Coordination of a Dirhodium(II) Center to Methionine and Cysteine Side Chains: Evidence from X-Ray Structure of the Adduct Formed by Dirhodium Tetraacetate with a C-Phycocyanin
Abstract
1. Introduction

2. Results and Discussion
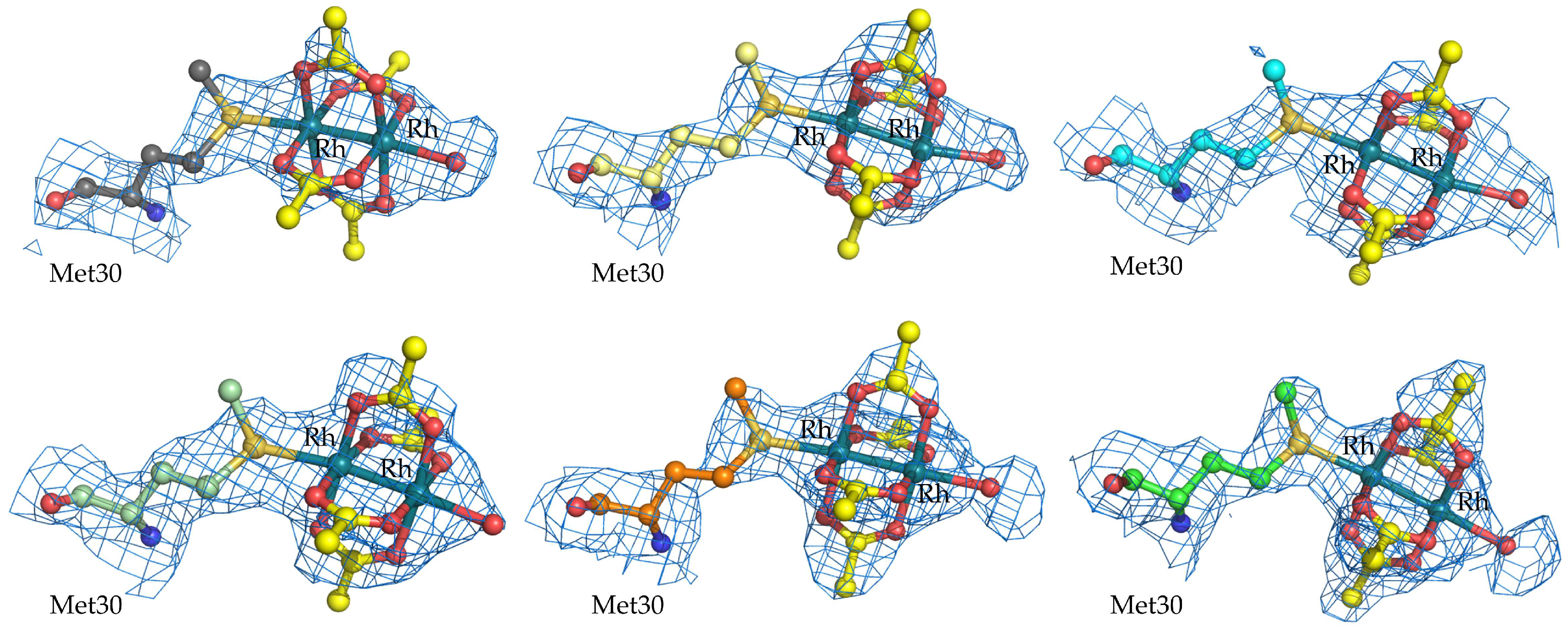
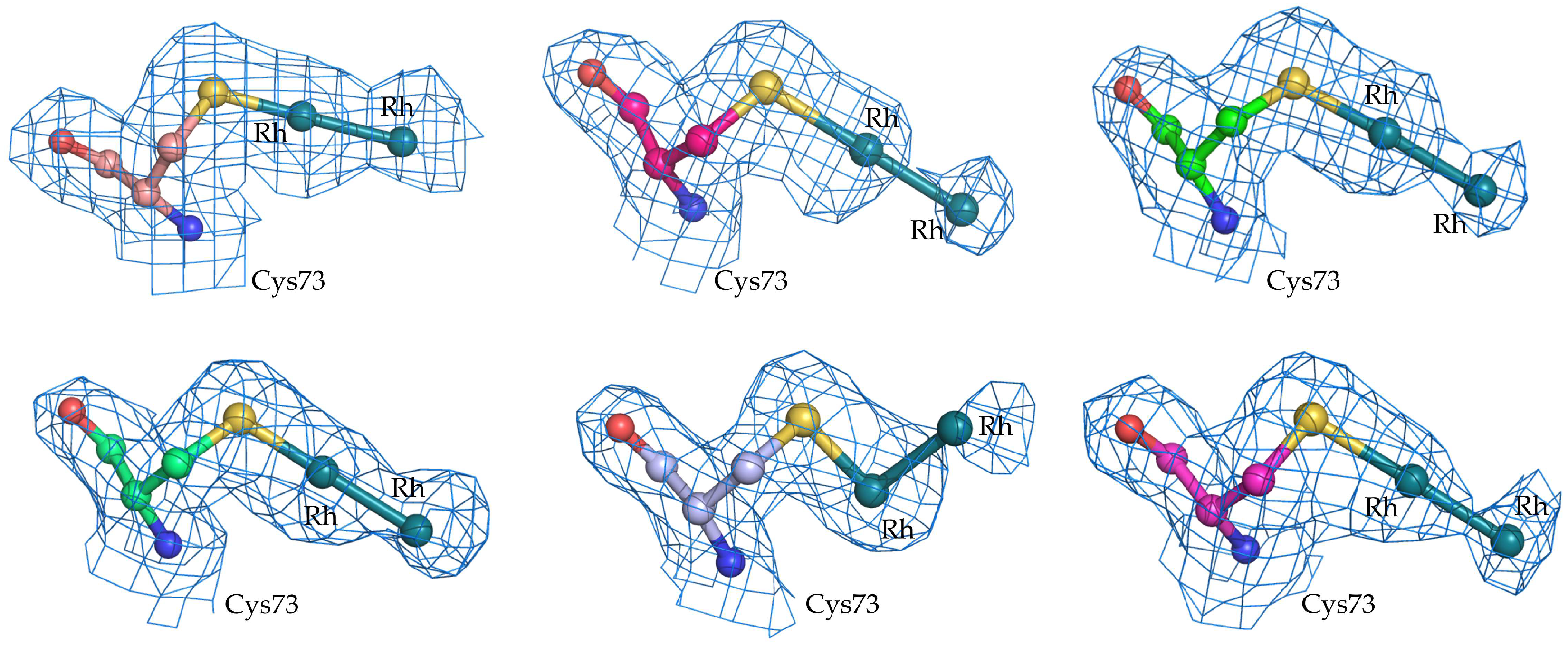
2.1. Structure of the diRh/GpPC Adduct
2.2. Comparison with Literature Data
2.3. In Solution Secondary Structure and Thermal Stability Analysis of diRh/GpPC Adduct
3. Materials and Methods
3.1. Crystallization of GpPC and Formation of the diRh/GpPC Adduct
3.2. Data Collection, Structure Solution, and Refinement
3.3. Circular Dichroism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| bpy | Bipyridine |
| diRh | dirhodium |
| ESI-MS | Electrospray ionization mass spectrometry |
| EXAFS | extended X-ray absorption fine structure |
| GpPC | C-phycocyanin from Galdiera phlegrea |
| HEWL | Hen Egg White Lysozyme |
| HSA | Human serum albumin |
| MS | Mass spectrometry |
| PCB | phycocyanobilin |
| PDB | Protein Data Bank |
References
- Hrdina, R. Dirhodium(II,II) Paddlewheel Complexes. Eur. J. Inorg. Chem. 2021, 2021, 501–528. [Google Scholar] [CrossRef]
- Cotton, F.A.; Murillo, C.A.; Walton, R.A. (Eds.) Multiple Bonds Between Metal Atoms; Springer: Boston, MA, USA, 2005; ISBN 978-0-387-25084-7. [Google Scholar]
- Paulissen, R.; Reimlinger, H.; Hayez, E.; Hubert, A.J.; Teyssié, P. Transition Metal Catalysed Reactions of Diazocompounds—II Insertion in the Hydroxylic Bond. Tetrahedron Lett. 1973, 14, 2233–2236. [Google Scholar] [CrossRef]
- Breslow, R.; Gellman, S.H. Intramolecular Nitrene Carbon-Hydrogen Insertions Mediated by Transition-Metal Complexes as Nitrogen Analogs of Cytochrome P-450 Reactions. J. Am. Chem. Soc. 1983, 105, 6728–6729. [Google Scholar] [CrossRef]
- Davies, H.M.L.; Manning, J.R. Catalytic C–H Functionalization by Metal Carbenoid and Nitrenoid Insertion. Nature 2008, 451, 417–424. [Google Scholar] [CrossRef]
- Davies, H.M.L.; Beckwith, R.E.J. Catalytic Enantioselective C−H Activation by Means of Metal−Carbenoid-Induced C−H Insertion. Chem. Rev. 2003, 103, 2861–2904. [Google Scholar] [CrossRef]
- Liu, W.; Kuang, Y.; Wang, Z.; Zhu, J.; Wang, Y. Dirhodium(II)-Catalyzed [3 + 2] Cycloaddition of N-Arylaminocyclopropane with Alkyne Derivatives. Beilstein J. Org. Chem. 2019, 15, 542–550. [Google Scholar] [CrossRef]
- Doyle, M.P.; Devora, G.A.; Nefedov, A.O.; High, K.G. Addition/Elimination in the Rhodium(II) Perfluorobutyrate Catalyzed Hydrosilylation of 1-Alkenes. Rhodium Hydride Promoted Isomerization and Hydrogenation. Organometallics 1992, 11, 549–555. [Google Scholar] [CrossRef]
- Doyle, M.P.; High, K.G.; Nesloney, C.L.; Clayton, T.W.; Lin, J. Rhodium(II) Perfluorobutyrate Catalyzed Hydrosilylation of 1-Alkynes. Trans Addition and Rearrangement to Allylsilanes. Organometallics 1991, 10, 1225–1226. [Google Scholar] [CrossRef]
- Kataoka, Y.; Yano, N.; Handa, M.; Kawamoto, T. Intrinsic Hydrogen Evolution Capability and a Theoretically Supported Reaction Mechanism of a Paddlewheel-Type Dirhodium Complex. Dalton Trans. 2019, 48, 7302–7312. [Google Scholar] [CrossRef]
- Davies, H.M.L.; Liao, K. Dirhodium Tetracarboxylates as Catalysts for Selective Intermolecular C–H Functionalization. Nat. Rev. Chem. 2019, 3, 347–360. [Google Scholar] [CrossRef]
- Keipour, H.; Carreras, V.; Ollevier, T. Recent Progress in the Catalytic Carbene Insertion Reactions into the Silicon–Hydrogen Bond. Org. Biomol. Chem. 2017, 15, 5441–5456. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.; Miel, H.; Ring, A.; Slattery, C.N.; Maguire, A.R.; McKervey, M.A. Modern Organic Synthesis with α-Diazocarbonyl Compounds. Chem. Rev. 2015, 115, 9981–10080. [Google Scholar] [CrossRef]
- Maas, G. New Syntheses of Diazo Compounds. Angew. Chem. Int. Ed. 2009, 48, 8186–8195. [Google Scholar] [CrossRef] [PubMed]
- Szilvágyi, G.; Hollósi, M.; Tölgyesi, L.; Frelek, J.; Majer, Z. Dirhodium Complexes of Amino Acid Derivatives: Separation and Characterization by Circular Dichroism Spectroscopy. Tetrahedron Asymmetry 2008, 19, 2594–2599. [Google Scholar] [CrossRef]
- Srivastava, P.; Yang, H.; Ellis-Guardiola, K.; Lewis, J.C. Engineering a Dirhodium Artificial Metalloenzyme for Selective Olefin Cyclopropanation. Nat. Commun. 2015, 6, 7789. [Google Scholar] [CrossRef]
- Lo Schiavo, S.; Cardiano, P.; Donato, N.; Latino, M.; Neri, G. A Dirhodium(II,II) Complex as a Highly Selective Molecular Material for Ammonia Detection: QCM Studies. J. Mater. Chem. 2011, 21, 18034. [Google Scholar] [CrossRef]
- Hilderbrand, S.A.; Lim, M.H.; Lippard, S.J. Dirhodium Tetracarboxylate Scaffolds as Reversible Fluorescence-Based Nitric Oxide Sensors. J. Am. Chem. Soc. 2004, 126, 4972–4978. [Google Scholar] [CrossRef]
- Zyngier, S.; Kimura, E.; Najjar, R. Antitumor Effects of Rhodium (II) Citrate in Mice Bearing Ehrlich Tumors. Braz. J. Med. Biol. Res. Rev. Bras. Pesqui. Medicas E Biol. 1989, 22, 397–401. [Google Scholar]
- Reibscheid, E.M.; Zyngier, S.; Maria, D.A.; Mistrone, R.J.; Sinisterra, R.D.; Couto, L.G.; Najjar, R. Antitumor Effects of Rhodium (II) Complexes on Mice Bearing Ehrlich Tumors. Braz. J. Med. Biol. Res. Rev. Bras. Pesqui. Med. E Biol. 1994, 27, 91–94. [Google Scholar]
- Chifotides, H.T.; Fu, P.K.-L.; Dunbar, K.R.; Turro, C. Effect of Equatorial Ligands of Dirhodium(II,II) Complexes on the Efficiency and Mechanism of Transcription Inhibition in Vitro. Inorg. Chem. 2004, 43, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Erck, A.; Rainen, L.; Whileyman, J.; Chang, I.-M.; Kimball, A.P.; Bear, J. Studies of Rhodium(II) Carboxylates as Potential Antitumor Agents. Exp. Biol. Med. 1974, 145, 1278–1283. [Google Scholar] [CrossRef]
- Chifotides, H.T.; Dunbar, K.R. Interactions of Metal−Metal-Bonded Antitumor Active Complexes with DNA Fragments and DNA. Acc. Chem. Res. 2005, 38, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Chifotides, H.T.; Koomen, J.M.; Kang, M.; Tichy, S.E.; Dunbar, K.R.; Russell, D.H. Binding of DNA Purine Sites to Dirhodium Compounds Probed by Mass Spectrometry. Inorg. Chem. 2004, 43, 6177–6187. [Google Scholar] [CrossRef]
- Bear, J.L.; Gray, H.B.; Rainen, L.; Chang, I.M.; Howard, R.; Serio, G.; Kimball, A.P. Interaction of Rhodium(II) Carboxylates with Molecules of Biologic Importance. Cancer Chemother. Rep. 1975, 59, 611–620. [Google Scholar]
- Dunham, S.U.; Chifotides, H.T.; Mikulski, S.; Burr, A.E.; Dunbar, K.R. Covalent Binding and Interstrand Cross-Linking of Duplex DNA by Dirhodium(II,II) Carboxylate Compounds. Biochemistry 2005, 44, 996–1003. [Google Scholar] [CrossRef]
- Aoki, K.; Salam, M.A. Interligand Interactions Affecting Specific Metal Bonding to Nucleic Acid Bases. A Case of [Rh2(OAc)4], [Rh2(HNOCCF3)4], and [Rh2(OAc)2(HNOCCF3)2] toward Purine Nucleobases and Nucleosides. Inorganica Chim. Acta 2002, 339, 427–437. [Google Scholar] [CrossRef]
- Kang, M.; Chifotides, H.T.; Dunbar, K.R. 2D NMR Study of the DNA Duplex d(CTCTC*A*ACTTCC)·d(GGAAGTTGAGAG) Cross-Linked by the Antitumor-Active Dirhodium(II,II) Unit at the Cytosine−Adenine Step. Biochemistry 2008, 47, 2265–2276. [Google Scholar] [CrossRef]
- Aguirre, J.D.; Angeles-Boza, A.M.; Chouai, A.; Pellois, J.-P.; Turro, C.; Dunbar, K.R. Live Cell Cytotoxicity Studies: Documentation of the Interactions of Antitumor Active Dirhodium Compounds with Nuclear DNA. J. Am. Chem. Soc. 2009, 131, 11353–11360. [Google Scholar] [CrossRef]
- Cotton, F.A.; DeBoer, B.G.; LaPrade, M.D.; Pipal, J.R.; Ucko, D.A. The Crystal and Molecular Structures of Dichromium Tetraacetate Dihydrate and Dirhodium Tetraacetate Dihydrate. Acta Crystallogr. B 1971, 27, 1664–1671. [Google Scholar] [CrossRef]
- Tito, G.; Troisi, R.; Ferraro, G.; Geri, A.; Massai, L.; Messori, L.; Sica, F.; Merlino, A. Dirhodium Tetraacetate Binding to a B-DNA Double Helical Dodecamer Probed by X-Ray Crystallography and Mass Spectrometry. Dalton Trans. 2023, 52, 6992–6996. [Google Scholar] [CrossRef] [PubMed]
- Chválová, K.; Brabec, V.; Kašpárková, J. Mechanism of the Formation of DNA–Protein Cross-Links by Antitumor Cisplatin. Nucleic Acids Res. 2007, 35, 1812–1821. [Google Scholar] [CrossRef]
- Troisi, R.; Tito, G.; Ferraro, G.; Sica, F.; Massai, L.; Geri, A.; Cirri, D.; Messori, L.; Merlino, A. On the Mechanism of Action of Arsenoplatins: Arsenoplatin-1 Binding to a B-DNA Dodecamer. Dalton Trans. 2024, 53, 3476–3483. [Google Scholar] [CrossRef]
- Popp, B.V.; Chen, Z.; Ball, Z.T. Sequence-Specific Inhibition of a Designed Metallopeptide Catalyst. Chem. Commun. 2012, 48, 7492. [Google Scholar] [CrossRef] [PubMed]
- Loreto, D.; Merlino, A. The Interaction of Rhodium Compounds with Proteins: A Structural Overview. Coord. Chem. Rev. 2021, 442, 213999. [Google Scholar] [CrossRef]
- Enriquez Garcia, A.; Jalilehvand, F.; Niksirat, P.; Gelfand, B.S. Methionine Binding to Dirhodium(II) Tetraacetate. Inorg. Chem. 2018, 57, 12787–12799. [Google Scholar] [CrossRef]
- Głaszczka, R.; Jaźwiński, J.; Kamieński, B.; Kamińska, M. Adducts of Rhodium(II) Tetraacylates with Methionine and Its Derivatives: 1H and 13C Nuclear Magnetic Resonance Spectroscopy and Chiral Recognition. Tetrahedron Asymmetry 2010, 21, 2346–2355. [Google Scholar] [CrossRef]
- Wong, D.L.; Stillman, M.J. Destructive Interactions of Dirhodium(II) Tetraacetate with β Metallothionein Rh1a. Chem. Commun. 2016, 52, 5698–5701. [Google Scholar] [CrossRef]
- Scheller, J.S.; Irvine, G.W.; Stillman, M.J. Unravelling the Mechanistic Details of Metal Binding to Mammalian Metallothioneins from Stoichiometric, Kinetic, and Binding Affinity Data. Dalton Trans. 2018, 47, 3613–3637. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.L.; Stillman, M.J. Metallothionein: An Aggressive Scavenger—The Metabolism of Rhodium(II) Tetraacetate (Rh2 (CH3 CO2)4). ACS Omega 2018, 3, 16314–16327. [Google Scholar] [CrossRef] [PubMed]
- Trynda-Lemiesz, L.; Pruchnik, F.P. Studies on the Interaction between Human Serum Albumin and [Rh2(OAc)2(Bpy)2(H2O)2](OAc)2. J. Inorg. Biochem. 1997, 66, 187–192. [Google Scholar] [CrossRef]
- Jalilehvand, F.; Enriquez Garcia, A.; Niksirat, P.; Finfrock, Y.Z.; Gelfand, B.S. Binding of Histidine and Human Serum Albumin to Dirhodium(II) Tetraacetate. J. Inorg. Biochem. 2021, 224, 111556. [Google Scholar] [CrossRef]
- Chen, J.; Kostic, N.M. Binuclear Transition-Metal Complexes as New Reagents for Selective Cross-Linking of Proteins. Coordination of Cytochrome c to Dirhodium(II).Mu.-Tetraacetate. Inorg. Chem. 1988, 27, 2682–2687. [Google Scholar] [CrossRef]
- Loreto, D.; Ferraro, G.; Merlino, A. Unusual Structural Features in the Adduct of Dirhodium Tetraacetate with Lysozyme. Int. J. Mol. Sci. 2021, 22, 1496. [Google Scholar] [CrossRef] [PubMed]
- Loreto, D.; Esposito, A.; Demitri, N.; Guaragna, A.; Merlino, A. Reactivity of a Fluorine-Containing Dirhodium Tetracarboxylate Compound with Proteins. Dalton Trans. 2022, 51, 3695–3705. [Google Scholar] [CrossRef] [PubMed]
- Loreto, D.; Esposito, A.; Demitri, N.; Guaragna, A.; Merlino, A. Digging into Protein Metalation Differences Triggered by Fluorine Containing-Dirhodium Tetracarboxylate Analogues. Dalton Trans. 2022, 51, 7294–7304. [Google Scholar] [CrossRef]
- Tito, G.; Ferraro, G.; Merlino, A. Dirhodium Tetraacetate Binding to Lysozyme at Body Temperature. Int. J. Mol. Sci. 2025, 26, 6582. [Google Scholar] [CrossRef]
- Ferraro, G.; Pratesi, A.; Messori, L.; Merlino, A. Protein Interactions of Dirhodium Tetraacetate: A Structural Study. Dalton Trans. 2020, 49, 2412–2416. [Google Scholar] [CrossRef]
- Loreto, D.; Maity, B.; Morita, T.; Nakamura, H.; Merlino, A.; Ueno, T. Cross-Linked Crystals of Dirhodium Tetraacetate/RNase A Adduct Can Be Used as Heterogeneous Catalysts. Inorg. Chem. 2023, 62, 7515–7524. [Google Scholar] [CrossRef]
- Loreto, D.; Fasulo, F.; Muñoz-García, A.B.; Pavone, M.; Merlino, A. Unexpected Imidazole Coordination to the Dirhodium Center in a Protein Environment: Insights from X-Ray Crystallography and Quantum Chemistry. Inorg. Chem. 2022, 61, 8402–8405. [Google Scholar] [CrossRef]
- Ferraro, G.; Merlino, A. Investigation of Metallodrug/Protein Interaction by X-Ray Crystallography and Complementary Biophysical Techniques. Inorg. Chem. Front. 2025, 12, 3345–3366. [Google Scholar] [CrossRef]
- Sarrou, I.; Feiler, C.G.; Falke, S.; Peard, N.; Yefanov, O.; Chapman, H. C-Phycocyanin as a Highly Attractive Model System in Protein Crystallography: Unique Crystallization Properties and Packing-Diversity Screening. Acta Crystallogr. Sect. Struct. Biol. 2021, 77, 224–236. [Google Scholar] [CrossRef]
- Ferraro, G.; Imbimbo, P.; Marseglia, A.; Lucignano, R.; Monti, D.M.; Merlino, A. X-Ray Structure of C-Phycocyanin from Galdieria Phlegrea: Determinants of Thermostability and Comparison with a C-Phycocyanin in the Entire Phycobilisome. Biochim. Biophys. Acta BBA-Bioenerg. 2020, 1861, 148236. [Google Scholar] [CrossRef]
- Ferraro, G.; Imbimbo, P.; Marseglia, A.; Illiano, A.; Fontanarosa, C.; Amoresano, A.; Olivieri, G.; Pollio, A.; Monti, D.M.; Merlino, A. A Thermophilic C-Phycocyanin with Unprecedented Biophysical and Biochemical Properties. Int. J. Biol. Macromol. 2020, 150, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Tolbatov, I.; Marrone, A. Reaction of Dirhodium and Diruthenium Paddlewheel Tetraacetate Complexes with Nucleophilic Protein Sites: A Computational Study. Inorganica Chim. Acta 2022, 530, 120684. [Google Scholar] [CrossRef]
- Vonrhein, C.; Flensburg, C.; Keller, P.; Sharff, A.; Smart, O.; Paciorek, W.; Womack, T.; Bricogne, G. Data Processing and Analysis with the autoPROC Toolbox. Acta Crystallogr. Sect. D Biol. Crystallogr. 2011, 67, 293–302. [Google Scholar] [CrossRef]
- McCoy, A.J.; Grosse-Kunstleve, R.W.; Adams, P.D.; Winn, M.D.; Storoni, L.C.; Read, R.J. Phaser Crystallographic Software. J. Appl. Crystallogr. 2007, 40, 658–674. [Google Scholar] [CrossRef] [PubMed]
- Murshudov, G.N.; Skubák, P.; Lebedev, A.A.; Pannu, N.S.; Steiner, R.A.; Nicholls, R.A.; Winn, M.D.; Long, F.; Vagin, A.A. REFMAC 5 for the Refinement of Macromolecular Crystal Structures. Acta Crystallogr. Sect. D Biol. Crystallogr. 2011, 67, 355–367. [Google Scholar] [CrossRef]
- Winn, M.D.; Ballard, C.C.; Cowtan, K.D.; Dodson, E.J.; Emsley, P.; Evans, P.R.; Keegan, R.M.; Krissinel, E.B.; Leslie, A.G.W.; McCoy, A.; et al. Overview of the CCP 4 Suite and Current Developments. Acta Crystallogr. Sect. D Biol. Crystallogr. 2011, 67, 235–242. [Google Scholar] [CrossRef]
- Emsley, P.; Cowtan, K. Coot: Model-Building Tools for Molecular Graphics. Acta Crystallogr. Sect. D Biol. Crystallogr. 2004, 60, 2126–2132. [Google Scholar] [CrossRef] [PubMed]
- Enriquez Garcia, A.; Jalilehvand, F.; Niksirat, P. Reactions of Rh2 (CH3 COO)4 with Thiols and Thiolates: A Structural Study. J. Synchrotron Radiat. 2019, 26, 450–461. [Google Scholar] [CrossRef]



| PDB Deposition Code | 9T05 |
|---|---|
| Crystallization conditions | 0.10–0.20 M magnesium chloride, 0.10 M Hepes, pH 6.5, and 9.0–10.0% (w/v) PEG 4000 |
| Crystallization temperature (K) | 293 |
| Soaking temperature (K) | 293 |
| Data collection | |
| Data collection temperature (K) | 100 |
| Wavelength (Å) | 0.9537 |
| a (Å)/b (Å)/c (Å) | 60.586/188.500/207.212 |
| (αβ) per asymmetric unit | 6 (12 chains: A, C, E, G, I, K for the α-chains and B, D, F, H, J, L for the β-chains) |
| Resolution range (Å) | 188.5–2.17 (2.31–2.17) |
| Unique reflections | 102,746 (5138) |
| Completeness (%) | 94.2 (63.6) |
| Redundancy | 12.9 (11.6) |
| † Rmerge (%) | 0.340 (2.13) |
| Rpim | 0.100 (0.656) |
| Average I/σ(I) | 6.6 (1.4) |
| CC1/2 | 0.996 (0.564) |
| Anomalous completeness (%) | 94.2 (65.5) |
| Anomalous redundancy | 6.7 (6.0) |
| Refinement | |
| Resolution range (Å) | 139.83–2.17 |
| N. of reflections (working set) | 97,759 |
| N. of reflections (test set) | 5000 |
| R-factor/R-free (%) | 22.7/26.3 |
| N. of non-H atoms | 16,492 |
| Rh occupancy at Met30β binding site | 0.50/0.50–B/0.50/0.50–D/0.50/0.50–F/0.50/0.50–H/0.50/0.50–J/0.60/0.60–L |
| Rh occupancy at Cys73α binding site | 0.40/0.40–A/0.25/0.25–C/0.30/0.30–E/0.35/0.35–G/0.20/0.20–I/0.25/0.25–K |
| Average B-factors (Å2) All atoms | 35.7 |
| B-factors (Å2) of Rh atoms at Met30β binding site | 44.0/48.8–B/47.7/49.1–D/58.6/59.7–F/43.5/49.6–H/33.8/34.1–J/24.0/26.7–L |
| B-factors (Å2) of Rh atoms at Cys73α binding site | 37.2/62.5–A/47.8/52.8–C/48.4/61.8–E/40.7/65.7–G/30.9/57.0–I/54.0/58.5–K |
| R.m.s. deviations | |
| Bond lengths (Å) | 0.008 |
| Bond angles (°) | 1.73 |
| Ramachandran statistics (Coot analysis) | |
| Favored regions (%)/Outliers(%) | 96.9/0.63 |
| Dirhodium/GpPC Adduct | |||||||
|---|---|---|---|---|---|---|---|
| Chain B | Chain D | Chain F | Chain H | Chain J | Chain L | ||
| Met30β | Rh—Rh (Å) | 2.38 | 2.38 | 2.39 | 2.38 | 2.41 | 2.36 |
| Rh—SD (Å) | 2.47 | 2.39 | 2.51 | 2.51 | 2.44 | 2.44 | |
| Rh—Oeq (Å) | 2.04 ± 0.01 | 2.04 ± 0.01 | 2.05 ± 0.01 | 2.05 ± 0.01 | 2.05 ± 0.01 | 2.04 ± 0.01 | |
| Rh—Oax a (Å) | 2.33 | 2.33 | 2.33 | 2.33 | 2.34 | 2.34 | |
| SD—Rh—Rh (°) | 170.5 | 172.8 | 177.1 | 172.1 | 173.3 | 176.6 | |
| Oeq—Rh—Rh b (°) | 87.4 ± 4.8 | 87.1 ± 6.0 | 88.2 ± 0.7 | 87.8 ± 3.2 | 87.6 ± 2.4 | 86.5 ± 2.8 | |
| Oeq—Rh—SD (°) | 92.2 ± 10.3 | 92.5 ± 7.0 | 91.6 ± 2.5 | 92.0 ± 9.6 | 92.2 ± 7.6 | 93.6 ± 2.3 | |
| Oax a—Rh—Rh (°) | 175.3 | 161.5 | 169.4 | 173.5 | 177.1 | 168.8 | |
| Chain A | Chain C | Chain E | Chain G | Chain I | Chain K | ||
| Cys73α | Rh—Rh (Å) | 2.41 | 2.40 | 2.40 | 2.40 | 2.38 | 2.39 |
| Rh—SG (Å) | 2.13 | 2.47 | 2.26 | 2.14 | 2.08 | 2.47 | |
| SG—Rh—Rh (°) | 179.3 | 179.8 | 177.8 | 178.1 | 90.0 | 178.8 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferraro, G.; Imbimbo, P.; Troisi, R.; Monti, D.M.; Merlino, A. Coordination of a Dirhodium(II) Center to Methionine and Cysteine Side Chains: Evidence from X-Ray Structure of the Adduct Formed by Dirhodium Tetraacetate with a C-Phycocyanin. Int. J. Mol. Sci. 2025, 26, 11492. https://doi.org/10.3390/ijms262311492
Ferraro G, Imbimbo P, Troisi R, Monti DM, Merlino A. Coordination of a Dirhodium(II) Center to Methionine and Cysteine Side Chains: Evidence from X-Ray Structure of the Adduct Formed by Dirhodium Tetraacetate with a C-Phycocyanin. International Journal of Molecular Sciences. 2025; 26(23):11492. https://doi.org/10.3390/ijms262311492
Chicago/Turabian StyleFerraro, Giarita, Paola Imbimbo, Romualdo Troisi, Daria Maria Monti, and Antonello Merlino. 2025. "Coordination of a Dirhodium(II) Center to Methionine and Cysteine Side Chains: Evidence from X-Ray Structure of the Adduct Formed by Dirhodium Tetraacetate with a C-Phycocyanin" International Journal of Molecular Sciences 26, no. 23: 11492. https://doi.org/10.3390/ijms262311492
APA StyleFerraro, G., Imbimbo, P., Troisi, R., Monti, D. M., & Merlino, A. (2025). Coordination of a Dirhodium(II) Center to Methionine and Cysteine Side Chains: Evidence from X-Ray Structure of the Adduct Formed by Dirhodium Tetraacetate with a C-Phycocyanin. International Journal of Molecular Sciences, 26(23), 11492. https://doi.org/10.3390/ijms262311492










