Gut Microbiota and Short-Chain Fatty Acids: Key Factors in Pediatric Obesity and Therapeutic Targets
Abstract
1. Introduction
2. Materials and Methods
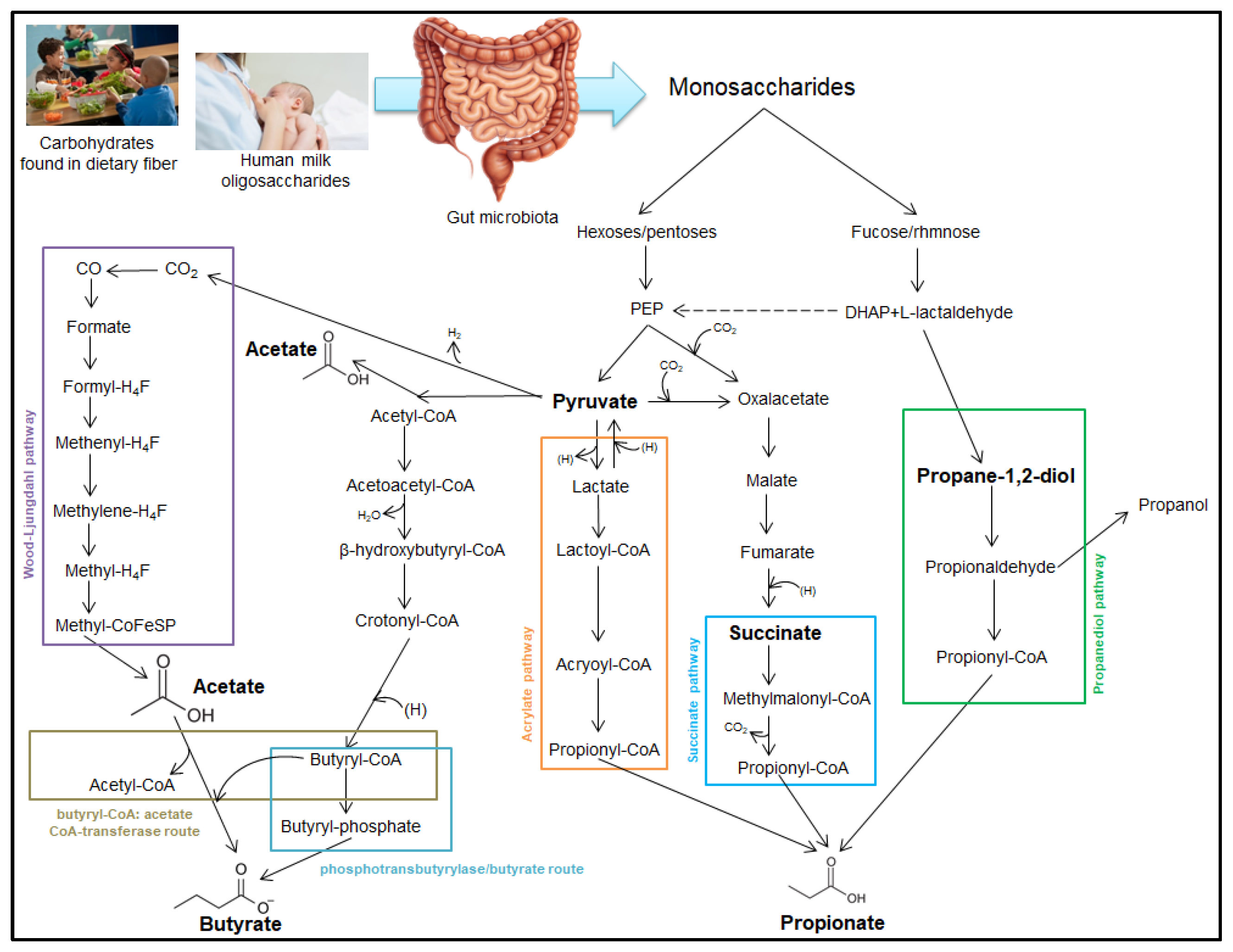
3. Short-Chain Fatty Acid Production
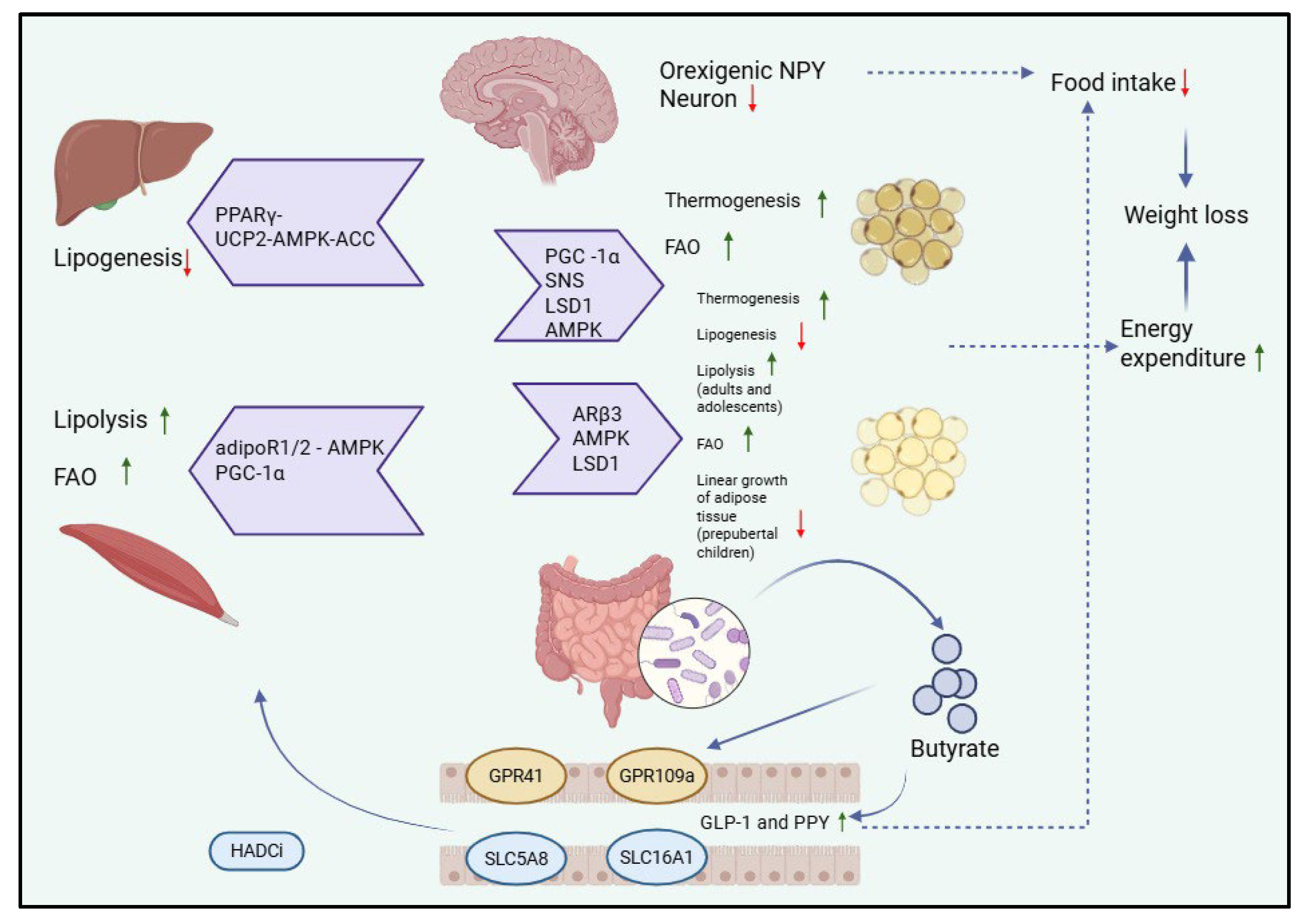
4. SCFAs and Obesity
4.1. SCFAs
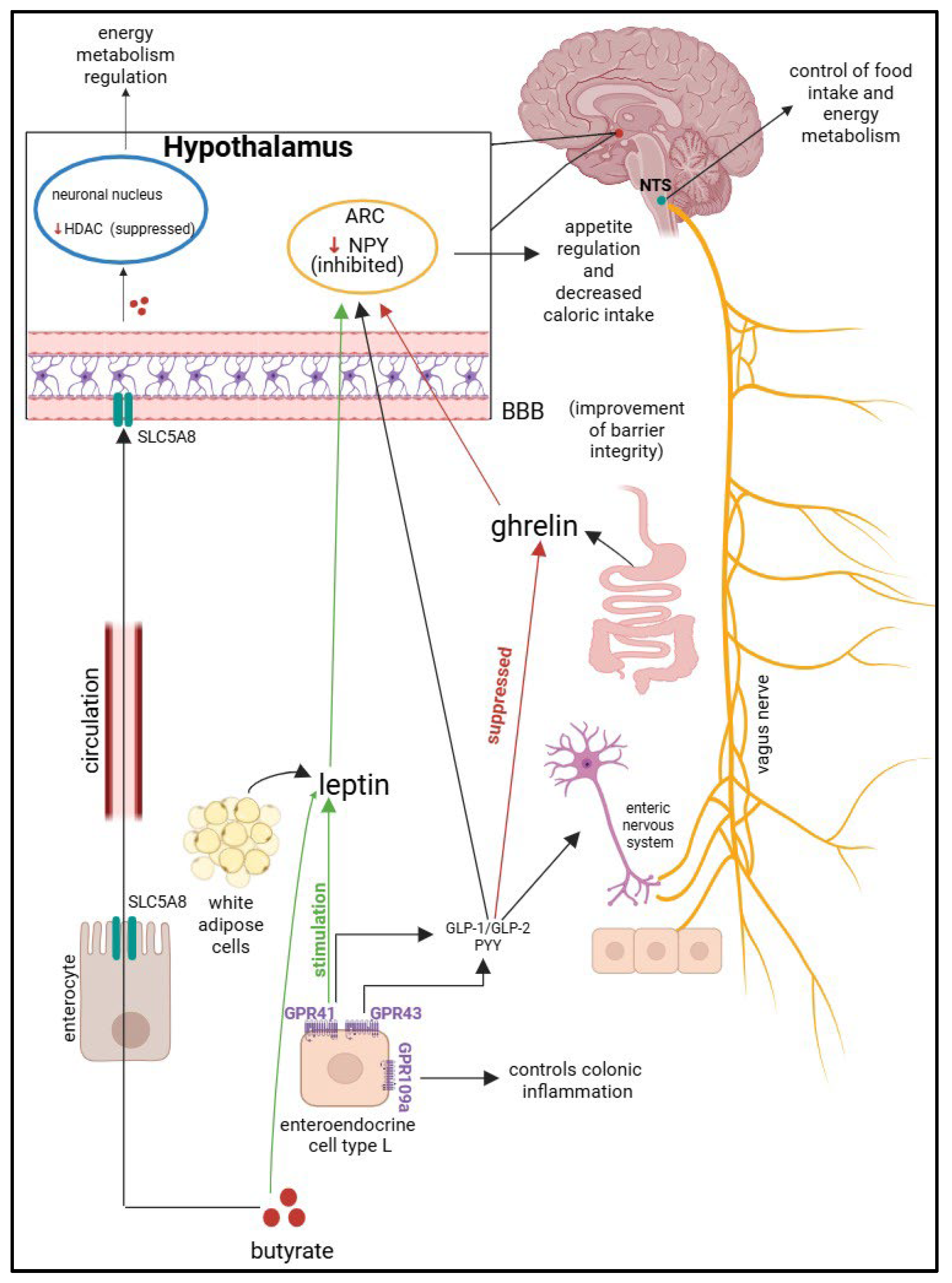
4.1.1. Physiological Roles of SCFAs in Pediatric Health
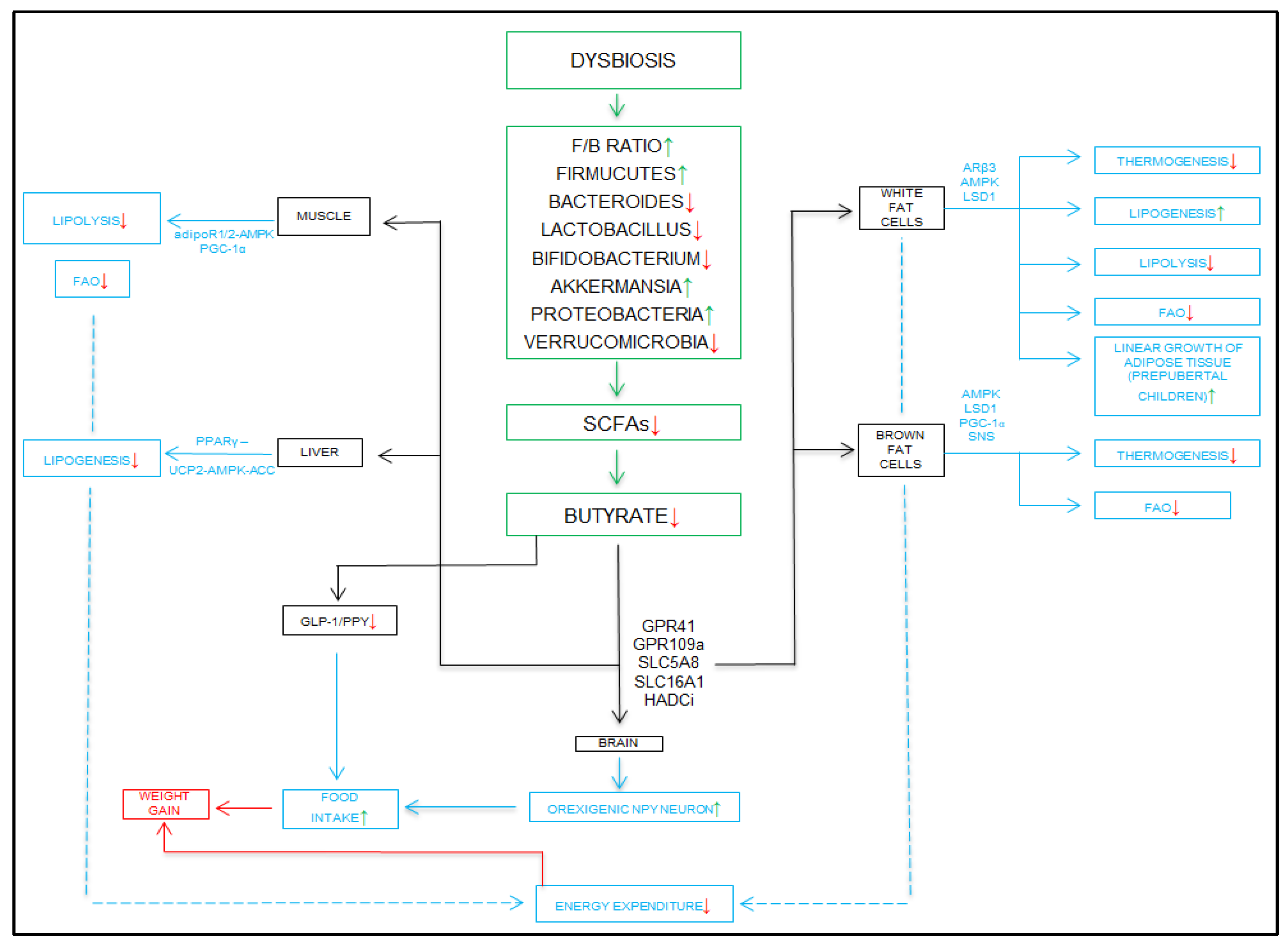
4.1.2. SCFAs in the Pathogenesis of Pediatric Obesity
4.1.3. Special Populations: Maternal SCFAs and Neonatal Obesity Risk
4.2. Other Metabolites and Their Involvement in Obesity
5. Dysbiosis in Obesity
5.1. Analysis of the Relationship Between Obesity and the Microbiome
5.2. Analysis of the Relationship Between Obesity and SCFAs
5.2.1. Analysis of the Relationship
5.2.2. Research Consensus and Controversy
5.3. Analysis of the Particular Relationship Between the Oral and Digestive Microbiomes
6. Therapeutic Strategies
6.1. Advanced Genomic Sequencing Technologies and Metaproteomics
6.2. Diete and Exercise
6.3. Supplements Containing SCFAs
6.4. Fecal Transplantation
6.5. Prebiotics, Probiotics, and Synbiotics
6.6. Implementation Challenges
7. Clinical Trial Progress and Future Challenges
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Faienza, M.F.; Chiarito, M.; Molina-Molina, E.; Shanmugam, H.; Lammert, F.; Krawczyk, M.; D’Amato, G.; Portincasa, P. Childhood obesity, cardiovascular and liver health: A growing epidemic with age. World J. Pediatr. 2020, 16, 438–445. [Google Scholar] [CrossRef]
- Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 17 September 2025).
- Kerr, J.A.; Patton, G.C.; Cini, K.I.; Abate, Y.H.; Abbas, N.; Abd Al Magied, A.H.A.; Abd ElHafeez, S.; Abd-Elsalam, S.; Abdollahi, A.; Abdoun, M.; et al. Global, regional, and national prevalence of child and adolescent overweight and obesity, 1990–2021, with forecasts to 2050: A forecasting study for the Global Burden of Disease Study 2021. Lancet 2025, 405, 785–812. [Google Scholar] [CrossRef]
- Poorolajal, J.; Sahraei, F.; Mohamdadi, Y.; Doosti-Irani, A.; Moradi, L. Behavioral factors influencing childhood obesity: A systematic review and meta-analysis. Obes. Res. Clin. Pract. 2020, 14, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Dong, Y.; Chen, H.; Ma, L.; Jia, L.; Luo, J.; Liu, Q.; Hu, Y.; Ma, J.; Song, Y. Determinants of childhood obesity in China. Lancet Public Health 2024, 9, e1105–e1114. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, B.; Taveras, E.; Allender, S.; Strugnell, C. Sleep and obesity among children: A systematic review of multiple sleep dimensions. Pediatr. Obes. 2020, 15, e12619. [Google Scholar] [CrossRef] [PubMed]
- Meneses-Echavez, J.F.; Iglesias-Gonzalez, L.E.; Loaiza-Betancur, A.F.; Guapo, N.C. Sedentary behavior and sleep for children and adolescents with obesity: A systematic review. Ann. New York Acad. Sci. 2025, 1545, 66–75. [Google Scholar] [CrossRef]
- Kumari, S.; Shukla, S.; Acharya, S. Childhood Obesity: Prevalence and Prevention in Modern Society. Cureus 2022, 14, e31640. [Google Scholar] [CrossRef]
- Colella, M.; Charitos, I.A.; Ballini, A.; Cafiero, C.; Topi, S.; Palmirotta, R.; Santacroce, L. Microbiota revolution: How gut microbes regulate our lives. World J. Gastroenterol. 2023, 29, 4368–4383. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, J.; Wang, L. Role and Mechanism of Gut Microbiota in Human Disease. Front. Cell. Infect. Microbiol. 2021, 11, 625913. [Google Scholar] [CrossRef]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef]
- Haran, J.P.; McCormick, B.A. Aging, Frailty, and the Microbiome-How Dysbiosis Influences Human Aging and Disease. Gastroenterology 2021, 160, 507–523. [Google Scholar] [CrossRef] [PubMed]
- Tarracchini, C.; Lugli, G.A.; Mancabelli, L.; van Sinderen, D.; Turroni, F.; Ventura, M.; Milani, C. Exploring the vitamin biosynthesis landscape of the human gut microbiota. mSystems 2024, 9, e0092924. [Google Scholar] [CrossRef]
- Pham, V.T.; Dold, S.; Rehman, A.; Bird, J.K.; Steinert, R.E. Vitamins, the gut microbiome and gastrointestinal health in humans. Nutr. Res. 2021, 95, 35–53. [Google Scholar] [CrossRef]
- Das, P.; Babaei, P.; Nielsen, J. Metagenomic analysis of microbe-mediated vitamin metabolism in the human gut microbiome. BMC Genomics. 2019, 20, 208. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Brown, E.M.; Clardy, J.; Xavier, R.J. Gut microbiome lipid metabolism and its impact on host physiology. Cell Host Microbe 2023, 31, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, X.; Liu, H.; Brown, M.A.; Qiao, S. Dietary Protein and Gut Microbiota Composition and Function. Curr. Protein Pept. Sci. 2019, 20, 145–154. [Google Scholar] [CrossRef]
- Alqudah, S.; Claesen, J. Mechanisms of gut bacterial metabolism of dietary polyphenols into bioactive compounds. Gut Microbes 2024, 16, 2426614. [Google Scholar] [CrossRef]
- Dikeocha, I.J.; Al-Kabsi, A.M.; Miftahussurur, M.; Alshawsh, M.A. Pharmacomicrobiomics: Influence of gut microbiota on drug and xenobiotic metabolism. FASEB J. 2022, 36, e22350. [Google Scholar] [CrossRef] [PubMed]
- Di Tommaso, N.; Gasbarrini, A.; Ponziani, F.R. Intestinal Barrier in Human Health and Disease. Int. J. Environ. Res. Public Health 2021, 18, 12836. [Google Scholar] [CrossRef]
- Riva, A.; Borgo, F.; Lassandro, C.; Verduci, E.; Morace, G.; Borghi, E.; Berry, D. Pediatric obesity is associated with an altered gut microbiota and discordant shifts in Firmicutes populations. Environ. Microbiol. 2017, 19, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Kim, S.; Seo, S.-U.; Kweon, M.-N. Gut microbiota-derived metabolites tune host homeostasis fate. Semin. Immunopathol. 2024, 46, 2. [Google Scholar] [CrossRef]
- Yoon, S.J.; Yu, J.S.; Min, B.H.; Gupta, H.; Won, S.-M.; Park, H.J.; Han, S.H.; Kim, B.-Y.; Kim, K.H.; Kim, B.K.; et al. Bifidobacterium-derived short-chain fatty acids and indole compounds attenuate nonalcoholic fatty liver disease by modulating gut-liver axis. Front. Microbiol. 2023, 14, 1129904. [Google Scholar] [CrossRef]
- Holmes, Z.C.; Silverman, J.D.; Dressman, H.K.; Wei, Z.; Dallow, E.P.; Armstrong, S.C.; Seed, P.C.; Rawls, J.F.; David, L.A. Short-Chain Fatty Acid Production by Gut Microbiota from Children with Obesity Differs According to Prebiotic Choice and Bacterial Community Composition. mBio 2020, 11, e00914-20. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.J.; Lee, J.; Lee, H.S. Metabolic changes of the acetogen Clostridium sp. AWRP through adaptation to acetate challenge. Front. Microbiol. 2022, 13, 982442. [Google Scholar] [CrossRef]
- Zhang, J.-Z.; Li, Y.-Z.; Xi, Z.-N.; Gao, H.-P.; Zhang, Q.; Liu, L.-C.; Li, F.-L.; Ma, X.-Q. Engineered acetogenic bacteria as microbial cell factory for diversified biochemicals. Front. Bioeng. Biotechnol. 2024, 12, 1395540. [Google Scholar] [CrossRef]
- Firrman, J.; Liu, L.; Mahalak, K.; Tanes, C.; Bittinger, K.; Tu, V.; Bobokalonov, J.; Mattei, L.; Zhang, H.; Van den Abbeele, P. The impact of environmental pH on the gut microbiota community structure and short chain fatty acid production. FEMS Microbiol. Ecol. 2022, 98, fiac038. [Google Scholar] [CrossRef]
- Seethaler, B.; Lehnert, K.; Yahiaoui-Doktor, M.; Basrai, M.; Vetter, W.; Kiechle, M.; Bischoff, S.C. Omega-3 polyunsaturated fatty acids improve intestinal barrier integrity-albeit to a lesser degree than short-chain fatty acids: An exploratory analysis of the randomized controlled LIBRE trial. Eur. J. Nutr. 2023, 62, 2779–2791. [Google Scholar] [CrossRef] [PubMed]
- Zou, B.; Zhao, D.; Zhou, S.; Kang, J.X.; Wang, B. Insight into the effects of Omega-3 fatty acids on gut microbiota: Impact of a balanced tissue Omega-6/Omega-3 ratio. Front. Nutr. 2025, 12, 1575323. [Google Scholar] [CrossRef]
- Uthaiah, N.M.; Venkataramareddy, S.R.; Mudhol, S.; Sheikh, A.Y. EPA-rich Nannochloropsis oceanica biomass regulates gut microbiota, alleviates inflammation and ameliorates liver fibrosis in rats. Food Res. Int. 2025, 202, 115733. [Google Scholar] [CrossRef]
- Sălcudean, A.; Cîmpian, D.-M.; Popovici, R.-A.; Forna, N.; Corodan-Comiati, D.-M.; Sasu, A.-B.; Cozma, M.-M.; Bodo, C.-R.; Enache, E.-C.; Păcurar, M.; et al. Dietary Habits and Their Influence on the Microbiome and Mental Health in Adolescents. Nutrients 2025, 17, 1496. [Google Scholar] [CrossRef]
- Via, E.; Contreras-Rodríguez, O. Binge-Eating Precursors in Children and Adolescents: Neurodevelopment, and the Potential Contribution of Ultra-Processed Foods. Nutrients 2023, 15, 2994. [Google Scholar] [CrossRef] [PubMed]
- Facchin, S.; Bertin, L.; Bonazzi, E.; Lorenzon, G.; De Barba, C.; Barberio, B.; Zingone, F.; Maniero, D.; Scarpa, M.; Ruffolo, C.; et al. Short-Chain Fatty Acids and Human Health: From Metabolic Pathways to Current Therapeutic Implications. Life 2024, 14, 559. [Google Scholar] [CrossRef] [PubMed]
- Moubareck, C.A. Human Milk Microbiota and Oligosaccharides: A Glimpse into Benefits, Diversity, and Correlations. Nutrients 2021, 13, 1123. [Google Scholar] [CrossRef] [PubMed]
- Kotlyarov, S. Role of Short-Chain Fatty Acids Produced by Gut Microbiota in Innate Lung Immunity and Pathogenesis of the Heterogeneous Course of Chronic Obstructive Pulmonary Disease. Int. J. Mol. Sci. 2022, 23, 4768. [Google Scholar] [CrossRef]
- Notting, F.; Pirovano, W.; Sybesma, W.; Kort, R. The butyrate-producing and spore-forming bacterial genus Coprococcus as a potential biomarker for neurological disorders. Gut. Microb. 2023, 4, e16. [Google Scholar] [CrossRef]
- Lange, O.; Proczko-Stepaniak, M.; Mika, A. Short-Chain Fatty Acids—A Product of the Microbiome and Its Participation in Two-Way Communication on the Microbiome-Host Mammal Line. Curr. Obes. Rep. 2023, 12, 108–126. [Google Scholar] [CrossRef]
- May, K.S.; den Hartigh, L.J. Gut Microbial-Derived Short Chain Fatty Acids: Impact on Adipose Tissue Physiology. Nutrients 2023, 15, 272. [Google Scholar] [CrossRef]
- Cani, P.D.; Van Hul, M. Gut microbiota in overweight and obesity: Crosstalk with adipose tissue. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 164–183. [Google Scholar] [CrossRef]
- Frampton, J.; Murphy, K.G.; Frost, G.; Chambers, E.S. Short-chain fatty acids as potential regulators of skeletal muscle metabolism and function. Nat. Metab. 2020, 2, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Münte, E.; Hartmann, P. The Role of Short-Chain Fatty Acids in Metabolic Dysfunction-Associated Steatotic Liver Disease and Other Metabolic Diseases. Biomolecules 2025, 15, 469. [Google Scholar] [CrossRef]
- Tan, J.K.; Macia, L.; Mackay, C.R. Dietary fiber and SCFAs in the regulation of mucosal immunity. J. Allergy Clin. Immunol. 2023, 151, 361–370. [Google Scholar] [CrossRef]
- Yu, M.; Yu, B.; Chen, D. The effects of gut microbiota on appetite regulation and the underlying mechanisms. Gut Microbes 2024, 16, 2414796. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, J.; Qin, Y.; Yuan, J.; Yu, Z.; Ma, R.; Liu, F.; Zhao, J. Linking Short-Chain Fatty Acids to Systemic Homeostasis: Mechanisms, Therapeutic Potential, and Future Directions. J. Nutr. Metab. 2025, 2025, 8870958. [Google Scholar] [CrossRef]
- Luu, M.; Riester, Z.; Baldrich, A.; Reichardt, N.; Yuille, S.; Busetti, A.; Klein, M.; Wempe, A.; Leister, H.; Raifer, H.; et al. Microbial short-chain fatty acids modulate CD8+ T cell responses and improve adoptive immunotherapy for cancer. Nat. Commun. 2021, 12, 4077. [Google Scholar] [CrossRef] [PubMed]
- Ney, L.-M.; Wipplinger, M.; Grossmann, M.; Engert, N.; Wegner, V.D.; Mosig, A.S. Short chain fatty acids: Key regulators of the local and systemic immune response in inflammatory diseases and infections. Open Biol. 2023, 13, 230014. [Google Scholar] [CrossRef]
- Pham, N.H.T.; Joglekar, M.V.; Wong, W.K.M.; Nassif, N.T.; Simpson, A.M.; Hardikar, A.A. Short-chain fatty acids and insulin sensitivity: A systematic review and meta-analysis. Nutr. Rev. 2024, 82, 193–209. [Google Scholar] [CrossRef]
- Tang, R.; Li, L. Modulation of Short-Chain Fatty Acids as Potential Therapy Method for Type 2 Diabetes Mellitus. Can. J. Infect. Dis. Med. Microbiol. 2021, 2021, 6632266. [Google Scholar] [CrossRef]
- Pérez-Reytor, D.; Puebla, C.; Karahanian, E.; García, K. Use of Short-Chain Fatty Acids for the Recovery of the Intestinal Epithelial Barrier Affected by Bacterial Toxins. Front. Physiol. 2021, 12, 650313. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Wen, S.; Long-Kun, D.; Man, Y.; Chang, S.; Min, Z.; Shuang-Yu, L.; Xin, Q.; Jie, M.; Liang, W. Three important short-chain fatty acids (SCFAs) attenuate the inflammatory response induced by 5-FU and maintain the integrity of intestinal mucosal tight junction. BMC Immunol. 2022, 23, 19. [Google Scholar] [CrossRef] [PubMed]
- Saadh, M.J.; Allela, O.Q.B.; Ballal, S.; Mahdi, M.S.; Chahar, M.; Verma, R.; Al-Hussein, R.K.A.; Adil, M.; Jawad, M.J.; Al-Nuaimi, A.M.A. The effects of microbiota-derived short-chain fatty acids on T lymphocytes: From autoimmune diseases to cancer. Semin. Oncol. 2025, 52, 152398. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Duan, Y.; Luo, S.; Zhou, F.; Wu, Q.; Lu, Z. Short-chain fatty acids and cancer. Trends Cancer 2025, 11, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Fock, E.; Parnova, R. Mechanisms of Blood-Brain Barrier Protection by Microbiota-Derived Short-Chain Fatty Acids. Cells 2023, 12, 657. [Google Scholar] [CrossRef]
- Ju, S.; Shin, Y.; Han, S.; Kwon, J.; Choi, T.G.; Kang, I.; Kim, S.S. The Gut-Brain Axis in Schizophrenia: The Implications of the Gut Microbiome and SCFA Production. Nutrients 2023, 15, 4391. [Google Scholar] [CrossRef]
- Yin, X.; Duan, C.; Zhang, L.; Zhu, Y.; Qiu, Y.; Shi, K.; Wang, S.; Zhang, X.; Zhang, H.; Hao, Y.; et al. Microbiota-derived acetate attenuates neuroinflammation in rostral ventrolateral medulla of spontaneously hypertensive rats. J. Neuroinflammation 2024, 21, 101. [Google Scholar] [CrossRef]
- Abdel-Sater, K.A.; Hassan, H.A. Gut microbiota and stress ulcers: Unraveling the neurotransmitter connection. Front. Neurosci. 2025, 19, 1594179. [Google Scholar] [CrossRef]
- Cao, Y.; Cheng, Y.; Pan, W.; Diao, J.; Sun, L.; Meng, M. Gut microbiota variations in depression and anxiety: A systematic review. BMC Psychiatry 2025, 25, 443. [Google Scholar] [CrossRef]
- Kowalski, K.; Szponar, B.; Bochen, P.; Żebrowska-Różańska, P.; Łaczmański, Ł.; Samochowiec, J.; Misiak, B. Altered levels of fecal short-chain fatty acids are associated with subclinical inflammation and worse cognitive performance in patients with schizophrenia. J. Psychiatr. Res. 2023, 165, 298–304, Erratum in J. Psychiatr. Res. 2024, 169, 191. [Google Scholar] [CrossRef]
- Peng, K.; Dong, W.; Luo, T.; Tang, H.; Zhu, W.; Huang, Y.; Yang, X. Butyrate and obesity: Current research status and future prospect. Front. Endocrinol. 2023, 14, 1098881. [Google Scholar] [CrossRef]
- Murugesan, S.; Nirmalkar, K.; Hoyo-Vadillo, C.; García-Espitia, M.; Ramírez-Sánchez, D.; García-Mena, J. Gut microbiome production of short-chain fatty acids and obesity in children. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 621–625. [Google Scholar] [CrossRef]
- Bridgeman, S.C.; Northrop, W.; Melton, P.E.; Ellison, G.C.; Newsholme, P.; Mamotte, C.D.S. Butyrate generated by gut microbiota and its therapeutic role in metabolic syndrome. Pharmacol. Res. 2020, 160, 105174. [Google Scholar] [CrossRef] [PubMed]
- Orsso, C.E.; Colin-Ramirez, E.; Field, C.J.; Madsen, K.L.; Prado, C.M.; Haqq, A.M. Adipose Tissue Development and Expansion from the Womb to Adolescence: An Overview. Nutrients 2020, 12, 2735. [Google Scholar] [CrossRef] [PubMed]
- Pinzariu, A.C.; Pasca, S.A.; Sindilar, A.; Drochioi, C.; Balan, M.; Oboroceanu, T.; Niculescu, S.; Crauciuc, D.V.; Crauciuc, E.G.; Luca, A.; et al. Adipose Tissue Remodeling by Prolonged Administration of High Dose of Vitamin D3 in Rats Treated to Prevent Sarcopenia. Rev. Chim. 2017, 68, 2139–2143. [Google Scholar] [CrossRef]
- Kardan, R.; Hemmati, J.; Nazari, M.; Ahmadi, A.; Asghari, B.; Azizi, M.; Khaledi, M.; Arabestani, M.R. Novel therapeutic strategy for obesity through the gut microbiota-brain axis: A review article. Casp. J. Intern. Med. 2024, 15, 215–227. [Google Scholar] [CrossRef]
- Pinzariu, A.C.; Oboroceanu, T.; Eloae, F.Z.; Hristov, I.; Costan, V.V.; Labusca, L.; Cianga, P.; Verestiuc, L.; Hanganu, B.; Crauciuc, D.V.; et al. Vitamin D as a Regulator of Adipocyte Differentiation Effects in vivo and in vitro. Rev. Chim. 2018, 69, 731–734. [Google Scholar] [CrossRef]
- Butcovan, D.; Oboroceanu, T.; Cimpeanu, C.; Mironescu, A.; Haliga, R.E.; Pinzariu, A.C.; Lupusoru, R.V.; Popescu, E.; Mocanu, V. The Involvement of Epicardial Adiposity and Inflammation in Postoperatory Atrial Fibrilation—Immunohistochemical Qualitative and Quantitative Assessment. Rev. Chim. 2017, 68, 886–889. [Google Scholar] [CrossRef]
- Saban Güler, M.; Arslan, S.; Ağagündüz, D.; Cerqua, I.; Pagano, E.; Berni Canani, R.; Capasso, R. Butyrate: A potential mediator of obesity and microbiome via different mechanisms of actions. Food Res. Int. 2025, 199, 115420. [Google Scholar] [CrossRef]
- Badulescu, O.; Sirbu, P.; Ungureanu, C.; Pȋnzariu, A.; Cojocaru, E.; Filip, N.; Bararu-Bojan, I.; Vladeanu, M.; Ciocoiu, M. Orthopedic surgery in hemophilic patients with musculoskeletal disorders: A systematic review. Exp. Ther. Med. 2021, 22, 995. [Google Scholar] [CrossRef]
- Haliga, R.; Zugun-Eloae, F.; Oboroceanu, T.; Pînzariu, A.; Mocanu, V. Vitamin D and Tissular Expression of Vitamin D Receptor in Obesity. Rev. Med. Chir. Soc. Med. Nat. Iasi. 2016, 120, 404–408. [Google Scholar] [PubMed]
- Kopczyńska, J.; Kowalczyk, M. The potential of short-chain fatty acid epigenetic regulation in chronic low-grade inflammation and obesity. Front. Immunol. 2024, 15, 1380476. [Google Scholar] [CrossRef]
- Toader, M.; Branisteanu, D.; Glod, M.; Esanu, I.; Branisteanu, C.; Capsa, M.-S.; Dimitriu, A.; Nicolescu, A.; Pinzariu, A.; Branisteanu, D. Mucocutaneous lesions associated with SARS-CoV-2 infection (Review). Exp. Ther. Med. 2022, 23, 258. [Google Scholar] [CrossRef]
- Coppola, S.; Avagliano, C.; Calignano, A.; Berni Canani, R. The Protective Role of Butyrate against Obesity and Obesity-Related Diseases. Molecules 2021, 26, 682. [Google Scholar] [CrossRef]
- Loh, J.S.; Mak, W.Q.; Tan, L.K.S.; Ng, C.X.; Chan, H.H.; Yeow, S.H.; Foo, J.B.; Ong, Y.S.; How, C.W.; Khaw, K.Y. Microbiota–gut–brain axis and its therapeutic applications in neurodegenerative diseases. Signal Transduct. Target. Ther. 2024, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Asadi, A.; Shadab Mehr, N.; Mohamadi, M.H.; Shokri, F.; Heidary, M.; Sadeghifard, N.; Khoshnood, S. Obesity and gut–microbiota–brain axis: A narrative review. Clin. Lab. Anal. 2022, 36, e24420. [Google Scholar] [CrossRef]
- Dehghani, A.; Wang, L.; Garssen, J.; Styla, E.; Leusink-Muis, T.; van Ark, I.; Folkerts, G.; van Bergenhenegouwen, J.; Braber, S. Pregnancy exacerbates neutrophil responses in murine lungs and alters gut microbiota composition after cigarette smoke exposure. Front. Immunol. 2025, 16, 1590290. [Google Scholar] [CrossRef] [PubMed]
- Zubcevic, J.; Watkins, J.; Lin, C.; Bautista, B.; Hatch, H.M.; Tevosian, S.G.; Hayward, L.F. Nicotine Exposure during Rodent Pregnancy Alters the Composition of Maternal Gut Microbiota and Abundance of Maternal and Amniotic Short Chain Fatty Acids. Metabolites 2022, 12, 735. [Google Scholar] [CrossRef]
- Biagioli, V.; Matera, M.; Ramenghi, L.A.; Falsaperla, R.; Striano, P. Microbiome and Pregnancy Dysbiosis: A Narrative Review on Offspring Health. Nutrients 2025, 17, 1033. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Chen, S.; Wang, P. Investigating the Impact of Short-Chain Fatty Acids (SCFAs) from the Maternal Microbiome on Pregnancy and Fetal Health of C57BL/6J Mice: A Research Protocol. URNCST J. 2025, 9, 1–9. [Google Scholar] [CrossRef]
- Ejtahed, H.-S.; Angoorani, P.; Soroush, A.-R.; Hasani-Ranjbar, S.; Siadat, S.-D.; Larijani, B. Gut microbiota-derived metabolites in obesity: A systematic review. Biosci. Microbiota Food Health 2020, 39, 65–76. [Google Scholar] [CrossRef]
- Mao, Z.-H.; Gao, Z.-X.; Liu, D.-W.; Liu, Z.-S.; Wu, P. Gut microbiota and its metabolites—Molecular mechanisms and management strategies in diabetic kidney disease. Front. Immunol. 2023, 14, 1124704. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Yin, Y.; Li, Z.; Zhang, W. Gut Microbiota-Derived Components and Metabolites in the Progression of Non-Alcoholic Fatty Liver Disease (NAFLD). Nutrients 2019, 11, 1712. [Google Scholar] [CrossRef] [PubMed]
- Bondia-Pons, I.; Maukonen, J.; Mattila, I.; Rissanen, A.; Saarela, M.; Kaprio, J.; Hakkarainen, A.; Lundbom, J.; Lundbom, N.; Hyötyläinen, T.; et al. Metabolome and fecal microbiota in monozygotic twin pairs discordant for weight: A Big Mac challenge. FASEB J. 2014, 28, 4169–4179. [Google Scholar] [CrossRef]
- Liu, R.; Hong, J.; Xu, X.; Feng, Q.; Zhang, D.; Gu, Y.; Shi, J.; Zhao, S.; Liu, W.; Wang, X.; et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat. Med. 2017, 23, 859–868. [Google Scholar] [CrossRef]
- Ottosson, F.; Brunkwall, L.; Ericson, U.; Nilsson, P.M.; Almgren, P.; Fernandez, C.; Melander, O.; Orho-Melander, M. Connection Between BMI-Related Plasma Metabolite Profile and Gut Microbiota. J. Clin. Endocrinol. Metab. 2018, 103, 1491–1501. [Google Scholar] [CrossRef] [PubMed]
- Org, E.; Blum, Y.; Kasela, S.; Mehrabian, M.; Kuusisto, J.; Kangas, A.J.; Soininen, P.; Wang, Z.; Ala-Korpela, M.; Hazen, S.L.; et al. Relationships between gut microbiota, plasma metabolites, and metabolic syndrome traits in the METSIM cohort. Genome Biol. 2017, 18, 70. [Google Scholar] [CrossRef]
- Druart, C.; Dewulf, E.M.; Cani, P.D.; Neyrinck, A.M.; Thissen, J.; Delzenne, N.M. Gut Microbial Metabolites of Polyunsaturated Fatty Acids Correlate with Specific Fecal Bacteria and Serum Markers of Metabolic Syndrome in Obese Women. Lipids 2014, 49, 397–402. [Google Scholar] [CrossRef]
- Sayin, S.I.; Wahlström, A.; Felin, J.; Jäntti, S.; Marschall, H.-U.; Bamberg, K.; Angelin, B.; Hyötyläinen, T.; Orešič, M.; Bäckhed, F. Gut Microbiota Regulates Bile Acid Metabolism by Reducing the Levels of Tauro-beta-muricholic Acid, a Naturally Occurring FXR Antagonist. Cell Metab. 2013, 17, 225–235. [Google Scholar] [CrossRef]
- Pallister, T.; Jackson, M.A.; Martin, T.C.; Zierer, J.; Jennings, A.; Mohney, R.P.; MacGregor, A.; Steves, C.J.; Cassidy, A.; Spector, T.D.; et al. Hippurate as a metabolomic marker of gut microbiome diversity: Modulation by diet and relationship to metabolic syndrome. Sci. Rep. 2017, 7, 13670. [Google Scholar] [CrossRef]
- Cho, K.Y. Lifestyle modifications result in alterations in the gut microbiota in obese children. BMC Microbiol. 2021, 21, 10. [Google Scholar] [CrossRef]
- Visuthranukul, C.; Sriswasdi, S.; Tepaamorndech, S.; Joyjinda, Y.; Saengpanit, P.; Kwanbunbumpen, T.; Panichsillaphakit, E.; Uaariyapanichkul, J.; Chomtho, S. Association of Human Intestinal Microbiota with Lifestyle Activity, Adiposity, and Metabolic Profiles in Thai Children with Obesity. J. Nutr. Metab. 2022, 1–14. [Google Scholar] [CrossRef]
- Li, S.; Ma, X.; Mei, H.; Chang, X.; He, P.; Sun, L.; Xiao, H.; Wang, S.; Li, R. Association between gut microbiota and short-chain fatty acids in children with obesity. Sci. Rep. 2025, 15, 483. [Google Scholar] [CrossRef]
- Gyarmati, P.; Song, Y.; Dotimas, J.; Yoshiba, G.; Christison, A. Cross-sectional comparisons of gut microbiome and short-chain fatty acid levels among children with varied weight classifications. Pediatr. Obes. 2021, 16, e12750. [Google Scholar] [CrossRef]
- Petraroli, M.; Castellone, E.; Patianna, V.; Esposito, S. Gut Microbiota and Obesity in Adults and Children: The State of the Art. Front. Pediatr. 2021, 9, 657020. [Google Scholar] [CrossRef] [PubMed]
- Indiani, C.M.D.S.P.; Rizzardi, K.F.; Castelo, P.M.; Ferraz, L.F.C.; Darrieux, M.; Parisotto, T.M. Childhood Obesity and Firmicutes/Bacteroidetes Ratio in the Gut Microbiota: A Systematic Review. Child. Obes. 2018, 14, 501–509. [Google Scholar] [CrossRef]
- Burananat, T.; Wilantho, A.; Kulalert, P.; Nanthapisal, S.; Tonglim, J.; Deetienin, W.; Wangkumhang, P.; Tongsima, S.; Thaweekul, P. The role of gut microbiota in obesity severity and metabolic risk in pediatric populations. Nutr. Metab. Cardiovasc. Dis. 2025, 35, 103970. [Google Scholar] [CrossRef]
- Liang, C.; Guo, M.; Liu, T.; Zhou, X.; Gong, P.; Lyu, L.; Niu, H.; Wu, Y.; Chen, S.; Han, X.; et al. Profiles of gut microbiota in children with obesity from Harbin, China and screening of strains with anti-obesity ability in vitro and in vivo. J. Appl. Microbiol. 2020, 129, 728–737. [Google Scholar] [CrossRef]
- Araujo, D.S.; Klein, M.I.; Scudine, K.G.d.O.; de Sales Leite, L.; Parisotto, T.M.; Ferreira, C.M.; Fonseca, F.L.A.; Perez, M.M.; Castelo, P.M. Salivary Microbiological and Gingival Health Status Evaluation of Adolescents With Overweight and Obesity: A Cluster Analysis. Front. Pediatr. 2020, 8, 429. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-M.; Lv, Q.; Chen, Y.-J.; Yan, L.-B.; Xiong, X. Association between childhood obesity and gut microbiota: 16S rRNA gene sequencing-based cohort study. World J. Gastroenterol. 2024, 30, 2249–2257. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.F.; Wang, Y.Y.; Peng, H.; Li, R.; Zhang, F.; Wang, N.; Shao, Q.W.; Jiang, Q. Association between obesity with the diversity and genus of gut microbiota in school-aged children. Zhonghua Liu Xing Bing Xue Za Zhi 2022, 43, 260–268. [Google Scholar] [CrossRef]
- Ismail, H.M.; Perera, D.; Mandal, R.; DiMeglio, L.A.; Evans-Molina, C.; Hannon, T.; Petrosino, J.; Javornik Cregeen, S.; Schmidt, N.W. Gut Microbial Changes Associated With Obesity in Youth With Type 1 Diabetes. J. Clin. Endocrinol. Metab. 2025, 110, 364–373. [Google Scholar] [CrossRef]
- Da Silva, C.C.; Monteil, M.A.; Davis, E.M. Overweight and Obesity in Children Are Associated with an Abundance of Firmicutes and Reduction of Bifidobacterium in Their Gastrointestinal Microbiota. Child. Obes. 2020, 16, 204–210. [Google Scholar] [CrossRef]
- Yuan, X.; Chen, R.; Zhang, Y.; Lin, X.; Yang, X.; McCormick, K.L. Gut Microbiota of Chinese Obese Children and Adolescents With and Without Insulin Resistance. Front. Endocrinol. 2021, 12, 636272. [Google Scholar] [CrossRef]
- Carrizales-Sánchez, A.K.; García-Cayuela, T.; Hernández-Brenes, C.; Senés-Guerrero, C. Gut microbiota associations with metabolic syndrome and relevance of its study in pediatric subjects. Gut Microbes 2021, 13, 1960135. [Google Scholar] [CrossRef]
- Moran-Ramos, S.; Lopez-Contreras, B.E.; Villarruel-Vazquez, R.; Ocampo-Medina, E.; Macias-Kauffer, L.; Martinez-Medina, J.N.; Villamil-Ramirez, H.; León-Mimila, P.; Del Rio-Navarro, B.E.; Ibarra-Gonzalez, I.; et al. Environmental and intrinsic factors shaping gut microbiota composition and diversity and its relation to metabolic health in children and early adolescents: A population-based study. Gut Microbes 2020, 11, 900–917. [Google Scholar] [CrossRef]
- Wang, L.; Yi, Q.; Xu, H.; Liu, H.; Tan, B.; Deng, H.; Chen, Y.; Wang, R.; Tang, F.; Cheng, X.; et al. Alterations in the gut microbiota community are associated with childhood obesity and precocious puberty. BMC Microbiol 2024, 24, 311. [Google Scholar] [CrossRef]
- Qian, Y.; Fang, X.; Chen, Y.; Ding, M.; Gong, M. Gut flora influences the hypothalamic-gonadal axis to regulate the pathogenesis of obesity-associated precocious puberty. Sci. Rep. 2024, 14, 28844. [Google Scholar] [CrossRef]
- Gallardo-Becerra, L.; Cornejo-Granados, F.; García-López, R.; Valdez-Lara, A.; Bikel, S.; Canizales-Quinteros, S.; López-Contreras, B.E.; Mendoza-Vargas, A.; Nielsen, H.; Ochoa-Leyva, A. Metatranscriptomic analysis to define the Secrebiome, and 16S rRNA profiling of the gut microbiome in obesity and metabolic syndrome of Mexican children. Microb. Cell Factories 2020, 19, 61. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Liang, J.; Su, Y.; Wang, J.; Amakye, W.K.; Pan, J.; Chu, X.; Ma, B.; Song, Y.; Li, Y.; et al. The associations of the gut microbiome composition and short-chain fatty acid concentrations with body fat distribution in children. Clin. Nutr. 2021, 40, 3379–3390. [Google Scholar] [CrossRef] [PubMed]
- Nandy, D.; Craig, S.J.C.; Cai, J.; Tian, Y.; Paul, I.M.; Savage, J.S.; Marini, M.E.; Hohman, E.E.; Reimherr, M.L.; Patterson, A.D.; et al. Metabolomic profiling of stool of two-year old children from the INSIGHT study reveals links between butyrate and child weight outcomes. Pediatr. Obes. 2022, 17, e12833. [Google Scholar] [CrossRef] [PubMed]
- Jaimes, J.D.; Slavíčková, A.; Hurych, J.; Cinek, O.; Nichols, B.; Vodolánová, L.; Černý, K.; Havlík, J. Stool metabolome-microbiota evaluation among children and adolescents with obesity, overweight, and normal-weight using 1H NMR and 16S rRNA gene profiling. PLoS ONE 2021, 16, e0247378. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Dang, Y. Roles of gut microbiota and metabolites in overweight and obesity of children. Front. Endocrinol. 2022, 13, 994930. [Google Scholar] [CrossRef]
- Li, Z.; Li, J.; Fu, R.; Liu, J.; Wen, X.; Zhang, L. Halitosis: Etiology, prevention, and the role of microbiota. Clin. Oral Investig. 2023, 27, 6383–6393. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Wu, Z.; Lin, J.; Shan, C.; Abasijiang, A.; Zhao, J. Characterization of the oral and gut microbiome in children with obesity aged 3 to 5 years. Front. Cell. Infect. Microbiol. 2023, 13, 1102650. [Google Scholar] [CrossRef]
- Mervish, N.A.; Hu, J.; Hagan, L.A.; Arora, M.; Frau, C.; Choi, J.; Attaie, A.; Ahmed, M.; Teitelbaum, S.L.; Wolff, M.S. Associations of the Oral Microbiota with Obesity and Menarche in Inner City Girls. J. Child. Obes. 2019, 4, 2. [Google Scholar] [CrossRef]
- Kwiatkowska, M.; Gołębiewski, M.; Sikora, M.; Rycharska, E.Ł.; Krogulska, A. The oral cavity and intestinal microbiome in children with functional constipation. Sci. Rep. 2024, 14, 8283. [Google Scholar] [CrossRef]
- Rahman, B.; Al-Marzooq, F.; Saad, H.; Benzina, D.; Al Kawas, S. Dysbiosis of the Subgingival Microbiome and Relation to Periodontal Disease in Association with Obesity and Overweight. Nutrients 2023, 15, 826. [Google Scholar] [CrossRef]
- Salman, U.; Dabdoub, S.M.; Reyes, A.; Sidahmed, A.; Weber-Gasperoni, K.; Brown, R.; Evans, I.A.; Taylor, E.; Mangalam, A.; Kanner, L.; et al. Dysbiotic Microbiome–Metabolome Axis in Childhood Obesity and Metabolic Syndrome. J. Dent. Res. 2025, 104, 1314–1323. [Google Scholar] [CrossRef]
- Reis, R.M.; Carlo, H.L.; Santos, R.L.D.; Sabella, F.M.; Parisotto, T.M.; Carvalho, F.G.D. Possible Relationship Between the Oral and Gut Microbiome, Caries Development, and Obesity in Children During the COVID-19 Pandemic. Front. Oral. Health 2022, 3, 887765. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Chu, C.; Peng, Y.; Zhang, N.; Yang, Z.; You, J.; Wei, F. Correlations between gastrointestinal and oral microbiota in children with cerebral palsy and epilepsy. Front. Pediatr. 2022, 10, 988601. [Google Scholar] [CrossRef]
- De Lemos, G.M.; Resende, C.M.M.; Campello, C.P.; Ribeiro, I.S.; Mendes, A.K.; De Lima, E.L.S.; De Oliveira, R.M.D.C.; Barbosa Filho, V.C.; Correia, M.J.; Muniz, M.T.C. Is oral microbiota associated with overweight and obesity in children and adolescents? A systematic review. Crit. Rev. Food Sci. Nutr. 2024, 64, 4275–4285. [Google Scholar] [CrossRef]
- Mameli, C.; Cattaneo, C.; Panelli, S.; Comandatore, F.; Sangiorgio, A.; Bedogni, G.; Bandi, C.; Zuccotti, G.; Pagliarini, E. Taste perception and oral microbiota are associated with obesity in children and adolescents. PLoS ONE 2019, 14, e0221656. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, B.; Selvaraju, V.; Chen, J.; Ayine, P.; Yang, L.; Ramesh Babu, J.; Geetha, T.; Taneja, V. Ethnic variability associating gut and oral microbiome with obesity in children. Gut Microbes 2021, 13, 1882926. [Google Scholar] [CrossRef]
- Rizzardi, K.F.; Indiani, C.M.D.S.P.; Mattos-Graner, R.D.O.; De Sousa, E.T.; Nobre-dos-Santos, M.; Parisotto, T.M. Firmicutes Levels in the Mouth Reflect the Gut Condition With Respect to Obesity and Early Childhood Caries. Front. Cell. Infect. Microbiol. 2021, 11, 593734. [Google Scholar] [CrossRef] [PubMed]
- Wernroth, M.-L.; Peura, S.; Hedman, A.M.; Hetty, S.; Vicenzi, S.; Kennedy, B.; Fall, K.; Svennblad, B.; Andolf, E.; Pershagen, G.; et al. Development of gut microbiota during the first 2 years of life. Sci. Rep. 2022, 12, 9080. [Google Scholar] [CrossRef] [PubMed]
- Dombrowska-Pali, A.; Wiktorczyk-Kapischke, N.; Chrustek, A.; Olszewska-Słonina, D.; Gospodarek-Komkowska, E.; Socha, M.W. Human Milk Microbiome—A Review of Scientific Reports. Nutrients 2024, 16, 1420. [Google Scholar] [CrossRef]
- Exclusive Breastfeeding to Reduce the Risk of Childhood Overweight and Obesity. Available online: https://www.who.int/tools/elena/interventions/breastfeeding-childhood-obesity (accessed on 17 September 2025).
- Zhang, M.; Ding, L.; Strodl, E.; Yin, X.; Wen, G.; Sun, D.; Xian, D.; Zhao, Y.; Zheng, Y.; Liu, F.; et al. Early supplement of probiotics reduces the risk of obesity among preschool children: A real-world observational study. Front. Nutr. 2025, 12, 1597894. [Google Scholar] [CrossRef]
- Soroceanu, R.P.; Timofte, D.V.; Timofeiov, S.; Vlasceanu, V.I.; Maxim, M.; Miler, A.A.; Iordache, A.G.; Moscalu, R.; Moscalu, M.; Văcărean-Trandafir, I.C.; et al. The Impact of Bariatric Surgery on Gut Microbiota Composition and Diversity: A Longitudinal Analysis Using 16S rRNA Sequencing. Int. J. Mol. Sci. 2025, 26, 7933. [Google Scholar] [CrossRef]
- Hu, T.; Chitnis, N.; Monos, D.; Dinh, A. Next-generation sequencing technologies: An overview. Hum. Immunol. 2021, 82, 801–811. [Google Scholar] [CrossRef]
- Nguyen Tran, T.; Luong, T.V.; Nguyen, N.V.D.; Dang, H.N.N. Complex relationship between childhood obesity and the gut microbiota. World J. Clin. Pediatr. 2025, 14, 100975. [Google Scholar] [CrossRef]
- Moorlag, S.J.C.F.M.; Coolen, J.P.M.; van den Bosch, B.; Jin, E.H.-M.; Buil, J.B.; Wertheim, H.F.L.; Melchers, W.J.G. Targeting the 16S rRNA Gene by Reverse Complement PCR Next-Generation Sequencing: Specific and Sensitive Detection and Identification of Microbes Directly in Clinical Samples. Microbiol. Spectr. 2023, 11, e0448322. [Google Scholar] [CrossRef] [PubMed]
- Buetas, E.; Jordán-López, M.; López-Roldán, A.; D’Auria, G.; Martínez-Priego, L.; De Marco, G.; Carda-Diéguez, M.; Mira, A. Full-length 16S rRNA gene sequencing by PacBio improves taxonomic resolution in human microbiome samples. BMC Genom. 2024, 25, 310. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, Y. Full-Length 16S rRNA Gene Analysis Using Long-Read Nanopore Sequencing for Rapid Identification of Bacteria from Clinical Specimens. Methods Mol. Biol. 2023, 2632, 193–213. [Google Scholar] [CrossRef]
- Weinroth, M.D.; Belk, A.D.; Dean, C.; Noyes, N.; Dittoe, D.K.; Rothrock, M.J.; Ricke, S.C.; Myer, P.R.; Henniger, M.T.; Ramírez, G.A.; et al. Considerations and best practices in animal science 16S ribosomal RNA gene sequencing microbiome studies. J. Anim. Sci. 2022, 100, skab346. [Google Scholar] [CrossRef]
- Solito, A.; Bozzi Cionci, N.; Calgaro, M.; Caputo, M.; Vannini, L.; Hasballa, I.; Archero, F.; Giglione, E.; Ricotti, R.; Walker, G.E.; et al. Supplementation with Bifidobacterium breve BR03 and B632 strains improved insulin sensitivity in children and adolescents with obesity in a cross-over, randomized double-blind placebo-controlled trial. Clin. Nutr. 2021, 40, 4585–4594. [Google Scholar] [CrossRef]
- Reyman, M.; van Houten, M.A.; Watson, R.L.; Chu, M.L.J.N.; Arp, K.; de Waal, W.J.; Schiering, I.; Plötz, F.B.; Willems, R.J.L.; van Schaik, W.; et al. Effects of early-life antibiotics on the developing infant gut microbiome and resistome: A randomized trial. Nat. Commun. 2022, 13, 893. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, X.; Wang, T.; Huang, R. Exploring the effects of short-course antibiotics on children’s gut microbiota by using 16S rRNA gene sequencing: A case-control study. BMC Pediatr. 2024, 24, 562. [Google Scholar] [CrossRef] [PubMed]
- Mou, Y.; Santos, S.; Lara, M.; Derks, I.P.M.; Jaddoe, V.W.V.; Gaillard, R.; Felix, J.F.; Steegers, E.; Voortman, T.; van IJzendoorn, M.H.; et al. Identifying Key Predictors of Mid-Childhood Obesity in a Population-Based Cohort Study: An Evidence Synthesis and Predictive Modeling Study. Obes. Rev. 2025, 26, e13958. [Google Scholar] [CrossRef]
- Regueira-Iglesias, A.; Balsa-Castro, C.; Blanco-Pintos, T.; Tomás, I. Critical review of 16S rRNA gene sequencing workflow in microbiome studies: From primer selection to advanced data analysis. Mol. Oral. Microbiol. 2023, 38, 347–399. [Google Scholar] [CrossRef]
- Davidson, R.M.; Epperson, L.E. Microbiome Sequencing Methods for Studying Human Diseases. Methods Mol. Biol. 2018, 1706, 77–90. [Google Scholar] [CrossRef]
- Milani, G.P.; Silano, M.; Mazzocchi, A.; Bettocchi, S.; De Cosmi, V.; Agostoni, C. Personalized nutrition approach in pediatrics: A narrative review. Pediatr. Res. 2021, 89, 384–388. [Google Scholar] [CrossRef]
- Van Den Bossche, T.; Armengaud, J.; Benndorf, D.; Blakeley-Ruiz, J.A.; Brauer, M.; Cheng, K.; Creskey, M.; Figeys, D.; Grenga, L.; Griffin, T.J.; et al. The microbiologist’s guide to metaproteomics. iMeta 2025, 4, e70031. [Google Scholar] [CrossRef] [PubMed]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.-C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Tanca, A.; Uzzau, S. Contribution of metaproteomics to unveiling the functional role of the gut microbiome in human physiology and metabolism. Expert Rev. Proteom. 2025, 10, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Valdés-Mas, R.; Leshem, A.; Zheng, D.; Cohen, Y.; Kern, L.; Zmora, N.; He, Y.; Katina, C.; Eliyahu-Miller, S.; Yosef-Hevroni, T.; et al. Metagenome-informed metaproteomics of the human gut microbiome, host, and dietary exposome uncovers signatures of health and inflammatory bowel disease. Cell 2025, 188, 1062–1083.e36. [Google Scholar] [CrossRef]
- Levi Mortera, S.; Marzano, V.; Rapisarda, F.; Marangelo, C.; Pirona, I.; Vernocchi, P.; Di Michele, M.; Del Chierico, F.; Quintero, M.A.; Fernandez, I.; et al. Metaproteomics reveals diet-induced changes in gut microbiome function according to Crohn’s disease location. Microbiome 2024, 12, 217. [Google Scholar] [CrossRef]
- Zhou, M.; Peng, C.; Miao, Z.; Wang, K.; Zhou, H.; Li, Y.; Xiao, G.; Wu, X. Improved diet-based nutritional interventions can improve childhood obesity with the synergistic regulation of gut microbiota. Benef. Microbes 2024, 15, 495–513. [Google Scholar] [CrossRef]
- Maiuolo, J.; Bulotta, R.M.; Ruga, S.; Nucera, S.; Macrì, R.; Scarano, F.; Oppedisano, F.; Carresi, C.; Gliozzi, M.; Musolino, V.; et al. The Postbiotic Properties of Butyrate in the Modulation of the Gut Microbiota: The Potential of Its Combination with Polyphenols and Dietary Fibers. Int. J. Mol. Sci. 2024, 25, 6971. [Google Scholar] [CrossRef]
- Alsharairi, N.A. The Role of Short-Chain Fatty Acids in Mediating Very Low-Calorie Ketogenic Diet-Infant Gut Microbiota Relationships and Its Therapeutic Potential in Obesity. Nutrients 2021, 13, 3702. [Google Scholar] [CrossRef]
- López-Gil, J.F.; García-Hermoso, A.; Sotos-Prieto, M.; Cavero-Redondo, I.; Martínez-Vizcaíno, V.; Kales, S.N. Mediterranean Diet-Based Interventions to Improve Anthropometric and Obesity Indicators in Children and Adolescents: A Systematic Review with Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2023, 14, 858–869. [Google Scholar] [CrossRef]
- Seral-Cortes, M.; Larruy-García, A.; De Miguel-Etayo, P.; Labayen, I.; Moreno, L.A. Mediterranean Diet and Genetic Determinants of Obesity and Metabolic Syndrome in European Children and Adolescents. Genes 2022, 13, 420. [Google Scholar] [CrossRef]
- Singh, R.P.; Bhardwaj, A. β-glucans: A potential source for maintaining gut microbiota and the immune system. Front. Nutr. 2023, 10, 1143682. [Google Scholar] [CrossRef]
- Akkerman, R.; Logtenberg, M.J.; An, R.; Van Den Berg, M.A.; de Haan, B.J.; Faas, M.M.; Zoetendal, E.; de Vos, P.; Schols, H.A. Endo-1,3(4)-β-Glucanase-Treatment of Oat β-Glucan Enhances Fermentability by Infant Fecal Microbiota, Stimulates Dectin-1 Activation and Attenuates Inflammatory Responses in Immature Dendritic Cells. Nutrients 2020, 12, 1660. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, Y.; Zhao, Y.; Li, L.; Zhang, Z.; Hettinga, K.; Yang, H.; Deng, J. A Comprehensive Review on Dietary Polysaccharides as Prebiotics, Synbiotics, and Postbiotics in Infant Formula and Their Influences on Gut Microbiota. Nutrients 2024, 16, 4122. [Google Scholar] [CrossRef]
- Annunziata, G.; Arnone, A.; Ciampaglia, R.; Tenore, G.C.; Novellino, E. Fermentation of Foods and Beverages as a Tool for Increasing Availability of Bioactive Compounds. Focus on Short-Chain Fatty Acids. Foods 2020, 9, 999. [Google Scholar] [CrossRef]
- Skowron, K.; Budzyńska, A.; Grudlewska-Buda, K.; Wiktorczyk-Kapischke, N.; Andrzejewska, M.; Wałecka-Zacharska, E.; Gospodarek-Komkowska, E. Two Faces of Fermented Foods-The Benefits and Threats of Its Consumption. Front. Microbiol. 2022, 13, 845166. [Google Scholar] [CrossRef] [PubMed]
- Acevedo-Román, A.; Pagán-Zayas, N.; Velázquez-Rivera, L.I.; Torres-Ventura, A.C.; Godoy-Vitorino, F. Insights into Gut Dysbiosis: Inflammatory Diseases, Obesity, and Restoration Approaches. Int. J. Mol. Sci. 2024, 25, 9715. [Google Scholar] [CrossRef]
- Bellicha, A.; van Baak, M.A.; Battista, F.; Beaulieu, K.; Blundell, J.E.; Busetto, L.; Carraça, E.V.; Dicker, D.; Encantado, J.; Ermolao, A.; et al. Effect of exercise training on weight loss, body composition changes, and weight maintenance in adults with overweight or obesity: An overview of 12 systematic reviews and 149 studies. Obes. Rev. 2021, 22 (Suppl. S4), e13256. [Google Scholar] [CrossRef] [PubMed]
- Lee, J. Influences of exercise interventions on overweight and obesity in children and adolescents. Public Health Nurs. 2021, 38, 502–516. [Google Scholar] [CrossRef]
- Migueles, J.H.; Cadenas-Sanchez, C.; Lubans, D.R.; Henriksson, P.; Torres-Lopez, L.V.; Rodriguez-Ayllon, M.; Plaza-Florido, A.; Gil-Cosano, J.J.; Henriksson, H.; Escolano-Margarit, M.V.; et al. Effects of an Exercise Program on Cardiometabolic and Mental Health in Children With Overweight or Obesity: A Secondary Analysis of a Randomized Clinical Trial. JAMA Netw. Open 2023, 6, e2324839. [Google Scholar] [CrossRef]
- Kononova, S.; Kashparov, M.; Xue, W.; Bobkova, N.; Leonov, S.; Zagorodny, N. Gut Microbiome Dysbiosis as a Potential Risk Factor for Idiopathic Toe-Walking in Children: A Review. Int. J. Mol. Sci. 2023, 24, 13204. [Google Scholar] [CrossRef]
- Morgado, M.C.; Sousa, M.; Coelho, A.B.; Costa, J.A.; Seabra, A. Exploring Gut Microbiota and the Influence of Physical Activity Interventions on Overweight and Obese Children and Adolescents: A Systematic Review. Healthcare 2023, 11, 2459. [Google Scholar] [CrossRef]
- Pérez-Prieto, I.; Plaza-Florido, A.; Ubago-Guisado, E.; Ortega, F.B.; Altmäe, S. Physical activity, sedentary behavior and microbiome: A systematic review and meta-analysis. J. Sci. Med. Sport 2024, 27, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Goldiș, A.; Dragomir, R.; Mercioni, M.A.; Sirca, D.; Goldiș, C.; Enatescu, I.; Olariu, L.; Belei, O. Clinical Efficacy of Sodium Butyrate in Managing Pediatric Inflammatory Bowel Disease. Life 2025, 15, 902. [Google Scholar] [CrossRef] [PubMed]
- Coppola, S.; Nocerino, R.; Paparo, L.; Bedogni, G.; Calignano, A.; Di Scala, C.; De Giovanni Di Santa Severina, A.F.; De Filippis, F.; Ercolini, D.; Berni Canani, R. Therapeutic Effects of Butyrate on Pediatric Obesity: A Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e2244912. [Google Scholar] [CrossRef]
- Fahim, S.M.; Huey, S.L.; Palma Molina, X.E.; Agarwal, N.; Ridwan, P.; Ji, N.; Kibbee, M.; Kuriyan, R.; Finkelstein, J.L.; Mehta, S. Gut microbiome-based interventions for the management of obesity in children and adolescents aged up to 19 years. Cochrane Database Syst. Rev. 2025, 7, CD015875. [Google Scholar] [CrossRef]
- Mohamed Elfadil, O.; Mundi, M.S.; Abdelmagid, M.G.; Patel, A.; Patel, N.; Martindale, R. Butyrate: More Than a Short Chain Fatty Acid. Curr. Nutr. Rep. 2023, 12, 255–262. [Google Scholar] [CrossRef]
- Mueller, N.T.; Differding, M.K.; Zhang, M.; Maruthur, N.M.; Juraschek, S.P.; Miller, E.R.; Appel, L.J.; Yeh, H.-C. Metformin Affects Gut Microbiome Composition and Function and Circulating Short-Chain Fatty Acids: A Randomized Trial. Diabetes Care 2021, 44, 1462–1471. [Google Scholar] [CrossRef]
- Popa, G.T. Ingrith Miron Pediatrie Volumul 2, 2nd ed.; UMF Iași: Iași, Romania, 2023; ISBN 978-606-544-906-0. [Google Scholar]
- Mo, X.; Shen, L.; Wang, X.; Ni, W.; Li, L.; Xia, L.; Liu, H.; Cheng, R.; Wen, L.; Xu, J.; et al. Melatonin Mitigates Sarcopenic Obesity via Microbiota and Short-Chain Fatty Acids: Evidence From Epidemiologic and In Vivo Studies. J. Cachexia Sarcopenia Muscle 2025, 16, e13869. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Zhu, H.; Chen, S.; Du, M.; Wan, X.; Liu, Y.; Hu, S.; Xu, Y. Anti-Obesity and Gut Microbiota Regulation Effects of Phospholipids from the Eggs of Crab, Portunus Trituberculatus, in High Fat Diet-Fed Mice. Mar. Drugs 2022, 20, 411. [Google Scholar] [CrossRef]
- Luqman, A.; Hassan, A.; Ullah, M.; Naseem, S.; Ullah, M.; Zhang, L.; Din, A.U.; Ullah, K.; Ahmad, W.; Wang, G. Role of the intestinal microbiome and its therapeutic intervention in cardiovascular disorder. Front. Immunol. 2024, 15, 1321395. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Khachatryan, L.G.; Younis, N.K.; Mustafa, M.A.; Ahmad, N.; Athab, Z.H.; Polyanskaya, A.V.; Kasanave, E.V.; Mirzaei, R.; Karampoor, S. Microbiota-derived short chain fatty acids in pediatric health and diseases: From gut development to neuroprotection. Front. Microbiol. 2024, 15, 1456793. [Google Scholar] [CrossRef]
- Recharla, N.; Geesala, R.; Shi, X.-Z. Gut Microbial Metabolite Butyrate and Its Therapeutic Role in Inflammatory Bowel Disease: A Literature Review. Nutrients 2023, 15, 2275. [Google Scholar] [CrossRef]
- Firoozi, D.; Masoumi, S.J.; Mohammad-Kazem Hosseini Asl, S.; Labbe, A.; Razeghian-Jahromi, I.; Fararouei, M.; Lankarani, K.B.; Dara, M. Effects of short-chain fatty acid-butyrate supplementation on expression of circadian-clock genes, sleep quality, and inflammation in patients with active ulcerative colitis: A double-blind randomized controlled trial. Lipids Health Dis. 2024, 23, 216. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, Z.; Ye, B.; Ma, J.H.; Ji, S.; Sheng, W.; Ye, S.; Ou, Y.; Peng, Y.; Yang, X.; et al. Sodium butyrate ameliorates diabetic retinopathy in mice via the regulation of gut microbiota and related short-chain fatty acids. J. Transl. Med. 2023, 21, 451. [Google Scholar] [CrossRef]
- Chadchan, S.B.; Popli, P.; Ambati, C.R.; Tycksen, E.; Han, S.J.; Bulun, S.E.; Putluri, N.; Biest, S.W.; Kommagani, R. Gut microbiota–derived short-chain fatty acids protect against the progression of endometriosis. Life Sci. Alliance 2021, 4, e202101224. [Google Scholar] [CrossRef]
- Zhou, Y.; Xie, L.; Schröder, J.; Schuster, I.S.; Nakai, M.; Sun, G.; Sun, Y.B.Y.; Mariño, E.; Degli-Esposti, M.A.; Marques, F.Z.; et al. Dietary Fiber and Microbiota Metabolite Receptors Enhance Cognition and Alleviate Disease in the 5xFAD Mouse Model of Alzheimer’s Disease. J. Neurosci. 2023, 43, 6460–6475. [Google Scholar] [CrossRef] [PubMed]
- Karunaratne, T.B.; Okereke, C.; Seamon, M.; Purohit, S.; Wakade, C.; Sharma, A. Niacin and Butyrate: Nutraceuticals Targeting Dysbiosis and Intestinal Permeability in Parkinson’s Disease. Nutrients 2020, 13, 28. [Google Scholar] [CrossRef]
- Wang, F.; Qian, F.; Zhang, Q.; Zhao, J.; Cen, J.; Zhang, J.; Zhou, J.; Luo, M.; Jia, C.; Rong, X.; et al. The reduced SCFA-producing gut microbes are involved in the inflammatory activation in Kawasaki disease. Front. Immunol. 2023, 14, 1124118. [Google Scholar] [CrossRef] [PubMed]
- Leong, K.S.W.; Jayasinghe, T.N.; Wilson, B.C.; Derraik, J.G.B.; Albert, B.B.; Chiavaroli, V.; Svirskis, D.M.; Beck, K.L.; Conlon, C.A.; Jiang, Y.; et al. Effects of Fecal Microbiome Transfer in Adolescents With Obesity: The Gut Bugs Randomized Controlled Trial. JAMA Netw. Open 2020, 3, e2030415. [Google Scholar] [CrossRef]
- Leong, K.S.W.; Jayasinghe, T.N.; Derraik, J.G.B.; Albert, B.B.; Chiavaroli, V.; Svirskis, D.M.; Beck, K.L.; Conlon, C.A.; Jiang, Y.; Schierding, W.; et al. Protocol for the Gut Bugs Trial: A randomised double-blind placebo-controlled trial of gut microbiome transfer for the treatment of obesity in adolescents. BMJ Open 2019, 9, e026174. [Google Scholar] [CrossRef]
- Yadegar, A.; Bar-Yoseph, H.; Monaghan, T.M.; Pakpour, S.; Severino, A.; Kuijper, E.J.; Smits, W.K.; Terveer, E.M.; Neupane, S.; Nabavi-Rad, A.; et al. Fecal microbiota transplantation: Current challenges and future landscapes. Clin. Microbiol. Rev. 2024, 37, e0006022. [Google Scholar] [CrossRef] [PubMed]
- Porcari, S.; Benech, N.; Valles-Colomer, M.; Segata, N.; Gasbarrini, A.; Cammarota, G.; Sokol, H.; Ianiro, G. Key determinants of success in fecal microbiota transplantation: From microbiome to clinic. Cell Host Microbe 2023, 31, 712–733. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, W.; Zhang, F. The Next Generation Fecal Microbiota Transplantation: To Transplant Bacteria or Virome. Adv. Sci. 2023, 10, 2301097. [Google Scholar] [CrossRef] [PubMed]
- Borka Balas, R.; Meliț, L.E.; Lupu, A.; Lupu, V.V.; Mărginean, C.O. Prebiotics, Probiotics, and Synbiotics—A Research Hotspot for Pediatric Obesity. Microorganisms 2023, 11, 2651. [Google Scholar] [CrossRef] [PubMed]
- Nicolucci, A.C.; Reimer, R.A. Prebiotics as a modulator of gut microbiota in paediatric obesity. Pediatr. Obes. 2017, 12, 265–273. [Google Scholar] [CrossRef]
- Bozzi Cionci, N.; Baffoni, L.; Gaggìa, F.; Di Gioia, D. Therapeutic Microbiology: The Role of Bifidobacterium breve as Food Supplement for the Prevention/Treatment of Paediatric Diseases. Nutrients 2018, 10, 1723. [Google Scholar] [CrossRef]
- Visuthranukul, C.; Sriswasdi, S.; Tepaamorndech, S.; Chamni, S.; Leelahavanichkul, A.; Joyjinda, Y.; Aksornkitti, V.; Chomtho, S. Enhancing gut microbiota and microbial function with inulin supplementation in children with obesity. Int. J. Obes. 2024, 48, 1696–1704. [Google Scholar] [CrossRef]
- Koller, A.M.; Săsăran, M.O.; Mărginean, C.O. Small Intestinal Bacterial Overgrowth and Pediatric Obesity—A Systematic Review. Nutrients 2025, 17, 1499. [Google Scholar] [CrossRef]
- Khongtan, S.; Sivamaruthi, B.; Thangaleela, S.; Kesika, P.; Bharathi, M.; Sirilun, S.; Choeisoongnern, T.; Peerajan, S.; Sittiprapaporn, P.; Chaiyasut, C. The Influence of Probiotic Supplementation on the Obesity Indexes, Neuroinflammatory and Oxidative Stress Markers, Gut Microbial Diversity, and Working Memory in Obese Thai Children. Foods 2023, 12, 3890. [Google Scholar] [CrossRef]
- Kilic Yildirim, G.; Dinleyici, M.; Vandenplas, Y.; Dinleyici, E.C. Effects of synbiotic supplementation on intestinal microbiota composition in children and adolescents with exogenous obesity: (Probesity-2 trial). Gut Pathog. 2023, 15, 36. [Google Scholar] [CrossRef]
- Cani, P.D.; Possemiers, S.; Van de Wiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.; Neyrinck, A.; Lambert, D.M.; et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009, 58, 1091–1103. [Google Scholar] [CrossRef]
- Wang, Y.; Salonen, A.; Jian, C. Can prebiotics help tackle the childhood obesity epidemic? Front. Endocrinol. 2023, 14, 1178155. [Google Scholar] [CrossRef]
- Rodriguez, J.; Delzenne, N.M. Modulation of the gut microbiota-adipose tissue-muscle interactions by prebiotics. J. Endocrinol. 2021, 249, R1–R23. [Google Scholar] [CrossRef]
- Gan, D.; Chen, J.; Tang, X.; Xiao, L.; Martoni, C.J.; Leyer, G.; Huang, G.; Li, W. Impact of a probiotic chewable tablet on stool habits and microbial profile in children with functional constipation: A randomized controlled clinical trial. Front. Microbiol. 2022, 13, 985308. [Google Scholar] [CrossRef]
- Hurley, K.F.; Fitzpatrick, E.A.; Xie, J.; Urquhart, S.; Farion, K.J.; Gouin, S.; Schuh, S.; Poonai, N.; Freedman, S.B. Predictors of Adherence to Short-Course Probiotics Among Children with Gastroenteritis who are Enrolled in a Clinical Trial. Clin. Investig. Med. 2023, 46, E15–E23. [Google Scholar] [CrossRef]
- Depoorter, L.; Vandenplas, Y. Probiotics in Pediatrics. A Review and Practical Guide. Nutrients 2021, 13, 2176. [Google Scholar] [CrossRef] [PubMed]
- Danilova, N.A.; Abdulkhakov, S.R.; Grigoryeva, T.V.; Markelova, M.I.; Vasilyev, I.Y.; Boulygina, E.A.; Ardatskaya, M.D.; Pavlenko, A.V.; Tyakht, A.V.; Odintsova, A.K.; et al. Markers of dysbiosis in patients with ulcerative colitis and Crohn’s disease. Ter. Arkhiv 2019, 91, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Nishida, A.; Fujimoto, T.; Fujii, M.; Shioya, M.; Imaeda, H.; Inatomi, O.; Bamba, S.; Sugimoto, M.; Andoh, A. Reduced Abundance of Butyrate-Producing Bacteria Species in the Fecal Microbial Community in Crohn’s Disease. Digestion 2016, 93, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, D.; Li, D.; Zeng, F.; Chen, C.; Bai, F. Microbiome characterization of patients with Crohn disease and the use of fecal microbiota transplantation: A review. Medicine 2025, 104, e41262. [Google Scholar] [CrossRef]
- Xu, H.-M.; Zhao, H.-L.; Guo, G.-J.; Xu, J.; Zhou, Y.-L.; Huang, H.-L.; Nie, Y.-Q. Characterization of short-chain fatty acids in patients with ulcerative colitis: A meta-analysis. BMC Gastroenterol. 2022, 22, 117. [Google Scholar] [CrossRef]
- Zakerska-Banaszak, O.; Tomczak, H.; Gabryel, M.; Baturo, A.; Wolko, L.; Michalak, M.; Malinska, N.; Mankowska-Wierzbicka, D.; Eder, P.; Dobrowolska, A.; et al. Dysbiosis of gut microbiota in Polish patients with ulcerative colitis: A pilot study. Sci. Rep. 2021, 11, 2166. [Google Scholar] [CrossRef]
- Gargari, G.; Mantegazza, G.; Taverniti, V.; Gardana, C.; Valenza, A.; Rossignoli, F.; Barbaro, M.R.; Marasco, G.; Cremon, C.; Barbara, G.; et al. Fecal short-chain fatty acids in non-constipated irritable bowel syndrome: A potential clinically relevant stratification factor based on catabotyping analysis. Gut Microbes 2023, 15, 2274128. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Wu, J.; Xin, L.; Yu, C.; Shen, Z. The Role of Short Chain Fatty Acids in Irritable Bowel Syndrome. J. Neurogastroenterol. Motil. 2022, 28, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Okumura, S.; Konishi, Y.; Narukawa, M.; Sugiura, Y.; Yoshimoto, S.; Arai, Y.; Sato, S.; Yoshida, Y.; Tsuji, S.; Uemura, K.; et al. Gut bacteria identified in colorectal cancer patients promote tumourigenesis via butyrate secretion. Nat. Commun. 2021, 12, 5674. [Google Scholar] [CrossRef] [PubMed]
- Hodgkinson, K.; El Abbar, F.; Dobranowski, P.; Manoogian, J.; Butcher, J.; Figeys, D.; Mack, D.; Stintzi, A. Butyrate’s role in human health and the current progress towards its clinical application to treat gastrointestinal disease. Clin. Nutr. 2023, 42, 61–75. [Google Scholar] [CrossRef]
- Gasaly, N.; Hermoso, M.A.; Gotteland, M. Butyrate and the Fine-Tuning of Colonic Homeostasis: Implication for Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2021, 22, 3061. [Google Scholar] [CrossRef]
- Kalyanaraman, B.; Cheng, G.; Hardy, M. The role of short-chain fatty acids in cancer prevention and cancer treatment. Arch. Biochem. Biophys. 2024, 761, 110172. [Google Scholar] [CrossRef]
- Son, M.-Y.; Cho, H.-S. Anticancer Effects of Gut Microbiota-Derived Short-Chain Fatty Acids in Cancers. J. Microbiol. Biotechnol. 2023, 33, 849–856. [Google Scholar] [CrossRef]
- Feitelson, M.A.; Arzumanyan, A.; Medhat, A.; Spector, I. Short-chain fatty acids in cancer pathogenesis. Cancer Metastasis Rev. 2023, 42, 677–698. [Google Scholar] [CrossRef]
- González-Bosch, C.; Zunszain, P.A.; Mann, G.E. Control of Redox Homeostasis by Short-Chain Fatty Acids: Implications for the Prevention and Treatment of Breast Cancer. Pathogens 2023, 12, 486. [Google Scholar] [CrossRef] [PubMed]
- Młynarska, E.; Barszcz, E.; Budny, E.; Gajewska, A.; Kopeć, K.; Wasiak, J.; Rysz, J.; Franczyk, B. The Gut-Brain-Microbiota Connection and Its Role in Autism Spectrum Disorders. Nutrients 2025, 17, 1135. [Google Scholar] [CrossRef]
- Dicks, L.M.T. Gut Bacteria and Neurotransmitters. Microorganisms 2022, 10, 1838. [Google Scholar] [CrossRef]
- Wiefels, M.D.; Furar, E.; Eshraghi, R.S.; Mittal, J.; Memis, I.; Moosa, M.; Mittal, R.; Eshraghi, A.A. Targeting Gut Dysbiosis and Microbiome Metabolites for the Development of Therapeutic Modalities for Neurological Disorders. Curr. Neuropharmacol. 2024, 22, 123–139. [Google Scholar] [CrossRef]
- Kadiyska, T.; Vassilev, D.; Tourtourikov, I.; Ciurinskiene, S.; Madzharova, D.; Savcheva, M.; Stoynev, N.; Mileva-Popova, R.; Tafradjiiska-Hadjiolova, R.; Mitev, V. Age-Dependent Gut Microbiome Dysbiosis in Autism Spectrum Disorder and the Role of Key Bacterial Ratios. Nutrients 2025, 17, 1775. [Google Scholar] [CrossRef]
- van de Wouw, M.; Wang, Y.; Workentine, M.L.; Vaghef-Mehrabani, E.; Dewey, D.; Reimer, R.A.; Tomfohr-Madsen, L.; Giesbrecht, G.F. Associations Between the Gut Microbiota and Internalizing Behaviors in Preschool Children. Psychosom. Med. 2022, 84, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Bruun, C.F.; Haldor Hansen, T.; Vinberg, M.; Kessing, L.V.; Coello, K. Associations between short-chain fatty acid levels and mood disorder symptoms: A systematic review. Nutr. Neurosci. 2024, 27, 899–912. [Google Scholar] [CrossRef]
- Vitetta, L.; Bambling, M.; Strodl, E. Probiotics and Commensal Bacteria Metabolites Trigger Epigenetic Changes in the Gut and Influence Beneficial Mood Dispositions. Microorganisms 2023, 11, 1334. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Cao, H.; Baranova, A.; Zhang, F. Evaluating Causal Effects of Gut Microbiome on Bipolar Disorder. Bipolar Disord. 2025, 1–6. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C.; Yuan, B.; Liu, L.; Zhang, H.; Zhu, M.; Chai, H.; Peng, J.; Huang, Y.; Zhou, S.; et al. Akkermansia muciniphila and its metabolite propionic acid maintains neuronal mitochondrial division and autophagy homeostasis during Alzheimer’s disease pathologic process via GPR41 and GPR43. Microbiome 2025, 13, 16. [Google Scholar] [CrossRef]
- Marizzoni, M.; Coppola, L.; Festari, C.; Luongo, D.; Salamone, D.; Naviglio, D.; Soricelli, A.; Mirabelli, P.; Salvatore, M.; Cattaneo, A.; et al. Circulating short chain fatty acids in Alzheimer’s disease: A cross-sectional observational study. J. Alzheimer’s Dis. 2025, 106, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.-X.; Wang, F.; Liu, J.-Y.; Liu, C.-F. Relationship Between Short-chain Fatty Acids and Parkinson’s Disease: A Review from Pathology to Clinic. Neurosci. Bull. 2024, 40, 500–516. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Ouyang, L.; Li, D.; Li, Z.; Yuan, L.; Fan, L.; Liao, A.; Li, J.; Wei, Y.; Yang, Z.; et al. Short-chain fatty acids in patients with schizophrenia and ultra-high risk population. Front. Psychiatry 2022, 13, 977538. [Google Scholar] [CrossRef] [PubMed]
- Paniagua, G.; Couce-Sánchez, M.; González-Blanco, L.; Sabater, C.; García-Fernández, A.; Rodríguez-Revuelta, J.; Sáiz, P.A.; Bobes, J.; Margolles, A.; García-Portilla, M.P. Comparative analysis of gut microbiome-derived short-chain fatty acids in patients with severe mental disorder: Insights from schizophrenia and bipolar disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2025, 138, 111345. [Google Scholar] [CrossRef]
- Saadh, M.J.; Ahmed, H.H.; Kareem, R.A.; Sanghvi, G.; Ganesan, S.; Agarwal, M.; Kaur, P.; Taher, W.M.; Alwan, M.; Jawad, M.J.; et al. Short-chain fatty acids in Huntington’s disease: Mechanisms of action and their therapeutic implications. Pharmacol. Biochem. Behav. 2025, 249, 173972. [Google Scholar] [CrossRef]
- Yang, L.L.; Stiernborg, M.; Skott, E.; Gillberg, T.; Landberg, R.; Giacobini, M.; Lavebratt, C. Lower plasma concentrations of short-chain fatty acids (SCFAs) in patients with ADHD. J. Psychiatr. Res. 2022, 156, 36–43. [Google Scholar] [CrossRef]
- Boonchooduang, N.; Louthrenoo, O.; Likhitweerawong, N.; Kunasol, C.; Thonusin, C.; Sriwichaiin, S.; Nawara, W.; Chattipakorn, N.; Chattipakorn, S.C. Impact of psychostimulants on microbiota and short-chain fatty acids alterations in children with attention-deficit/hyperactivity disorder. Sci. Rep. 2025, 15, 3034. [Google Scholar] [CrossRef]
| Authors | Study Type | Main Objective | Outcome | Integrative Interpretation | Key Limitation |
|---|---|---|---|---|---|
| Ky Young Cho et al. [91], 2021 | Observational and intervention (obese children, 7–18 years, 2-month weight-change program) | To investigate changes in gut microbiota composition, richness, and predicted functional profiles in obese children undergoing lifestyle modification (diet + physical activity). | Obese children showed dysbiotic baseline vs. controls; in the “fat loss” group after intervention: altered composition and predicted functional pathways (e.g., nitrate reduction, aspartate superpathway) though diversity changes were limited. | Lifestyle modification in childhood obesity can modify microbial composition and functional potential, suggesting microbiota plasticity and a pathway by which behavioral change may mediate metabolic risk. | Short intervention (2 months) with modest sample and no randomized control; predicted functions (16S) rather than shotgun/functional assays limit causal inference. |
| Chonnikant Visuthranukul et al. [92], 2022 | Cross-sectional study (obese Thai children 7–15 years) | To compare the gut microbiome of obese children vs. healthy controls and examine associations with lifestyle activity, adiposity, and metabolic profiles. | Obese children had lower abundance of Bacteroidetes and Actinobacteria, altered beta-diversity; lifestyle and adiposity metrics correlated with microbiota composition. | The link between lifestyle behavior, adiposity, and gut microbiota in obese youth highlights a behavior–microbiome–metabolism axis, emphasizing that modifiable habits shape microbial profiles and hence metabolic risk. | Cross-sectional design cannot establish causality between lifestyle, microbiota, and obesity. |
| Li S. et al. [93], 2025 | Observational study (children with obesity vs. normal weight) | To evaluate associations between gut microbiota composition and fecal SCFA concentrations in obese children. | Obese children showed depleted butyrate-producing bacteria (e.g., Oscillibacter, Alistipes) and elevated Gram-negative bacteria (Proteobacteria, Escherichia coli), with lower plasma butyrate/isobutyrate and higher caproate levels linked to obesity. | The depletion of butyrate- and propionate-producing bacteria indicates reduced fermentative capacity and energy imbalance. The strong correlation between SCFA alterations and metabolic markers supports the microbiota–SCFA–obesity axis as a key pathogenic mechanism in childhood obesity. | Single time-point observational design; diet/medication not tightly controlled; fecal SCFAs are correlative and may not reflect systemic exposure. |
| Gyarmati P. et al. [94], 2021 | Cross-sectional study (83 children, 5–12 years, normal weight vs. overweight/severe obesity) | To evaluate microbial diversity, F/B ratio, and SCFA levels by weight classification. | Increased Proteobacteria, decreased Verrucomicrobia, no significant F/B ratio change; dysbiosis associated with severe obesity. | Higher Proteobacteria abundance with obesity severity implies inflammation-driven dysbiosis, highlighting that BMI alone does not predict microbial shifts. | Modest sample (n = 83) and cross-sectional design limit generalizability and causal inference; potential dietary and socioeconomic confounding. |
| Petraroli M. et al. [95], 2021 | Systematic review (children and adults) | To review microbiota, F/B ratio, and SCFA roles in obesity. | Increased F/B ratio, reduced Bacteroides; SCFAs regulate energy metabolism in obesity. | Evidence across age groups confirms SCFA-mediated energy regulation; inconsistent F/B ratios limit its utility as a biomarker. | High heterogeneity (populations, sequencing methods, obesity definitions) and publication bias; reliance on F/B ratio oversimplifies community structure. |
| Indiani C.M.D.S.P. et al. [96], 2018 | Systematic review (children) | To analyze F/B ratio and bacterial changes in childhood obesity. | Increased F/B ratio, reduced Bacteroides and Bifidobacterium; dysbiosis impacts metabolism. | Early studies identified increased F/B and reduced Bacteroides/Bifidobacterium, supporting metabolic impact despite methodological variability. | Older, small, and heterogeneous primary studies; limited longitudinal/interventional evidence; potential publication bias. |
| Burananat T. et al. [97], 2025 | Observational study (pediatric populations) | To evaluate microbiota, F/B ratio, and metabolic risk in obesity severity. | Variable Actinobacteriota and Bacteroidota abundance, altered F/B ratio; dysbiosis linked to metabolic risk. | Variations in Actinobacteriota/Bacteroidota balance with obesity severity suggest community composition, not single taxa, determines metabolic risk. | Observational design with heterogeneous cohorts and methods; residual confounding (diet, antibiotics, puberty) and limited external validity beyond study populations. |
| Liang C. et al. [98], 2020 | Observational and in vitro/in vivo study (99 children, 5–15 years, Harbin, China) | To identify microbiota differences, F/B ratio, and test anti-obesity bacterial strains. | Decreased F/B ratio, reduced Lactobacillus and Bifidobacterium, increased Akkermansia in obesity; anti-obesity effects of strains in HFD mice. | Decreased Lactobacillus and Bifidobacterium with increased Akkermansia indicate microbial imbalance; validation in mice suggests potential causal and probiotic effects. | Human data are cross-sectional; causality inferred from mouse models may not translate to children; single-region cohort limits generalizability. |
| Araujo D.S. et al. [99], 2020 | Observational study (46 children, 5–13 years, Korea) | To evaluate salivary microbiota, bacterial diversity, and gingival health in obese adolescents. | Reduced Bacteroides in salivary microbiota; no F/B ratio or SCFA data; linked to gingival inflammation. | Salivary dysbiosis mirrors gut microbial imbalance, indicating shared dietary and inflammatory drivers across body sites. | Small sample and cross-sectional design; salivary microbiota may not reflect gut; oral hygiene/dental disease and diet are potential confounders. |
| Li X.-M. et al. [100], 2024 | Cohort study (children, 16S rRNA sequencing) | To associate childhood obesity with microbiota diversity, F/B ratio, and bacterial changes. | Decreased diversity, reduced Bacteroides, variable F/B ratio; dysbiosis correlated with obesity. | Consistently reduced diversity and Bacteroides confirm a core dysbiotic signature; variability in the F/B ratio reflects diet, geography, and methodology. | Observational cohort without intervention; 16S rRNA limits taxonomic/functional resolution; unmeasured diet/antibiotics may confound associations. |
| Jiang L.F. et al. [101], 2022 | Observational study (school-aged children) | To associate microbiota diversity, F/B ratio, and obesity. | Decreased diversity, reduced Bacteroides, altered F/B ratio; dysbiosis linked to higher BMI. | Reduced microbial diversity and Bacteroides consistently associate with higher BMI, confirming diversity loss as a hallmark of pediatric obesity. | Cross-sectional design; potential reporting bias for lifestyle/diet; limited functional (metabolite) data. |
| Ismail H.M. et al. [102], 2025 | Observational study (youth with type 1 diabetes and obesity) | To evaluate microbiota changes, F/B ratio, and SCFA levels in obese youth with type 1 diabetes. | Reduced Bifidobacterium, altered F/B ratio, and lower SCFA levels in obesity; dysbiosis linked to metabolic impact. | Type 1 diabetes with obesity intensifies dysbiosis and SCFA loss, showing endocrine–microbiota interdependence in metabolic outcomes. | Disease- and treatment-related confounding (autoimmunity, insulin therapy); cross-sectional design; generalizability limited beyond T1D. |
| Da Silva C.C. et al. [103], 2020 | Observational study (obese children) | To analyze gastrointestinal microbiota, F/B ratio, and bacterial changes in childhood obesity. | Increased Firmicutes, reduced Bifidobacterium, higher F/B ratio; dysbiosis linked to fat accumulation. | Enrichment of Firmicutes and loss of Bifidobacterium support an energy-harvesting, pro-inflammatory microbial pattern associated with obesity. | Cross-sectional, likely modest sample; potential confounding by diet/antibiotics; emphasis on F/B ratio limits granularity. |
| Yuan X. et al. [104], 2021 | Observational study (Chinese children and adolescents, obese with/without insulin resistance) | To compare gut microbiota in obese children with and without insulin resistance, focusing on bacterial diversity and the F/B ratio. | Lower microbial diversity, increased Firmicutes, and altered F/B ratio in obese children with insulin resistance; dysbiosis linked to metabolic disorders. | Dysbiosis with increased Firmicutes and reduced diversity suggests energy-harvesting capacity; insulin resistance modulates the microbial–metabolic relationship beyond the F/B ratio alone. | Cross-sectional comparisons cannot address directionality; potential confounding from diet, medications, and puberty status. |
| Carrizales-Sánchez A.K. et al. [105], 2021 | Systematic review (children) | To analyze microbiota, F/B ratio, and SCFA roles in metabolic syndrome and obesity in children. | Increased Firmicutes, reduced Bifidobacterium, altered F/B ratio; SCFAs influence energy metabolism in metabolic syndrome. | Microbiota shifts (increased Firmicutes, decreased Bifidobacterium) and SCFA dysregulation underline a mechanistic link between gut metabolism and pediatric metabolic syndrome. | Heterogeneous case definitions and methods; few longitudinal/interventional pediatric studies; risk of publication and language bias. |
| Moran-Ramos S. et al. [106], 2020 | Population-based study (children and early adolescents) | To evaluate environmental/intrinsic factors shaping microbiota, F/B ratio, and metabolic health. | Increased Firmicutes, reduced Bifidobacterium, altered F/B ratio; low-fiber diet linked to dysbiosis and metabolic risk. | Low-fiber diet and lifestyle factors appear central to microbiota alteration, emphasizing environmental modulation of obesity-related dysbiosis. | Predominantly cross-sectional associations; diet largely self-reported; socioeconomic and geographic factors may confound effects. |
| Wang L. et al. [107], 2024 | Observational study (children with obesity and precocious puberty) | To analyze microbiota changes, F/B ratio, and bacterial diversity in obesity and precocious puberty. | Increased Firmicutes, decreased Bacteroides, altered F/B ratio; dysbiosis linked to metabolic risk and precocious puberty. | Altered F/B ratio and reduced Bacteroides in obese children with precocious puberty suggest a microbiome–endocrine axis influencing early maturation. | Cross-sectional design with small subgroup sizes; hormonal status and treatment confounders; limited mechanistic assessment. |
| Qian Y. et al. [108], 2024 | Observational study (children with obesity and precocious puberty) | To analyze microbiota influence on obesity-associated precocious puberty and F/B ratio. | Dysbiosis with reduced Bacteroides, altered F/B ratio; impacts hypothalamic–gonadal axis and precocious puberty. | Altered microbiota composition in obese children with precocious puberty supports a link between gut dysbiosis and hormonal regulation. | Mechanistic claims drawn from correlative data; F/B ratio is a coarse metric; lack of longitudinal follow-up/intervention. |
| Gallardo-Becerra L. et al. [109], 2020 | Metatranscriptomic study (Mexican children with obesity/metabolic syndrome) | To define secrebiome, microbiota profile, and SCFA levels in obesity. | Reduced abundance of 9 bacteria, lower SCFA levels, altered F/B ratio; linked to metabolic syndrome. | Reduced SCFAs and bacterial abundance reveal a functional alignment between microbial metabolism and metabolic syndrome pathology. | Likely small, cross-sectional cohort; high technical variability in RNA-based assays; diet/timing of sampling may influence expression profiles. |
| Wei Y. et al. [110], 2021 | Observational study (children, body fat distribution) | To assess microbiota composition, F/B ratio, and SCFA levels in relation to body fat distribution. | Reduced Bacteroides and SCFA levels (e.g., butyrate) linked to higher body fat; altered F/B ratio; dysbiosis tied to metabolic risk. | Reduced Bacteroides and SCFA (butyrate) levels correlate with adiposity, suggesting a metabolic dependency between fermentation capacity and fat accumulation. | Cross-sectional design; fecal SCFAs may not capture host absorption; imaging/body-fat assessment and diet variability may confound results. |
| Nandy D. et al. [111], 2022 | Metabolomic study (2-year-old children) | To analyze butyrate levels, microbiota, and weight outcomes. | Lower butyrate levels, altered microbiota, and F/B ratio; dysbiosis linked to early obesity. | Early-life butyrate deficiency and dysbiosis precede obesity onset, highlighting a preventive window in early childhood. | Small cohort and observational design; rapid developmental/dietary changes at age 2 confound associations; limited taxonomic resolution alongside metabolomics. |
| Jaimes J.D. et al. [112], 2021 | Metabolomic and microbiomic study (children and adolescents) | To evaluate the stool metabolome, microbiota, and SCFA levels in obesity. | Reduced SCFA levels, lower Bacteroides, altered F/B ratio; dysbiosis linked to metabolic risk. | Combined metabolomic and microbiome data confirm that reduced SCFAs and Bacteroides underpin metabolic risk in pediatric obesity. | Cross-sectional design; 1H-NMR metabolomics has limited compound coverage; multi-site variability and diet not fully controlled. |
| Zhang S. & Dang Y. et al. [113], 2022 | Observational study | To analyze the F/B ratio as a biological parameter of microbiome structure in obesity. | Altered F/B ratio linked to dysbiosis and obesity. | The F/B ratio remains consistently altered in obesity but is context-dependent, serving as a coarse indicator rather than a diagnostic marker. | F/B ratio is an oversimplified metric sensitive to methods, age, and diet; observational evidence lacks mechanistic/causal confirmation. |
| Age Group | Intervention Type | Key Clinical Applications | Supporting Authors |
|---|---|---|---|
| Infants (0–2 years) | Probiotics | May reduce obesity risk through early microbiota modulation; e.g., perinatal supplementation reduces overweight up to 10 years by promoting beneficial bacteria and SCFAs. Potential for preventing metabolic complications via gut barrier enhancement. | Borka Balas et al., 2023 [188]; Petraroli et al., 2021 [95]; Bozzi Cionci et al., 2018 [190]. |
| Infants (0–2 years) | Synbiotics | Improves intestinal function in short bowel syndrome; supports gut barrier and reduces infections, indirectly aiding metabolic health. | Bozzi Cionci et al., 2018 [190]. |
| Children (3–12 years) | Prebiotics | Inulin supplementation (e.g., 30 g/day) increases microbiota diversity, promotes SCFA-producing bacteria (e.g., Bifidobacterium, Agathobacter), and correlates with reduced BMI Z-score and improved fat-free mass; no direct weight loss but potential for metabolic improvements. Oligofructose-enriched inulin reduces body fat, triglycerides, and inflammation. | Visuthranukul et al., 2024 [191]; Borka Balas et al., 2023 [188]; Wang et al., 2023 [182]; Koller et al., 2025 [192]. |
| Children (3–12 years) | Probiotics | Multi-strain probiotics (e.g., Lactobacillus, Bifidobacterium) reduce BMI, cholesterol, and inflammation and improve insulin sensitivity; e.g., B. breve strains improve glucose metabolism and weight management. Reduces NAFLD severity and liver enzymes. No consistent weight loss but aids comorbidities. | Borka Balas et al., 2023 [188]; Khongtan et al., 2023 [193]; Bozzi Cionci et al., 2018 [190]; Petraroli et al., 2021 [95]; Solito et al., 2021 [137]; Koller et al., 2025 [192]; Facchin et al., 2024 [35] (butyrate example). |
| Children (3–12 years) | Synbiotics | Multi-strain synbiotics (e.g., with FOS) improve anthropometric indices (e.g., waist–height ratio, BMI), body composition, and microbiota (e.g., increased Bacteroidetes); reduces inflammation and metabolic risks. | Borka Balas et al., 2023 [188]; Kilic Yildirim et al., 2023 [194]; Koller et al., 2025 [192]; Petraroli et al., 2021 [95]. |
| Children (3–12 years) | SCFAs/Gut Modulation | SCFAs (e.g., butyrate supplementation) improve glycemic control and obesity markers when combined with therapy; modulation via diet/probiotics increases SCFA levels, reduces neuroinflammation, and enhances antioxidant capacity. Altered SCFA profiles linked to dysbiosis; potential for reducing inflammation and metabolic dysfunction. | Facchin et al., 2024 [35]; Khongtan et al., 2023 [193]; Petraroli et al., 2021 [95]; Koller et al., 2025 [192]. |
| Adolescents (13–18 years) | Prebiotics | Limited consistent effects on weight; may reduce BMI, fat, and inflammation via microbiota shifts (e.g., increased Bifidobacterium). Potential adjunct for metabolic markers. | Wang et al., 2023 [182]; Koller et al., 2025 [192]. |
| Adolescents (13–18 years) | Probiotics | Improves insulin sensitivity, reduces BMI and waist circumference; e.g., B. breve strains enhance metabolic homeostasis and reduce inflammation. Aids NAFLD and metabolic syndrome. | Borka Balas et al., 2023 [188]; Bozzi Cionci et al., 2018 [190]; Solito et al., 2021 [137]; Petraroli et al., 2021 [95]; Koller et al., 2025 [192]. |
| Adolescents (13–18 years) | Synbiotics | Reduces BMI Z-score, waist circumference, and anthropometric indices; modulates microbiota (e.g., lower Firmicutes/Bacteroidetes ratio) for reduced energy harvest and inflammation. | Borka Balas et al., 2023 [188]; Kilic Yildirim et al., 2023 [194]; Koller et al., 2025 [192]; Petraroli et al., 2021 [95]. |
| Adolescents (13–18 years) | SCFAs/Gut Modulation | SCFAs modulate appetite, insulin sensitivity, and inflammation; potential for NAFLD improvement via microbiota restoration. | Petraroli et al., 2021 [95]; Koller et al., 2025 [192]. |
| Adults (>18 years) | Prebiotics | Increases SCFA production, improves insulin sensitivity and glucose metabolism, and reduces inflammation; e.g., inulin-type fructans for T2D and metabolic syndrome. | Facchin et al., 2024 [35]; Petraroli et al., 2021 [95]. |
| Adults (>18 years) | Probiotics | Multi-strain formulas elevate SCFAs, improve cardiometabolic health, and reduce inflammation; mixed effects on T2D. | Facchin et al., 2024 [35]; Petraroli et al., 2021 [95]. |
| Adults (>18 years) | Synbiotics | Reduces appetite, improves metabolic profiles; potential for obesity management. | Facchin et al., 2024 [35]; Petraroli et al., 2021 [95]. |
| Adults (>18 years) | SCFAs/Gut Modulation | Propionate/butyrate supplementation prevents weight gain, improves insulin sensitivity, and attenuates atherosclerosis; acetate shows no benefit. Gut modulation (e.g., FMT) enhances insulin sensitivity in metabolic syndrome. | Facchin et al., 2024 [35]; Petraroli et al., 2021 [95]. |
| General/Animal | Prebiotics | In mice, oligofructose reduces permeability, inflammation, and adiposity via GLP-2; improves gut barrier and metabolic endotoxemia. Potential for obesity prevention. | Cani et al., 2009 [195]; Facchin et al., 2024 [35]. |
| General/Animal | Probiotics | In mice, B. breve suppresses weight gain and improves lipid metabolism. | Bozzi Cionci et al., 2018 [190]. |
| General/Animal | Synbiotics | Enhanced effects in models for metabolic health. | Facchin et al., 2024 [35]. |
| General/Animal | SCFAs/Gut Modulation | SCFAs regulate energy homeostasis, appetite, and inflammation via receptors; higher levels associated with obesity but protective in models. | Facchin et al., 2024 [35]; Petraroli et al., 2021 [95]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pînzariu, A.C.; Leonte, S.M.; Trofin, A.G.; Trandafir, L.M.; Moscalu, M.; Manole, L.M.; Moscalu, R.; Lazăr, C.I.; Confederat, L.G.; Vlăsceanu, V.I.; et al. Gut Microbiota and Short-Chain Fatty Acids: Key Factors in Pediatric Obesity and Therapeutic Targets. Int. J. Mol. Sci. 2025, 26, 11503. https://doi.org/10.3390/ijms262311503
Pînzariu AC, Leonte SM, Trofin AG, Trandafir LM, Moscalu M, Manole LM, Moscalu R, Lazăr CI, Confederat LG, Vlăsceanu VI, et al. Gut Microbiota and Short-Chain Fatty Acids: Key Factors in Pediatric Obesity and Therapeutic Targets. International Journal of Molecular Sciences. 2025; 26(23):11503. https://doi.org/10.3390/ijms262311503
Chicago/Turabian StylePînzariu, Alin Constantin, Sebastian Marian Leonte, Alexandra Gabriela Trofin, Laura Mihaela Trandafir, Mihaela Moscalu, Lorena Mihaela Manole, Roxana Moscalu, Cristina Iuliana Lazăr, Luminita Georgeta Confederat, Vlad Ionuț Vlăsceanu, and et al. 2025. "Gut Microbiota and Short-Chain Fatty Acids: Key Factors in Pediatric Obesity and Therapeutic Targets" International Journal of Molecular Sciences 26, no. 23: 11503. https://doi.org/10.3390/ijms262311503
APA StylePînzariu, A. C., Leonte, S. M., Trofin, A. G., Trandafir, L. M., Moscalu, M., Manole, L. M., Moscalu, R., Lazăr, C. I., Confederat, L. G., Vlăsceanu, V. I., Soroceanu, R. P., Timofte, D. V., Şerban, D. N., & Şerban, I. L. (2025). Gut Microbiota and Short-Chain Fatty Acids: Key Factors in Pediatric Obesity and Therapeutic Targets. International Journal of Molecular Sciences, 26(23), 11503. https://doi.org/10.3390/ijms262311503








