Ferroptosis Inhibition Enhances Osteoblast Activity: The Role of Liproxstatin-1 and Coenzyme Q10
Abstract
1. Introduction
2. Results
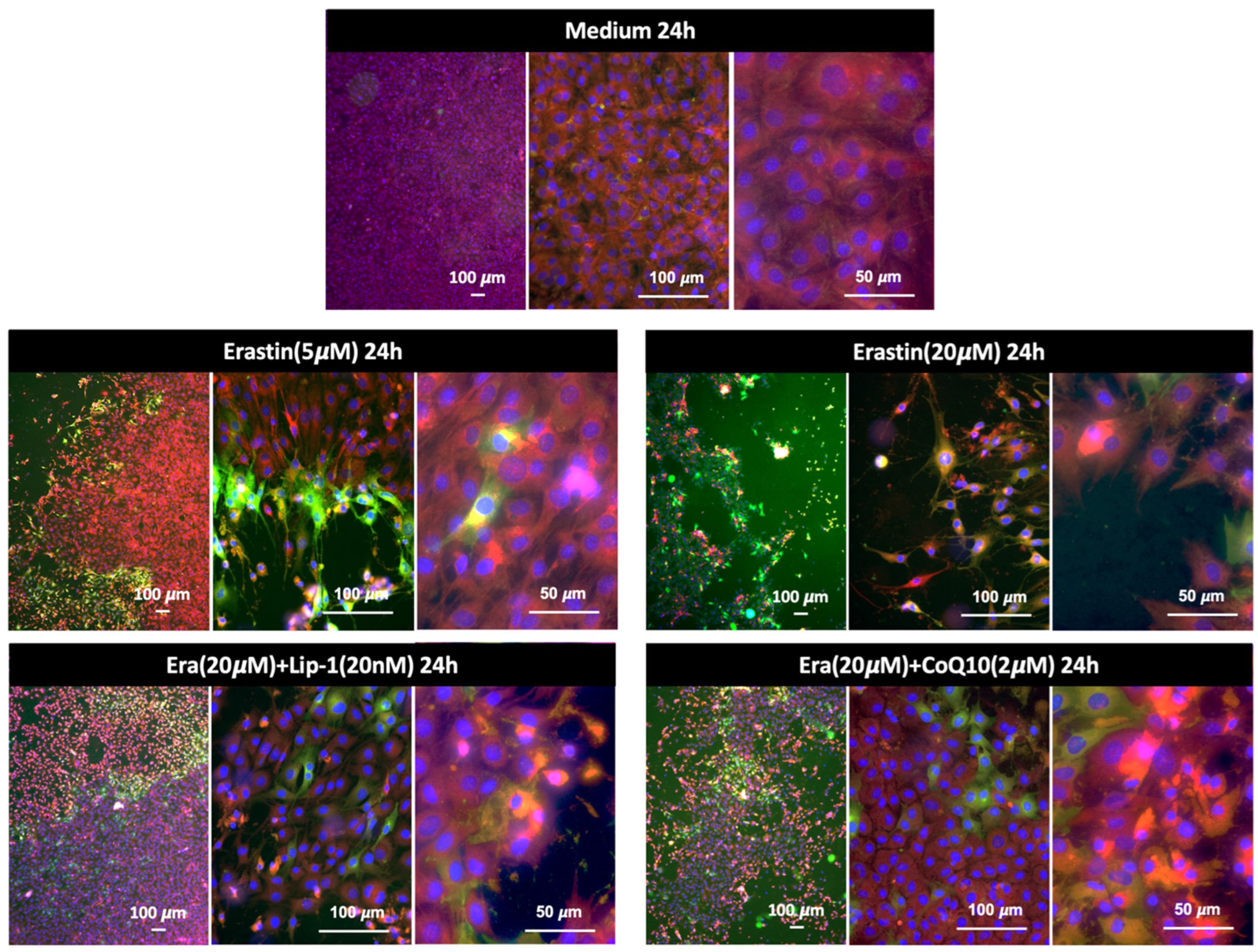
2.1. Erastin Induced Cell Death
2.2. Optimum Lip-1 and CoQ10 Concentration
2.3. Lip-1 and CoQ10 and Ferroptosis Inhibition
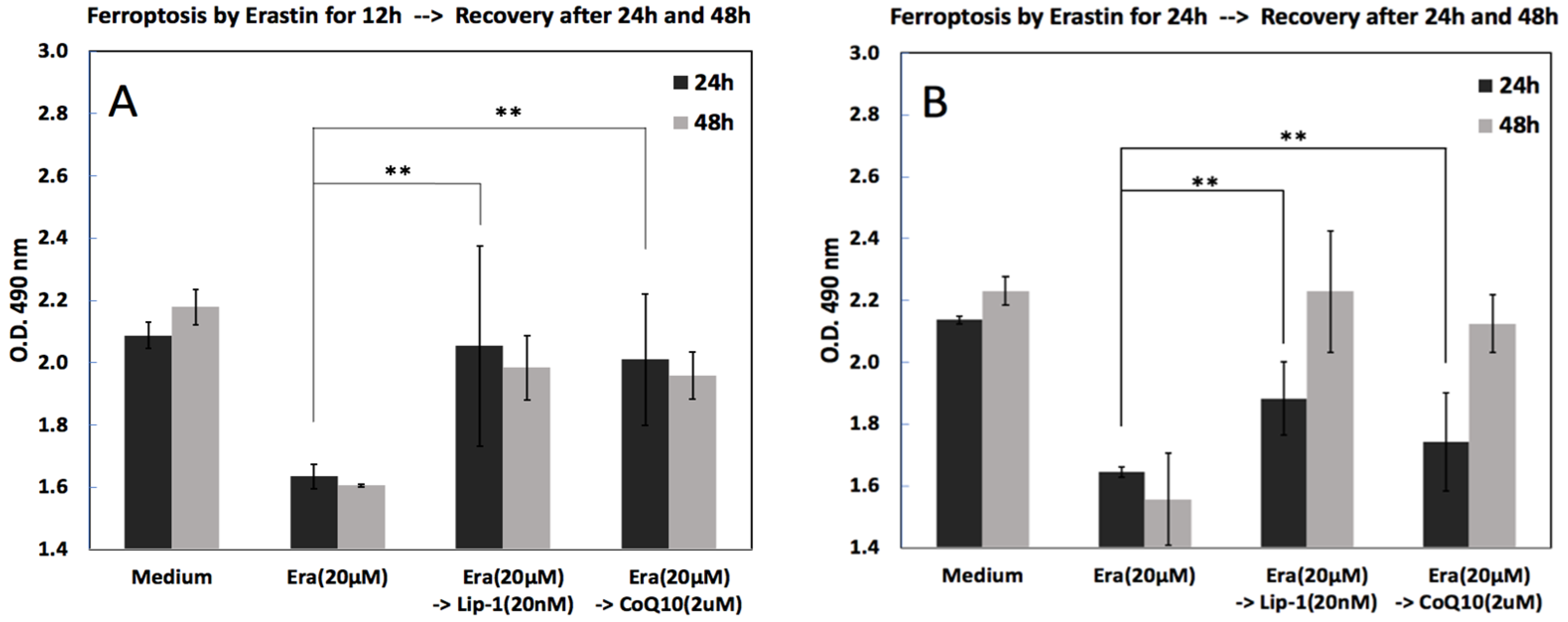
2.4. Cell Viability, Ferroptosis and Recovery
2.5. BODIPY™ 581/591 C11 and Hoechst®33342 Staining
2.5.1. Ferroptosis or Lipid Peroxidation Imaging
2.5.2. Quantitative Lipid Peroxidation
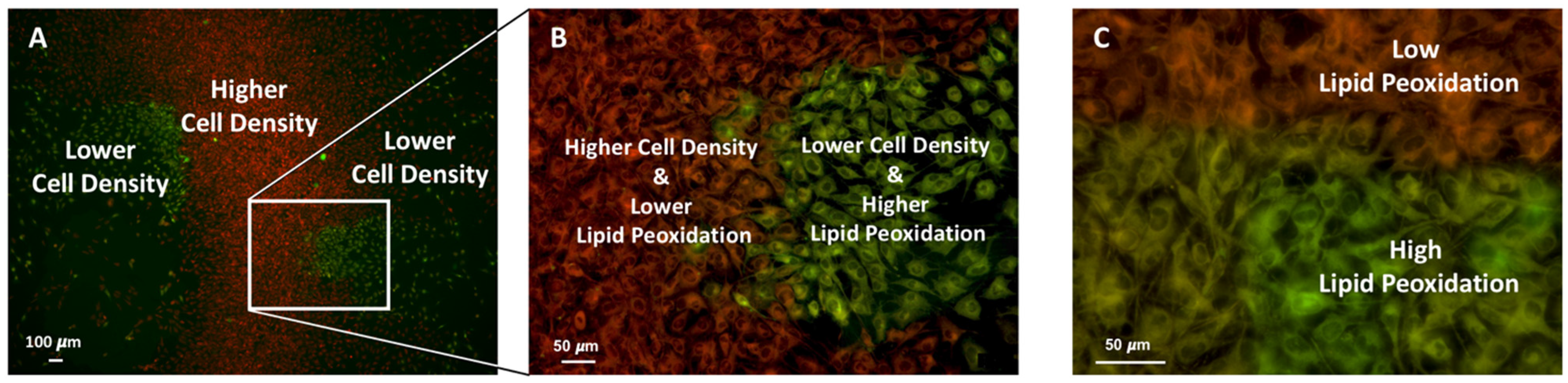
2.5.3. Ferroptosis or Lipid Peroxidation and Cell Density
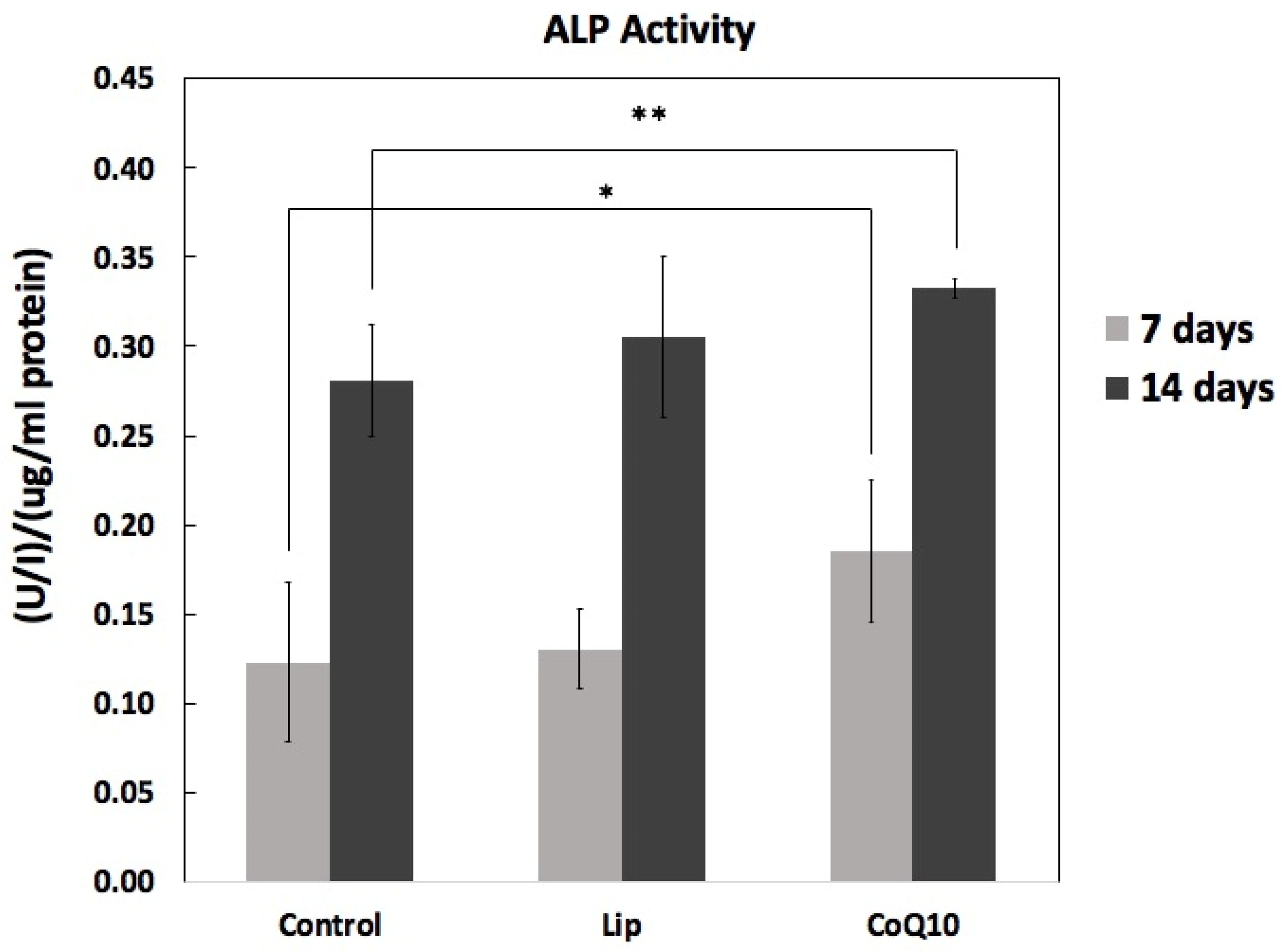
2.6. Differentiation: ALP Activity
3. Discussion
3.1. Cell Density–Dependent Modulation of Ferroptosis Sensitivity
3.2. Ferroptosis Inhibition and Recovery of Osteoblast Function
3.3. Implications for Osteogenic Differentiation
4. Materials and Methods
4.1. Materials
4.2. Cell Viability
4.2.1. Erastin Induced Cell Death
4.2.2. Optimum Lip-1 and CoQ10 Concentration
4.2.3. Lip-1 and CoQ10 and Ferroptosis Inhibition
4.2.4. Cell Viability, Ferroptosis and Recovery
4.3. BODIPY™ 581/591 C11: Ferroptosis or Lipid Peroxidation Imaging
4.4. Hoechst®33342: Live-Cell Imaging
4.5. Quantitative Lipid Peroxidation
4.6. Differentiation: ALP Activity
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McAllister, B.S.; Haghighat, K. Bone Augmentation Techniques. J. Periodontol. 2007, 78, 377–396. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Jiang, X.; Gu, W. Emerging Mechanisms and Disease Relevance of Ferroptosis. Trends Cell Biol. 2020, 30, 478–490. [Google Scholar] [CrossRef]
- Bertheloot, D.; Latz, E.; Franklin, B.S. Necroptosis, Pyroptosis and Apoptosis: An Intricate Game of Cell Death. Cell. Mol. Immunol. 2021, 18, 1106–1121. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.C.; Cullen, S.P.; Martin, S.J. Apoptosis: Controlled Demolition at the Cellular Level. Nat. Rev. Mol. Cell Biol. 2008, 9, 231–241. [Google Scholar] [CrossRef]
- Lee, E.; Song, C.H.; Bae, S.J.; Ha, K.T.; Karki, R. Regulated Cell Death Pathways and Their Roles in Homeostasis, Infection, Inflammation, and Tumorigenesis. Exp. Mol. Med. 2023, 55, 1632–1643. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Eleina, M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death Scott. NIH Public Access 2013, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Bayır, H.; Anthonymuthu, T.S.; Tyurina, Y.Y.; Patel, S.J.; Amoscato, A.A.; Lamade, A.M.; Yang, Q.; Vladimirov, G.K.; Philpott, C.C.; Kagan, V.E. Achieving Life through Death: Redox Biology of Lipid Peroxidation in Ferroptosis. Cell Chem. Biol. 2020, 27, 387–408. [Google Scholar] [CrossRef]
- Bartos, A.; Sikora, J.; Aldrovandi, M.; Bartos, A.; Sikora, J. Bioinorganic Modulators of Ferroptosis: A Review of Recent Findings. Int. J. Mol. Sci. 2023, 24, 3634. [Google Scholar] [CrossRef]
- Fleming, R.E.; Ponka, P. Iron Overload in Human Disease. N. Engl. J. Med. 2012, 366, 348–359, Erratum in N. Engl. J. Med. 2012, 366, 771. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Y.; Ye, T.; Yang, L.; Shen, Y.; Li, H. Ferroptosis Signaling and Regulators in Atherosclerosis. Front. Cell Dev. Biol. 2021, 9, 809457. [Google Scholar] [CrossRef]
- Meynard, D.; Babitt, J.L.; Lin, H.Y. The Liver: Conductor of Systemic Iron Balance. Blood 2014, 123, 168–176. [Google Scholar] [CrossRef]
- Battaglia, A.M.; Chirillo, R.; Aversa, I.; Sacco, A.; Costanzo, F.; Biamonte, F. Ferroptosis and Cancer: Mitochondria Meet the “Iron Maiden” Cell Death. Cells 2020, 9, 1505. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; An, P.; Xie, E.; Wu, Q.; Fang, X.; Gao, H.; Zhang, Z.; Li, Y.; Wang, X.; Zhang, J.; et al. Characterization of Ferroptosis in Murine Models of Hemochromatosis. Hepatology 2017, 66, 449–465. [Google Scholar] [CrossRef]
- Tian, Q.; Qin, B.; Gu, Y.; Zhou, L.; Chen, S.; Zhang, S.; Zhang, S.; Han, Q.; Liu, Y.; Wu, X. ROS-Mediated Necroptosis Is Involved in Iron Overload-Induced Osteoblastic Cell Death. Oxidative Med. Cell. Longev. 2020, 2020, 1295382. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, H.; Qi, G.; Jiang, C.; Chen, K.; Yan, Z. Iron Overload-Induced Ferroptosis of Osteoblasts Inhibits Osteogenesis and Promotes Osteoporosis: An in Vitro and in Vivo Study. IUBMB Life 2022, 74, 1052–1069. [Google Scholar] [CrossRef]
- Jing, X.; Du, T.; Chen, K.; Guo, J.; Xiang, W.; Yao, X.; Sun, K.; Ye, Y.; Guo, F. Icariin Protects against Iron Overload-Induced Bone Loss via Suppressing Oxidative Stress. J. Cell. Physiol. 2019, 234, 10123–10137. [Google Scholar] [CrossRef] [PubMed]
- Jeney, V. Clinical Impact and Cellular Mechanisms of Iron Overload-Associated Bone Loss. Front. Pharmacol. 2017, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Ru, Q.; Li, Y.; Xie, W.; Ding, Y.; Chen, L.; Xu, G.; Wu, Y.; Wang, F. Fighting Age-Related Orthopedic Diseases: Focusing on Ferroptosis. Bone Res. 2023, 11, 12. [Google Scholar] [CrossRef]
- Wang, D.; Shen, J.; Wang, Y.; Cui, H.; Li, Y.; Zhou, L.; Li, G.; Wang, Q.; Feng, X.; Qin, M.; et al. Mechanisms of Ferroptosis in Bone Disease: A New Target for Osteoporosis Treatment. Cell. Signal. 2025, 127, 111598. [Google Scholar] [CrossRef]
- Valanezhad, A.; Odatsu, T.; Abe, S.; Watanabe, I. Bone Formation Ability and Cell Viability Enhancement of Mc3t3-E1 Cells by Ferrostatin-1 a Ferroptosis Inhibitor of Cancer Cells. Int. J. Mol. Sci. 2021, 22, 12259. [Google Scholar] [CrossRef]
- Xu, Y.; Sang, W.; Zhong, Y.; Xue, S.; Yang, M.; Wang, C.; Lu, H.; Huan, R.; Mao, X.; Zhu, L.; et al. CoCrMo-Nanoparticles Induced Peri-Implant Osteolysis by Promoting Osteoblast Ferroptosis via Regulating Nrf2-ARE Signalling Pathway. Cell Prolif. 2021, 54, e13142. [Google Scholar] [CrossRef]
- Xiao, W.; Beibei, F.; Guangsi, S.; Yu, J.; Wen, Z.; Xi, H.; Youjia, X. Iron Overload Increases Osteoclastogenesis and Aggravates the Effects of Ovariectomy on Bone Mass. J. Endocrinol. 2015, 226, 121–134. [Google Scholar] [CrossRef]
- Zilka, O.; Shah, R.; Li, B.; Friedmann Angeli, J.P.; Griesser, M.; Conrad, M.; Pratt, D.A. On the Mechanism of Cytoprotection by Ferrostatin-1 and Liproxstatin-1 and the Role of Lipid Peroxidation in Ferroptotic Cell Death. ACS Cent. Sci. 2017, 3, 232–243. [Google Scholar] [CrossRef]
- Hadian, K. Ferroptosis Suppressor Protein 1 (FSP1) and Coenzyme Q10 Cooperatively Suppress Ferroptosis. Biochemistry 2020, 59, 637–638. [Google Scholar] [CrossRef]
- Deshwal, S.; Onishi, M.; Tatsuta, T.; Bartsch, T.; Cors, E.; Ried, K.; Lemke, K.; Nolte, H.; Giavalisco, P.; Langer, T. Mitochondria Regulate Intracellular Coenzyme Q Transport and Ferroptotic Resistance via STARD7. Nat. Cell Biol. 2023, 25, 246–257. [Google Scholar] [CrossRef]
- Fikry, H.; Saleh, L.A.; Mahmoud, F.A.; Gawad, S.A.; Abd-Alkhalek, H.A. CoQ10 Targeted Hippocampal Ferroptosis in a Status Epilepticus Rat Model. Cell Tissue Res. 2024, 396, 371–397. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Cui, C.; Yu, M.; Li, X.; Wang, L.; Chen, X.; Lin, Y. Coenzyme Q10 Promotes Osteoblast Proliferation and Differentiation and Protects against Ovariectomy-Induced Osteoporosis. Mol. Med. Rep. 2018, 17, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liang, S.; Zhu, X.; Wu, X.; Dong, Z. CoQ10 Suppression of Oxidative Stress and Cell Senescence Increases Bone Mass in Orchiectomized Mice. Am. J. Transl. Res. 2020, 12, 4314. [Google Scholar] [PubMed]
- Naguib, Y.M.A. A Fluorometric Method for Measurement of Peroxyl Radical Scavenging Activities of Lipophilic Antioxidants. Anal. Biochem. 1998, 265, 290–298. [Google Scholar] [CrossRef]
- Drummen, G.P.C.; Van Liebergen, L.C.M.; Op den Kamp, J.A.F.; Post, J.A. C11-BODIPY581/591, an Oxidation-Sensitive Fluorescent Lipid Peroxidation Probe: (Micro)Spectroscopic Characterization and Validation of Methodology. Free Radic. Biol. Med. 2002, 33, 473–490. [Google Scholar] [CrossRef]
- Drummen, G.P.C.; Gadella, B.M.; Post, J.A.; Brouwers, J.F. Mass Spectrometric Characterization of the Oxidation of the Fluorescent Lipid Peroxidation Reporter Molecule C11-BODIPY581/591. Free Radic. Biol. Med. 2004, 36, 1635–1644. [Google Scholar] [CrossRef]
- MacDonald, M.L.; Murray, I.V.J.; Axelsen, P.H. Mass Spectrometric Analysis Demonstrates That BODIPY 581/591 C11 Overestimates and Inhibits Oxidative Lipid Damage. Free Radic. Biol. Med. 2007, 42, 1392–1397. [Google Scholar] [CrossRef]
- Panzilius, E.; Holstein, F.; Dehairs, J.; Planque, M.; von Toerne, C.; Koenig, A.-C.; Doll, S.; Bannier-Hélaouët, M.; Ganz, H.M.; Hauck, S.M.; et al. Cell Density-Dependent Ferroptosis in Breast Cancer Is Induced by Accumulation of Polyunsaturated Fatty Acid-Enriched Triacylglycerides. bioRxiv 2019. bioRxiv:417949. [Google Scholar] [CrossRef]
- Tang, D.; Chen, X.; Kang, R.; Kroemer, G. Ferroptosis: Molecular Mechanisms and Health Implications. Cell Res. 2021, 31, 107–125. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.-f.; Zou, T.; Tuo, Q.-z.; Xu, S.; Li, H.; Belaidi, A.A.; Lei, P. Ferroptosis: Mechanisms and Links with Diseases. Signal Transduct. Target. Ther. 2021, 6, 49. [Google Scholar] [CrossRef] [PubMed]
- Pap, E.H.W.; Drummen, G.P.C.; Winter, V.J.; Kooij, T.W.A.; Rijken, P.; Wirtz, K.W.A.; Op Den Kamp, J.A.F.; Hage, W.J.; Post, J.A. Ratio-Fluorescence Microscopy of Lipid Oxidation in Living Cells Using C11-BODIPY581/591. FEBS Lett. 1999, 453, 278–282. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valanezhad, A.; Odatsu, T.; Valanezhad, F.; Abe, S.; Watanabe, I. Ferroptosis Inhibition Enhances Osteoblast Activity: The Role of Liproxstatin-1 and Coenzyme Q10. Int. J. Mol. Sci. 2025, 26, 12059. https://doi.org/10.3390/ijms262412059
Valanezhad A, Odatsu T, Valanezhad F, Abe S, Watanabe I. Ferroptosis Inhibition Enhances Osteoblast Activity: The Role of Liproxstatin-1 and Coenzyme Q10. International Journal of Molecular Sciences. 2025; 26(24):12059. https://doi.org/10.3390/ijms262412059
Chicago/Turabian StyleValanezhad, Alireza, Tetsurou Odatsu, Farzaneh Valanezhad, Shigeaki Abe, and Ikuya Watanabe. 2025. "Ferroptosis Inhibition Enhances Osteoblast Activity: The Role of Liproxstatin-1 and Coenzyme Q10" International Journal of Molecular Sciences 26, no. 24: 12059. https://doi.org/10.3390/ijms262412059
APA StyleValanezhad, A., Odatsu, T., Valanezhad, F., Abe, S., & Watanabe, I. (2025). Ferroptosis Inhibition Enhances Osteoblast Activity: The Role of Liproxstatin-1 and Coenzyme Q10. International Journal of Molecular Sciences, 26(24), 12059. https://doi.org/10.3390/ijms262412059





