An ATF3 Inducer Ameliorates Metabolic Dysfunction-Associated Steatotic Liver Disease Through the AMPK and PKA Pathways
Abstract
1. Introduction
2. Results
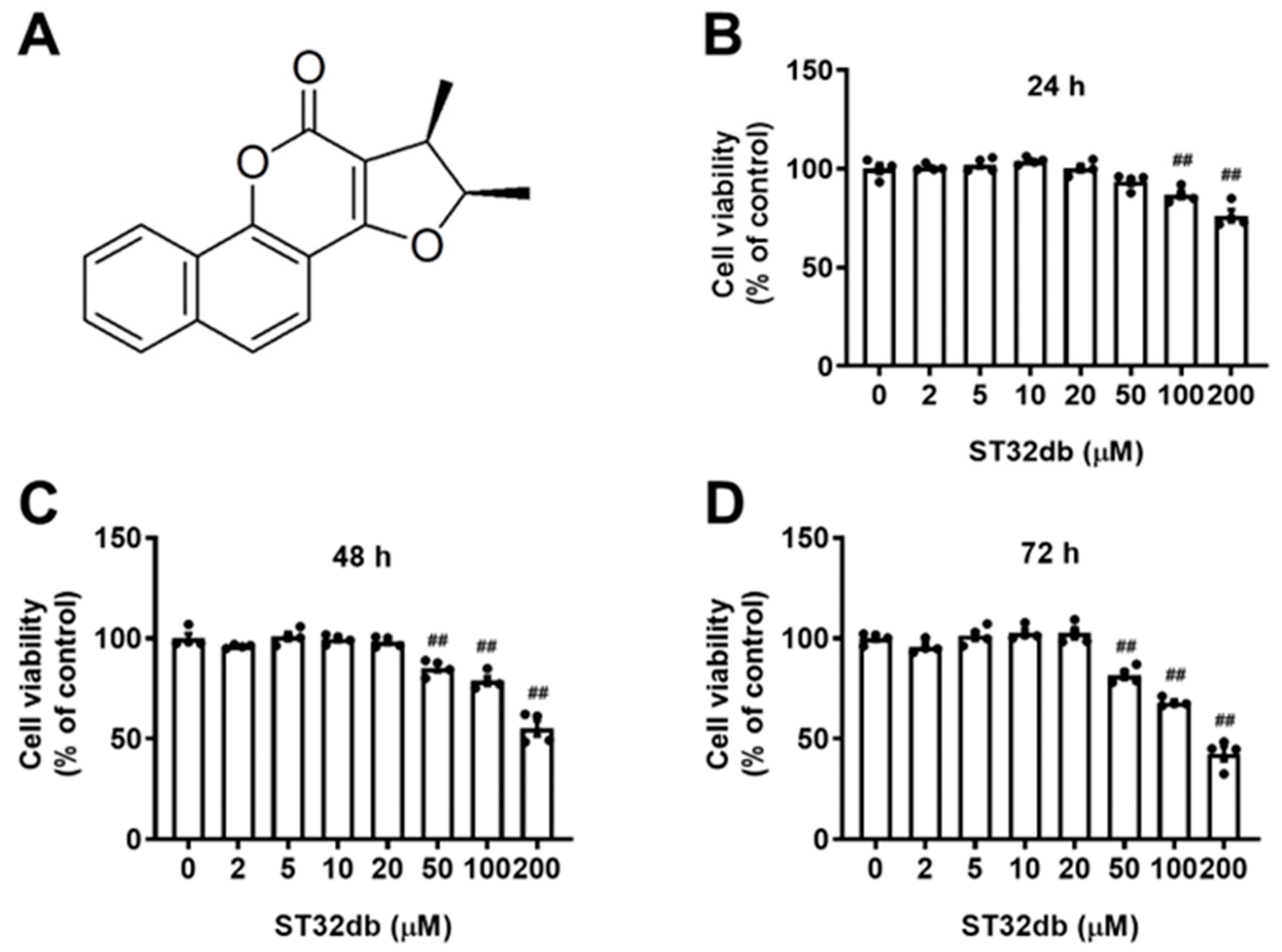
2.1. Effects of ST32db, an ATF3 Inducer, on the Viability of HepG2 Cells
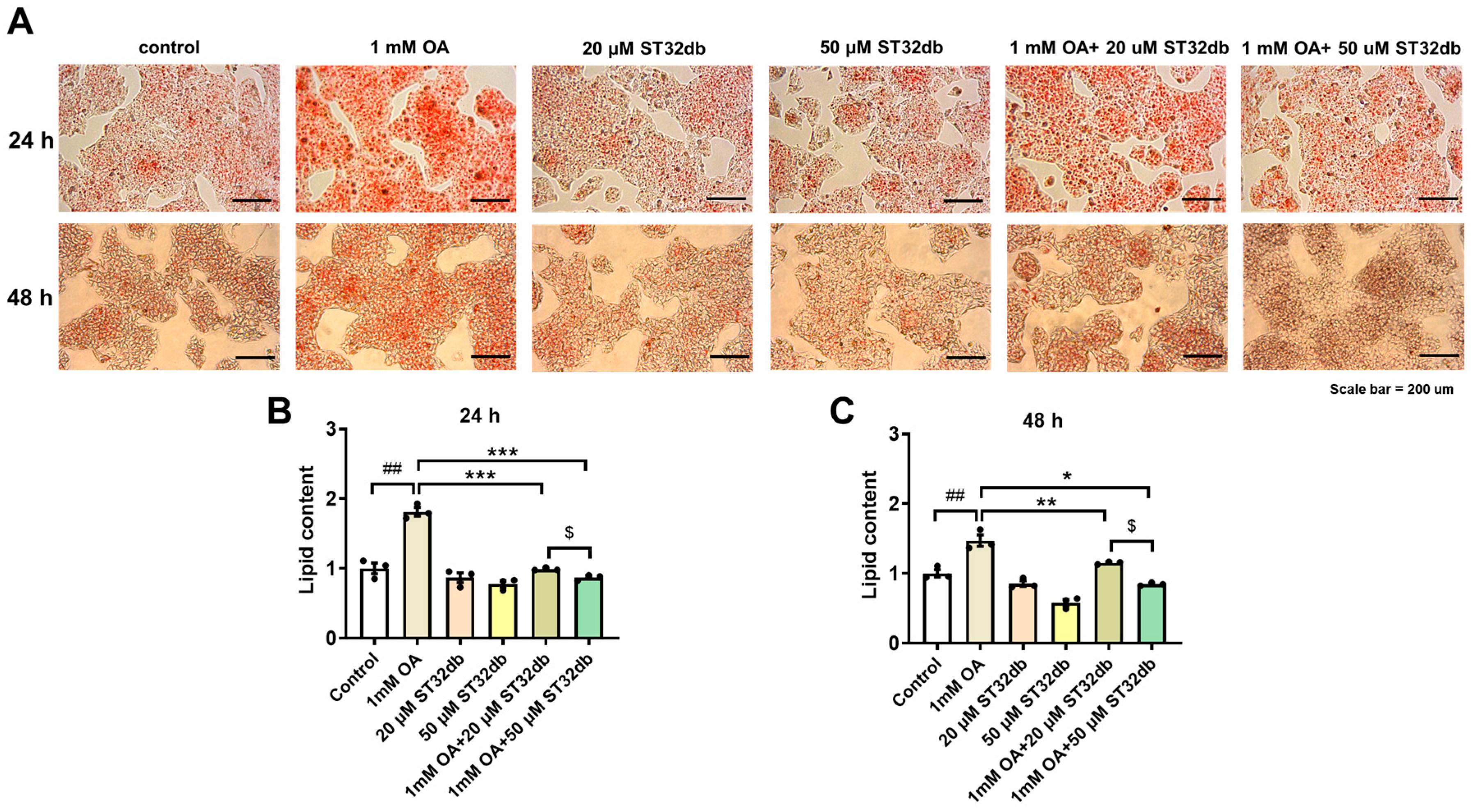
2.2. ST32db Decreases Lipid Accumulation in OA-Treated HepG2 Cells
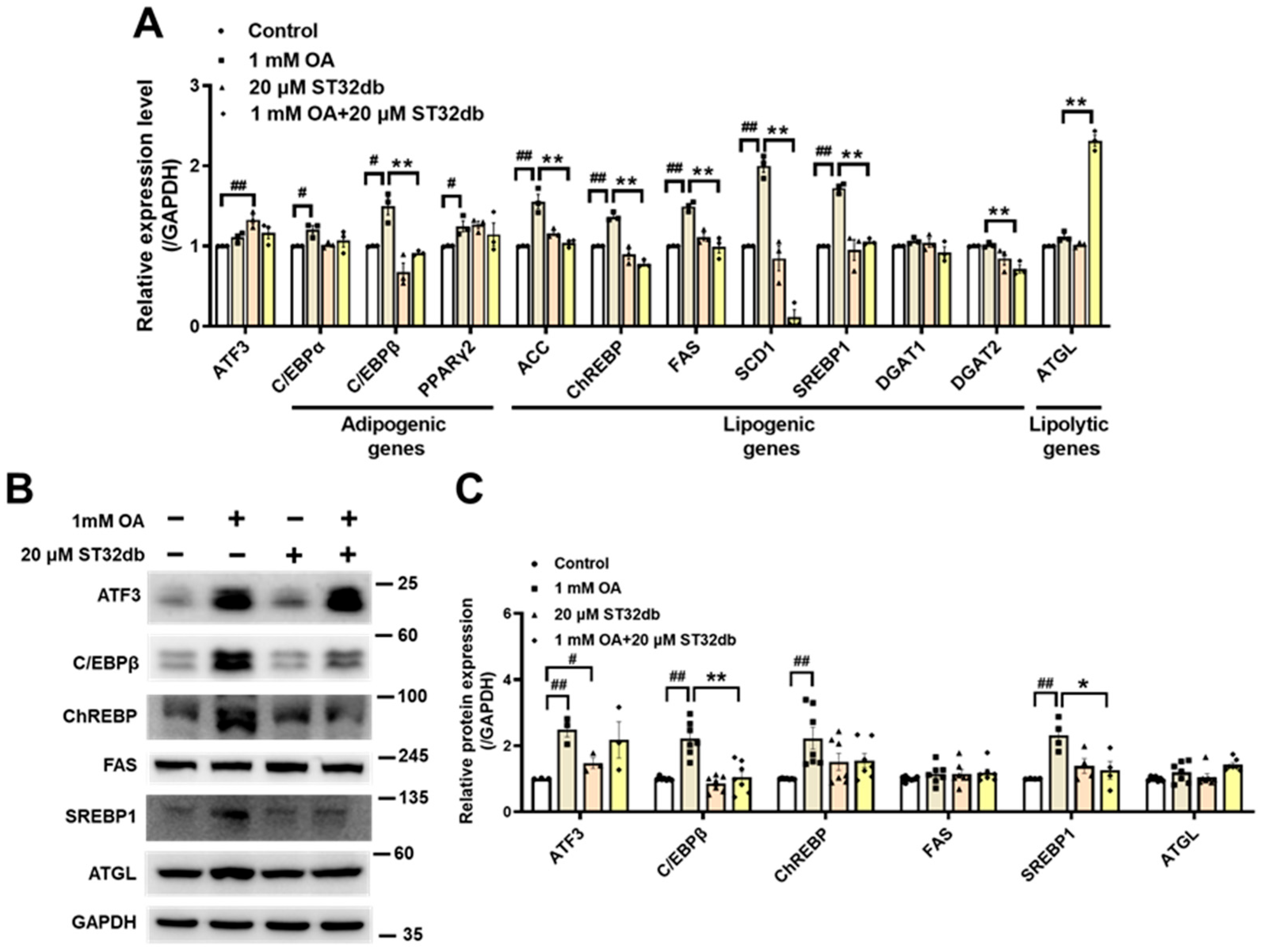
2.3. ST32db Regulates mRNA and Protein Levels of Genes Related to Adipogenesis, Lipogenesis, and Lipolysis in OA-Treated HepG2 Cells
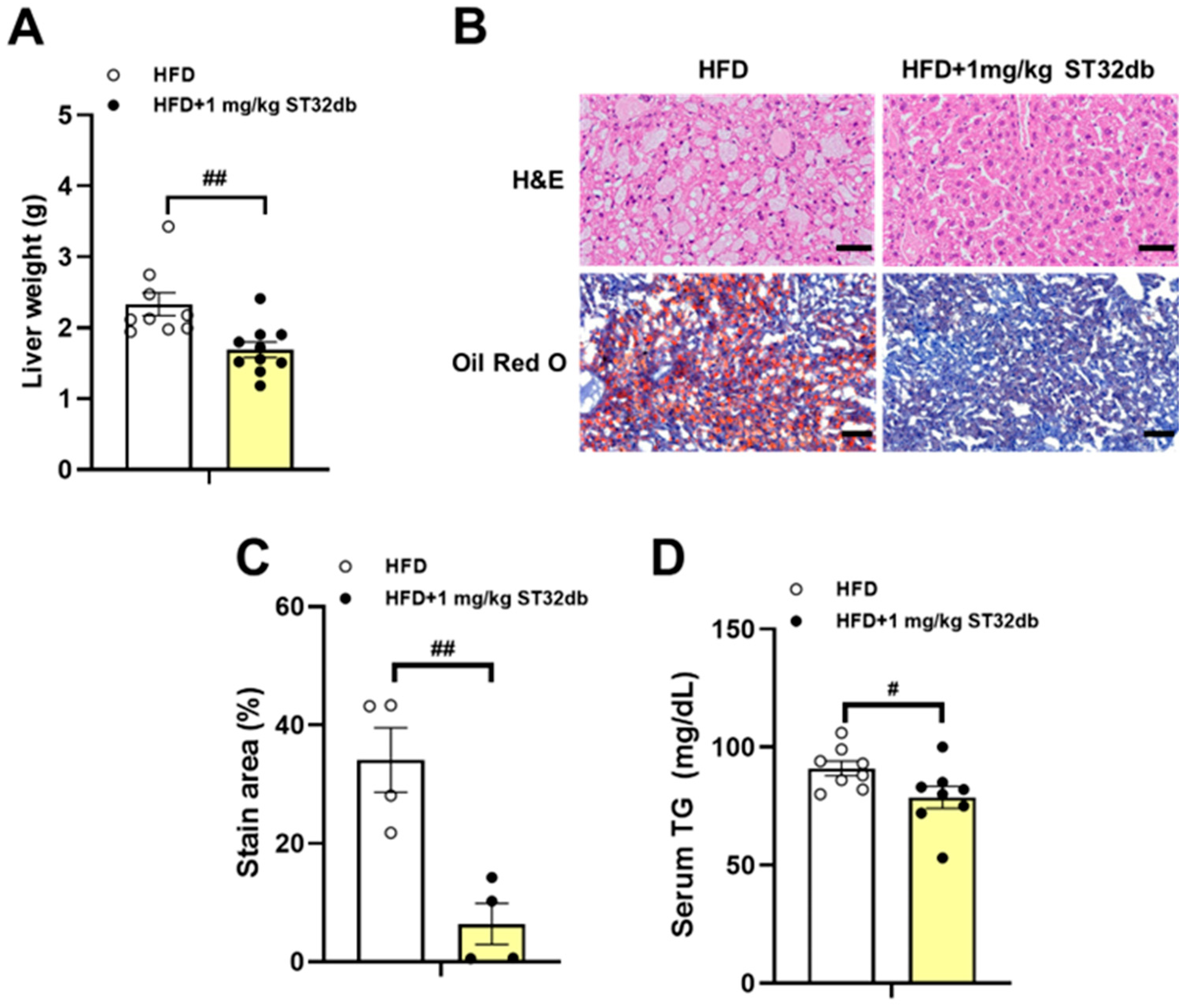
2.4. ST32db Ameliorates HFD-Induced Hepatic Steatosis
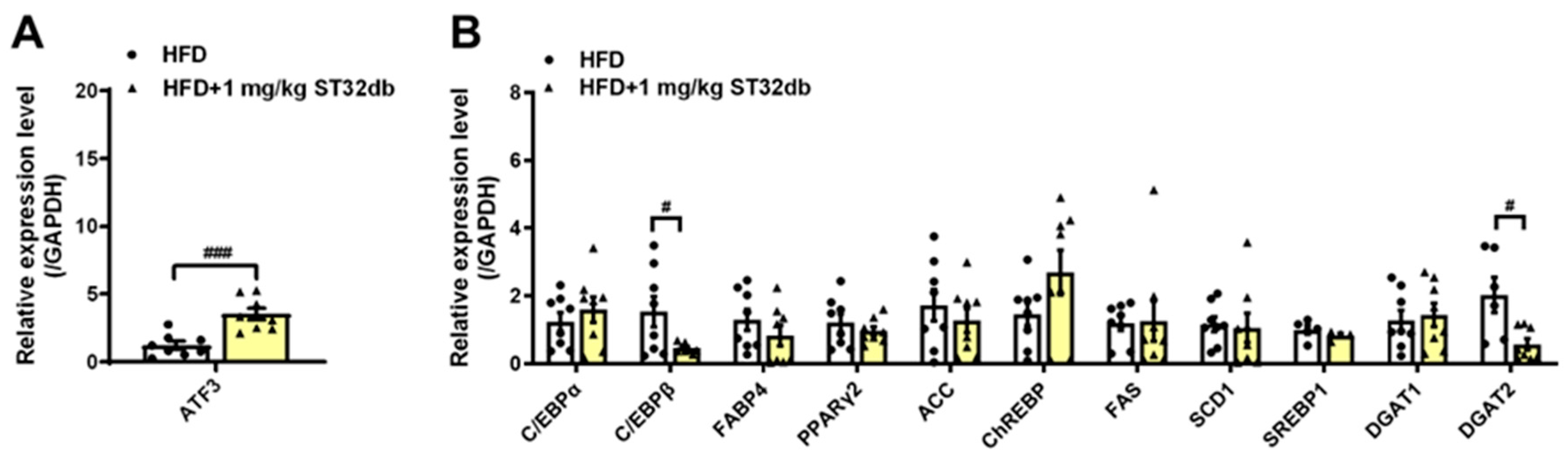
2.5. ST32db Increases ATF3 mRNA Levels but Decreases Adipogenesis- and Lipogenesis-Related mRNA in Mouse Liver
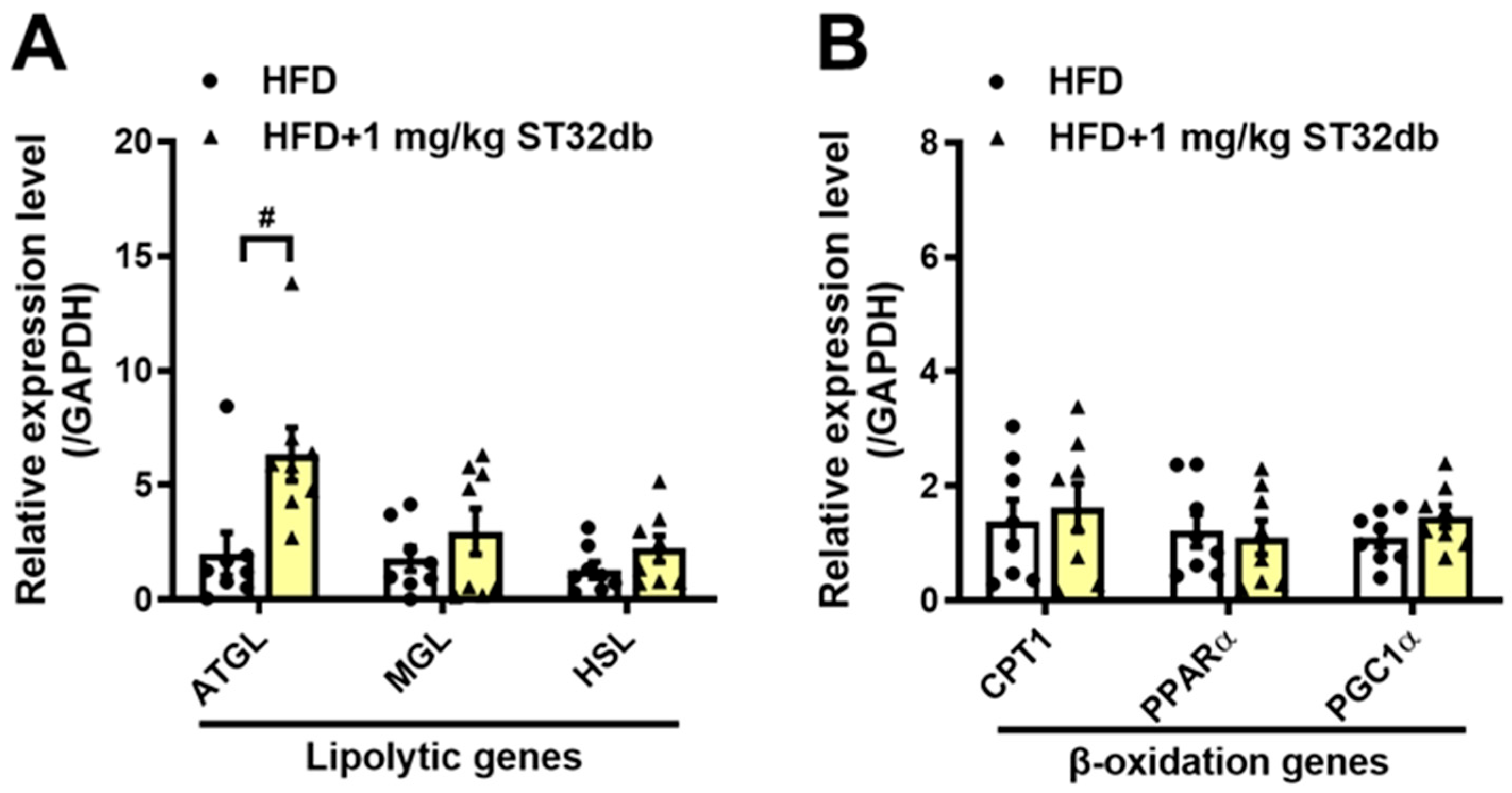
2.6. ST32db Promotes Lipolysis in the Mouse Liver
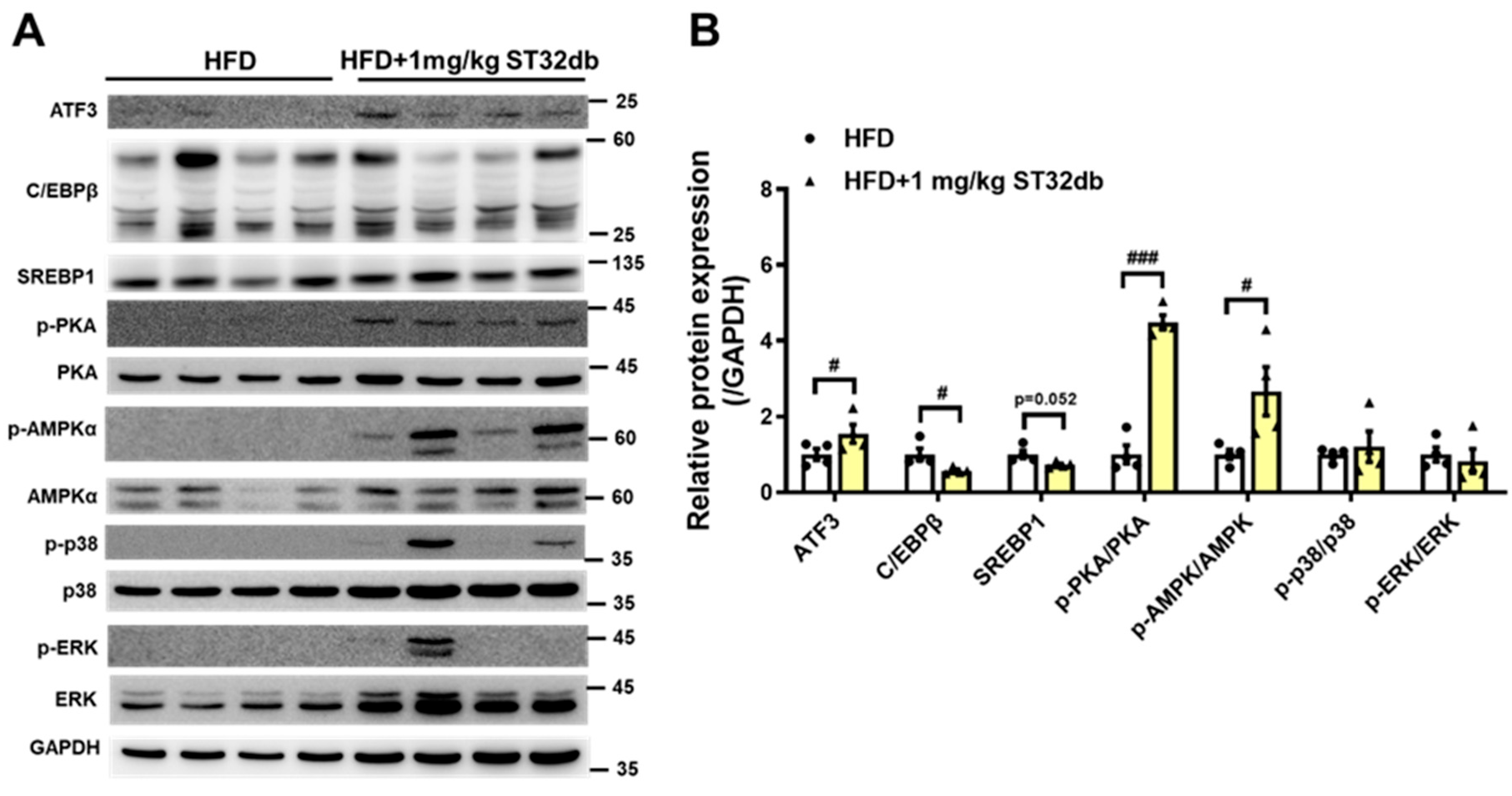
2.7. ST32db Increases Levels of ATF3, Phospho-PKA, and Phospho-AMPK Proteins but Decreases C/EBPβ Protein Levels in Mouse Liver
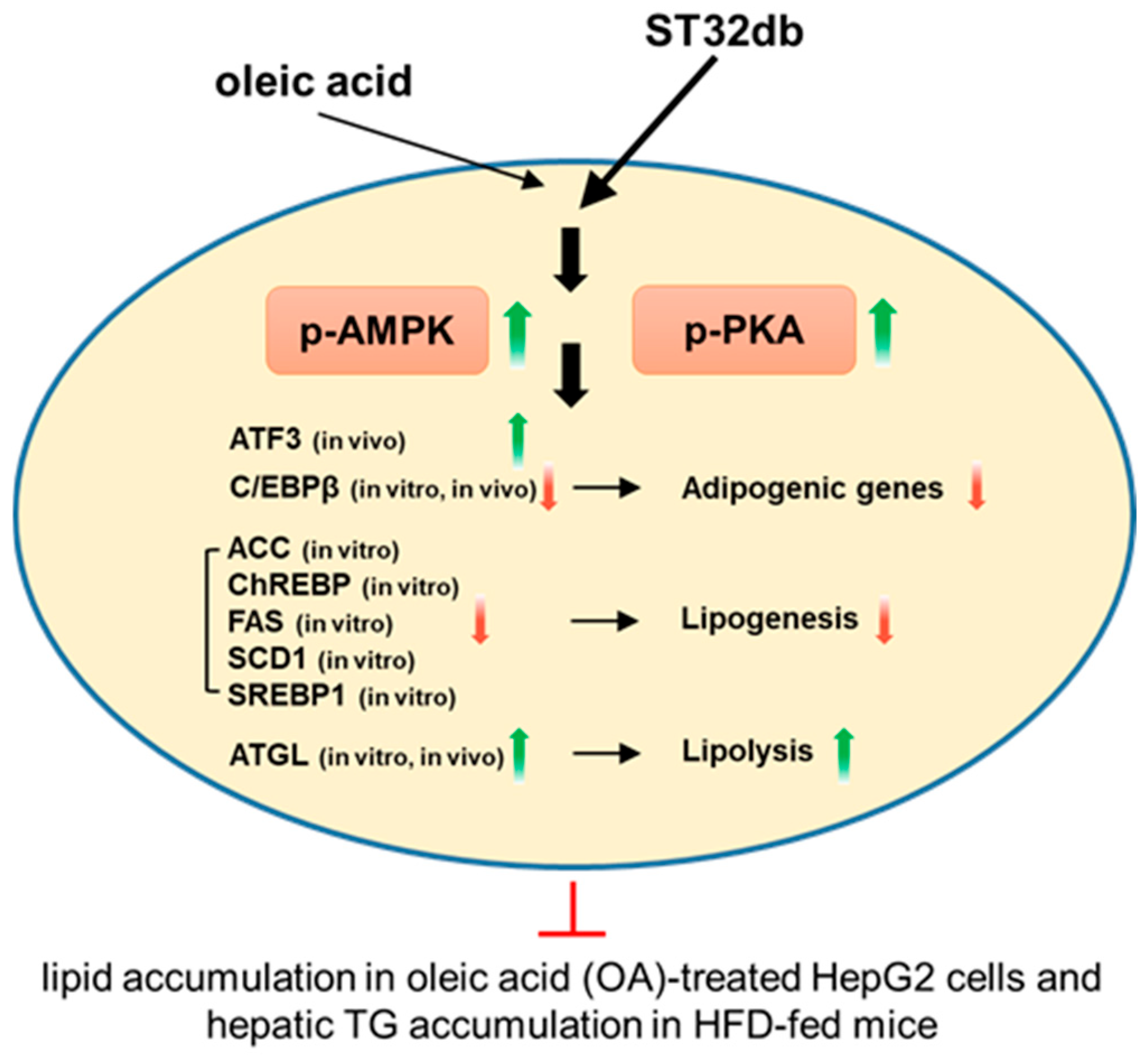
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Cell Culture
4.3. Analysis of Cell Viability
4.4. Oil Red O Staining
4.5. Real-Time Quantitative PCR
4.6. Western Blot Analysis
4.7. Animal Studies
4.8. Histopathological Assessments of Livers
4.9. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACC | Acetyl-CoA carboxylase |
| AMPK | AMP-activated protein kinase |
| ATF | Activating transcription factor |
| ATGL | Adipose triglyceride lipase |
| C/EBPα | CCAAT/enhancer-binding protein α |
| ChREBP | Carbohydrate-responsive element-binding protein |
| CREB | cAMP response element-binding protein |
| DGAT1 | Diacylglycerol acyltransferase 1 |
| DMEM | Dulbecco’s modified eagle medium |
| FAS | Fatty acid synthase |
| H&E | Hematoxylin–eosin |
| HFD | High-fat diet |
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| OA | Oleic acid |
| PKA | Protein kinase A |
| PPAR | Peroxisome proliferator-activated receptor |
| SCD1 | Stearoyl-CoA desaturase 1 |
| SREBP1 | Sterol regulatory element binding protein 1 |
| TG | Triglyceride |
References
- Riazi, K.; Azhari, H.; Charette, J.H.; Underwood, F.E.; King, J.A.; Afshar, E.E.; Swain, M.G.; Congly, S.E.; Kaplan, G.G.; Shaheen, A.A. The prevalence and incidence of NAFLD worldwide: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2022, 7, 851–861. [Google Scholar] [CrossRef]
- Tilg, H.; Petta, S.; Stefan, N.; Targher, G. Metabolic Dysfunction–Associated Steatotic Liver Disease in Adults: A Review. JAMA, 2025; epub ahead of print. [Google Scholar] [CrossRef]
- Chan, W.K.; Chuah, K.H.; Rajaram, R.B.; Lim, L.L.; Ratnasingam, J.; Vethakkan, S.R. Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A State-of-the-Art Review. J. Obes. Metab. Syndr. 2023, 32, 197–213. [Google Scholar] [CrossRef]
- Guo, X.; Yin, X.; Liu, Z.; Wang, J. Non-Alcoholic Fatty Liver Disease (NAFLD) Pathogenesis and Natural Products for Prevention and Treatment. Int. J. Mol. Sci. 2022, 23, 15489. [Google Scholar] [CrossRef]
- Ipsen, D.H.; Lykkesfeldt, J.; Tveden-Nyborg, P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell. Mol. Life Sci. CMLS 2018, 75, 3313–3327. [Google Scholar] [CrossRef]
- Stefan, N.; Kantartzis, K.; Häring, H.-U. Causes and Metabolic Consequences of Fatty Liver. Endocr. Rev. 2008, 29, 939–960. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Li, Y.; Bai, M.; Huang, Y.; Yang, H.; Liu, L.; Wang, S.; Yu, C.; Song, Z.; Bao, Y.; et al. Hypericin attenuates nonalcoholic fatty liver disease and abnormal lipid metabolism via the PKA-mediated AMPK signaling pathway in vitro and in vivo. Pharmacol. Res. 2020, 153, 104657. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Saltiel, A.R. From overnutrition to liver injury: AMP-activated protein kinase in nonalcoholic fatty liver diseases. J. Biol. Chem. 2020, 295, 12279–12289. [Google Scholar] [CrossRef]
- Montminy, M.R.; Bilezikjian, L.M. Binding of a nuclear protein to the cyclic-AMP response element of the somatostatin gene. Nature 1987, 328, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Ku, H.C.; Chan, T.Y.; Chung, J.F.; Kao, Y.H.; Cheng, C.F. The ATF3 inducer protects against diet-induced obesity via suppressing adipocyte adipogenesis and promoting lipolysis and browning. Biomed. Pharmacother. Biomed. Pharmacother. 2022, 145, 112440. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Y.; Jadhav, K.; Pan, X.; Zhu, Y.; Hu, S.; Chen, S.; Chen, L.; Tang, Y.; Wang, H.H.; et al. Hepatocyte ATF3 protects against atherosclerosis by regulating HDL and bile acid metabolism. Nat. Metab. 2021, 3, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Hu, S.; Jadhav, K.; Zhu, Y.; Pan, X.; Bawa, F.C.; Yin, L.; Zhang, Y. Hepatocytic Activating Transcription Factor 3 Protects Against Steatohepatitis via Hepatocyte Nuclear Factor 4α. Diabetes 2021, 70, 2506–2517. [Google Scholar] [CrossRef]
- Li, H.; Meng, Q.; Xiao, F.; Chen, S.; Du, Y.; Yu, J.; Wang, C.; Guo, F. ATF4 deficiency protects mice from high-carbohydrate-diet-induced liver steatosis. Biochem. J. 2011, 438, 283–289. [Google Scholar] [CrossRef]
- Xiao, G.; Zhang, T.; Yu, S.; Lee, S.; Calabuig-Navarro, V.; Yamauchi, J.; Ringquist, S.; Dong, H.H. ATF4 protein deficiency protects against high fructose-induced hypertriglyceridemia in mice. J. Biol. Chem. 2013, 288, 25350–25361. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.P.; Yu, X.; Song, G.Y.; Zhang, P.; Sun, L.N.; Chen, S.C.; Hu, Z.J.; Zhang, X.M. Impact of activating transcription factor 4 signaling on lipogenesis in HepG2 cells. Mol. Med. Rep. 2016, 14, 1649–1658. [Google Scholar] [CrossRef]
- Usui, M.; Yamaguchi, S.; Tanji, Y.; Tominaga, R.; Ishigaki, Y.; Fukumoto, M.; Katagiri, H.; Mori, K.; Oka, Y.; Ishihara, H. Atf6α-null mice are glucose intolerant due to pancreatic β-cell failure on a high-fat diet but partially resistant to diet-induced insulin resistance. Metab. Clin. Exp. 2012, 61, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Lu, M.; Mori, K.; Luo, S.; Lee, A.S.; Zhu, Y.; Shyy, J.Y. ATF6 modulates SREBP2-mediated lipogenesis. EMBO J. 2004, 23, 950–958, Erratum in EMBO J. 2008, 27, 2941. [Google Scholar] [CrossRef]
- Dong, B.; Sun, Y.; Cheng, B.; Xue, Y.; Li, W.; Sun, X. Activating transcription factor (ATF) 6 upregulates cystathionine β synthetase (CBS) expression and hydrogen sulfide (H2S) synthesis to ameliorate liver metabolic damage. Eur. J. Med. Res. 2023, 28, 540. [Google Scholar] [CrossRef]
- Howarth, D.L.; Lindtner, C.; Vacaru, A.M.; Sachidanandam, R.; Tsedensodnom, O.; Vasilkova, T.; Buettner, C.; Sadler, K.C. Activating transcription factor 6 is necessary and sufficient for alcoholic fatty liver disease in zebrafish. PLoS Genet. 2014, 10, e1004335. [Google Scholar] [CrossRef]
- Ku, H.C.; Cheng, C.F. Master Regulator Activating Transcription Factor 3 (ATF3) in Metabolic Homeostasis and Cancer. Front. Endocrinol. 2020, 11, 556. [Google Scholar] [CrossRef]
- Fabbrini, E.; Sullivan, S.; Klein, S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology 2010, 51, 679–689. [Google Scholar] [CrossRef]
- Cominguez, D.C.; Park, Y.J.; Kang, Y.M.; Nugroho, A.; Kim, S.; An, H.J. Clitorin ameliorates western diet-induced hepatic steatosis by regulating lipogenesis and fatty acid oxidation in vivo and in vitro. Sci. Rep. 2022, 12, 4154. [Google Scholar] [CrossRef]
- Osborne, T.F. Sterol regulatory element-binding proteins (SREBPs): Key regulators of nutritional homeostasis and insulin action. J. Biol. Chem. 2000, 275, 32379–32382. [Google Scholar] [CrossRef] [PubMed]
- Eichmann, T.O.; Grumet, L.; Taschler, U.; Hartler, J.; Heier, C.; Woblistin, A.; Pajed, L.; Kollroser, M.; Rechberger, G.; Thallinger, G.G.; et al. ATGL and CGI-58 are lipid droplet proteins of the hepatic stellate cell line HSC-T6. J. Lipid Res. 2015, 56, 1972–1984. [Google Scholar] [CrossRef] [PubMed]
- Mello, T.; Nakatsuka, A.; Fears, S.; Davis, W.; Tsukamoto, H.; Bosron, W.F.; Sanghani, S.P. Expression of carboxylesterase and lipase genes in rat liver cell-types. Biochem. Biophys. Res. Commun. 2008, 374, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Turpin, S.M.; Hoy, A.J.; Brown, R.D.; Rudaz, C.G.; Honeyman, J.; Matzaris, M.; Watt, M.J. Adipose triacylglycerol lipase is a major regulator of hepatic lipid metabolism but not insulin sensitivity in mice. Diabetologia 2011, 54, 146–156. [Google Scholar] [CrossRef]
- Reid, B.N.; Ables, G.P.; Otlivanchik, O.A.; Schoiswohl, G.; Zechner, R.; Blaner, W.S.; Goldberg, I.J.; Schwabe, R.F.; Chua, S.C., Jr.; Huang, L.S. Hepatic overexpression of hormone-sensitive lipase and adipose triglyceride lipase promotes fatty acid oxidation, stimulates direct release of free fatty acids, and ameliorates steatosis. J. Biol. Chem. 2008, 283, 13087–13099. [Google Scholar] [CrossRef]
- Kato, M.; Higuchi, N.; Enjoji, M. Reduced hepatic expression of adipose tissue triglyceride lipase and CGI-58 may contribute to the development of non-alcoholic fatty liver disease in patients with insulin resistance. Scand. J. Gastroenterol. 2008, 43, 1018–1019. [Google Scholar] [CrossRef]
- Ladiges, W.; Enns, L. Targeting PKA Signaling to Prevent Metabolic Syndrome and Delay Aging. In Medical Complications of Type 2 Diabetes; Croniger, C.M., Ed.; IntechOpen: London, UK, 2011. [Google Scholar]
- Yan, F.; Wang, Q.; Lu, M.; Chen, W.; Song, Y.; Jing, F.; Guan, Y.; Wang, L.; Lin, Y.; Bo, T.; et al. Thyrotropin increases hepatic triglyceride content through upregulation of SREBP-1c activity. J. Hepatol. 2014, 61, 1358–1364. [Google Scholar] [CrossRef]
- Kohjima, M.; Higuchi, N.; Kato, M.; Kotoh, K.; Yoshimoto, T.; Fujino, T.; Yada, M.; Yada, R.; Harada, N.; Enjoji, M.; et al. SREBP-1c, regulated by the insulin and AMPK signaling pathways, plays a role in nonalcoholic fatty liver disease. Int. J. Mol. Med. 2008, 21, 507–511. [Google Scholar] [CrossRef]
- Feng, S.; Reuss, L.; Wang, Y. Potential of Natural Products in the Inhibition of Adipogenesis through Regulation of PPARγ Expression and/or Its Transcriptional Activity. Molecules 2016, 21, 1278. [Google Scholar] [CrossRef] [PubMed]
| Gene Name | Forward Primer 5′-3′ | Reverse Primer 5′-3′ | Gene Name | Forward Primer 5′-3′ | Reverse Primer 5′-3′ |
|---|---|---|---|---|---|
| mATF3 | CTCCTGGGTCACTGGTATTTG | CCGATGGCAGAGGTGTTTAT | mPPARα | GTCCTCAGTGCTTCCAGAGG | GGTCACCTACGAGTGGCATT |
| mC/EBPα | GTAACCTTGTGCCTTGGATACT | GGAAGCAGGAATCCTCCAAATA | mGAPDH | GGAGCCAAACGGGTCATCATCTC | GAGGGGCCATCCACAGTCTTCT |
| mC/EBPβ | CTTGATGCAATCCGGATCAAAC | CCCGCAGGAACATCTTTAAGT | hATF3 | CTGGAAAGTGTGAATGCTGAAC | ATTCTGAGCCCGGACAATAC |
| mPPARγ2 | CTGGCCTCCCTGATGAATAAAG | AGGCTCCATAAAGTCACCAAAG | hC/EBPα | GAAGTCGGTGGACAAGAACA | TCATTGTCACTGGTCAGCTC |
| mFABP4 | GCTCCTCCTCGAAGGTTTAC | CCCACTCCCACTTCTTTCAT | hC/EBPβ | CGCGACAAGGCCAAGAT | GCTGCTCCACCTTCTTCTG |
| mACC | TGATGGTGGCCTGCTCTTGTCTTA | CAGCAAACACATGTCCGCCATCTT | hPPARγ2 | GCCTGCATCTCCACCTTATTA | ATCTCCACAGACACGACATTC |
| mFAS | AGACCCGAACTCCAAGTTATTC | GCAGCTCCTTGTATACTTCTCC | hACC | CAGAAGTGACAGACTACAGG | ATCCATGGCTTCCAGGAGTA |
| mDGAT1 | GGCCTTACTGGTTGAGTCTATC | GTTGACATCCCGGTAGGAATAA | hFAS | TGGTCACGGACGATGACCGTCG | GCGGCAGTACCCATTCCCCGC |
| mDGAT2 | GAAGGGCTTCTCTTCTCTTCAC | CTTTCTCCCAACGCCTCATAA | hDGAT1 | CTGGTCCAGTCTTGGGGTCT | ACCAAGCTGGATAGATGGGG |
| mSCD1 | TGGGTTGGCTGCTTGTG | GCGTGGGCAGGATGAAG | hDGAT2 | CTGGAGAACCTCATCAAGTATGG | CAAAGACATTGGCCGCAATAA |
| mChREBP | TGTTCAGCATCCTCATCCGACCTT | TGAGTTGGCGAAGGGAATTCAGGA | hSCD1 | ACAACTACCACCACTCCTTTC | GGAGACTTTCTTCCGGTCATAG |
| mATGL | CATCCGTGGCTGTCTACTAAAG | GACGTTCTCTCCGTCTGAAAC | hChREBP | GGAAGAATTTCAAAGGCCTCAAG | CTCTTCCTCCGCTTCACATAC |
| mHSL | GGACGGTCCTAGGTTTGAATAC | GATGGGAAGGTCTGTGGTTAC | hSREBP1 | GAGCCATGGATTGCACTTTC | AGCATAGGGTGGGTCAAATAG |
| mMGL | GACAGAAAGAGTGTGGGAAGAG | CTGAGCACAGTAGTCTGGAATG | hATGL | AACACCAGCATCCAGTTCA | TATCCCTGCTTGCACATCTC |
| mPGC1α | CTAGCCATGGATGGCCTATTT | GTCTCGACACGGAGAGTTAAAG | hGAPDH | GGTGTGAACCATGAGAAGTATGA | GAGTCCTTCCACGATACCAAAG |
| mCPT1 | GAAGTGTCGGCAGACCTATTT | GTCCTCCTCTCTATATCCCTGTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, C.-F.; Yang, R.-B.; Chen, W.-T.; Chung, J.-F.; Ku, H.-C. An ATF3 Inducer Ameliorates Metabolic Dysfunction-Associated Steatotic Liver Disease Through the AMPK and PKA Pathways. Int. J. Mol. Sci. 2025, 26, 11877. https://doi.org/10.3390/ijms262411877
Cheng C-F, Yang R-B, Chen W-T, Chung J-F, Ku H-C. An ATF3 Inducer Ameliorates Metabolic Dysfunction-Associated Steatotic Liver Disease Through the AMPK and PKA Pathways. International Journal of Molecular Sciences. 2025; 26(24):11877. https://doi.org/10.3390/ijms262411877
Chicago/Turabian StyleCheng, Ching-Feng, Ruey-Bing Yang, Wen-Ting Chen, Jia-Fang Chung, and Hui-Chen Ku. 2025. "An ATF3 Inducer Ameliorates Metabolic Dysfunction-Associated Steatotic Liver Disease Through the AMPK and PKA Pathways" International Journal of Molecular Sciences 26, no. 24: 11877. https://doi.org/10.3390/ijms262411877
APA StyleCheng, C.-F., Yang, R.-B., Chen, W.-T., Chung, J.-F., & Ku, H.-C. (2025). An ATF3 Inducer Ameliorates Metabolic Dysfunction-Associated Steatotic Liver Disease Through the AMPK and PKA Pathways. International Journal of Molecular Sciences, 26(24), 11877. https://doi.org/10.3390/ijms262411877







