1. Introduction
Methamphetamine (MA) remains one of the most widely abused psychostimulants worldwide, with increasing prevalence across high- and middle-income countries [
1,
2]. In Taiwan, MA is consistently the most frequently detected illicit substance, representing a persistent public health challenge [
3,
4]. Its systemic toxicity affects multiple organ systems, particularly the cardiovascular, cerebrovascular, and central nervous systems (CNSs) [
5,
6,
7]. In addition to direct end-organ injury, MA induces complex neurocardiac interactions through dysregulation of the brain–heart–gut axis. Alterations in gut microbiome composition, impaired enteric nervous system (ENS) function, and systemic inflammation have been implicated in autonomic imbalance and pathological brain–heart–gut signaling, amplifying hemodynamic instability, arrhythmogenesis, and myocardial injury.
The incidence of ischemic stroke in younger adults (18–49 years) has risen substantially and accounts for approximately 15% of all cases [
8]. Illicit drug use, particularly MA, is considered a key contributor due to its potent sympathomimetic effects, arrhythmogenic potential, and chronic cardiotoxicity [
8,
9,
10]. While most existing research focuses on MA-induced neurovascular complications such as intracranial hemorrhage and vasospasm-related ischemia, a critical mechanism remains underrecognized: cardiogenic stroke resulting from left ventricular thrombus (LVT) secondary to methamphetamine-associated cardiomyopathy (MACM) [
9].
MACM is a distinct subtype of toxic cardiomyopathy arising from chronic MA exposure. It is characterized by severe left ventricular systolic dysfunction, biventricular dilatation, and malignant arrhythmias, which collectively create a prothrombotic environment conducive to LVT formation [
7]. Although MACM-related stroke may resemble atrial fibrillation (AF)–-associated stroke clinically, the underlying mechanisms differ fundamentally, reflecting a pathophysiologic continuum of autonomic dysregulation, sympathetic overactivation, and progressive myocardial remodeling. Despite increasing recognition of MACM and its association with ventricular thrombus and cardioembolic stroke, the mechanistic links between neuroautonomic dysregulation, myocardial remodeling, and thrombogenesis remain poorly synthesized.
This focused review synthesizes current evidence on brain–heart–gut axis disruption in MA users and its contribution to LVT formation and cardioembolic stroke. We outline relevant pathophysiological mechanisms, diagnostic challenges, and therapeutic considerations, and present a representative clinical case in a young adult. To contextualize the multi-organ impact of acute MA toxicity,
Figure 1 summarizes its effects across the central nervous, cardiovascular, gastrointestinal, and systemic axes. These acute pathophysiologic perturbations initiate a cascade culminating in myocardial dysfunction, arrhythmogenesis, and prothrombotic states that predispose to stroke. By integrating mechanistic insights with clinical observations, this work aims to improve recognition, diagnostic precision, and management of this increasingly prevalent yet frequently overlooked manifestation of MA toxicity.
2. Pathophysiology of MACM and Thrombus Formation
MA induces structural and functional myocardial injury through a spectrum of mechanisms tightly coupled to dysregulation of the brain–heart axis. After systemic absorption, MA exerts potent sympathomimetic effects in the CNS, producing sustained tachycardia, hypertension, and increased myocardial oxygen demand [
9]. Chronic exposure results in cardiomyocyte injury, oxidative stress, mitochondrial dysfunction, and progressive fibrosis, ultimately leading to ventricular dilatation and severe contractile impairment [
7]. Deterioration of left ventricular systolic function and chamber enlargement promote intracavitary stasis. The left ventricular apex is particularly susceptible to thrombus formation because it physiologically experiences lower shear stress and reduced intracavitary blood-flow velocity [
11]. When contractile dysfunction, such as akinesia or aneurysmal remodeling, is present, stasis is further accentuated [
7,
12]. Furthermore, in both stress-induced cardiomyopathy and MACM, catecholamine excess preferentially affects the apical segments, producing transient apical akinesia that amplifies local stasis and predisposes to LVT [
13].
Combined with MA-induced endothelial activation, upregulation of procoagulant mediators such as tissue factor and plasminogen activator inhibitor-1 (PAI-1), and enhanced platelet aggregation, contributing to a prothrombotic state that increases the risk of LVT and cardioembolic stroke [
7,
9,
14,
15].
Comparative evidence suggests that MACM progresses more rapidly and diffusely than idiopathic or alcoholic cardiomyopathies, often with greater biventricular involvement and more pronounced reductions in ejection fraction [
16]. Malignant arrhythmias and hemodynamic instability frequently occur, further increasing the risk of thromboembolism [
5,
16].
2.1. Role of CNS
MA is highly lipophilic and rapidly penetrates the blood–brain barrier, where it profoundly alters central monoaminergic neurotransmission. Acute exposure increases dopaminergic and noradrenergic signaling, producing heightened arousal, euphoria, and psychomotor activation [
17,
18]. In contrast, repeated or high-dose use depletes presynaptic monoamines and impairs striatal and limbic circuits [
17].
In addition to neurotransmitter dysregulation, chronic MA exposure disrupts higher-order autonomic networks in the medial prefrontal cortex, anterior cingulate cortex, insular cortex, amygdala, hypothalamus, and brainstem autonomic nuclei [
1,
9,
18]. Persistent activation of these regions produces chronic sympathetic hyperactivity, impaired cardiovascular rhythm regulation, and long-term hemodynamic instability [
2,
5,
9]. This top-down dysregulation drives cycles of hyperexcitability and myocardial exhaustion, predisposing to arrhythmias, remodeling, and blood stasis.
2.2. Peripheral Nerve–Myocardium Interface
Excessive norepinephrine release from cardiac sympathetic terminals reinforces sympathetic predominance and diminishes parasympathetic tone, leading to autonomic imbalance and impaired electrophysiological stability [
2,
18,
19]. Sustained β-adrenergic stimulation induces oxidative injury, mitochondrial dysfunction, and apoptosis in cardiomyocytes, accompanied by interstitial fibrosis and contractile decline [
5,
6]. Persistent peripheral sympathetic activation may additionally promote local inflammatory responses, further exacerbating myocardial vulnerability [
5].
Damage at this peripheral interface represents an essential downstream component of brain–heart axis dysfunction. Initiated centrally and amplified peripherally, this process fosters electrical instability, left ventricular deterioration, and a prothrombotic substrate, providing a mechanistic link between MA exposure and cardiogenic stroke in MACM.
2.3. Bidirectional Disruption of the Brain–Heart Axis: From Central Autonomic Circuits to Cardiac Afferents
In MACM, myocardial dilatation, ischemia, and inflammation may abnormally activate vagal and spinal sensory fibers, altering neuroplasticity and autonomic regulation within brainstem centers [
20]. Dysregulated afferent input alters baroreflex sensitivity (BRS) and autonomic regulation, leading to desensitization and increased arrhythmic susceptibility [
2,
19]. This bottom–up feedback further destabilizes central control, reinforcing sympathetic overactivity and creating a bidirectional failure of the brain–heart axis.
2.4. MA Effects on Gut Microbiota and ENS
Emerging evidence suggests that MA alters the composition of the gut microbiota and the ENS. Individuals with long-term MA use demonstrate reduced microbial diversity, characterized by the depletion of beneficial taxa, such as
Bacteroidaceae and
Faecalibacterium, and an increased abundance of pro-inflammatory taxa, including
Sphingomonadales,
Romboutsia, and
Lachnospiraceae [
21,
22]. These compositional shifts are associated with impaired intestinal barrier integrity, which facilitates the translocation of endotoxins into the systemic circulation and amplifies inflammatory responses.
Beyond localized gut effects, MA influences CNS function through ENS and vagal afferent pathways. Animal and human studies have shown that MA downregulates tight junction proteins, increases circulating levels of inflammatory mediators such as lipopolysaccharide (LPS) and tumor necrosis factor-alpha (TNF-α), and potentially activates the gut–brain inflammatory axis [
22,
23,
24].
Microbial metabolites derived from dysbiotic gut communities may contribute to heightened thrombotic risk. Among these, trimethylamine N-oxide (TMAO) has emerged as a key mediator. TMAO promotes vascular inflammation by upregulating pro-inflammatory cytokines and adhesion molecules, as well as activating the nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome, ultimately leading to endothelial dysfunction [
25,
26]. It also impairs antioxidant defense mechanisms and increases the production of reactive oxygen species (ROS), further exacerbating vascular injury [
26,
27]. TMAO concurrently enhances stimulus-dependent platelet activation by promoting intracellular Ca
2+ release, thereby increasing platelet aggregation and accelerating thrombus formation [
25,
26,
27]. These interconnected mechanisms place TMAO at the intersection of gut dysbiosis and cardiovascular complications in MACM.
2.5. Molecular Mechanisms of MA-Induced Myocardial Injury and Thrombogenesis
MA-induced myocardial injury arises from a multifaceted cascade involving mitochondrial dysfunction, oxidative stress, apoptotic signaling, and pro-fibrotic remodeling. Preclinical studies demonstrate that MA suppresses Sigma-1 receptor activity, reducing cAMP response element-binding protein-mediated transcription and downregulating mitochondrial fission protein-1. This impairs mitochondrial dynamics, disrupts the membrane potential, and diminishes ATP production, thereby increasing susceptibility to apoptosis [
17,
28].
In parallel, MA activates NADPH oxidase-2, markedly elevating ROS levels. Excess ROS further damages mitochondrial membranes and activates intrinsic apoptotic pathways, including the Bcl-2/Bax imbalance, cytochrome c release, and caspase-3 activation [
1,
17].
Chronic exposure also triggers pro-fibrotic molecular remodeling. MA upregulates periostin, α-smooth muscle actin, and collagen-synthesizing fibroblast markers, consistent with activation of trace amine-associated receptor-1/cAMP signaling pathways [
1,
7,
17]. This results in the persistent deposition of extracellular matrix and the stiffening of the myocardial interstitium.
In addition to direct cardiomyocyte toxicity, MA exerts prothrombotic effects at the molecular level. Experimental models show that amphetamine derivatives increase endothelial tissue factor expression, suppress tissue factor pathway inhibitor activity, and elevate PAI-1 levels, shifting the coagulation cascade toward thrombogenesis [
15].
MA-induced oxidative stress upregulates intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1), facilitating leukocyte adhesion and platelet–endothelial interactions that initiate thrombus formation [
1].
Collectively, these alterations culminate in a maladaptive myocardial environment with impaired energetics, structural disarray, and heightened thrombogenic potential, establishing the pathophysiological foundation for LVT formation in MACM [
1,
17]
2.6. Integrated Pathophysiological Framework
MA abuse disrupts the brain–heart–gut axis through converging mechanisms. Central sympathetic overactivation triggers β-adrenergic signaling and ROS generation in cardiomyocytes; peripheral sympathetic terminals sustain oxidative injury and fibrosis; gut-derived inflammatory mediators amplify myocardial vulnerability; and pathological cardiac afferents destabilize central autonomic control. These mechanisms form an interconnected, multi-level cascade linking central autonomic dysregulation, peripheral neural-myocardial injury, gut-derived inflammation, endothelial dysfunction, and molecular mitochondrial toxicity.
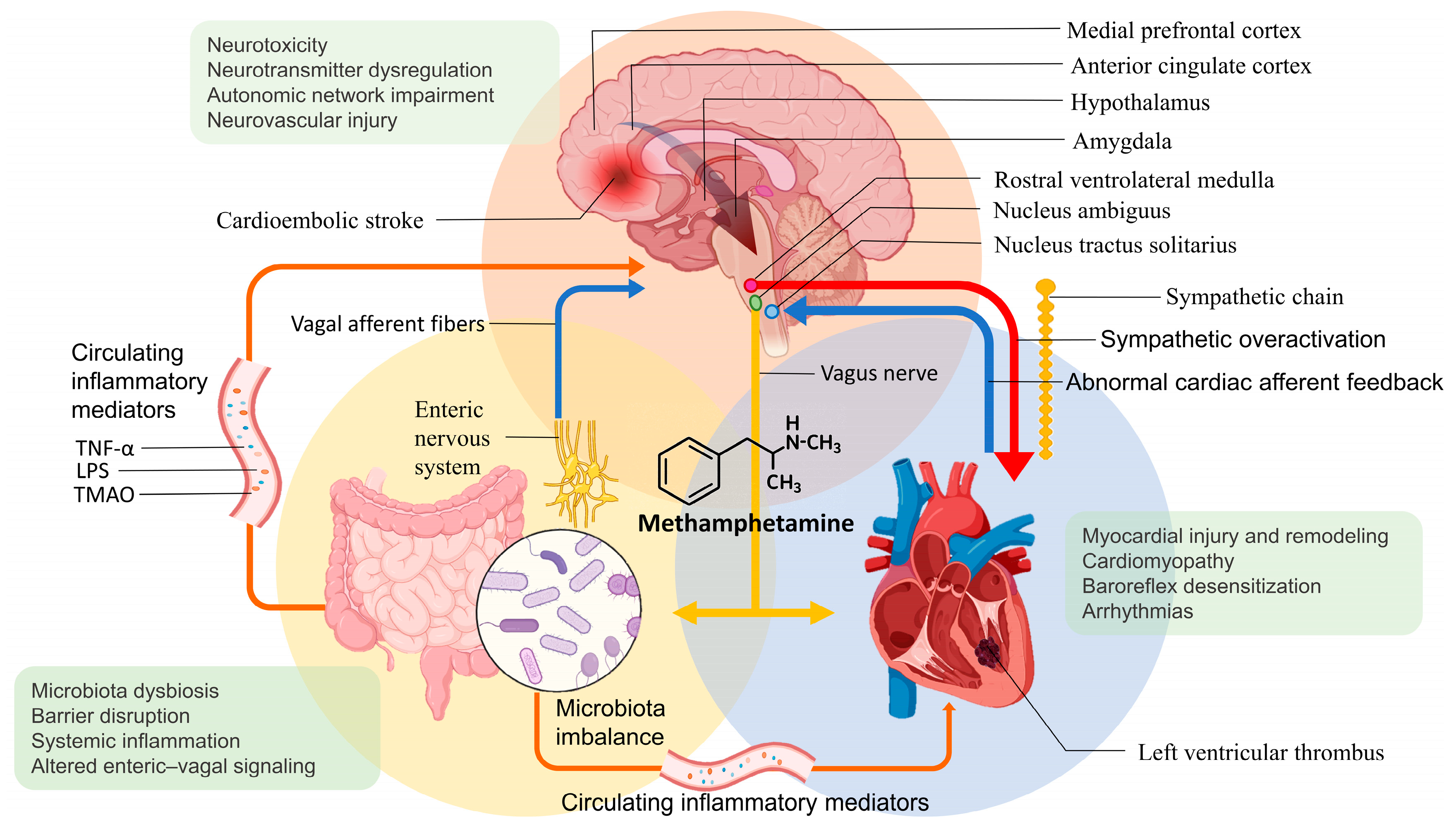
Figure 2 summarizes a proposed integrative model of the brain–heart–gut axis in chronic MA exposure leading to ventricular remodeling, thrombogenesis, and cardioembolic stroke.
3. Diagnostic Evaluation of MACM and LVT
Patients with suspected MACM commonly present with acute or chronic heart failure, malignant arrhythmias, or a marked reduction in systolic function on initial imaging. Because rapid ventricular dilation and low-flow states are frequent in this population, early evaluation for intracardiac thrombus should be incorporated into the initial diagnostic workup. Accordingly, clinical assessment must address both the severity of cardiomyopathy and the presence of thrombotic complications.
3.1. Imaging Modalities
Detection of LVT relies predominantly on cardiac imaging. Transthoracic echocardiography (TTE) remains the first-line modality due to its wide availability, noninvasiveness, and cost-effectiveness; however, its sensitivity is limited, particularly without contrast enhancement. Small, mural, and apex-adherent thrombi may be overlooked, leading to underdiagnosis [
29,
30].
LVT should be strongly suspected in the presence of severe left ventricular systolic dysfunction, defined as a left ventricular ejection fraction (LVEF) of less than 30–40%, and regional wall motion abnormalities such as apical akinesia or aneurysm. In such cases, advanced imaging techniques with higher spatial resolution are recommended for definitive evaluation [
31].
3.2. Advanced Imaging Modalities
Cardiac computed tomography (CCT) and cardiac magnetic resonance imaging (CMR) offer superior sensitivity and improved visualization for the detection of LVT. Among these, late gadolinium enhancement CMR (LGE-CMR) is regarded as the diagnostic gold standard, given its high spatial resolution and excellent tissue contrast, which reliably distinguishes thrombus from surrounding myocardium [
30].
CCT represents a valuable alternative in clinical settings where echocardiographic findings are inconclusive or when detailed anatomical assessment of the cardiac chambers is required. It also serves as an appropriate option for patients who are unable to undergo CMR due to renal impairment, implanted devices, or other contraindications [
32]. In suspected cases of LVT, the use of high-resolution imaging tools for diagnostic confirmation not only improves detection rates but also aids in guiding subsequent anticoagulation strategies.
3.3. Laboratory and Biomarker Indicators
Although the definitive diagnosis of LVT relies on imaging, laboratory parameters may provide adjunctive value for risk stratification and longitudinal monitoring. Conventional cardiac biomarkers, including troponin, B-type natriuretic peptide (BNP), and N-terminal pro-B-type natriuretic peptide (NT-proBNP), remain central to assessing the severity of heart failure and disease progression. BNP and NT-proBNP are widely used cardiac biomarkers that reflect myocardial stretch and hemodynamic stress. Inflammatory markers such as high-sensitivity C-reactive protein (hs-CRP) may also reflect ongoing myocardial inflammation or a prothrombotic state [
7,
33,
34,
35].
Beyond traditional markers, cancer antigen-125 (CA-125) has emerged as a surrogate indicator of cardiac volume overload and congestion. Elevated CA-125 levels are associated with increased mortality and rehospitalization and correlate with NT-proBNP, LVEF, and pulmonary artery pressure, suggesting value as an indirect marker of pathological hemodynamic stress [
34,
35].
Despite their clinical utility, no circulating biomarker provides sufficient sensitivity or specificity for definitive LVT diagnosis, underscoring the essential role of imaging confirmation.
3.4. Autonomic Nervous Function Assessment
Autonomic evaluation provides a physiologic dimension that complements structural imaging and biomarker analysis in patients with suspected MACM. Heart rate variability (HRV) is the most widely used non-invasive index of autonomic nervous system activity. Reduced HRV has been consistently linked to worsening heart failure, arrhythmogenesis, and increased mortality [
36]. In individuals with chronic MA exposure, HRV typically declines with an elevated low-frequency/high-frequency (LF/HF) ratio, indicating sympathetic predominance and parasympathetic withdrawal [
19]. Notably, autonomic dysfunction appears to be at least partially reversible. In abstinent MA users, an eight-week aerobic intervention improved the standard deviation of normal-to-normal intervals (SDNN) by approximately 34% and the root mean square of successive differences (RMSSD) by 63%, while reducing the LF/HF ratio, underscoring autonomic plasticity and potential for rehabilitation [
37].
BRS offers additional insight into neurocardiac regulation by quantifying cardiac responses to changes in blood pressure. Reduced BRS has been reported in acute ischemic stroke and correlates with worse functional outcomes [
38]. Low BRS in combination with abnormal HRV indices may represent a more advanced form of autonomic imbalance and has been associated with a higher risk of adverse cardiovascular events [
38,
39].
Although the prognostic value of HRV and BRS in MACM-related thromboembolism remains to be fully defined, both metrics demonstrate potential as physiological markers of brain–heart–gut axis dysregulation. Incorporating autonomic parameters into multimodal risk stratification, alongside biochemical and imaging data, may improve early identification of high-risk patients and support individualized management strategies.
4. Embolic Patterns and Clinical Presentation: Differences Between MACM and Conventional Cardioembolic Stroke
4.1. MACM vs. AF: Clinical and Radiographic Differences
Cardioembolic strokes secondary to MACM exhibit distinct clinical and radiographic features compared to strokes related to AF (
Table 1). These events frequently arise during episodes of acute decompensated heart failure, where patients demonstrate marked systolic dysfunction and substantial reductions in LVEF, occasionally progressing to cardiogenic shock [
5]. MA users are also predisposed to arrhythmias and infective endocarditis, both of which further heighten ischemic stroke risk. Continued MA use has been associated with increased cardiovascular mortality, recurrent stroke, and rehospitalization, underscoring the link between progressive cardiac dysfunction and embolic events [
7,
9].
Importantly, MACM-related strokes can occur in the absence of documented arrhythmia. Although MA use increases the risk of tachyarrhythmias, QT prolongation, and AF, prolonged electrocardiography (ECG) monitoring often fails to identify AF episodes. This suggests that LVT in MACM may precipitate stroke without overt electrical abnormalities, reinforcing the need for heightened diagnostic vigilance [
9].
The anatomical origin and morphology of thrombi also differ between MACM and AF. AF-related emboli typically arise from the left atrial appendage and are small and friable, leading to multifocal small-vessel occlusions. In contrast, MACM is characterized by pronounced ventricular dilatation and impaired contractility, which promote the formation of larger and structurally stable apical thrombi, and in some cases, biventricular thrombi. These emboli are more likely to cause a single large-vessel occlusion [
7,
9,
14].
Unfavorable prognoses are common in patients with MACM, who often present with chamber enlargement, abnormal cardiac biomarkers, and higher rates of emergency department visits, hospital admissions, and prolonged hospital stays. Cardiac function may improve after MA cessation, whereas continued use is strongly associated with myocardial fibrosis and persistent systolic failure [
5,
40].
Neuroimaging findings further differentiate these etiologies: AF-related strokes typically appear as scattered multifocal infarcts, whereas MACM-related events more commonly involve a single large cerebral artery with a visible apical thrombus source [
7,
9,
14,
41]. These distinctions have significant implications for diagnostic strategy and anticoagulation selection, underscoring the need for personalized management in this population.
4.2. Diagnostic Pitfalls: MACM and Embolic Stroke of Undetermined Source (ESUS)
Despite these characteristic clinical and imaging features, MACM-related strokes can be misclassified in clinical practice. In young ischemic stroke patients lacking traditional vascular risk factors, embolic-pattern infarcts may be labeled as ESUS when no embolic source is initially identified. In this context, systematic screening for substance use and toxicology testing is an essential component of evaluating patients for potential MACM-related LVT [
9]. Based on established criteria, imaging-confirmed LVT or significant left ventricular systolic dysfunction constitutes a primary cardioembolic source and therefore excludes ESUS categorization [
9,
41].
The diagnostic workflow for suspected MACM differs from the exclusion-based ESUS evaluation and instead benefits proactive, thrombus-directed assessment. At the initial neurological assessment, stimulant use evaluation may be appropriately paired with high-sensitivity cardiac imaging, including CMR or CCT, to detect subtle or mural LVT often missed by routine TTE [
5]. This active and confirmatory diagnostic strategy helps reduce the risk of ESUS misclassification, facilitates recognition of LVT arising from severe drug-related cardiomyopathy, and supports timely initiation of anticoagulation therapy (
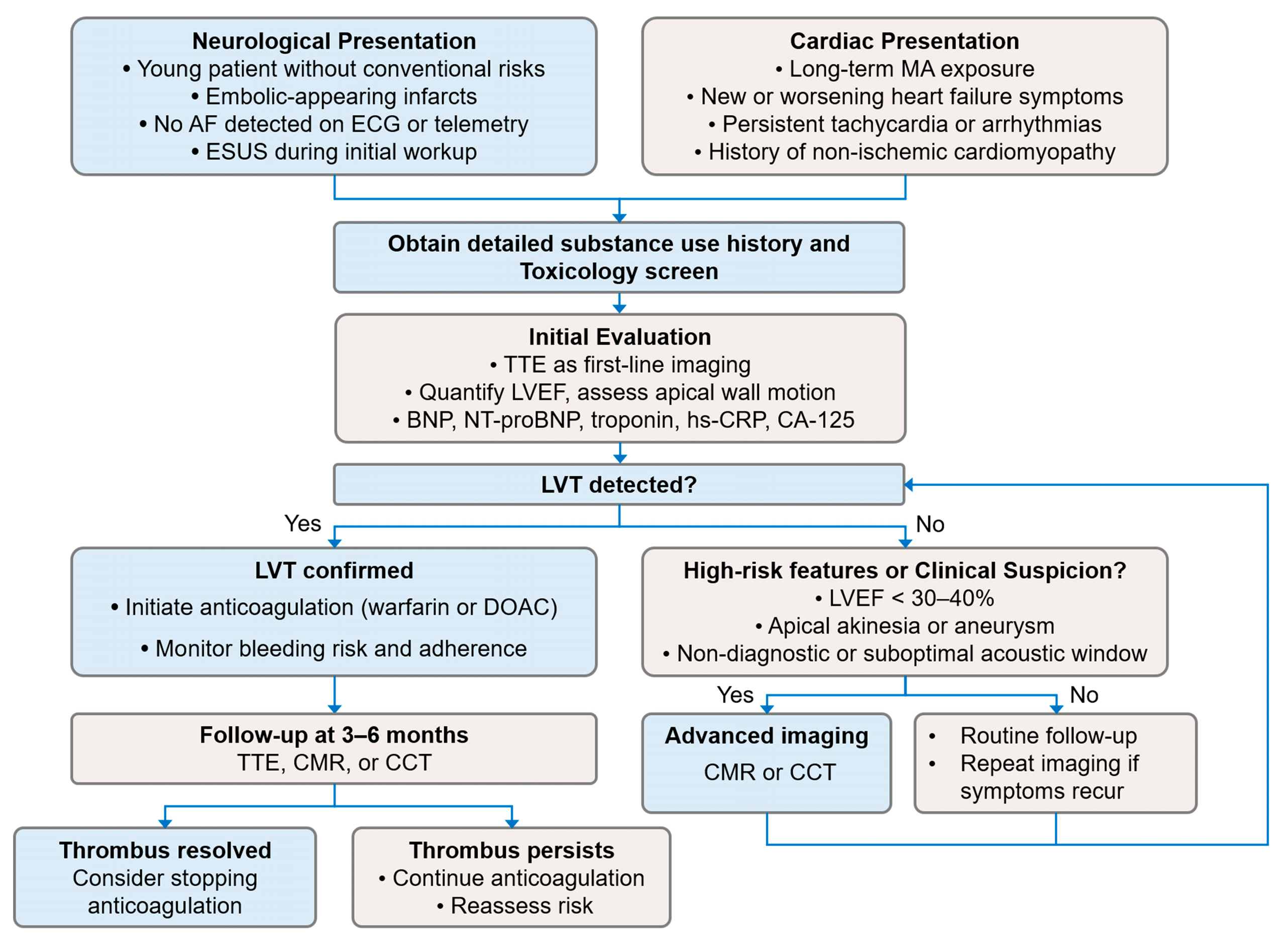
Figure 3).
5. Clinical Management of MACM with LVT
Management of MACM with LVT requires simultaneous treatment of heart failure, arrhythmias, and thromboembolism. Clinical care should integrate hemodynamic stabilization, guideline-directed medical therapy, structured substance cessation, and multidisciplinary support.
5.1. Cardiac Stabilization: Heart Failure and Arrhythmia Management
Guideline-directed heart failure therapy remains the cornerstone of clinical management. β-adrenergic blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor–neprilysin inhibitors, mineralocorticoid receptor antagonists, and sodium–glucose cotransporter-2 inhibitors should be initiated when hemodynamically tolerated, consistent with guideline-directed therapy for dilated cardiomyopathy and severe systolic dysfunction [
5,
42]. Diuretics provide symptomatic relief from congestion, while short-term inotropes or vasopressors may be required in cases of cardiogenic shock. Benzodiazepines are favored for managing sympathetic overactivity associated with stimulant withdrawal or severe agitation [
5,
42].
Arrhythmia management is critical, as ventricular tachyarrhythmias and AF are frequently encountered in MACM [
9]. β-blockers serve dual roles in rate control and sympathetic suppression, whereas refractory cases may require amiodarone or catheter ablation [
5].
Intra-aortic balloon pump counterpulsation, Impella microaxial flow pumps, and veno-arterial extracorporeal membrane oxygenation can serve as a bridge to myocardial recovery in acute cardiogenic shock [
5,
7]. The presence of LVT warrants prompt anticoagulation to mitigate embolic complications.
5.2. Anticoagulation Strategies for MACM with LVT
Evidence specific to anticoagulation in MACM complicated by LVT remains scarce, with current management generally extrapolated from studies of LVT in other forms of non-ischemic cardiomyopathy and post-myocardial infarction populations [
5,
7]. In non-ischemic cardiomyopathy, routine oral anticoagulation for primary prevention is not recommended for patients in sinus rhythm; anticoagulation is generally reserved for confirmed LVT or selected high-risk subtypes such as left ventricular noncompaction [
12]. Accordingly, in MACM, reduced LVEF alone without evidence of thrombus is not an indication for routine anticoagulation. Individualized consideration is reasonable when marked regional wall motion abnormalities or apical aneurysms raise concern for stasis [
5,
7,
12].
Vitamin K antagonists, particularly warfarin, have historically served as standard therapy for LVT. Recent systematic reviews and meta-analyses indicate that direct oral anticoagulants (DOACs) demonstrate comparable efficacy to warfarin in thrombus resolution, prevention of systemic embolism, and overall safety, with a potentially lower bleeding risk in selected populations [
12,
43,
44].
LVT morphology may add complexity to treatment decisions; however, current evidence does not support morphology-specific anticoagulation strategies. Newly identified mural and protuberant thrombi are typically managed similarly with oral anticoagulation, and no robust data exist to guide differences in drug selection [
12].
Substance use, irregular follow-up, and inconsistent adherence complicate anticoagulant selection in MACM. DOACs are often favored when international normalized ratio (INR) monitoring is unfeasible or when frequent laboratory testing is uncertain [
12,
45].
Overall, DOACs represent a reasonable alternative to warfarin, particularly when INR control is suboptimal. However, no specific clinical or imaging-based thresholds exist to favor DOACs in MACM-related LVT. Treatment decisions should be individualized, taking into account thrombus characteristics, bleeding risk, likelihood of adherence, and feasibility of imaging follow-up, ideally within the context of shared decision-making.
5.3. Anticoagulation Duration and Monitoring
Duration and monitoring strategies for MACM-related LVT have not been prospectively studied. The 2022 AHA scientific statement recommends anticoagulation for 3 to 6 months with serial imaging to confirm thrombus resolution. Discontinuation may be considered if the thrombus has completely resolved and high-risk features, such as severely reduced LVEF or persistent regional wall motion abnormalities, are absent. Conversely, prolonged therapy is warranted if thrombus persists or high-risk structural abnormalities remain [
5,
12] (
Figure 3).
When embolic events occur despite therapeutic anticoagulation, rigorous reassessment is required. Verifying anticoagulant compliance and screening for substance relapse are the requisite initial steps, as nonadherence is the predominant cause of therapeutic failure in this population [
5,
7]. If compliance is confirmed and events persist, repeat cardiac imaging is warranted to reassess thrombus burden, mobility, and structural status. Treatment modifications with anticoagulant class-switching should follow standard secondary stroke prevention guidelines: transitioning from DOACs to warfarin, or from warfarin (despite therapeutic INR) to low-molecular-weight heparin or an alternative DOAC [
5,
12,
46]. Use of concomitant antiplatelet is discouraged unless clearly indicated (e.g., recent coronary stent placement) to avoid excess bleeding risk [
46].
For acute ischemic stroke, management follows standard guidelines, prioritizing revascularization (intravenous thrombolysis or mechanical thrombectomy) when eligibility criteria are met, with subsequent cautious blood pressure control to reduce reperfusion injury [
46].
In the long term, patients with persistent LVEF ≤ 35% despite optimized medical therapy and MA abstinence, implantable cardioverter–defibrillator evaluation is warranted to mitigate the risk of sudden cardiac death [
5].
Recurrent embolism in MACM often reflects persistent dysfunction, thrombotic burden, and unstable adherence. Management must integrate anticoagulant strategy, structural heart care, and substance use intervention through coordinated, multidisciplinary care [
5,
7,
9].
5.4. Unique Challenges and Integrated Care Considerations
MACM-related stroke predominantly affects young adult males, who frequently demonstrate ongoing MA use, polysubstance exposure, unstable housing, unemployment, and poor medical adherence [
5,
7]. These factors, together with a higher prevalence of hypertension and alcohol misuse, contribute to attenuated myocardial recovery, recurrent decompensation, and suboptimal neurorehabilitation outcomes [
42,
47].
Absolute MA abstinence is a critical component of therapy, as sustained cessation has been associated with partial or even full recovery of cardiac function. Myocardial function can improve significantly after months of sobriety, especially if instituted before irreversible fibrosis has developed [
7,
48,
49,
50]. Adherence to anticoagulation is undermined by socioeconomic and behavioral instability. Furthermore, comorbidities elevate the risk of major bleeding events such as gastrointestinal or intracranial hemorrhage [
9,
51,
52]. In this context, pharmacologic management alone is insufficient to achieve durable clinical benefit.
Multidisciplinary care is imperative. Neuromodulatory interventions, particularly repetitive transcranial magnetic stimulation (rTMS) and intermittent theta burst stimulation (iTBS) targeting the dorsolateral prefrontal cortex, have demonstrated reductions in craving intensity and improvements in executive control in randomized clinical trials [
53,
54]. Complementary psychological therapies, including cognitive behavioral therapy, contingency management, mindfulness-based interventions, and group counseling, have been shown to improve adherence and support sustained abstinence. Social work involvement is similarly critical for facilitating access to housing, employment resources, and community support networks, thereby enhancing stability and continuity of care [
55,
56].
The long-term prognosis in MACM is closely tied to psychosocial factors. Integrating cardiovascular and cerebrovascular care with behavioral and social interventions offers the best opportunity to reduce recurrence and improve outcomes.
6. Clinical Case Illustration
A 34-year-old man with no significant past medical history presented to the emergency department with sudden-onset left-sided weakness and dysarthria. Upon admission, his blood pressure was 153/99 mmHg, and his heart rate was 101 beats per minute. Neurological examination revealed right gaze deviation, left visual field deficit, left facial droop, and severe left hemiplegia. The initial National Institutes of Health Stroke Scale (NIHSS) score was 16, consistent with severe ischemic stroke.
Non-contrast brain CT revealed a large hypodense lesion in the right middle cerebral artery (MCA) territory (
Figure 4A). Subsequent diffusion-weighted magnetic resonance imaging (DWI) confirmed acute ischemic infarction (
Figure 4B), and magnetic resonance angiography (MRA) demonstrated complete occlusion of the right internal carotid artery (ICA) (
Figure 4C). Urine toxicology was positive for MA, and the patient reported daily use for over five years.
Cardiothoracic CT revealed a large thrombus within the left ventricular cavity (
Figure 4D). TTE showed four-chamber enlargement and severe systolic dysfunction, with a LVEF of 24%, consistent with MACM. Continuous 24 h ECG monitoring did not detect AF. Laboratory tests revealed an elevated Factor VIII level at 257% (reference range: 60–150%) and a CA-125 level of 168.8 U/mL (normal range: <35.0 U/mL), indicating a hypercoagulable state and increased cardiac filling pressures.
On hospital day 5, the patient developed a new right cerebellar infarction (
Figure 4E), indicating ongoing embolic activity. Initial anticoagulation with warfarin was complicated by gastrointestinal bleeding, prompting a switch to rivaroxaban, which was continued without further thromboembolic or bleeding events during hospitalization.
He was discharged on day 26 with persistent neurological deficits (NIHSS 18, modified Rankin Scale [mRS] of 4), requiring 24 h care, and was transferred to a long-term care facility. At the three-month follow-up, his mRS score remained unchanged, and no recurrent stroke events were reported. Given severe post-stroke disability and transition to long-term care, no follow-up cardiac imaging was obtained.
This case highlights the substantial cardiovascular and cerebrovascular risk associated with MACM. In young patients with ischemic stroke, the coexistence of severe left ventricular systolic dysfunction and a hypercoagulable profile should prompt consideration of drug-related cardiomyopathy as a cardioembolic source. Cardiac and thoracic imaging facilitates early identification of LVT, particularly when arrhythmia is absent. The markedly elevated CA-125 level in this patient may reflect cardiac congestion and warrants further investigation as a potential adjunctive biomarker for disease severity and monitoring.
The successful transition from warfarin to a DOAC in the setting of gastrointestinal bleeding illustrates a feasible alternative in high-risk patients, despite the lack of randomized trial data in MACM-related LVT. This case underscores the importance of individualized anticoagulation strategies and highlights the urgent need for dedicated clinical evidence to guide optimal management and long-term prognosis in this growing population.
7. Literature Context: Published Evidence on MACM with Thrombotic Complications
Current evidence on MACM remains limited and heterogeneous, with most publications consisting of small observational cohorts supplemented by single-patient case reports. Using a predefined search strategy and selection criteria (
Supplementary Table S1), we synthesized these data into
Table 2, which integrates both cohort-level findings and detailed case reports. [
7,
10,
14,
45,
48,
49,
50,
57,
58,
59,
60,
61,
62,
63,
64,
65,
66,
67,
68,
69].
Across published studies, affected individuals are typically young, predominantly male, and frequently demonstrate marked left ventricular systolic dysfunction, with reported mean LVEF ranging from 19% to 30%. Studies incorporating systematic cardiac imaging have identified a meaningful prevalence of LVT, approximately one-third of patients in Schürer (2017) [
7] and 12% in Zhao (2020) [
49], raising concern that the true burden may be underestimated in settings where surveillance imaging is not routinely performed. Abstinence consistently correlates with improved outcomes, including higher follow-up LVEF, fewer heart failure hospitalizations, and lower mortality [
48,
49,
50,
58]. Even among individuals with severe systolic dysfunction, abstinence was associated with meaningful LVEF recovery [
50]. In stroke populations, as demonstrated by Lee et al. (2024) [
10], MACM was strongly associated with impaired systolic function, LVT burden, and cardioembolic mechanisms, reinforcing the heart–brain interaction in this disease process.
The case-level data in
Table 2 further expand this spectrum, illustrating infrequent but clinically important presentations, including biventricular thrombi [
60,
65], massive apical thrombi [
65], and recurrent stroke occurring in the context of MACM [
14,
68]. These reports also highlight the broader systemic embolic burden associated with MACM, encompassing aortic and limb ischemia [
61,
66,
69], renal infarction [
61,
69], pulmonary embolism [
60,
65], and cardiogenic shock resulting from extensive ventricular thrombi [
61]. Management strategies varied substantially, ranging from unfractionated heparin, DOACs, and warfarin bridging to mechanical thrombectomy and circulatory support, reflecting the absence of consensus on antithrombotic selection, timing, or duration. Follow-up imaging protocols were inconsistently reported, limiting evaluation of thrombus resolution, embolic recurrence, and criteria for anticoagulation tapering.
8. Limitations
This review is narrative in scope and therefore limited to observational-level evidence. Interpretation of existing studies is constrained by substantial methodological heterogeneity. Diagnostic criteria for MACM are not standardized, and the use of advanced cardiac imaging varies widely across reports. Anticoagulation practices also demonstrate considerable variability in drug selection, treatment duration, and criteria for discontinuation, resulting in inconsistent outcome reporting.
Long-term follow-up data are scarce, particularly regarding thrombus resolution, recurrence, bleeding risk, and outcomes after withdrawal of therapy. Definitions of embolic endpoints and clinical deterioration differ between studies, further preventing pooled analysis or quantitative synthesis.
Given these limitations, current knowledge relies heavily on small cohorts and case-level evidence. The available literature is also likely affected by publication bias, as severe or recurrent embolic events are more frequently reported, whereas patients with milder ventricular dysfunction or transient thrombi may be underrepresented. Prospective registries, standardized imaging protocols, and harmonized outcome definitions will be crucial for generating MACM-specific evidence and informing future clinical practice.
9. Conclusions
MACM has emerged as an important cause of heart failure and cardioembolic stroke in younger adults, yet it remains underrecognized in clinical practice. Chronic MA exposure disrupts regulatory pathways within the brain–heart–gut axis, leading to dysregulation of dopaminergic and noradrenergic signaling. This results in autonomic imbalance and sustained sympathetic overactivation. Additionally, alterations in gut microbiota and systemic inflammation promote oxidative stress, microvascular injury, and a prothrombotic milieu. These neuro-immune mechanisms contribute to myocardial fibrosis, ventricular remodeling, and impaired intracardiac flow, thereby increasing the propensity for LVT formation and systemic embolization.
From a diagnostic perspective, MACM should be considered in the differential diagnosis of cardioembolic or cryptogenic stroke in young adults, particularly in the presence of positive toxicology screens or unexplained left ventricular systolic dysfunction. Early utilization of high-resolution cardiac imaging may improve the detection of LVT and guide anticoagulation decisions. Although DOACs have demonstrated favorable safety and efficacy profiles in mixed-etiology LVT, no randomized evidence exists specifically for MACM, and treatment decisions currently require individualized risk assessment and close imaging surveillance.
Clinical outcomes in MACM are also heavily influenced by behavioral and psychosocial factors, including continued substance use, limited healthcare access, inadequate addiction support, and poor medication adherence. These factors contribute to persistent ventricular dysfunction, interruption of anticoagulation, and recurrent thromboembolism, underscoring that cardiac therapy alone is insufficient. Integrated care models centered on addiction treatment, longitudinal follow-up, and supportive services are essential to achieve durable improvement.
Early recognition, cessation of MA use, individualized anticoagulation guided by multimodal cardiac imaging, and integration of addiction treatment into stroke and heart failure pathways are essential to improving outcomes. Prospective registries and multicenter studies are needed to establish MACM-specific risk stratification tools, standardized diagnostic protocols, and comprehensive treatment strategies.
Author Contributions
P.-J.L.: methodology, literature screening, data curation, analysis and interpretation, writing—original draft preparation, and visualization. C.-H.W.: literature screening, data curation, and writing—original draft preparation. J.-H.H.: data interpretation, writing—review and editing. J.H.B.M.: literature screening, data curation, and writing—review and editing. C.-T.H.: methodology, supervision, and writing—review and editing. L.C.: methodology, supervision, and writing—review and editing. C.-C.C.: conceptualization, methodology, data curation, literature screening, writing—review and editing, and visualization. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Joint Institutional Review Board of Taipei Medical University (TMU-JIRB Approval No. N202406005 on 21 June 2024).
Informed Consent Statement
Patient consent was waived by the Joint Institutional Review Board of Taipei Medical University due to the retrospective design and minimal risk to the participant.
Data Availability Statement
All data cited in this review were obtained from publicly available, peer-reviewed sources indexed in PubMed and Scopus. No new data were generated or analyzed in the course of this work. Therefore, data sharing is not applicable.
Acknowledgments
The authors gratefully acknowledge the contributions of researchers whose work has advanced understanding in this field. Due to space limitations, it was not possible to cite all relevant studies, and the authors extend their appreciation and respect to those whose important work could not be referenced in this review. We sincerely thank the editors and reviewers for their valuable insights, which strengthened the manuscript from both clinical and conceptual perspectives. We appreciate the time and expertise they devoted to this work.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AF | Atrial fibrillation |
| BNP | B-type natriuretic peptide |
| BRS | Baroreflex Sensitivity |
| CA-125 | Cancer antigen-125 |
| CCT | Cardiac computed tomography |
| CMR | Cardiac magnetic resonance imaging |
| CNS | Central nervous system |
| DOAC | Direct oral anticoagulant |
| DWI | Diffusion-weighted imaging |
| ENS | Enteric nervous system |
| ESUS | Embolic Stroke of Undetermined Source |
| GDMT | Guideline-directed medical therapy |
| HRV | Heart rate variability |
| LPS | Lipopolysaccharide |
| LVEF | Left ventricular ejection fraction |
| LVT | Left ventricular thrombus |
| MA | Methamphetamine |
| MACM | Methamphetamine-associated cardiomyopathy |
| MRA | Magnetic resonance angiography |
| MRI | magnetic resonance imaging |
| mRS | Modified Rankin Scale |
| NIHSS | National Institutes of Health Stroke Scale |
| NT-proBNP | N-terminal pro-B-type natriuretic peptide |
| ROS | Reactive oxygen species |
| TMAO | Trimethylamine N-oxide |
| TTE | Transthoracic echocardiography |
References
- Kevil, C.G.; Goeders, N.E.; Woolard, M.D.; Bhuiyan, M.S.; Dominic, P.; Kolluru, G.K.; Arnold, C.L.; Traylor, J.G.; Orr, A.W. Methamphetamine Use and Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1739–1746. [Google Scholar] [CrossRef]
- Dominic, P.; Ahmad, J.; Awwab, H.; Bhuiyan, M.S.; Kevil, C.G.; Goeders, N.E.; Murnane, K.S.; Patterson, J.C.; Sandau, K.E.; Gopinathannair, R.; et al. Stimulant Drugs of Abuse and Cardiac Arrhythmias. Circ. Arrhythmia Electrophysiol. 2022, 15, e010273. [Google Scholar] [CrossRef]
- Lin, H.H.-H.; Wang, Y.-H.; Liu, J.I.W.W.; Hsieh, M.-C.; Huang, S.-J.; Lien, E.; Huang, L.-W.; Lin, A.Y.-C. Evaluation of spatial and temporal changes in illicit drug use in the Taipei metropolitan area via wastewater-based epidemiology. Sci. Total Environ. 2024, 934, 173313. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Wong, B.-Y.; Lo, M.-T.; Chiu, Y.-C.; Lin, Y.-H. Development of an addiction index and delineation 15-year trends of illicit drugs from the Taiwan national drug enhancement database. J. Psychiatr. Res. 2020, 120, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.K.V.; Ng, T.M.H.; Oh, E.E.; Moady, G.; Elkayam, U. Clinical Characteristics and Management of Methamphetamine-Associated Cardiomyopathy: State-of-the-Art Review. J. Am. Heart Assoc. 2020, 9, e016704. [Google Scholar] [CrossRef]
- Jayanthi, S.; Daiwile, A.P.; Cadet, J.L. Neurotoxicity of methamphetamine: Main effects and mechanisms. Exp. Neurol. 2021, 344, 113795. [Google Scholar] [CrossRef]
- Schürer, S.; Klingel, K.; Sandri, M.; Majunke, N.; Besler, C.; Kandolf, R.; Lurz, P.; Luck, M.; Hertel, P.; Schuler, G.; et al. Clinical Characteristics, Histopathological Features, and Clinical Outcome of Methamphetamine-Associated Cardiomyopathy. JACC Heart Fail. 2017, 5, 435–445, Erratum in JACC Heart Fail. 2017, 5, 620. [Google Scholar] [CrossRef]
- George, M.G. Risk Factors for Ischemic Stroke in Younger Adults. Stroke 2020, 51, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Hemphill, K.; Tierney, S.; Tirschwell, D.; Davis, A.P. A review of methamphetamine use and stroke in the young. Front. Neurol. 2024, 15, 1397677. [Google Scholar] [CrossRef]
- Lee, S.J.; Liu, S.; Blackwill, H.; Stradling, D.; Shafie, M.; Yu, W. Cardiomyopathy in Patients With Acute Ischemic Stroke and Methamphetamine Use: Relevance for Cardioembolic Stroke and Outcome. J. Am. Heart Assoc. 2024, 13, e033667. [Google Scholar] [CrossRef]
- Benito, Y.; Martinez-Legazpi, P.; Rossini, L.; Pérez del Villar, C.; Yotti, R.; Martín Peinador, Y.; Rodríguez-Pérez, D.; Desco, M.M.; Medrano, C.; Antoranz, J.C.; et al. Age-Dependence of Flow Homeostasis in the Left Ventricle. Front. Physiol. 2019, 10, 485. [Google Scholar] [CrossRef]
- Levine, G.N.; McEvoy, J.W.; Fang, J.C.; Ibeh, C.; McCarthy, C.P.; Misra, A.; Shah, Z.I.; Shenoy, C.; Spinler, S.A.; Vallurupalli, S.; et al. Management of Patients at Risk for and With Left Ventricular Thrombus: A Scientific Statement From the American Heart Association. Circulation 2022, 146, e205–e223. [Google Scholar] [CrossRef]
- Paur, H.; Wright, P.T.; Sikkel, M.B.; Tranter, M.H.; Mansfield, C.; O’Gara, P.; Stuckey, D.J.; Nikolaev, V.O.; Diakonov, I.; Pannell, L.; et al. High Levels of Circulating Epinephrine Trigger Apical Cardiodepression in a β2-Adrenergic Receptor/Gi–Dependent Manner. Circulation 2012, 126, 697–706. [Google Scholar] [CrossRef]
- Dhaliwal, J.S.S.; Ansari, S.A.; Ghosh, S.; Chitkara, A.; Khizer, U. Duet of Death: Biventricular Thrombus in a Methamphetamine User. Cureus 2023, 15, e39917. [Google Scholar] [CrossRef]
- Gebhard, C.; Breitenstein, A.; Akhmedov, A.; Gebhard, C.E.; Camici, G.G.; Lüscher, T.F.; Tanner, F.C. Amphetamines induce tissue factor and impair tissue factor pathway inhibitor: Role of dopamine receptor type 4. Eur. Heart J. 2010, 31, 1780–1791. [Google Scholar] [CrossRef]
- Reddy, P.; O’Meara, K.; Patel, S.; Chau, E.; Chukumerije, M.; Mehra, A.; Ng, T.; Elkayam, U. Clinical Differences between Methamphetamine and Non-Methamphetamine Associated Non-Ischemic Dilated Cardiomyopathy. J. Card. Fail. 2019, 25, S62–S63. [Google Scholar] [CrossRef]
- Ramli, F.F.; Rejeki, P.S.; Ibrahim, N.; Abdullayeva, G.; Halim, S. A Mechanistic Review on Toxicity Effects of Methamphetamine. Int. J. Med. Sci. 2025, 22, 482–507. [Google Scholar] [CrossRef]
- Ferrucci, M.; Limanaqi, F.; Ryskalin, L.; Biagioni, F.; Busceti, C.L.; Fornai, F. The Effects of Amphetamine and Methamphetamine on the Release of Norepinephrine, Dopamine and Acetylcholine from the Brainstem Reticular Formation. Front. Neuroanat. 2019, 13, 48. [Google Scholar] [CrossRef] [PubMed]
- Henry, B.L.; Minassian, A.; Perry, W. Effect of methamphetamine dependence on heart rate variability. Addict. Biol. 2012, 17, 648–658. [Google Scholar] [CrossRef] [PubMed]
- van Weperen, V.Y.H.; Vaseghi, M. Cardiac vagal afferent neurotransmission in health and disease: Review and knowledge gaps. Front. Neurosci. 2023, 17, 1192188. [Google Scholar] [CrossRef] [PubMed]
- Borrego-Ruiz, A.; Borrego, J.J. The Bidirectional Interplay Between Substances of Abuse and Gut Microbiome Homeostasis. Life 2025, 15, 834. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, X.; Liu, X.; Liu, G.; Zeng, K.; Wang, G. Altered fecal microbiota composition in individuals who abuse methamphetamine. Sci. Rep. 2021, 11, 18178. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Kong, D.; Sun, J.-x.; Ma, Z.-x.; Wang, G.-q.; Ma, X.-f.; Sun, L.; Luo, H.-y.; Xu, Y.; Wang, K.-h. Intestinal barrier damage caused by addictive substance use disorder. Eur. J. Med. Res. 2025, 30, 226. [Google Scholar] [CrossRef]
- Simpson, S.; McLellan, R.; Wellmeyer, E.; Matalon, F.; George, O. Drugs and Bugs: The Gut-Brain Axis and Substance Use Disorders. J. Neuroimmune Pharmacol. 2022, 17, 33–61. [Google Scholar] [CrossRef]
- Zhu, W.; Gregory, J.C.; Org, E.; Buffa, J.A.; Gupta, N.; Wang, Z.; Li, L.; Fu, X.; Wu, Y.; Mehrabian, M.; et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell 2016, 165, 111–124. [Google Scholar] [CrossRef]
- Chen, M.L.; Zhu, X.H.; Ran, L.; Lang, H.D.; Yi, L.; Mi, M.T. Trimethylamine-N-Oxide Induces Vascular Inflammation by Activating the NLRP3 Inflammasome Through the SIRT3-SOD2-mtROS Signaling Pathway. J. Am. Heart Assoc. 2017, 6, e006347. [Google Scholar] [CrossRef] [PubMed]
- Chou, R.H.; Chen, C.Y.; Chen, I.C.; Huang, H.L.; Lu, Y.W.; Kuo, C.S.; Chang, C.C.; Huang, P.H.; Chen, J.W.; Lin, S.J. Trimethylamine N-Oxide, Circulating Endothelial Progenitor Cells, and Endothelial Function in Patients with Stable Angina. Sci. Rep. 2019, 9, 4249. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, C.S.; Aishwarya, R.; Alam, S.; Morshed, M.; Remex, N.S.; Nitu, S.; Kolluru, G.K.; Traylor, J.; Miriyala, S.; Panchatcharam, M.; et al. Methamphetamine induces cardiomyopathy by Sigmar1 inhibition-dependent impairment of mitochondrial dynamics and function. Commun. Biol. 2020, 3, 682. [Google Scholar] [CrossRef]
- Weinsaft, J.W.; Kim, R.J.; Ross, M.; Krauser, D.; Manoushagian, S.; LaBounty, T.M.; Cham, M.D.; Min, J.K.; Healy, K.; Wang, Y.; et al. Contrast-enhanced anatomic imaging as compared to contrast-enhanced tissue characterization for detection of left ventricular thrombus. JACC Cardiovasc. Imaging 2009, 2, 969–979. [Google Scholar] [CrossRef]
- Chaosuwannakit, N.; Makarawate, P. Left Ventricular Thrombi: Insights from Cardiac Magnetic Resonance Imaging. Tomography 2021, 7, 180–188. [Google Scholar] [CrossRef]
- Kwok, C.S.; Bennett, S.; Borovac, J.A.; Schwarz, K.; Lip, G.Y.H. Predictors of left ventricular thrombus after acute myocardial infarction: A systematic review and meta-analysis. Coron. Artery Dis. 2023, 34, 250–259. [Google Scholar] [CrossRef]
- Ghozy, S.; Liu, M.; Kobeissi, H.; Mortezaei, A.; Amoukhteh, M.; Abbas, A.S.; Dmytriw, A.A.; Kadirvel, R.; Rabinstein, A.A.; Kallmes, D.F.; et al. Cardiac CT vs Echocardiography for Intracardiac Thrombus Detection in Ischemic Stroke. Neurology 2024, 103, e209771. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Yu, S. The Value of Combined Detection of Serum BNP, Cardiac Troponin-I and Dynamic Electrocardiogram in Early Clinical Diagnosis and Prognosis of Patients with Acute Myocardial Infarction. Discov. Med. 2024, 36, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Marinescu, M.C.; Oprea, V.D.; Munteanu, S.N.; Nechita, A.; Tutunaru, D.; Nechita, L.C.; Romila, A. Carbohydrate Antigen 125 (CA 125): A Novel Biomarker in Acute Heart Failure. Diagnostics 2024, 14, 795. [Google Scholar] [CrossRef]
- Núñez, J.; de la Espriella, R.; Miñana, G.; Santas, E.; Llácer, P.; Núñez, E.; Palau, P.; Bodí, V.; Chorro, F.J.; Sanchis, J.; et al. Antigen carbohydrate 125 as a biomarker in heart failure: A narrative review. Eur. J. Heart Fail. 2021, 23, 1445–1457. [Google Scholar] [CrossRef]
- Jarczok, M.N.; Weimer, K.; Braun, C.; Williams, D.P.; Thayer, J.F.; Gündel, H.O.; Balint, E.M. Heart rate variability in the prediction of mortality: A systematic review and meta-analysis of healthy and patient populations. Neurosci. Biobehav. Rev. 2022, 143, 104907. [Google Scholar] [CrossRef]
- Dolezal, B.A.; Chudzynski, J.; Dickerson, D.; Mooney, L.; Rawson, R.A.; Garfinkel, A.; Cooper, C.B. Exercise training improves heart rate variability after methamphetamine dependency. Med. Sci. Sports Exerc. 2014, 46, 1057–1066. [Google Scholar] [CrossRef]
- Tsai, W.C.; Lin, H.C.; Lai, Y.R.; Hsu, C.W.; Huang, C.C.; Wang, H.C.; Su, C.M.; Su, Y.J.; Lin, W.C.; Cheng, B.C.; et al. The Effect of Stroke Subtypes on Baroreceptor Sensitivity, a Predict for Acute Stroke Outcome. Biomed. Res. Int. 2019, 2019, 7614828. [Google Scholar] [CrossRef]
- Lin, C.H.; Yen, C.C.; Hsu, Y.T.; Chen, H.H.; Cheng, P.W.; Tseng, C.J.; Lo, Y.K.; Chan, J.Y.H. Baroreceptor Sensitivity Predicts Functional Outcome and Complications after Acute Ischemic Stroke. J. Clin. Med. 2019, 8, 300. [Google Scholar] [CrossRef]
- Islam, M.N.; Kuroki, H.; Hongcheng, B.; Ogura, Y.; Kawaguchi, N.; Onishi, S.; Wakasugi, C. Cardiac lesions and their reversibility after long term administration of methamphetamine. Forensic Sci. Int. 1995, 75, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, J.; Oak, S.; Tiongson, J.; Vigilante, N.; Frost, E.; Penckofer, M.; Thau, L.; Iezzi, Z.; Patel, P.; Siegler, J.E. Embolic infarct topology differs between atrial fibrillation subtypes and embolic stroke of undetermined source. J. Stroke Cerebrovasc. Dis. 2022, 31, 106782. [Google Scholar] [CrossRef]
- Osekowski, M.; Trytell, A.; La Gerche, A.; Prior, D.; MacIsaac, A.; Paratz, E.D. A Comprehensive Approach to Managing Methamphetamine-Associated Cardiomyopathy. Am. J. Cardiovasc. Drugs 2022, 22, 385–393. [Google Scholar] [CrossRef]
- Vorla, M.; Kalra, D.K. Meta-Analysis of the Safety and Efficacy of Direct Oral Anticoagulants for the Treatment of Left Ventricular Thrombus. Pharmaceuticals 2024, 17, 708. [Google Scholar] [CrossRef] [PubMed]
- Attachaipanich, T.; Thanyaratsarun, T.; Attachaipanich, S.; Danpanichkul, P.; Kaewboot, K. Efficacy of direct oral anticoagulants vs. warfarin in left ventricular thrombus in myocardial infarction: Systematic review and meta-analysis. J. Cardiovasc. Med. 2025, 26, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Schwarzbach, V.; Lenk, K.; Laufs, U. Methamphetamine-related cardiovascular diseases. ESC Heart Fail. 2020, 7, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Kernan, W.N.; Ovbiagele, B.; Black, H.R.; Bravata, D.M.; Chimowitz, M.I.; Ezekowitz, M.D.; Fang, M.C.; Fisher, M.; Furie, K.L.; Heck, D.V.; et al. Guidelines for the Prevention of Stroke in Patients with Stroke and Transient Ischemic Attack. Stroke 2014, 45, 2160–2236, Correction in Stroke 2015, 46, e54 https://www.ahajournals.org/doi/10.1161/STR.0000000000000059. [Google Scholar] [CrossRef]
- Zhao Susan, X.; Kwong, C.; Swaminathan, A.; Gohil, A.; Crawford Michael, H. Clinical Characteristics and Outcome of Methamphetamine-Associated Pulmonary Arterial Hypertension and Dilated Cardiomyopathy. JACC Heart Fail. 2018, 6, 209–218. [Google Scholar] [CrossRef]
- Sliman, S.; Waalen, J.; Shaw, D. Methamphetamine-Associated Congestive Heart Failure: Increasing Prevalence and Relationship of Clinical Outcomes to Continued Use or Abstinence. Cardiovasc. Toxicol. 2016, 16, 381–389. [Google Scholar] [CrossRef]
- Zhao, S.X.; Seng, S.; Deluna, A.; Yu, E.C.; Crawford, M.H. Comparison of Clinical Characteristics and Outcomes of Patients with Reversible Versus Persistent Methamphetamine-Associated Cardiomyopathy. Am. J. Cardiol. 2020, 125, 127–134. [Google Scholar] [CrossRef]
- Bhatia, H.S.; Nishimura, M.; Dickson, S.; Adler, E.; Greenberg, B.; Thomas, I.C. Clinical and echocardiographic outcomes in heart failure associated with methamphetamine use and cessation. Heart 2021, 107, 741–747. [Google Scholar] [CrossRef]
- Suto, D.J.; Pott, E.; Brennan, J.; Jackson, M.; Thomas, I.; Coyne, C.J. Risk Factors and Emergency Department Outcomes in Methamphetamine-Associated Cardiomyopathy: A Case-Control Study. J. Emerg. Med. 2024, 67, e188–e197. [Google Scholar] [CrossRef]
- Cai, Y.; Dai, Z.; Wen, S.; Bhandari, R. Risk factors associated with infection of blood-borne virus among people who used methamphetamine. BMC Infect. Dis. 2020, 20, 742. [Google Scholar] [CrossRef]
- Gay, A.; Cabe, J.; De Chazeron, I.; Lambert, C.; Defour, M.; Bhoowabul, V.; Charpeaud, T.; Tremey, A.; Llorca, P.-M.; Pereira, B.; et al. Repetitive Transcranial Magnetic Stimulation (rTMS) as a Promising Treatment for Craving in Stimulant Drugs and Behavioral Addiction: A Meta-Analysis. J. Clin. Med. 2022, 11, 624. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, Y.; Wang, L.; Mu, L.; Zhu, L.; Ding, D.; Ren, Z.; Yang, D.; Tang, H.; Zhang, L.; et al. High-frequency repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex reduces drug craving and improves decision-making ability in methamphetamine use disorder. Psychiatry Res. 2022, 317, 114904. [Google Scholar] [CrossRef]
- Roberts, K.; Smith, E.; Sousa, C.; Young, J.E.; Corley, A.G.; Szczotka, D.; Sepanski, A.; Hartoch, A. Centering persons who use drugs: Addressing social determinants of health among patients hospitalized with substance use disorders. Soc. Work. Health Care 2024, 63, 19–34. [Google Scholar] [CrossRef]
- Danaee-far, M.; Maarefvand, M.; Rafiey, H. Effectiveness of a Brief Home-Based Social Work Motivational Intervention for Male Methamphetamine Users in Tehran: A Randomized Clinical Trial. Subst. Use Misuse 2016, 51, 1863–1869. [Google Scholar] [CrossRef]
- Voskoboinik, A.; Ihle, J.F.; Bloom, J.E.; Kaye, D.M. Methamphetamine-associated cardiomyopathy: Patterns and predictors of recovery. Intern. Med. J. 2016, 46, 723–727. [Google Scholar] [CrossRef]
- Bhatia, H.S.; Nishimura, M.; Martinez, A.; Vanam, S.; Kahn, A.M.; DeMaria, A.; Thomas, I.C. Systolic dysfunction in patients with methamphetamine use and heart failure with preserved ejection fraction. Int. J. Cardiol. 2022, 348, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Yew, K.L.; Go, C.S.; Razali, F.; Rajendran, P.; Ooi, P.S.; Anum, A. Methamphetamine-associated reversible cardiomyopathy and stroke risk. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2403–2404. [Google Scholar] [PubMed]
- Janardhanan, R.; Kannan, A. Methamphetamine Cardiotoxicity: Unique Presentation with Multiple Bi-Ventricular Thrombi. Am. J. Med. 2016, 129, e3–e4. [Google Scholar] [CrossRef] [PubMed]
- Eliveha, J.; Vindhyal, S.; Vindhyal, M. Cardiac and Systemic Thrombus Caused by Drug Abuse. Case Rep. Cardiol. 2019, 2019, 5083624. [Google Scholar] [CrossRef]
- Navid, H.; Soleimani, H.; Hosseini, K. Wild at heart: 34-year-old male with new onset dyspnea, heart failure and history of amphetamine use; a case report. Egypt. Heart J. 2019, 71, 20. [Google Scholar] [CrossRef]
- Patel, K.R.; Kassir, M.; Patel, M.; Eichorn, W. Left Ventricular Thrombus Formation in a Young Female with a Severely Reduced Left Ventricular Ejection Fraction and a Recent Non-ST Segment Elevation-Acute Coronary Syndrome. Cureus 2021, 13, e17804. [Google Scholar] [CrossRef]
- Del Rio-Pertuz, G.; Morataya, C.; Iskandir, M.; Argueta-Sosa, E. Acute Myocardial Infarction Associated with a Mobile Left Ventricular Thrombi. J. Investig. Med. High Impact Case Rep. 2022, 10, 23247096221078704. [Google Scholar] [CrossRef]
- Zaidi, S.H.; Khan, U.A.; Sagheer, S.; Sheikh, A.; Garcia, M.E. Multiple Biventricular Thrombi Associated with Methamphetamine-Associated Cardiomyopathy. Ochsner J. 2022, 22, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Newman, E. Acute aortic occlusion: A point-of-care ultrasound case report. Australas J. Ultrasound Med. 2024, 27, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Zuern, C.S.; Sticherling, C.; Krisai, P.; Haaf, P. Methamphetamine-associated cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2024, 25, e147. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, T.; Higashida, K.; Hatayama, N.; Nagashima, N.; Shimada, Y.; Fukasaka, I.; Shimizu, M.; Sumikura, H.; Hoshi, T.; Takasugi, J.; et al. Mechanical Thrombectomy for Methamphetamine-Associated Cardiomyopathy with Left Ventricular Thrombus: A Case Report. J. Neuroendovasc. Ther. 2025, 19, cr.2025-0003. [Google Scholar] [CrossRef]
- Moayerifar, M.; Moayerifar, M.; Gholipour, M.; Samidoust, P.; Poursadrolah, S.; Ghasemzadeh, G.; Radmoghadam, M. Multiple cardioembolic attacks with a huge ventricular thrombus in dilated cardiomyopathy: A case report and comprehensive review. J. Int. Med. Res. 2025, 53, 03000605251324593. [Google Scholar] [CrossRef]
Figure 1.
Acute MA toxicity across major organ systems. The schematic depicts the principal acute toxic effects of methamphetamine involving the central nervous, cardiovascular, gastrointestinal, and systemic organ systems.
Figure 1.
Acute MA toxicity across major organ systems. The schematic depicts the principal acute toxic effects of methamphetamine involving the central nervous, cardiovascular, gastrointestinal, and systemic organ systems.
Figure 2.
Proposed integrative model of brain–heart–gut axis dysregulation in chronic MA use to cardioembolic stroke. The schematic illustration depicts the multi-level neurocardiac and systemic pathways through which chronic MA exposure contributes to LVT formation and cardioembolic stroke. MA induces excessive release of dopamine and norepinephrine, leading to sustained activation of cortical (medial prefrontal and anterior cingulate cortices), subcortical (amygdala and hypothalamus), and brainstem autonomic centers (nucleus tractus solitarius, nucleus ambiguus, and rostral ventrolateral medulla). This central autonomic dysfunction drives sympathetic overexcitation and hemodynamic stress, promoting arrhythmias, myocardial injury and remodeling, MACM, baroreflex desensitization, and a hypercoagulable state, thereby predisposing to LVT and cardioembolic stroke. In parallel, MA-induced gut microbiota dysbiosis and intestinal barrier disruption exacerbate systemic inflammation and alter enteric–vagal signaling, thereby further amplifying neurocardiac dysregulation through the circulation of inflammatory mediators (e.g., TNF-α, LPS). These interacting brain–heart–gut feedback loops collectively establish a maladaptive cycle linking autonomic imbalance, myocardial injury, and systemic inflammation in the pathogenesis of MA-related cardioembolic events. Abbreviations: LPS, lipopolysaccharide; LVT, left ventricular thrombus; MACM, methamphetamine-associated cardiomyopathy; MA, methamphetamine; TMAO, trimethylamine N-oxide; TNF-α, tumor necrosis factor-alpha.
Figure 2.
Proposed integrative model of brain–heart–gut axis dysregulation in chronic MA use to cardioembolic stroke. The schematic illustration depicts the multi-level neurocardiac and systemic pathways through which chronic MA exposure contributes to LVT formation and cardioembolic stroke. MA induces excessive release of dopamine and norepinephrine, leading to sustained activation of cortical (medial prefrontal and anterior cingulate cortices), subcortical (amygdala and hypothalamus), and brainstem autonomic centers (nucleus tractus solitarius, nucleus ambiguus, and rostral ventrolateral medulla). This central autonomic dysfunction drives sympathetic overexcitation and hemodynamic stress, promoting arrhythmias, myocardial injury and remodeling, MACM, baroreflex desensitization, and a hypercoagulable state, thereby predisposing to LVT and cardioembolic stroke. In parallel, MA-induced gut microbiota dysbiosis and intestinal barrier disruption exacerbate systemic inflammation and alter enteric–vagal signaling, thereby further amplifying neurocardiac dysregulation through the circulation of inflammatory mediators (e.g., TNF-α, LPS). These interacting brain–heart–gut feedback loops collectively establish a maladaptive cycle linking autonomic imbalance, myocardial injury, and systemic inflammation in the pathogenesis of MA-related cardioembolic events. Abbreviations: LPS, lipopolysaccharide; LVT, left ventricular thrombus; MACM, methamphetamine-associated cardiomyopathy; MA, methamphetamine; TMAO, trimethylamine N-oxide; TNF-α, tumor necrosis factor-alpha.
![Ijms 26 11908 g002 Ijms 26 11908 g002]()
Figure 3.
The proposed diagnostic and management algorithm for suspected MACM and LVT. The flowchart illustrates a diagnostic pathway initiated from two distinct clinical scenarios: (Left) neurological presentations and (Right) cardiac presentations. The algorithm emphasizes the importance of obtaining a detailed substance use history and performing a toxicology screen early in the assessment. Initial cardiac evaluation with TTE is recommended, followed by advanced imaging (CMR or CCT) when clinical suspicion for LVT remains high or when TTE findings are non-diagnostic. Management recommendations, including initiation of anticoagulation and follow-up imaging, are based on established guidelines for LVT detection and treatment. Abbreviations: AF, atrial fibrillation; BNP, B-type natriuretic peptide; CA-125, cancer antigen-125; CCT, cardiac computed tomography; CMR, cardiac magnetic resonance imaging; DOAC, direct oral anticoagulant; ECG, electrocardiogram; ESUS, embolic stroke of undetermined source; hs-CRP, high-sensitivity C-reactive protein; LVEF, left ventricular ejection fraction; LVT, left ventricular thrombus; MA, methamphetamine; NT-proBNP, N-terminal pro-B-type natriuretic peptide; TTE, transthoracic echocardiography.
Figure 3.
The proposed diagnostic and management algorithm for suspected MACM and LVT. The flowchart illustrates a diagnostic pathway initiated from two distinct clinical scenarios: (Left) neurological presentations and (Right) cardiac presentations. The algorithm emphasizes the importance of obtaining a detailed substance use history and performing a toxicology screen early in the assessment. Initial cardiac evaluation with TTE is recommended, followed by advanced imaging (CMR or CCT) when clinical suspicion for LVT remains high or when TTE findings are non-diagnostic. Management recommendations, including initiation of anticoagulation and follow-up imaging, are based on established guidelines for LVT detection and treatment. Abbreviations: AF, atrial fibrillation; BNP, B-type natriuretic peptide; CA-125, cancer antigen-125; CCT, cardiac computed tomography; CMR, cardiac magnetic resonance imaging; DOAC, direct oral anticoagulant; ECG, electrocardiogram; ESUS, embolic stroke of undetermined source; hs-CRP, high-sensitivity C-reactive protein; LVEF, left ventricular ejection fraction; LVT, left ventricular thrombus; MA, methamphetamine; NT-proBNP, N-terminal pro-B-type natriuretic peptide; TTE, transthoracic echocardiography.
![Ijms 26 11908 g003 Ijms 26 11908 g003]()
Figure 4.
(A) Brain CT initially revealed a right middle cerebral artery territory with a large hypodensity (star). (B) Brain MRI showed hyperintensity on DWI in the same area. (C) MRA showed total occlusion of the right internal carotid artery (arrow). (D) Contrast-enhanced CT of the chest revealed a left ventricular thrombus (arrow). (E) Follow-up brain CT showed a new hypodensity in the right cerebellum (star). CT, computed tomography; DWI, diffusion-weighted imaging; MRA, magnetic resonance angiography; MRI, magnetic resonance imaging.
Figure 4.
(A) Brain CT initially revealed a right middle cerebral artery territory with a large hypodensity (star). (B) Brain MRI showed hyperintensity on DWI in the same area. (C) MRA showed total occlusion of the right internal carotid artery (arrow). (D) Contrast-enhanced CT of the chest revealed a left ventricular thrombus (arrow). (E) Follow-up brain CT showed a new hypodensity in the right cerebellum (star). CT, computed tomography; DWI, diffusion-weighted imaging; MRA, magnetic resonance angiography; MRI, magnetic resonance imaging.
Table 1.
Key distinctions between MACM- and AF-related cardioembolic stroke.
Table 1.
Key distinctions between MACM- and AF-related cardioembolic stroke.
| Feature | MACM-Related Stroke | AF-Related Stroke |
|---|
| Underlying mechanism | MA-induced toxic cardiomyopathy with severe LV dysfunction | Atrial arrhythmia causing stasis in the LA |
| Typical cardiac finding | LV dilatation, apical thrombus, may occur without AF | AF on ECG; LA appendage thrombus |
| Thrombus location | LV (often apical) | Left atrial appendage |
| Infarct pattern | Single large-vessel occlusion | Multiple small or scattered infarcts |
| Clinical context | Occurs during decompensated heart failure or MACM | Occurs during or after an AF episode |
| Prognosis | Worse outcomes; improvement with MA cessation | Variable; depends on AF and anticoagulation control |
Table 2.
Published cohorts and case-level reports of MACM, with associated cardiac or systemic thrombotic complications and clinical outcomes.
Table 2.
Published cohorts and case-level reports of MACM, with associated cardiac or systemic thrombotic complications and clinical outcomes.
| Author (Year) | Population/Demographics | LVEF | LVT | Stroke/Embolic Event | Treatments | Clinical Outcome |
|---|
| Yew (2014) [59] | 33-year-old male | Severely reduced | No (spontaneous echo contrast) | Left anterior circulation infarction | Antiplatelets; statin; antihypertensives | Abstinent; neurologic recovery; NYHA I at 6 months; LVEF normalized at 1 year. |
| Janardhanan (2016) [60] | 35-year-old male | 23% | Yes—LV and RV | Pulmonary embolism | IV heparin; warfarin; ticagrelor; HF therapy | Abstinent; LVEF improved to 30% at 3 months; no recurrent embolic events. |
| Sliman (2016) [48] | 141 MA-associated HF (mean 52 ± 9 years; 79% male) | Mean 29.9% | Not reported | Not reported | Standard HF therapy | Abstinence associated with improved NYHA class, better functional status, and trend toward LVEF recovery. |
| Voskoboinik (2016) [57] | 20 MACM (mean 35 ± 9 years; 70% male) | Mean 19.7% | Not reported | Not reported | Inotropes; ICU care; ECMO/IABP; GDMT | Six of 19 achieved LVEF normalization within 6 weeks; reverse-Takotsubo morphology associated with early recovery. |
| Schürer (2017) [7] | 30 MACM (mean 30 ± 9 years; 77% male) | Mean 19% | Yes—single or multiple (10 cases) | Yes—non-fatal stroke reported (composite outcome) | ECMO/IABP; valve surgery; ICD; wearable cardioverter–defibrillator; anticoagulation; GDMT | Abstinence associated with higher follow-up LVEF and fewer composite adverse events (death, nonfatal stroke, and rehospitalization). |
| Eliveha (2019) [61] | 24-year-old female | 15% | Yes—single | Aortic occlusion; superior mesenteric artery thrombus; bilateral renal infarcts; limb ischemia | Surgical thrombectomy; vasopressors; supportive care | Not abstinent; died from cardiogenic shock with end-organ failure. |
| Navid (2019) [62] | 34-year-old male | 10–15% | No (spontaneous echo contrast) | No embolic event; spontaneous coronary artery dissection | GDMT; aspirin; clopidogrel | Not abstinent; persistent severe LV dysfunction; ineligible for transplant. |
| Schwarzbach (2020) [45] | 23-year-old male | 20% | No | Not reported | Inotropes; GDMT; wearable cardioverter–defibrillator vest | Not abstinent; no LV recovery; HF readmission. |
| Zhao (2020) [49] | 357 MACM (mean 47 ± 10 years; 83% male) | ≤40% | 12% overall | Not reported | GDMT; ICD/CRT when indicated | Abstinence was associated higher LVEF and lower HF hospitalization and mortality. |
| Bhatia (2021) [50] | 56 MACM: reduced LVEF group (n = 28, 51 ± 9 years, 82% male); preserved LVEF group (n = 28, 50 ± 8 years, 61% male) | Mean 30.2% in reduced LVEF group; mean 66.2% in preserved LVEF group | Not reported | Not reported | GDMT | Abstinence associated with improved LVEF and fewer HF hospitalizations. |
| Patel (2021) [63] | 30-year-old female | 20% | Yes—single | Stroke-like symptoms without infarction | Apixaban; GDMT | Neurologic symptoms improved; discharged on apixaban; lost to follow-up. |
| Bhatia (2022) [58] | 31 MA-associated preserved LVEF (mean 49 ± 10 years; 55% male) | Median 66% | Not reported | Not reported | Standard HF therapy | Abstinence at 1 year associated with improved GLS and reduced HF admissions. |
| Del Rio-Pertuz (2022) [64] | 33-year-old female | 3–20% | Yes—multiple | STEMI due to coronary embolism | Anticoagulation; GDMT; mechanical aspiration | Persistent low LVEF and LVT. |
| Zaidi (2022) [65] | 48-year-old female | 18% | Yes—multiple; LV and RV | Pulmonary embolism | IV heparin; warfarin | Not abstinent; HF readmission; decreased LVT burden; RV thrombus resolution or embolization. |
| Dhaliwal (2023) [14] | 60-year-old male | 20–25% | Yes—LV and RV | Cortical infarcts | IV thrombolysis; heparin; GDMT; rivaroxaban | Abstinent; NIHSS 0 at discharge; medication compliant. |
| Lee (2024) [10] | 46 MA-associated acute ischemic stroke (mean 52.8 ± 9.6 years; 78.3% male); 14 with MACM | Mean 26% in MACM | Yes—LV thrombus (4 patients) | All patients had acute ischemic stroke | GDMT; antithrombotic therapy based on stroke guidelines; revascularization when indicated | mRS 0–2 at 3 months in 64.3% of MACM subgroup; 2 deaths; MACM strongly associated with cardioembolic stroke (92.9%). |
| Newman (2024) [66] | 39-year-old male | 14% initially; 8% after deterioration | Yes—LV and RV | Aortic occlusion; bilateral limb ischemia | Aortoiliac thrombectomy; IV heparin; rivaroxaban; GDMT | Not abstinent; successful reperfusion; neurologic recovery; ambulatory. |
| Zuern (2024) [67] | 49-year-old male | 35% | Yes—single | No embolic event | Oral anticoagulation; GDMT | LVT resolved; LVEF normalized. |
| Fujioka (2025) [68] | 54-year-old male | 24.1% | Yes—single | Left internal carotid artery occlusion | Warfarin; UFH bridging; mechanical thrombectomy; diuretics; antihypertensives; antiseizure medication | Complete reperfusion; cerebral hyperperfusion; mRS 3 at day 90; LVEF improved to 53%. |
| Moayerifar (2025) [69] | Early-40 s male | 5–10% initially; 10–15% at discharge | Yes—giant thrombus | Left common iliac artery thrombosis; renal infarct; transient dysarthria | IV heparin; warfarin; GDMT; iliac artery stenting | Abstinent; LVT markedly reduced with improved LV size at 3 months; limb ischemia improved; LVEF 35%. |
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).