Arthrospira platensis Preserves Uterine Function by Modulating Electromechanical Coupling and Redox Pathways During Resistance Training in Female Rats
Abstract
1. Introduction
2. Results
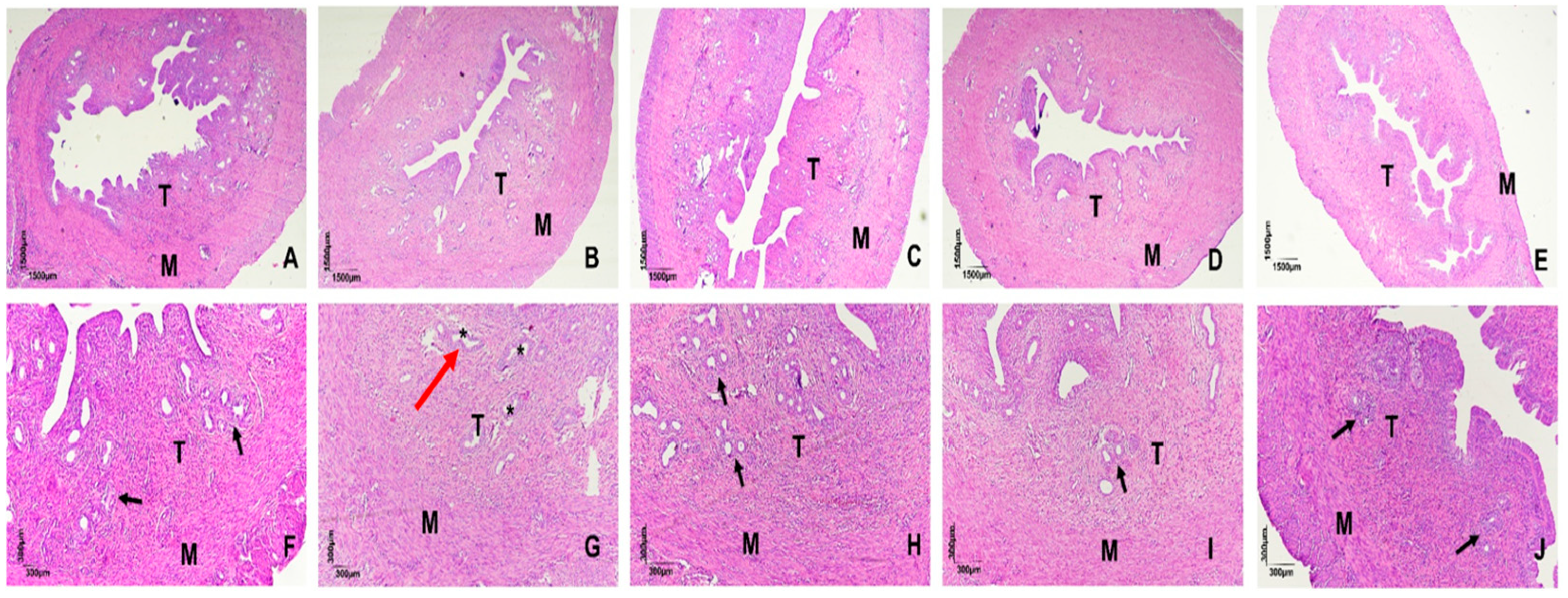
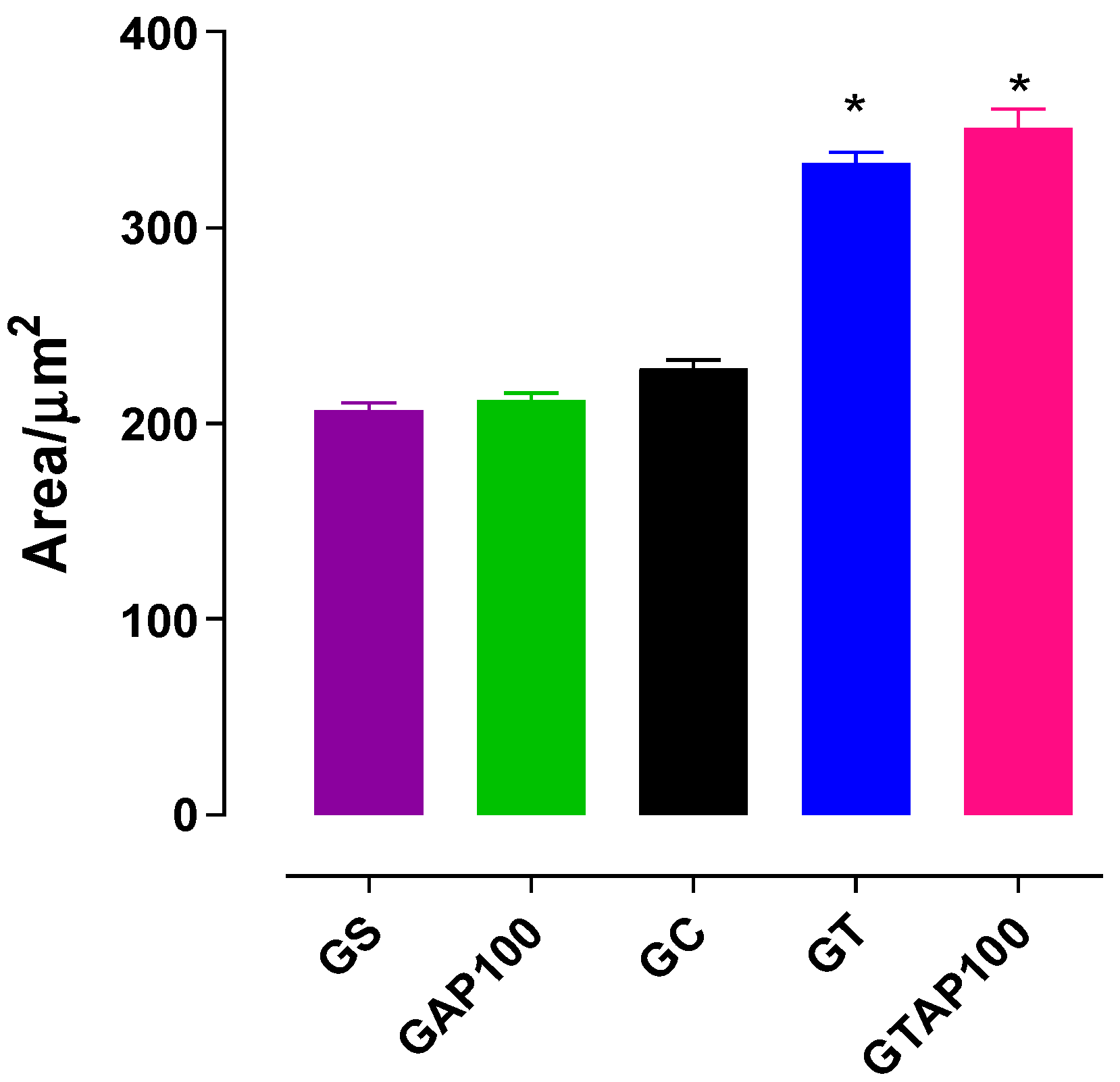
2.1. Histomorphometric Evaluation of Uterine Tissue
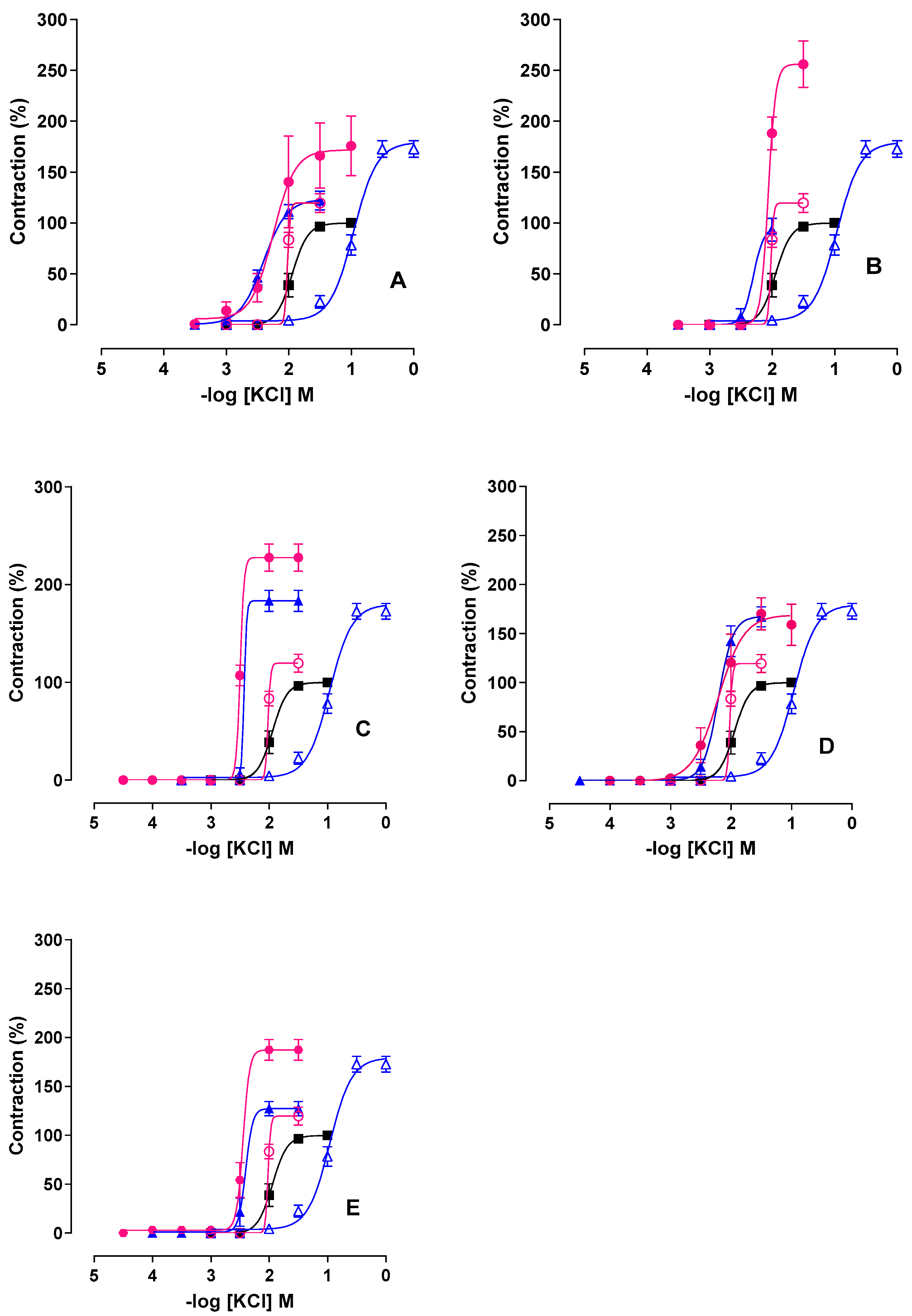
2.2. Effect of PST and A. platensis on KCl-Induced Contractility in the Presence of L-NAME
2.3. Effect of PST and A. platensis in the Presence of Indomethacin
2.4. Combined Effect of L-NAME and Indomethacin
2.5. Effect of NADPH Oxidase Inhibition with Apocynin
2.6. Effect of Tempol (SOD Mimetic) on Contractility
3. Discussion
4. Methods and Materials
4.1. Obtaining and Preparing Arthrospira Platensis
4.2. Animals and Experimental Groups
- 1.
- Sedentary control (GS): Did not undergo either adaptation or training and remained completely sedentary, receiving only saline by gavage.
- 2.
- Sedentary supplemented with A. platensis (GAP100, 100 mg/kg): Did not undergo exercise intervention or an adaptation period and received A. platensis at 100 mg/kg by gavage.
- 3.
- Adapted control (GC): Underwent the one-week adaptation protocol only, which consisted of light water-jumping sessions with reduced overload (50% of body weight) and limited repetitions. This adaptation was designed to familiarize animals with the aquatic environment and apparatus, minimize stress, and prevent confounding acute effects of exercise initiation. Importantly, GC animals did not undergo subsequent progressive strength training, allowing the differentiation between physiological effects of the adaptation process itself and those induced by the full training program.
- 4.
- Trained (GT): Underwent the adaptation week followed by 8 weeks of progressive resistance training and received saline by gavage.
- 5.
- Trained supplemented with A. platensis (GTAP100, 100 mg/kg): Underwent the adaptation week followed by 8 weeks of progressive resistance training and received A. platensis at 100 mg/kg by gavage.
4.3. Progressive Strength Training Program (PST)
4.4. Reagents and Solutions
4.5. Nutrient Solutions
4.6. Histological Analysis
4.7. Investigation of the Preventive Mechanism of A. platensis Supplementation on Contractile Changes Induced by Strength Training
4.7.1. Preparation of Isolated Rat Uterus
4.7.2. Investigation of the Participation of the Nitric Oxide Pathway and Cyclooxygenases
Obtaining Cumulative Concentration–Response Curves to KCl, in the Absence and Presence of L-NAME or Indomethacin
4.7.3. Investigation of the Participation of the Enzymes Nicotinamide Adenine Dinucleotide Phosphate Oxidase and Superoxide Dismutase
Obtaining Cumulative Concentration–Response Curves to KCl, in the Absence and Presence of Apocynin and Tempol
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| AP | Arthrospira platensis |
| AQP | Aquaporins |
| ATP | Adenosine triphosphate |
| CAPES | Coordination for the Improvement of Higher Education Personnel (Brazil) |
| CAT | Catalase |
| CLC3 | Chloride channel 3 |
| CNPq | National Council for Scientific and Technological Development (Brazil) |
| CO2 | Carbon dioxide |
| COX | Cyclooxygenase |
| cPLA2 | Cytosolic phospholipase A2 |
| CYP450 | Cytochrome P450 monooxygenases |
| ERK | Extracellular signal-regulated kinase |
| EtOH | Ethanol |
| FSH | Follicle-stimulating hormone |
| GAP100 | Sedentary group supplemented with A. platensis (100 mg/kg) |
| GC | Control group |
| GS | Saline group |
| GT | Trained group |
| GTAP100 | Trained group supplemented with A. platensis (100 mg/kg) |
| GTSP100 | Trained group supplemented with Spirulina platensis (100 mg/kg) |
| HCl | Hydrochloric acid |
| HIIT | High-intensity interval training |
| IL-1β | Interleukin-1 beta |
| iNOS | Inducible nitric oxide synthase |
| IP3R | Inositol trisphosphate receptor |
| LH | Luteinizing hormone |
| LOX | Lipoxygenase |
| L-NAME | Nω-Nitro-L-arginine methyl ester hydrochloride |
| MAPK | Mitogen-activated protein kinase |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NBF | Neutral buffered formalin |
| NO | Nitric oxide |
| NOS | Nitric oxide synthase |
| O2•− | Superoxide anion |
| PGE2 | Prostaglandin E2 |
| PGF2α | Prostaglandin F2α |
| PLA2 | Phospholipase A2 |
| PVC | Polyvinyl chloride |
| PST | Progressive strength training |
| qPCR | Quantitative polymerase chain reaction |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| SEM | Standard error of the mean |
| SOD | Superoxide dismutase |
| UFPB | Federal University of Paraíba (Brazil) |
| UPA | Animal Production Unit (UFPB) |
| XO | Xanthine oxidase |
References
- McGregor, R.A.; Cameron-Smith, D.; Poppitt, S.D. It is not just muscle mass: A review of muscle quality, composition and metabolism during ageing as determinants of muscle function and mobility in later life. Clin. Nutr. 2014, 33, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Cartee, G.D.; Hepple, R.T.; Bamman, M.M.; Zierath, J.R. Exercise promotes healthy aging of skeletal muscle. Cell Metab. 2016, 23, 1034–1047. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.C.; Ellefsen, S.; Baar, K. Adaptations to endurance and strength training. Cold Spring Harb. Perspect. Med. 2018, 8, a029769. [Google Scholar] [CrossRef]
- Zampieri, S.; Pietrangelo, L.; Loefler, S.; Fruhmann, H.; Vogelauer, M.; Burggraf, S.; Pond, A.; Grim-Stieger, M.; Cvecka, J.; Sedliak, M.; et al. Lifelong physical exercise delays age-associated skeletal muscle decline. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 163–173. [Google Scholar] [CrossRef]
- Dudley, G.A.; Tesch, P.A.; Miller, B.J.; Buchanan, P. Importance of eccentric actions in performance adaptations to resistance training. Aviat. Space Environ. Med. 1991, 62, 543–550. [Google Scholar] [PubMed]
- Powers, S.K.; Radak, Z.; Ji, L.L.; Jackson, M. Reactive oxygen species promote endurance exercise-induced adaptations in skeletal muscles. J. Sport Health Sci. 2024, 13, 780–792. [Google Scholar] [CrossRef]
- Karolkiewicz, J.; Szczêsniak, L.; Deskur-Smielecka, E.; Nowak, A.; Stemplewski, R.; Szeklicki, R. Oxidative stress and antioxidant defense system in healthy, elderly men: Relationship to physical activity. Aging Male. 2003, 6, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.S.; Spagnol, A.R.; Luciano, E.; Leme, J.A.C.A. Influence of aerobic exercise training on serum markers of oxidative stress in diabetic rats. J. Phys. Educ. 2016, 27, e2726. [Google Scholar] [CrossRef]
- Murrell, G.A.; Francis, M.J.; Bromley, L. Modulation of fibroblast proliferation by oxygen free radicals. Biochem. J. 1990, 265, 659–665. [Google Scholar] [CrossRef]
- Warren, M.P.; Perlroth, N.E. The effects of intense exercise on the female reproductive system. J. Endocrinol. 2001, 170, 3–11. [Google Scholar] [CrossRef]
- Soltaninejad, F.; Shafiei, G.; Rafie, F.; Nematollahi-Mahani, S.N.; Pourjafari, F.; Haghpanah, T.; Afarinesh, M.R. Ovarian epigenetics modifications following lifestyle interventions by exercise and alternate-day feeding in letrozole-induced PCOS rats. Sci. Rep. 2025, 15, 25557. [Google Scholar] [CrossRef]
- Di Meo, S.; Iossa, S.; Venditti, P. Skeletal muscle insulin resistance: Role of mitochondria and other ROS sources. J. Endocrinol. 2017, 233, R15–R42. [Google Scholar] [CrossRef] [PubMed]
- De Souza, M.J.; Williams, N.I. Physiological aspects and clinical sequelae of energy deficiency and hypoestrogenism in exercising women. Hum. Reprod. Update 2004, 10, 433–448. [Google Scholar] [CrossRef] [PubMed]
- Belza, B.; Warms, C. Physical activity and exercise in women’s health. Nurs. Clin. N. Am. 2004, 39, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Hamanaka, R.B.; Chandel, N.S. Mitochondrial reactive oxygen species regulate hypoxic signaling. Curr. Opin. Cell Biol. 2009, 21, 894–899. [Google Scholar] [CrossRef]
- Lee, P.; Chandel, N.S.; Simon, M.C. Cellular Adaptation to Hypoxia through Hypoxia Inducible Factors and Beyond. Nat. Rev. Mol. Cell Biol. 2020, 21, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Olson, S.L.; Akbar, R.J.; Gorniak, A.; Fuhr, L.I.; Borahay, M.A. Hypoxia in uterine fibroids: Role in pathobiology and therapeutic opportunities. Oxygen 2024, 4, 236–252. [Google Scholar] [CrossRef]
- Žitkutė, V.; Kvietkauskas, M.; Maskoliūnaitė, V.; Leber, B.; Ramašauskaitė, D.; Strupas, K.; Stiegler, P.; Schemmer, P. Melatonin and Glycine Reduce Uterus Ischemia/Reperfusion Injury in a Rat Model of Warm Ischemia. Int. J. Mol. Sci. 2021, 22, 8373. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, S.; Sharma, R.K. Role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2005, 3, 28. [Google Scholar] [CrossRef]
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Amani, X.; Gupta, S. The effects of oxidative stress on female reproduction: A review. Reprod. Biol. Endocrinol. 2012, 10, 49. [Google Scholar] [CrossRef]
- Lara, L.A.; Ramos, F.K.; Kogure, G.S.; Costa, R.S.; Silva de Sá, M.F.; Ferriani, R.A.; dos Reis, R.M. Impact of Physical Resistance Training on the Sexual Function of Women with Polycystic Ovary Syndrome. J. Sex. Med. 2015, 12, 1584–1590. [Google Scholar] [CrossRef] [PubMed]
- Jourkesh, M.; Soori, R.; Earnest, C.P.; Mirheidari, L.; Ravasi, A.A.; Stannard, S.R.; Monsalves-Alvarez, M. Effects of six weeks of resistance-endurance training on microRNA-29 expression in the heart of ovariectomised rats. Prz. Menopauzalny 2018, 17, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Streuli, I.; Santulli, P.; Chouzenoux, S.; Chapron, C.; Batteux, F. Activation of the MAPK/ERK cell-signaling pathway in uterine smooth muscle cells of women with adenomyosis. Reprod. Sci. 2015, 22, 1549–1560. [Google Scholar] [CrossRef]
- Chen, C.; Zhu, S.; Bai, L.; Sui, M.; Chen, D. The role of formyl peptide receptor 1 in uterine contraction during parturition. Front. Pharmacol. 2021, 12, 696697. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.J.; Geary, M.P.; Hayes, B.C. Intrapartum uterine activity and neonatal outcomes: A systematic review. BMC Pregnancy Childbirth 2020, 20, 532. [Google Scholar] [CrossRef]
- Hellman, K.M.; Yu, P.Y.; Oladosu, F.A.; Segel, C.; Han, A.; Prasad, P.V.; Tu, F.F. The effects of platelet-activating factor on uterine contractility, perfusion, hypoxia, and pain in mice. Reprod. Sci. 2018, 25, 384–394. [Google Scholar] [CrossRef]
- Tian, R.; Li, X.; Su, J.; Yu, H.; Fei, J.; Xu, C.; Du, X.; Yu, B.; Cao, Y.; Yin, Z. Regional uterine contractility differences during pregnancy: The role of hypoxia and ferroptosis in vitro. Life Sci. 2025, 371, 123603. [Google Scholar] [CrossRef]
- Turner, J.M.; Mitchell, M.D.; Kumar, S.S. The physiology of intrapartum fetal compromise at term. Am. J. Obstet. Gynecol. 2020, 222, 17–26. [Google Scholar] [CrossRef]
- Critchley, H.O.; Kelly, R.W.; Baird, D.T.; Brenner, R.M. Regulation of human endometrial function: Mechanisms relevant to uterine bleeding. Reprod. Biol. Endocrinol. 2006, 4 (Suppl. 1), S5. [Google Scholar] [CrossRef]
- Lashley, C.J.; Supik, D.A.; Atkinson, J.T.; Murphy, R.J.; O’Hagan, K.P. Effect of pregnancy on the uterine vasoconstrictor response to exercise in rats. Physiol. Rep. 2015, 3, e12337. [Google Scholar] [CrossRef]
- Ferreira, P.B.; Diniz, A.F.A.; Lacerda Júnior, F.F.; Silva, M.d.C.C.; Cardoso, G.A.; Silva, A.S.; da Silva, B.A. Supplementation with Spirulina platensis Prevents Uterine Diseases Related to Muscle Reactivity and Oxidative Stress in Rats Undergoing Strength Training. Nutrients 2021, 13, 3763. [Google Scholar] [CrossRef]
- Diniz, A.F.A.; Claudino, B.F.O.; Francelino, D.M.C.; Junior, E.B.A.; Ferreira, P.B.; Lacerda-Júnior, F.F.L.; Barros, B.C.; Arruda, R.R.A.; Alves, A.F.; Batista, L.M.; et al. Arthrospira platensis prevents oxidative stress and suppresses IL-1β expression in the ileum of rats fed a hypercaloric diet. J. Funct. Foods 2023, 106, 105586. [Google Scholar] [CrossRef]
- Araujo, L.C.C.; Brito, A.F.; Souza, I.L.L.; Ferreira, P.B.; Vasconcelos, L.H.C.; Silva, A.S.; Silva, B.A. Spirulina platensis Supplementation Coupled to Strength Exercise Improves Redox Balance and Reduces Intestinal Contractile Reactivity in Rat Ileum. Mar. Drugs 2020, 18, 89. [Google Scholar] [CrossRef]
- Souza, I.L.L.d.; Barros, B.C.; Ferreira, E.d.S.; Queiroga, F.R.; Vasconcelos, L.H.C.; Toscano, L.d.L.T.; Silva, A.S.; Silva, P.M.d.; Cavalcante, F.d.A.; Silva, B.A.d. Supplementation with Spirulina platensis Prevents Damage to Rat Erections in a Model of Erectile Dysfunction Promoted by Hypercaloric Diet-Induced Obesity. Mar. Drugs 2022, 20, 467. [Google Scholar] [CrossRef]
- Diniz, A.F.A.; Souza, I.L.L.; Ferreira, E.S.; Carvalho, M.T.L.; Barros, B.C.; Ferreira, P.B.; Silva, M.C.C.; Lacerda-Júnior, F.F.; Toscano, L.L.T.; Silva, A.S.; et al. Potential Therapeutic Role of Dietary Supplementation with Spirulina platensis on the Erectile Function of Obese Rats Fed a Hypercaloric Diet. Oxidative Med. Cell. Longev. 2020, 2020, 3293065. [Google Scholar] [CrossRef] [PubMed]
- Brito, A.F.; Silva, A.S.; Souza, A.A.; Ferreira, P.B.; Souza, I.L.L.; Araujo, L.C.C.; Félix, G.S.; Sampaio, R.S.; Silva, M.C.C.; Tavares, R.L.; et al. Aortic Response to Strength Training and Spirulina platensis Dependent on Nitric Oxide and Antioxidants. Front. Physiol. 2018, 9, 1522. [Google Scholar] [CrossRef] [PubMed]
- Brito, A.F.; Silva, A.S.; Souza, I.L.L.; Pereira, J.C.; Silva, B.A. Intensity of Swimming Exercise Influences Aortic Reactivity in Rats. Braz. J. Med. Biol. Res. 2015, 48, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Kruk, J.; Aboul-Enein, H.Y.; Kładna, A.; Bowser, J.E. Oxidative stress in biological systems and its relation with pathophysiological functions: The effect of physical activity on cellular redox homeostasis. Free Radic. Res. 2019, 53, 497–521. [Google Scholar] [CrossRef]
- Fisher-Wellman, K.H.; Bloomer, R.J. Acute exercise and oxidative stress: A 30 year history. Dyn. Med. 2009, 8, 1. [Google Scholar] [CrossRef]
- Brito, A.F.; Silva, A.S.; de Oliveira, C.V.C.; de Souza, A.A.; Ferreira, P.B.; de Souza, I.L.L.; da Cunha Araujo, L.C.; da Silva Félix, G.; de Souza Sampaio, R.; Tavares, R.L.; et al. Spirulina platensis prevents oxidative stress and inflammation promoted by strength training in rats: Dose-response relation study. Sci. Rep. 2020, 10, 6382. [Google Scholar] [CrossRef]
- Mosavat, M.; Ooi, F.K.; Mohamed, M. Stress hormone and reproductive system in response to honey supplementation combined with different jumping exercise intensities in female rats. BioMed Res. Int. 2014, 2014, 123640. [Google Scholar] [CrossRef] [PubMed]
- Mosavat, M.; Ooi, F.K.; Mohamed, M. Effects of honey supplementation combined with different jumping exercise intensities on bone mass, serum bone metabolism markers and gonadotropins in female rats. BMC Complement. Altern. Med. 2014, 14, 126. [Google Scholar] [CrossRef] [PubMed]
- Lindström, P.; Källskog, O.; Wadström, J.; Persson, A.E. Blood flow distribution during elevated intraperitoneal pressure in the rat. Acta Physiol. Scand. 2003, 177, 149–156. [Google Scholar] [CrossRef]
- McCulloch, P.F. Animal models for investigating the central control of the Mammalian diving response. Front. Physiol. 2012, 3, 169. [Google Scholar] [CrossRef]
- Gilbert, J.S.; Banek, C.T.; Bauer, A.J.; Gingery, A.; Needham, K. Exercise training attenuates placental ischemia-induced hypertension and angiogenic imbalance in the rat. Hypertension 2012, 60, 1545–1551. [Google Scholar] [CrossRef]
- Gilbert, J.S.; Banek, C.T.; Bauer, A.J.; Gingery, A.; Dreyer, H.C. Placental and vascular adaptations to exercise training before and during pregnancy in the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 303, R520–R526. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.J.; Bin, P.S.; Han, M. Di-(2-ethylhexyl)-phthalate induces oxidative stress in human endometrial stromal cells in vitro. Mol. Cell. Endocrinol. 2015, 407, 9–17. [Google Scholar] [CrossRef]
- Neubig, R.R.; Spedding, M.; Kenakin, T.; Christopoulos, A. International Union of Pharmacology Committee on Receptor Nomenclature and Drug Classification. XXXVIII. Update on terms and symbols in quantitative pharmacology. Pharmacol. Rev. 2003, 55, 597–606. [Google Scholar] [CrossRef]
- Merry, T.L.; Ristow, M. Do antioxidant supplements interfere with skeletal muscle adaptation to exercise training? J. Physiol. 2016, 594, 5135–5147. [Google Scholar] [CrossRef]
- Petersen, A.M.W.; Pedersen, B.K. The anti-inflammatory effect of exercise. J. Appl. Physiol. 2006, 98, 1154–1162. [Google Scholar] [CrossRef]
- Fernandes, T.; Hashimoto, N.Y.; Magalhães, F.C.; Fernandes, F.B.; Casarini, D.E.; Carmona, A.K.; Krieger, J.E.; Phillips, M.I.; Oliveira, E.M. Aerobic exercise training-induced left ventricular hypertrophy involves regulatory microRNAs, decreased angiotensin-converting enzyme-angiotensin II, and activation of the PI3K-Akt-mTOR signaling pathway. Hypertension 2011, 58, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Caramori, P.R.A.; Zago, A.J. Endothelial Dysfunction and Coronary Artery Disease. Arq. Bras. Cardiol. 2000, 75, 163–172. [Google Scholar] [CrossRef]
- Carrizzo, A.; Conte, G.M.; Sommella, E.; Damato, A.; Ambrosio, M.; Sala, M.; Scala, M.C.; Aquino, R.P.; De Lucia, M.; Madonna, M.; et al. Novel Potent Decameric Peptide of Spirulina platensis Reduces Blood Pressure Levels Through a PI3K/AKT/eNOS-Dependent Mechanism. Hypertension 2019, 73, 449–457. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, J.; Lu, Y.; Wang, R. The vasorelaxant effect of hydrogen sulfide as a novel endogenous gaseous KATP channel opener. EMBO J. 2002, 20, 6008–6016. [Google Scholar] [CrossRef]
- Nethery, D.; Stofan, D.; Callahan, L.A.; DiMarco, A.F.; Supinski, G.S. Formation of reactive oxygen species by the contracting diaphragm is PLA2 dependent. J. Appl. Physiol. 2000, 89, 216–224. [Google Scholar] [CrossRef]
- Bouvière, J.; Fortunato, R.; Dupuy, C.; Werneck-De-Castro, J.; Carvalho, D.; Louzada, R. Exercise-Stimulated ROS Sensitive Signaling Pathways in Skeletal Muscle. Antioxidants 2021, 10, 537. [Google Scholar] [CrossRef]
- Palmer, R.M.J.; Ferrige, A.G.; Moncada, S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 1987, 327, 524–526. [Google Scholar] [CrossRef]
- Trappe, T.A.; Fluckey, J.D.; White, F.; Lambert, C.P.; Evans, W.J. Skeletal muscle PGF2α and PGE2 in response to eccentric resistance exercise: Influence of ibuprofen acetaminophen. J. Clin. Endocrinol. Metab. 2001, 86, 5067–5070. [Google Scholar] [CrossRef]
- Richmond, G.S. Prostaglandins in smooth muscle. In Handbook of Experimental Pharmacology; Furchgott, R.F., Vane, J.R., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 217–232. [Google Scholar]
- Abdel-Moneim, A.E.; El-Saadony, M.T.; Shehata, A.M.; Saad, A.M.; Aldhumri, S.A.; Ouda, S.M.; Mesalam, N.M. Antioxidant and antimicrobial activities of Spirulina platensis extracts and biogenic selenium nanoparticles against selected pathogenic bacteria and fungi. Saudi J. Biol. Sci. 2022, 29, 1197–1209. [Google Scholar] [CrossRef] [PubMed]
- Cozza, K.L.; Costa, J.A.V. Lipídios em Spirulina.Spirulina. VETOR Rev. Ciências Exatas E Eng. 2007, 10, 69–80. [Google Scholar]
- Diniz, A.F.A.; de Oliveira Claudino, B.F.; Francelino, D.M.C.; da Silva, J.M.A.; Barros, B.C.; Arruda, R.R.A.; Melchiades, M.K.D.N.; Ferreira, P.B.; Júnior, F.F.L.; Abreu, L.S.; et al. Arthrospira platensis prevents contractile reactivity damage in obese rats fed a hypercaloric diet by positive modulating the Rho-A/Rho-kinase pathway, inflammation and oxidative stress. J. Funct. Foods 2024, 115, 106116. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, Y.; Zhang, R.; Cai, T.; Cai, Y. Medical Application of Spirulina platensis Derived C-Phycocyanin. Evid. Based Complement. Altern. Med. 2016, 2016, 7803846. [Google Scholar] [CrossRef]
- Slemmer, J.E.; Shacka, J.J.; Sweeney, M.I.; Weber, J.T. Antioxidants and free radical scavengers for the treatment of stroke, traumatic brain injury and aging. Curr. Med. Chem. 2008, 15, 404–414. [Google Scholar] [CrossRef]
- Laemmel, E.; Bonnardel-Phu, E.; Hou, X.; Seror, J.; Vicaut, E. Interaction between nitric oxide and prostanoids in arterioles of rat cremaster muscle in vivo. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H1254–H1260. [Google Scholar] [CrossRef]
- Kennedy, B.P.; Payette, P.; Mudgett, J.; Vadas, P.; Pruzanski, W.; Kwan, M.; Tang, C.; Rancourt, D.E.; Cromlish, W.A. A natural disruption of the secretory group II phospholipase A2 gene in inbred mouse strains. J. Biol. Chem. 1995, 270, 22378–22385. [Google Scholar] [CrossRef]
- Hajjar, D.P.; Lander, H.M.; Pearce, S.F.A.; Upmacis, R.; Pomerantz, K.B. Nitric oxide enhances prostaglandin-H synthase-1 activity by a hemeindependent mechanism: Evidence implicating nitrosothiols. J. Am. Chem. Soc. 1995, 117, 3340–3346. [Google Scholar] [CrossRef]
- Kim, S.F.; Huri, D.A.; Snyder, S.H. Inducible nitric oxide synthase binds, S-nitrosylates, and activates cyclooxygenase-2. Science 2005, 310, 1966–1970. [Google Scholar] [CrossRef] [PubMed]
- Davidge, S.T.; Baker, P.N.; Mclaughlin, M.K.; Roberts, J.M. Nitric oxide produced by endothelial cells increases production of eicosanoids through activation of prostaglandin H synthase. Circ. Res. 1995, 77, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Duarte, J.; Kavazis, A.N.; Talbert, E.E. Reactive oxygen species are signalling molecules for skeletal muscle adaptation. Exp. Physiol. 2010, 95, 1–9. [Google Scholar] [CrossRef]
- Fridovich, I. Superoxide radical and superoxide dismutases. Annu. Rev. Biochem. 1995, 64, 97–112. [Google Scholar] [CrossRef]
- Balarini, C.M.; Leal, M.A.; Gomes, I.B.; Pereira, T.M.; Gava, A.L.; Meyrelles, S.S.; Vasquez, E.C. Sildenafil restores endothelial function in the apolipoprotein E knockout mouse. J. Transl. Med. 2013, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Liu, L.; Miron, A.; Klímová, B.; Wan, D.; Kuca, K. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: An overview. Arch. Toxicol. 2016, 90, 1817–1840. [Google Scholar] [CrossRef]
- Khalil, S.R.; Salem, H.F.A.; Metwally, M.M.M.; Emad, R.M.; Elbohi, K.M.; Ali, S.A. Protective effect of Spirulina platensis against physiological, ultrastructural and cell proliferation damage induced by furan in kidney and liver of rat. Ecotoxicol. Environ. Saf. 2020, 192, 110256. [Google Scholar] [CrossRef]
- Ristow, M.; Zarse, K.; Oberbach, A.; Klöting, N.; Birringer, M.; Kiehntopf, M.; Stumvoll, M.; Kahn, C.R.; Blüher, M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc. Natl. Acad. Sci. USA 2009, 106, 8665–8670. [Google Scholar] [CrossRef] [PubMed]
- Meitzler, J.L.; Antony, S.; Wu, Y.; Juhasz, A.; Liu, H.; Jiang, G.; Lu, J.; Roy, K.; Doroshow, J.H. NADPH oxidases: A perspective on reactive oxygen species production in tumor biology. Antioxid. Redox Signal. 2014, 20, 2873–2889. [Google Scholar] [CrossRef] [PubMed]
- Revuelta, A.V.; Cantabrana, B.; Hidalgo, A. Depolarization-dependent effect of flavonoids in rat uterine smooth muscle contraction elicited by CaCl2. Gen. Pharmacol. Vasc. Syst. 1997, 29, 847–857. [Google Scholar] [CrossRef]
- Caliari, M.V. Principles of Digital Morphometry: KS300 for Beginners; UFMG Press: Belo Horizonte, Brazil, 1997; p. 149. [Google Scholar]
- Vignozzi, L.; Morelli, A.; Filippi, S.; Vannelli, G.B.; Mungai, S.; Marini, M.; Boddi, V.; Forti, G.; Maggi, M. Effect of sildenafil administration on penile hypoxia induced by cavernous neurotomy in the rat. Int. J. Impot. Res. 2008, 20, 60–67. [Google Scholar] [CrossRef]
- Cartledge, J.J.; Eardley, I.; Morrison, J.F. Impairment of corpus cavernosal smooth muscle relaxation by glycosylated human haemoglobin. Br. J. Urol. 2000, 85, 735–741. [Google Scholar] [CrossRef]
- Côco, H.; Pernomian, L.; Marchia, K.C.; Gomes, M.S.; Andrade, C.R.; Ramalho, L.N.Z.; Tirapelli, C.R.; Oliveira, A.M. Consequence of hyperhomocysteinaemia on α1-adrenoceptor-mediated contraction in the rat corpus cavernosum: The role of reactive oxygen species. J. Pharm. Pharmacol. 2016, 68, 63–75. [Google Scholar] [CrossRef]
- Peixoto, E.B.; Pessoa, B.S.; Biskwas, S.K.; Faria, J.B.L. Antioxidant SOD mimetic prevents NADPH oxidase-induced oxidative stress and renal damage in the early stage of experimental diabetes and hypertension. Am. J. Nephrol. 2009, 29, 309–318. [Google Scholar] [CrossRef] [PubMed]

| Inhibitor | GC | GT | GT + Inhibitor | GTAP100 | GTAP100 + Inhibitor |
|---|---|---|---|---|---|
| Emax (%) L-NAME | 122.1 ± 9.2 # | 203.0 ± 27.8 *$ | |||
| Indomethacin L-NAME + Indomethacin | 100 | 172.7 ± 8.1 * | 93.4 ± 11.3 # 183.5 ± 10.85 * | 119.7 ± 9.1 | 256.0 ± 22.9 *$ 227.8 ± 14.0 *$ |
| Apocynin Tempol | 167.0 ± 10.3 * 127.2 ± 7.3 # | 170.2 ± 16.3 *$ 187.3 ± 10.6 *$ | |||
| pCE50 L-NAME | 2.4 ± 0.05 *# | 2.1 ± 0.2 | |||
| Indomethacin L-NAME + Indomethacin | 2.0 ± 0.07 | 1.0 ± 0.03 * | 2.2 ± 0.06 *# 2.3 ± 0.05 # | 2.0 ± 0.05 | 2.0 ± 0.03 2.5 ± 0.005 *$ |
| Apocynin Tempol | 2.2 ± 0.06 # 2.3 ± 0.06 *# | 2.2 ± 0.1 2.4 ± 0.06 *$ |
| Adaptation (Days) | Weeks | |||||
|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 1st and 2nd | 3rd and 4th | 5th and 6th | 7th and 8th |
| 2 series × | 4 series × | 4 series × | 4 series × | 4 series × | 4 series × | 4 series × |
| 5 jumps | 5 jumps | 9 jumps | 10 jumps | 10 jumps | 10 jumps | 12 jumps |
| 50% | 50% | 50% | 50% | 60% | 80% | 80% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barros, B.C.; Diniz, A.F.A.; Lacerda-Júnior, F.F.; Souza, P.P.d.S.; Alves, A.F.; Ferreira, P.B.; Cavalcante, F.d.A.; da Silva, B.A. Arthrospira platensis Preserves Uterine Function by Modulating Electromechanical Coupling and Redox Pathways During Resistance Training in Female Rats. Int. J. Mol. Sci. 2025, 26, 11440. https://doi.org/10.3390/ijms262311440
Barros BC, Diniz AFA, Lacerda-Júnior FF, Souza PPdS, Alves AF, Ferreira PB, Cavalcante FdA, da Silva BA. Arthrospira platensis Preserves Uterine Function by Modulating Electromechanical Coupling and Redox Pathways During Resistance Training in Female Rats. International Journal of Molecular Sciences. 2025; 26(23):11440. https://doi.org/10.3390/ijms262311440
Chicago/Turabian StyleBarros, Bárbara Cavalcanti, Anderson Fellyp Avelino Diniz, Francisco Fernandes Lacerda-Júnior, Petruska Pessoa da Silva Souza, Adriano Francisco Alves, Paula Benvindo Ferreira, Fabiana de Andrade Cavalcante, and Bagnólia Araújo da Silva. 2025. "Arthrospira platensis Preserves Uterine Function by Modulating Electromechanical Coupling and Redox Pathways During Resistance Training in Female Rats" International Journal of Molecular Sciences 26, no. 23: 11440. https://doi.org/10.3390/ijms262311440
APA StyleBarros, B. C., Diniz, A. F. A., Lacerda-Júnior, F. F., Souza, P. P. d. S., Alves, A. F., Ferreira, P. B., Cavalcante, F. d. A., & da Silva, B. A. (2025). Arthrospira platensis Preserves Uterine Function by Modulating Electromechanical Coupling and Redox Pathways During Resistance Training in Female Rats. International Journal of Molecular Sciences, 26(23), 11440. https://doi.org/10.3390/ijms262311440







