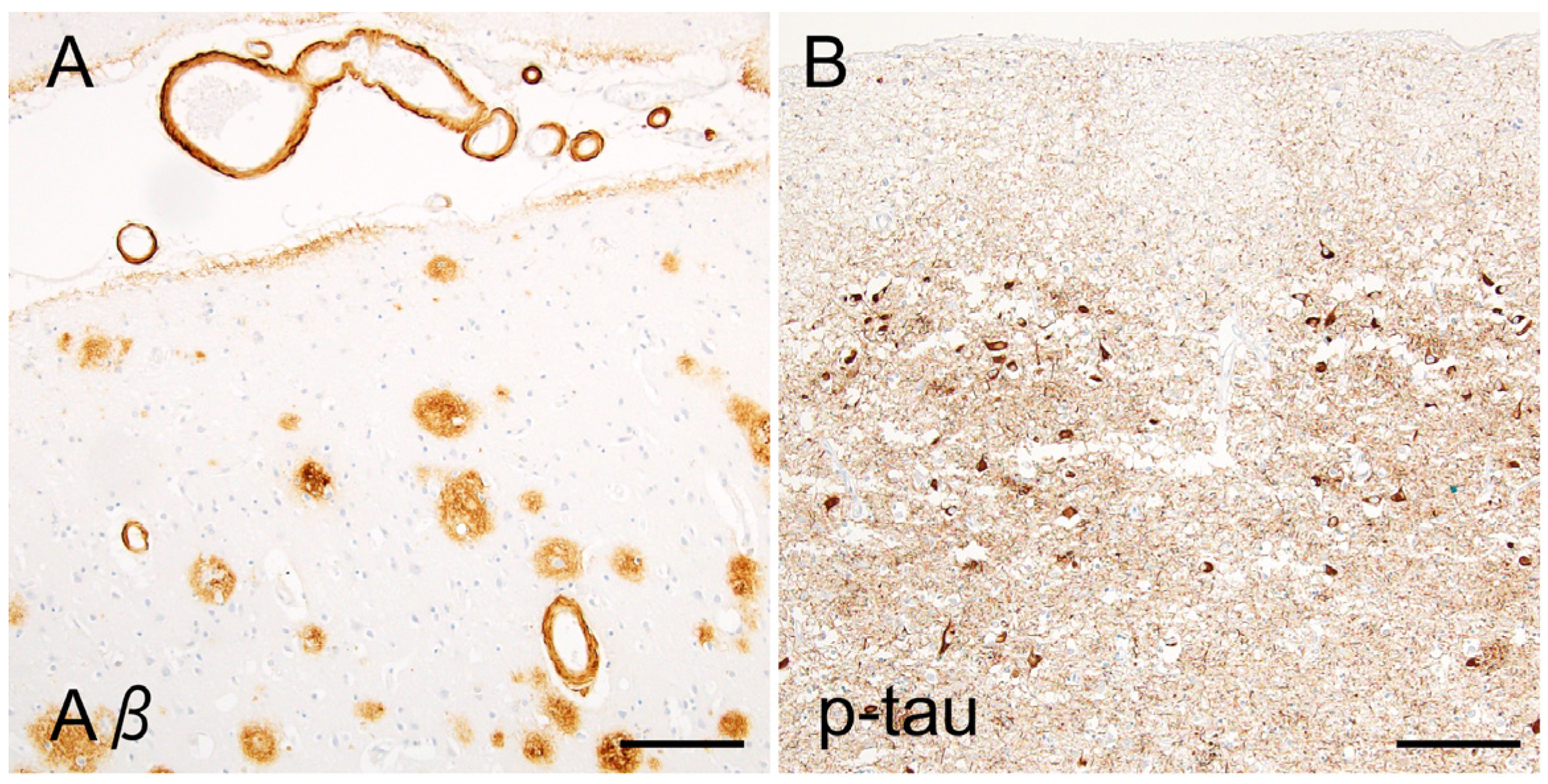
2.1. Neuropathology of Alzheimer’s Disease
2.2. Is Tau a Bystander of Aβ in AD Pathology?
3. Controversies of Tau Pathology in AD
3.1. Spatiotemporal Discrepancy Between Senile Plaque and NFTs in the Human Brain, from the Viewpoint of the ‘Amyloid Cascade’
3.2. Is AD a System Degeneration of Memory Circuits? From the Viewpoint of ‘Tauopathy’
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| Aβ | amyloid-β |
| APP | amyloid precursor protein |
| ALS | amyotrophic lateral sclerosis |
| CAA | cerebral amyloid angiopathy |
| CBD | corticobasal degeneration |
| FTDP | frontotemporal dementia with parkinsonism |
| FTLD | frontotemporal lobar degeneration |
| MAPT | microtubule-associated protein tau |
| MSA | multiple system atrophy |
| NFT | neurofibrillary tangle |
| PART | primary aging-related tauopathy |
| PSP | progressive supranuclear palsy |
| PSEN1 | presenilin-1 |
| SDNFT | senile dementia with NFT |
| TDP-43 | TAR DNA-binding protein 43kDa |
| 3R/4R-tau | 3-repeat/4-repeat tau |
References
- GBD 2021 Nervous System Disorders Collaborators. Global, regional, and national burden of disorders affecting the nervous system, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Neurol. 2024, 23, 344–381, Correction in Lancet Neurol. 2024, 23, e9. Correction in Lancet Neurol. 2024, 23, e11. [Google Scholar] [CrossRef] [PubMed]
- Gracner, T.; Chaturvedi, R.; Nguyen, P.; Heun-Johnson, H.; Tysinger, B.; Goldman, D.; Lakdawalla, D. The burden of cognitive impairment. Alzheimers Dement. 2025, 21, e70436. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer, A.; Stelzmann, R.A.; Schnitzlein, H.N.; Murtagh, F.R. An English translation of Alzheimer’s 1907 paper, “Uber eine eigenartige Erkankung der Hirnrinde”. Clin. Anat. 1995, 8, 429–431. [Google Scholar] [CrossRef]
- Fischer, O. Miliare Nekrosen mit drusingen Wucherungen der Neurofibrillen, eine regelmässige Veränderung der Hirnrinde bei seniler Demenz. Monattsschrift Psychiatr. Neurol. 1907, 22, 361–372. [Google Scholar] [CrossRef]
- Beach, T.G. A History of Senile Plaques: From Alzheimer to Amyloid Imaging. J. Neuropathol. Exp. Neurol. 2022, 81, 387–413. [Google Scholar] [CrossRef]
- Blocq, F.; Marinesco, C. Sur les lésions et la pathogénie de l’épilepsie dite essentielle. Sem. Med. 1892, 12, 445. [Google Scholar]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s disease: The amyloid cascade hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef]
- Tarawneh, R.; Head, D.; Allison, S.; Buckles, V.; Fagan, A.M.; Ladenson, J.H.; Morris, J.C.; Holtzman, D.M. Cerebrospinal Fluid Markers of Neurodegeneration and Rates of Brain Atrophy in Early Alzheimer Disease. JAMA Neurol. 2015, 72, 656–665. [Google Scholar] [CrossRef]
- Therriault, J.; Janelidze, S.; Benedet, A.L.; Ashton, N.J.; Arranz Martínez, J.; Gonzalez-Escalante, A.; Bellaver, B.; Alcolea, D.; Vrillon, A.; Karim, H.; et al. Diagnosis of Alzheimer’s disease using plasma biomarkers adjusted to clinical probability. Nat. Aging 2024, 4, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liao, W.; Wang, L.; Li, J.; Huang, D.; Cheng, W.; Tian, J.; Luan, P. Advance and Prospect of Positron Emission Tomography in Alzheimer’s disease research. Mol. Psychiatry 2025, 30, 4899–4909. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Alafuzoff, I.; Arzberger, T.; Kretzschmar, H.; Del Tredici, K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006, 112, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Thal, D.R.; Rüb, U.; Orantes, M.; Braak, H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology 2002, 58, 1791–1800. [Google Scholar] [CrossRef] [PubMed]
- Del Tredici, K.; Braak, H. Neuropathology and neuroanatomy of TDP-43 amyotrophic lateral sclerosis. Curr. Opin. Neurol. 2022, 35, 660–671. [Google Scholar] [CrossRef]
- Arnold, F.J.; Putka, A.F.; Raychaudhuri, U.; Hsu, S.; Bedlack, R.S.; Bennett, C.L.; La Spada, A.R. Revisiting Glutamate Excitotoxicity in Amyotrophic Lateral Sclerosis and Age-Related Neurodegeneration. Int. J. Mol. Sci. 2024, 25, 5587. [Google Scholar] [CrossRef] [PubMed]
- Montine, T.J.; Phelps, C.H.; Beach, T.G.; Bigio, E.H.; Cairns, N.J.; Dickson, D.W.; Duyckaerts, C.; Frosch, M.P.; Masliah, E.; Mirra, S.S.; et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: A practical approach. Acta Neuropathol. 2012, 123, 1–11. [Google Scholar] [CrossRef]
- Braak, H.; Braak, E. Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol. Aging 1995, 16, 271–278; discussion 278–284. [Google Scholar] [CrossRef]
- Mirra, S.S.; Heyman, A.; McKeel, D.; Sumi, S.M.; Crain, B.J.; Brownlee, L.M.; Vogel, F.S.; Hughes, J.P.; van Belle, G.; Berg, L. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of A45Alzheimer’s disease. Neurology 1991, 41, 479–486. [Google Scholar] [CrossRef]
- Brion, J.P. Immunological demonstration of tau protein in neurofibrillary tangles of Alzheimer’s disease. J. Alzheimers Dis. 2006, 9, 177–185. [Google Scholar] [CrossRef]
- Soeda, Y.; Hayashi, E.; Nakatani, N.; Ishigaki, S.; Takaichi, Y.; Tachibana, T.; Riku, Y.; Chambers, J.K.; Koike, R.; Mohammad, M.; et al. A novel monoclonal antibody generated by immunization with granular tau oligomers binds to tau aggregates at 423-430 amino acid sequence. Sci. Rep. 2024, 14, 16391. [Google Scholar] [CrossRef]
- Tatsumi, S.; Uchihara, T.; Aiba, I.; Iwasaki, Y.; Mimuro, M.; Takahashi, R.; Yoshida, M. Ultrastructural differences in pretangles between Alzheimer disease and corticobasal degeneration revealed by comparative light and electron microscopy. Acta Neuropathol. Commun. 2014, 2, 161. [Google Scholar] [CrossRef]
- Wang, Y.; Mandelkow, E. Tau in physiology and pathology. Nat. Rev. Neurosci. 2016, 17, 5–21. [Google Scholar] [CrossRef]
- de Silva, R.; Lashley, T.; Gibb, G.; Hanger, D.; Hope, A.; Reid, A.; Bandopadhyay, R.; Utton, M.; Strand, C.; Jowett, T.; et al. Pathological inclusion bodies in tauopathies contain distinct complements of tau with three or four microtubule-binding repeat domains as demonstrated by new specific monoclonal antibodies. Neuropathol. Appl. Neurobiol. 2003, 29, 288–302. [Google Scholar] [CrossRef]
- Hara, M.; Hirokawa, K.; Kamei, S.; Uchihara, T. Isoform transition from four-repeat to three-repeat tau underlies dendrosomatic and regional progression of neurofibrillary pathology. Acta Neuropathol. 2013, 125, 565–579. [Google Scholar] [CrossRef]
- Buée, L.; Bussière, T.; Buée-Scherrer, V.; Delacourte, A.; Hof, P.R. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res. Brain Res. Rev. 2000, 33, 95–130. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Maat-Schieman, M.L.; van Duinen, S.G.; Prins, F.A.; Neeskens, P.; Natté, R.; Roos, R.A. Amyloid beta protein (Abeta) starts to deposit as plasma membrane-bound form in diffuse plaques of brains from hereditary cerebral hemorrhage with amyloidosis-Dutch type, Alzheimer disease and nondemented aged subjects. J. Neuropathol. Exp. Neurol. 2000, 59, 723–732. [Google Scholar] [CrossRef]
- Le, T.V.; Crook, R.; Hardy, J.; Dickson, D.W. Cotton wool plaques in non-familial late-onset Alzheimer disease. J. Neuropathol. Exp. Neurol. 2001, 60, 1051–1061. [Google Scholar] [CrossRef]
- Boon, B.D.C.; Bulk, M.; Jonker, A.J.; Morrema, T.H.J.; van den Berg, E.; Popovic, M.; Walter, J.; Kumar, S.; van der Lee, S.J.; Holstege, H.; et al. The coarse-grained plaque: A divergent Aβ plaque-type in early-onset Alzheimer’s disease. Acta Neuropathol. 2020, 140, 811–830. [Google Scholar] [CrossRef]
- Dickson, T.C.; Vickers, J.C. The morphological phenotype of beta-amyloid plaques and associated neuritic changes in Alzheimer’s disease. Neuroscience 2001, 105, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Nelson, P.T.; Alafuzoff, I.; Bigio, E.H.; Bouras, C.; Braak, H.; Cairns, N.J.; Castellani, R.J.; Crain, B.J.; Davies, P.; Del Tredici, K.; et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: A review of the literature. J. Neuropathol. Exp. Neurol. 2012, 71, 362–381. [Google Scholar] [CrossRef] [PubMed]
- Brendza, R.P.; Bacskai, B.J.; Cirrito, J.R.; Simmons, K.A.; Skoch, J.M.; Klunk, W.E.; Mathis, C.A.; Bales, K.R.; Paul, S.M.; Hyman, B.T.; et al. Anti-Abeta antibody treatment promotes the rapid recovery of amyloid-associated neuritic dystrophy in PDAPP transgenic mice. J. Clin. Investig. 2005, 115, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Luehmann, M.; Coomaraswamy, J.; Bolmont, T.; Kaeser, S.; Schaefer, C.; Kilger, E.; Neuenschwander, A.; Abramowski, D.; Frey, P.; Jaton, A.L.; et al. Exogenous induction of cerebral beta-amyloidogenesis is governed by agent and host. Science 2006, 313, 1781–1784. [Google Scholar] [CrossRef] [PubMed]
- Tsering, W.; Prokop, S. Neuritic Plaques—Gateways to Understanding Alzheimer’s Disease. Mol. Neurobiol. 2024, 61, 2808–2821. [Google Scholar] [CrossRef]
- Deczkowska, A.; Keren-Shaul, H.; Weiner, A.; Colonna, M.; Schwartz, M.; Amit, I. Disease-Associated Microglia: A Universal Immune Sensor of Neurodegeneration. Cell 2018, 173, 1073–1081. [Google Scholar] [CrossRef]
- Baligács, N.; Albertini, G.; Borrie, S.C.; Serneels, L.; Pridans, C.; Balusu, S.; De Strooper, B. Homeostatic microglia initially seed and activated microglia later reshape amyloid plaques in Alzheimer’s Disease. Nat. Commun. 2024, 15, 10634. [Google Scholar] [CrossRef]
- Brier, M.R.; Gordon, B.; Friedrichsen, K.; McCarthy, J.; Stern, A.; Christensen, J.; Owen, C.; Aldea, P.; Su, Y.; Hassenstab, J.; et al. Tau and Aβ imaging, CSF measures, and cognition in Alzheimer’s disease. Sci. Transl. Med. 2016, 8, 338ra66. [Google Scholar] [CrossRef]
- Murray, M.E.; Graff-Radford, N.R.; Ross, O.A.; Petersen, R.C.; Duara, R.; Dickson, D.W. Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: A retrospective study. Lancet Neurol. 2011, 10, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Ossenkoppele, R.; Salvadó, G.; Janelidze, S.; Pichet Binette, A.; Bali, D.; Karlsson, L.; Palmqvist, S.; Mattsson-Carlgren, N.; Stomrud, E.; Therriault, J.; et al. Plasma p-tau217 and tau-PET predict future cognitive decline among cognitively unimpaired individuals: Implications for clinical trials. Nat. Aging 2025, 5, 883–896. [Google Scholar] [CrossRef]
- Roberson, E.D.; Scearce-Levie, K.; Palop, J.J.; Yan, F.; Cheng, I.H.; Wu, T.; Gerstein, H.; Yu, G.Q.; Mucke, L. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer’s disease mouse model. Science 2007, 316, 750–754. [Google Scholar] [CrossRef]
- Leroy, K.; Ando, K.; Laporte, V.; Dedecker, R.; Suain, V.; Authelet, M.; Héraud, C.; Pierrot, N.; Yilmaz, Z.; Octave, J.N.; et al. Lack of tau proteins rescues neuronal cell death and decreases amyloidogenic processing of APP in APP/PS1 mice. Am. J. Pathol. 2012, 181, 1928–1940. [Google Scholar] [CrossRef]
- Müller, U.; Winter, P.; Graeber, M.B. A presenilin 1 mutation in the first case of Alzheimer’s disease. Lancet Neurol. 2013, 12, 129–130. [Google Scholar] [CrossRef]
- Hanger, D.P.; Brion, J.P.; Gallo, J.M.; Cairns, N.J.; Luthert, P.J.; Anderton, B.H. Tau in Alzheimer’s disease and Down’s syndrome is insoluble and abnormally phosphorylated. Biochem. J. 1991, 275, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, D.E.; Molina-Porcel, L.; Iba, M.; Aboagye, A.K.; Paul, S.M.; Trojanowski, J.Q.; Lee, V.M. A-beta accelerates the spatiotemporal progression of tau pathology and augments tau amyloidosis in an Alzheimer mouse model. Am. J. Pathol. 2010, 177, 1977–1988. [Google Scholar] [CrossRef]
- van Swieten, J.; Spillantini, M.G. Hereditary frontotemporal dementia caused by Tau gene mutations. Brain Pathol. 2007, 17, 63–73. [Google Scholar] [CrossRef]
- Jaunmuktane, Z.; Mead, S.; Ellis, M.; Wadsworth, J.D.; Nicoll, A.J.; Kenny, J.; Launchbury, F.; Linehan, J.; Richard-Loendt, A.; Walker, A.S.; et al. Evidence for human transmission of amyloid-β pathology and cerebral amyloid angiopathy. Nature 2015, 525, 247–250, Erratum in Nature 2015, 526, 595. [Google Scholar] [CrossRef]
- Hervé, D.; Porché, M.; Cabrejo, L.; Guidoux, C.; Tournier-Lasserve, E.; Nicolas, G.; Adle-Biassette, H.; Plu, I.; Chabriat, H.; Duyckaerts, C. Fatal Aβ cerebral amyloid angiopathy 4 decades after a dural graft at the age of 2 years. Acta Neuropathol. 2018, 135, 801–803. [Google Scholar] [CrossRef]
- Ritchie, D.L.; Adlard, P.; Peden, A.H.; Lowrie, S.; Le Grice, M.; Burns, K.; Jackson, R.J.; Yull, H.; Keogh, M.J.; Wei, W.; et al. Amyloid-β accumulation in the CNS in human growth hormone recipients in the UK. Acta Neuropathol. 2017, 134, 221–240. [Google Scholar] [CrossRef] [PubMed]
- Duyckaerts, C.; Sazdovitch, V.; Ando, K.; Seilhean, D.; Privat, N.; Yilmaz, Z.; Peckeu, L.; Amar, E.; Comoy, E.; Maceski, A.; et al. Neuropathology of iatrogenic Creutzfeldt-Jakob disease and immunoassay of French cadaver-sourced growth hormone batches suggest possible transmission of tauopathy and long incubation periods for the transmission of Abeta pathology. Acta Neuropathol. 2018, 135, 201–212. [Google Scholar] [CrossRef]
- Banerjee, G.; Farmer, S.F.; Hyare, H.; Jaunmuktane, Z.; Mead, S.; Ryan, N.S.; Schott, J.M.; Werring, D.J.; Rudge, P.; Collinge, J. Iatrogenic Alzheimer’s disease in recipients of cadaveric pituitary-derived growth hormone. Nat. Med. 2024, 30, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Kane, M.D.; Lipinski, W.J.; Callahan, M.J.; Bian, F.; Durham, R.A.; Schwarz, R.D.; Roher, A.E.; Walker, L.C. Evidence for seeding of beta -amyloid by intracerebral infusion of Alzheimer brain extracts in beta -amyloid precursor protein-transgenic mice. J. Neurosci. 2000, 20, 3606–3611. [Google Scholar] [CrossRef]
- Jin, M.; Shepardson, N.; Yang, T.; Chen, G.; Walsh, D.; Selkoe, D.J. Soluble amyloid beta-protein dimers isolated from Alzheimer cortex directly induce Tau hyperphosphorylation and neuritic degeneration. Proc. Natl. Acad. Sci. USA 2011, 108, 5819–5824. [Google Scholar] [CrossRef] [PubMed]
- Götz, J.; Chen, F.; van Dorpe, J.; Nitsch, R.M. Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Abeta 42 fibrils. Science 2001, 293, 1491–1495. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.; Dickson, D.W.; Lin, W.L.; Chisholm, L.; Corral, A.; Jones, G.; Yen, S.H.; Sahara, N.; Skipper, L.; Yager, D.; et al. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science 2001, 293, 1487–1491. [Google Scholar] [CrossRef] [PubMed]
- Héraud, C.; Goufak, D.; Ando, K.; Leroy, K.; Suain, V.; Yilmaz, Z.; De Decker, R.; Authelet, M.; Laporte, V.; Octave, J.N.; et al. Increased misfolding and truncation of tau in APP/PS1/tau transgenic mice compared to mutant tau mice. Neurobiol. Dis. 2014, 62, 100–112. [Google Scholar] [CrossRef]
- He, Z.; Guo, J.L.; McBride, J.D.; Narasimhan, S.; Kim, H.; Changolkar, L.; Zhang, B.; Gathagan, R.J.; Yue, C.; Dengler, C.; et al. Amyloid-β plaques enhance Alzheimer’s brain tau-seeded pathologies by facilitating neuritic plaque tau aggregation. Nat. Med. 2018, 24, 29–38. [Google Scholar] [CrossRef]
- Vergara, C.; Houben, S.; Suain, V.; Yilmaz, Z.; De Decker, R.; Vanden Dries, V.; Boom, A.; Mansour, S.; Leroy, K.; Ando, K.; et al. Amyloid-β pathology enhances pathological fibrillary tau seeding induced by Alzheimer PHF in vivo. Acta Neuropathol. 2019, 137, 397–412. [Google Scholar] [CrossRef]
- Duyckaerts, C.; Braak, H.; Brion, J.P.; Buée, L.; Del Tredici, K.; Goedert, M.; Halliday, G.; Neumann, M.; Spillantini, M.G.; Tolnay, M.; et al. PART is part of Alzheimer disease. Acta Neuropathol. 2015, 129, 749–756. [Google Scholar] [CrossRef]
- Yamada, M. Senile dementia of the neurofibrillary tangle type (tangle-only dementia): Neuropathological criteria and clinical guidelines for diagnosis. Neuropathology 2003, 23, 311–317. [Google Scholar] [CrossRef]
- Spina, S.; Schonhaut, D.R.; Boeve, B.F.; Seeley, W.W.; Ossenkoppele, R.; O’Neil, J.P.; Lazaris, A.; Rosen, H.J.; Boxer, A.L.; Perry, D.C.; et al. Frontotemporal dementia with the V337M MAPT mutation: Tau-PET and pathology correlations. Neurology 2017, 88, 758–766. [Google Scholar] [CrossRef]
- Qi, C.; Lövestam, S.; Murzin, A.G.; Peak-Chew, S.; Franco, C.; Bogdani, M.; Latimer, C.; Murrell, J.R.; Cullinane, P.W.; Jaunmuktane, Z.; et al. Tau filaments with the Alzheimer fold in human MAPT mutants V337M and R406W. Nat. Struct. Mol. Biol. 2025, 32, 1297–1304. [Google Scholar] [CrossRef]
- Sepulveda-Falla, D.; Sanchez, J.S.; Almeida, M.C.; Boassa, D.; Acosta-Uribe, J.; Vila-Castelar, C.; Ramirez-Gomez, L.; Baena, A.; Aguillon, D.; Villalba-Moreno, N.D.; et al. Distinct tau neuropathology and cellular profiles of an APOE3 Christchurch homozygote protected against autosomal dominant Alzheimer’s dementia. Acta Neuropathol. 2022, 144, 589–601. [Google Scholar] [CrossRef]
- Gibbons, G.S.; Banks, R.A.; Kim, B.; Changolkar, L.; Riddle, D.M.; Leight, S.N.; Irwin, D.J.; Trojanowski, J.Q.; Lee, V.M.Y. Detection of Alzheimer Disease (AD)-Specific Tau Pathology in AD and NonAD Tauopathies by Immunohistochemistry with Novel Conformation-Selective Tau Antibodies. J. Neuropathol. Exp. Neurol. 2018, 77, 216–228. [Google Scholar] [CrossRef]
- Kaufman, S.K.; Del Tredici, K.; Thomas, T.L.; Braak, H.; Diamond, M.I. Tau seeding activity begins in the transentorhinal/entorhinal regions and anticipates phospho-tau pathology in Alzheimer’s disease and PART. Acta Neuropathol. 2018, 136, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Murzin, A.G.; Falcon, B.; Epstein, A.; Machin, J.; Tempest, P.; Newell, K.L.; Vidal, R.; Garringer, H.J.; Sahara, N.; et al. Cryo-EM structures of tau filaments from Alzheimer’s disease with PET ligand APN-1607. Acta Neuropathol. 2021, 141, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Browne, D.F.; Smirnov, D.S.; Coughlin, D.; Peng, I.; Standke, H.G.; Kim, Y.; Pizzo, D.P.; Unapanta, A.; Andreasson, T.; Hiniker, A.; et al. Early Alzheimer’s Disease with frequent neuritic plaques harbors neocortical tau seeds distinct from primary age-related tauopathy. Nat. Commun. 2025, 16, 1851. [Google Scholar] [CrossRef]
- Masliah, E.; Mallory, M.; Deerinck, T.; DeTeresa, R.; Lamont, S.; Miller, A.; Terry, R.D.; Carragher, B.; Ellisman, M. Re-evaluation of the structural organization of neuritic plaques in Alzheimer’s disease. J. Neuropathol. Exp. Neurol. 1993, 52, 619–632. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.L.; Lee, V.M.; Trojanowski, J.Q. Comparative epitope analysis of neuronal cytoskeletal proteins in Alzheimer’s disease senile plaque neurites and neuropil threads. Lab. Investig. 1991, 64, 352–357. [Google Scholar]
- Thierry, M.; Boluda, S.; Delatour, B.; Marty, S.; Seilhean, D.; Brainbank Neuro-CEB Neuropathology Network; Potier, M.C.; Duyckaerts, C. Human subiculo-fornico-mamillary system in Alzheimer’s disease: Tau seeding by the pillar of the fornix. Acta Neuropathol. 2020, 139, 443–461. [Google Scholar] [CrossRef]
- Ando, K.; Laborde, Q.; Lazar, A.; Godefroy, D.; Youssef, I.; Amar, M.; Pooler, A.; Potier, M.C.; Delatour, B.; Duyckaerts, C. Inside Alzheimer brain with CLARITY: Senile plaques, neurofibrillary tangles and axons in 3-D. Acta Neuropathol. 2014, 128, 457–459. [Google Scholar] [CrossRef]
- Probst, A.; Anderton, B.H.; Brion, J.P.; Ulrich, J. Senile plaque neurites fail to demonstrate anti-paired helical filament and anti-microtubule-associated protein-tau immunoreactive proteins in the absence of neurofibrillary tangles in the neocortex. Acta Neuropathol. 1989, 77, 430–436. [Google Scholar] [CrossRef]
- Schmidt, M.L.; Murray, J.M.; Trojanowski, J.Q. Continuity of neuropil threads with tangle-bearing and tangle-free neurons in Alzheimer disease cortex. A confocal laser scanning microscopy study. Mol. Chem. Neuropathol. 1993, 18, 299–312. [Google Scholar] [CrossRef]
- Braak, H.; Mayer, B.; Feldengut, S.; Schön, M.; Del Tredici, K. Sequence and trajectory of early Alzheimer’s disease-related tau inclusions in the hippocampal formation of cases without amyloid-β deposits. Acta Neuropathol. 2025, 149, 50. [Google Scholar] [CrossRef]
- Senut, M.C.; Roudier, M.; Davous, P.; Fallet-Bianco, C.; Lamour, Y. Senile dementia of the Alzheimer type: Is there a correlation between entorhinal cortex and dentate gyrus lesions? Acta Neuropathol. 1991, 82, 306–315. [Google Scholar] [CrossRef]
- Yilmazer-Hanke, D.M.; Hanke, J. Progression of Alzheimer-related neuritic plaque pathology in the entorhinal region, perirhinal cortex and hippocampal formation. Dement. Geriatr. Cogn. Disord. 1999, 10, 70–76. [Google Scholar] [CrossRef]
- Liu, A.K.; Chang, R.C.; Pearce, R.K.; Gentleman, S.M. Nucleus basalis of Meynert revisited: Anatomy, history and differential involvement in Alzheimer’s and Parkinson’s disease. Acta Neuropathol. 2015, 129, 527–540. [Google Scholar] [CrossRef]
- Kovacs, G.G.; Katsumata, Y.; Wu, X.; Aung, K.Z.; Fardo, D.W.; Forrest, S.L.; Alzheimer’s Disease Genetics Consortium; Nelson, P.T. Amyloid-β predominant Alzheimer’s disease neuropathologic change. Brain 2025, 148, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Duyckaerts, C.; Christen, Y. Connections, Cognition and Alzheimer diseaseons, Cognition and Alzheimer disease. In Research and Perspective in Alzheimer’s Disease; Springer: Berlin/Heidelberg, Germany, 1997. [Google Scholar]
- Duyckaerts, C.; Delatour, B.; Potier, M.C. Classification and basic pathology of Alzheimer disease. Acta Neuropathol. 2009, 118, 5–36. [Google Scholar] [CrossRef] [PubMed]
- Hyman, B.T.; Van Hoesen, G.W.; Damasio, A.R.; Barnes, C.L. Alzheimer’s disease: Cell-specific pathology isolates the hippocampal formation. Science 1984, 225, 1168–1170. [Google Scholar] [CrossRef]
- Hornberger, M.; Wong, S.; Tan, R.; Irish, M.; Piguet, O.; Kril, J.; Hodges, J.R.; Halliday, G. In vivo and post-mortem memory circuit integrity in frontotemporal dementia and Alzheimer’s disease. Brain 2012, 135, 3015–3025. [Google Scholar] [CrossRef] [PubMed]
- Ince, P.G.; Lowe, J.; Shaw, P.J. Amyotrophic lateral sclerosis: Current issues in classification, pathogenesis and molecular pathology. Neuropathol. Appl. Neurobiol. 1998, 24, 104–117. [Google Scholar] [CrossRef]
- Riku, Y.; Watanabe, H.; Yoshida, M.; Tatsumi, S.; Mimuro, M.; Iwasaki, Y.; Katsuno, M.; Iguchi, Y.; Masuda, M.; Senda, J.; et al. Lower motor neuron involvement in TAR DNA-binding protein of 43 kDa-related frontotemporal lobar degeneration and amyotrophic lateral sclerosis. JAMA Neurol. 2014, 71, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Seeley, W.W. The Salience Network: A Neural System for Perceiving and Responding to Homeostatic Demands. J. Neurosci. 2019, 39, 9878–9882. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Nakatani, H.; Ueno, K.; Asamizuya, T.; Cheng, K.; Tanaka, K. The neural basis of intuitive best next-move generation in board game experts. Science 2011, 331, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Riku, Y.; Watanabe, H.; Yoshida, M.; Mimuro, M.; Iwasaki, Y.; Masuda, M.; Ishigaki, S.; Katsuno, M.; Sobue, G. Marked Involvement of the Striatal Efferent System in TAR DNA-Binding Protein 43 kDa-Related Frontotemporal Lobar Degeneration and Amyotrophic Lateral Sclerosis. J. Neuropathol. Exp. Neurol. 2016, 75, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Riku, Y.; Watanabe, H.; Yoshida, M.; Mimuro, M.; Iwasaki, Y.; Masuda, M.; Ishigaki, S.; Katsuno, M.; Sobue, G. Pathologic Involvement of Glutamatergic Striatal Inputs from the Cortices in TAR DNA-Binding Protein 43 kDa-Related Frontotemporal Lobar Degeneration and Amyotrophic Lateral Sclerosis. J. Neuropathol. Exp. Neurol. 2017, 76, 759–768. [Google Scholar] [CrossRef]
- Iwabuchi, K.; Koyano, S.; Yagishita, S. Simple and clear differentiation of spinocerebellar degenerations: Overview of macroscopic and low-power view findings. Neuropathology 2022, 42, 379–393. [Google Scholar] [CrossRef]
- Plowey, E.D.; Ziskin, J.L. Hippocampal phospho-tau/MAPT neuropathology in the fornix in Alzheimer disease: An immunohistochemical autopsy study. Acta Neuropathol. Commun. 2016, 4, 114. [Google Scholar] [CrossRef]
- Takeda, T.; Uchihara, T.; Arai, N.; Mizutani, T.; Iwata, M. Progression of hippocampal degeneration in amyotrophic lateral sclerosis with or without memory impairment: Distinction from Alzheimer disease. Acta Neuropathol. 2009, 117, 35–44. [Google Scholar] [CrossRef]
- Hof, P.R.; Morrison, J.H. The cellular basis of cortical disconnection in Alzheimer disease and related dementing conditions. In Alzheimer Disease, 2nd ed.; Terry, R.D., Katzman, R., Bick, K.L., Sisodia, S.S., Eds.; Lipincott Williams & Wilkins: Philadelphia, PA, USA, 1999; pp. 210–214. [Google Scholar]
- Duyckaerts, C.; Uchihara, T.; Seilhean, D.; He, Y.; Hauw, J.J. Dissociation of Alzheimer type pathology in a disconnected piece of cortex. Acta Neuropathol. 1997, 93, 501–507. [Google Scholar] [CrossRef]
- Mudher, A.; Colin, M.; Dujardin, S.; Medina, M.; Dewachter, I.; Alavi Naini, S.M.; Mandelkow, E.M.; Mandelkow, E.; Buée, L.; Goedert, M.; et al. What is the evidence that tau pathology spreads through prion-like propagation? Acta Neuropathol. Commun. 2017, 5, 99. [Google Scholar] [CrossRef]
- Miyoshi, E.; Bilousova, T.; Melnik, M.; Fakhrutdinov, D.; Poon, W.W.; Vinters, H.V.; Miller, C.A.; Corrada, M.; Kawas, C.; Bohannan, R.; et al. Exosomal tau with seeding activity is released from Alzheimer’s disease synapses, and seeding potential is associated with amyloid beta. Lab. Investig. 2021, 101, 1605–1617. [Google Scholar] [CrossRef]
- Takeda, S.; Wegmann, S.; Cho, H.; DeVos, S.L.; Commins, C.; Roe, A.D.; Nicholls, S.B.; Carlson, G.A.; Pitstick, R.; Nobuhara, C.K.; et al. Neuronal uptake and propagation of a rare phosphorylated high-molecular-weight tau derived from Alzheimer’s disease brain. Nat. Commun. 2015, 6, 8490. [Google Scholar] [CrossRef]
- Boluda, S.; Iba, M.; Zhang, B.; Raible, K.M.; Lee, V.M.; Trojanowski, J.Q. Differential induction and spread of tau pathology in young PS19 tau transgenic mice following intracerebral injections of pathological tau from Alzheimer’s disease or corticobasal degeneration brains. Acta Neuropathol. 2015, 129, 221–237. [Google Scholar] [CrossRef]
- Clavaguera, F.; Bolmont, T.; Crowther, R.A.; Abramowski, D.; Frank, S.; Probst, A.; Fraser, G.; Stalder, A.K.; Beibel, M.; Staufenbiel, M.; et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat. Cell Biol. 2009, 11, 909–913. [Google Scholar] [CrossRef]
- Audouard, E.; Houben, S.; Masaracchia, C.; Yilmaz, Z.; Suain, V.; Authelet, M.; De Decker, R.; Buée, L.; Boom, A.; Leroy, K.; et al. High-Molecular-Weight Paired Helical Filaments from Alzheimer Brain Induces Seeding of Wild-Type Mouse Tau into an Argyrophilic 4R Tau Pathology in Vivo. Am. J. Pathol. 2016, 186, 2709–2722. [Google Scholar] [CrossRef]
- McGeachan, R.I.; Keavey, L.; Simzer, E.M.; Chang, Y.Y.; Rose, J.L.; Spires-Jones, M.P.; Gilmore, M.; Holt, K.; Meftah, S.; Ravingerova, N.; et al. Evidence for trans-synaptic propagation of oligomeric tau in human progressive supranuclear palsy. Nat. Neurosci. 2025, 28, 1622–1634. [Google Scholar] [CrossRef]
- Kar, A.; Havlioglu, N.; Tarn, W.Y.; Wu, J.Y. RBM4 interacts with an intronic element and stimulates tau exon 10 inclusion. J. Biol. Chem. 2006, 281, 24479–24488. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, L.; Tse, S.W.; Andreadis, A. Heterogeneous nuclear ribonucleoprotein E3 modestly activates splicing of tau exon 10 via its proximal downstream intron, a hotspot for frontotemporal dementia mutations. Gene 2010, 451, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Storbeck, M.; Hupperich, K.; Gaspar, J.A.; Meganathan, K.; Martínez Carrera, L.; Wirth, R.; Sachinidis, A.; Wirth, B. Neuronal-specific deficiency of the splicing factor Tra2b causes apoptosis in neurogenic areas of the developing mouse brain. PLoS ONE 2014, 9, e89020. [Google Scholar] [CrossRef] [PubMed]
- Riku, Y.; Iwasaki, Y.; Ishigaki, S.; Akagi, A.; Hasegawa, M.; Nishioka, K.; Li, Y.; Riku, M.; Ikeuchi, T.; Fujioka, Y.; et al. Motor neuron TDP-43 proteinopathy in progressive supranuclear palsy and corticobasal degeneration. Brain 2022, 145, 2769–2784. [Google Scholar] [CrossRef] [PubMed]
- Ishigaki, S.; Fujioka, Y.; Okada, Y.; Riku, Y.; Udagawa, T.; Honda, D.; Yokoi, S.; Endo, K.; Ikenaka, K.; Takagi, S.; et al. Altered Tau Isoform Ratio Caused by Loss of FUS and SFPQ Function Leads to FTLD-like Phenotypes. Cell Rep. 2017, 18, 1118–1131. [Google Scholar] [CrossRef]
- Hyman, B.T.; Augustinack, J.C.; Ingelsson, M. Transcriptional and conformational changes of the tau molecule in Alzheimer’s disease. Biochim. Biophys. Acta 2005, 1739, 150–157. [Google Scholar] [CrossRef]
- Ginsberg, S.D.; Che, S.; Counts, S.E.; Mufson, E.J. Shift in the ratio of three-repeat tau and four-repeat tau mRNAs in individual cholinergic basal forebrain neurons in mild cognitive impairment and Alzheimer’s disease. J. Neurochem. 2006, 96, 1401–1408. [Google Scholar] [CrossRef]
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef]
- Li, L.L.; Wang, R.Z.; Wang, Z.; Hu, H.; Xu, W.; Zhu, L.; Sun, Y.; Chen, K.L.; Chen, S.F.; He, X.Y.; et al. Safety and short-term outcomes of lecanemab for Alzheimer’s disease in China: A multicentre study. Brain 2025, epub ahead print. [Google Scholar] [CrossRef] [PubMed]
- McDade, E.; Cummings, J.L.; Dhadda, S.; Swanson, C.J.; Reyderman, L.; Kanekiyo, M.; Koyama, A.; Irizarry, M.; Kramer, L.D.; Bateman, R.J. Lecanemab in patients with early Alzheimer’s disease: Detailed results on biomarker, cognitive, and clinical effects from the randomized and open-label extension of the phase 2 proof-of-concept study. Alzheimers Res. Ther. 2022, 14, 191. [Google Scholar] [CrossRef]
- Chen, S.; Ou, R.; Wei, Q.; Li, C.; Song, W.; Zhao, B.; Yang, J.; Fu, J.; Ma, Y.; Liu, J.; et al. Lecanemab treatment for Alzheimer’s Disease of varying severities and associated plasma biomarkers monitoring: A multi-center real-world study in China. Alzheimers Dement. 2025, 21, e70750. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Fan, S.; Zhao, H.; Sun, C.; Liu, L.; Wang, X.; Zou, L. Combined 64-channel EEG and PET evaluation of lecanemab efficacy: Two case reports. J. Alzheimers Dis. Rep. 2025, 9, 25424823251395615. [Google Scholar] [CrossRef] [PubMed]
- Angioni, D.; Middleton, L.; Bateman, R.; Aisen, P.; Boxer, A.; Sha, S.; Zhou, J.; Gerlach, I.; Raman, R.; Fillit, H.; et al. Challenges and opportunities for novel combination therapies in Alzheimer’s disease: A report from the EU/US CTAD Task Force. J. Prev. Alzheimers Dis. 2025, 12, 100163. [Google Scholar] [CrossRef] [PubMed]
- Fischer, D.L.; Grinberg, L.T.; Ahrendsen, J.T.; Beach, T.G.; Bieniek, K.F.; Castellani, R.J.; Chkheidze, R.; Cobos, I.; Cohen, M.; Crary, J.F.; et al. Celebrating neuropathology’s contributions to Alzheimer’s Disease Research Centers. Alzheimers Dement. 2025, 21, e70734. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riku, Y.; Brion, J.-P.; Ando, K.; Uchihara, T.; Iwasaki, Y. The Determinant of Tau Spreading in Alzheimer’s Disease: Dependent on Senile Plaque, Neural Circuits, or Spatial Proximity? Int. J. Mol. Sci. 2025, 26, 12088. https://doi.org/10.3390/ijms262412088
Riku Y, Brion J-P, Ando K, Uchihara T, Iwasaki Y. The Determinant of Tau Spreading in Alzheimer’s Disease: Dependent on Senile Plaque, Neural Circuits, or Spatial Proximity? International Journal of Molecular Sciences. 2025; 26(24):12088. https://doi.org/10.3390/ijms262412088
Chicago/Turabian StyleRiku, Yuichi, Jean-Pierre Brion, Kunie Ando, Toshiki Uchihara, and Yasushi Iwasaki. 2025. "The Determinant of Tau Spreading in Alzheimer’s Disease: Dependent on Senile Plaque, Neural Circuits, or Spatial Proximity?" International Journal of Molecular Sciences 26, no. 24: 12088. https://doi.org/10.3390/ijms262412088
APA StyleRiku, Y., Brion, J.-P., Ando, K., Uchihara, T., & Iwasaki, Y. (2025). The Determinant of Tau Spreading in Alzheimer’s Disease: Dependent on Senile Plaque, Neural Circuits, or Spatial Proximity? International Journal of Molecular Sciences, 26(24), 12088. https://doi.org/10.3390/ijms262412088





