2. Results and Discussion
Consider a poorly soluble (hydrophobic) molecule
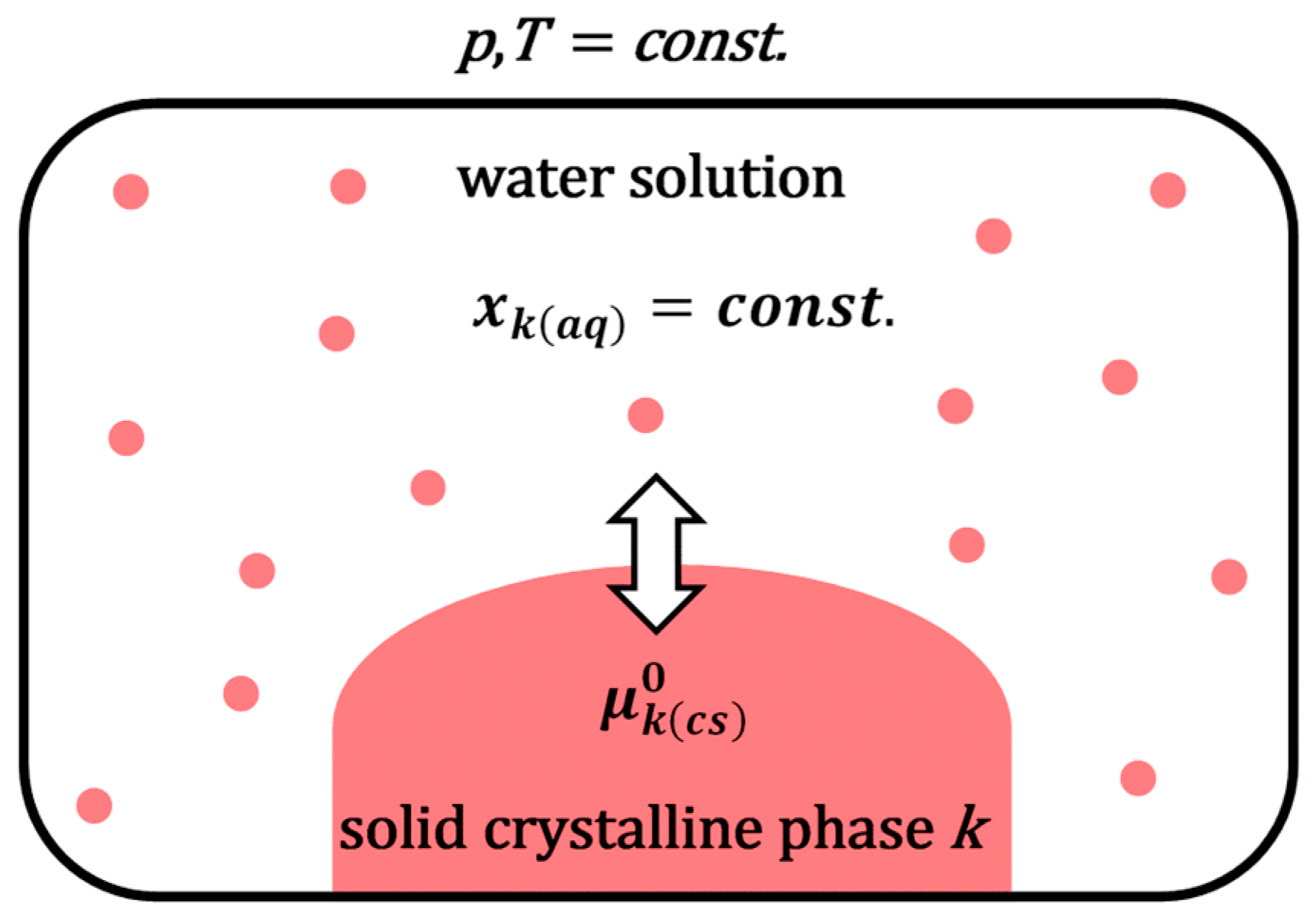
k (drug) in water. Suppose the solid crystalline phase of the hydrophobic molecule
k is in equilibrium with an aqueous solution of the same molecule. In that case, the system simultaneously contains a solid (undissolved) phase of the molecule
k and its infinitely diluted aqueous solution (
Figure 1). The chemical potentials of the hydrophobic molecule
k in the undissolved solid crystalline phase and aqueous solution are mutually equal:
where
is the chemical potential of the molecule
k in the solid crystalline phase (i.e., state,
cs), and
is the standard (reference) chemical potential of the solubilizate
k in a water solution (the reference state is chosen to be the hypothetical state of the unit mole fraction extrapolated along the Henry’s law line, i.e., from the infinitely diluted aqueous solution of the solubilizate)
represents the solubility (i.e., molar fractions) of
k in a water phase. At constant temperature (
T) and pressure (
p), it can be defined as follows [
25,
26]:
According to Equation (1), the standard molar Gibbs free energy (
) of solubilization
k from the crystalline state is
Since the solubility of the hydrophobic drug molecule in aqueous solution is low, the activity coefficient in Equation (3) has a value of one in the infinitely (ideally) diluted state of the solution [
25,
26].
The standard molar Gibbs free energy of the solubilization of a crystalline, poorly water-soluble molecule,
k (
), is the fundamental reference value for evaluating the efficiency of HME technology in increasing solubility [
3,
27,
28].
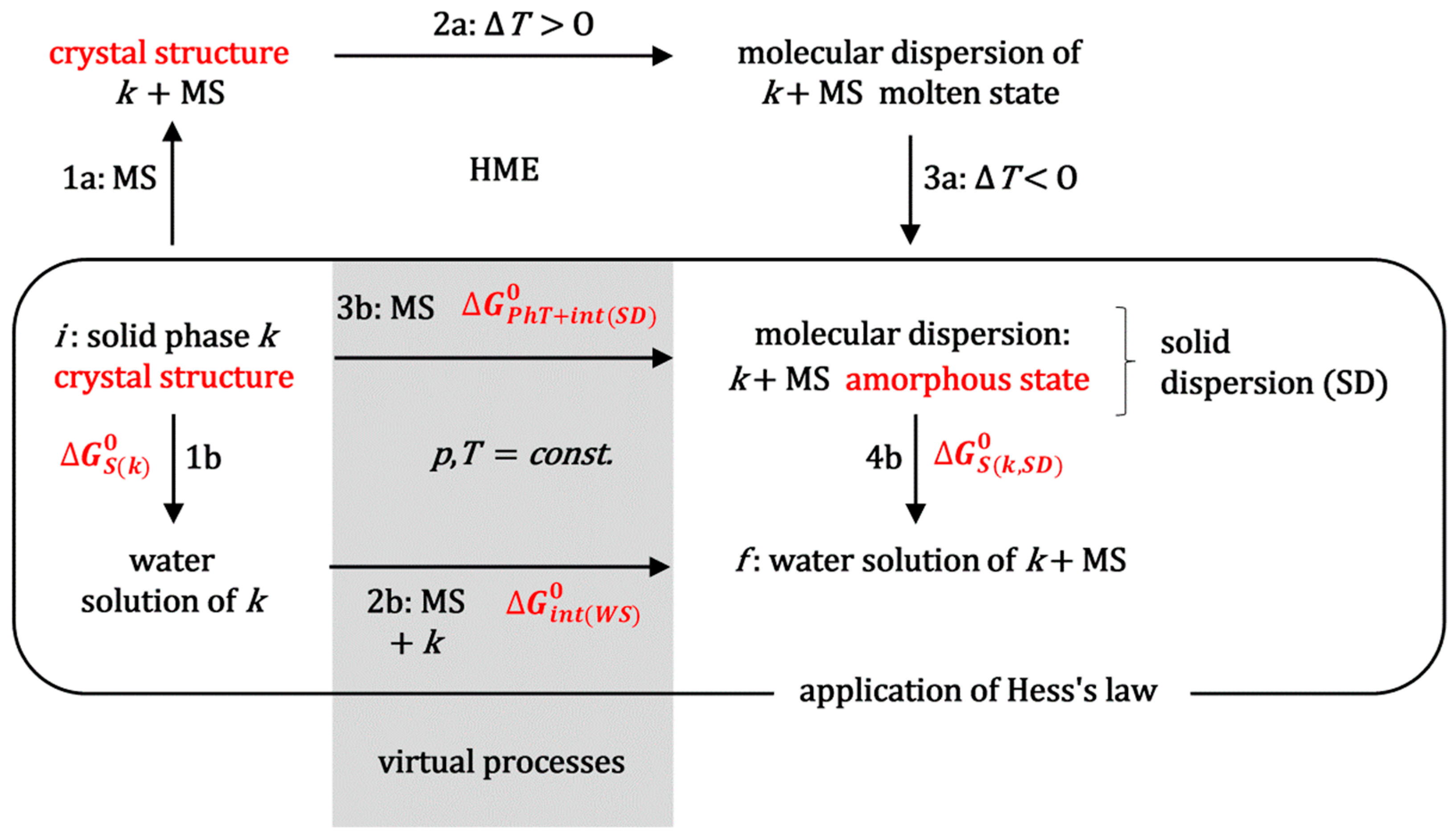
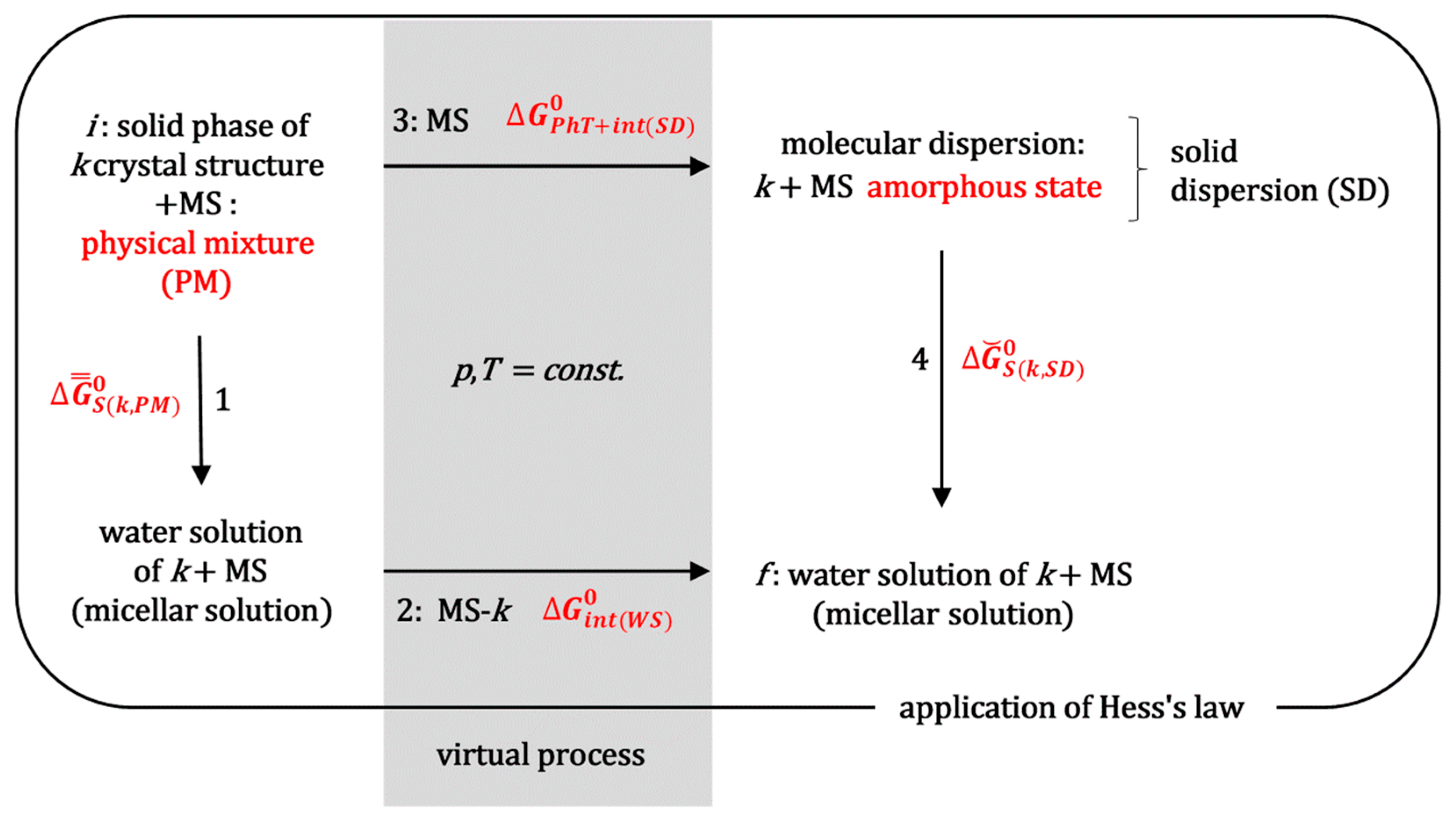
The standard molar Gibbs energy, which indicates the efficiency of hot-melt extrusion technology in enhancing the solubilization of the hydrophobic molecule
k during the saturation experiments, can be determined by applying Hess’s law [
29] to the thermodynamic process outlined in
Figure 2. This figure illustrates the simultaneous non-spontaneous thermodynamic process involved in HME, specifically the transformation of a crystalline substance (
k) into the amorphous state within a solid dispersion [
1,
2]. Process 1a (
Figure 2) represents obtaining a physical mixture between the powder of crystalline substance
k and the matrix substance (MS). Let the MS be a block copolymer surfactant, MS = PS, e.g., a polymer from the Poloxamer group, i.e., a polymer that forms micellar aggregates in aqueous solution.
During process 2a (
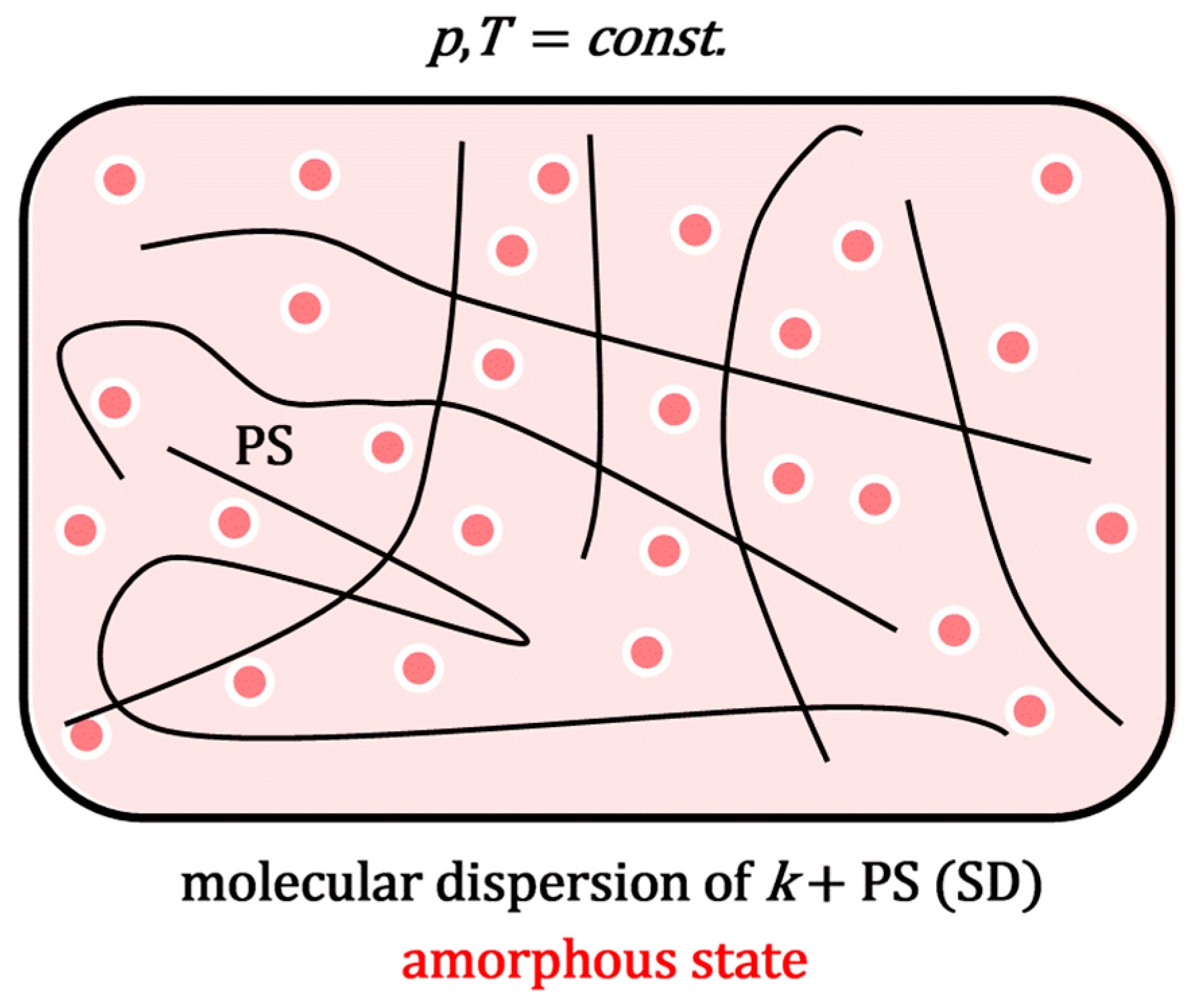
Figure 2), the system (extruder) is heated to above the melting point of the polymeric surfactant, while, with mixing, a molecular dispersion is formed in the molten state. In process 3a, the melt forms an amorphous solid solution upon cooling (SD,
Figure 3).
When comparing the saturation solubilization of the solid crystalline substance
k (process 1b,
Figure 2) with the saturation solubilization of an amorphous solid dispersion (SD) of
k (process 4b,
Figure 2), the temperature (
T), pressure (
p), and characteristics of the aqueous solution (pH and ionic strength) are the same for both processes. The change in the standard molar Gibbs free energy for process 1b (
) is described by Equation (3). In process 4b, during the dissolution of the amorphous SD, polymeric surfactants first dissolve in the aqueous solution. Upon reaching a critical micellar concentration, these surfactants form micellar aggregates. These aggregates then incorporate hydrophobic molecules
k into their hydrophobic interiors (
Figure 4) [
21]. However, process 4b can be represented as the sum of the dissolution process of the amorphous (solid) phase
k (without polymer surfactants) in the aqueous solution and the distribution process of hydrophobic molecules
k between the aqueous phase and micellar aggregates; the last process in the thermodynamic model is viewed as the distribution of
k between the aqueous phase and micellar pseudophase [
14].
The change in the standard molar Gibbs free energy (
) for the dissolution of the amorphous solid phase of molecules
k in an aqueous solution is obtained in an analogous way to Equation (3) (
Appendix A):
where
represents the mole fraction (solubility) of solute (i.e., solubilizate) in aqueous solution when dissolving the amorphous solid phase of
k. However, the chemical potentials of
k molecules in the crystalline (
cs) and amorphous state (
as) differ from each other:
Therefore, it follows that the changes in the standard molar Gibbs free energies of dissolution, as well as the solubility values, also differ:
In an aqueous solution of polymer micelles and hydrophobic molecules
k during the distribution of
k between micellar particles and water, equilibrium is established, whereby the set of micellar particles is viewed as a separate phase (micellar pseudophase [
30]) where hydrophobic molecules
k have
chemical potential, i.e.,
standard chemical potential. Simultaneously,
is the mole fraction of solubilizates
k in the micellar pseudophase (
Figure 4).
Equation (8) represents the change in the standard molar Gibbs free energy that follows the distribution (partition) of one mole of hydrophobic particles
k between the micellar and aqueous phases. As
at constant temperature and pressure, for the mole fraction
k from the aqueous phase (generally from any of the two), the value of
defined by Equation (4) can be arbitrarily taken, whereby the value of
is then adjusted to the value of
to obtain a constant value for
. According to the detailed balance theory [
31,
32], the change in the standard molar Gibbs free energy
(process 4b,
Figure 2) can be represented as the sum of the Gibbs free energies of the elementary processes that make up process 4b (solubilization of the amorphous solid
k and the distribution of particles
k between the micellar pseudophase and aqueous phase [
33],
Figure 4):
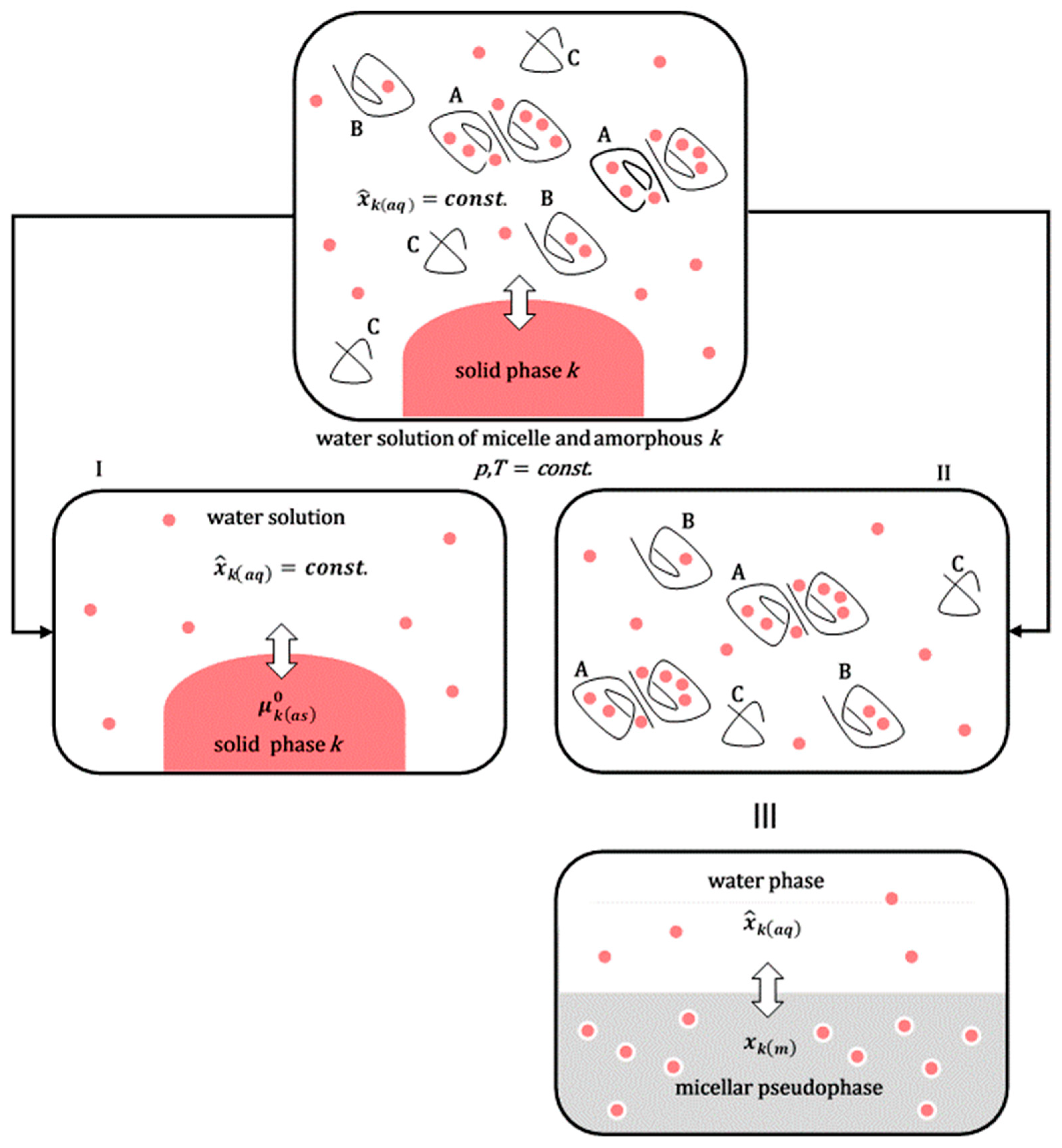
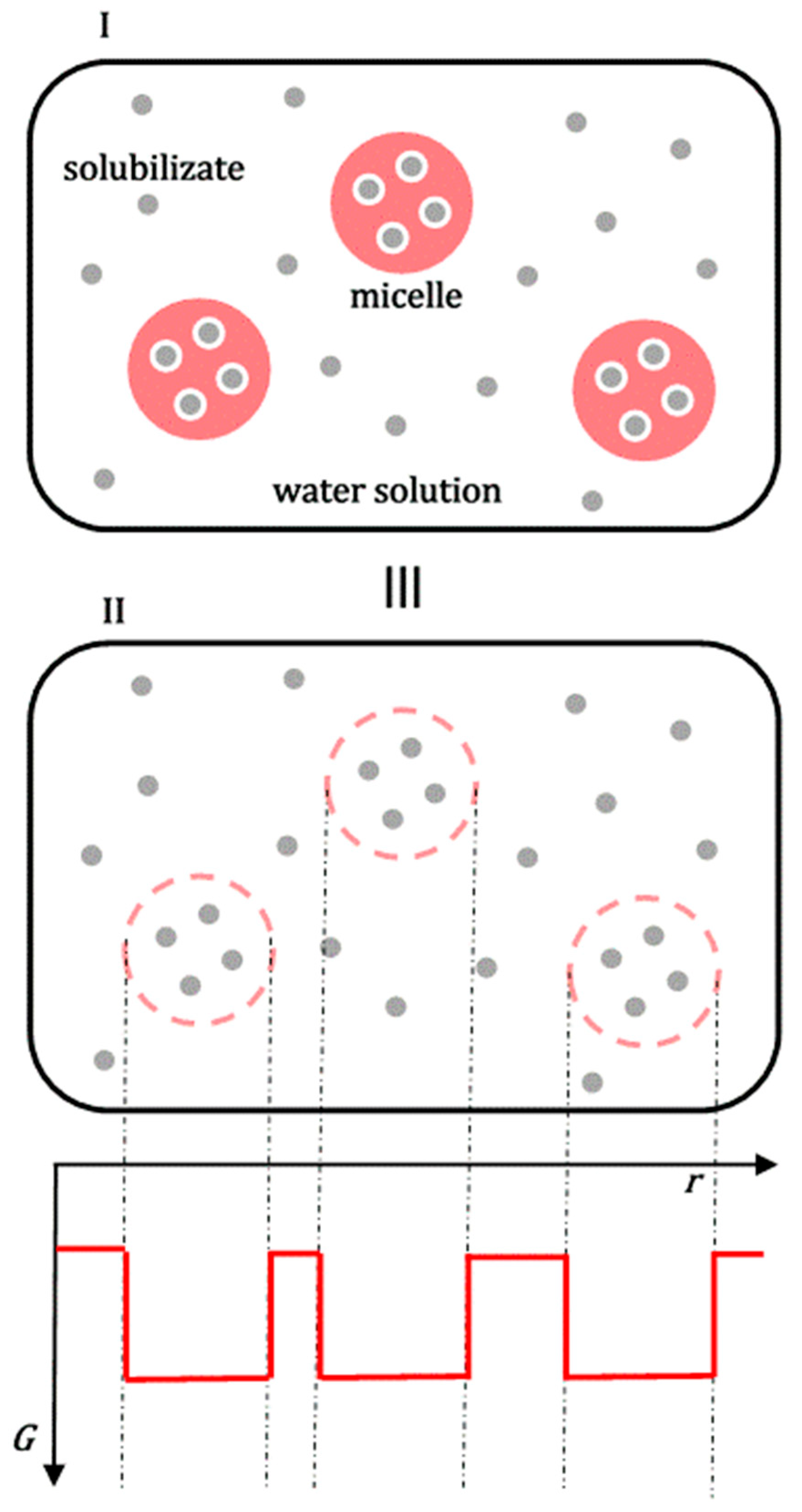
Figure 4.
The solubilization of the amorphous solid phase of the hydrophobic molecule
k in the micellar aqueous solution of the polymeric surfactant can be viewed as the sum of the process of solubilization of the amorphous solid phase in the aqueous solution without surfactant (
I) and the distribution of hydrophobic solute
k between the aqueous solution and micellar aggregates (
II): micelles with solubilizates
k (A), polymeric surfactants in an unassociated state to whose hydrophobic regions solubilizate
k can also bind (B). There are also shorter polymer chains (C) without solubilizates (polymeric surfactants exhibit polydispersity in polymer chain length [
33]; (
III) two graphical representations of an identical physical state).
Figure 4.
The solubilization of the amorphous solid phase of the hydrophobic molecule
k in the micellar aqueous solution of the polymeric surfactant can be viewed as the sum of the process of solubilization of the amorphous solid phase in the aqueous solution without surfactant (
I) and the distribution of hydrophobic solute
k between the aqueous solution and micellar aggregates (
II): micelles with solubilizates
k (A), polymeric surfactants in an unassociated state to whose hydrophobic regions solubilizate
k can also bind (B). There are also shorter polymer chains (C) without solubilizates (polymeric surfactants exhibit polydispersity in polymer chain length [
33]; (
III) two graphical representations of an identical physical state).
By introducing Equations (4) and (8) into (9), we obtain
In order to apply Hess’s law (
Figure 2) between the final (
f) and initial state (
i), in addition to the real solubilization processes 1b and 4b, it is necessary to introduce the imaginary processes 2b and 3b (
Figure 2). In the imaginary process 2b, a polymeric surfactant and solubilizate
k are introduced into a saturated aqueous solution of hydrophobic solubilizate
k in order to obtain the final state, which is obtained during the solubilization of a solid dispersion (SD) of hydrophobic molecule
k (in an amorphous state) and polymeric surfactant (
k is introduced from the aqueous solution where it is in equilibrium with the imaginary amorphous form whose solubility is many times higher than the real amorphous state from the SD, so that there is an imaginary state where the concentration of solubilizate
k is many times higher than in the final state; this way hydrated particles
k are provided). The change in the standard molar Gibbs energy (
) in the imaginary process 2b represents the change in the Gibbs free energy due to intermolecular interactions between hydrophobic particles
k and polymer surfactants (mostly) in the micellar state (if in the final state, the concentration of the polymer surfactant is higher than the critical micellar concentration). Since the incorporation of hydrophobic solute into the hydrophobic core of the micelle is an (irreversible) spontaneous process with an entropic driving force around a temperature of 298.15 K (dehydration of the hydrophobic molecular surfaces of the solute
k and the departure of water molecules from the hydration shell above the hydrophobic surface into the interior of the aqueous solution—the molar entropy of the water molecules inside the solution is greater than the molar entropy of the water in the hydration layer above the hydrophobic surface) [
34], it can be defined as
In the imaginary process 3b (
Figure 2), at the same temperature as the initial state, the incorporation of the polymer surfactant destroys the crystal structure. It forms an amorphous state of molecular dispersion. Since during a relatively long time interval, the amorphous SD phase decomposes into the crystalline phase of the hydrophobic molecule
k (initial state) and the solid phase of the polymeric surfactant [
3], it follows that in the amorphous SD state the hydrophobic molecule
k is in a thermodynamically less stable state than in the initial crystalline state, despite the formation of molecular dispersion (the Gibbs free energy of the formation of an ideal dispersion is always less than zero [
29,
31]) and possible intermolecular interactions between the polymeric surfactant and particles
k. It further follows that the change in the standard molar Gibbs free energy for the imaginary process 3b (phase transformation: crystalline state → amorphous state (
PhT) and intermolecular interactions in SD with polymeric surfactants or with the matrix in general (
int(
SD))
is valid, i.e.,
represents the thermodynamic destabilization of the hydrophobic molecule
k in the amorphous SD state in relation to the initial crystalline state.
According to Hess’s law, the change in Gibbs free energy along the path that includes processes 1b and 2b is equal to the change in Gibbs energy along the path that includes processes 3b and 4b if both paths connect the same initial and final state (
Figure 2):
In Equation (14),
represents the standard molar Gibbs free energy as a measure for increasing the degree of spontaneity in the solubilization of hydrophobic molecules
k from the amorphous SD relative to the solubilization of crystalline
k (referent state). By introducing Equations (3) and (10) into (4), we obtain
If after HME it can be proven by structural instrumental methods that polymeric surfactants (PSs) from SDs do not change their aggregation abilities (i.e., no conformational changes occurred in the PS during HME) and if the total amount of PS from the SD dissolves in an aqueous solution, then knowing the values of the critical micellar concentration and the total concentration of the surfactant in the aqueous solution (i.e., how many times the total concentration is greater than the critical micellar concentration),
from Equation (15) can be expressed with the molar solubilization ratio (MSR,
Appendix B) [
26]. Simultaneously,
, from Equation (15), can be expressed in terms of the solubility value (
, in molar concentration) of the solubilizate
k in aqueous solution (without polymeric surfactants,
Appendix B). Accordingly, Equation (16) is
If the critical micellar concentration is not known for the PS or during HME, the aggregation behavior of the polymeric surfactant changes, so the MSR values cannot be determined, and it is necessary to determine
experimentally. However, there is a difficulty in determining the mole fraction of the hydrophobic solubilizate
k in the micellar pseudophase experimentally. Namely, during the determination of the solubilizate in micellar solubilization, the micelles are destroyed (by dilution). The total amount of solute
k is determined together with the amount that was already present in the water, in addition to the micellar solubilized amount (the micellar pseudophase, i.e., the set of all micelles could be determined (separated from the aqueous solution) by gel filtration or ultracentrifuge if the micelles are large enough). Therefore, for practical reasons, it is more convenient to avoid the micellar pseudophase and to observe the equilibrium between the hydrophobic molecule
k in the amorphous state (
as) and the hydrophobic molecule
k from the aqueous micellar solution with mole fraction
(it refers to the micellar aqueous solution of the solubilizate and not only the micellar pseudophase and is calculated as the ratio of the number of moles of solubilizate
k (
) to the number of water moles (
) from the aqueous micellar phase (
), while the amount of polymeric surfactants is not taken into account). The activity coefficient (
) describes the interactions between the solubilizate
k and the micellar aggregates or amphiphilic polymers [
25,
26]; in this imaginary model, micelles represent Gibbs free energy points, i.e., centers of intermolecular and hydrophobic interactions (
Figure 5 and
Figure 6):
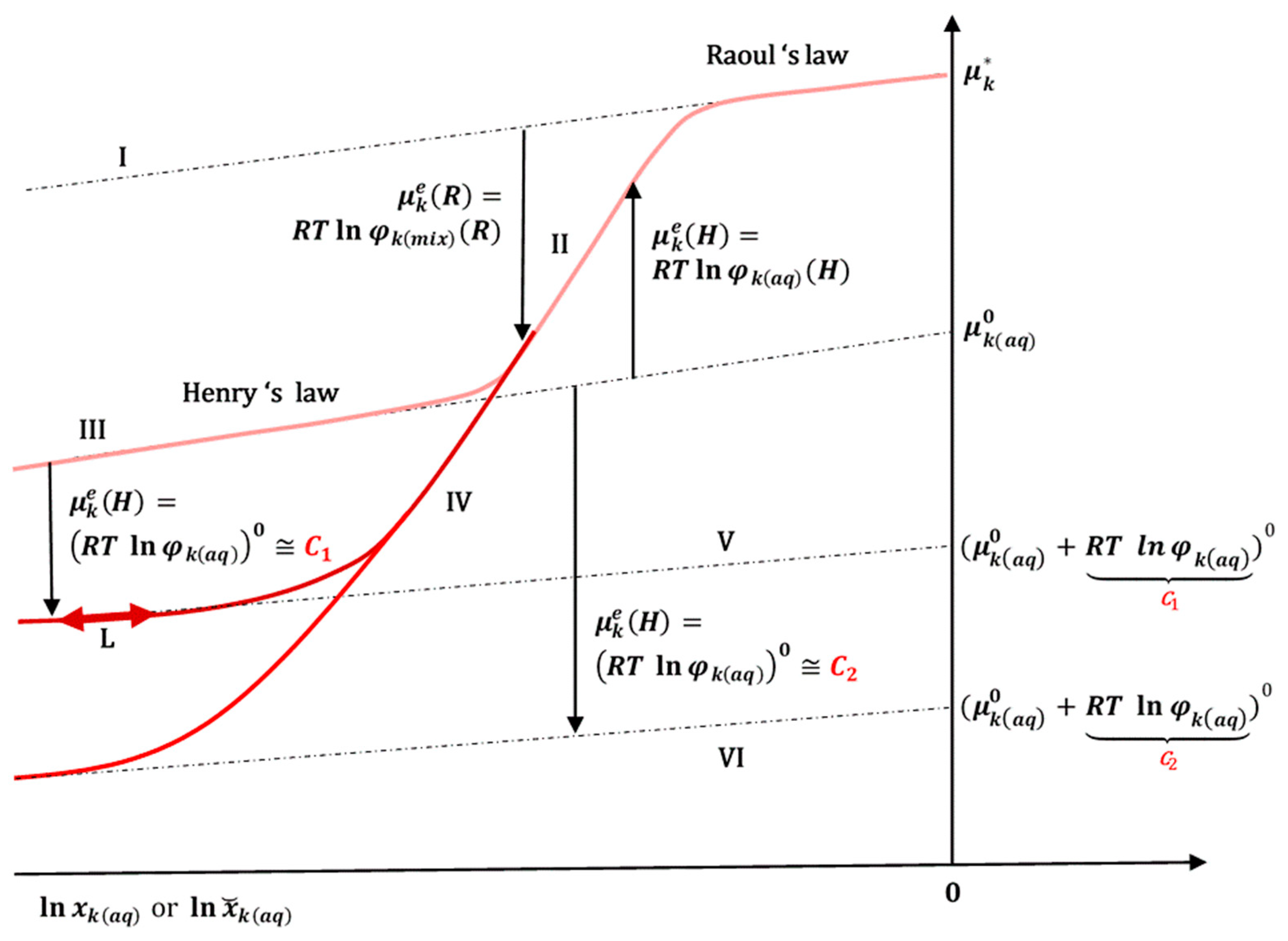
If we take the approximation that the excess chemical potential
is constant in a diluted micellar solution of solubilizate
k (
Figure 5) [
35]—the total concentration of the polymeric surfactant (PS) is constant (above the critical micellar concentration)—then for every constant total concentration of PS there is a constant
:
from which follows a new imaginary reference state in the chemical potential that obeys Henry’s law (17) (
Figure 5 and
Appendix C):
In the equilibrium state,
can fluctuates according to the linear function:
(
Figure 5, L). Polymeric surfactants from the Pluronic Block Copolymer group usually have critical micellar concentrations in the range of 0.001 mM to 0.1 mM [
36], so that they form micelles in the region of the infinite dilute solution and intermolecular interactions between the micelles themselves can be neglected.
Taking into account Equations (17) and (19) for the chemical potential, the change in the standard molar Gibbs free energy upon dissolution of the SD is
Applying Hess’s law (13), the molar Gibbs free energy of the change in the degree of spontaneity in the solubilization of the hydrophobic particle
k from the amorphous (SD) state is
If the ratio of mole fractions in Equation (21) is
then
, which means that the degree of spontaneity increases during the solubilization of amorphous
k from the SD in relation to the solubilization of crystalline
k. From Equation (21), it can be concluded that
is more negative if
and has the highest possible value, and also if
and has the highest possible absolute value. Therefore,
is as negative as possible if the hydrophobic molecule
k in the amorphous state has a higher energy content than in the crystalline (reference) state, i.e., is more thermodynamically destabilized, and if there are additional intermolecular interactions (and/or hydrophobic effect) between the solubilizate
k and the amphiphilic polymers or the micellar polymer surfactants in the aqueous solution, the solubilizate
k is in a thermodynamically more stable state than in pure water (
Figure 7).
However, the question can be raised as to whether the thermodynamic destabilization of the molecule
k in the amorphous state compared to the (reference) crystalline state is more important to the values of
than the thermodynamic stabilization of the hydrophobic solubilizate
k in the aqueous solution of micellar polymeric surfactants (or amphiphilic polymers) [
3]. Therefore, it is necessary to observe the solubility of crystalline
k from a physical mixture (PM) with a matrix substance (amphiphilic polymers or polymeric surfactants). As the matrix polymers are soluble in water, they dissolve first. Suppose the polymer surfactants are present and their concentration is above the critical micellar concentration. In that case, they form micelles, so that the crystalline substance
k dissolves in the micellar solution of the polymeric surfactant (process 1,
Figure 8). Applying equilibrium conditions, i.e., the equality between the chemical potential of
k in the crystalline solid state and the chemical potential of
k in the micellar aqueous solution (or solution of amphiphilic polymers), leads to a change in the standard molar Gibbs free energy in process 1 (
Figure 8), analogous to Equation (20):
where
represents the mole fraction of solubilizate
k in aqueous solution when dissolving the physical mixture (PM).
Figure 8 presents a scheme of the thermodynamic processes (including the dissolution of a physical mixture) whose changes in standard molar Gibbs free energies are defined in the thermodynamic scheme from
Figure 2. They are defined by the following expressions: process 2 (11), process 3 (12), and process 4 (20). Applying Hess’s law to the thermodynamic scheme in
Figure 2, we obtain
By introducing Equations (20) and (22), the following expression is obtained:
If in Equation (25), ; then, applies, which means that the dissolution of the amorphous solid phase of molecules k in a micellar aqueous solution of polymeric surfactants is a more thermodynamically spontaneous process than the dissolution of a crystalline substance k from a physical mixture in the same micellar solution, i.e., the thermodynamic destabilization of the particle k in the amorphous state relative to the particle k from the crystalline structure is the leading phenomenon that increases the solubility of k.
In the literature [
27,
28], there is an empirical equation for
(applied without deriving and proving the equation):
in which, instead of the mole fractions of hydrophobic molecules
k, the solubilities of substance
k per unit volume of aqueous solution are used (
= the solubility of the crystalline solid phase of
k in water, and
= the solubility of amorphous
k from a solid dispersion in water). In Equation (21), which was obtained by applying thermodynamic formalism, the mole fractions can be represented as follows (
or
amount of substance
k;
= amount of water):
If the observed system is a dilute solution so that conditions
and
apply, then the mole fractions can be approximated by
and be represented by equations (
and
= mass of solubilizate
k,
= molecular weight of solubilizate
k,
= molecular weight of water,
= water density, and
= the volume of aqueous solution which is identical when dissolving crystalline
k and amorphous
k from the SD):
Therefore,
equates to (
and
If it is true that the solution is diluted, i.e., approximations (28) are acceptable, then it holds that
is approximately equal to
. Similar to Equations (25) and (31), they can be presented identically with the solubilities of the hydrophobic substance
k from the physical mixture (PM) and the solid dispersion (SD):
If in a solid dispersion obtained by HMT technology, the ratio of a poorly water-soluble drug
k and the polymer matrix remains constant; however, the composition within the polymer matrix is modified by adding a non-polymeric surfactant or another polymer surfactant or a polymer that does not build micelles, then the efficiency of solubilization of
k in a solid dispersion with a modified polymer matrix can be compared with the solubilization of
k from a solid dispersion with the reference polymer matrix. Applying Hess’s law to a thermodynamic scheme that is analogous to the schemes in
Figure 1 or
Figure 8, and where one of the real processes is the reference process of solubilization of amorphous
k from SD with a reference polymer matrix, while the other real process is the solubilization of
k from SD with a changed composition of the polymer matrix, then for
an Equation is obtained that is related to Equation (32):
where
represents the solubilization of
k from SD with the reference polymer matrix, while
is the solubilization of the same molecule with a modified polymer matrix. To illustrate the values for
, Equation (33),
Table 1 presents the solubilization values of the drug lornoxicam in an aqueous solution [
27], where the reference polymer matrix is the polymer surfactant Soluplus
®, while the modified polymer matrices of the mixture are: Soluplus
®-Lutrol F127, Soluplus
®-Lutrol F68, and Soluplus
®-polyethylene Glycol 400.
The value means that the process of solubilization of amorphous solid phase k with a modified polymer matrix (PM) is a thermodynamically less spontaneous process than the dissolution of amorphous k with a reference polymer matrix. In contrast, means that the dissolution of amorphous k with a modified PM is a more spontaneous thermodynamic process than the same process with a reference PM.
The saturation solubility experiment is conducted under constant temperature and pressure conditions, with the thermoreservoir serving as the environment. According to De Donder’s equation (
= total differential entropy change in the composite system: system and environment,
[
37]), we can derive the following expression (considering Equations (31) and (32)):
In this context, represents the increase in total entropy (for ) in the total entropy change of the composite system in which amorphous k from SD is dissolved relative to the total entropy change in the reference composite system (dissolution of crystalline k or physical mixture).
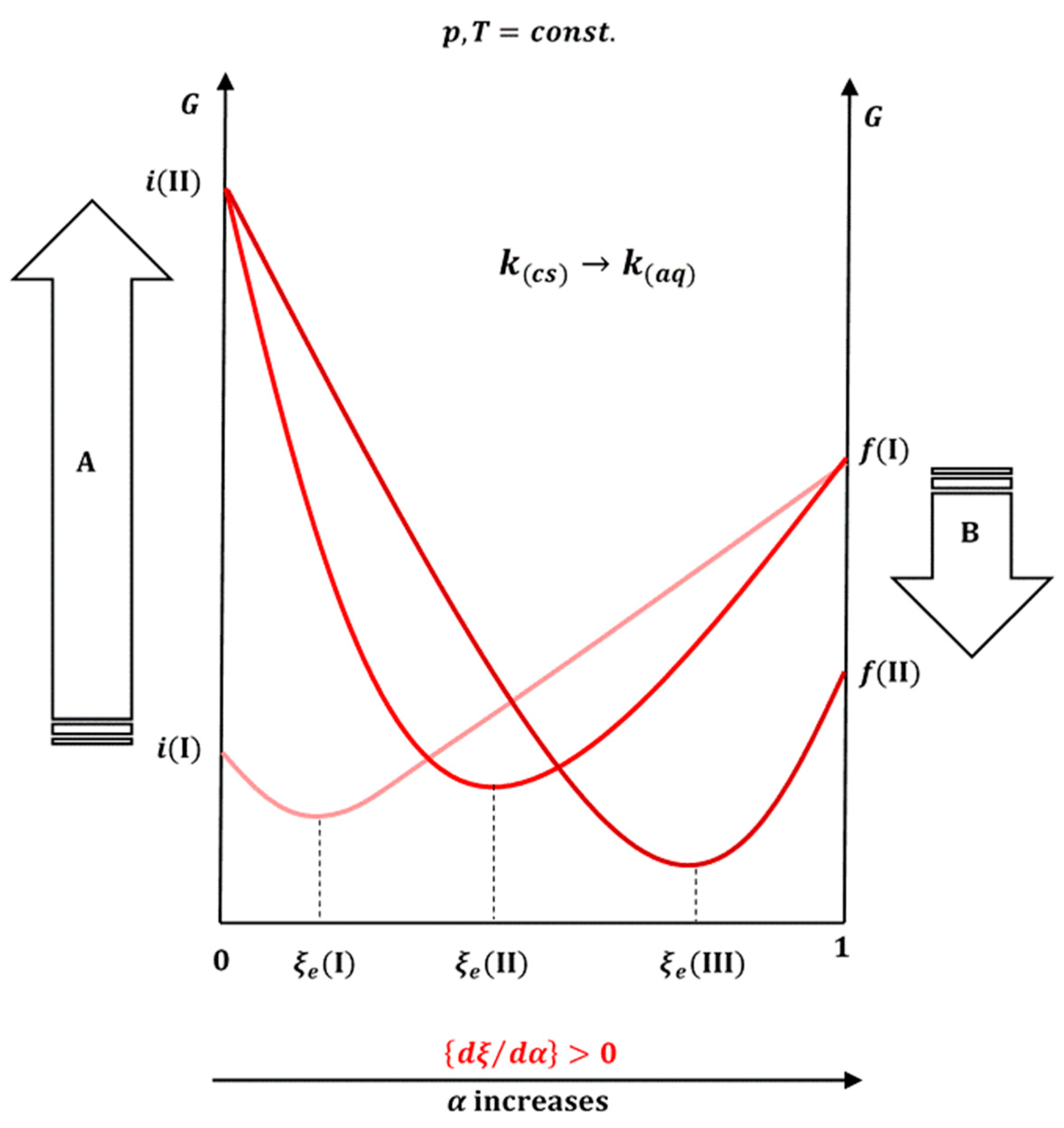
2.1. Dissolution Process as a Chemical Reaction
The differential change in Gibbs free energy is [
38]
that is, at constant temperature and pressure:
Consider the dissolution process as a chemical reaction, i.e., dissolution (transfer) of one mole of
k from the crystalline form into a saturated aqueous solution:
so that the degree of progress (ξ) in (37) can be defined [
39]. The differential change in the amount of molecules in Equation (36) can be expressed by the degree of progress in the reaction and with the stoichiometric number:
Therefore,
represents the slope of the dependence of the Gibbs free energy on the degree of progress in the reaction. In the state of equilibrium (
), Equation (39) is
where
represents the standard change in the Gibbs free energy of the reaction, while
K is the equilibrium constant. Equation (41) for the dissolution process of crystalline
k is identical to Equation (3). Let
(
p,
T =
const.), in addition to
ξ, also depend on the parameter
α [
40], which can be a measure for the presence of surfactants in different amounts (above the critical micellar concentration) and (or) the degree of translation of the crystalline form
k into the amorphous form. The total differential for
as a function of
is
In a state of equilibrium, it is
Let expression (43) be divided by
:
Given the definition of
(39), Equation (45) is
Taking into account the convexity of the function
G depending on ξ [
38,
40,
41,
42], it follows that
Therefore, the sign of
depends on the sign of
. The slope
(39) can be expressed as the ratio of the reaction quotient (
Q) [
29] and the reaction equilibrium constant:
Moreover, since during the reaction progress, it is
that follows
, i.e.,
. Inserting Equation (48) into Equation (46) yields
By converting the crystalline form
k into the amorphous form, the value of
K increases (the
ratio decreases), which means that with an increase in the proportion of the amorphous form, it is
. Therefore, with an increase in
α, the degree of progress of the reaction in the new equilibrium state is higher,
, i.e., the solubility of the hydrophobic molecule
k increases. An increase in the total surfactant concentration above the critical micellar concentration has a similar effect (
Figure 9).
2.2. Future Perspectives
In the dissolution process of the amorphous form of k from SD with polymeric surfactant (PS), for the purpose of applying thermodynamic models, it is important to determine experimentally whether the dissolution of PS can be considered instantaneous, so that the micellar solubilization of the one-component amorphous solid phase k is observed. It is important to determine, especially for polymeric surfactants, whether, during the HME technological process (after returning to the initial temperature), there are conformational changes in the polymer chains that would alter the critical micellar concentration. With the applicability of Equations (19) and (20) by molecular dynamic methods, the existence of a linear dependence between the excess chemical potential and lnx (infinitely diluted solutions in terms of solubilizates) at different total concentrations of polymeric surfactant (above the critical micellar concentration) could be established. The development of a thermodynamic model includes the activity coefficient which also takes into account intermicellar interactions, especially if the surfactant concentration is several times above the critical micellar concentration.
In HME technology used for obtaining a solid mixture (SD) of a hydrophobic drug and polymer matrix, in addition to polymer surfactants, non-polymeric (classical) surfactants and polymers that do not form micelles on their own could be included. However, suppose surfactants and polymers that form aggregates in aqueous solution are selected; upon dissolving SD, depending on the molar ratio of surfactant to polymer, different aggregates form. In that case, if the polymer chain is already saturated with surfactants, micelles form as well. Therefore, the saturation solubilization of a hydrophobic drug can be determined at different mole ratios of surfactant and polymer [
43,
44]. Similarly, in HME technology used for obtaining a solid mixture of a hydrophobic drug and polymer matrix, ionic surfactants with a simple counterion and a polyion with a simple counterion could also be included in the polymer matrix. When dissolving a SD, the surfactant ions and polyion (polymer) form a complex salt that can eventually solubilize the amorphous hydrophobic drug [
45].