Modification of Chitosan with (−)-Gossypol and (−)-Gossypol Acetic Acid Using Free-Radical Grafting Method
Abstract
1. Introduction
2. Results and Discussion
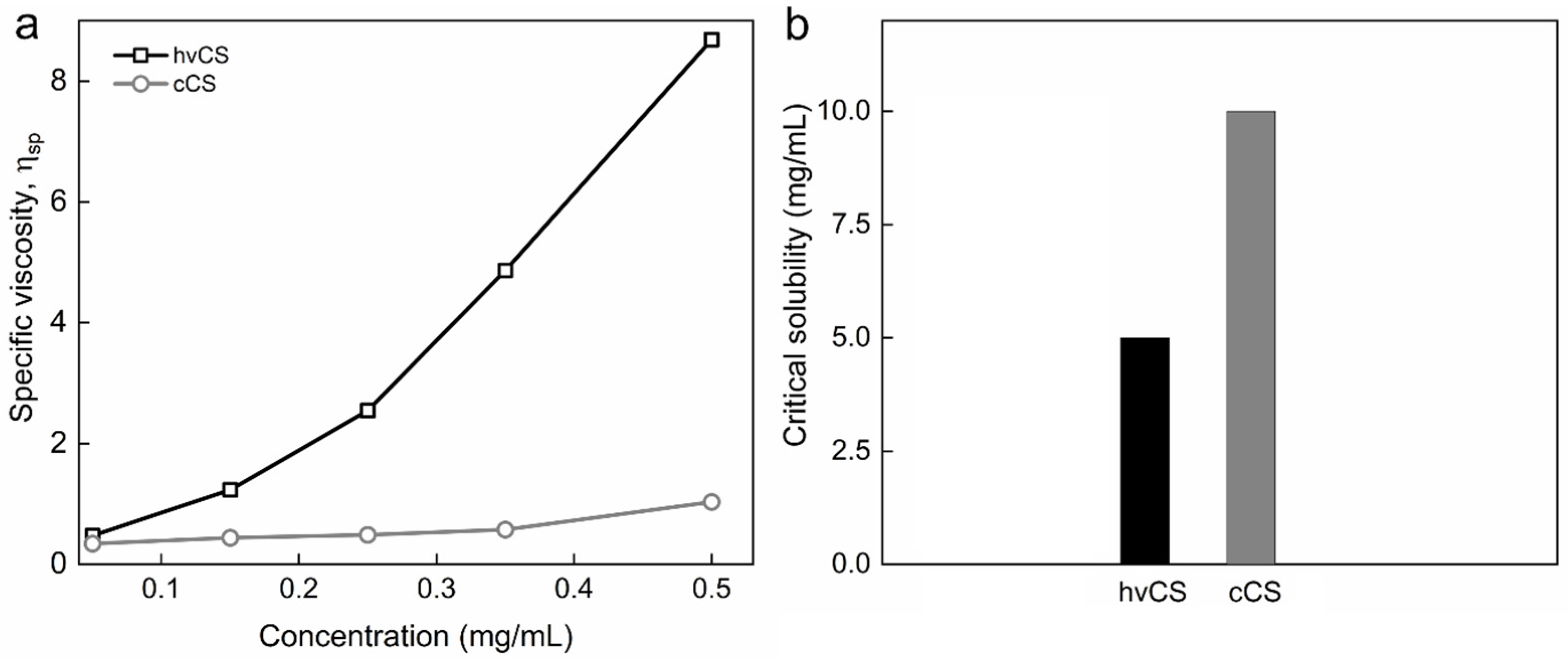
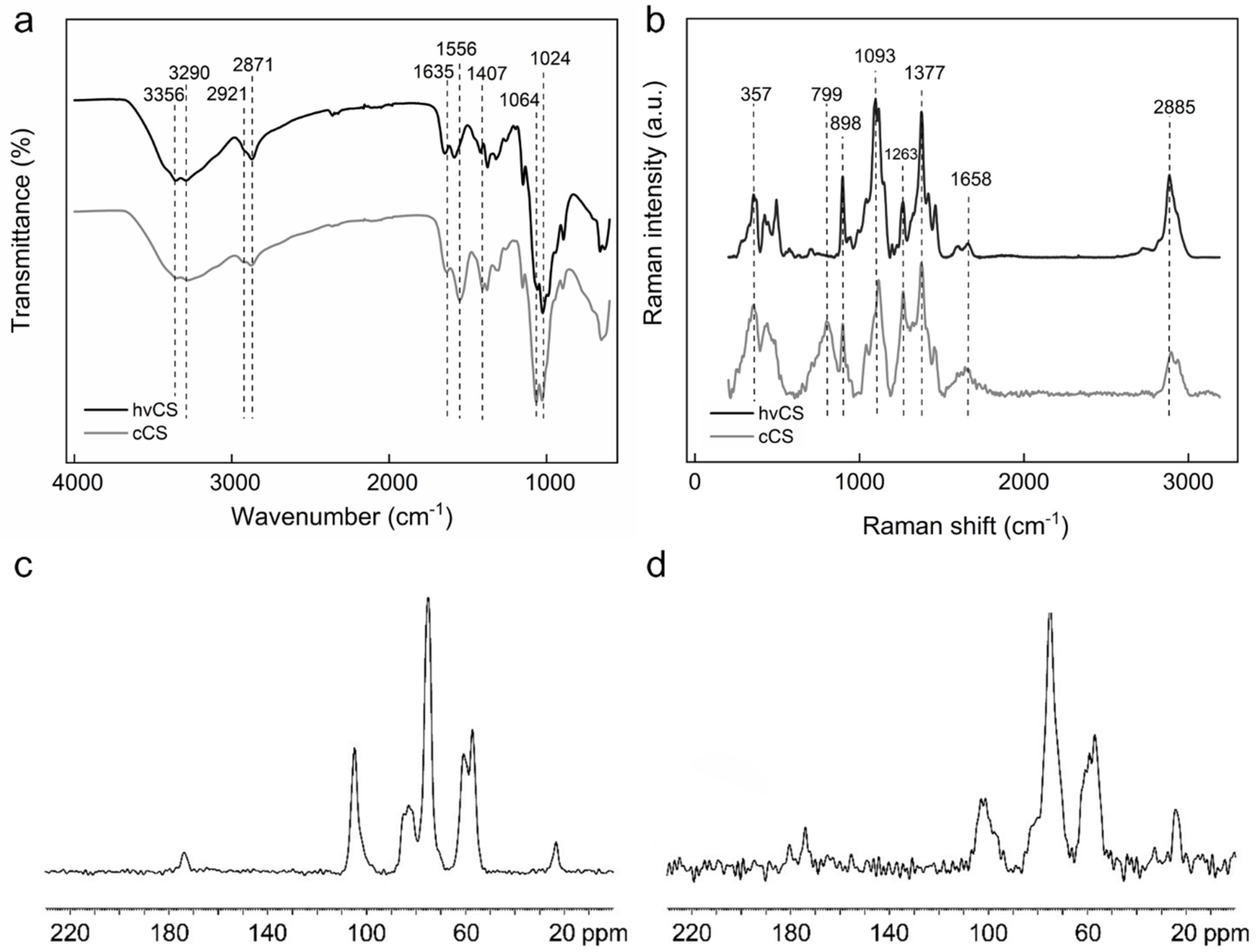
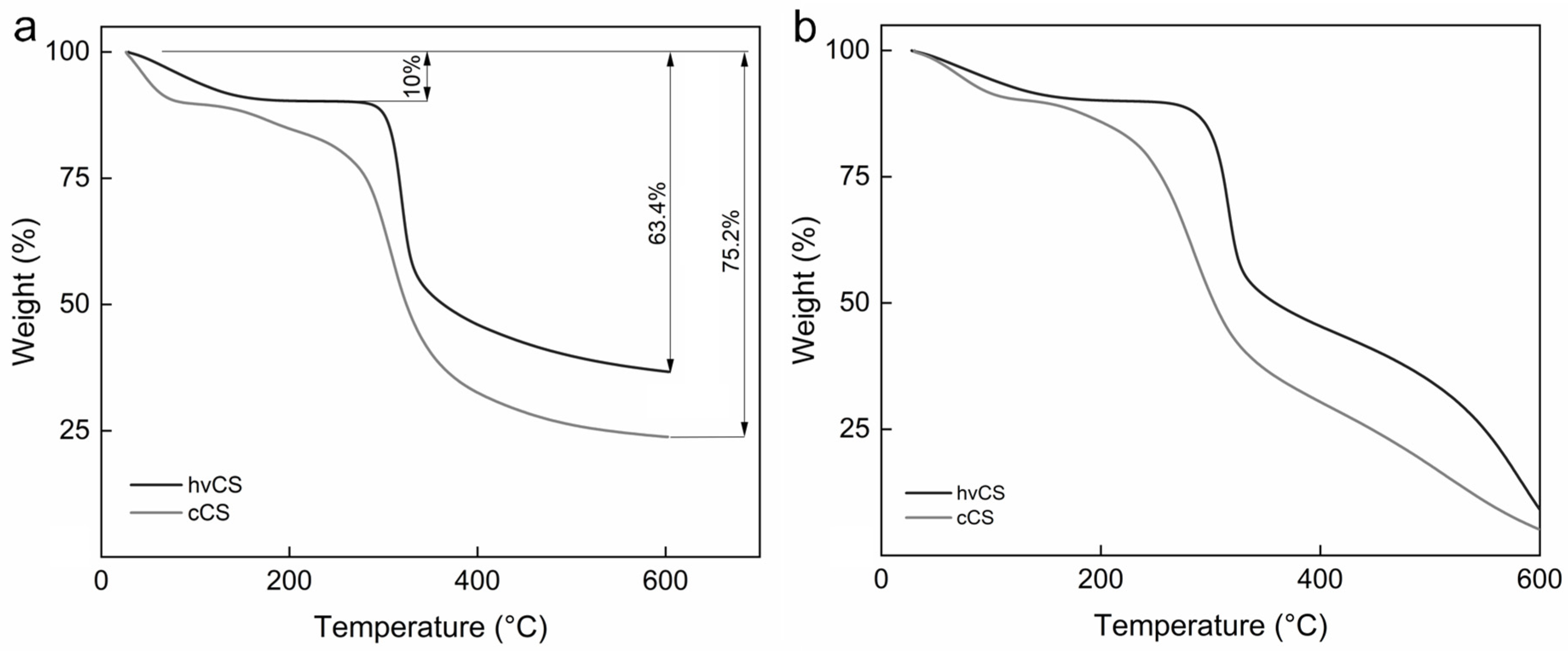
2.1. Characterization of Highly Viscous Chitosan (hvCS) and Control Chitosan (cCS)
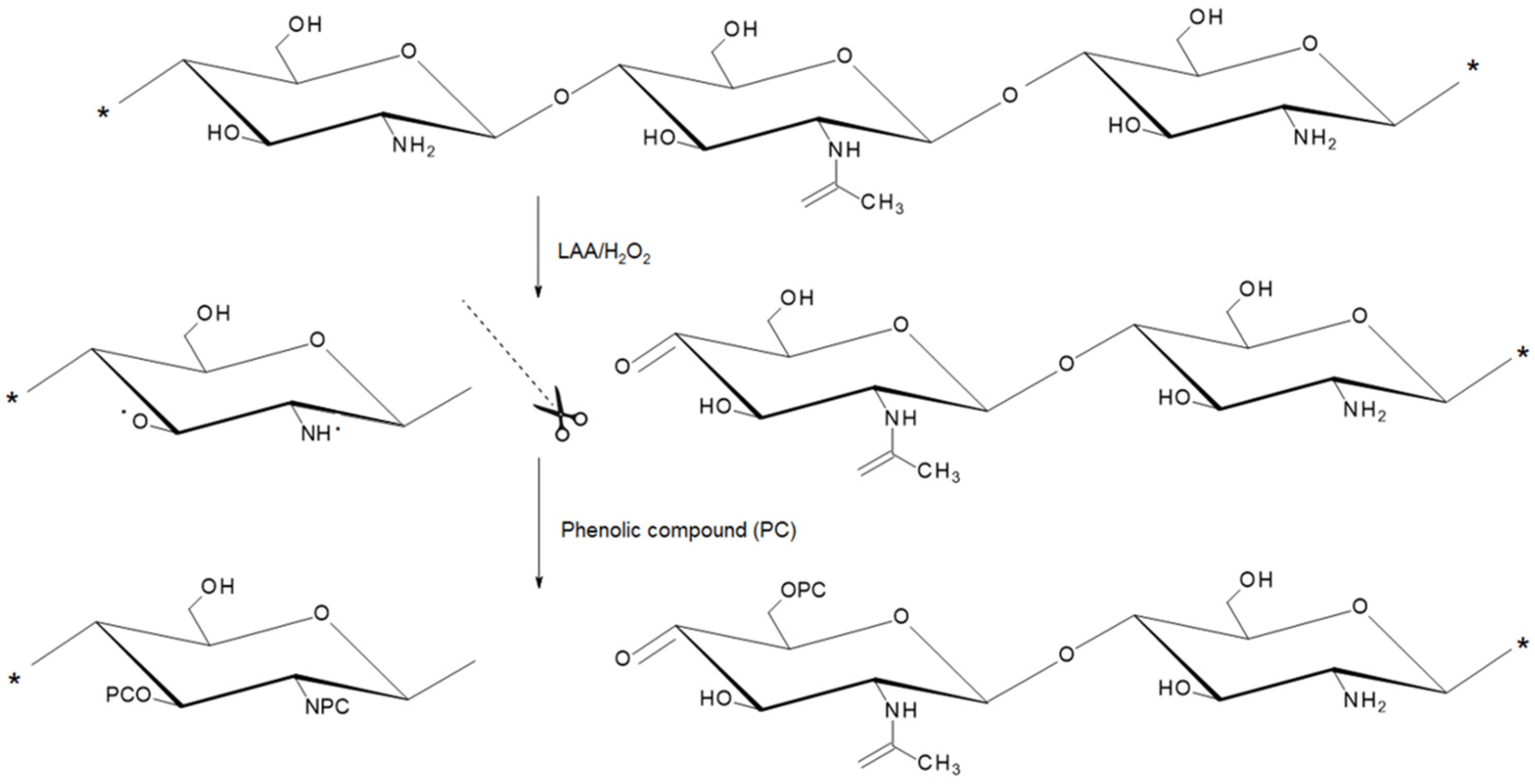
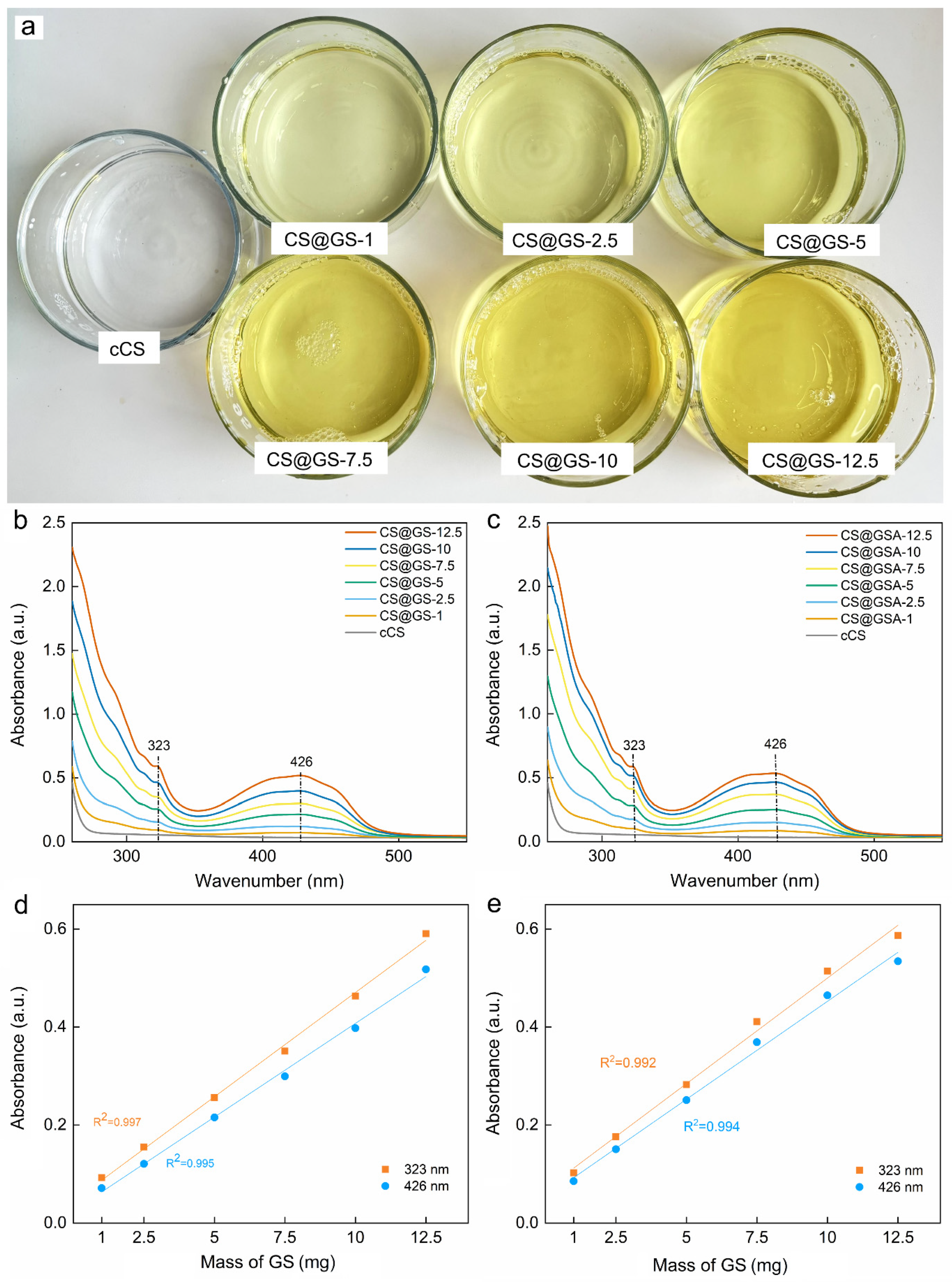
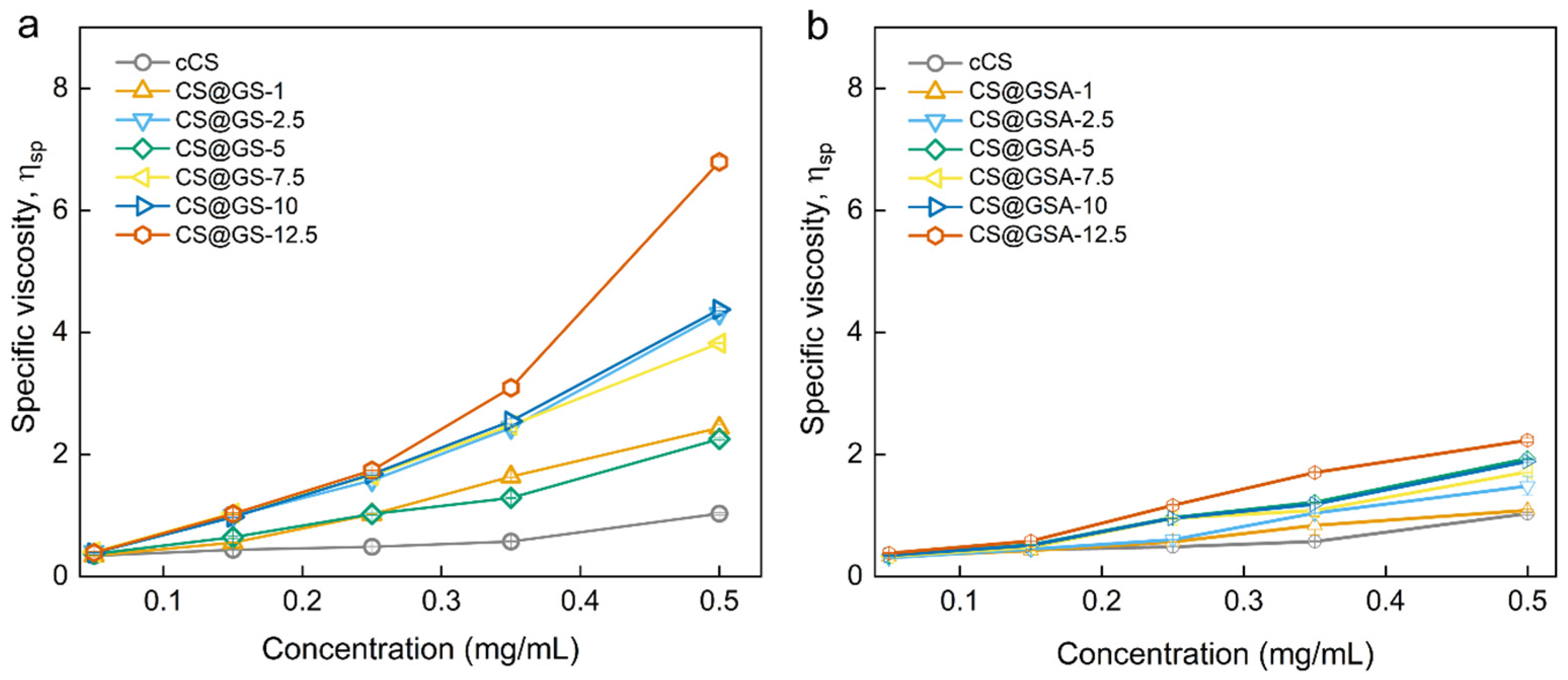
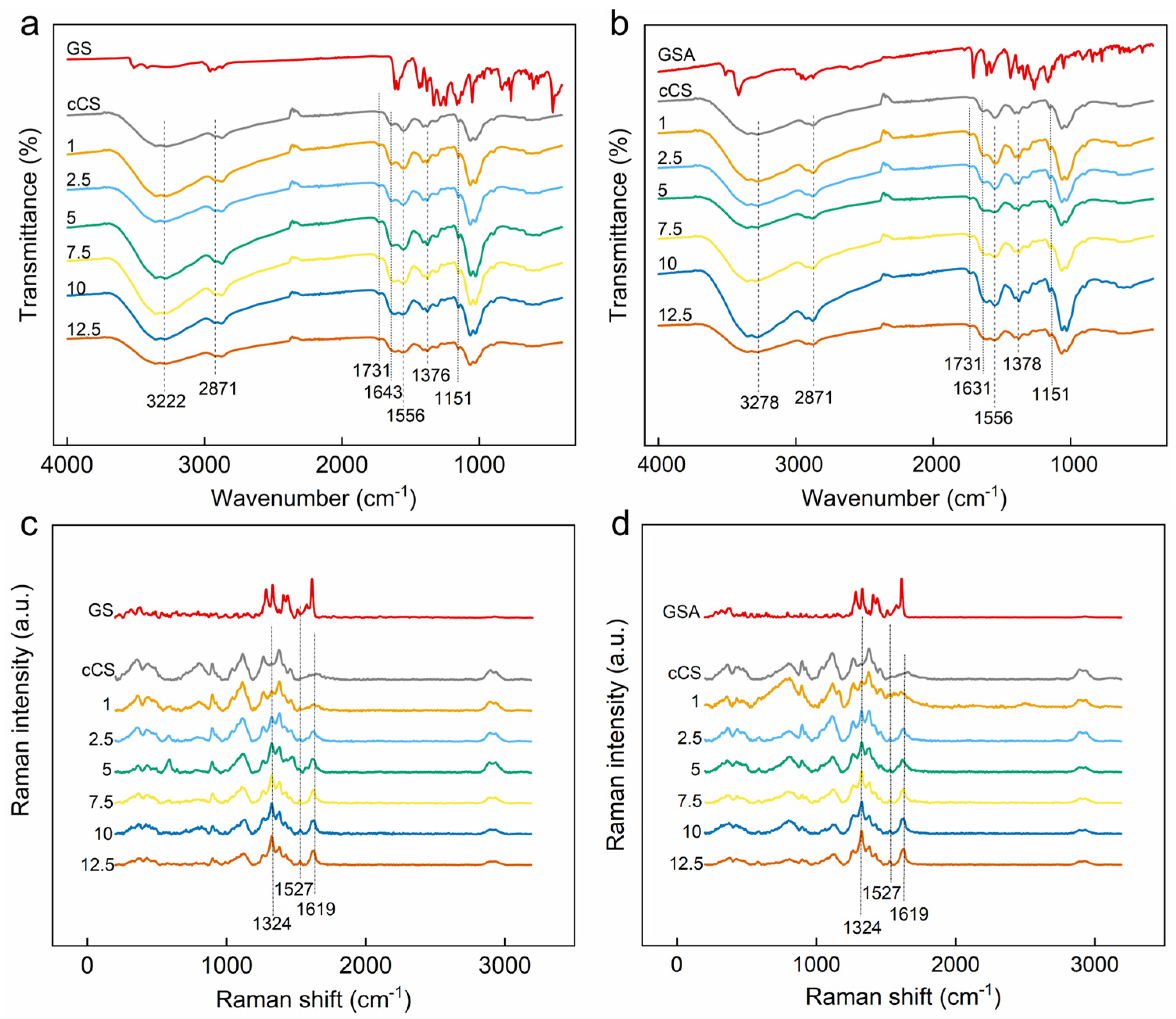
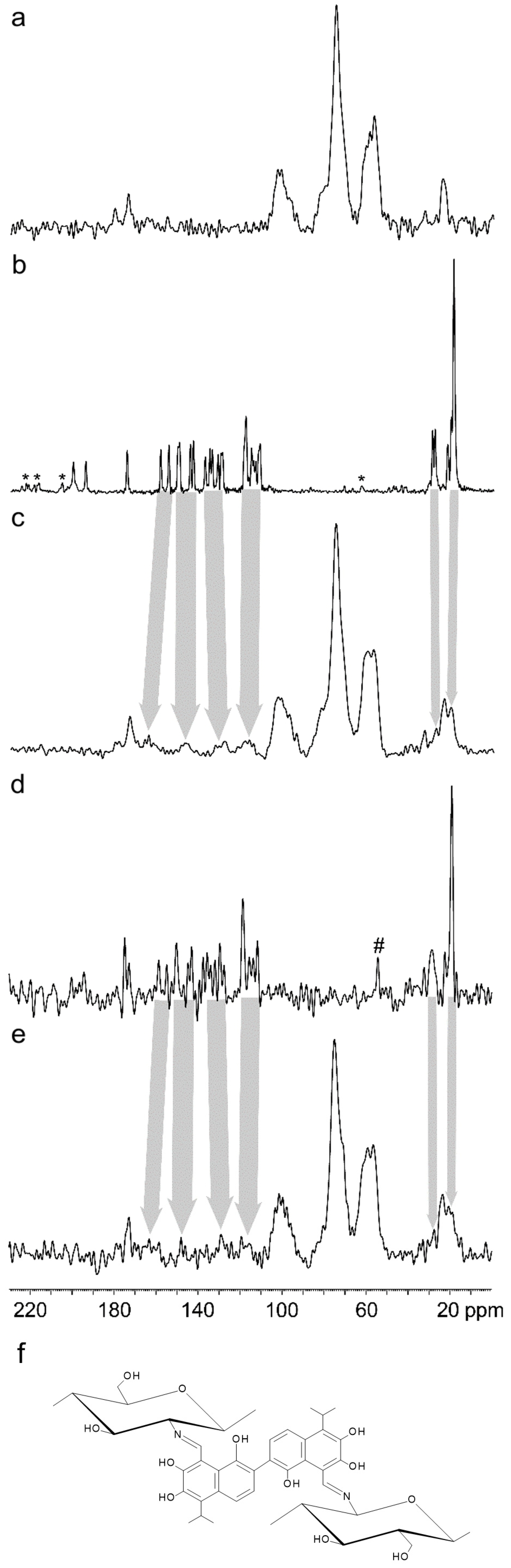
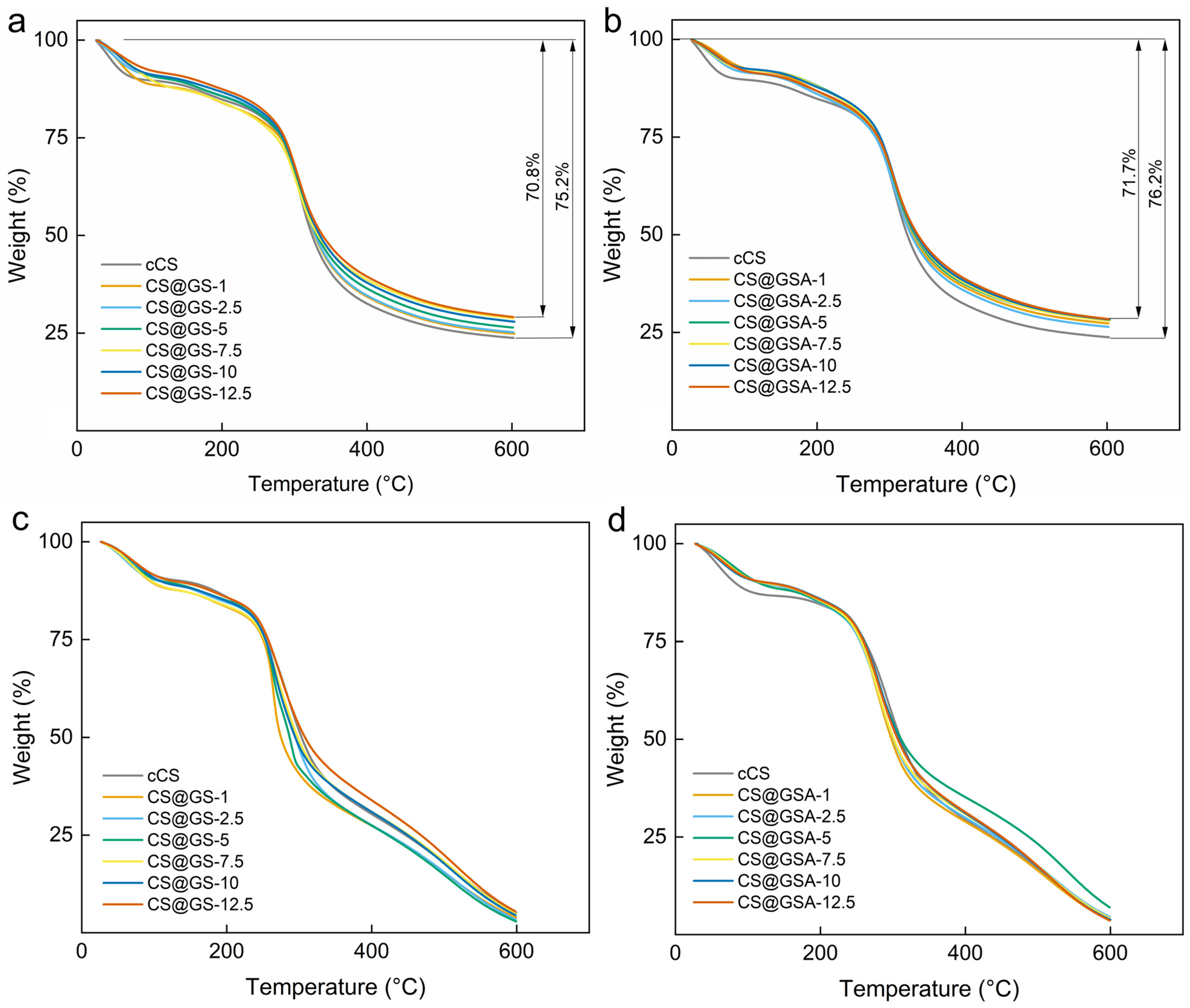
2.2. Characterization of the GS-Modified CS Derivatives
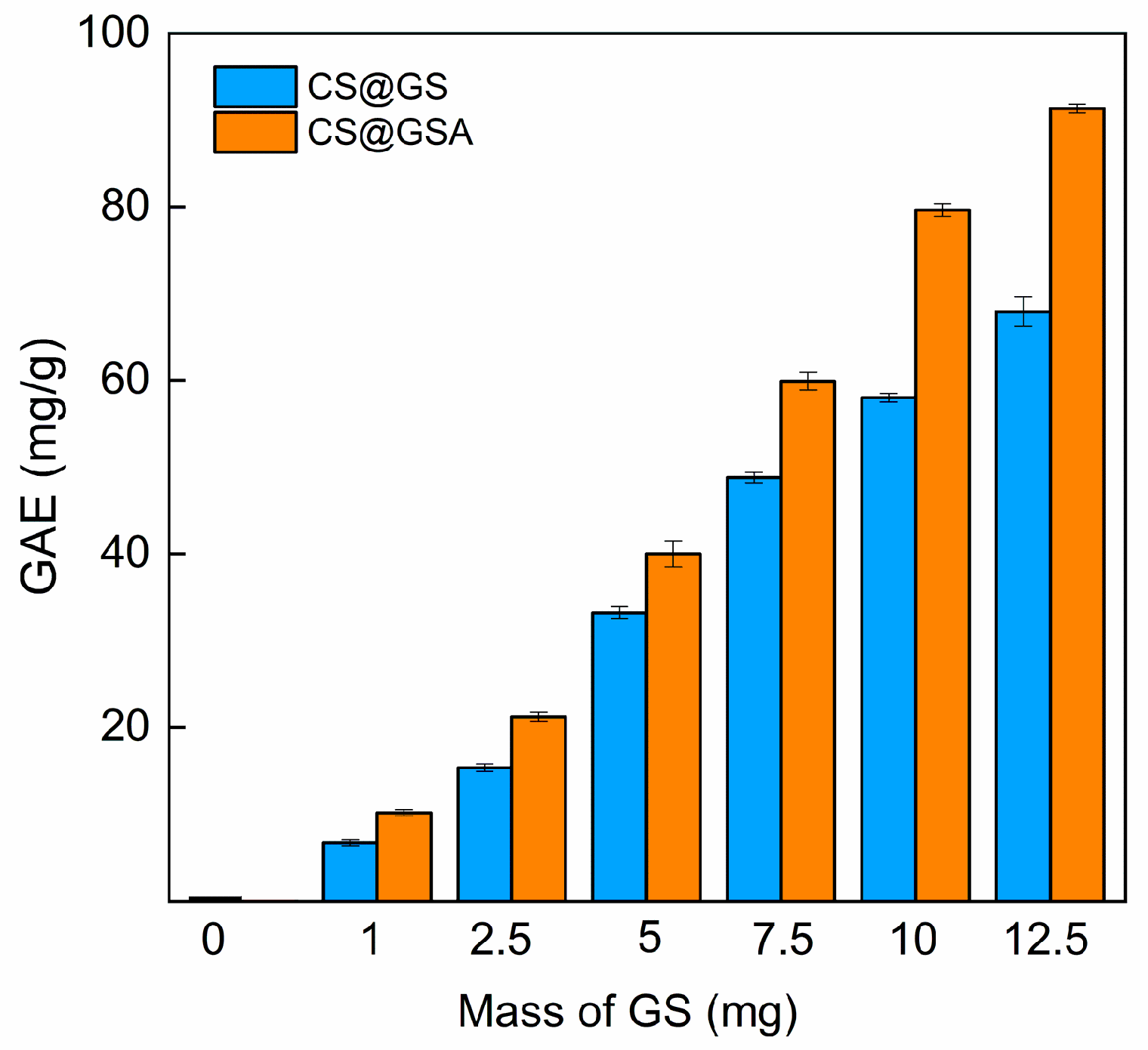
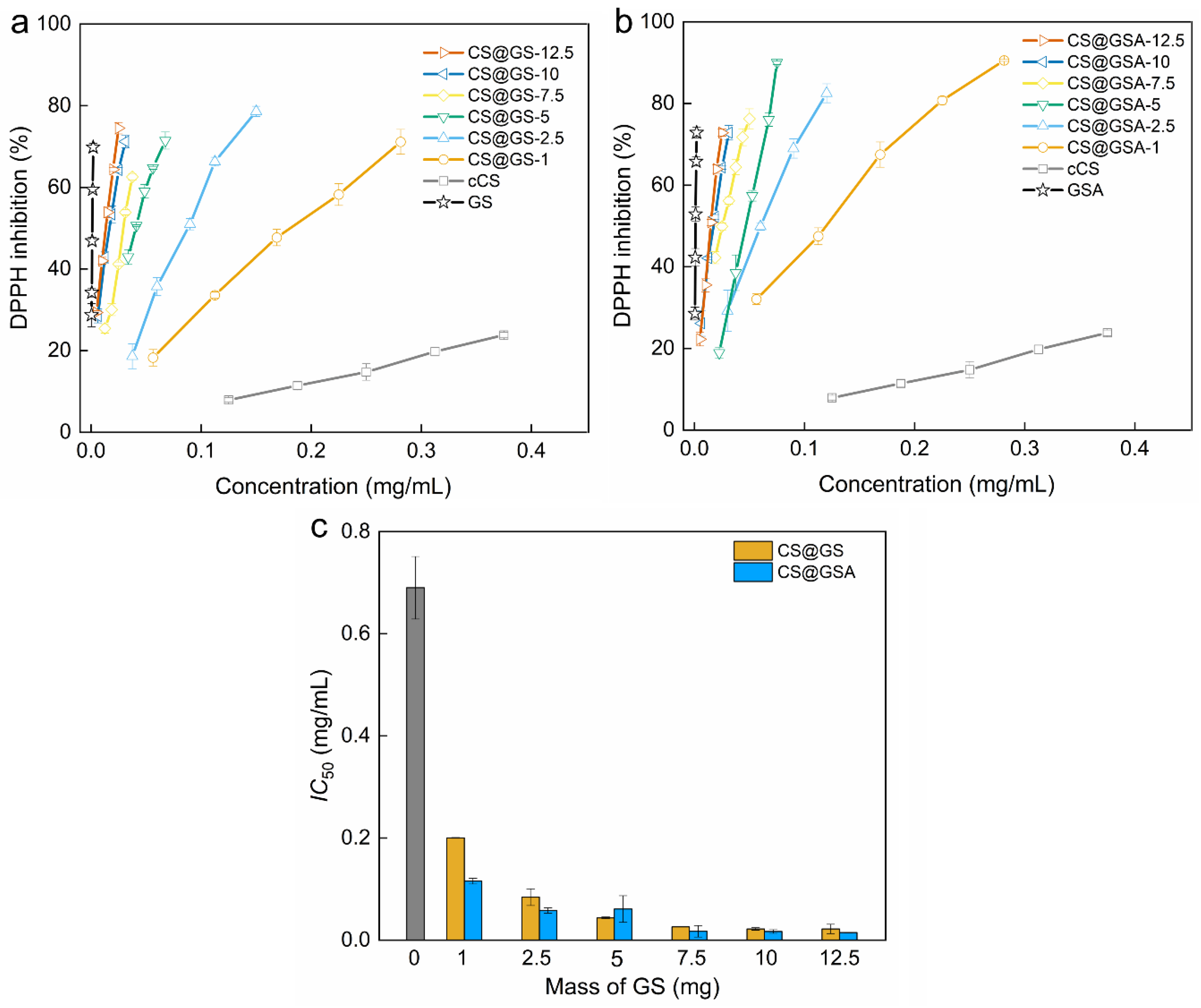
2.3. Antioxidant Properties of GS-Modified CS Derivatives
3. Materials and Methods
3.1. Materials
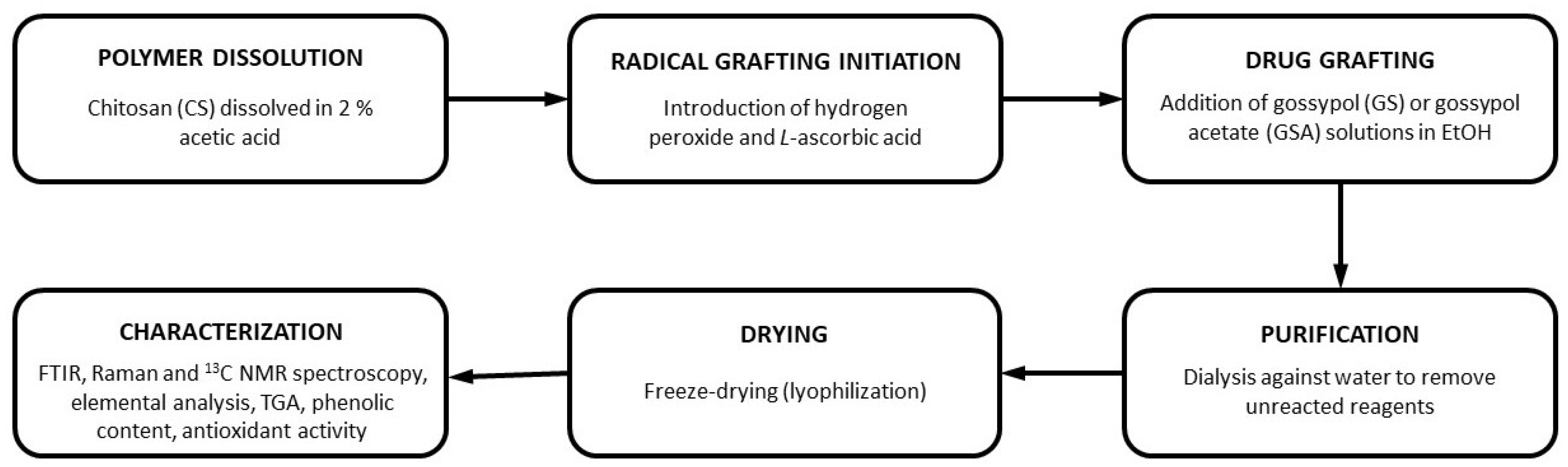
3.2. Methods
3.2.1. Synthesis of Chitosan Derivatives
3.2.2. Physicochemical Characterization of the Polymers
3.2.3. Characterization of Antioxidant Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATR-FTIR | Attenuated total reflectance Fourier-transform infrared spectroscopy |
| AA | Acetic acid |
| CS | Chitosan |
| CS@GS(A) | Gossypol-modified chitosan prepared using GS or GSA |
| cCS | Control chitosan |
| DDA | Degree of deacetylation |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| EtOH | Ethanol |
| F-C | Folin–Ciocalteu |
| hvCS | Highly viscous chitosan |
| IC50 | Half-maximal inhibitory concentration |
| GAE | Gallic acid equivalent |
| GS | Gossypol |
| GSA | Gossypol acetate |
| LAA | L-ascorbic acid |
| NMR | Nuclear magnetic resonance |
| RT | Room temperature |
| SD | Standard deviation |
| TGA | Thermogravimetric analysis |
| ηsp | Specific viscosity |
References
- De Oliveira, I.; Santos-Buelga, C.; Aquino, Y.; Barros, L.; Heleno, S.A. New frontiers in the exploration of phenolic compounds and other bioactives as natural preservatives. Food Biosci. 2025, 68, 106571. [Google Scholar] [CrossRef]
- Paunovic, D.; Rajkovic, J.; Novakovic, R.; Grujic-Milanovic, J.; Mekky, R.H.; Popa, D.; Calina, D.; Sharifi-Rad, J. The potential roles of gossypol as anticancer agent: Advances and future directions. Chin. Med. 2023, 18, 163. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, L.; Zhao, L.; Zhang, Y. Structure, properties of gossypol and its derivatives—From physiological activities to drug discovery and drug design. Nat. Prod. Rep. 2022, 39, 1282–1304. [Google Scholar] [CrossRef]
- Bera, R.; Bandyopadhyay, R.; Debnath, B.; Dutta, G.; Sugumaran, A. Review on various activator-assisted polymer grafting techniques for smart drug delivery applications. RSC Adv. 2025, 15, 23025–23044. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.S.A.; Naveed, M.; Jost, N. Polysaccharides; Classification, chemical properties, and future perspective applications in fields of pharmacology and biological medicine (A review of current applications and upcoming potentialities). J. Polym. Environ. 2021, 29, 2359–2371. [Google Scholar] [CrossRef] [PubMed]
- Kołodziejska, M.; Jankowska, K.; Klak, M.; Wszoła, M. Chitosan as an underrated polymer in modern tissue engineering. Nanomaterials 2021, 11, 3019. [Google Scholar] [CrossRef]
- Li, X.; Dong, W.; Nalin, A.P.; Wang, Y.; Pan, P.; Xu, B.; Zhang, Y.; Tun, S.; Zhang, J.; Wang, L.-S.; et al. The natural product chitosan enhances the anti-tumor activity of natural killer cells by activating dendritic cells. Oncoimmunology 2018, 7, e1431085. [Google Scholar] [CrossRef]
- Wimardhani, Y.S.; Suniarti, D.F.; Freisleben, H.J.; Wanandi, S.I.; Siregar, N.C.; Ikeda, M.A. Chitosan exerts anticancer activity through induction of apoptosis and cell cycle arrest in oral cancer cells. J. Oral Sci. 2014, 56, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yu, X.; Su, C.; Zhao, L.; Shi, Y. Chitosan nanoparticles induced the antitumor effect in hepatocellular carcinoma cells by regulating ROS-mediated mitochondrial damage and endoplasmic reticulum stress. Artif. Cells Nanomed. Biotechnol. 2019, 47, 747–756. [Google Scholar] [CrossRef]
- Hussain Shah, S.N.; Zulcaif Syed, A.; Syed, A.; Aslam, A.; Zafar, N.; Arif, A. Development of film forming gel for the delivery of 5-flurouracil: In-vitro/ex-vivo evaluation. Polym. Bull. 2024, 81, 7121–7137. [Google Scholar] [CrossRef]
- Chen, S.; Deng, J.; Zhang, L.-M. Cationic nanoparticles self-assembled from amphiphilic chitosan derivatives containing poly(amidoamine) dendrons and deoxycholic acid as a vector for co-delivery of doxorubicin and gene. Carbohydr. Polym. 2021, 258, 117706. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Gao, Y.; Heng, L.; Liu, Y.; Yao, G.; Wang, Y.; Liu, Y. Amphiphilic N-(2,3-dihydroxypropyl)–chitosan–cholic acid micelles for paclitaxel delivery. Carbohydr. Polym. 2013, 94, 394–399. [Google Scholar] [CrossRef]
- Sood, A.; Gupta, A.; Bharadwaj, R.; Ranganath, P.; Silverman, N.; Agrawal, G. Biodegradable disulfide crosslinked chitosan/stearic acid nanoparticles for dual drug delivery for colorectal cancer. Carbohydr. Polym. 2022, 294, 119833. [Google Scholar] [CrossRef] [PubMed]
- Wan Yusof, W.R.; Awang, N.Y.F.; Azhar Laile, M.A.; Azizi, J.; Awang Husaini, A.A.S.; Seeni, A.; Wilson, L.D.; Sabar, S. Chemically modified water-soluble chitosan derivatives: Modification strategies, biological activities, and applications. Polym.-Plast. Technol. Mater. 2023, 62, 2182–2220. [Google Scholar] [CrossRef]
- Oliver, S.; Vittorio, O.; Cirillo, G.; Boyer, C. Enhancing the therapeutic effects of polyphenols with macromolecules. Polym. Chem. 2016, 7, 1529–1544. [Google Scholar] [CrossRef]
- Świętek, M.; Lu, Y.-C.; Konefał, R.; Ferreira, L.P.; Cruz, M.M.; Ma, Y.-H.; Horák, D. Scavenging of reactive oxygen species by phenolic compound-modified maghemite nanoparticles. Beilstein J. Nanotechnol. 2019, 10, 1073–1088. [Google Scholar] [CrossRef]
- Liu, J.; Wen, X.-Y.; Lu, J.-F.; Kan, J.; Jin, C.-H. Free radical mediated grafting of chitosan with caffeic and ferulic acids: Structures and antioxidant activity. Int. J. Biol. Macromol. 2014, 65, 97–106. [Google Scholar] [CrossRef]
- Diao, Y.; Yu, X.; Zhang, C.; Jing, Y. Quercetin-grafted chitosan prepared by free radical grafting: Characterization and evaluation of antioxidant and antibacterial properties. J. Food Sci. Technol. 2020, 57, 2259–2268. [Google Scholar] [CrossRef]
- Wu, T.; Wu, C.; Xiang, Y.; Huang, J.; Luan, L.; Chena, S.; Hu, Y. Kinetics and mechanism of degradation of chitosan by combining sonolysis with H2O2/ascorbic acid. RSC Adv. 2016, 6, 76280–76287. [Google Scholar] [CrossRef]
- Liu, J.; Pu, H.; Zhang, X.; Xiao, L.; Kan, J.; Jin, C. Effects of ascorbate and hydroxyl radical degradations on the structural, physicochemical, antioxidant and film forming properties of chitosan. Int. J. Biol. Macromol. 2018, 114, 1086–1093. [Google Scholar] [CrossRef]
- Mittal, A.; Singh, A.; Hong, H.; Benjakul, S. Chitooligosaccharides from shrimp shell chitosan prepared using H2O2 or ascorbic acid/H2O2 redox pair hydrolysis: Characteristics, antioxidant and antimicrobial activities. Int. J. Food Sci. Technol. 2023, 58, 2645–2660. [Google Scholar] [CrossRef]
- Tapia, A.; Seña, R.; Zambrano, H.; Paredes, V. Extraction and characterization of chitosan obtained from shells of crab (Callinectes bocourti and Callinectes sapidus). Int. J. Biol. Macromol. 2025, 320, 145963. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Choi, H.; Jo, S.; Kim, M.-J.; Lee, S.; Ahn, S.; Lee, J. Modification of chitosan using hydrogen peroxide and ascorbic acid and its physicochemical properties including water solubility, oil entrapment and in vitro lipase activity. Int. J. Food Sci. Technol. 2019, 54, 2300–2308. [Google Scholar] [CrossRef]
- Zając, A.; Hanuza, J.; Wandas, M.; Dymińska, L. Determination of N-acetylation degree in chitosan using Raman spectroscopy. Spectrochim. Act. Part A Mol. Biomol. Spectrosc. 2015, 134, 114–120. [Google Scholar] [CrossRef]
- Zhang, J.; Xia, W.; Liu, P.; Cheng, Q.; Tahirou, T.; Gu, W.; Li, B. Chitosan modification and pharmaceutical/biomedical applications. Mar. Drugs 2010, 8, 1962–1987. [Google Scholar] [CrossRef]
- Farion, I.A.; Burdukovskii, V.F.; Kholkhoev, B.C.; Timashev, P.S.; Chailakhyan, R.K. Functionalization of chitosan with carboxylic acids and derivatives of them: Synthesis issues and prospects of practical use: A review. Express Polym. Lett. 2018, 12, 1081–1105. [Google Scholar] [CrossRef]
- Ziegler-Borowska, M.; Chełminiak, D.; Kaczmarek, H. Thermal stability of magnetic nanoparticles coated by blends of modified chitosan and poly(quaternary ammonium) salt. J. Therm. Anal. Calorim. 2015, 119, 499–506. [Google Scholar] [CrossRef]
- Beda, A.; Yamada, H.; Egunov, A.; Ghimbeu, C.M.; Malval, J.-P.; Saito, Y.; Luchnikov, V. Carbon microtubes derived from self-rolled chitosan acetate films and graphitized by joule heating. J. Mater. Sci. 2019, 54, 11345–11356. [Google Scholar] [CrossRef]
- Moreno-Vásquez, M.J.; Valenzuela-Buitimea, E.L.; Plascencia-Jatomea, M.; Encinas-Encinas, J.C.; Rodríguez-Félix, F.; Sánchez-Valdes, S.; Carina Rosas-Burgos, E.; Ocaño-Higuera, V.M.; Graciano-Verdugo, A.Z. Functionalization of chitosan by a free radical reaction: Characterization, antioxidant and antibacterial potential. Carbohyd. Polym. 2017, 155, 117–127. [Google Scholar] [CrossRef]
- Qin, C.Q.; Du, Y.M.; Xiao, L. Effect of hydrogen peroxide treatment on the molecular weight and structure of chitosan. Polym. Degrad. Stab. 2002, 76, 211–218. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Zhang, Y.; Yasin, A.; Zhang, L. Investigating stability and tautomerization of gossypol—A spectroscopy study. Molecules 2019, 24, 1286. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, J.; Li, Q.; Liu, C.; Niu, F.; Yue, R.; Zhang, Y.; Zhu, H.; Ma, C.; Deng, S. Free radical-mediated grafting of natural polysaccharides such as chitosan, starch, inulin, and pectin with some polyphenols: Synthesis, structural characterization, bioactivities, and applications—A review. Foods 2023, 12, 3688. [Google Scholar] [CrossRef] [PubMed]
- Przybylski, P.; Bejcar, G.; Huczyński, A.; Schroeder, G.; Brzezinski, B.; Franz, B. 1H- and 13C-NMR, FTIR, UV-VIS, ESI-MS, and PM5 studies as well as emission properties of a new Schiff base of gossypol with 5-methoxytryptamine and a new hydrazone of gossypol with dansylhydrazine. Biopolymers 2006, 82, 521–535. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Cortés, S.; García-Ramos, J.V. Adsorption and chemical modification of phenols on a silver surface. J. Colloid. Interface Sci. 2000, 231, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Demetgül, C.; Beyazit, N. Synthesis, characterization and antioxidant activity of chitosan-chromone derivatives. Carbohyd. Polym. 2018, 181, 812–817. [Google Scholar] [CrossRef]
- Beyazit, N.; Çakran, H.S.; Cabir, A.; Akışcan, Y.; Demetgül, C. Synthesis, characterization and antioxidant activity of chitosan Schiff base derivatives bearing (−)-gossypol. Carbohyd. Polym. 2020, 240, 16333. [Google Scholar] [CrossRef]
- Lawag, I.L.; Nolden, E.S.; Schaper, A.A.M.; Lim, L.Y.; Locher, C. A modified Folin-Ciocalteu assay for the determination of total phenolics content in honey. Appl. Sci. 2023, 13, 2135. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Amer, N.M.; Abdallah, H.M.I.; Saleh, M.S. A comprehensive tool in recycling plant-waste of Gossypium barbadense L agricultural and industrial waste extracts containing gossypin and gossypol: Hepatoprotective, anti-inflammatory and antioxidant effects. Plant Methods 2024, 20, 54. [Google Scholar] [CrossRef]
- Morcombe, C.R.; Zilm, K.W. Chemical shift referencing in MAS solid state NMR. J. Magn. Reson. 2003, 162, 479–486. [Google Scholar] [CrossRef]
- Harish Prashanth, K.V.; Kittur, F.S.; Tharanathan, R.N. Solid state structure of chitosan prepared under different N-deacetylating conditions. Carbohyd. Polym. 2002, 50, 27–33. [Google Scholar] [CrossRef]
- Gupta, K.C.; Jabrail, F.B. Effects of degree of deacetylation and cross-linking on physical characteristics, swelling and release behavior of chitosan microspheres. Carbohyd. Polym. 2006, 66, 43–54. [Google Scholar] [CrossRef]
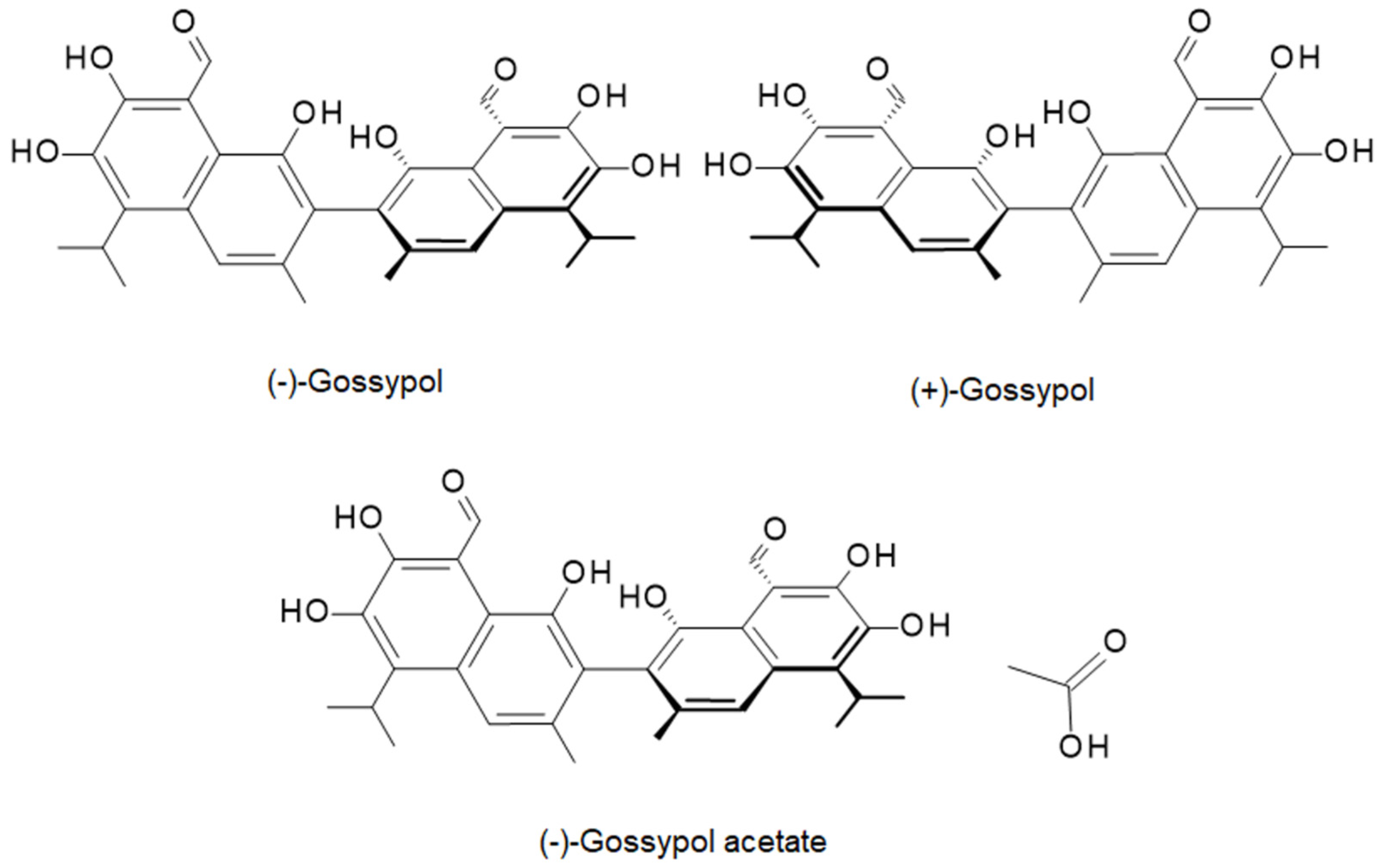
| Polymer | C (wt.%) | H (wt.%) | N (wt.%) | C/N |
|---|---|---|---|---|
| hvCS | 40.78 | 6.99 | 7.42 | 5.49 |
| cCS | 37.17 | 6.47 | 6.67 | 5.57 |
| CS@GS-1 | 37.33 | 6.38 | 6.50 | 5.74 |
| CS@GS-2.5 | 36.11 | 6.02 | 5.74 | 6.29 |
| CS@GS-5 | 35.61 | 6.40 | 5.64 | 6.32 |
| CS@GS-7.5 | 37.85 | 6.39 | 5.86 | 6.46 |
| CS@GS-10 | 35.22 | 6.15 | 4.84 | 7.28 |
| CS@GS-12.5 | 31.16 | 6.53 | 4.21 | 7.41 |
| CS@GSA-1 | 36.55 | 5.96 | 6.12 | 5.98 |
| CS@GSA-2.5 | 35.95 | 6.18 | 5.70 | 6.31 |
| CS@GSA-5 | 33.97 | 5.88 | 4.88 | 6.96 |
| CS@GSA-7.5 | 34.55 | 6.39 | 5.07 | 6.82 |
| CS@GSA-10 | 35.98 | 6.52 | 5.33 | 6.75 |
| CS@GSA-12.5 | 31.69 | 6.35 | 4.08 | 7.78 |
| Polymer | hvCS (mg) | GS (mg) | GSA (mg) * |
|---|---|---|---|
| cCS | 100 | 0 | —————— |
| CS@GS-1 | 100 | 1 | —————— |
| CS@GS-2.5 | 100 | 2.5 | —————— |
| CS@GS-5 | 100 | 5 | —————— |
| CS@GS-7.5 | 100 | 7.5 | —————— |
| CS@GS-10 | 100 | 10 | —————— |
| CS@GSA-12.5 | 100 | 12.5 | —————— |
| CS@GSA-1 | 100 | —————— | 1.12 |
| CS@GSA-2.5 | 100 | —————— | 2.78 |
| CS@GSA-5 | 100 | —————— | 5.58 |
| CS@GSA-7.5 | 100 | —————— | 8.37 |
| CS@GSA-10 | 100 | —————— | 11.16 |
| CS@GSA-12.5 | 100 | —————— | 13.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hlukhaniuk, A.; Świętek, M.; Kołodziej, A.; Wesełucha-Birczyńska, A.; Mahun, A.; Kobera, L.; Horák, D. Modification of Chitosan with (−)-Gossypol and (−)-Gossypol Acetic Acid Using Free-Radical Grafting Method. Int. J. Mol. Sci. 2025, 26, 11721. https://doi.org/10.3390/ijms262311721
Hlukhaniuk A, Świętek M, Kołodziej A, Wesełucha-Birczyńska A, Mahun A, Kobera L, Horák D. Modification of Chitosan with (−)-Gossypol and (−)-Gossypol Acetic Acid Using Free-Radical Grafting Method. International Journal of Molecular Sciences. 2025; 26(23):11721. https://doi.org/10.3390/ijms262311721
Chicago/Turabian StyleHlukhaniuk, Anna, Małgorzata Świętek, Anna Kołodziej, Aleksandra Wesełucha-Birczyńska, Andrii Mahun, Libor Kobera, and Daniel Horák. 2025. "Modification of Chitosan with (−)-Gossypol and (−)-Gossypol Acetic Acid Using Free-Radical Grafting Method" International Journal of Molecular Sciences 26, no. 23: 11721. https://doi.org/10.3390/ijms262311721
APA StyleHlukhaniuk, A., Świętek, M., Kołodziej, A., Wesełucha-Birczyńska, A., Mahun, A., Kobera, L., & Horák, D. (2025). Modification of Chitosan with (−)-Gossypol and (−)-Gossypol Acetic Acid Using Free-Radical Grafting Method. International Journal of Molecular Sciences, 26(23), 11721. https://doi.org/10.3390/ijms262311721









