Clinical Strategies for Counteracting Human Ovarian Aging: Molecular Background, Update, and Outlook
Abstract
1. Introduction
2. Basic Mechanisms of OA as Possible Therapeutic Targets
2.1. Targeting the HPO Axis
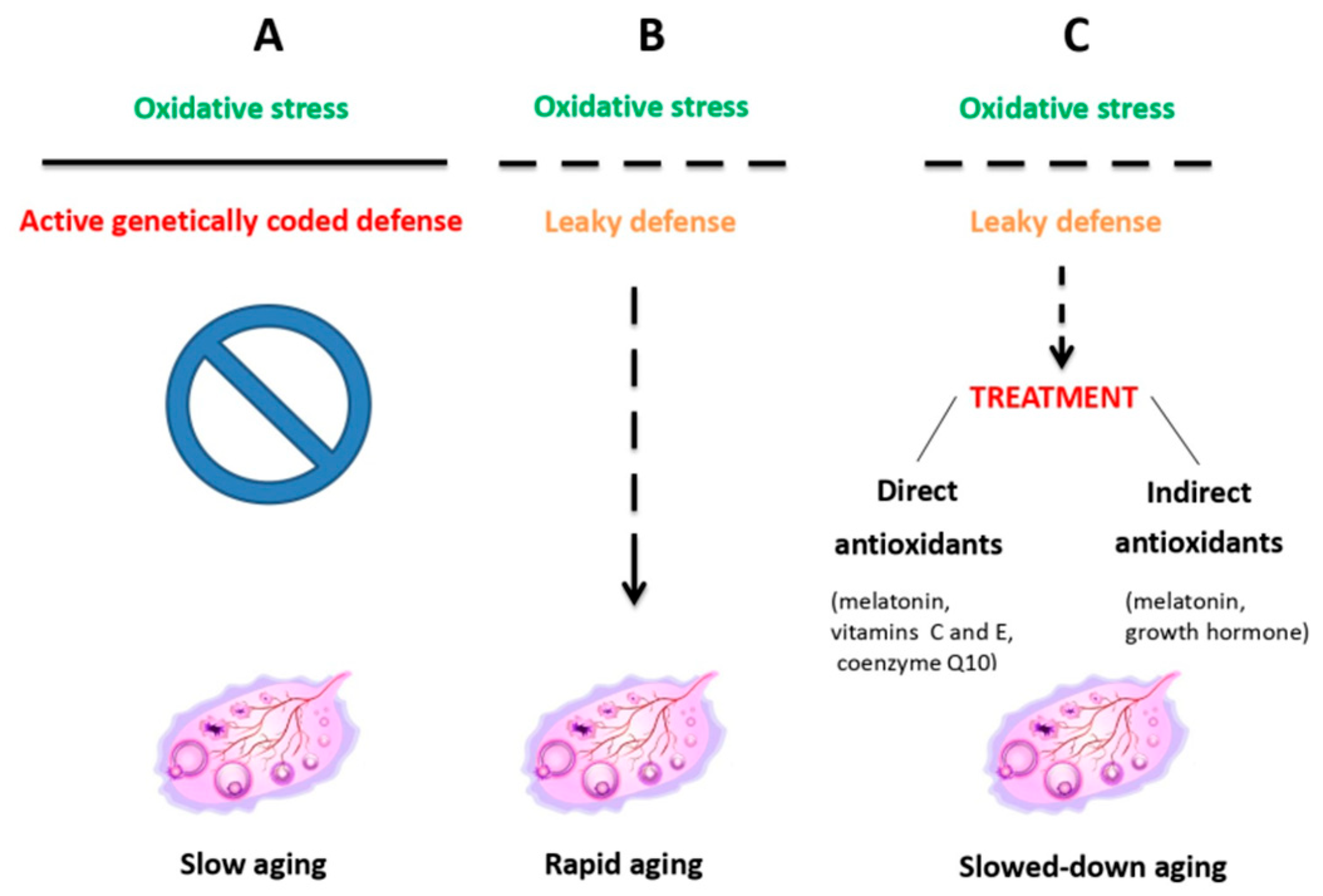
2.2. Targeting HPO-Independent Ovarian Decay
3. Update on Existing Clinical Strategies Evaluated in Women
3.1. Antioxidant and Mitochondrial Therapies
3.2. GnRH Agonists and Antagonists
3.3. Hormones and Growth Factors
3.4. More Invasive Treatments
4. Outlook
4.1. Biomaterials
4.2. Metal-Based Biomedicines
4.3. Natural Biomedicines
4.4. Perspectives and Precautions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Legro, R.S.; Wong, I.L.; Paulson, R.J.; Lobo, R.A.; Sauer, M.V. Recipient’s age does not adversely affect pregnancy outcome after oocyte donation. Am. J. Obstet. Gynecol. 1995, 172, 96–100. [Google Scholar] [CrossRef]
- Tesarik, J. Endocrinology of primary ovarian insufficiency: Diagnostic and therapeutic clues. Endocrines 2025, 6, 18. [Google Scholar] [CrossRef]
- Gubbels Bupp, M.R. Sex, the aging immune system, and chronic disease. Cell. Immunol. 2015, 294, 102–110. [Google Scholar] [CrossRef]
- Szmuilowicz, E.D.; Manson, J.E.; Rossouw, J.E.; Howard, B.V.; Margolis, K.L.; Greep, N.C.; Brzyski, R.G.; Stefanick, M.L.; O’Sullivan, M.J.; Wu, C.; et al. Vasomotor symptoms and cardiovascular events in postmenopausal women. Menopause 2011, 18, 603–610. [Google Scholar] [CrossRef]
- Gray, K.E.; Katon, J.G.; LeBlanc, E.S.; Woods, N.F.; Bastian, L.A.; Reiber, G.E.; Weitlauf, J.C.; Nelson, K.M.; LaCroix, A.Z. Vasomotor symptom characteristics: Are they risk factors for incident diabetes? Menopause 2018, 25, 520–530. [Google Scholar] [CrossRef]
- Benayoun, B.A.; Kochersberger, A.; Garrison, J.L. Studying ovarian aging and its health impacts: Modern tools and approaches. Genes Dev. 2025, 39, 975–990. [Google Scholar] [CrossRef]
- Recognizing the importance of ovarian aging research. Nat. Aging 2022, 2, 1071–1072. [CrossRef] [PubMed]
- Pelosi, E.; Forabosco, A.; Schlessinger, D. Genetics of the ovarian reserve. Front. Genet. 2015, 6, 308. [Google Scholar] [CrossRef] [PubMed]
- Migale, R.; Neumann, M.; Mitter, R.; Rafiee, M.R.; Wood, S.; Olsen, J.; Lovell-Badge, R. FOXL2 interaction with different binding partners regulates the dynamics of ovarian development. Sci. Adv. 2024, 10, eadl0788. [Google Scholar] [CrossRef]
- Federici, S.; Rossetti, R.; Moleri, S.; Munari, E.V.; Frixou, M.; Bonomi, M.; Persani, L. Primary ovarian insufficiency: Update on clinical and genetic findings. Front. Endocrinol. 2024, 15, 1464803. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Xiang, W. Mechanisms of ovarian aging in women: A review. J. Ovarian Res. 2023, 16, 67. [Google Scholar] [CrossRef]
- Bochynska, S.; Garcia-Perez, M.A.; Tarin, J.J.; Szeliga, A.; Meczekalski, B.; Cano, A. The final phases of ovarian aging: A tale of diverging functional trajectories. J. Clin. Med. 2025, 14, 5834. [Google Scholar] [CrossRef]
- Hirano, M.; Onodera, T.; Takasaki, K.; Takahashi, Y.; Ichinose, T.; Nishida, H.; Hiraike, H.; Nagasaka, K. Ovarian aging: Pathophysiology and recent developments in maintaining ovarian reserve. Front. Endocrinol. 2025, 16, 1619516. [Google Scholar] [CrossRef]
- Peng, M.T.; Huang, H.H. Aging of hypothalamic-pituitary-ovarian function in the rat. Fertil. Steril. 1972, 23, 535–542. [Google Scholar] [CrossRef]
- Neal-Perry, G.; Nejat, E.; Dicken, C. The neuroendocrine physiology of female reproductive aging: An update. Maturitas 2010, 67, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Goldsmith, L.T.; Weiss, G. Age-related changes in the regulation of luteinizing hormone secretion by estrogen in women. Exp. Biol. Med. 2002, 227, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Ahmed Ebbiary, N.A.; Lenton, E.A.; Cooke, I.D. Hypothalamic-pituitary ageing: Progressive increase in FSH and LH concentrations throughout the reproductive life in regularly menstruating women. Clin. Endocrinol. 1994, 41, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.R., Jr.; Porter, J.C. Luteinizing hormone-releasing hormone and thyrotropin-releasing hormone in the hypothalamus of women: Effects of age and reproductive status. J. Clin. Endocrinol. Metab. 1984, 58, 488–491. [Google Scholar] [CrossRef]
- Lenton, E.A.; Sexton, L.; Lee, S.; Cooke, I.D. Progressive changes in LH and FSH and LH: FSH ratio in women throughout reproductive life. Maturitas 1988, 10, 35–43. [Google Scholar] [CrossRef]
- Hrabovszky, E.; Turi, G.F.; Kalló, I.; Liposits, Z. Expression of vesicular glutamate transporter-2 in gonadotropin-releasing hormone neurons of the adult male rat. Endocrinology 2004, 145, 4018–4021. [Google Scholar] [CrossRef][Green Version]
- Yin, W.; Sun, Z.; Mendenhall, J.M.; Walker, D.M.; Riha, P.D.; Bezner, K.S.; Gore, A.C. Expression of Vesicular Glutamate Transporter 2 (vGluT2) on Large Dense-Core Vesicles within GnRH Neuroterminals of Aging Female Rats. PLoS ONE 2015, 10, e0129633. [Google Scholar] [CrossRef]
- Zuo, Z.; Mahesh, V.B.; Zamorano, P.L.; Brann, D.W. Decreased gonadotropin-releasing hormone neurosecretory response to glutamate agonists in middle-aged female rats on proestrus afternoon: A possible role in reproductive aging? Endocrinology 1996, 137, 2334–2338. [Google Scholar] [CrossRef]
- Jung, H.; Shannon, E.M.; Fritschy, J.M.; Ojeda, S.R. Several GABAA receptor subunits are expressed in LHRH neurons of juvenile female rats. Brain Res. 1998, 780, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Moenter, S.M.; DeFazio, R.A. Endogenous gamma-aminobutyric acid can excite gonadotropin-releasing hormone neurons. Endocrinology 2005, 146, 5374–5379. [Google Scholar] [CrossRef]
- Neal-Perry, G.S.; Zeevalk, G.D.; Shu, J.; Etgen, A.M. Restoration of the luteinizing hormone surge in middle-aged female rats by altering the balance of GABA and glutamate transmission in the medial preoptic area. Biol. Reprod. 2008, 79, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Seminara, S.B.; Messager, S.; Chatzidaki, E.E.; Thresher, R.R.; Acierno, J.S., Jr.; Shagoury, J.K.; Bo-Abbas, Y.; Kuohung, W.; Schwinof, K.M.; Hendrick, A.G.; et al. The GPR54 gene as a regulator of puberty. N. Engl. J. Med. 2003, 349, 1614–1627. [Google Scholar] [CrossRef] [PubMed]
- Topaloglu, A.K.; Reimann, F.; Guclu, M.; Yalin, A.S.; Kotan, L.D.; Porter, K.M.; Serin, A.; Mungan, N.O.; Cook, J.R.; Imamoglu, S.; et al. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat. Genet. 2009, 41, 354–358. [Google Scholar] [CrossRef]
- Lehman, M.N.; Coolen, L.M.; Goodman, R.L. Minireview: Kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: A central node in the control of gonadotropin-releasing hormone secretion. Endocrinology 2010, 151, 3479–3489. [Google Scholar] [CrossRef]
- Herbison, A.E. The gonadotropin-releasing hormone pulse generator. Endocrinology 2018, 159, 3723–3736. [Google Scholar] [CrossRef]
- Rance, N.E.; Young, W.S., 3rd. Hypertrophy and increased gene expression of neurons containing neurokinin-B and substance-P messenger ribonucleic acids in the hypothalami of postmenopausal women. Endocrinology 1991, 128, 2239–2247. [Google Scholar] [CrossRef]
- Rometo, A.M.; Krajewski, S.J.; Voytko, M.L.; Rance, N.E. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J. Clin. Endocrinol. Metab. 2007, 92, 2744–2750. [Google Scholar] [CrossRef] [PubMed]
- Rometo, A.M.; Rance, N.E. Changes in prodynorphin gene expression and neuronal morphology in the hypothalamus of postmenopausal women. J. Neuroendocrinol. 2008, 20, 1376–1381. [Google Scholar] [CrossRef]
- Eghlidi, D.H.; Haley, G.E.; Noriega, N.C.; Kohama, S.G.; Urbanski, H.F. Influence of age and 17beta-estradiol on kisspeptin, neurokinin B, and prodynorphin gene expression in the arcuate-median eminence of female rhesus macaques. Endocrinology 2010, 151, 3783–3794. [Google Scholar] [CrossRef]
- Wallace, W.H.; Kelsey, T.W. Human ovarian reserve from conception to the menopause. PLoS ONE 2010, 5, e8772. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Barbacioru, C.; Wang, Y.; Nordman, E.; Lee, C.; Xu, N.; Wang, X.; Bodeau, J.; Tuch, B.B.; Siddiqui, A.; et al. mRNA-Seq whole-transcriptome analysis of a single cell. Nat. Methods 2009, 6, 377–382. [Google Scholar] [CrossRef]
- Liang, J.; Gai, S.; Na, X.; Hu, J.; Zhao, Z.; Zi, D.; Na, Z.; Gao, W.; Bi, F.; Li, D. Ovarian aging at single-cell resolution: Current paradigms and perspectives. Ageing Res. Rev. 2025, 110, 102807. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, Y.; Li, J.; Yu, Y.; Zhang, W.; Song, M.; Liu, Z.; Min, Z.; Hu, H.; Jing, Y.; et al. Single-cell transcriptomic atlas of primate ovarian aging. Cell 2020, 180, 585–600.e19. [Google Scholar] [CrossRef]
- Isola, J.V.V.; Ocañas, S.R.; Hubbart, C.R.; Ko, S.; Mondal, S.A.; Hense, J.D.; Carter, H.N.C.; Schneider, A.; Kovats, S.; Alberola-Ila, J.; et al. A single-cell atlas of the aging mouse ovary. Nat. Aging 2024, 4, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Rooda, I.; Méar, L.; Hassan, J.; Damdimopoulou, P. The adult ovary at single cell resolution: An expert review. Am. J. Obstet. Gynecol. 2025, 232, S95.e1–S95.e16. [Google Scholar] [CrossRef]
- Bucala, R.; Cerami, A. Advanced glycosylation: Chemistry, biology, and implications for diabetes and aging. Adv. Pharmacol. 1992, 23, 1–34. [Google Scholar] [CrossRef]
- Papachroni, K.K.; Piperi, C.; Levidou, G.; Korkolopoulou, P.; Pawelczyk, L.; Diamanti-Kandarakis, E.; Papavassiliou, A.G. Lysyl oxidase interacts with AGE signalling to modulate collagen synthesis in polycystic ovarian tissue. J. Cell. Mol. Med. 2010, 14, 2460–2469. [Google Scholar] [CrossRef]
- Rodríguez-Varela, C.; Labarta, E. Clinical application of antioxidants to improve human oocyte mitochondrial function: A review. Antioxidants 2020, 9, 1197. [Google Scholar] [CrossRef]
- Wang, L.; Tang, J.; Wang, L.; Tan, F.; Song, H.; Zhou, J.; Li, F. Oxidative stress in oocyte aging and female reproduction. J. Cell. Physiol. 2021, 236, 7966–7983. [Google Scholar] [CrossRef]
- Tesarik, J.; Galán-Lázaro, M.; Mendoza-Tesarik, R. Ovarian aging: Molecular mechanisms and medical management. Int. J. Mol. Sci. 2021, 22, 1371. [Google Scholar] [CrossRef] [PubMed]
- Duval, C.; Wyse, B.A.; Fuchs Weizman, N.; Kuznyetsova, I.; Madjunkova, S.; Librach, C.L. Cannabis impacts female fertility as evidenced by an in vitro investigation and a case-control study. Nat. Commun. 2025, 16, 8185. [Google Scholar] [CrossRef]
- Tesarik, J. Towards personalized antioxidant use in female infertility: Need for more molecular and clinical studies. Biomedicines 2021, 9, 1933. [Google Scholar] [CrossRef]
- Tesarik, J.; Mendoza Tesarik, R. Melatonin in the treatment of female infertility: Update on biological and clinical findings. Biomedicines 2025, 13, 2434. [Google Scholar] [CrossRef]
- Glode, L.M.; Robinson, J.; Gould, S.F. Protection from cyclophosphamide-induced testicular damage with an analogue of gonadotropin-releasing hormone. Lancet 1981, 1, 1132–1134. [Google Scholar] [CrossRef]
- Ataya, K.; Rao, L.V.; Lawrence, E.; Kimmel, R. Luteinizing hormone-releasing hormone agonist inhibits cyclophosphamide-induced ovarian follicular depletion in rhesus monkeys. Biol. Reprod. 1995, 52, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Elgindy, E.; Sibai, H.; Abdelghani, A.; Mostafa, M. Protecting ovaries during chemotherapy through gonad suppression: A systematic review and meta-analysis. Obstet. Gynecol. 2015, 126, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Lambertini, M.; Horicks, F.; Del Mastro, L.; Partridge, A.H.; Demeestere, I. Ovarian protection with gonadotropin-releasing hormone agonists during chemotherapy in cancer patients: From biological evidence to clinical application. Cancer Treat. Rev. 2019, 72, 65–77. [Google Scholar] [CrossRef]
- Blumenfeld, Z. Fertility preservation using GnRH agonists: Rationale, possible mechanisms, and explanation of controversy. Clin. Med. Insights Reprod. Health 2019, 13, 1179558119870163. [Google Scholar] [CrossRef]
- Guo, Y.; Xue, L.; Tang, W.; Xiong, J.; Chen, D.; Dai, Y.; Wu, C.; Wei, S.; Dai, J.; Wu, M.; et al. Ovarian microenvironment: Challenges and opportunities in protecting against chemotherapy-associated ovarian damage. Hum. Reprod. Update 2024, 30, 614–647. [Google Scholar] [CrossRef]
- Yilmaz, N.; Uygur, D.; Inal, H.; Gorkem, U.; Cicek, N.; Mollamahmutoglu, L. Dehydroepiandrosterone supplementation improves predictive markers for diminished ovarian reserve: Serum AMH, inhibin B and antral follicle count. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 169, 257–260. [Google Scholar] [CrossRef]
- Tesarik, J.; Hazout, A.; Mendoza, C. Improvement of delivery and live birth rates after ICSI in women aged >40 years by ovarian co-stimulation with growth hormone. Hum. Reprod. 2005, 20, 2536–2541. [Google Scholar] [CrossRef] [PubMed]
- Tesarik, J.; Yovich, J.L.; Menezo, Y. Editorial: Growth hormone in fertility and infertility: Physiology, pathology, diagnosis and treatment. Front. Endocrinol. 2021, 12, 621722. [Google Scholar] [CrossRef] [PubMed]
- Tesarik, J. Editorial: Growth hormone in fertility and infertility: Physiology, pathology, diagnosis and treatment, volume II. Front. Endocrinol. 2024, 15, 1446734. [Google Scholar] [CrossRef]
- Yang, S.; Luo, W.; Sun, Y.; Wang, S. Novel perspectives on growth hormone regulation of ovarian function: Mechanisms, formulations, and therapeutic applications. Front. Endocrinol. 2025, 16, 1576333. [Google Scholar] [CrossRef]
- Sadeghpour, S.; Maleki, F.; Hajizadeh-Sharafabad, F.; Ghasemnejad-Berenji, H. Evaluation of intraovarian injection of platelet-rich plasma for enhanced ovarian function and reproductive success in women with POI and POR: A systematic review and meta-analysis. Eur. J. Med. Res. 2025, 30, 610. [Google Scholar] [CrossRef]
- Karimizadeh, Z.; Saltanatpour, Z.; Tarafdari, A.; Rezaeinejad, M.; Hamidieh, A.A. Ovarian tissue cryopreservation: A narrative review on cryopreservation and transplantation techniques, and the clinical outcomes. Ther. Adv. Reprod. Health 2025, 19, 26334941251340517. [Google Scholar] [CrossRef]
- Khattak, H.; Malhas, R.; Craciunas, L.; Afifi, Y.; Amorim, C.A.; Fishel, S.; Silber, S.; Gook, D.; Demeestere, I.; Bystrova, O.; et al. Fresh and cryopreserved ovarian tissue transplantation for preserving reproductive and endocrine function: A systematic review and individual patient data meta-analysis. Hum. Reprod. Update 2022, 28, 400–416, Erratum in: Hum. Reprod. Update 2022, 28, 455. https://doi.org/10.1093/humupd/dmac015. [Google Scholar] [CrossRef] [PubMed]
- McFarland, R.; Hyslop, L.A.; Feeney, C.; Pillai, R.N.; Blakely, E.L.; Moody, E.; Prior, M.; Devlin, A.; Taylor, R.W.; Herbert, M.; et al. Mitochondrial donation in a reproductive care pathway for mtDNA disease. N. Engl. J. Med. 2025, 393, 461–468. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, S.; Li, D.; Liang, H.; Yao, Y.; Xia, X.; Yu, H.; Jiang, M.; Yang, Y.; Gao, M.; et al. The cutting-edge progress of novel biomedicines in ovulatory dysfunction therapy. Acta Pharm. Sin. B 2025, 15, 5145–5166. [Google Scholar] [CrossRef]
- Wu, M.; Guo, Y.; Wei, S.; Xue, L.; Tang, W.; Chen, D.; Xiong, J.; Huang, Y.; Fu, F.; Wu, C.; et al. Biomaterials and advanced technologies for the evaluation and treatment of ovarian aging. J. Nanobiotechnology 2022, 20, 374. [Google Scholar] [CrossRef]
- Jiang, Y.; Shi, Y.; Lv, M.; Wang, T.; Wang, P.; Yuan, X.; Gao, F.; Ma, B. Celastrol modulates IRS1 expression to alleviate ovarian aging and to enhance follicular development. Cell Biol. Toxicol. 2025, 41, 129, Correction in Cell Biol. Toxicol. 2025, 41, 148. [Google Scholar] [CrossRef]
- Shen, H.H.; Zhang, X.Y.; Liu, N.; Zhang, Y.Y.; Wu, H.H.; Xie, F.; Wang, W.J.; Li, M.Q. Chitosan alleviates ovarian aging by enhancing macrophage phagocyte-mediated tissue homeostasis. Immun. Ageing 2024, 21, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chen, T.; Hu, J.; Zhang, W.; Shen, M.; Yu, Q.; Chen, Y.; Xie, J. Collagen (peptide) extracted from sturgeon swim bladder: Physicochemical characterization and protective effects on cyclophosphamide-induced premature ovarian failure in mice. Food Chem. 2025, 466, 142217. [Google Scholar] [CrossRef]
- Tang, W.; Wang, K.; Feng, Y.; Tsui, K.H.; Singh, K.K.; Stout, M.B.; Wang, S.; Wu, M. Exploration of the mechanism and therapy of ovarian aging by targeting cellular senescence. Life Med. 2025, 4, lnaf004. [Google Scholar] [CrossRef]
- Sampaio, O.G.M.; Santos, S.A.A.R.; Damasceno, M.B.M.V.; Joventino, L.B.; Schneider, A.; Masternak, M.M.; Campos, A.R.; Cavalcante, M.B. Impact of repeated ovarian hyperstimulation on the reproductive function. J. Reprod. Immunol. 2024, 164, 104277. [Google Scholar] [CrossRef] [PubMed]


| Targeting HPO Axis | Targeting Ovarian Cells | ||
|---|---|---|---|
| Glutamate receptors | Antioxidants | Hormones and GFs | |
| GABA receptors | |||
| GnRH agonists | Direct | Indirect | GH |
| GnRH antagonists | DHEA | ||
| Melatonin | Melatonin | IGF-1 | |
| CoQ10 | VEGF | ||
| Vitamin C | PRP (PDGF) | ||
| Vitamin E | |||
| Folic acid | |||
| Others | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tesarik, J.; Mendoza Tesarik, R. Clinical Strategies for Counteracting Human Ovarian Aging: Molecular Background, Update, and Outlook. Int. J. Mol. Sci. 2025, 26, 11973. https://doi.org/10.3390/ijms262411973
Tesarik J, Mendoza Tesarik R. Clinical Strategies for Counteracting Human Ovarian Aging: Molecular Background, Update, and Outlook. International Journal of Molecular Sciences. 2025; 26(24):11973. https://doi.org/10.3390/ijms262411973
Chicago/Turabian StyleTesarik, Jan, and Raquel Mendoza Tesarik. 2025. "Clinical Strategies for Counteracting Human Ovarian Aging: Molecular Background, Update, and Outlook" International Journal of Molecular Sciences 26, no. 24: 11973. https://doi.org/10.3390/ijms262411973
APA StyleTesarik, J., & Mendoza Tesarik, R. (2025). Clinical Strategies for Counteracting Human Ovarian Aging: Molecular Background, Update, and Outlook. International Journal of Molecular Sciences, 26(24), 11973. https://doi.org/10.3390/ijms262411973







