Anti-Cancer Outcome of Glucocorticoid Receptor Transrepression by Synephrine Derivatives in Hematological Malignancies
Abstract
1. Introduction
2. Results
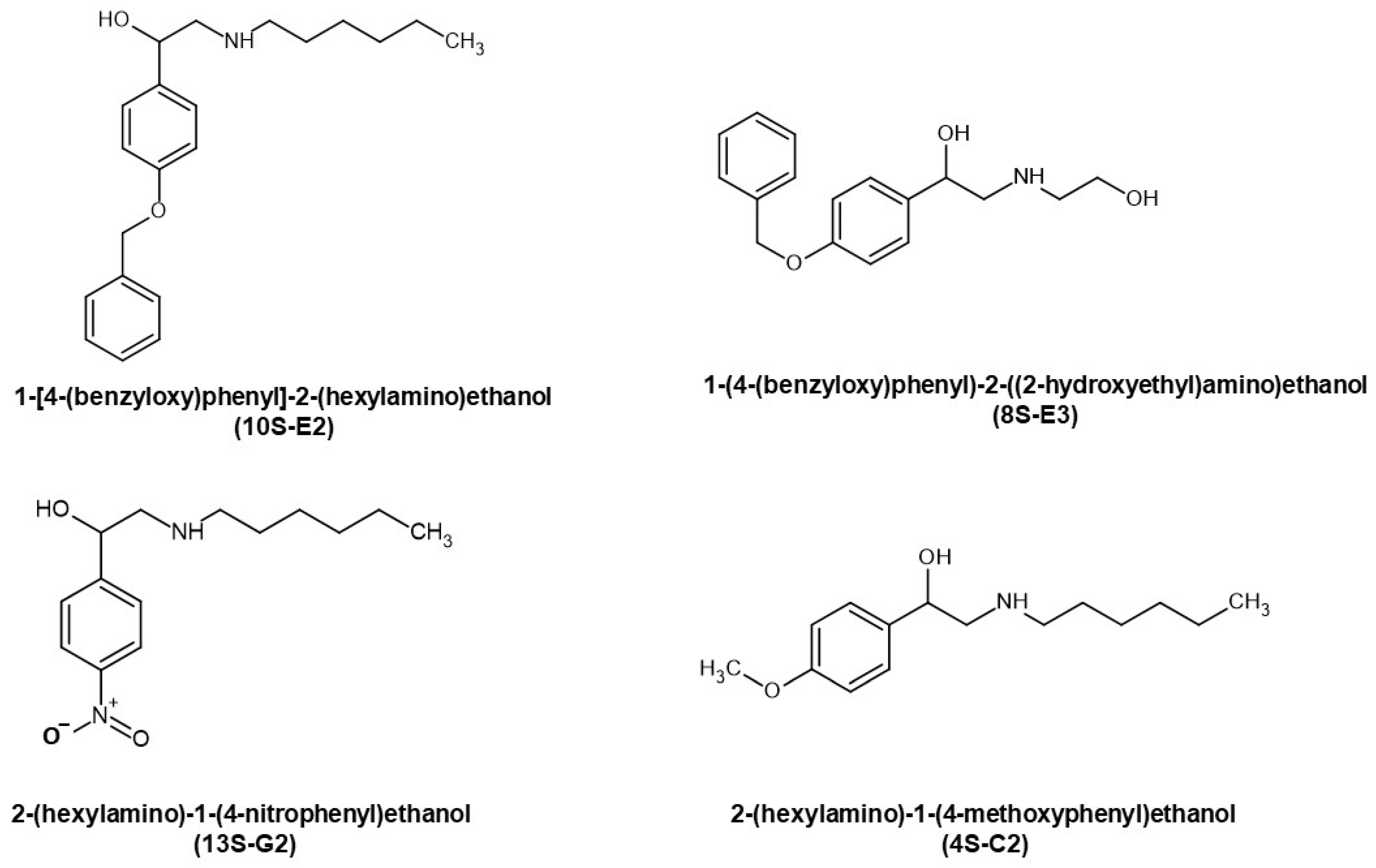
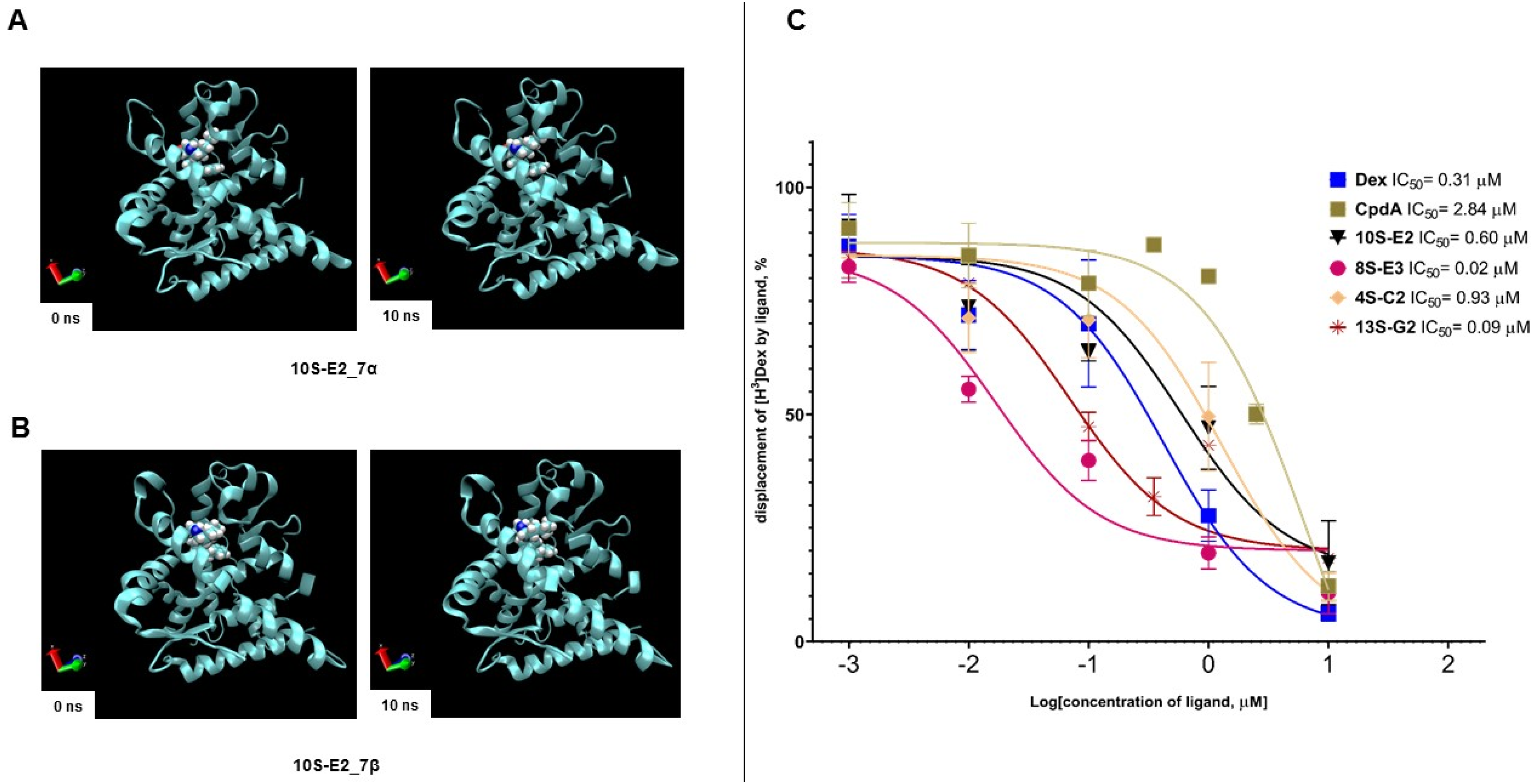
2.1. Evaluation of Affinity of Synephrine Derivatives to GR
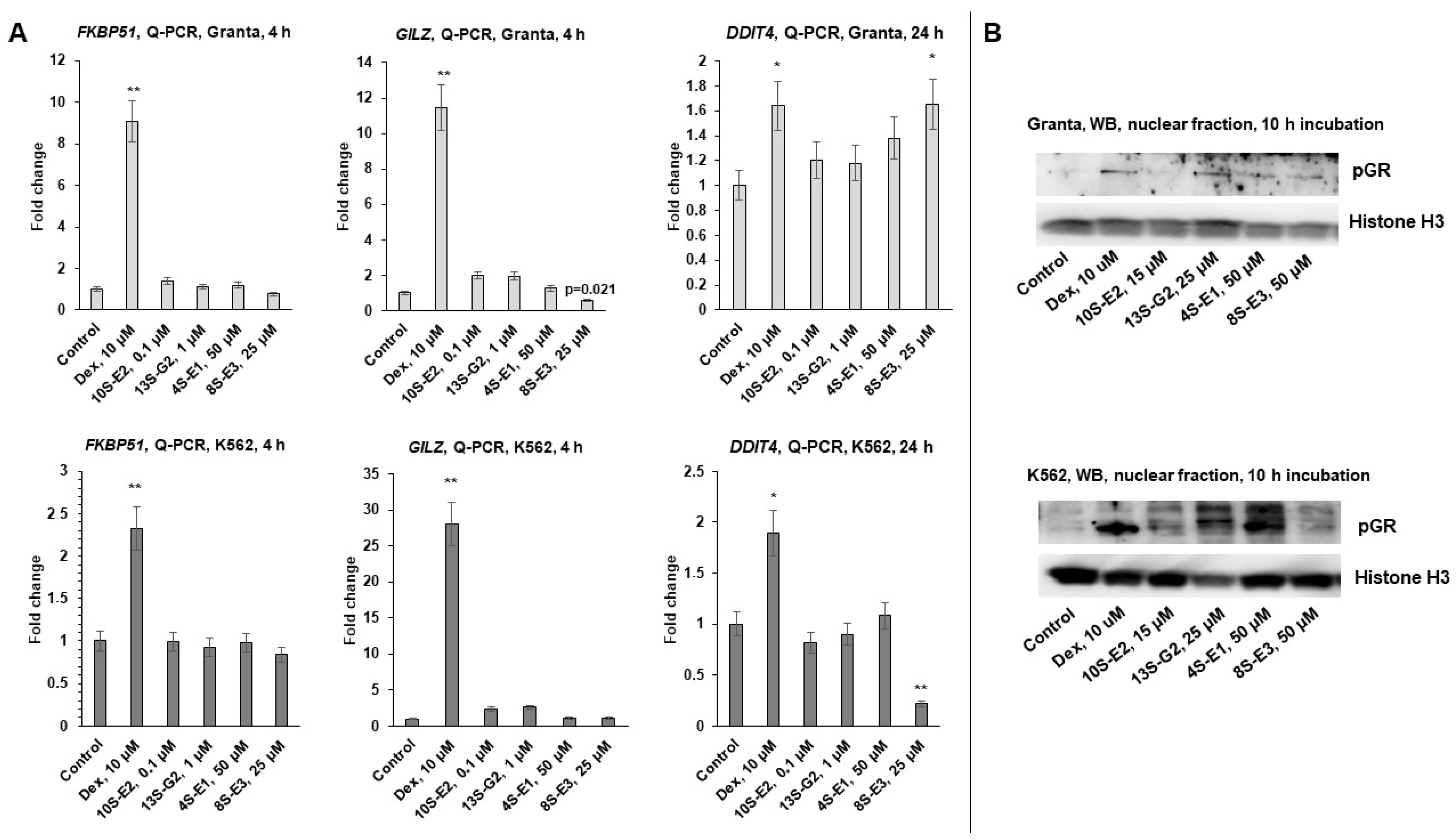
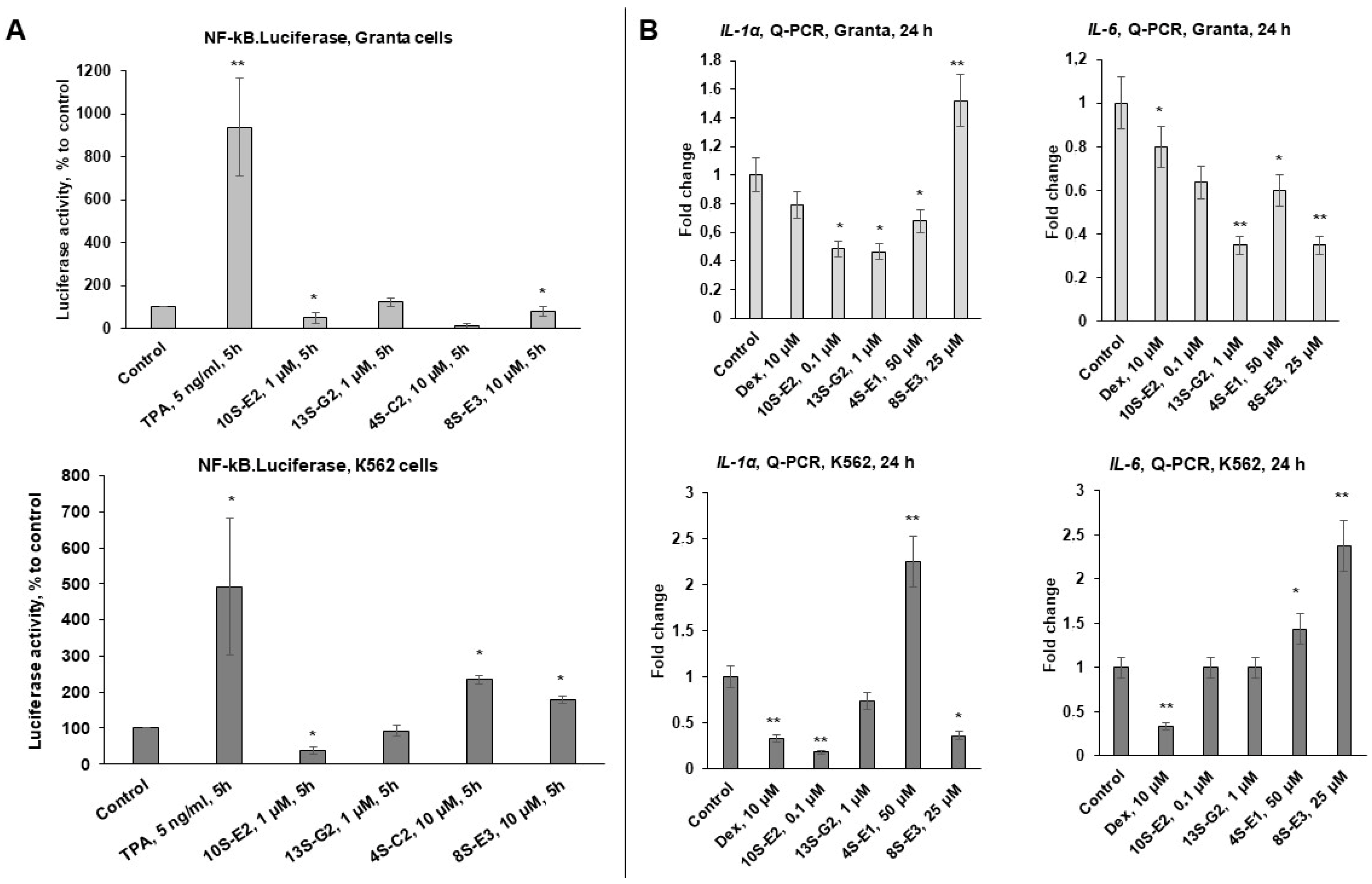
2.2. Effects of Synephrine Derivatives on GR Functions
2.3. Pro-Apoptotic and Anti-Cancer Effects of Synephrine Derivatives In Vitro and In Vivo
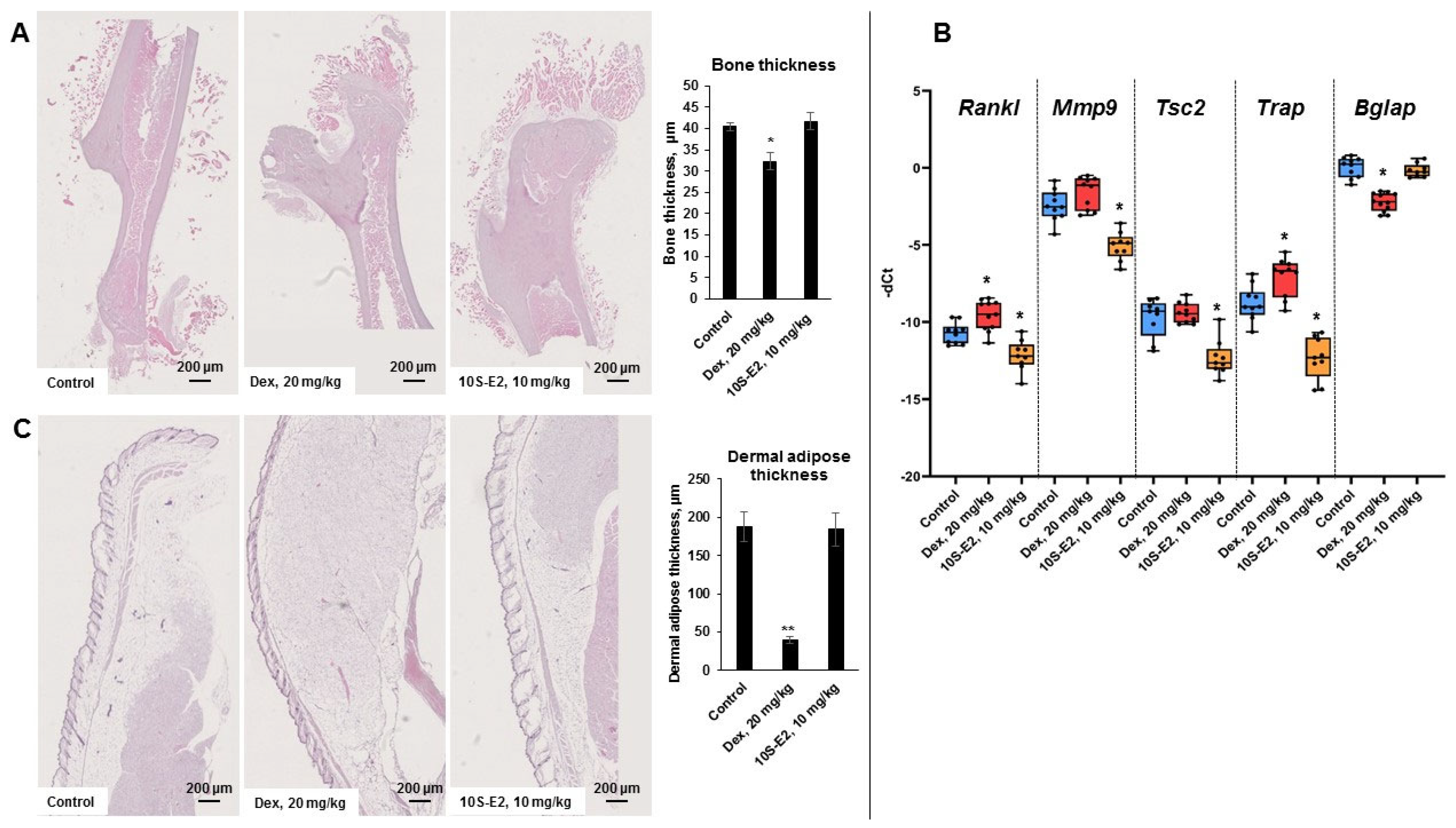
2.4. 10S-E2 Did Not Induce Atrophic Changes in Skin and Bone Tissue Compared to Dex
3. Discussion
4. Materials and Methods
4.1. Cells and Treatments
4.2. Cell Cycle Analysis
4.3. Western Blot Analysis
4.4. Human Peripheral Blood Mononuclear Cell (PBMC) Isolation and Culture
4.5. Resazurin Cytotoxicity Assay
4.6. RNA Extraction and Q-PCR
4.7. Lentivirus Preparation and Cell Transduction
4.8. Luciferase Assay
4.9. Molecular Docking and Molecular Dynamics Simulation
4.10. GR Binding Affinity Assay
4.11. Anti-Cancer Study In Vivo
4.12. Glucocorticoid-Induced Osteoporosis
4.13. Histology and Morphometry
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALL | acute lymphoblastic leukemia |
| Bglap | bone gamma-carboxyglutamic acid-containing protein |
| CMV | cytomegalovirus |
| CpdA | compound A |
| DDIT4 | DNA-damage-inducible transcript 4 |
| Dex | dexamethasone |
| FKBP51 | FK506-binding protein 51 |
| GC | glucocorticoid |
| GILZ | glucocorticoid-induced leucine zipper |
| GIOP | glucocorticoid-induced osteoporosis |
| GR | glucocorticoid receptor |
| GRE | glucocorticoid-responsive element |
| H&E | hematoxylin and eosin |
| HEK | human embryonic kidney |
| IC50 | 50% inhibitory concentration |
| IL1-α | interleukin 1-α |
| IL6 | interleukin 6 |
| Mmp9 | matrix metalloproteinase-9 |
| PBMC | peripheral bone marrow cell |
| Q-PCR | quantitative polymerase chain reaction |
| Rankl | receptor activator of nuclear factor kappa-Β |
| RMSD | root mean square deviation |
| RPL27 | ribosomal protein 27 |
| SEGRAM | selective glucocorticoid receptor agonist/modulator |
| TA | transactivation |
| TPA | 12-O-tetradecanoylphorbol-13-acetate |
| TR | transrepression |
| Trap | tartrate-resistant acid phosphatase |
| TSC2 | tuberoses sclerosis complex 2 |
References
- Buttgereit, F. Views on Glucocorticoid Therapy in Rheumatology: The Age of Convergence. Nat. Rev. Rheumatol. 2020, 16, 239–246. [Google Scholar] [CrossRef]
- Sundahl, N.; Bridelance, J.; Libert, C.; De Bosscher, K.; Beck, I.M. Selective Glucocorticoid Receptor Modulation: New Directions with Non-Steroidal Scaffolds. Pharmacol. Ther. 2015, 152, 28–41. [Google Scholar] [CrossRef]
- Eiers, A.-K.; Vettorazzi, S.; Tuckermann, J.P. Journey through Discovery of 75 Years Glucocorticoids: Evolution of Our Knowledge of Glucocorticoid Receptor Mechanisms in Rheumatic Diseases. Ann. Rheum. Dis. 2024, 83, 1603–1613. [Google Scholar] [CrossRef]
- Cain, D.W.; Cidlowski, J.A. Specificity and Sensitivity of Glucocorticoid Signaling in Health and Disease. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Jakob, F.; Hennen, S.; Gautrois, M.; Khalil, F.; Lockhart, A. Novel Selective Glucocorticoid Receptor Modulator GRM-01 Demonstrates Dissociation of Anti-Inflammatory Effects from Adverse Effects on Glucose and Bone Metabolism. Front. Pharmacol. 2025, 16, 1542351. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-X.; Cummins, C.L. Fresh Insights into Glucocorticoid-Induced Diabetes Mellitus and New Therapeutic Directions. Nat. Rev. Endocrinol. 2022, 18, 540–557. [Google Scholar] [CrossRef] [PubMed]
- Van Staa, T.P. The Pathogenesis, Epidemiology and Management of Glucocorticoid-Induced Osteoporosis. Calcif. Tissue Int. 2006, 79, 129–137. [Google Scholar] [CrossRef]
- De Bosscher, K.; Haegeman, G. Minireview: Latest Perspectives on Antiinflammatory Actions of Glucocorticoids. Mol. Endocrinol. 2009, 23, 281–291. [Google Scholar] [CrossRef]
- Bhattacharyya, T.; Das, P.; Banerjee, R. Targeting Steroid Hormone Receptors for Anti-Cancer Therapy. In Vitamins and Hormones; Elsevier: Amsterdam, The Netherlands, 2025; Volume 129, pp. 1–59. ISBN 978-0-443-29548-5. [Google Scholar]
- Permpoon, U.; Moon, J.; Kim, C.Y.; Nam, T. Glucocorticoid-Mediated Skeletal Muscle Atrophy: Molecular Mechanisms and Potential Therapeutic Targets. Int. J. Mol. Sci. 2025, 26, 7616. [Google Scholar] [CrossRef]
- Pufall, M.A. Glucocorticoids and Cancer. In Glucocorticoid Signaling; Wang, J.-C., Harris, C., Eds.; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2015; Volume 872, pp. 315–333. ISBN 978-1-4939-2894-1. [Google Scholar]
- Lucafò, M.; Franzin, M.; Decorti, G.; Stocco, G. A Patent Review of Anticancer Glucocorticoid Receptor Modulators (2014-Present). Expert Opin. Ther. Pat. 2020, 30, 313–324. [Google Scholar] [CrossRef]
- Scheijen, B. Molecular Mechanisms Contributing to Glucocorticoid Resistance in Lymphoid Malignancies. CDR 2019, 2, 647–664. [Google Scholar] [CrossRef]
- Zhidkova, E.M.; Tilova, L.R.; Fetisov, T.I.; Kirsanov, K.I.; Kulikov, E.P.; Enikeev, A.D.; Budunova, I.V.; Badun, G.A.; Chernysheva, M.G.; Shirinian, V.Z.; et al. Synthesis and Anti-Cancer Activity of the Novel Selective Glucocorticoid Receptor Agonists of the Phenylethanolamine Series. Int. J. Mol. Sci. 2024, 25, 8904. [Google Scholar] [CrossRef]
- Hsu, S.-J.; He, M.; Salomé-Abarca, L.F.; Choi, Y.H.; Wang, M. Uncovering Anti-Inflammatory Activity of Ginsenoside Rg1 in a Wound-Inured Zebrafish Model by GC-MS-Based Chemical Profiling. Planta Med. 2025, 91, 609–620. [Google Scholar] [CrossRef]
- Lesovaya, E.A.; Chudakova, D.; Baida, G.; Zhidkova, E.M.; Kirsanov, K.I.; Yakubovskaya, M.G.; Budunova, I.V. The Long Winding Road to the Safer Glucocorticoid Receptor (GR) Targeting Therapies. Oncotarget 2022, 13, 408–424. [Google Scholar] [CrossRef]
- Zhidkova, E.M.; Lylova, E.S.; Savinkova, A.V.; Mertsalov, S.A.; Kirsanov, K.I.; Belitsky, G.A.; Yakubovskaya, M.G.; Lesovaya, E.A. A Brief Overview of the Paradoxical Role of Glucocorticoids in Breast Cancer. Breast Cancer 2020, 14, 1178223420974667. [Google Scholar] [CrossRef] [PubMed]
- Lesovaya, E.; Yemelyanov, A.; Swart, A.C.; Swart, P.; Haegeman, G.; Budunova, I. Discovery of Compound A—A Selective Activator of the Glucocorticoid Receptor with Anti-Inflammatory and Anti-Cancer Activity. Oncotarget 2015, 6, 30730–30744. [Google Scholar] [CrossRef]
- Lesovaya, E.; Yemelyanov, A.; Kirsanov, K.; Popa, A.; Belitsky, G.; Yakubovskaya, M.; Gordon, L.I.; Rosen, S.T.; Budunova, I. Combination of a Selective Activator of the Glucocorticoid Receptor Compound A with a Proteasome Inhibitor as a Novel Strategy for Chemotherapy of Hematologic Malignancies. Cell Cycle 2013, 12, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Buttgereit, F.; Elling, C.; Jakob, F. Design and Development of Glucocorticoid Receptor Modulators. Trends Pharmacol. Sci. 2025, 46, 771–791. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.N.; Fuhr, R.; Beier, J.; Su, H.-L.; Chen, Y.; Forsman, H.; Hamrén, U.W.; Jackson, H.; Aggarwal, A. Efficacy and Safety of AZD7594, an Inhaled Non-Steroidal Selective Glucocorticoid Receptor Modulator, in Patients with Asthma: A Phase 2a Randomized, Double Blind, Placebo-Controlled Crossover Trial. Respir. Res. 2019, 20, 37. [Google Scholar] [CrossRef]
- Cheng, F.; Shen, T.; Zhang, F.; Lei, C.; Zhu, Y.; Luo, G.; Xiao, D. Bioequivalence Study of Fluticasone Propionate Nebuliser Suspensions in Healthy Chinese Subjects. Front. Pharmacol. 2025, 15, 1452596. [Google Scholar] [CrossRef]
- Hunt, H.; Donaldson, K.; Strem, M.; Zann, V.; Leung, P.; Sweet, S.; Connor, A.; Combs, D.; Belanoff, J. Assessment of Safety, Tolerability, Pharmacokinetics, and Pharmacological Effect of Orally Administered CORT125134: An Adaptive, Double-Blind, Randomized, Placebo-Controlled Phase 1 Clinical Study. Clin. Pharmacol. Drug Dev. 2018, 7, 408–421. [Google Scholar] [CrossRef]
- Kuna, P.; Aurivillius, M.; Jorup, C.; Prothon, S.; Taib, Z.; Edsbäcker, S. Efficacy and Tolerability of an Inhaled Selective Glucocorticoid Receptor Modulator—AZD5423—In Chronic Obstructive Pulmonary Disease Patients: Phase II Study Results. Basic Clin. Pharmacol. Toxicol. 2017, 121, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Werkström, V.; Prothon, S.; Ekholm, E.; Jorup, C.; Edsbäcker, S. Safety, Pharmacokinetics and Pharmacodynamics of the Selective Glucocorticoid Receptor Modulator AZD5423 after Inhalation in Healthy Volunteers. Basic Clin. Pharmacol. Toxicol. 2016, 119, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Conrado, D.J.; Krishnaswami, S.; Shoji, S.; Kolluri, S.; Hey-Hadavi, J.; McCabe, D.; Rojo, R.; Tammara, B.K. Predicting the Probability of Successful Efficacy of a Dissociated Agonist of the Glucocorticoid Receptor from Dose–Response Analysis. J. Pharmacokinet. Pharmacodyn. 2016, 43, 325–341. [Google Scholar] [CrossRef] [PubMed]
- Bareille, P.; Hardes, K.; Donald, A.C. Efficacy and Safety of Once-Daily GW870086 a Novel Selective Glucocorticoid in Mild-Moderate Asthmatics: A Randomised, Two-Way Crossover, Controlled Clinical Trial. J. Asthma 2013, 50, 1077–1082. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, Y.; Li, X.; Ding, J.; Huang, J.; Lian, K.; Duan, P.; Hu, C.; Xu, J. Unraveling Ginsenoside Rg1’s Osteoprotective Pathways in Zebrafish Models of Glucocorticoid Induced Osteoporosis via Transcriptomics. Sci. Rep. 2025, 15, 30519. [Google Scholar] [CrossRef]
- Harcken, C.; Scholl, P.; Nabozny, G.; Thomson, D.; Bianchi, D. Clinical Profile of the Functionally Selective Glucocorticoid Receptor Agonist BI 653048 in Healthy Male Subjects. Expert Opin. Investig. Drugs 2019, 28, 489–496. [Google Scholar] [CrossRef]
- Weatherley, B.; McFadyen, L.; Tammara, B. Population Pharmacokinetics of Fosdagrocorat (PF-04171327), a Dissociated Glucocorticoid Receptor Agonist, in Patients With Rheumatoid Arthritis. Clin. Transl. Sci. 2018, 11, 54–62. [Google Scholar] [CrossRef]
- Dodonova, S.A.; Zhidkova, E.M.; Kryukov, A.A.; Valiev, T.T.; Kirsanov, K.I.; Kulikov, E.P.; Budunova, I.V.; Yakubovskaya, M.G.; Lesovaya, E.A. Synephrine and Its Derivative Compound A: Common and Specific Biological Effects. Int. J. Mol. Sci. 2023, 24, 17537. [Google Scholar] [CrossRef]
- Zhidkova, E.M.; Oleynik, E.S.; Mikhina, E.A.; Stepanycheva, D.V.; Grigoreva, D.D.; Grebenkina, L.E.; Gordeev, K.V.; Savina, E.D.; Matveev, A.V.; Yakubovskaya, M.G.; et al. Synthesis and Anti-Cancer Activity In Vitro of Synephrine Derivatives. Biomolecules 2024, 15, 2. [Google Scholar] [CrossRef]
- Brandon, D.D.; Kendall, J.W.; Alman, K.; Tower, P.; Loriaux, D.L. Inhibition of Dexamethasone Binding to Human Glucocorticoid Receptor by New World Primate Cell Extracts. Steroids 1995, 60, 463–466. [Google Scholar] [CrossRef]
- Yemelyanov, A.; Czwornog, J.; Gera, L.; Joshi, S.; Chatterton, R.T.; Budunova, I. Novel Steroid Receptor Phyto-Modulator Compound a Inhibits Growth and Survival of Prostate Cancer Cells. Cancer Res. 2008, 68, 4763–4773. [Google Scholar] [CrossRef]
- Ronacher, K.; Hadley, K.; Avenant, C.; Stubsrud, E.; Simons, S.S., Jr.; Louw, A.; Hapgood, J.P. Ligand-Selective Transactivation and Transrepression via the Glucocorticoid Receptor: Role of Cofactor Interaction. Mol. Cell. Endocrinol. 2009, 299, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Lesovaya, E.; Agarwal, S.; Readhead, B.; Vinokour, E.; Baida, G.; Bhalla, P.; Kirsanov, K.; Yakubovskaya, M.; Platanias, L.C.; Dudley, J.T.; et al. Rapamycin Modulates Glucocorticoid Receptor Function, Blocks Atrophogene REDD1, and Protects Skin from Steroid Atrophy. J. Investig. Dermatol. 2018, 138, 1935–1944. [Google Scholar] [CrossRef] [PubMed]
- Baida, G.; Bhalla, P.; Kirsanov, K.; Lesovaya, E.; Yakubovskaya, M.; Yuen, K.; Guo, S.; Lavker, R.M.; Readhead, B.; Dudley, J.T.; et al. REDD1 Functions at the Crossroads between the Therapeutic and Adverse Effects of Topical Glucocorticoids. EMBO Mol. Med. 2015, 7, 42–58. [Google Scholar] [CrossRef]
- Yang, N.; Baban, B.; Isales, C.M.; Shi, X.-M. Role of Glucocorticoid-Induced Leucine Zipper (GILZ) in Inflammatory Bone Loss. PLoS ONE 2017, 12, e0181133. [Google Scholar] [CrossRef]
- Fan, H.; Kao, W.; Yang, Y.H.; Gu, R.; Harris, J.; Fingerle-Rowson, G.; Bucala, R.; Ngo, D.; Beaulieu, E.; Morand, E.F. Macrophage Migration Inhibitory Factor Inhibits the Antiinflammatory Effects of Glucocorticoids via Glucocorticoid-Induced Leucine Zipper. Arthritis Rheumatol. 2014, 66, 2059–2070. [Google Scholar] [CrossRef]
- Agarwal, S.; Mirzoeva, S.; Readhead, B.; Dudley, J.T.; Budunova, I. PI3K Inhibitors Protect against Glucocorticoid-Induced Skin Atrophy. EBioMedicine 2019, 41, 526–537. [Google Scholar] [CrossRef]
- Carruthers, C.W.; Suh, J.H.; Gustafsson, J.-A.; Webb, P. Phosphorylation of Glucocorticoid Receptor Tau1c Transactivation Domain Enhances Binding to CREB Binding Protein (CBP) TAZ2. Biochem. Biophys. Res. Commun. 2015, 457, 119–123. [Google Scholar] [CrossRef]
- Tonsing-Carter, E.; Hernandez, K.M.; Kim, C.R.; Harkless, R.V.; Oh, A.; Bowie, K.R.; West-Szymanski, D.C.; Betancourt-Ponce, M.A.; Green, B.D.; Lastra, R.R.; et al. Glucocorticoid Receptor Modulation Decreases ER-Positive Breast Cancer Cell Proliferation and Suppresses Wild-Type and Mutant ER Chromatin Association. Breast Cancer Res. 2019, 21, 82. [Google Scholar] [CrossRef]
- Cai, L.; Hua, C.; Geng, Y.; Chen, Q.; Niu, L.; Tao, S.; Ni, Y.; Zhao, R. Chronic Dexamethasone Exposure Activates the TLR4-Mediated Inflammation Pathway and Induces Epithelial Apoptosis in the Goat Colon. Biochem. Biophys. Res. Commun. 2019, 518, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Cacheiro-Llaguno, C.; Hernández-Subirá, E.; Díaz-Muñoz, M.D.; Fresno, M.; Serrador, J.M.; Íñiguez, M.A. Regulation of Cyclooxygenase-2 Expression in Human T Cells by Glucocorticoid Receptor-Mediated Transrepression of Nuclear Factor of Activated T Cells. Int. J. Mol. Sci. 2022, 23, 13275. [Google Scholar] [CrossRef] [PubMed]
- Lesovaya, E.A.; Savinkova, A.V.; Morozova, O.V.; Lylova, E.S.; Zhidkova, E.M.; Kulikov, E.P.; Kirsanov, K.I.; Klopot, A.; Baida, G.; Yakubovskaya, M.G.; et al. A Novel Approach to Safer Glucocorticoid Receptor-Targeted Anti-Lymphoma Therapy via REDD1 (Regulated in Development and DNA Damage 1) Inhibition. Mol. Cancer Ther. 2020, 19, 1898–1908. [Google Scholar] [CrossRef] [PubMed]
- Dexamethasone Taw—Withdrawal Assessment Report EMA/78138/2021. 2020. Available online: https://www.ema.europa.eu/en/documents/withdrawal-report/withdrawal-assessment-report-dexamethasone-taw_en.pdf (accessed on 19 November 2025).
- Shimizu, N.; Yoshikawa, N.; Ito, N.; Maruyama, T.; Suzuki, Y.; Takeda, S.; Nakae, J.; Tagata, Y.; Nishitani, S.; Takehana, K.; et al. Crosstalk between Glucocorticoid Receptor and Nutritional Sensor mTOR in Skeletal Muscle. Cell Metab. 2011, 13, 170–182. [Google Scholar] [CrossRef]
- Frenkel, B.; White, W.; Tuckermann, J. Glucocorticoid-Induced Osteoporosis. In Glucocorticoid Signaling; Wang, J.-C., Harris, C., Eds.; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2015; Volume 872, pp. 179–215. ISBN 978-1-4939-2894-1. [Google Scholar]
- McLaughlin, F.; Mackintosh, J.; Hayes, B.P.; McLaren, A.; Uings, I.J.; Salmon, P.; Humphreys, J.; Meldrum, E.; Farrow, S.N. Glucocorticoid-Induced Osteopenia in the Mouse as Assessed by Histomorphometry, Microcomputed Tomography, and Biochemical Markers. Bone 2002, 30, 924–930. [Google Scholar] [CrossRef]
- Dodonova, S.A.; Zhidkova, E.M.; Kryukov, A.A.; Valiev, T.T.; Kulikov, E.P.; Yakubovskaya, M.G.; Lesovaya, E.A. Side Effects of Glucocorticoids: In Vivo Models and Underlying Mechanisms. Discov. Med. 2025, 2, 212. [Google Scholar] [CrossRef]
- Wu, T.; Wang, F.; Ai, C.; Li, L.; Wu, F. Tangeretin Suppresses RANKL-Induced Osteoclastogenesis and Alleviates Postmenopausal Osteoporosis by Inhibiting Notch Signaling. Regen. Ther. 2025, 30, 136–143. [Google Scholar] [CrossRef]
- Zhu, G.; Chen, W.; Tang, C.-Y.; McVicar, A.; Edwards, D.; Wang, J.; McConnell, M.; Yang, S.; Li, Y.; Chang, Z.; et al. Knockout and Double Knockout of Cathepsin K and Mmp9 Reveals a Novel Function of Cathepsin K as a Regulator of Osteoclast Gene Expression and Bone Homeostasis. Int. J. Biol. Sci. 2022, 18, 5522–5538. [Google Scholar] [CrossRef]
- Xiong, K.; Li, J.; Liu, Y.; Pan, Y.; Huang, Y.; Zhan, D.; Zhang, L.; Tang, M.; Li, J.; Sun, H. Lacticaseibacillus Rhamnosus LGG Suppresses Osteoclastogenesis via TLR6/NF-κB Modulation and Attenuates Ovariectomy-Induced Bone Loss in Mice. Probiotics Antimicrob. Proteins 2025, 1–17. [Google Scholar] [CrossRef]
- Salemdawod, A.; Cooper, B.; Liang, Y.; Walczak, P.; Vatter, H.; Maciaczyk, J.; Janowski, M. CRISPR-Cas9 Single Nucleotide Editing of Tuberous Sclerosis Complex 2 Gene in Mesenchymal Stem Cells. CRISPR J. 2025, 25731599251367059. [Google Scholar] [CrossRef]
- Komori, T. Regulation of Skeletal Development and Maintenance by Runx2 and Sp7. Int. J. Mol. Sci. 2024, 25, 10102. [Google Scholar] [CrossRef]
- Huang, Y.; Seitz, D.; Chevalier, Y.; Müller, P.E.; Jansson, V.; Klar, R.M. Synergistic Interaction of hTGF-β3 with hBMP-6 Promotes Articular Cartilage Formation in Chitosan Scaffolds with hADSCs: Implications for Regenerative Medicine. BMC Biotechnol. 2020, 20, 48. [Google Scholar] [CrossRef] [PubMed]
- Hua, G.; Ganti, K.P.; Chambon, P. Glucocorticoid-Induced Tethered Transrepression Requires SUMOylation of GR and Formation of a SUMO-SMRT/NCoR1-HDAC3 Repressing Complex. Proc. Natl. Acad. Sci. USA 2016, 113, E635–E643. [Google Scholar] [CrossRef] [PubMed]
- Baiula, M.; Bedini, A.; Baldi, J.; Cavet, M.E.; Govoni, P.; Spampinato, S. Mapracorat, a Selective Glucocorticoid Receptor Agonist, Causes Apoptosis of Eosinophils Infiltrating the Conjunctiva in Late-Phase Experimental Ocular Allergy. Drug Des. Dev. Ther. 2014, 8, 745–757. [Google Scholar] [CrossRef] [PubMed]
- De Bosscher, K.; Berghe, W.V.; Beck, I.M.E.; Van Molle, W.; Hennuyer, N.; Hapgood, J.; Libert, C.; Staels, B.; Louw, A.; Haegeman, G. A Fully Dissociated Compound of Plant Origin for Inflammatory Gene Repression. Proc. Natl. Acad. Sci. USA 2005, 102, 15827–15832. [Google Scholar] [CrossRef] [PubMed]
- Hua, G.; Zein, N.; Daubeuf, F.; Chambon, P. Glucocorticoid Receptor Modulators CpdX and CpdX-D3 Exhibit the Same In Vivo Antiinflammatory Activities as Synthetic Glucocorticoids. Proc. Natl. Acad. Sci. USA 2019, 116, 14191–14199. [Google Scholar] [CrossRef]
- Hua, G.; Zein, N.; Paulen, L.; Chambon, P. The Glucocorticoid Receptor Agonistic Modulators CpdX and CpdX-D3 Do Not Generate the Debilitating Effects of Synthetic Glucocorticoids. Proc. Natl. Acad. Sci. USA 2019, 116, 14200–14209. [Google Scholar] [CrossRef]
- Coghlan, M.J.; Jacobson, P.B.; Lane, B.; Nakane, M.; Lin, C.W.; Elmore, S.W.; Kym, P.R.; Luly, J.R.; Carter, G.W.; Turner, R.; et al. A Novel Antiinflammatory Maintains Glucocorticoid Efficacy with Reduced Side Effects. Mol. Endocrinol. 2003, 17, 860–869. [Google Scholar] [CrossRef]
- van Lierop, M.-J.C.; Alkema, W.; Laskewitz, A.J.; Dijkema, R.; van der Maaden, H.M.; Smit, M.J.; Plate, R.; Conti, P.G.M.; Jans, C.G.J.M.; Timmers, C.M.; et al. Org 214007-0: A Novel Non-Steroidal Selective Glucocorticoid Receptor Modulator with Full Anti-Inflammatory Properties and Improved Therapeutic Index. PLoS ONE 2012, 7, e48385. [Google Scholar] [CrossRef]
- De Bosscher, K.; Beck, I.M.; Haegeman, G. Classic Glucocorticoids versus Non-Steroidal Glucocorticoid Receptor Modulators: Survival of the Fittest Regulator of the Immune System? Brain Behav. Immun. 2010, 24, 1035–1042. [Google Scholar] [CrossRef]
- Karra, A.G.; Tziortziou, M.; Kylindri, P.; Georgatza, D.; Gorgogietas, V.A.; Makiou, A.; Krokida, A.; Tsialtas, I.; Kalousi, F.D.; Papadopoulos, G.E.; et al. Boswellic Acids and Their Derivatives as Potent Regulators of Glucocorticoid Receptor Actions. Arch. Biochem. Biophys. 2020, 695, 108656. [Google Scholar] [CrossRef] [PubMed]
- Leão, T.K.; Ribeiro, D.L.; Machado, A.R.T.; Costa, T.R.; Sampaio, S.V.; Antunes, L.M.G. Synephrine and Caffeine Combination Promotes Cytotoxicity, DNA Damage and Transcriptional Modulation of Apoptosis-Related Genes in Human HepG2 Cells. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2021, 868–869, 503375. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.L.; Machado, A.R.T.; Da Silva Machado, C.; Santos, P.W.D.S.; Aissa, A.F.; Barcelos, G.R.M.; Antunes, L.M.G. Analysis of the Cytotoxic, Genotoxic, Mutagenic, and pro-Oxidant Effect of Synephrine, a Component of Thermogenic Supplements, in Human Hepatic Cells In Vitro. Toxicology 2019, 422, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.L.; Machado, A.R.T.; Machado, C.; Ferro Aissa, A.; Dos Santos, P.W.; Barcelos, G.R.M.; Antunes, L.M.G. P-Synephrine Induces Transcriptional Changes via the cAMP/PKA Pathway but Not Cytotoxicity or Mutagenicity in Human Gastrointestinal Cells. J. Toxicol. Environ. Health Part A 2021, 84, 196–212. [Google Scholar] [CrossRef]
- Li, L.; Lou, Z.; Wang, L. The Role of FKBP5 in Cancer Aetiology and Chemoresistance. Br. J. Cancer 2011, 104, 19–23. [Google Scholar] [CrossRef]
- Pei, H.; Li, L.; Fridley, B.L.; Jenkins, G.D.; Kalari, K.R.; Lingle, W.; Petersen, G.; Lou, Z.; Wang, L. FKBP51 Affects Cancer Cell Response to Chemotherapy by Negatively Regulating Akt. Cancer Cell 2009, 16, 259–266. [Google Scholar] [CrossRef]
- Yemelyanov, A.; Czwornog, J.; Chebotaev, D.; Karseladze, A.; Kulevitch, E.; Yang, X.; Budunova, I. Tumor Suppressor Activity of Glucocorticoid Receptor in the Prostate. Oncogene 2007, 26, 1885–1896. [Google Scholar] [CrossRef]
- Lesovaya, E.A.; Yemelyanov, A.Y.; Kirsanov, K.I.; Yakubovskaya, M.G.; Budunova, I.V. Antitumor Effect of Non-Steroid Glucocorticoid Receptor Ligand CpdA on Leukemia Cell Lines CEM and K562. Biochemistry 2011, 76, 1242–1252. [Google Scholar] [CrossRef]
- Workman, P.; Aboagye, E.O.; Balkwill, F.; Balmain, A.; Bruder, G.; Chaplin, D.J.; Double, J.A.; Everitt, J.; Farningham, D.A.H.; Glennie, M.J.; et al. Guidelines for the Welfare and Use of Animals in Cancer Research. Br. J. Cancer 2010, 102, 1555–1577. [Google Scholar] [CrossRef]

| Time of Simulation | Compound | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 4 ns | Protein | Ligand | |||||||
| avr | sd | min | max | avr | sd | min | max | ||
| 4S-C2_7α | 1.043 | 0.048 | 0.399 | 1.128 | 0.822 | 0.125 | 0.138 | 1.194 | |
| 4S-C2_7β | 1.048 | 0.049 | 0.395 | 1.134 | 0.883 | 0.100 | 0.228 | 1.139 | |
| 8S-E3_7α | 1.055 | 0.055 | 0.388 | 1.150 | 0.474 | 0.067 | 0.09 | 0.678 | |
| 8S-E3_7β | 1.042 | 0.044 | 0.4 | 1.112 | 0.488 | 0.094 | 0.139 | 0.860 | |
| 10S-E2_7α | 1.042 | 0.042 | 0.387 | 1.106 | 0.565 | 0.117 | 0.120 | 0.999 | |
| 10S-E2_7β | 1.057 | 0.073 | 0.007 | 1.140 | 0.497 | 0.079 | 0.095 | 0.769 | |
| 13S-G2_7α | 1.062 | 0.049 | 0.392 | 1.136 | 0.550 | 0.122 | 0.140 | 0.886 | |
| 13S_G2_7β | 1.060 | 0.042 | 0.378 | 1.120 | 0.561 | 0.118 | 0.168 | 0.932 | |
| 10 ns | Protein | Ligand | |||||||
| avr | sd | min | max | avr | sd | min | max | ||
| 10S-E2_7α | 1.070 | 0.041 | 0.387 | 1.157 | 0.319 | 0.081 | 0.110 | 0.707 | |
| 10S-E2_7β | 1.089 | 0.041 | 0.397 | 1.175 | 0.550 | 0.118 | 0.100 | 0.939 |
| No | Patient Code | IC50, μM |
|---|---|---|
| 1 | 3 | 40.9 ± 3.12 |
| 2 | 4 | 43.2 ± 9.48 |
| 3 | 7 | 43.0 ± 0.71 |
| 4 | 8 | 60.6 ± 0.97 |
| 5 | 10 | 71.3 ± 5.59 |
| 6 | 12 | 75.7 ± 4.63 |
| 7 | 20 | 64.2 ± 0.55 |
| 8 | 21 | 49.4 ± 1.95 |
| 9 | 23 | 55.2 ± 2.25 |
| 10 | 24 | 70.3 ± 2.54 |
| 11 | 25 | 80.3 ± 4.09 |
| 12 | 30 | 52.4 ± 0.07 |
| 13 | 31 | 63.2 ± 1.42 |
| 14 | 33 | 67.6 ± 4.02 |
| 15 | 34 | 55.0 ± 1.91 |
| 16 | 35 | 65.9 ± 0.27 |
| 17 | 37 | 69.8 ± 3.66 |
| 18 | 39 | 71.3 ± 2.59 |
| 19 | 46 | 71.5 ± 2.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhidkova, E.M.; Savina, E.D.; Migaleva, D.V.; Vlasova, O.A.; Valiev, T.T.; Enikeev, A.D.; Badun, G.A.; Chernysheva, M.G.; Dodonova, S.A.; Kryukov, A.A.; et al. Anti-Cancer Outcome of Glucocorticoid Receptor Transrepression by Synephrine Derivatives in Hematological Malignancies. Int. J. Mol. Sci. 2025, 26, 11404. https://doi.org/10.3390/ijms262311404
Zhidkova EM, Savina ED, Migaleva DV, Vlasova OA, Valiev TT, Enikeev AD, Badun GA, Chernysheva MG, Dodonova SA, Kryukov AA, et al. Anti-Cancer Outcome of Glucocorticoid Receptor Transrepression by Synephrine Derivatives in Hematological Malignancies. International Journal of Molecular Sciences. 2025; 26(23):11404. https://doi.org/10.3390/ijms262311404
Chicago/Turabian StyleZhidkova, Ekaterina M., Ekaterina D. Savina, Daria V. Migaleva, Olga A. Vlasova, Timur T. Valiev, Adel D. Enikeev, Gennadii A. Badun, Maria G. Chernysheva, Svetlana A. Dodonova, Alexey A. Kryukov, and et al. 2025. "Anti-Cancer Outcome of Glucocorticoid Receptor Transrepression by Synephrine Derivatives in Hematological Malignancies" International Journal of Molecular Sciences 26, no. 23: 11404. https://doi.org/10.3390/ijms262311404
APA StyleZhidkova, E. M., Savina, E. D., Migaleva, D. V., Vlasova, O. A., Valiev, T. T., Enikeev, A. D., Badun, G. A., Chernysheva, M. G., Dodonova, S. A., Kryukov, A. A., Kusov, P. A., Gordeev, K. V., Yurchenko, E. A., Matveev, A. V., Yakubovskaya, M. G., & Lesovaya, E. A. (2025). Anti-Cancer Outcome of Glucocorticoid Receptor Transrepression by Synephrine Derivatives in Hematological Malignancies. International Journal of Molecular Sciences, 26(23), 11404. https://doi.org/10.3390/ijms262311404







