Synergistic Effects of Non-Ionizing Radiation in the Targeted Modification of Living Tissues
Abstract
1. Introduction
2. Physiological Requirements for Modification of Tissue Properties
3. Current Applications of Bosonic Concentrate Principles in Biology and Medicine
3.1. Phenomenon of Bosonic Concentrate in the Condensed Matter
3.2. Photons: Laser and Maser Sources
3.3. Acoustic Phonons
3.4. Other Particles and Quasiparticles
4. Nonlinear Interactions and Synergisms of the High-Density Non-Ionizing Irradiation
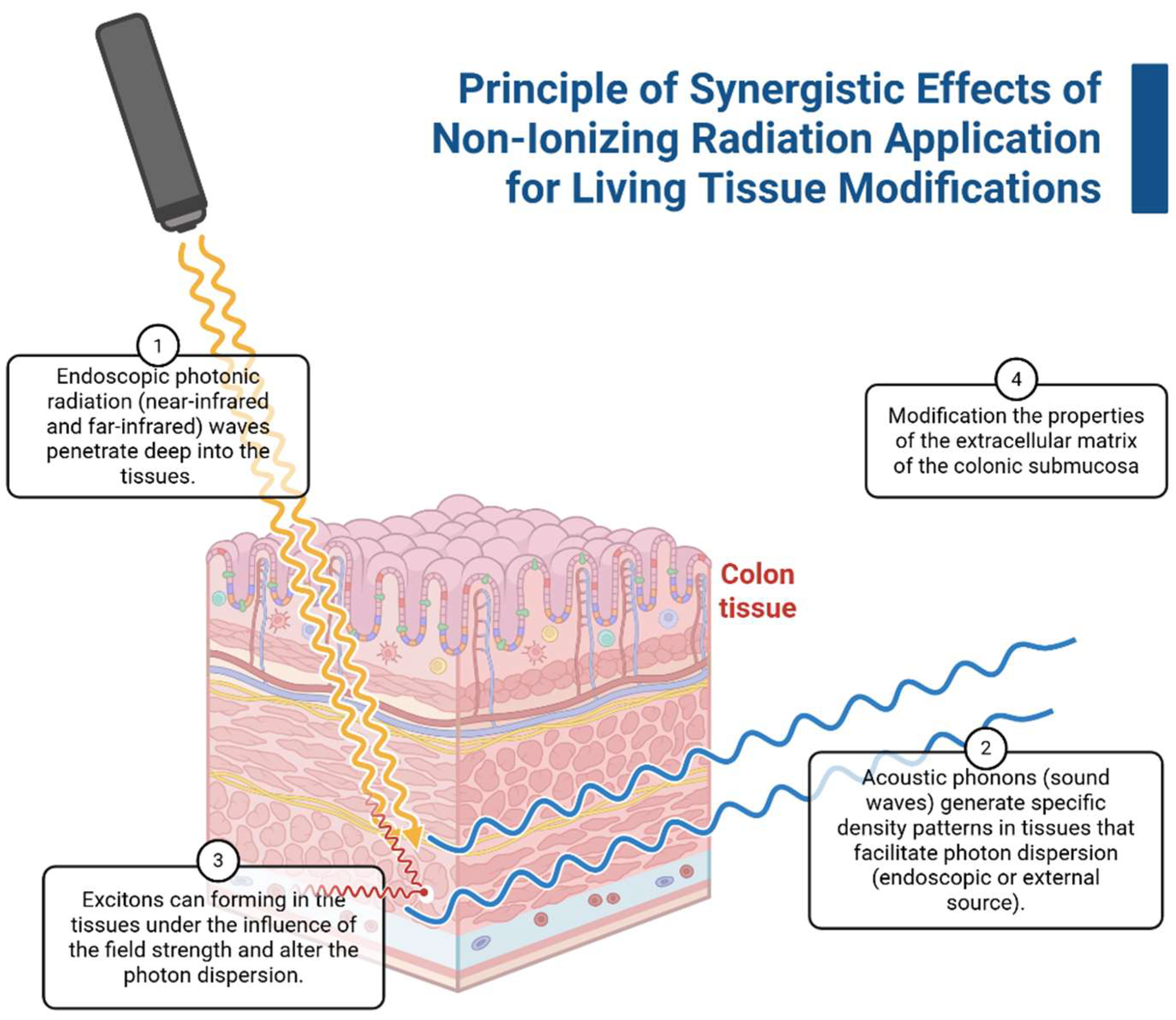
5. Application of Combined Non-Ionizing Irradiation In Vivo
Combined Effects of Irradiation on Biological Tissues
6. Limitations and Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ECM | Extracellular Matrix |
| FIR | Far-infrared radiation |
| NIR | Near-infrared radiation |
| SBS | Stimulated Brillouin Scattering |
| SRS | Stimulated Raman Scattering |
References
- Sherlock, B.E.; Chen, J.; Mansfield, J.C.; Green, E.; Winlove, C.P. Biophotonic tools for probing extracellular matrix mechanics. Matrix Biol. Plus 2021, 12, 100093. [Google Scholar] [CrossRef] [PubMed]
- Bakhshandeh, B.; Ranjbar, N.; Abbasi, A.; Amiri, E.; Abedi, A.; Mehrabi, M.; Dehghani, Z.; Pennisi, C.P. Recent progress in the manipulation of biochemical and biophysical cues for engineering functional tissues. Bioeng. Transl. Med. 2023, 8, e10383. [Google Scholar] [CrossRef]
- Alexandrovskaya, Y.M.; Baum, O.I.; Zaitsev, V.Y.; Sovetsky, A.A.; Matveyev, A.L.; Matveev, L.A.; Larin, K.V.; Sobol, E.N.; Tuchin, V.V. Optical and mechanical properties of cartilage during optical clearing. In Handbook of Tissue Optical Clearing; CRC Press: Boca Raton, FL, USA, 2022; pp. 185–198. [Google Scholar]
- Tuieng, R.J.; Cartmell, S.H.; Kirwan, C.C.; Sherratt, M.J. The effects of ionising and non-ionising electromagnetic radiation on extracellular matrix proteins. Cells 2021, 10, 3041. [Google Scholar] [CrossRef] [PubMed]
- Baranovskii, D.; Smirnova, A.; Yakimova, A.; Kisel, A.; Koryakin, S.; Atiakshin, D.; Ignatyuk, M.; Potievskiy, M.; Saburov, V.; Budnik, S.; et al. Tissue-Cultured Chondrocytes Survive After Irradiation in 1300 Gy Dose. Biomedicines 2025, 13, 2153. [Google Scholar] [CrossRef]
- Yusof, N. Gamma irradiation for sterilising tissue grafts for viral inactivation. Malays. J. Nucl. Sci. 2000, 18, 23–35. [Google Scholar]
- Ng, K.; Allam, N.; Neshatian, M.; Vaez, M.; Hirvonen, L.M.; Lam, E.; Vitkin, A.; Bozec, L. Effects of Ionizing Radiation on the Biophysical Properties of Type I Collagen Fibrils. PLoS ONE 2025, 20, e0319777. [Google Scholar] [CrossRef]
- Rödel, F.; Frey, B.; Gaipl, U.; Keilholz, L.; Fournier, C.; Manda, K.; Schollnberger, H.; Hildebrandt, G.; Rodel, C. Modulation of inflammatory immune reactions by low-dose ionizing radiation: Molecular mechanisms and clinical application. Curr. Med. Chem. 2012, 19, 1741–1750. [Google Scholar] [CrossRef]
- Tuieng, R.J.; Disney, C.; Cartmell, S.H.; Kirwan, C.C.; Eckersley, A.; Newham, E.; Gupta, H.S.; Hoyland, J.A.; Lee, P.D.; Sherratt, M.J. Impact of therapeutic X-ray exposure on collagen I and associated proteins. Acta Biomater. 2025, 197, 294–311. [Google Scholar] [CrossRef]
- Mottareale, R.; Frascogna, C.; La Verde, G.; Arrichiello, C.; Muto, P.; Netti, P.A.; Fusco, S.; Panzetta, V.; Pugliese, M. Impact of ionizing radiation on cell-ECM mechanical crosstalk in breast cancer. Front. Bioeng. Biotechnol. 2024, 12, 1408789. [Google Scholar] [CrossRef]
- Harrell, C.R.; Djonov, V.; Fellabaum, C.; Volarevic, V. Risks of using sterilization by gamma radiation: The other side of the coin. Int. J. Med. Sci. 2018, 15, 274. [Google Scholar] [CrossRef] [PubMed]
- Spyratou, E.; Kokkinogoulis, K.; Tsigaridas, G.; Kareliotis, G.; Platoni, K.; Makropoulou, M.; Efstathopoulos, E.P. Novel Biophotonic Techniques for Phototherapy Enhancement: Cerenkov Radiation as a Bridge between Ionizing and Non-Ionizing Radiation Treatment. J. Nanotheranostics 2023, 4, 86–105. [Google Scholar] [CrossRef]
- Zalesskaya, G.A.; Sambor, E.G.; Kuchinskii, A.V. Effect of intravenous laser irradiation on the molecular structure of blood and blood components. J. Appl. Spectrosc. 2006, 73, 115–122. [Google Scholar] [CrossRef]
- Stadler, I.; Evans, R.; Kolb, B.; Naim, J.O.; Narayan, V.; Buehner, N.; Lanzafame, R.J. In vitro effects of low-level laser irradiation at 660 nm on peripheral blood lymphocytes. Lasers Surg. Med. 2000, 27, 255–261. [Google Scholar] [CrossRef]
- Baranovskii, D.; Demner, J.; Nürnberger, S.; Lyundup, A.; Redl, H.; Hilpert, M.; Pigeot, S.; Krasheninnikov, M.; Krasilnikova, O.; Klabukov, I.; et al. Engineering of tracheal grafts based on recellularization of laser-engraved human airway cartilage substrates. Cartilage 2022, 13, 19476035221075951. [Google Scholar] [CrossRef]
- Saran, R.; Ginjupalli, K.; George, S.D.; Chidangil, S.; Unnikrishnan, V.K. LASER as a tool for surface modification of dental biomaterials: A review. Heliyon 2023, 9, e17457. [Google Scholar] [CrossRef]
- Sitnikov, D.S.; Ilina, I.V.; Revkova, V.A.; Rodionov, S.A.; Gurova, S.A.; Shatalova, R.O.; Kovalev, A.V.; Ovchinnikov, A.V.; Chefonov, O.V.; Konoplyannikov, M.A.; et al. Effects of high intensity non-ionizing terahertz radiation on human skin fibroblasts. Biomed. Opt. Express 2021, 12, 7122–7138. [Google Scholar] [CrossRef] [PubMed]
- Il’ina, I.V.; Sitnikov, D.S.; Agranat, M.B. State-of-the-art of studies of the effect of terahertz radiation on living biological systems. High Temp. 2018, 56, 789–810. [Google Scholar] [CrossRef]
- Geboers, B.; Scheffer, H.J.; Graybill, P.M.; Ruarus, A.H.; Nieuwenhuizen, S.; Puijk, R.S.; van den Tol, P.M.; Davalos, R.V.; Rubinsky, B.; De Gruijl, T.D.; et al. High-voltage electrical pulses in oncology: Irreversible electroporation, electrochemotherapy, gene electrotransfer, electrofusion, and electroimmunotherapy. Radiology 2020, 295, 254–272. [Google Scholar] [CrossRef]
- Petrov, A.A.; Moraleva, A.A.; Antipova, N.V.; Amirov, R.K.; Samoylov, I.S.; Savinov, S.Y. The action of the pulsed electric field of the subnanosecond range on human tumor cells. Bioelectromagnetics 2022, 43, 327–335. [Google Scholar] [CrossRef]
- Smye, S.W.; Chamberlain, J.M.; Fitzgerald, A.J.; Berry, E. The interaction between terahertz radiation and biological tissue. Phys. Med. Biol. 2001, 46, R101. [Google Scholar] [CrossRef] [PubMed]
- Pogorelsky, I.V.; Polyanskiy, M.N. Harnessing Ultra-Intense Long-Wave Infrared Lasers: New Frontiers in Fundamental and Applied Research. Photonics 2025, 12, 221. [Google Scholar] [CrossRef]
- Liu, Y.; Lai, P.; Ma, C.; Xu, X.; Grabar, A.A.; Wang, L.V. Optical focusing deep inside dynamic scattering media with near-infrared time-reversed ultrasonically encoded (TRUE) light. Nat. Commun. 2015, 6, 5904. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Dong, B.; Mahapatra, S.; Ma, S. Advances and future trends in real-time precision optical control of chemical processes in live cells. npj Imaging 2025, 3, 23. [Google Scholar] [CrossRef]
- Razek, A. Analysis of the Interaction Effects of Electromagnetic Fields with Major Living Tissues—One Health Concept Numerical Evaluation Strategy. Digit. Technol. Res. Appl. 2024, 3, 18–34. [Google Scholar] [CrossRef]
- Hielscher, A.H.; Tittel, F.K.; Jacques, S.L. Photon density wave diffraction tomography. In Advances in Optical Imaging and Photon Migration; Optica Publishing Group: Washington, DC, USA, 1994; p. APMPDWI-78. [Google Scholar] [CrossRef]
- Abbott, R.D.; Kaplan, D.L. Strategies for improving the physiological relevance of human engineered tissues. Trends Biotechnol. 2015, 33, 401–407. [Google Scholar] [CrossRef]
- Villani, C.; Murugan, P.; George, A. Exosome-Laden Hydrogels as Promising Carriers for Oral and Bone Tissue Engineering: Insight into Cell-Free Drug Delivery. Int. J. Mol. Sci. 2024, 25, 11092. [Google Scholar] [CrossRef]
- Klabukov, I.; Tenchurin, T.; Shepelev, A.; Baranovskii, D.; Mamagulashvili, V.; Dyuzheva, T.; Krasilnikova, O.; Balyasin, M.; Lyundup, A.; Krasheninnikov, M.; et al. Biomechanical behaviors and degradation properties of multilayered polymer scaffolds: The phase space method for bile duct design and bioengineering. Biomedicines 2023, 11, 745. [Google Scholar] [CrossRef]
- Nitti, P.; Narayanan, A.; Pellegrino, R.; Villani, S.; Madaghiele, M.; Demitri, C. Cell-Tissue Interaction: The Biomimetic Approach to Design Tissue Engineered Biomaterials. Bioengineering 2023, 10, 1122. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M. Biocompatibility and the relationship to standards: Meaning and scope of biomaterials testing. Compr. Biomater. 2011, 4, 7–26. [Google Scholar] [CrossRef]
- Shestakova, V.A.; Klabukov, I.D.; Baranovskii, D.S.; Shegay, P.V.; Kaprin, A.D. Assessment of immunological responses-a novel challenge in tissue engineering and regenerative medicine. Biomed. Res. Ther. 2022, 9, 5384–5386. [Google Scholar] [CrossRef]
- Angeletti, A.; Cantarelli, C.; Cravedi, P. Immune responses towards bioengineered tissues and strategies to control them. Curr. Opin. Organ. Transplant. 2019, 24, 582–589. [Google Scholar] [CrossRef]
- Andorko, J.I.; Jewell, C.M. Designing biomaterials with immunomodulatory properties for tissue engineering and regenerative medicine. Bioeng. Transl. Med. 2017, 2, 139–155. [Google Scholar] [CrossRef] [PubMed]
- Boehler, R.M.; Graham, J.G.; Shea, L.D. Tissue engineering tools for modulation of the immune response. Biotechniques 2011, 51, 239–254. [Google Scholar] [CrossRef]
- Dziki, J.L.; Badylak, S.F. Targeting the host immune response for tissue engineering and regenerative medicine applications. Princ. Tissue Eng. 2020, 363–368. [Google Scholar] [CrossRef]
- Han, F.; Zhu, C.; Guo, Q.; Yang, H.; Li, B. Cellular modulation by the elasticity of biomaterials. J. Mater. Chem. B 2016, 4, 9–26. [Google Scholar] [CrossRef]
- Garvin, K.A.; VanderBurgh, J.; Hocking, D.C.; Dalecki, D. Controlling collagen fiber microstructure in three-dimensional hydrogels using ultrasound. J. Acoust. Soc. Am. 2013, 134, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, M.; Mechanic, G.L. Cross-linking of collagen. In Collagen; CRC Press: Boca Raton, FL, USA, 2018; pp. 157–172. [Google Scholar]
- Depalle, B.; Qin, Z.; Shefelbine, S.J.; Buehler, M.J. Influence of cross-link structure, density and mechanical properties in the mesoscale deformation mechanisms of collagen fibrils. J. Mech. Behav. Biomed. Mater. 2015, 52, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Redden, R.A.; Doolin, E.J. Collagen crosslinking and cell density have distinct effects on fibroblast-mediated contraction of collagen gels. Skin Res. Technol. 2003, 9, 290–293. [Google Scholar] [CrossRef]
- Nair, M.; Best, S.M.; Cameron, R.E. Crosslinking Collagen Constructs: Achieving Cellular Selectivity Through Modifications of Physical and Chemical Properties. Appl. Sci. 2020, 10, 6911. [Google Scholar] [CrossRef]
- Chai, D.; Juhasz, T.; Brown, D.J.; Jester, J.V. Nonlinear optical collagen cross-linking and mechanical stiffening: A possible photodynamic therapeutic approach to treating corneal ectasia. J. Biomed. Opt. 2013, 18, 038003. [Google Scholar] [CrossRef]
- Halsey, G.; Sinha, D.; Dhital, S.; Wang, X.; Vyavahare, N. Role of elastic fiber degradation in disease pathogenesis. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166706. [Google Scholar] [CrossRef]
- Sherratt, M.J. Tissue elasticity and the ageing elastic fibre. Age 2009, 31, 305–325. [Google Scholar] [CrossRef]
- Heinz, A. Elastic fibers during aging and disease. Ageing Res. Rev. 2021, 66, 101255. [Google Scholar] [CrossRef] [PubMed]
- Schmelzer, C.E.H.; Duca, L. Elastic fibers: Formation, function, and fate during aging and disease. FEBS J. 2022, 289, 3704–3730. [Google Scholar] [CrossRef]
- Wang, M.; McGraw, K.R.; Monticone, R.E.; Pintus, G. Unraveling Elastic Fiber-Derived Signaling in Arterial Aging and Related Arterial Diseases. Biomolecules 2025, 15, 153. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, S.A.; Serra, A.J.; Stancker, T.G.; Simões, M.C.B.; Vieira, M.A.d.S.; Leal-Junior, E.C.; Prokic, M.; Vasconsuelo, A.; Santos, S.S.; Carvalho, P.d.T.C.d. Effects of Photobiomodulation Therapy on Oxidative Stress in Muscle Injury Animal Models: A Systematic Review. Oxid. Med. Cell Longev. 2017, 2017, 5273403. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, L.S.; Medrado, A.P.; Reis, S.R.; Andrade Zde, A. The influence of low-level laser therapy on biomodulation of collagen and elastic fibers. Pesqui. Odontol. Bras. 2003, 17, 307–313. [Google Scholar] [CrossRef]
- Liu, S.; Liu, J.; Wang, Y.; Deng, F.; Deng, Z. Oxidative Stress: Signaling Pathways, Biological Functions, and Disease. MedComm 2025, 6, e70268. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Okajima, F. Regulation of inflammation by extracellular acidification and proton-sensing GPCRs. Cell Signal 2013, 25, 2263–2271. [Google Scholar] [CrossRef]
- Liu, H.; Wang, S.; Wang, J.; Guo, X.; Song, Y.; Fu, K.; Gao, Z.; Liu, D.; He, W.; Yang, L.-L. Energy metabolism in health and diseases. Signal Transduct. Target. Ther. 2025, 10, 69. [Google Scholar] [CrossRef]
- Schulz, E.; Münzel, T. Intracellular pH: A fundamental modulator of vascular function. Circulation 2011, 124, 1806–1807. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017, 4, 337–361. [Google Scholar] [CrossRef]
- Yue, B. Biology of the extracellular matrix: An overview. J. Glaucoma. 2014, 23 (Suppl. S1), S20–S23. [Google Scholar] [CrossRef]
- Sanford-Crane, H.; Abrego, J.; Sherman, M.H. Fibroblasts as Modulators of Local and Systemic Cancer Metabolism. Cancers 2019, 11, 619. [Google Scholar] [CrossRef]
- Sakai, Y.; Koike, M.; Kawahara, D.; Hasegawa, H.; Murai, T.; Yamanouchi, K.; Soyama, A.; Hidaka, M.; Takatsuki, M.; Fujita, F.; et al. Controlled cell morphology and liver-specific function of engineered primary hepatocytes by fibroblast layer cell densities. J. Biosci. Bioeng. 2018, 126, 249–257. [Google Scholar] [CrossRef]
- Danalache, M.; Jacobi, L.F.; Schwitalle, M.; Hofmann, U.K. Assessment of biomechanical properties of the extracellular and pericellular matrix and their interconnection throughout the course of osteoarthritis. J. Biomech. 2019, 97, 109409. [Google Scholar] [CrossRef] [PubMed]
- Lata, J.P.; Guo, F.; Guo, J.; Huang, P.H.; Yang, J.; Huang, T.J. Surface Acoustic Waves Grant Superior Spatial Control of Cells Embedded in Hydrogel Fibers. Adv. Mater. 2016, 28, 8632–8638. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, D.V.; Reichert, P.; Zvick, J.; Labouesse, C.; Künzli, V.; Dudaryeva, O.; Bar-Nur, O.; Tibbitt, M.W.; Dual, J. Continuous production of acoustically patterned cells within hydrogel fibers for musculoskeletal tissue engineering. Adv. Funct. Mater. 2022, 32, 2113038. [Google Scholar] [CrossRef]
- Saraswathibhatla, A.; Indana, D.; Chaudhuri, O. Cell-extracellular matrix mechanotransduction in 3D. Nat. Rev. Mol. Cell Biol. 2023, 24, 495–516. [Google Scholar] [CrossRef]
- Davis, G.E. Matricryptic sites control tissue injury responses in the cardiovascular system: Relationships to pattern recognition receptor regulated events. J. Mol. Cell Cardiol. 2010, 48, 454–460. [Google Scholar] [CrossRef]
- Love, R.J.; Jones, K.S. The recognition of biomaterials: Pattern recognition of medical polymers and their adsorbed biomolecules. J. Biomed. Mater. Res. A 2013, 101, 2740–2752. [Google Scholar] [CrossRef]
- Kechagia, J.Z.; Ivaska, J.; Roca-Cusachs, P. Integrins as biomechanical sensors of the microenvironment. Nat. Rev. Mol. Cell Biol. 2019, 20, 457–473. [Google Scholar] [CrossRef] [PubMed]
- Tiskratok, W.; Chuinsiri, N.; Limraksasin, P.; Kyawsoewin, M.; Jitprasertwong, P. Extracellular Matrix Stiffness: Mechanotransduction and Mechanobiological Response-Driven Strategies for Biomedical Applications Targeting Fibroblast Inflammation. Polymers 2025, 17, 822. [Google Scholar] [CrossRef] [PubMed]
- Mierke, C.T. Extracellular matrix cues regulate mechanosensing and mechanotransduction of cancer cells. Cells 2024, 13, 96. [Google Scholar] [CrossRef]
- Chu, Y.C.; Lim, J.; Chien, A.; Chen, C.C.; Wang, J.L. Activation of Mechanosensitive Ion Channels by Ultrasound. Ultrasound Med. Biol. 2022, 48, 1981–1994. [Google Scholar] [CrossRef] [PubMed]
- Kubanek, J.; Shukla, P.; Das, A.; Baccus, S.A.; Goodman, M.B. Ultrasound Elicits Behavioral Responses through Mechanical Effects on Neurons and Ion Channels in a Simple Nervous System. J. Neurosci. 2018, 38, 3081–3091. [Google Scholar] [CrossRef]
- Kologrivova, I.; Shtatolkina, M.; Suslova, T.; Ryabov, V. Cells of the Immune System in Cardiac Remodeling: Main Players in Resolution of Inflammation and Repair After Myocardial Infarction. Front. Immunol. 2021, 12, 664457. [Google Scholar] [CrossRef]
- Smigiel, K.S.; Parks, W.C. Macrophages, Wound Healing, and Fibrosis: Recent Insights. Curr. Rheumatol. Rep. 2018, 20, 17. [Google Scholar] [CrossRef]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef]
- Martin, K.E.; García, A.J. Macrophage phenotypes in tissue repair and the foreign body response: Implications for biomaterial-based regenerative medicine strategies. Acta Biomater. 2021, 133, 4–16. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, J.; Sun, H.; Zhang, Y.; Zou, D. New insights into fibrosis from the ECM degradation perspective: The macrophage-MMP-ECM interaction. Cell Biosci. 2022, 12, 117. [Google Scholar] [CrossRef]
- de Brito Sousa, K.; Rodrigues, M.F.S.D.; de Souza Santos, D.; Mesquita-Ferrari, R.A.; Nunes, F.D.; Silva, D.d.F.T.d.; Bussadori, S.K.; Fernandes, K.P.S. Differential expression of inflammatory and anti-inflammatory mediators by M1 and M2 macrophages after photobiomodulation with red or infrared lasers. Lasers Med. Sci. 2020, 35, 337–343. [Google Scholar] [CrossRef]
- Yan, H.; Cheng, Q.; Si, J.; Wang, S.; Wan, Y.; Kong, X.; Wang, T.; Zheng, W.; Rafique, M.; Li, X.; et al. Functionalization of in vivo tissue-engineered living biotubes enhance patency and endothelization without the requirement of systemic anticoagulant administration. Bioact. Mater. 2023, 26, 292–305. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Xu, L.; Liu, S.; Li, J.; Xia, J.; Qin, X.; Li, Y.; Gao, T.; Tang, X. The state-of-the-art and perspectives of laser ablation for tumor treatment. Cyborg Bionic Syst. 2024, 5, 0062. [Google Scholar] [CrossRef] [PubMed]
- Khalkhal, E.; Rezaei-Tavirani, M.; Zali, M.R.; Akbari, Z. The evaluation of laser application in surgery: A review article. J. Lasers Med. Sci. 2019, 10 (Suppl. S1), S104. [Google Scholar] [CrossRef]
- Cloutier, G.; Destrempes, F.; Yu, F.; Tang, A. Quantitative ultrasound imaging of soft biological tissues: A primer for radiologists and medical physicists. Insights Imaging 2021, 12, 127. [Google Scholar] [CrossRef]
- Sarvazyan, A.P.; Urban, M.W.; Greenleaf, J.F. Acoustic waves in medical imaging and diagnostics. Ultrasound Med. Biol. 2013, 39, 1133–1146. [Google Scholar] [CrossRef] [PubMed]
- Vighetto, V.; Pascucci, E.; Savino, G.; Rosso, G.; Percivalle, N.M.; Conte, M.; Dumontel, B.; Balboni, A.; Mesiano, G.; Masoero, A.; et al. The Multifunctional Purposes of Ultrasound in 3D Models. Adv. Ther. 2024, 7, 2400161. [Google Scholar] [CrossRef]
- Norris, E.G.; Dalecki, D.; Hocking, D.C. Acoustic modification of collagen hydrogels facilitates cellular remodeling. Mater. Today Bio 2019, 3, 100018. [Google Scholar] [CrossRef]
- Del Giudice, E.; Doglia, S.; Milani, M.; Vitiello, G. A quantum field theoretical approach to the collective behaviour of biological systems. Nucl. Phys. B 1985, 251, 375–400. [Google Scholar] [CrossRef]
- Popp, F.A.; Chang, J.J.; Herzog, A.; Yan, Z.; Yan, Y. Evidence of non-classical (squeezed) light in biological systems. Phys. Lett. A 2002, 293, 98–102. [Google Scholar] [CrossRef]
- Zhu, Z. Decoherence in Photosynthetic Energy Transfer: Based on a Spin Bath Model. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 2009. [Google Scholar]
- Gordon, G.A. Extrinsic electromagnetic fields, low frequency (phonon) vibrations, and control of cell function: A non-linear resonance system. J. Biomed. Sci. Eng. 2008, 1, 152. [Google Scholar] [CrossRef]
- Walski, T.; Dąbrowska, K.; Drohomirecka, A.; Jędruchniewicz, N.; Trochanowska-Pauk, N.; Witkiewicz, W.; Komorowska, M. The effect of red-to-near-infrared (R/NIR) irradiation on inflammatory processes. Int. J. Radiat. Biol. 2019, 95, 1326–1336. [Google Scholar] [CrossRef] [PubMed]
- Markelz, A.G.; Mittleman, D.M. Perspective on terahertz applications in bioscience and biotechnology. ACS Photonics 2022, 9, 1117–1126. [Google Scholar] [CrossRef]
- Zaytsev, K.I.; Dolganova, I.N.; Chernomyrdin, N.V.; Katyba, G.M.; Gavdush, A.A.; Cherkasova, O.P.; A Komandin, G.; A Shchedrina, M.; Khodan, A.N.; Ponomarev, D.S.; et al. The progress and perspectives of terahertz technology for diagnosis of neoplasms: A review. J. Opt. 2019, 22, 013001. [Google Scholar] [CrossRef]
- Emerick, J.; Roy, C.; Branković, Z.; Rostovtsev, Y. Quantum control of quantum systems: From room-temperature masers to generation of entanglement photons. Eur. Phys. J. Spec. Top. 2023, 232, 3359–3367. [Google Scholar] [CrossRef]
- Wongkasem, N. Electromagnetic pollution alert: Microwave radiation and absorption in human organs and tissues. Electromagn. Biol. Med. 2021, 40, 236–253. [Google Scholar] [CrossRef]
- Teklu, A.; Declercq, N.F.; McPherson, M. Acousto-optic Bragg imaging of biological tissue. J. Acoust. Soc. Am. 2014, 136, 634–637. [Google Scholar] [CrossRef]
- Feng, G.; Zhang, G.Q.; Ding, D. Design of superior phototheranostic agents guided by Jablonski diagrams. Chem. Soc. Rev. 2020, 49, 8179–8234. [Google Scholar] [CrossRef]
- Pu, K.; Shuhendler, A.J.; Jokerst, J.V.; Mei, J.; Gambhir, S.S.; Bao, Z.; Rao, J. Semiconducting polymer nanoparticles as photoacoustic molecular imaging probes in living mice. Nat. Nanotech. 2014, 9, 233–239. [Google Scholar] [CrossRef]
- Wang, S.; Larin, K.V. Noncontact depth-resolved micro-scale optical coherence elastography of the cornea. Biomed. Opt. Express 2014, 5, 3807–3821. [Google Scholar] [CrossRef]
- Mishra, R.K.; Bhaumik, K.; Mathur, S.C.; Mitra, S. Excitons and Bose–Einstein condensation in living systems. Int. J. Quantum Chem. 1979, 16, 691–706. [Google Scholar] [CrossRef]
- Zhou, R.; Sui, L.; Liu, X.; Liu, K.; Guo, D.; Zhao, W.; Song, S.; Lv, C.; Chen, S.; Jiang, T.; et al. Multiphoton excited singlet/triplet mixed self-trapped exciton emission. Nat. Commun. 2023, 14, 1310. [Google Scholar] [CrossRef] [PubMed]
- Davydov, A.S. Excitons and solitons in molecular systems. Int. Rev. Cytol. 1987, 106, 183–225. [Google Scholar] [PubMed]
- Bergholt, M.S.; Serio, A.; Albro, M.B. Raman spectroscopy: Guiding light for the extracellular matrix. Front. Bioeng. Biotechnol. 2019, 7, 303. [Google Scholar] [CrossRef]
- Palombo, F.; Winlove, C.P.; Edginton, R.S.; Green, E.; Stone, N.; Caponi, S.; Fioretto, D. Biomechanics of fibrous proteins of the extracellular matrix studied by Brillouin scattering. J. R. Soc. Interface 2014, 11, 20140739. [Google Scholar] [CrossRef]
- Evstratova, E.S.; Petin, V.G. Biophysical interpretation of the dependence of synergy on the intensity of applied agents. Biophysics 2018, 63, 959–966. [Google Scholar] [CrossRef]
- Janson, N.B. Non-linear dynamics of biological systems. Contemp. Phys. 2012, 53, 137–168. [Google Scholar] [CrossRef]
- Akhmanova, M.; Osidak, E.; Domogatsky, S.; Rodin, S.; Domogatskaya, A. Physical, spatial, and molecular aspects of extracellular matrix of in vivo niches and artificial scaffolds relevant to stem cells research. Stem Cells Int. 2015, 2015, 167025. [Google Scholar] [CrossRef]
- Salem, N.M. Thermal Effects of Photon-Phonon interaction on a simple tissue. Environmentalist 2005, 25, 241–246. [Google Scholar] [CrossRef]
- Takemoto, K. Optical manipulation of molecular function by chromophore-assisted light inactivation. Proc. Jpn. Acad. Ser. B 2021, 97, 197–209. [Google Scholar] [CrossRef]
- Kishore, P.; Kumar, S. Analytical investigation of non-Fourier bioheat transfer in the axisymmetric living tissue exposed to pulsed laser heating using finite integral transform technique. J. Heat Transf. 2021, 143, 121201. [Google Scholar] [CrossRef]
- Campbell, K.R.; Campagnola, P.J. Wavelength-dependent second harmonic generation circular dichroism for differentiation of Col I and Col III isoforms in stromal models of ovarian cancer based on intrinsic chirality differences. J. Phys. Chem. B 2017, 121, 1749–1757. [Google Scholar] [CrossRef]
- Shcheslavskiy, V.; Heathcote, R.D.; Yakovlev, V.V. Nonlinear-optical photothermal modification of collagen. In Laser Interaction with Tissue and Cells XV; SPIE: Bellingham, WA, USA, 2004; Volume 5319, pp. 378–384. [Google Scholar]
- Bashkatov, A.N.; Genina, E.A.; Kochubey, V.I.; Tuchin, V.V. Optical properties of human skin, subcutaneous and mucous tissues in the wavelength range from 400 to 2000 nm. J. Phys. D Appl. Phys. 2005, 38, 2543. [Google Scholar] [CrossRef]
- Iannucci, L.E.; Riak, M.B.; Lake, S.P. The effect of extracellular matrix properties on polarized light-based analysis of collagen fiber alignment in soft tissues. Polariz. Light Opt. Angular Momentum Biomed. Diagn. 2022, 11963, 14–21. [Google Scholar]
- Jacques, S.L. Optical properties of biological tissues: A review. Phys. Med. Biol. 2013, 58, R37–R61, Correction in Phys. Med. Biol. 2013, 58, 5007. [Google Scholar] [CrossRef] [PubMed]
- Piazena, H.; Vaupel, P. Hyperhydration of breast and skin cancers: Effects on thermophysical tissue properties in clinical hyperthermia with water-filtered infrared-A radiation (wIRA)—An updated review. Int. J. Hyperth. 2025, 42, 2519352. [Google Scholar] [CrossRef] [PubMed]
- Gouarderes, S.; Mingotaud, A.F.; Vicendo, P.; Gibot, L. Vascular and extracellular matrix remodeling by physical approaches to improve drug delivery at the tumor site. Expert Opin. Drug Deliv. 2020, 17, 1703–1726. [Google Scholar] [CrossRef]
- Tuchin, V.V. Polarized light interaction with tissues. J. Biomed. Opt. 2016, 21, 71114. [Google Scholar] [CrossRef]
- Pshenichnyuk, S.A.; Modelli, A.; Komolov, A.S. Interconnections between dissociative electron attachment and electron-driven biological processes. Int. Rev. Phys. Chem. 2018, 37, 125–170. [Google Scholar] [CrossRef]
- Yakovlev, V.V. Laser tissue interactions: Are quantum effects important? In Optical Interactions with Tissue and Cells XXXIII; and Advanced Photonics in Urology; SPIE: Bellingham, WA, USA, 2023; p. PC1195806. [Google Scholar]
- Cai, N.; Lai, A.C.K.; Liao, K.; Corridon, P.R.; Graves, D.J.; Chan, V. Recent advances in fluorescence recovery after photobleaching for decoupling transport and kinetics of biomacromolecules in cellular physiology. Polymers 2022, 14, 1913. [Google Scholar] [CrossRef] [PubMed]
- Heikal, A.A. Intracellular coenzymes as natural biomarkers for metabolic activities and mitochondrial anomalies. Biomark. Med. 2010, 4, 241–263. [Google Scholar] [CrossRef]
- Karrobi, K.; Tank, A.; Fuzail, M.A.; Kalidoss, M.; Tilbury, K.; Zaman, M.; Ferruzzi, J.; Roblyer, D. Fluorescence Lifetime Imaging Microscopy (FLIM) reveals spatial-metabolic changes in 3D breast cancer spheroids. Sci. Rep. 2023, 13, 3624. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Liang, Z.; Zhang, J.; Zuo, X.; Sun, J.; Zheng, Q.; Song, J.; Ding, T.; Hu, X.; Wang, Z. Attenuation of the inflammatory response and polarization of macrophages by photobiomodulation. Lasers Med. Sci. 2020, 35, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Skala, M.C.; Riching, K.M.; Gendron-Fitzpatrick, A.; Eickhoff, J.; Eliceiri, K.W.; White, J.G.; Ramanujam, N. In vivo multiphoton microscopy of NADH and FAD redox states, fluorescence lifetimes, and cellular morphology in precancerous epithelia. Proc. Natl. Acad. Sci. USA 2007, 104, 19494–19499. [Google Scholar] [CrossRef]
- Martins, I.S.; Silva, H.F.; Lazareva, E.N.; Chernomyrdin, N.V.; Zaytsev, K.I.; Oliveira, L.M.; Tuchin, V.V. Measurement of tissue optical properties in a wide spectral range: A review. Biomed. Opt. Express 2022, 14, 249–298. [Google Scholar] [CrossRef]
- Subasinghe, S.A.A.S.; Pautler, R.G.; Samee, M.A.H.; Yustein, J.T.; Allen, M.J. Dual-Mode Tumor Imaging Using Probes That Are Responsive to Hypoxia-Induced Pathological Conditions. Biosensors 2022, 12, 478. [Google Scholar] [CrossRef]
- Svoboda, K.; Yasuda, R. Principles of two-photon excitation microscopy and its applications to neuroscience. Neuron 2006, 50, 823–839. [Google Scholar] [CrossRef]
- Zhi, W.; Li, Y.; Wang, L.; Hu, X. Advancing Neuroscience and Therapy: Insights into Genetic and Non-Genetic Neuromodulation Approaches. Cells 2025, 14, 122. [Google Scholar] [CrossRef]
- Abdollahian, P.; Sui, K.; Li, G.; Berg, R.W.; Meneghetti, M.; Markos, C. Evaluating safe infrared neural stimulation parameters: Calcium dynamics and excitotoxicity thresholds in dorsal root ganglia neurons. J. Neurosci. Methods 2025, 421, 110484. [Google Scholar] [CrossRef]
- Baranovskii, D.; Klabukov, I.; Isaev, E.; Shegay, P.; Kaprin, A. Auricular Cartilage Tissue-Engineering with Gentle Laser-Micropore Technique. Tissue Eng. Part A 2022, 28 (Suppl. S2), 341–342. [Google Scholar]
- Klabukov, I.; Atiakshin, D.; Kogan, E.; Ignatyuk, M.; Krasheninnikov, M.; Zharkov, N.; Yakimova, A.; Grinevich, V.; Pryanikov, P.; Parshin, V.; et al. Post-implantation inflammatory responses to xenogeneic tissue-engineered cartilage implanted in rabbit trachea: The role of cultured chondrocytes in the modification of inflammation. Int. J. Mol. Sci. 2023, 24, 16783. [Google Scholar] [CrossRef] [PubMed]
- Kisel, A.A.; Stepanov, V.A.; Isaeva, E.V.; Demyashkin, G.A.; Isaev, E.I.; Smirnova, E.I.; Yatsenko, E.M.; Afonin, G.V.; Ivanov, S.A.; Atiakshin, D.A.; et al. Cartilage Laser Engraving for Fast-Track Tissue Engineering of Auricular Grafts. Int. J. Mol. Sci. 2024, 25, 11538. [Google Scholar] [CrossRef] [PubMed]
- Myakishev-Rempel, M.; Stadler, I.; Brondon, P.; Axe, D.R.; Friedman, M.; Nardia, F.B.; Lanzafame, R. A preliminary study of the safety of red light phototherapy of tissues harboring cancer. Photomed. Laser Surg. 2012, 30, 551–558. [Google Scholar] [CrossRef]
- Felician, M.C.P.; Belotto, R.; Tardivo, J.P.; Baptista, M.S.; Martins, W.K. Photobiomodulation: Cellular, molecular, and clinical aspects. J. Photochem. Photobiol. 2023, 17, 100197. [Google Scholar] [CrossRef]
- Klabukov, I.; Smirnova, A.; Evstratova, E.; Baranovskii, D. Development of a biodegradable prosthesis through tissue engineering: The lack of the physiological abstractions prevents bioengineering innovations. Ann. Hepatol. 2025, 30, 101587. [Google Scholar] [CrossRef]
- Ebata, H.; Kidoaki, S. Avoiding tensional equilibrium in cells migrating on a matrix with cell-scale stiffness-heterogeneity. Biomaterials 2021, 274, 120860. [Google Scholar] [CrossRef]
- Nakamura, N. Reexamining the role of tissue inflammation in radiation carcinogenesis: A hypothesis to explain an earlier onset of cancer. Int. J. Radiat. Biol. 2021, 97, 1341–1351. [Google Scholar] [CrossRef]
- Batool, F.; Özçelik, H.; Stutz, C.; Gegout, P.Y.; Benkirane-Jessel, N.; Petit, C.; Huck, O. Modulation of immune-inflammatory responses through surface modifications of biomaterials to promote bone healing and regeneration. J. Tissue Eng. 2021, 12, 20417314211041428. [Google Scholar] [CrossRef]
- Lahir, Y.K. Non-ionizing radiations and their biochemical and biomedical impacts: A review. J. Radiat. Cancer Res. 2023, 14, 53–66. [Google Scholar] [CrossRef]
- Chang, L.; Zhang, L.; Cheng, Z.; Zhang, N.; Wang, C.; Wang, Y.; Liu, W. Effectiveness of collagen cross-linking induced by two-photon absorption properties of a femtosecond laser in ex vivo human corneal stroma. Biomed. Opt. Express 2022, 13, 5067–5081. [Google Scholar] [CrossRef]
- da Silva Sergio, L.P.; da Fonseca, A.D.S.; Mencalha, A.L.; de Paoli, F. Photobiomodulation on extracellular matrix. Laser Phys. 2023, 33, 033001. [Google Scholar] [CrossRef]
- Ahad, I.U.l.; Bartnik, A.; Fiedorowicz, H.; Kostecki, J.; Korczyc, B.; Ciach, T.; Brabazon, D. Surface modification of polymers for biocompatibility via exposure to extreme ultraviolet radiation. J. Biomed. Mater. Res. Part A 2014, 102, 3298–3310. [Google Scholar] [CrossRef]
- Klabukov, I.; Smirnova, A.; Yakimova, A.; Kabakov, A.E.; Atiakshin, D.; Petrenko, D.; Shestakova, V.A.; Sulina, Y.; Yatsenko, E.; Stepanenko, V.N.; et al. Oncomatrix: Molecular Composition and Biomechanical Properties of the Extracellular Matrix in Human Tumors. J. Mol. Pathol. 2024, 5, 437–453. [Google Scholar] [CrossRef]
- Jia, H.; Janjanam, J.; Wu, S.C.; Wang, R.; Pano, G.; Celestine, M.; Martinot, O.; Breeze-Jones, H.; Clayton, G.; Garcin, C.; et al. The tumor cell-secreted matricellular protein WISP 1 drives pro-metastatic collagen linearization. EMBO J. 2019, 38, e101302. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.Q.; Wang, S.B.; Liu, J.H.; Jin, L.; Liu, Y.; Li, C.Y.; Su, Y.R.; Liu, Y.R.; Sang, X.; Wan, Q.; et al. Modifying the tumour microenvironment and reverting tumour cells: New strategies for treating malignant tumours. Cell Prolif. 2020, 53, e12865. [Google Scholar] [CrossRef] [PubMed]
- Klabukov, I.; Kabakov, A.E.; Yakimova, A.; Baranovskii, D.; Sosin, D.; Atiakshin, D.; Ignatyuk, M.; Yatsenko, E.; Rybachuk, V.; Evstratova, E.; et al. Tumor-Associated Extracellular Matrix Obstacles for CAR-T Cell Therapy: Approaches to Overcoming. Curr. Oncol. 2025, 32, 79. [Google Scholar] [CrossRef]
- El-Mashtoly, S.F.; Gerwert, K. Diagnostics and therapy assessment using label-free Raman imaging. Anal. Chem. 2021, 94, 120–142. [Google Scholar] [CrossRef] [PubMed]
- Gulyaev, Y.V.; Proklov, V.V.; Shkerdin, G.N. Diffraction of light by sound in solids. Sov. Phys. Uspekhi 1978, 21, 29. [Google Scholar] [CrossRef]
- Brown, E.; Brunker, J.; Bohndiek, S.E. Photoacoustic imaging as a tool to probe the tumour microenvironment. Dis. Models mechanisms. 2019, 12, dmm039636. [Google Scholar] [CrossRef]
- Marozik, P.; Mosse, I.; Mothersill, C.; Seymour, C. Protection by chemicals against radiation-induced bystander effects. In Multiple Stressors: A Challenge for the Future; Springer: Dordrecht, The Netherlands, 2007; pp. 247–262. [Google Scholar]
- Fitzgerald, A.J.; Berry, E.; Zinov’ev, N.N.; Homer-Vanniasinkam, S.; Miles, R.E.; Chamberlain, J.M.; Smith, M.A. Catalogue of human tissue optical properties at terahertz frequencies. J. Biol. Phys. 2003, 29, 123–128. [Google Scholar] [CrossRef]
- Madl, P.; Renati, P. Quantum Electrodynamics Coherence and Hormesis: Foundations of Quantum Biology. Int. J. Mol. Sci. 2023, 24, 14003. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Müller, C.A.; Nørrehvedde Jensen, B.; Chen, M. Embracing Remote Fields as the Fourth Dimension of Tissue Biofabrication. Adv. Funct. Mater. 2024, 34, 2401654. [Google Scholar] [CrossRef]
- Parousis-Paraskevas, O.; Gkikoudi, A.; Al-Qaaod, A.; Vasilopoulos, S.N.; Manda, G.; Beinke, C.; Haghdoost, S.; Terzoudi, G.I.; Krasniqi, F.; Georgakilas, A.G. Combined Radiations: Biological Effects of Mixed Exposures Across the Radiation Spectrum. Biomolecules 2025, 15, 1282. [Google Scholar] [CrossRef]
- Gkikoudi, A.; Manda, G.; Beinke, C.; Giesen, U.; Al-Qaaod, A.; Dragnea, E.-M.; Dobre, M.; Neagoe, I.V.; Sangsuwan, T.; Haghdoost, S.; et al. Synergistic Effects of UVB and Ionizing Radiation on Human Non-Malignant Cells: Implications for Ozone Depletion and Secondary Cosmic Radiation Exposure. Biomolecules 2025, 15, 536. [Google Scholar] [CrossRef] [PubMed]
- Menozzi, L.; Yao, J. Deep tissue photoacoustic imaging with light and sound. npj Imaging 2024, 2, 44. [Google Scholar] [CrossRef]
- Englert, L.; Jüstel, D.; Ntziachristos, V. The need for optoacoustic microscopy. Rev. Mod. Phys. 2025, 97, 015005. [Google Scholar] [CrossRef]
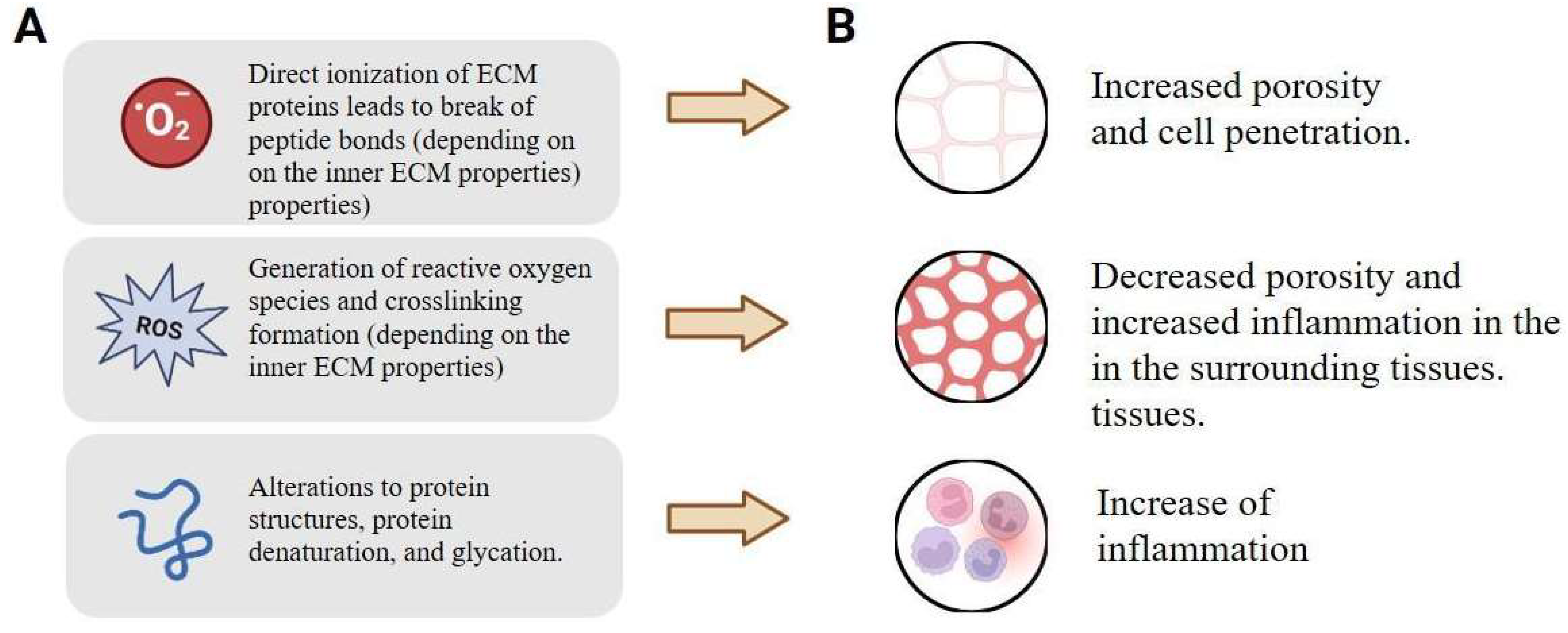
| Factor | Source of Differences | Effect | Refs |
|---|---|---|---|
| ECM organization | The presence of spatial patterns, preferred direction and linear organization of fibers. | Stimulating a specific optical image and density pattern. | [104,110,111] |
| ECM density | Reduced density leads to a reduction in the proportion of macromolecules and increases hydration. | Increased water content leads to local overheating and reduces the radiation penetration. | [112,113,114] |
| Specific metabolites presence | Excess presence of electron donors. | Specific effects of radiation scattering. | [106,115,116] |
| Cellular Structure | Changes in the structure and composition of membrane lipids, organelle density, and cytoskeletal organization. | Increased multiphoton absorption and FRET efficiency, leading to enhanced nonlinear signaling and potential cellular stress responses. | [115,116,117,118] |
| Metabolic States | Significant differences in ATP levels, enzyme activity and redox potential in different tissues. | Modulation of photon–phonon thermal effects, causing uneven heating and changes in metabolic rate, synergistically combined with resonant energy transfer for targeted biomodulation. | [119,120,121] |
| Tissue oxygenation | Heterogeneous oxygen gradients caused by perfusion and hypoxia in various areas. | Influence on the scattering and absorption of boson concentrates, leading to local oxidative stress or increased penetration into hypoxic areas. | [122,123,124] |
| Neural connections | Changes in synaptic density and neurotransmitter profiles in neural tissue. | Enhancement of nonlinear signal transmission via FRET and multiphoton absorption, leading to altered neuromodulation or excitotoxicity. | [56,125,126,127] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klabukov, I.; Eygel, D.; Isaeva, E.; Kisel, A.; Isaev, E.I.; Potievskiy, M.; Atiakshin, D.; Shestakova, V.; Baranovskii, D.; Akhmedov, B.; et al. Synergistic Effects of Non-Ionizing Radiation in the Targeted Modification of Living Tissues. Int. J. Mol. Sci. 2025, 26, 11415. https://doi.org/10.3390/ijms262311415
Klabukov I, Eygel D, Isaeva E, Kisel A, Isaev EI, Potievskiy M, Atiakshin D, Shestakova V, Baranovskii D, Akhmedov B, et al. Synergistic Effects of Non-Ionizing Radiation in the Targeted Modification of Living Tissues. International Journal of Molecular Sciences. 2025; 26(23):11415. https://doi.org/10.3390/ijms262311415
Chicago/Turabian StyleKlabukov, Ilya, Daria Eygel, Elena Isaeva, Anastas Kisel, Evgeny I. Isaev, Mikhail Potievskiy, Dmitrii Atiakshin, Victoria Shestakova, Denis Baranovskii, Bagavdin Akhmedov, and et al. 2025. "Synergistic Effects of Non-Ionizing Radiation in the Targeted Modification of Living Tissues" International Journal of Molecular Sciences 26, no. 23: 11415. https://doi.org/10.3390/ijms262311415
APA StyleKlabukov, I., Eygel, D., Isaeva, E., Kisel, A., Isaev, E. I., Potievskiy, M., Atiakshin, D., Shestakova, V., Baranovskii, D., Akhmedov, B., Sulina, Y., Skornyakova, E., Shegay, P., & Kaprin, A. D. (2025). Synergistic Effects of Non-Ionizing Radiation in the Targeted Modification of Living Tissues. International Journal of Molecular Sciences, 26(23), 11415. https://doi.org/10.3390/ijms262311415









