Polystyrene Nanoplastics in Human Gastrointestinal Models—Cellular and Molecular Mechanisms of Toxicity
Abstract
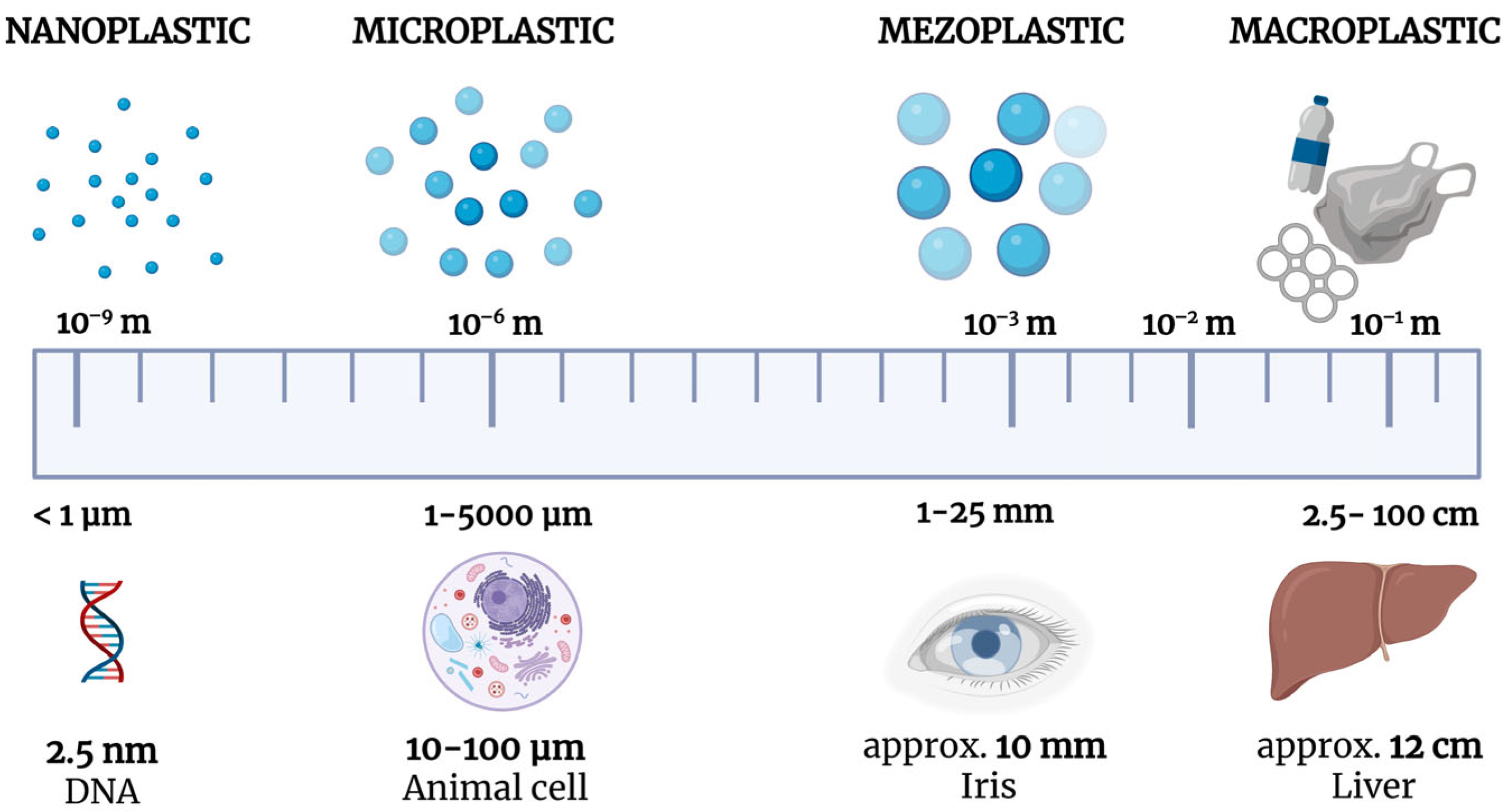
1. Introduction
- -
- Polyethylene Terephthalate (PET)—often used for bottles, containers, and trays. It is the most widely recycled plastic and is valued for its strength, clarity, and resistance to impact.
- -
- High-Density Polyethylene (HDPE)—commonly used for milk jugs, juice bottles, and grocery bags. It is tough, resistant to chemicals, and is often recycled.
- -
- Polyvinyl Chloride (PVC)—used for shrink wraps, food containers, and plastic pipes. Its versatility makes it useful for a range of food packaging, but it is less common for direct contact with food due to concerns over chemical leaching.
- -
- Low-Density Polyethylene (LDPE)—found in grocery bags, bread bags, and some food wraps. It is flexible, durable, and resistant to impact.
- -
- Polypropylene (PP)—widely used for yogurt containers, straws, and microwaveable food trays. It is heat-resistant and has a higher melting point, making it suitable for hot food applications.
- -
- Polystyrene (PS)—used in disposable cutlery, foam cups, and trays. It is lightweight and inexpensive, but also one of the most controversial due to concerns over its environmental impact and potential leaching of styrene into food.
2. Physicochemical Properties of Polystyrene Nanoparticles
3. Mechanisms of Nanoplastics Entering the Human Body
3.1. Gastrointestinal Uptake of Nanoplastics
3.2. Other Exposure Routes
3.2.1. Entry of Nanoplastics Through the Respiratory Tract
3.2.2. Dermal Uptake and Cellular Response to Nanoplastics
4. Bioaccumulation of Micro- and Nanoplastics in Human Tissues, Organs, and Cells
4.1. Micro- and Nanoplastics in Human Tissues and Organs
4.2. Localization of Nanoplastics in Cellular Organelles
5. The Impact of Nanoplastics on the Structure and Function of Cell Membranes
6. Cellular Metabolism and Metabolic Reprogramming Induced by PS-NPs
7. Mitochondrial Dysfunction and Oxidative Stress Induced by Nanoplastics in Gastrointestinal Epithelial Models
8. Molecular Mechanisms Underlying Nanoplastic-Induced DNA Damage and Repair in Gastrointestinal Cell Lines
9. Interactions of Nanoplastics with Ion Channels
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 8-OHdG | 8-hydroxy-2′-deoxyguanosine |
| A431 | Human epidermoid carcinoma keratinocyte cells |
| A549 | Human alveolar basal epithelial cells |
| AgNPs | Silver nanoparticles |
| ALI | Air liquid interface |
| AMPK | AMP-activated protein kinase |
| ATM | Ataxia-telangiectasia mutated |
| ATR | Ataxia-telangiectasia and Rad3-related |
| ATF4 | Activating transcription factor 4 |
| ATP | Adenosine triphosphate |
| BPA | Bisphenol A |
| BEAS-2B | Human bronchial epithelial cells |
| BER | Base excision repair |
| BNIP3 | BCL2/adenovirus E1B 19 kDa-interacting protein 3 |
| Caco-2 | Human colorectal adenocarcinoma enterocyte-like cells |
| CAT | Catalase |
| Cav-1 | Caveolin-1 |
| CFTR | Cystic fibrosis transmembrane conductance regulator |
| CHOP | C/EBP homologous protein |
| CME | Clathrin-mediated endocytosis |
| COOH | Carboxyl functional group (COOH) |
| cGAS | Cyclic GMP-AMP synthase |
| DAF-16 | FOXO transcription factor homolog (abnormal dauer formation protein 16) |
| DNA | Deoxyribonucleic acid |
| DNA-PKcs | DNA-dependent protein kinase catalytic subunit |
| DSB(s) | Double-strand break(s) |
| EDX | Energy-dispersive X-ray spectroscopy |
| ENMs | Engineered nanomaterials |
| ER (stress) | Endoplasmic reticulum stress |
| ERK | Extracellular signal-regulated kinase |
| FTIR | Fourier-transform infrared spectroscopy |
| GALT | Gut-associated lymphoid tissue |
| GES-1 | Human gastric epithelial cells |
| GSH-Px | Glutathione peroxidase |
| HaCaT | Human immortalized keratinocytes |
| HEEC | Human esophageal epithelial cells |
| HepG2 | Human hepatocellular carcinoma cells |
| HET-1A | Human esophageal epithelial cells |
| HKC | Human kidney cells |
| HL-7702 (L-02) | Human normal liver cell line |
| HR | Homologous recombination |
| HT-29 | Human colorectal adenocarcinoma goblet-like cells |
| IEC-6 | Rat intestinal epithelial cells |
| IL-6 | Interleukin-6 |
| IL-17C | Interleukin-17C |
| JC-1 | JC-1 mitochondrial membrane potential dye |
| LC-MS | Liquid chromatography mass spectrometry |
| LTCCs | L-type calcium channels |
| MAECM | Mouse alveolar epithelial cell monolayers |
| MAPK | Mitogen-activated protein kinase |
| MDA | Malondialdehyde |
| MMR | Mismatch repair |
| M cells | Microfold cells |
| MPs | Microplastics |
| mtROS | Mitochondrial reactive oxygen species |
| mTOR | Mechanistic target of rapamycin |
| mTORC1 | Mechanistic target of rapamycin complex 1 |
| NCM460 | Normal human colon mucosal epithelial cells |
| NER | Nucleotide excision repair |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NHEJ | Non-homologous end joining |
| NGEC | Normal gastric epithelial cells |
| NH2 | Amine functional group (NH2) |
| NIX | BNIP3-like protein |
| NPs | Nanoparticles/nanoplastics |
| NRVMs | Neonatal rat ventricular myocytes |
| PA | Polyamide |
| PAN | Polyacrylonitrile |
| PC | Polycarbonate |
| PE | Polyethylene |
| PERK | Protein kinase RNA-like ER kinase |
| PET | Polyethylene terephthalate |
| PIEZO | Piezo-type mechanosensitive ion channel component |
| PMMA | Polymethyl methacrylate |
| POM | Polyoxymethylene |
| PP | Polypropylene |
| PPAR | Peroxisome proliferator-activated receptor |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| PS | Polystyrene |
| PS-NPs | Polystyrene nanoplastics |
| PTFE | Polytetrafluoroethylene |
| PU | Polyurethane |
| PVC | Polyvinyl chloride |
| Py-GC/MS | Pyrolysis gas chromatography mass spectrometry |
| pPS-NP | Positively charged polystyrene nanoparticle |
| Raman | Raman microspectroscopy |
| RNAi | RNA interference |
| ROS | Reactive oxygen species |
| SCFAs | Short-chain fatty acids |
| SEBS | Styrene ethylene butylene styrene |
| SEM | Scanning electron microscopy |
| SOD | Superoxide dismutase |
| SREBP-1 | Sterol regulatory element-binding protein 1 |
| SSB(s) | Single-strand break(s) |
| STAT | Signal transducer and activator of transcription |
| STING | Stimulator of interferon genes |
| TEM | Transmission electron microscopy |
| THP-1 | Human monocytic cells |
| TNF-α | Tumor necrosis factor alpha |
| TPE | Thermoplastic elastomer |
| ULK1 | Unc-51-like kinase 1 |
| UV | Ultraviolet |
| γ-H2AX | Phosphorylated H2A.X histone variant |
| ↓ | Decrease |
| ↑ | Increase |
References
- Luan, X.; Cui, X.; Zhang, L.; Chen, X.; Li, X.; Feng, X.; Chen, L.; Liu, W.; Cui, Z. Dynamic Material Flow Analysis of Plastics in China from 1950 to 2050. J. Clean. Prod. 2021, 327, 129492. [Google Scholar] [CrossRef]
- Kumar, R.; Verma, A.; Shome, A.; Sinha, R.; Sinha, S.; Jha, P.K.; Kumar, R.; Kumar, P.; Shubham; Das, S.; et al. Impacts of Plastic Pollution on Ecosystem Services, Sustainable Development Goals, and Need to Focus on Circular Economy and Policy Interventions. Sustainability 2021, 13, 9963. [Google Scholar] [CrossRef]
- Babaremu, K.O.; Okoya, S.A.; Hughes, E.; Tijani, B.; Teidi, D.; Akpan, A.; Igwe, J.; Karera, S.; Oyinlola, M.; Akinlabi, E.T. Sustainable Plastic Waste Management in a Circular Economy. Heliyon 2022, 8, e09984. [Google Scholar] [CrossRef]
- Li, M.; Pan, Y.; Hou, Z.; Wu, Z.; Zeng, Z.; Wang, B. Plastic or Plastic-Free Life: From Formation to Removal. Sci. Total Environ. 2023, 890, 164359. [Google Scholar] [CrossRef]
- Lamberti, F.M.; Román-Ramírez, L.A.; Wood, J. Recycling of Bioplastics: Routes and Benefits. J. Polym. Environ. 2020, 28, 2551–2571. [Google Scholar] [CrossRef]
- Du, H.; Xie, Y.; Wang, J. Microplastic Degradation Methods and Corresponding Degradation Mechanism: Research Status and Future Perspectives. J. Hazard. Mater. 2021, 418, 126377. [Google Scholar] [CrossRef]
- Olajire, A.A.; Mohammed, A.A. Bio-Directed Synthesis of Gold Nanoparticles Using Ananas Comosus Aqueous Leaf Extract and Their Photocatalytic Activity for LDPE Degradation. Adv. Powder Technol. 2021, 32, 600–610. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, T.; Zhang, D.; Shi, Z.; Zhang, X.; Li, C.; Wang, L.; Song, J.; Lin, Q. Enhanced Photodegradability of PVC Plastics Film by Codoping Nano-Graphite and TiO2. Polym. Degrad. Stab. 2020, 181, 109332. [Google Scholar] [CrossRef]
- Lu, L.; Li, W.; Cheng, Y.; Liu, M. Chemical Recycling Technologies for PVC Waste and PVC-Containing Plastic Waste: A Review. Waste Manag. 2023, 166, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Astner, A.F.; Hayes, D.G.; O’Neill, H.; Evans, B.R.; Pingali, S.V.; Urban, V.S.; Young, T.M. Mechanical Formation of Micro- and Nano-Plastic Materials for Environmental Studies in Agricultural Ecosystems. Sci. Total Environ. 2019, 685, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Afshar, S.V.; Boldrin, A.; Astrup, T.F.; Daugaard, A.E.; Hartmann, N.B. Degradation of Biodegradable Plastics in Waste Management Systems and the Open Environment: A Critical Review. J. Clean. Prod. 2024, 434, 140000. [Google Scholar] [CrossRef]
- Kabir, A.H.M.E.; Sekine, M.; Imai, T.; Yamamoto, K.; Kanno, A.; Higuchi, T. Assessing Small-Scale Freshwater Microplastics Pollution, Land-Use, Source-to-Sink Conduits, and Pollution Risks: Perspectives from Japanese Rivers Polluted with Microplastics. Sci. Total Environ. 2021, 768, 144655. [Google Scholar] [CrossRef]
- Alimba, C.G.; Faggio, C.; Sivanesan, S.; Ogunkanmi, A.L.; Krishnamurthi, K. Micro(Nano)-Plastics in the Environment and Risk of Carcinogenesis: Insight into Possible Mechanisms. J. Hazard. Mater. 2021, 416, 126143. [Google Scholar] [CrossRef]
- Xu, J.L.; Lin, X.; Wang, J.J.; Gowen, A.A. A Review of Potential Human Health Impacts of Micro- and Nanoplastics Exposure. Sci. Total Environ. 2022, 851, 158111. [Google Scholar] [CrossRef]
- Hirt, N.; Body-Malapel, M. Immunotoxicity and Intestinal Effects of Nano- and Microplastics: A Review of the Literature. Part. Fibre Toxicol. 2020, 17, 57. [Google Scholar] [CrossRef]
- Fröhlich, E.; Meindl, C.; Roblegg, E.; Ebner, B.; Absenger, M.; Pieber, T.R. Action of Polystyrene Nanoparticles of Different Sizes on Lysosomal Function and Integrity. Part. Fibre Toxicol. 2012, 9, 26. [Google Scholar] [CrossRef]
- Choi, D.; Bang, J.; Kim, T.; Oh, Y.; Hwang, Y.; Hong, J. In Vitro Chemical and Physical Toxicities of Polystyrene Microfragments in Human-Derived Cells. J. Hazard. Mater. 2020, 400, 123308. [Google Scholar] [CrossRef]
- Jeon, S.; Clavadetscher, J.; Lee, D.K.; Chankeshwara, S.V.; Bradley, M.; Cho, W.S. Surface Charge-Dependent Cellular Uptake of Polystyrene Nanoparticles. Nanomaterials 2018, 8, 1028. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Jiang, C.; Wu, L.; Bai, X.; Zhai, S. Cytotoxicity-Related Bioeffects Induced by Nanoparticles: The Role of Surface Chemistry. Front. Bioeng. Biotechnol. 2019, 7, 414. [Google Scholar] [CrossRef]
- Banerjee, A.; Billey, L.O.; Shelver, W.L. Uptake and Toxicity of Polystyrene Micro/Nanoplastics in Gastric Cells: Effects of Particle Size and Surface Functionalization. PLoS ONE 2021, 16, e0260803. [Google Scholar] [CrossRef] [PubMed]
- Kik, K.; Bukowska, B.; Krokosz, A.; Sicińska, P. Oxidative Properties of Polystyrene Nanoparticles with Different Diameters in Human Peripheral Blood Mononuclear Cells (In Vitro Study). Int. J. Mol. Sci. 2021, 22, 4406. [Google Scholar] [CrossRef] [PubMed]
- Kik, K.; Bukowska, B.; Sicińska, P. Polystyrene Nanoparticles: Sources, Occurrence in the Environment, Distribution in Tissues, Accumulation and Toxicity to Various Organisms. Environ. Pollut. 2020, 262, 114297. [Google Scholar] [CrossRef]
- Shi, X.; Wang, X.; Huang, R.; Tang, C.; Hu, C.; Ning, P.; Wang, F. Cytotoxicity and Genotoxicity of Polystyrene Micro-and Nanoplastics with Different Size and Surface Modification in A549 Cells. Int. J. Nanomed. 2022, 17, 4509–4523. [Google Scholar] [CrossRef]
- Bochicchio, D.; Panizon, E.; Monticelli, L.; Rossi, G. Interaction of Hydrophobic Polymers with Model Lipid Bilayers. Sci. Rep. 2017, 7, 6357. [Google Scholar] [CrossRef]
- Lee, H. Effect of Protein Corona on Nanoparticle-Lipid Membrane Binding: The Binding Strength and Dynamics. Langmuir 2021, 37, 3751–3760. [Google Scholar] [CrossRef]
- Yoshito, O.; Kobayashi, T.; Asabe, H.M.; Nobunao, M.; Ono, K. Biodegradation of Low-Density Polyethylene, Polystyrene, Polyvinyl Chloride, and Urea Formaldehyde Resin Buried Under Soil for Over 32 Years. J. Appl. Polym. Sci. 1995, 56, 1789–1796. [Google Scholar]
- Zhang, Y.; Pedersen, J.N.; Eser, B.E.; Guo, Z. Biodegradation of Polyethylene and Polystyrene: From Microbial Deterioration to Enzyme Discovery. Biotechnol. Adv. 2022, 60, 107991. [Google Scholar] [CrossRef]
- Mohanan, N.; Montazer, Z.; Sharma, P.K.; Levin, D.B. Microbial and Enzymatic Degradation of Synthetic Plastics. Front. Microbiol. 2020, 11, 580709. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Majumder, E.L.W. Potential for and Distribution of Enzymatic Biodegradation of Polystyrene by Environmental Microorganisms. Materials 2021, 14, 503. [Google Scholar] [CrossRef] [PubMed]
- Danso, D.; Chow, J.; Streita, W.R. Plastics: Environmental and Biotechnological Perspectives on Microbial Degradation. Appl. Environ. Microbiol. 2019, 85, e01095-19. [Google Scholar] [CrossRef]
- Di Gennaro, P.; Colmegna, A.; Galli, E.; Sello, G.; Pelizzoni, F.; Bestetti, G. A New Biocatalyst for Production of Optically Pure Aryl Epoxides by Styrene Monooxygenase from Pseudomonas Fluorescens ST. 1999, Volume 65. Available online: https://journals.asm.org/journal/aem (accessed on 25 November 2025).
- Prata, J.C. Airborne Microplastics: Consequences to Human Health? Environ. Pollut. 2018, 234, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Schwabl, P.; Koppel, S.; Konigshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of Various Microplastics in Human Stool: A Prospective Case Series. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef]
- Brouwer, H.; Porbahaie, M.; Boeren, S.; Busch, M.; Bouwmeester, H. The in Vitro Gastrointestinal Digestion-Associated Protein Corona of Polystyrene Nano- and Microplastics Increases Their Uptake by Human THP-1-Derived Macrophages. Part. Fibre Toxicol. 2024, 21, 4. [Google Scholar] [CrossRef]
- Cao, J.; Yang, Q.; Jiang, J.; Dalu, T.; Kadushkin, A.; Singh, J.; Fakhrullin, R.; Wang, F.; Cai, X.; Li, R. Coronas of Micro/Nano Plastics: A Key Determinant in Their Risk Assessments. Part. Fibre Toxicol. 2022, 19, 55. [Google Scholar] [CrossRef]
- Ali, N.; Katsouli, J.; Marczylo, E.L.; Gant, T.W.; Wright, S.; Bernardino de la Serna, J. The Potential Impacts of Micro-and-Nano Plastics on Various Organ Systems in Humans. EBioMedicine 2024, 99, 104901. [Google Scholar] [CrossRef]
- Placci, A.; Fadda, M.; Coralli, I.; Wang, J.; Zattoni, A.; Costa, A.L.; Portela, R.; Giovannozzi, A.M.; Fabbri, D.; Melucci, D.; et al. Multitechnique Characterization of Eco-Corona Formation on Airborne Nanoplastics. RSC Adv. 2025, 15, 30849–30864. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Chai, Z.; Chen, S.; Zhang, H.; Zhang, X.; Zhou, Y. Intestinal Mucus Components and Secretion Mechanisms: What We Do and Do Not Know. Exp. Mol. Med. 2023, 55, 681–691. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, Q.; Hu, M.; Zhou, X.; Guan, T.; Wu, N.; Zhu, C.; Wang, H.; Wang, G.; Li, J. Toxic Mechanisms of Nanoplastics Exposure at Environmental Concentrations on Juvenile Red Swamp Crayfish (Procambarus clarkii): From Multiple Perspectives. Environ. Pollut. 2024, 352, 124125. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Liu, J.; Guo, Y.; Zhang, Q.; Cao, A.; Wang, H. Interactions between Polystyrene Nanoparticles and Human Intestinal Epithelial Caco-2 Cells. NanoImpact 2025, 38, 100559. [Google Scholar] [CrossRef]
- Męczyńska-Wielgosz, S.; Sikorska, K.; Czerwińska, M.; Kapka-Skrzypczak, L.; Kruszewski, M. Uptake and Toxicity of Polystyrene NPs in Three Human Cell Lines. Int. J. Mol. Sci. 2025, 26, 4783. [Google Scholar] [CrossRef]
- Bannunah, A.; Cavanagh, R.; Shubber, S.; Vllasaliu, D.; Stolnik, S. Difference in Endocytosis Pathways Used by Differentiated Versus Nondifferentiated Epithelial Caco-2 Cells to Internalize Nanosized Particles. Mol. Pharm. 2024, 21, 3603–3612. [Google Scholar] [CrossRef]
- Delon, L.C.; Faria, M.; Jia, Z.; Johnston, S.; Gibson, R.; Prestidge, C.A.; Thierry, B. Capturing and Quantifying Particle Transcytosis with Microphysiological Intestine-on-Chip Models. Small Methods 2023, 7, e2200989. [Google Scholar] [CrossRef] [PubMed]
- Parton, R.G.; Del Pozo, M.A. Caveolae as Plasma Membrane Sensors, Protectors and Organizers. Nat. Rev. Mol. Cell Biol. 2013, 14, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Deng, S.; Luo, X.; Liu, Y.; Xu, W.; Pan, J.; Wang, M.; Xia, Z. Evaluation of Intestinal Absorption Mechanism and Pharmacokinetics of Curcumin-Loaded Galactosylated Albumin Nanoparticles. Int. J. Nanomed. 2019, 14, 9721–9730. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, Z.; Gao, X.; Zhao, J.; Zhang, H. Polystyrene Nanoparticles Induced Mammalian Intestine Damage Caused by Blockage of BNIP3/NIX-Mediated Mitophagy and Gut Microbiota Alteration. Sci. Total Environ. 2024, 907, 168064. [Google Scholar] [CrossRef]
- Bianchi, M.G.; Casati, L.; Sauro, G.; Taurino, G.; Griffini, E.; Milani, C.; Ventura, M.; Bussolati, O.; Chiu, M. Biological Effects of Micro-/Nano-Plastics in Macrophages. Nanomaterials 2025, 15, 394. [Google Scholar] [CrossRef]
- Awaad, A.; Nakamura, M.; Ishimura, K. Histochemical and Biochemical Analysis of the Size-Dependent Nanoimmunoresponse in Mouse Peyer’s Patches Using Fluorescent Organosilica Particles. Int. J. Nanomed. 2012, 7, 1423–1439. [Google Scholar] [CrossRef]
- Zhang, Q.; Hao, S.; Li, L.; Liu, M.; Huo, C.; Bao, W.; Cheng, H.; Fung, H.; Wong, T.; Wu, W.; et al. M Cells of Mouse and Human Peyer’s Patches Mediate the Lymphatic Absorption of an Astragalus Hyperbranched Heteroglycan. Carbohydr. Polym. 2022, 296, 120952. [Google Scholar] [CrossRef]
- Wright, S.L.; Kelly, F.J. Plastic and Human Health: A Micro Issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef]
- Yan, L.; Yu, Z.; Lin, P.; Qiu, S.; He, L.; Wu, Z.; Ma, L.; Gu, Y.; He, L.; Dai, Z.; et al. Polystyrene Nanoplastics Promote the Apoptosis in Caco-2 Cells Induced by Okadaic Acid More than Microplastics. Ecotoxicol. Environ. Saf. 2023, 249, 114375. [Google Scholar] [CrossRef]
- Stock, V.; Böhmert, L.; Lisicki, E.; Block, R.; Cara-Carmona, J.; Pack, L.K.; Selb, R.; Lichtenstein, D.; Voss, L.; Henderson, C.J.; et al. Uptake and Effects of Orally Ingested Polystyrene Microplastic Particles in Vitro and in Vivo. Arch. Toxicol. 2019, 93, 1817–1833. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Mirande, C.; Mandin, C.; Guerrouache, M.; Langlois, V.; Tassin, B. A First Overview of Textile Fibers, Including Microplastics, in Indoor and Outdoor Environments. Environ. Pollut. 2017, 221, 453–458. [Google Scholar] [CrossRef]
- Narmadha, V.V.; Jose, J.; Patil, S.; Farooqui, M.O.; Srimuruganandam, B.; Saravanadevi, S.; Krishnamurthi, K. Assessment of Microplastics in Roadside Suspended Dust from Urban and Rural Environment of Nagpur, India. Int. J. Environ. Res. 2020, 14, 629–640. [Google Scholar] [CrossRef]
- Gasperi, J.; Wright, S.L.; Dris, R.; Collard, F.; Mandin, C.; Guerrouache, M.; Langlois, V.; Kelly, F.J.; Tassin, B. Microplastics in Air: Are We Breathing It In? Curr. Opin. Environ. Sci. Health 2018, 1, 1–5. [Google Scholar] [CrossRef]
- Kuhn, D.A.; Vanhecke, D.; Michen, B.; Blank, F.; Gehr, P.; Petri-Fink, A.; Rothen-Rutishauser, B. Different Endocytotic Uptake Mechanisms for Nanoparticles in Epithelial Cells and Macrophages. Beilstein J. Nanotechnol. 2014, 5, 1625–1636. [Google Scholar] [CrossRef]
- Gou, Z.; Wu, H.; Li, S.; Liu, Z.; Zhang, Y. Airborne Micro- and Nanoplastics: Emerging Causes of Respiratory Diseases. Part. Fibre Toxicol. 2024, 21, 50. [Google Scholar] [CrossRef] [PubMed]
- Doryab, A.; Taskin, M.B.; Stahlhut, P.; Schröppel, A.; Orak, S.; Voss, C.; Ahluwalia, A.; Rehberg, M.; Hilgendorff, A.; Stöger, T.; et al. A Bioinspired in Vitro Lung Model to Study Particokinetics of Nano-/Microparticles Under Cyclic Stretch and Air-Liquid Interface Conditions. Front. Bioeng. Biotechnol. 2021, 9, 616830. [Google Scholar] [CrossRef]
- Fazlollahi, F.; Kim, Y.H.; Sipos, A.; Hamm-Alvarez, S.F.; Borok, Z.; Kim, K.J.; Crandall, E.D. Nanoparticle Translocation across Mouse Alveolar Epithelial Cell Monolayers: Species-Specific Mechanisms. Nanomedicine 2013, 9, 786–794. [Google Scholar] [CrossRef]
- Braakhuis, H.M.; Kloet, S.K.; Kezic, S.; Kuper, F.; Park, M.V.D.Z.; Bellmann, S.; van der Zande, M.; Le Gac, S.; Krystek, P.; Peters, R.J.B. Progress and Future of in Vitro Models to Study Translocation of Nanoparticles. Arch. Toxicol. 2015, 89, 1469–1495. [Google Scholar] [CrossRef] [PubMed]
- da Silva Brito, W.A.; Mutter, F.; Wende, K.; Cecchini, A.L.; Schmidt, A.; Bekeschus, S. Consequences of Nano and Microplastic Exposure in Rodent Models: The Known and Unknown. Part. Fibre Toxicol. 2022, 19, 28. [Google Scholar] [CrossRef] [PubMed]
- Cartiera, M.S.; Johnson, K.M.; Rajendran, V.; Caplan, M.J.; Saltzman, W.M. The Uptake and Intracellular Fate of PLGA Nanoparticles in Epithelial Cells. Biomaterials 2009, 30, 2790–2798. [Google Scholar] [CrossRef]
- Ji, Y.; Wang, Y.; Shen, D.; Kang, Q.; Chen, L. Mucin Corona Delays Intracellular Trafficking and Alleviates Cytotoxicity of Nanoplastic-Benzopyrene Combined Contaminant. J. Hazard. Mater. 2021, 406, 124306. [Google Scholar] [CrossRef]
- Dong, X.; Yang, B.; Xie, Y.; Li, M.; Chen, J.; Li, H.; Ruan, H.; Wang, Q. Study on the Accumulation Characteristics of Nanoplastics in SD Rats under Inhalation Exposure Route. J. Environ. Eng. Technol. 2024, 14, 764–768. [Google Scholar] [CrossRef]
- Rakowski, M.; Grzelak, A. A New Occupational and Environmental Hazard–Nanoplastic. Med. Pr. 2020, 71, 743–756. [Google Scholar] [CrossRef]
- Alvarez-Román, R.; Naik, A.; Kalia, Y.N.; Guy, R.H.; Fessi, H. Skin Penetration and Distribution of Polymeric Nanoparticles. J. Control. Release 2004, 99, 53–62. [Google Scholar] [CrossRef]
- Campbell, C.S.J.; Contreras-Rojas, L.R.; Delgado-Charro, M.B.; Guy, R.H. Objective Assessment of Nanoparticle Disposition in Mammalian Skin after Topical Exposure. J. Control. Release 2012, 162, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Vogt, A.; Hadam, S.; Deckert, I.; Schmidt, J.; Stroux, A.; Afraz, Z.; Rancan, F.; Lademann, J.; Combadiere, B.; Blume-Peytavi, U. Hair Follicle Targeting, Penetration Enhancement and Langerhans Cell Activation Make Cyanoacrylate Skin Surface Stripping a Promising Delivery Technique for Transcutaneous Immunization with Large Molecules and Particle-Based Vaccines. Exp. Dermatol. 2015, 24, 73–75. [Google Scholar] [CrossRef] [PubMed]
- Phuc, L.T.M.; Taniguchi, A. Epidermal Growth Factor Enhances Cellular Uptake of Polystyrene Nanoparticles by Clathrin-Mediated Endocytosis. Int. J. Mol. Sci. 2017, 18, 1301. [Google Scholar] [CrossRef] [PubMed]
- Vogt, A.; Combadiere, B.; Hadam, S.; Stieler, K.M.; Lademann, J.; Schaefer, H.; Autran, B.; Sterry, W.; Blume-Peytavi, U. 40 Nm, but Not 750 or 1,500 Nm, Nanoparticles Enter Epidermal CD1a+ Cells after Transcutaneous Application on Human Skin. J. Investig. Dermatol. 2006, 126, 1316–1322. [Google Scholar] [CrossRef]
- Gopinath, P.M.; Twayana, K.S.; Ravanan, P.; Thomas, J.; Mukherjee, A.; Jenkins, D.F.; Chandrasekaran, N. Prospects on the Nano-Plastic Particles Internalization and Induction of Cellular Response in Human Keratinocytes. Part. Fibre Toxicol. 2021, 18, 35. [Google Scholar] [CrossRef]
- Döge, N.; Hadam, S.; Volz, P.; Wolf, A.; Schönborn, K.H.; Blume-Peytavi, U.; Alexiev, U.; Vogt, A. Identification of Polystyrene Nanoparticle Penetration across Intact Skin Barrier as Rare Event at Sites of Focal Particle Aggregations. J. Biophotonics 2018, 11, e201700169. [Google Scholar] [CrossRef] [PubMed]
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and Quantification of Plastic Particle Pollution in Human Blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Abbasi, S.; Turner, A. Human Exposure to Microplastics: A Study in Iran. J. Hazard. Mater. 2021, 403, 123799. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Greer, M.L.; Salehi, M. Impact of Micro- and Nanoplastics Exposure on Human Health: Focus on Neurological Effects from Ingestion. Front. Public Health 2025, 13, 1681776. [Google Scholar] [CrossRef] [PubMed]
- Barone, M.; Dimante-Deimantovica, I.; Busmane, S.; Koistinen, A.; Poikane, R.; Saarni, S.; Stivrins, N.; Tylmann, W.; Uurasjärvi, E.; Viksna, A. What to Monitor? Microplastics in a Freshwater Lake—From Seasonal Surface Water to Bottom Sediments. Environ. Adv. 2024, 17, 100577. [Google Scholar] [CrossRef]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First Evidence of Microplastics in Human Placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef]
- Jenner, L.C.; Rotchell, J.M.; Bennett, R.T.; Cowen, M.; Tentzeris, V.; Sadofsky, L.R. Detection of Microplastics in Human Lung Tissue Using ΜFTIR Spectroscopy. Sci. Total Environ. 2022, 831, 154907. [Google Scholar] [CrossRef]
- Amato-Lourenço, L.F.; Carvalho-Oliveira, R.; Júnior, G.R.; dos Santos Galvão, L.; Ando, R.A.; Mauad, T. Presence of Airborne Microplastics in Human Lung Tissue. J. Hazard. Mater. 2021, 416, 126124. [Google Scholar] [CrossRef]
- Cortés, C.; Domenech, J.; Salazar, M.; Pastor, S.; Marcos, R.; Hernández, A. Nanoplastics as a Potential Environmental Health Factor: Effects of Polystyrene Nanoparticles on Human Intestinal Epithelial Caco-2 Cells. Environ. Sci. Nano 2020, 7, 272–285. [Google Scholar] [CrossRef]
- Wang, H.; Shi, X.; Gao, Y.; Zhang, X.; Zhao, H.; Wang, L.; Zhang, X.; Chen, R. Polystyrene Nanoplastics Induce Profound Metabolic Shift in Human Cells as Revealed by Integrated Proteomic and Metabolomic Analysis. Environ. Int. 2022, 166, 107349. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Meng, R.; Chen, G.; Lai, T.; Qing, R.; Hao, S.; Deng, J.; Wang, B. Distinct Accumulation of Nanoplastics in Human Intestinal Organoids. Sci. Total Environ. 2022, 838, 155811. [Google Scholar] [CrossRef]
- Li, Y.; Guo, M.; Niu, S.; Shang, M.; Chang, X.; Sun, Z.; Zhang, R.; Shen, X.; Xue, Y. ROS and DRP1 Interactions Accelerate the Mitochondrial Injury Induced by Polystyrene Nanoplastics in Human Liver HepG2 Cells. Chem. Biol. Interact. 2023, 379, 110502. [Google Scholar] [CrossRef]
- Domenech, J.; Cortés, C.; Vela, L.; Marcos, R.; Hernández, A. Polystyrene Nanoplastics as Carriers of Metals. Interactions of Polystyrene Nanoparticles with Silver Nanoparticles and Silver Nitrate, and Their Effects on Human Intestinal Caco-2 Cells. Biomolecules 2021, 11, 859. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, J.B.; Haigis, M.C. The Multifaceted Contributions of Mitochondria to Cellular Metabolism. Nat. Cell Biol. 2018, 20, 745–754. [Google Scholar] [CrossRef]
- Ali, O.; Szabó, A. Review of Eukaryote Cellular Membrane Lipid Composition, with Special Attention to the Fatty Acids. Int. J. Mol. Sci. 2023, 24, 15693. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Liu, J.; Wu, H.; Zhang, Q.; Tang, X.R.; Li, D.; Li, C.S.; Liu, Y.; Cao, A.; Wang, H. Endocytosis, Distribution, and Exocytosis of Polystyrene Nanoparticles in Human Lung Cells. Nanomaterials 2023, 13, 84. [Google Scholar] [CrossRef]
- Wang, Y.; Mo, Y.; Sun, Y.; Li, J.; An, Y.; Feng, N.; Liu, Y. Intestinal Nanoparticle Delivery and Cellular Response: A Review of the Bidirectional Nanoparticle-Cell Interplay in Mucosa Based on Physiochemical Properties. J. Nanobiotechnol. 2024, 22, 669. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Luo, S.; Xu, M.; He, Q.; Xie, J.; Wu, J.; Huang, Y. Transepithelial Transport of Nanoparticles in Oral Drug Delivery: From the Perspective of Surface and Holistic Property Modulation. Acta Pharm. Sin. B 2024, 14, 3876–3900. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Li, X.; Li, J.; Zhang, Y.; Wei, X.; Hu, H.; Zhang, B.; Du, H.; Zhao, M.; Zhu, R.; et al. Polystyrene Nanoplastics Induce Glycolipid Metabolism Disorder via NF-ΚB and MAPK Signaling Pathway in Mice. J. Environ. Sci. 2024, 137, 553–566. [Google Scholar] [CrossRef]
- Peng, M.; Vercauteren, M.; Grootaert, C.; Rajkovic, A.; Boon, N.; Janssen, C.; Asselman, J. Cellular and Bioenergetic Effects of Polystyrene Microplastic in Function of Cell Type, Differentiation Status and Post-Exposure Time. Environ. Pollut. 2023, 337, 122550. [Google Scholar] [CrossRef]
- Brandts, I.; Solà, R.; Garcia-Ordoñez, M.; Gella, A.; Quintana, A.; Martin, B.; Esteve-Codina, A.; Teles, M.; Roher, N. Polystyrene Nanoplastics Target Lysosomes Interfering with Lipid Metabolism through the PPAR System and Affecting Macrophage Functionalization. Environ. Sci. Nano 2023, 10, 2245–2258. [Google Scholar] [CrossRef]
- Lv, J.; Liu, G.; Wang, Z.; Zhang, J.; Li, Y.; Wang, Y.; Liu, N.; Altyn, S.; Jiang, Z. Internalized polystyrene nanoplastics trigger testicular damage and promote ferroptosis via CISD1 downregulation in mouse spermatocyte. J. Nanobiotechnol. 2025, 23, 537. [Google Scholar] [CrossRef]
- He, Y.; Yu, T.; Li, H.; Sun, Q.; Chen, M.; Lin, Y.; Dai, J.; Wang, W.; Li, Q.; Ju, S. Polystyrene Nanoplastic Exposure Actives Ferroptosis by Oxidative Stress-Induced Lipid Peroxidation in Porcine Oocytes during Maturation. J. Anim. Sci. Biotechnol. 2024, 15, 117. [Google Scholar] [CrossRef]
- Yu, Z.; Fan, X.; Zhao, X.; He, T.; Li, X.; Du, H.; Zhao, M.; Zhu, R.; Li, M.; Zhang, Z.; et al. Polystyrene Nanoplastics Induce Lipid Metabolism Disorder by Activating the PERK-ATF4 Signaling Pathway in Mice. ACS Appl. Mater. Interfaces 2024, 16, 34524–34537. [Google Scholar] [CrossRef]
- Song, W.; Popp, L.; Yang, J.; Kumar, A.; Gangoli, V.S.; Segatori, L. The Autophagic Response to Polystyrene Nanoparticles Is Mediated by Transcription Factor EB and Depends on Surface Charge. J. Nanobiotechnol. 2015, 13, 87. [Google Scholar] [CrossRef]
- Liang, X.; Zeng, Y.; Zhang, P.; Zhu, B.; Feng, J.; Deng, T.; Fu, Z.; Liu, C.; Chen, C.; Zhang, Y. Polystyrene Nanoplastics Trigger Pyroptosis in Dopaminergic Neurons through TSC2/TFEB-Mediated Disruption of Autophagosome-Lysosome Fusion in Parkinson’s Disease. J. Transl. Med. 2025, 23, 631. [Google Scholar] [CrossRef]
- Huang, Y.; Liang, B.; Li, Z.; Zhong, Y.; Wang, B.; Zhang, B.; Du, J.; Ye, R.; Xian, H.; Min, W.; et al. Polystyrene Nanoplastic Exposure Induces Excessive Mitophagy by Activating AMPK/ULK1 Pathway in Differentiated SH-SY5Y Cells and Dopaminergic Neurons in Vivo. Part. Fibre Toxicol. 2023, 20, 44. [Google Scholar] [CrossRef] [PubMed]
- Malinowska, K.; Sicińska, P.; Michałowicz, J.; Bukowska, B. The Effects of Non-Functionalized Polystyrene Nanoparticles of Different Diameters on the Induction of Apoptosis and MTOR Level in Human Peripheral Blood Mononuclear Cells. Chemosphere 2023, 335, 139137. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Shao, Y.; Hua, X.; Wang, D. Activated Hedgehog and Insulin Ligands by Decreased Transcriptional Factor DAF-16 Mediate Transgenerational Nanoplastic Toxicity in Caenorhabditis elegans. J. Hazard. Mater. 2024, 480, 135909. [Google Scholar] [CrossRef] [PubMed]
- Nunnari, J.; Suomalainen, A. Mitochondria: In Sickness and in Health. Cell 2012, 148, 1145–1159. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, Y.; He, L.; Zhou, C.; Hong, P.; Qian, Z.-J.; Ju, X.; Sun, S.; Li, C. Nanoplastics Induce More Severe Apoptosis Through Mitochondrial Damage in Caco-2 Cells Compared to Sub-Micron Plastics. 2022. Available online: https://www.researchsquare.com/article/rs-1417473/v1 (accessed on 25 November 2025).
- Bu, W.; Cui, Y.; Jin, Y.; Wang, X.; Jiang, M.; Huang, R.; Egbobe, J.P.O.; Zhao, X.; Tang, J. Unmasking the Invisible Threat: Biological Impacts and Mechanisms of Polystyrene Nanoplastics on Cells. Toxics 2024, 12, 908. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, H.; Wang, C.; Su, X.L.; Song, Y.; Wu, P.; Yang, Z.; Wong, M.H.; Cai, Z.; Zheng, C. Metabolomics Reveal Nanoplastic-Induced Mitochondrial Damage in Human Liver and Lung Cells. Environ. Sci. Technol. 2022, 56, 12483–12493. [Google Scholar] [CrossRef]
- Kadac-Czapska, K.; Ośko, J.; Knez, E.; Grembecka, M. Microplastics and Oxidative Stress—Current Problems and Prospects. Antioxidants 2024, 13, 579. [Google Scholar] [CrossRef]
- Mahmud, F.; Sarker, D.B.; Jocelyn, J.A.; Sang, Q.X.A. Molecular and Cellular Effects of Microplastics and Nanoplastics: Focus on Inflammation and Senescence. Cells 2024, 13, 1788. [Google Scholar] [CrossRef] [PubMed]
- Sadique, S.A.; Konarova, M.; Niu, X.; Szilagyi, I.; Nirmal, N.; Li, L. Impact of Microplastics and Nanoplastics on Human Health: Emerging Evidence and Future Directions. Emerg. Contam. 2025, 11, 100545. [Google Scholar] [CrossRef]
- Agrawal, M.; Vianello, A.; Picker, M.; Simon-Sánchez, L.; Chen, R.; Estevinho, M.M.; Weinstein, K.; Lykkemark, J.; Jess, T.; Peter, I.; et al. Micro- and Nano-Plastics, Intestinal Inflammation, and Inflammatory Bowel Disease: A Review of the Literature. Sci. Total Environ. 2024, 953, 176228. [Google Scholar] [CrossRef]
- Wang, Q.; Bai, J.; Ning, B.; Fan, L.; Sun, T.; Fang, Y.; Wu, J.; Li, S.; Duan, C.; Zhang, Y.; et al. Effects of Bisphenol A and Nanoscale and Microscale Polystyrene Plastic Exposure on Particle Uptake and Toxicity in Human Caco-2 Cells. Chemosphere 2020, 254, 126788. [Google Scholar] [CrossRef]
- Cheng, Y.; Chen, J.; Fu, R.; Zhang, P.; Chen, H.; Cao, H.; Jiang, Z.; Hong, Y.; Li, Y.; He, C.; et al. Molecular Mechanism Differences between Nanoplastics and Microplastics in Colon Toxicity: Nanoplastics Induce Ferroptosis-Mediated Immunogenic Cell Death, While Microplastics Cause Cell Metabolic Reprogramming. J. Nanobiotechnol. 2025, 23, 505. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.; Chen, Y.; Shi, X.; Li, Y.; Yu, Y.; Sun, Z.; Duan, J. Size-Dependent Toxicity of Polystyrene Microplastics on the Gastrointestinal Tract: Oxidative Stress Related-DNA Damage and Potential Carcinogenicity. Sci. Total Environ. 2024, 912, 169514. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, Y.; Zu, D.; Liu, H.; He, H.; Bao, Q.; He, Y.; Liang, C.; Luo, G.; Teng, Y.; et al. PMMA Nanoplastics Induce Gastric Epithelial Cellular Senescence and CGAS-STING-Mediated Inflammation via ROS Overproduction and NHEJ Suppression. Ecotoxicol. Environ. Saf. 2024, 287, 117284. [Google Scholar] [CrossRef]
- Guanglin, L.; Shuqin, W. Polystyrene Nanoplastics Exposure Causes Inflammation and Death of Esophageal Cell. Ecotoxicol. Environ. Saf. 2024, 269, 115819. [Google Scholar] [CrossRef]
- Kustra, A.; Zając, M.; Bednarczyk, P.; Maliszewska-Olejniczak, K. Exposure to Polystyrene Nanoparticles Leads to Dysfunction in DNA Repair Mechanisms in Caco-2 Cells. Biol. Res. 2025, 58, 49. [Google Scholar] [CrossRef]
- Toprani, S.M.; Bitounis, D.; Huang, Q.; Oliveira, N.; Ng, K.W.; Tay, C.Y.; Nagel, Z.D.; Demokritou, P. High-Throughput Screening Platform for Nanoparticle-Mediated Alterations of DNA Repair Capacity. ACS Nano 2021, 15, 4728–4746. [Google Scholar] [CrossRef]
- Tian, S.; Li, R.; Li, J.; Zou, J. Polystyrene Nanoplastics Promote Colitis-Associated Cancer by Disrupting Lipid Metabolism and Inducing DNA Damage. Environ. Int. 2025, 195, 109258. [Google Scholar] [CrossRef]
- Jackson, S.P.; Bartek, J. The DNA-Damage Response in Human Biology and Disease. Nature 2009, 461, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Lee, S.; Ahn, J.; Kim, J.H.; Lee, E.; Lee, I.; Byun, S. Transcriptomic and Metabolomic Analysis Unveils Nanoplastic-Induced Gut Barrier Dysfunction via STAT1/6 and ERK Pathways. Environ. Res. 2024, 249, 118437. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Deng, Y.; Huang, Y.; Zhong, Y.; Li, Z.; Du, J.; Ye, R.; Feng, Y.; Bai, R.; Fan, B.; et al. Supplemental Information Fragile Guts Make Fragile Brains: Intestinal Epithelial Nrf2 Deficiency Exacerbates Neurotoxicity Induced by Polystyrene Nanoplastics. ACS Nano 2024, 18, 24044–24059. [Google Scholar] [CrossRef]
- Jin, M.H.; Hu, J.N.; Zhang, M.; Meng, Z.; Shi, G.P.; Wang, Z.; Li, W. Maltol Attenuates Polystyrene Nanoplastic-Induced Enterotoxicity by Promoting AMPK/MTOR/TFEB-Mediated Autophagy and Modulating Gut Microbiota. Environ. Pollut. 2023, 322, 121202, Erratum in Environ. Pollut. 2023, 334, 122127. [Google Scholar] [CrossRef]
- Ashcroft, F.; Harrison, D.; Ashcroft, S. Glucose induces closure of single potassium channels in isolated rat pancreatic β-cells. Nature 1984, 312, 446–448. [Google Scholar] [CrossRef]
- Kass, R.S. The Channelopathies: Novel Insights into Molecular and Genetic Mechanisms of Human Disease. J. Clin. Investig. 2005, 115, 1986–1989. [Google Scholar] [CrossRef] [PubMed]
- Soloviev, A.; Zholos, A.; Ivanova, I.; Novokhatska, T.; Tishkin, S.; Raevska, A.; Stroyuk, A.; Yefanov, V. Plasmonic Gold Nanoparticles Possess the Ability to Open Potassium Channels in Rat Thoracic Aorta Smooth Muscles in a Remote Control Manner. Vascul Pharmacol. 2015, 72, 190–196. [Google Scholar] [CrossRef]
- Chen, C.; Bu, W.; Ding, H.; Li, Q.; Wang, D.; Bi, H.; Guo, D. Cytotoxic Effect of Zinc Oxide Nanoparticles on Murine Photoreceptor Cells via Potassium Channel Block and Na+/K+-ATPase Inhibition. Cell Prolif. 2017, 50, e12339. [Google Scholar] [CrossRef]
- Li, H.; Tan, X.Q.; Yan, L.; Zeng, B.; Meng, J.; Xu, H.Y.; Cao, J.M. Multi-Walled Carbon Nanotubes Act as a Chemokine and Recruit Macrophages by Activating the PLC/IP3/CRAC Channel Signaling Pathway. Sci. Rep. 2017, 7, 226. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, J.; Gong, X.; Nahirney, D.; Duszyk, M.; Radomski, M. Polystyrene Nanoparticles Activate Ion Transport in Human Airway Epithelial Cells. Int. J. Nanomed. 2011, 6, 1343–1356. [Google Scholar] [CrossRef] [PubMed]
- Şenol, O.; Sulukan, E.; Baran, A.; Bolat, İ.; Toraman, E.; Alak, G.; Yildirim, S.; Bilgin, G.; Ceyhun, S.B. Global Warming and Nanoplastic Toxicity; Small Temperature Increases Can Make Gill and Liver Toxicity More Dramatic, Which Affects Fillet Quality Caused by Polystyrene Nanoplastics in the Adult Zebrafish Model. Sci. Total Environ. 2023, 892, 164682. [Google Scholar] [CrossRef]
- Li, Y.; Ye, Y.; Zhu, X.; Yao, Z.; Li, Y.; Sun, Z.; Rihan, N.; Zhao, Y.; Lai, Q. Ion transport and metabolic regulation induced by nanoplastic toxicity in gill of Litopenaeus vannamei using proteomics. Environ. Sci. Nano 2025, 12, 3592–3608. [Google Scholar] [CrossRef]
- Wang, X.; Shao, S.; Zhang, T.; Zhang, Q.; Yang, D.; Zhao, J. Effects of Exposure to Nanoplastics on the Gill of Mussels Mytilus galloprovincialis: An Integrated Perspective from Multiple Biomarkers. Mar. Environ. Res. 2023, 191, 106174. [Google Scholar] [CrossRef]
- Qu, M.; Zhao, X.; Wang, Q.; Xu, X.; Chen, H.; Wang, Y. PIEZO Mediates a Protective Mechanism for Nematode Caenorhabditis elegans in Response to Nanoplastics Caused Dopaminergic Neurotoxicity at Environmentally Relevant Concentrations. Ecotoxicol. Environ. Saf. 2024, 269, 115738. [Google Scholar] [CrossRef] [PubMed]
- Roshanzadeh, A.; Oyunbaatar, N.E.; Ganjbakhsh, S.E.; Park, S.; Kim, D.S.; Kanade, P.P.; Lee, S.; Lee, D.W.; Kim, E.S. Exposure to Nanoplastics Impairs Collective Contractility of Neonatal Cardiomyocytes under Electrical Synchronization. Biomaterials 2021, 278, 121175. [Google Scholar] [CrossRef]
| Mechanism | Model Type | Cell Line | NP Characteristics | Exposure Conditions | Observed Effects | Reference |
|---|---|---|---|---|---|---|
| Clathrin-mediated endocytosis (CME) | In vitro | Caco-2 | PS-NPs (≈50–200 nm; surface unmodified/COOH) | 2–24 h; ±chlorpromazine | CME inhibition significantly reduces PS-NP uptake; internalized NPs localize in endosomes/lysosomes | [40] |
| Caveolae-mediated endocytosis | In vitro | Caco-2 | PS-NPs (small < 100 nm) | ±genistein or filipin | Caveolin inhibition decreases uptake of small/surface-modified NPs; caveolae support transcytosis | [42,45] |
| Macropinocytosis | In vitro | Caco-2 | PS-NPs ~100–200 nm | ±cytochalasin D, ±amiloride | Actin-dependent uptake contributes to internalization and enhances basolateral transport; inhibition reduces uptake | [40,51] |
| Differentiation-dependent uptake | In vitro | Caco-2 (differentiated vs. non-differentiated) | Polymer NPs (≈100 nm; +/− charge) | 2–3 h | Differentiated cells rely more on macropinocytosis; undifferentiated on dynamin-dependent uptake (CME/caveolae) | [52] |
| Transcytosis (vesicular) | In vitro | Caco-2 Transwell/intestine-on-chip | Fluorescent NPs ~100 nm | 2–24 h; static vs. flow | Shear stress increases NP translocation ~350× vs. static monolayer; energy-dependent pathway | [42,43] |
| Immune-associated uptake (M cells) | In vivo/ex vivo | Peyer’s patches | silica NPs (100–925 nm) | oral exposure | Particles < 200 nm efficiently transcytosed by M cells; initiate mucosal immune response | [48] |
| Mechanism | Plastic/NP Type | Size | Model Type | Cell Line/Organism | Exposure | Observed Effects | Reference |
|---|---|---|---|---|---|---|---|
| Clathrin-mediated endocytosis (CME) | PS, PLGA | 40–200 nm | In vitro | A549, BEAS-2B, MDCK | 4–24 h | CME inhibition (chlorpromazine) ↓ uptake; NPs accumulate in endosomes/lysosomes | [46,56,62] |
| Caveolae-mediated endocytosis | PS, PLGA | <100 nm | In vitro | A549, BEAS-2B | 4–24 h | Caveolae inhibition (genistein/filipin) ↓ uptake; lipid-raft dependent entry | [56,62,63] |
| Macropinocytosis | PS | 40–100 nm | In vitro | A549 | 4 h | Actin inhibition (cytochalasin D) ↓ uptake; macropinocytosis = major uptake route | [56,63] |
| Transcytosis (apical → basolateral) | PS | 20–120 nm | In vitro | Mouse alveolar epithelial monolayers (MAECM) | 2–24 h | Energy-dependent NP translocation across alveolar barrier; species-specific differences | [59] |
| Barrier-disruption dependent paracellular transport | PS | <100 nm | ALI in vitro | Human ALI lung model (cyclic stretch + inflammation) | TNF-α, ROS | TJ disruption ↑ NP passage; oxidative stress enhances leakage | [58] |
| Phagocytosis by alveolar macrophages | PS, PE | <200 nm | In vitro + primary cells | Alveolar macrophages | 24–48 h | Uptake induces ROS, TNF-α, IL-6; high inflammatory activation | [47] |
| Immune transport (lymphatic dissemination) | PS | <200 nm | In vivo | SD rat model | Inhalation | NPs detected in mediastinal lymph nodes; macrophage-mediated transport | [57,64] |
| Mechanism | Plastic Type | Modification | Size | Model (In Vitro/Ex Vivo) | Exposure | Observed Effects | Reference |
|---|---|---|---|---|---|---|---|
| Macropinocytosis | PS, PE | Protein corona | 30–300 nm | HaCaT keratinocytes (in vitro) | 24 h | Lysosomal accumulation, ROS↑, autophagy, senescence; dominant macropinocytosis pathway | [71] |
| Clathrin-mediated endocytosis (CME) | PS | None | 100 nm | A431 keratinocytes (in vitro) | 1–6 h | CME inhibition ↓ uptake by ~40%; EGF enhances CME-dependent NP internalization | [69] |
| Caveolae-mediated endocytosis + immune uptake | PS | None/surface-dependent | 20–50 nm | Primary keratinocytes + human skin (ex vivo) | <24 h | Rare penetration through intact barrier; uptake in keratinocytes and perifollicular tissue; partial uptake by Langerhans cells; surface chemistry influences penetration | [72] |
| Follicular penetration | PS | None | 20 nm | Porcine skin (ex vivo) | 6 h | Preferential accumulation in hair follicles; no dermal penetration | [66] |
| Follicular penetration (limited stratum corneum entry) | PS | None | 20–200 nm | Human skin (ex vivo) | 24 h | Limited entry (2–3 µm) into stratum corneum only | [67] |
| Phagocytosis by Langerhans cells | PS | Fluorescent | 40 nm | Human skin explants (ex vivo) | 24 h | Uptake by epidermal CD1a+ Langerhans cells | [70] |
| System | Human Tissue/Sample | Sample Collection | Model Type | Identified Polymers | Size | Detection Method | Reference |
|---|---|---|---|---|---|---|---|
| Digestive system | Blood | Healthy adult volunteers (n = 22) | Clinical (in vivo) | PE, PS, PP, PET, PMMA | 700 nm–2 µm | μ-FTIR + Py-GC/MS | [73] |
| Stool | Stool from volunteers (n = 8) | Clinical (in vivo) | PA, PC, PE, PET, POM, PP, PS, PU, PVC | 50–500 µm | μ-FTIR | [33] | |
| Saliva | Food-contact saliva sampling cohort | Clinical (in vivo) | PE-PET-PP fibers | <100 µm | Micro-Raman + microscopy | [74] | |
| Liver | Autopsy liver tissue (n = 28) | Autopsy (post-mortem) | PE | 1–5 µm | μ-FTIR + Raman | [75] | |
| Kidneys | Autopsy kidney tissue | Autopsy (post-mortem) | PE | 1–5 µm | μ-FTIR + Raman | [75] | |
| Respiratory system | Lungs | Surgically resected tissue (n = 13) | Clinical (surgical, in vivo exposure) | PAN, PE, PS, PET, PMMA, PP, PTFE, PUR, SEBS | ~23 µm | μ-FTIR | [78] |
| Lungs | Autopsy lungs (smokers + non-smokers) | Autopsy (post-mortem) | Polymeric particles, fibers | 1.6–5.56 µm | Raman | [79] | |
| Sputum | Patients with respiratory disease | Clinical (in vivo) | PET, PS, PVC, PE, PAN, PU | μm range | Raman + μ-FTIR | [74] | |
| Skin | Human skin | Ex vivo explants after NP application | Ex vivo | PS | 40 nm | Confocal, CD1a immunostaining, TEM | [70] |
| Reproductive system | Placenta | Placental tissue from pregnancies | Clinical (in vivo) | PP, PE, PVC | <10 µm | Raman | [77] |
| Nervous system | Brain | Human post-mortem brain tissue | Autopsy (post-mortem) | PS, PE, PET, PVC | 1–20 µm | Raman, SEM, Nano-FTIR | [76] |
| Cell Model | NP Type/Size | Model Type | Methods Used | Confirmed Localization | Main Functional Effects | References |
|---|---|---|---|---|---|---|
| Caco-2 | PS-NPs, 50–100 nm (plain, COOH, NH2) | in vitro | Confocal microscopy (Hoechst, LysoTracker), TEM | Endosomes, lysosomes, perinuclear region | ↑ROS, oxidative stress, tight junction disruption | [52] |
| HT-29 | PS-MPs 3–10 µm | in vitro | Confocal microscopy, TEM, flow cytometry, viability assays | Lysosomes, endosomes | Lysosomal membrane permeabilization, lysosomal stress, autophagy activation, oxidative stress, inflammatory response | [69] |
| HL-7702 | Polystyrene nanoplastics (PS-NPs), <100 nm | in vitro | Confocal microscopy (Hoechst staining), lysosomal staining, proteomics, metabolomics, Seahorse metabolic flux analysis | Cytoplasm, lysosomes (no nuclear localization) | Metabolic reprogramming, impaired oxidative and energy pathways, ↑mTORC1 activity, mitochondrial metabolic disturbance | [81] |
| HepG2 | PS-NPs 21.5 ± 2.7 nm | in vitro | JC-1 assay, ROS assay, TEM, LC-MS proteomics | Autophagosomes, lysosomes (no nuclear localization) | Mitochondrial dysfunction (↓ΔΨm, ↑ROS), ↑DRP1, ↓OPA1 | [83] |
| Human intestinal organoids | PS-NPs ~50 nm | ex vivo/in vitro hybrid | Confocal microscopy, TEM, fluorescence imaging | Cytoplasm, perinuclear region, lysosomes | Differential accumulation in specific intestinal cell types; differences in uptake pathways | [82] |
| Caco-2 + co-exposure with Ag | PS-NPs + AgNPs, ~40 nm | in vitro | Confocal microscopy (Hoechst), TEM-EDX | Rare nuclear localization events | DNA damage, oxidative stress, genotoxicity | [85] |
| Cell Line/Model | Type of NPs | Size | Model Type | Dose/Exposure Time | Effect on ΔΨm | ATP Changes | ROS/Other Effects | Reference |
|---|---|---|---|---|---|---|---|---|
| Caco-2 | PS-NPs vs. PS-MPs | 80 nm; 500 nm; 3 µm | in vitro | 10–100 µg/mL; 24 h | ΔΨm↓; stronger effect in presence of BPA | ↓ATP (stronger for 300–500 nm MPs) | ↑ROS, oxidative stress | [109] |
| Caco-2 | PS-NPs vs. PS-MPs | 80 nm; 500 nm | in vitro | 100–1000 µg/mL; 24–48 h | Strong ΔΨm depolarization (NPs > MPs) | ↓ATP | ↑ROS, caspase activation, apoptosis | [102] |
| Caco-2 + okadaic acid | PS-NPs vs. PS-MPs | 80 nm; 500 nm | in vitro | 100 µg/mL; 24 h | ΔΨm↓; NPs potentiate OA-induced damage | ↓ATP (not quantified) | ↑ROS, ↑ apoptosis | [95] |
| PBMC | PS-NPs (NH2 vs. COOH) | 50–100 nm | in vitro | 10–100 µg/mL; 24 h | Mild ΔΨm reduction; donor variability | n/a | ↑ROS, ↑ lipid peroxidation | [21] |
| L02 | PS-NPs | ~78 nm | in vitro | 100 µg/mL; 24 h | ΔΨm↓ associated with metabolic disruption | ↓ATP | Metabolic reprogramming, OXPHOS disruption | [104] |
| Gastrointestinal Cell Line | Model Type | Mechanism | Description | Reference |
|---|---|---|---|---|
| GES-1 NGEC Caco-2 | In vitro | oxidative stress and ROS | increased ROS production leading to DNA damage | [112,114,116] |
| GES-1 NGEC Caco-2 | In vitro | DNA repair inhibition | inhibition of NHEJ, HR and BER pathways, resulting in genomic instability | [114,116] |
| NCM460 | In vitro | lipid peroxidation and ferroptosis | induction of ferroptosis through lipid peroxidation | [110] |
| GES-1 NGEC | In vitro | inflammatory pathways activation | activation of cGAS-STING, NF-κB, and ERK1/2 pathways | [112,117] |
| HET-1A HEEC IEC-6 | In vitro | mitochondrial dysfunction | disruption of mitochondrial function and inhibition of mitophagy | [46,113] |
| Caco-2 | In vitro | gut microbiota disruption | alteration of gut microbiota and increased gut permeability | [116] |
| Caco-2 | In vitro | size-dependent effects | smaller particles induce more significant oxidative stress and DNA repair inhibition or DNA damage | [110,111,114] |
| GES-1 NGEC | In vitro | cell cycle arrest/senescence | induction of cell cycle arrest and senescence-associated activity | [116] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kustra, A.; Maliszewska-Olejniczak, K.; Sekrecka-Belniak, A.; Kulawiak, B.; Bednarczyk, P. Polystyrene Nanoplastics in Human Gastrointestinal Models—Cellular and Molecular Mechanisms of Toxicity. Int. J. Mol. Sci. 2025, 26, 11738. https://doi.org/10.3390/ijms262311738
Kustra A, Maliszewska-Olejniczak K, Sekrecka-Belniak A, Kulawiak B, Bednarczyk P. Polystyrene Nanoplastics in Human Gastrointestinal Models—Cellular and Molecular Mechanisms of Toxicity. International Journal of Molecular Sciences. 2025; 26(23):11738. https://doi.org/10.3390/ijms262311738
Chicago/Turabian StyleKustra, Agata, Kamila Maliszewska-Olejniczak, Anna Sekrecka-Belniak, Bogusz Kulawiak, and Piotr Bednarczyk. 2025. "Polystyrene Nanoplastics in Human Gastrointestinal Models—Cellular and Molecular Mechanisms of Toxicity" International Journal of Molecular Sciences 26, no. 23: 11738. https://doi.org/10.3390/ijms262311738
APA StyleKustra, A., Maliszewska-Olejniczak, K., Sekrecka-Belniak, A., Kulawiak, B., & Bednarczyk, P. (2025). Polystyrene Nanoplastics in Human Gastrointestinal Models—Cellular and Molecular Mechanisms of Toxicity. International Journal of Molecular Sciences, 26(23), 11738. https://doi.org/10.3390/ijms262311738





