Exploring the Mechanism of 2,4-Dichlorophenoxyacetic Acid in Causing Neurodegenerative Diseases Based on Network Toxicology and Molecular Docking
Abstract
1. Introduction
2. Results
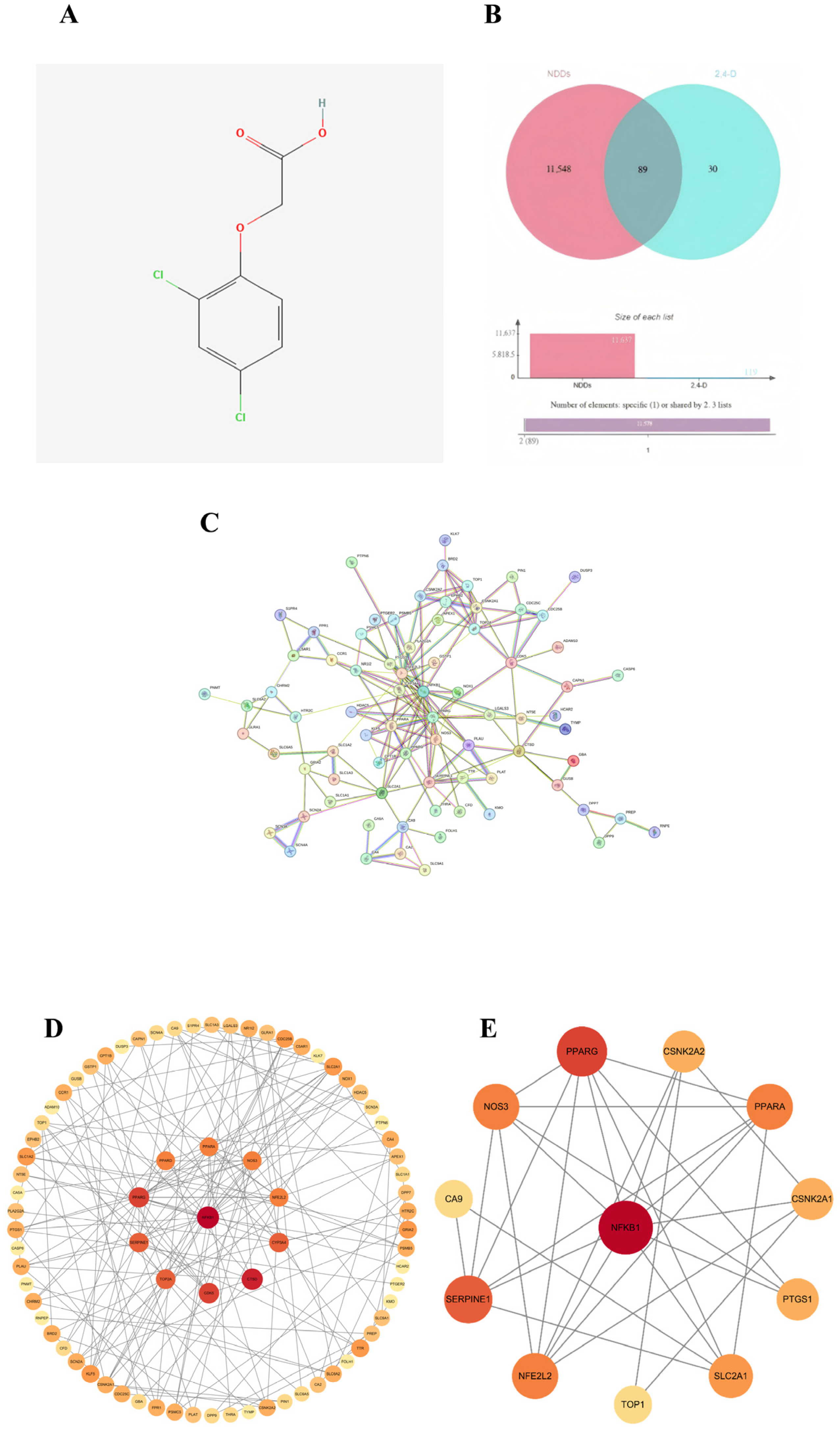
2.1. Screening of Common Targets
2.2. Construction of PPI Network and Identification of Core Targets
2.3. Gene Ontology (GO) Enrichment Analysis
2.4. KEGG Pathway Enrichment Analysis
2.5. Molecular Docking of 2,4-D with Core Targets of Neurodegenerative Diseases
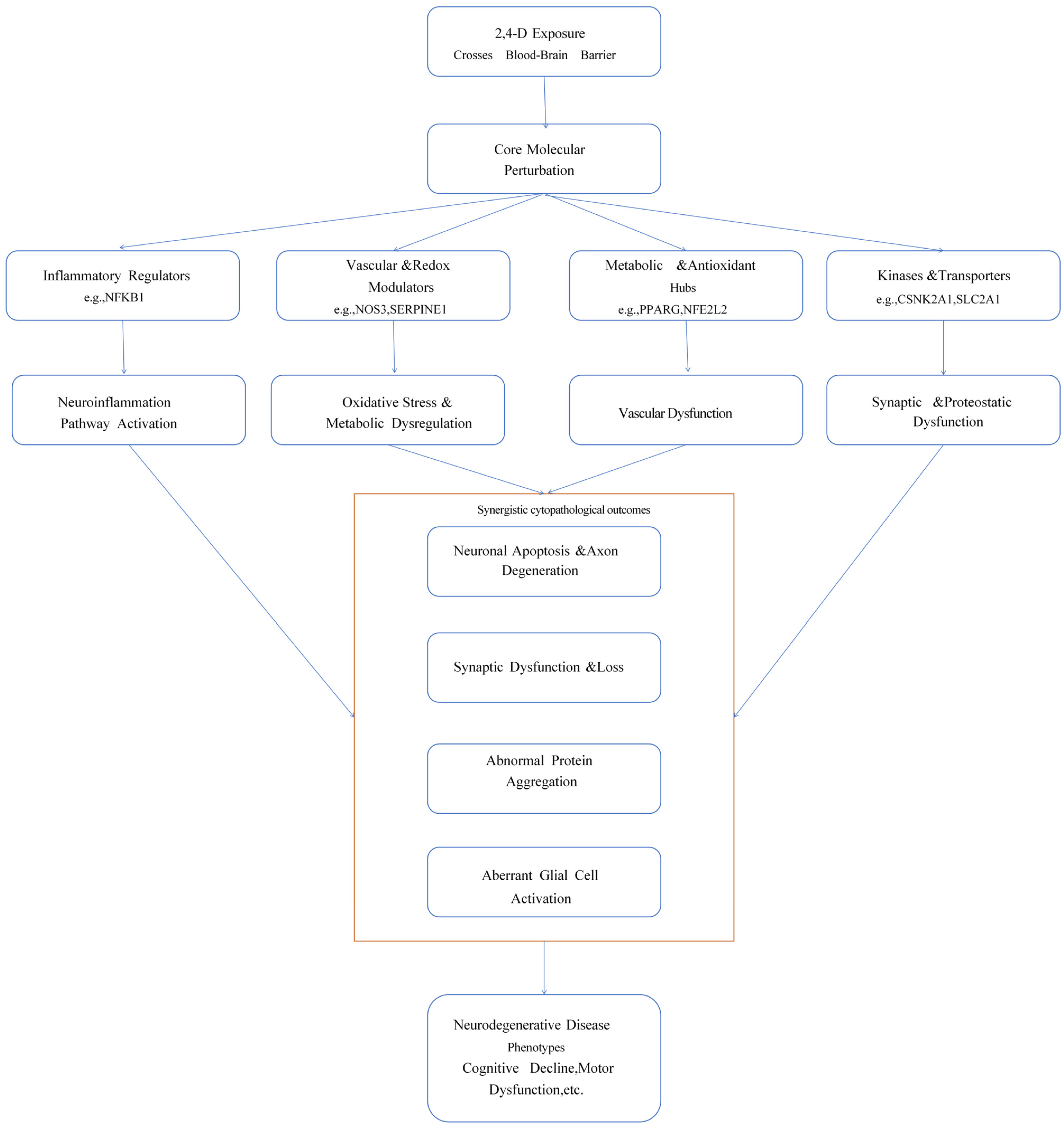
3. Discussion
Limitations and Future Perspectives
4. Materials and Methods
4.1. Identification of Potential Targets for 2,4-D
4.2. Screening of Neurodegenerative Disease-Associated Targets
4.3. Intersection of Toxicological Targets and Disease Targets
4.4. Protein–Protein Interaction (PPI) Network Construction
4.5. GO and KEGG Pathway Enrichment Analysis
4.6. Molecular Docking
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boon, D.; Burns, C.J. Biomonitoring of 2,4-dichlorophenoxyacetic acid (2,4-D) herbicide: A global view. Regul. Toxicol. Pharmacol. 2024, 152, 105687. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.P.; Morais, E.R.; Oliveira, E.C.; Ghisi, N.C. Does exposure to environmental 2,4-dichlorophenoxyacetic acid concentrations increase mortality rate in animals? A meta-analytic review. Environ. Pollut. 2022, 303, 119179. [Google Scholar] [CrossRef] [PubMed]
- Venners, S.A.; Khoshnood, N.; Jeronimo, M.; Sobkowicz, A.; Provencher, P.; Tang, G.; Chu, W.; Copes, R. Adult and child urinary 2,4-D in cities with and without cosmetic pesticide bylaws: A population-based cross-sectional pilot study. J. Expo. Sci. Environ. Epidemiol. 2017, 27, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, H.; Wang, F.; Wang, H.; Chai, R.; Li, J.; Jia, L.; Wang, K.; Zhang, P.; Zhu, L.; et al. Effects of 2,4-dichlorophenoxyacetic acid on the expression of NLRP3 inflammasome and autophagy-related proteins as well as the protective effect of Lycium barbarum polysaccharide in neonatal rats. Environ. Toxicol. 2021, 36, 2454–2466. [Google Scholar] [CrossRef]
- Zou, X.; Shi, Y.; Su, J.; Ye, Q.; Lin, F.; Cai, G. Association between 2,4-dichlorophenoxyacetic acid and cognitive impairment in older adults: A cross-sectional study from NHANES 2001-2002 and 2011-2014. J. Expo. Sci. Environ. Epidemiol. 2024, 34, 308–316. [Google Scholar] [CrossRef]
- Agnello, L.; Ciaccio, M. Neurodegenerative Diseases: From Molecular Basis to Therapy. Int. J. Mol. Sci. 2022, 23, 12854. [Google Scholar] [CrossRef]
- Andrew, A.; Zhou, J.; Gui, J.; Harrison, A.; Shi, X.; Li, M.; Guetti, B.; Nathan, R.; Tischbein, M.; Pioro, E.P.; et al. Pesticides applied to crops and amyotrophic lateral sclerosis risk in the U.S. Neurotoxicology 2021, 87, 128–135. [Google Scholar] [CrossRef]
- de la Monte, S.M.; Tong, M. Agent Orange Herbicidal Toxin-Initiation of Alzheimer-Type Neurodegeneration. J. Alzheimer’s Dis. 2024, 97, 1703–1726. [Google Scholar] [CrossRef]
- Song, S.; Kim, J.Y.; Lee, Y.; Jeong, H.; Kim, S.; Lee, E.E. Effects of defoliant exposure and medication use on the development of Parkinson’s disease in veterans. Age Ageing 2023, 52, afad192. [Google Scholar] [CrossRef]
- Shrestha, S.; Kamel, F.; Umbach, D.M.; Freeman, L.E.B.; Koutros, S.; Alavanja, M.; Blair, A.; Sandler, D.P.; Chen, H. High Pesticide Exposure Events and Olfactory Impairment among U.S. Farmers. Environ. Health Perspect. 2019, 127, 17005. [Google Scholar] [CrossRef]
- Cheng, M.; Li, M.; Zhang, Y.; Gu, X.; Gao, W.; Zhang, S.; Liu, J. Exploring the mechanism of PPCPs on human metabolic diseases based on network toxicology and molecular docking. Environ. Int. 2025, 196, 109324. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Shu, Y.; Shen, R.; Zhou, Y.; Pan, H.; He, L.; Fang, F.; Zhu, X.; Wang, X.; Wang, Y.; et al. The regulation of NFKB1 on CD200R1 expression and their potential roles in Parkinson’s disease. J. Neuroinflam. 2024, 21, 229. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Zhang, H.; Wang, C.; Jiao, F.; Xu, H.; Wang, X.; Luan, W.; Ma, F.; Ni, L.; Tang, X.; et al. Activation of ROS/MAPKs/NF-κB/NLRP3 and inhibition of efferocytosis in osteoclast-mediated diabetic osteoporosis. FASEB J. 2019, 33, 12515–12527. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Zhang, M.; Lv, L.; Zhang, X.; Zhang, P.; Zhou, Y. Inhibition of PTGS1 promotes osteogenic differentiation of adipose-derived stem cells by suppressing NF-kB signaling. Stem Cell Res. Ther. 2019, 10, 57. [Google Scholar] [CrossRef]
- Palma-Barqueros, V.; Bohdan, N.; Revilla, N.; Vicente, V.; Bastida, J.M.; Rivera, J. PTGS1 gene variations associated with bleeding and platelet dysfunction. Platelets 2021, 32, 710–716. [Google Scholar] [CrossRef]
- Yagmur, E.; Weiskirchen, R.; Schedel, A.; Bugert, P. PTGS1 compound heterozygosity impairs gene expression and platelet aggregation and is associated with severe bleeding complications. Thromb. Haemost. 2013, 110, 1083–1085. [Google Scholar] [CrossRef]
- Choi, S.H.; Aid, S.; Caracciolo, L.; Sakura Minami, S.; Niikura, T.; Matsuoka, Y.; Turner, R.S.; Mattson, M.P.; Bosetti, F. Cyclooxygenase-1 inhibition reduces amyloid pathology and improves memory deficits in a mouse model of Alzheimer’s disease. J. Neurochem. 2013, 124, 59–68. [Google Scholar] [CrossRef]
- Zhang, L.; Meng, Z.; Wang, H.; Miao, Y. Effect of PEAR1, PTGS1 gene polymorphisms on the recurrence of aspirin-treated patients with ischemic stroke in the Han population of China: A 4-year follow-up study. Medicine 2024, 103, e38031. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, J.W.; Xu, H.; Chen, X.; Gao, Y.; Jiang, H.G.; Wang, Y.; Wu, H.; Yang, L.; Wang, W.B.; et al. Therapy-induced senescent tumor cell-derived extracellular vesicles promote colorectal cancer progression through SERPINE1-mediated NF-κB p65 nuclear translocation. Mol. Cancer 2024, 23, 70. [Google Scholar] [CrossRef]
- Wang, N.; Gao, Y.; Wang, Y.; Dai, Y.; Tang, Y.; Huang, J.; Sun, L.; Qian, G.; Ma, J.; Li, X.; et al. Plasma proteomic profiling reveals that SERPINE1 is a potential biomarker associated with coronary artery lesions in Kawasaki disease. Int. Immunopharmacol. 2024, 139, 112698. [Google Scholar] [CrossRef]
- Gold, P.W. The PPARg System in Major Depression: Pathophysiologic and Therapeutic Implications. Int. J. Mol. Sci. 2021, 22, 9248. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Yu, Q.; Ou, J.; Lou, J.; Zhu, J.; Lin, Z. The Neuroprotective Mechanisms of PPAR-γ: Inhibition of Microglia-Mediated Neuroinflammation and Oxidative Stress in a Neonatal Mouse Model of Hypoxic-Ischemic White Matter Injury. CNS Neurosci. Ther. 2024, 30, e70081. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.P.; Romme, E.; van der Spek, P.J.; Dirven, C.M.; Willemsen, R.; Kros, J.M. Glut1/SLC2A1 is crucial for the development of the blood-brain barrier in vivo. Ann. Neurol. 2010, 68, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Veys, K.; Fan, Z.; Ghobrial, M.; Bouché, A.; García-Caballero, M.; Vriens, K.; Conchinha, N.V.; Seuwen, A.; Schlegel, F.; Gorski, T.; et al. Role of the GLUT1 Glucose Transporter in Postnatal CNS Angiogenesis and Blood-Brain Barrier Integrity. Circ. Res. 2020, 127, 466–482. [Google Scholar] [CrossRef]
- Zhu, Y.P.; Gao, X.Y.; Xu, G.H.; Qin, Z.F.; Ju, H.X.; Li, D.C.; Ma, D.N. Computational Dissection of the Role of Trp305 in the Regulation of the Death-Associated Protein Kinase-Calmodulin Interaction. Biomolecules 2022, 12, 1395. [Google Scholar] [CrossRef]
- Tilson, H.A.; Kodavanti, P.R. The neurotoxicity of polychlorinated biphenyls. Neurotoxicology 1998, 19, 517–525. [Google Scholar]
- Kong, A.M.; Idris, Z.A.; Urrutia-Cabrera, D.; Lees, J.G.; Phang, R.J.; Mitchell, G.M.; Wong, R.C.B.; Lim, S.Y. NOS3 regulates angiogenic potential of human induced pluripotent stem cell-derived endothelial cells. Biochem. Biophys. Rep. 2024, 40, 101876. [Google Scholar] [CrossRef]
- Austin, S.A.; Santhanam, A.V.; Hinton, D.J.; Choi, D.S.; Katusic, Z.S. Endothelial nitric oxide deficiency promotes Alzheimer’s disease pathology. J. Neurochem. 2013, 127, 691–700. [Google Scholar] [CrossRef]
- Dahiyat, M.; Cumming, A.; Harrington, C.; Wischik, C.; Xuereb, J.; Corrigan, F.; Breen, G.; Shaw, D.; St Clair, D. Association between Alzheimer’s disease and the NOS3 gene. Ann. Neurol. 1999, 46, 664–667. [Google Scholar] [CrossRef]
- Xiong, W.; MacColl Garfinkel, A.E.; Li, Y.; Benowitz, L.I.; Cepko, C.L. NRF2 promotes neuronal survival in neurodegeneration and acute nerve damage. J. Clin. Investig. 2015, 125, 1433–1445. [Google Scholar] [CrossRef]
- Bahn, G.; Jo, D.G. Therapeutic Approaches to Alzheimer’s Disease Through Modulation of NRF2. Neuromol. Med. 2019, 21, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Fu, C.; Wei, Y.; Xu, B.; Yang, R.; Li, C.; Qiu, M.; Yin, Y.; Qin, D. Ferroptosis-related biomarkers for Alzheimer’s disease: Identification by bioinformatic analysis in hippocampus. Front. Cell. Neurosci. 2022, 16, 1023947. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; He, Y.; Yang, S.; Xue, X.; Qin, H.; Sun, T.; Yang, W. CA9 knockdown enhanced ionizing radiation-induced ferroptosis and radiosensitivity of hypoxic glioma cells. Int. J. Radiat. Biol. 2023, 99, 1908–1924. [Google Scholar] [CrossRef] [PubMed]
- Leventhal, M.J.; Zanella, C.A.; Kang, B.; Peng, J.; Gritsch, D.; Liao, Z.; Bukhari, H.; Wang, T.; Pao, P.C.; Danquah, S.; et al. An integrative systems-biology approach defines mechanisms of Alzheimer’s disease neurodegeneration. bioRxiv 2024. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, H.; Guo, F.; Si, N.; Brantner, A.; Yang, J.; Han, L.; Wei, X.; Zhao, H.; Bian, B. Integrated Proteomics and Lipidomics Investigation of the Mechanism Underlying the Neuroprotective Effect of N-benzylhexadecanamide. Molecules 2018, 23, 2929. [Google Scholar] [CrossRef]
- Li, X.; Kong, Z.; Cai, K.; Qi, F.; Zhu, S. Neopterin mediates sleep deprivation-induced microglial activation resulting in neuronal damage by affecting YY1/HDAC1/TOP1/IL-6 signaling. J. Adv. Res. 2025, 72, 181–195. [Google Scholar] [CrossRef]


| Ingredient | Target | Binding Energy (kcal⋅mol−1) |
|---|---|---|
| 2,4-Dichlorophenoxyacetic acid | NFKB1 | −5.1 |
| 2,4-Dichlorophenoxyacetic acid | PPARG | −5.9 |
| 2,4-Dichlorophenoxyacetic acid | SERPINE1 | −6.2 |
| 2,4-Dichlorophenoxyacetic acid | PPARA | −6.1 |
| 2,4-Dichlorophenoxyacetic acid | NOS3 | −7.1 |
| 2,4-Dichlorophenoxyacetic acid | NFE2L2 | −5.2 |
| 2,4-Dichlorophenoxyacetic acid | SLC2A1 | −6.0 |
| 2,4-Dichlorophenoxyacetic acid | CSNK2A2 | −6.6 |
| 2,4-Dichlorophenoxyacetic acid | CSNK2A1 | −6.5 |
| 2,4-Dichlorophenoxyacetic acid | PTGS1 | −6.8 |
| 2,4-Dichlorophenoxyacetic acid | TOP1 | −5.7 |
| 2,4-Dichlorophenoxyacetic acid | CA9 | −6.4 |
| Core Target | Associated Pathways (KEGG) | Core Biological/Pathological Role |
|---|---|---|
| NFKB1 | Neuroactive ligand–receptor interaction; AGE-RAGE signaling pathway in diabetic complications | Inflammatory transcriptional regulator |
| PPARG | Insulin resistance; Adipocytokine signaling pathway; cAMP signaling pathway | Nuclear receptor for metabolism and inflammation regulation |
| SERPINE1 | Complement and coagulation cascades; Fluid shear stress and atherosclerosis | Inhibitor of the fibrinolytic system |
| PPARA | Insulin resistance; Adipocytokine signaling pathway | Nuclear receptor for fatty acid metabolism regulation |
| NOS3 | Nitrogen metabolism; Fluid shear stress and atherosclerosis | Nitric oxide synthase (endothelial) |
| NFE2L2 | Efferocytosis; AGE-RAGE signaling pathway in diabetic complications | Master regulator of antioxidant response |
| SLC2A1 | cAMP signaling pathway | Glucose transporter (GLUT1) |
| CSNK2A1 | Synaptic vesicle cycle; cAMP signaling pathway | Serine/threonine-protein kinase |
| CSNK2A2 | Synaptic vesicle cycle; cAMP signaling pathway | Serine/threonine-protein kinase subunit |
| PTGS1 | Complement and coagulation cascades; Fluid shear stress and atherosclerosis | Prostaglandin synthase (COX-1) |
| TOP1 | Efferocytosis | DNA topoisomerase I |
| CA9 | Nitrogen metabolism; Fluid shear stress and atherosclerosis | Carbonic anhydrase IX |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Y.; Luo, X.; Song, Y.; Gao, H.; Wang, Y.; Li, Y.; Yang, H.; Zhou, J. Exploring the Mechanism of 2,4-Dichlorophenoxyacetic Acid in Causing Neurodegenerative Diseases Based on Network Toxicology and Molecular Docking. Int. J. Mol. Sci. 2025, 26, 11980. https://doi.org/10.3390/ijms262411980
Yan Y, Luo X, Song Y, Gao H, Wang Y, Li Y, Yang H, Zhou J. Exploring the Mechanism of 2,4-Dichlorophenoxyacetic Acid in Causing Neurodegenerative Diseases Based on Network Toxicology and Molecular Docking. International Journal of Molecular Sciences. 2025; 26(24):11980. https://doi.org/10.3390/ijms262411980
Chicago/Turabian StyleYan, Yucheng, Xiaoqi Luo, Yanan Song, Haoxuan Gao, Yuwen Wang, Yiman Li, Huifang Yang, and Jian Zhou. 2025. "Exploring the Mechanism of 2,4-Dichlorophenoxyacetic Acid in Causing Neurodegenerative Diseases Based on Network Toxicology and Molecular Docking" International Journal of Molecular Sciences 26, no. 24: 11980. https://doi.org/10.3390/ijms262411980
APA StyleYan, Y., Luo, X., Song, Y., Gao, H., Wang, Y., Li, Y., Yang, H., & Zhou, J. (2025). Exploring the Mechanism of 2,4-Dichlorophenoxyacetic Acid in Causing Neurodegenerative Diseases Based on Network Toxicology and Molecular Docking. International Journal of Molecular Sciences, 26(24), 11980. https://doi.org/10.3390/ijms262411980







