Age-Related Transcriptomic Changes in the Vermiform Appendix
Abstract
1. Introduction
2. Results
2.1. Baseline Patient Characteristics
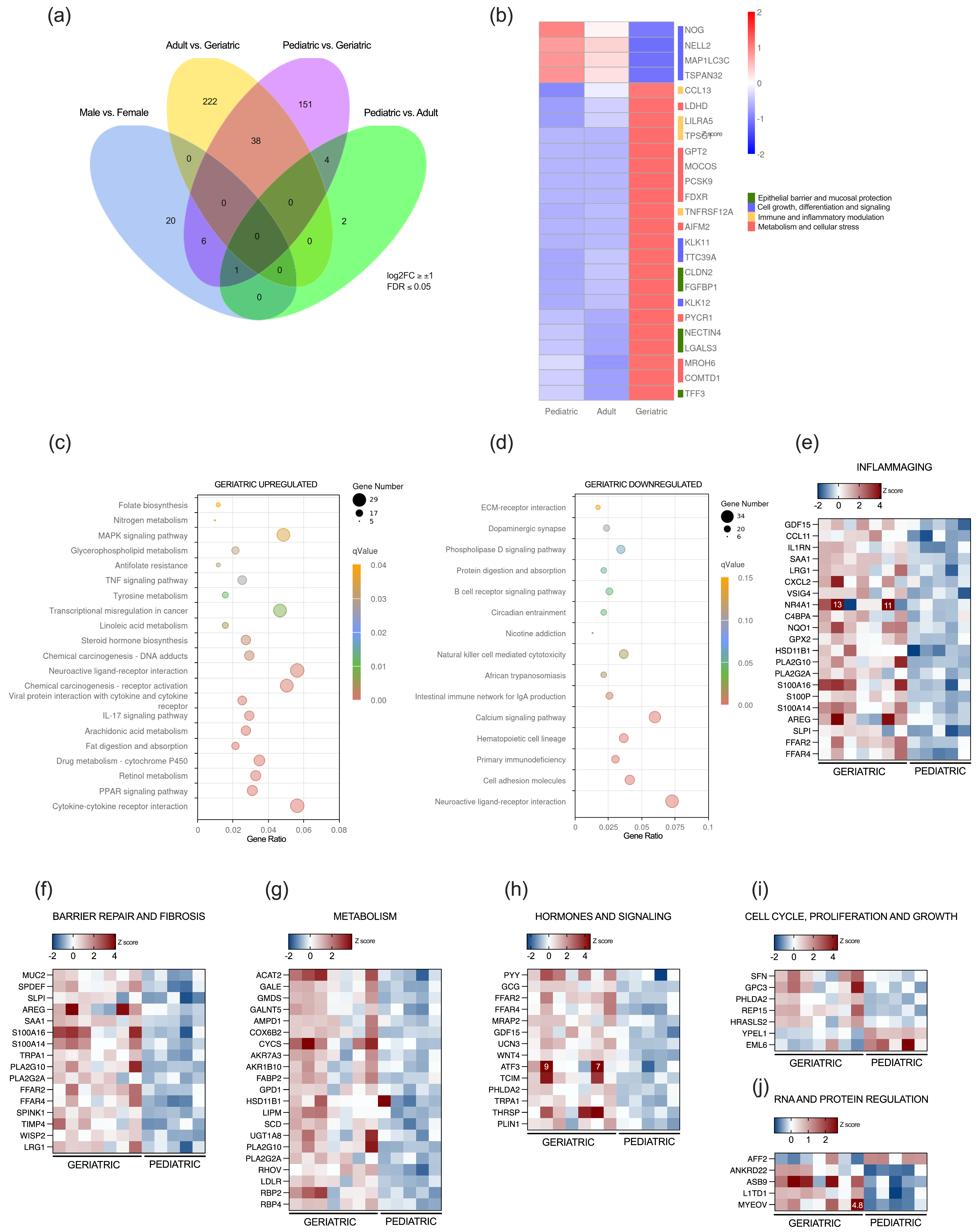
2.2. Transcriptomic Alterations in the Appendix of the Aged Population
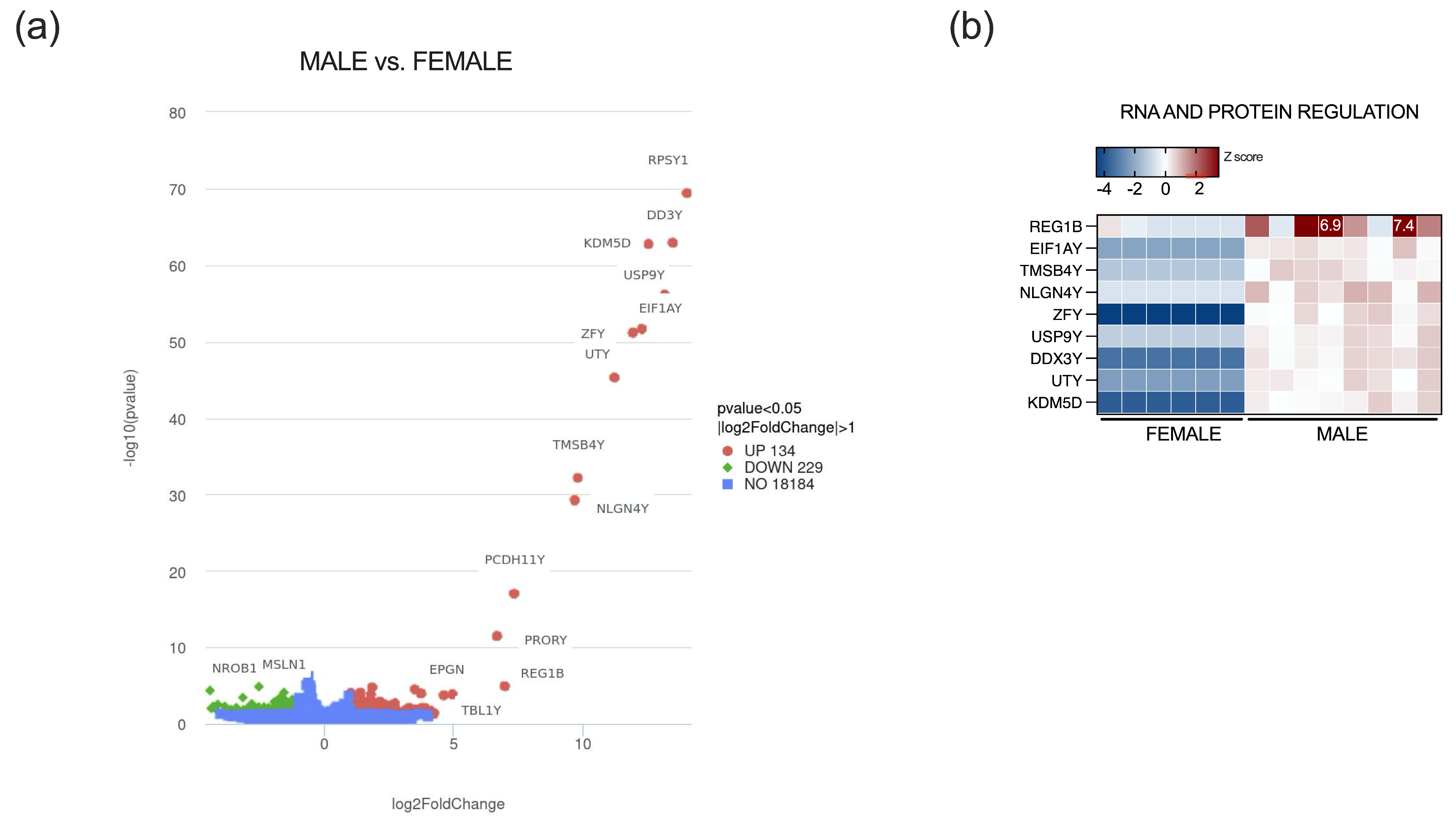
2.3. Sex-Specific Transcriptomic Alterations in Vermiform Appendix
2.4. Progressive Age-Dependent Differential Expression of Genes in the Appendix
3. Discussion
4. Materials and Methods
4.1. Patient Samples
4.2. Histological Analysis
4.3. RNA Preparation and Sequencing
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMPA | α-Amino-3-hydroxy-5-Methyl-4-isoxazolePropionic Acid |
| BMI | Body Mass Index |
| DEG | Differentially Expressed Gene |
| ENS | Enteric Nervous System |
| FDR | False Discovery Rate |
| GALT | Gut-Associated Lymphoid |
| GO | Gene Ontology |
| ISCs | Intestinal Stem Cells |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| PCA | Principal Component Analysis |
| ROS | Reactive Oxygen Species |
| TER | Transepithelial Electrical Resistance |
References
- Smith, H.F.; Fisher, R.E.; Everett, M.L.; Thomas, A.D.; Randal Bollinger, R.; Parker, W. Comparative Anatomy and Phylogenetic Distribution of the Mammalian Cecal Appendix. J. Evol. Biol. 2009, 22, 1984–1999. [Google Scholar] [CrossRef] [PubMed]
- Scott, G.B.D. The Primate Caecum and Appendix Vermiformis: A Comparative Study. J. Anat. 1980, 131, 549–563. [Google Scholar] [PubMed]
- Kooij, I.A.; Sahami, S.; Meijer, S.L.; Buskens, C.J.; te Velde, A.A. The Immunology of the Vermiform Appendix: A Review of the Literature. Clin. Exp. Immunol. 2016, 186, 1–9. [Google Scholar] [CrossRef]
- Bollinger, R.R.; Barbas, A.S.; Bush, E.L.; Lin, S.S.; Parker, W. Biofilms in the Large Bowel Suggest an Apparent Function of the Human Vermiform Appendix. J. Theor. Biol. 2007, 249, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Masahata, K.; Umemoto, E.; Kayama, H.; Kotani, M.; Nakamura, S.; Kurakawa, T.; Kikuta, J.; Gotoh, K.; Motooka, D.; Sato, S.; et al. Generation of Colonic IgA-Secreting Cells in the Caecal Patch. Nat. Commun. 2014, 5, 3704. [Google Scholar] [CrossRef]
- Rasmussen, T.; Fonnes, S.; Rosenberg, J. Long-Term Complications of Appendectomy: A Systematic Review. Scand. J. Surg. 2018, 107, 189–196. [Google Scholar] [CrossRef]
- Cosnes, J.; Carbonnel, F.; Beaugerie, L.; Blain, A.; Reijasse, D.; Gendre, J.P. Effects of Appendicectomy on the Course of Ulcerative Colitis. Gut 2002, 51, 803–807. [Google Scholar] [CrossRef]
- Myrelid, P.; Landerholm, K.; Nordenvall, C.; Pinkney, T.D.; Andersson, R.E. Appendectomy and the Risk of Colectomy in Ulcerative Colitis: A National Cohort Study. Am. J. Gastroenterol. 2017, 112, 1311–1319. [Google Scholar] [CrossRef]
- Funk, M.C.; Zhou, J.; Boutros, M. Ageing, Metabolism and the Intestine. EMBO Rep. 2020, 21, e50047. [Google Scholar] [CrossRef]
- Wang, Q.; Qi, Y.; Shen, W.; Xu, J.; Wang, L.; Chen, S.; Si, J. The Aged Intestine: Performance and Rejuvenation. Aging Dis. 2021, 12, 1693–1712. [Google Scholar] [CrossRef]
- Choi, J. Intestinal Stem Cells: Guardians of Homeostasis in Health and Aging amid Environmental Challenges. Exp. Mol. Med. 2024, 56, 495–500. [Google Scholar] [CrossRef]
- Nefzger, C.M.; Jardé, T.; Srivastava, A.; Schroeder, J.; Rossello, F.J.; Horvay, K.; Prasko, M.; Paynter, J.M.; Chen, J.; Weng, C.F.; et al. Intestinal Stem Cell Aging Signature Reveals a Reprogramming Strategy to Enhance Regenerative Potential. NPJ Regen. Med. 2022, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Ikuno, Y.; Kakeya, Y.; Ikeno, S.; Taniura, H.; Kurono, M. Age-Related Dysfunction of the DNA Damage Response in Intestinal Stem Cells. Inflamm. Regen. 2019, 39, 8. [Google Scholar] [CrossRef] [PubMed]
- France, M.M.; Turner, J.R. The Mucosal Barrier at a Glance. J. Cell Sci. 2017, 130, 307–314. [Google Scholar] [CrossRef]
- Tsukita, S.; Tanaka, H.; Tamura, A. The Claudins: From Tight Junctions to Biological Systems. Trends Biochem. Sci. 2019, 44, 141–152. [Google Scholar] [CrossRef]
- Günzel, D.; Yu, A.S.L. Claudins and the Modulation of Tight Junction Permeability. Physiol. Rev. 2013, 93, 525–569. [Google Scholar] [CrossRef] [PubMed]
- Biragyn, A.; Ferrucci, L. Gut Dysbiosis: A Potential Link between Increased Cancer Risk in Ageing and Inflammaging. Lancet Oncol. 2018, 19, e295–e304. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, Oxidative Stress and the Biology of Ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Lin, M.T.; Beal, M.F. Mitochondrial Dysfunction and Oxidative Stress in Neurodegenerative Diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef]
- Zuo, L.; Prather, E.R.; Stetskiv, M.; Garrison, D.E.; Meade, J.R.; Peace, T.I.; Zhou, T. Inflammaging and Oxidative Stress in Human Diseases: From Molecular Mechanisms to Novel Treatments. Int. J. Mol. Sci. 2019, 20, 4472. [Google Scholar] [CrossRef]
- Elmentaite, R.; Kumasaka, N.; Roberts, K.; Fleming, A.; Dann, E.; King, H.W.; Kleshchevnikov, V.; Dabrowska, M.; Pritchard, S.; Bolt, L.; et al. Cells of the Human Intestinal Tract Mapped across Space and Time. Nature 2021, 597, 250–255. [Google Scholar] [CrossRef]
- Drokhlyansky, E.; Smillie, C.S.; Van Wittenberghe, N.; Ericsson, M.; Griffin, G.K.; Eraslan, G.; Dionne, D.; Cuoco, M.S.; Goder-Reiser, M.N.; Sharova, T.; et al. The Human and Mouse Enteric Nervous System at Single-Cell Resolution. Cell 2020, 182, 1606–1622.e23. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, P.; Guo, K.; Schirrick, G.; Gill, J.S.; Weis, J.; Lund Da Costa, A.; Rahman, M.; Mehta, H.; Fleecs, J.; et al. Age-Related Dysregulation of Intestinal Epithelium Fucosylation Is Linked to an Increased Risk of Colon Cancer. JCI Insight 2024, 9, e167676. [Google Scholar] [CrossRef]
- Choi, J.; Houston, M.; Wang, R.; Ye, K.; Li, W.; Zhang, X.; Huffman, D.M.; Augenlicht, L.H. Intestinal Stem Cell Aging at Single-cell Resolution: Transcriptional Perturbations Alter Cell Developmental Trajectory Reversed by Gerotherapeutics. Aging Cell 2023, 22, e13802. [Google Scholar] [CrossRef]
- Jing, Y.; Wang, Q.; Bai, F.; Li, Z.; Li, Y.; Liu, W.; Yan, Y.; Zhang, S.; Gao, C.; Yu, Y. Age-Related Alterations in Gut Homeostasis Are Microbiota Dependent. NPJ Biofilms Microbiomes 2025, 11, 51. [Google Scholar] [CrossRef]
- Meng, L.; Yang, Y.; He, S.; Chen, H.; Zhan, Y.; Yang, R.; Li, Z.; Zhu, J.; Zhou, J.; Li, Y.; et al. Single-Cell Sequencing of the Vermiform Appendix during Development Identifies Transcriptional Relationships with Appendicitis in Preschool Children. BMC Med. 2024, 22, 383. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, M.; Vohra, H.; Singh, P. Histomorphometry of Human Vermiform Appendix. Int. J. Res. Med. Sci. 2019, 7, 405. [Google Scholar] [CrossRef]
- Berry, R.J.A.; Lack, L.A.H. The Vermiform Appendix of Man, and the Structural Changes Therein Coincident with Age. J. Anat. Physiol. 1906, 40, 247. [Google Scholar] [PubMed]
- Gebbers, J.; Laissue, J. Bacterial Translocation in the Normal Human Appendix Parallels the Development of the Local Immune System. Ann. N. Y Acad. Sci. 2004, 1029, 337–343. [Google Scholar] [CrossRef]
- Dincel, O.; Göksu, M.; Türk, B.A.; Pehlivanoğlu, B.; İşler, S. Incidental Findings in Routine Histopathological Examination of Appendectomy Specimens; Retrospective Analysis of 1970 Patients. Indian J. Surg. 2018, 80, 48–53. [Google Scholar] [CrossRef]
- Şengiz Erhan, S.; Dobral, A.; Kulduk, G.; Alemdar, A. Unusual Histopathological Findings in Cases with a Preliminary Clinical Diagnosis of Acute Appendicitis: What Was Expected, What Did We Discover? Istanb. Med. J. 2022, 23, 247–253. [Google Scholar] [CrossRef]
- Emre, A.; Akbulut, S.; Bozdag, Z.; Yilmaz, M.; Kanlioz, M.; Emre, R.; Sahin, N. Routine Histopathologic Examination of Appendectomy Specimens: Retrospective Analysis of 1255 Patients. Int. Surg. 2013, 98, 354–362. [Google Scholar] [CrossRef]
- Albright, J.B.; Fakhre, P.G.; Nields, W.W.; Metzger, P.P. Incidental Appendectomy: 18-Year Pathologic Survey and Cost Effectiveness in the Nonmanaged-Care Setting. J. Am. Coll. Surg. 2007, 205, 298–306. [Google Scholar] [CrossRef]
- Takeuchi, T. Factors Involved in the Degeneration of Lymphoid Tissue in the Appendix. Kurume Med. J. 2018, 65, 123–127. [Google Scholar] [CrossRef]
- Jang, I.S.; Chae, K.R.; Cho, J.S. Effects of Age and Strain on Small Intestinal and Hepatic Antioxidant Defense Enzymes in Wistar and Fisher 344 Rats. Mech. Ageing Dev. 2001, 122, 561–570. [Google Scholar] [CrossRef]
- Xu, X.; Pang, Y.; Fan, X. Mitochondria in Oxidative Stress, Inflammation and Aging: From Mechanisms to Therapeutic Advances. Signal Transduct. Target. Ther. 2025, 10, 190. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Hu, M.; Gao, L.; Yuan, H.; Chong, B.; Liu, Y.; Zhang, R.; Gong, Y.; Du, D.; Zhang, Y.; et al. Low-Input Redoxomics Facilitates Global Identification of Metabolic Regulators of Oxidative Stress in the Gut. Signal Transduct. Target. Ther. 2025, 10, 8. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, C.; Wang, Q.; Feng, S.; Fang, Y.; Zhang, S. The Impact of Aging on Intestinal Mucosal Immune Function and Clinical Applications. Front. Immunol. 2022, 13, 1029948. [Google Scholar] [CrossRef] [PubMed]
- Homolak, J. Gastrointestinal Redox Homeostasis in Ageing. Biogerontology 2023, 24, 741–752. [Google Scholar] [CrossRef]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 Signaling in Oxidative and Reductive Stress. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef]
- Jaiswal, A.K. Regulation of Genes Encoding NAD(P)H:Quinone Oxidoreductases. Free Radic. Biol. Med. 2000, 29, 254–262. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, H.; Davies, K.J.A.; Forman, H.J. Aging-Related Decline in the Induction of Nrf2-Regulated Antioxidant Genes in Human Bronchial Epithelial Cells. Redox Biol. 2017, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.H.; Shenvi, S.V.; Dixon, B.M.; Liu, H.; Jaiswal, A.K.; Liu, R.M.; Hagen, T.M. Decline in Transcriptional Activity of Nrf2 Causes Age-Related Loss of Glutathione Synthesis, Which Is Reversible with Lipoic Acid. Proc. Natl. Acad. Sci. USA 2004, 101, 3381–3386. [Google Scholar] [CrossRef]
- Gounder, S.S.; Kannan, S.; Devadoss, D.; Miller, C.J.; Whitehead, K.S.; Odelberg, S.J.; Firpo, M.A.; Paine, R.; Hoidal, J.R.; Abel, E.D.; et al. Impaired Transcriptional Activity of Nrf2 in Age-Related Myocardial Oxidative Stress Is Reversible by Moderate Exercise Training. PLoS ONE 2012, 7, e45697, https://doi.org/10.1371/JOURNAL.PONE.0045697. Erratum in PLoS ONE 2012, 7. Erratum in PLoS ONE 2013, 8. [Google Scholar]
- Kruse, M.L.; Friedrich, M.; Arlt, A.; Röcken, C.; Egberts, J.H.; Sebens, S.; Schäfer, H. Colonic Lamina Propria Inflammatory Cells from Patients with IBD Induce the Nuclear Factor-E2 Related Factor-2 Thereby Leading to Greater Proteasome Activity and Apoptosis Protection in Human Colonocytes. Inflamm. Bowel Dis. 2016, 22, 2593–2606. [Google Scholar] [CrossRef]
- Yagishita, Y.; McCallum, M.L.; Kensler, T.W.; Wakabayashi, N. Constitutive Activation of Nrf2 in Mice Expands Enterogenesis in Small Intestine Through Negative Regulation of Math1. CMGH 2021, 11, 503–524. [Google Scholar] [CrossRef]
- Piechota-Polanczyk, A.; Mariwani, Z.; Fichna, J.; Polanczyk, A.; Jozkowicz, A. Chemical Inhibition of NRF2 Transcriptional Activity Influences Colon Function and Oestrogen Receptor Expression in Mice at Different Ages. Int. J. Mol. Sci. 2024, 25, 13647. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Baumann, P.; Tüscher, O.; Schick, S.; Endres, K. The Aging Enteric Nervous System. Int. J. Mol. Sci. 2023, 24, 9471. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Li, D.; Hu, S.; Huang, L.; Sun, H.; Yang, S.; Wu, B.; Ji, F.; Zhou, D. Aging-Dependent Decrease in the Numbers of Enteric Neurons, Interstitial Cells of Cajal and Expression of Connexin43 in Various Regions of Gastrointestinal Tract. Aging 2018, 10, 3851. [Google Scholar] [CrossRef]
- Perea, D.; Guiu, J.; Hudry, B.; Konstantinidou, C.; Milona, A.; Hadjieconomou, D.; Carroll, T.; Hoyer, N.; Natarajan, D.; Kallijärvi, J.; et al. Ret Receptor Tyrosine Kinase Sustains Proliferation and Tissue Maturation in Intestinal Epithelia. EMBO J. 2017, 36, 3029–3045. [Google Scholar] [CrossRef]
- Yu, W.; Zhang, H.; Shin, M.R.; Sesti, F. Oxidation of KCNB1 Potassium Channels in the Murine Brain during Aging Is Associated with Cognitive Impairment. Biochem. Biophys. Res. Commun. 2019, 512, 665–669, https://doi.org/10.1016/J.BBRC.2019.03.130. Erratum in Biochem. Biophys. Res. Commun. 2022, 632, 206–207. [Google Scholar] [PubMed]
- Urrutia, J.; Arrizabalaga-Iriondo, A.; Sanchez-del-Rey, A.; Martinez-Ibargüen, A.; Gallego, M.; Casis, O.; Revuelta, M. Therapeutic Role of Voltage-Gated Potassium Channels in Age-Related Neurodegenerative Diseases. Front. Cell Neurosci. 2024, 18, 1406709. [Google Scholar] [CrossRef] [PubMed]
- Delfino, G.; Briand, J.B.; Oullier, T.; Nienkemper, L.; Greig, J.; Veziers, J.; Neunlist, M.; Derkinderen, P.; Paillusson, S. Characterization of Mitochondria-Associated ER Membranes in the Enteric Nervous System under Physiological and Pathological Conditions. Am. J. Physiol. Gastrointest. Liver Physiol. 2024, 326, G330–G343. [Google Scholar] [CrossRef]
- Görlach, A.; Bertram, K.; Hudecova, S.; Krizanova, O. Calcium and ROS: A Mutual Interplay. Redox Biol. 2015, 6, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Seifi, M.; Swinny, J.D. Immunolocalization of AMPA Receptor Subunits within the Enteric Nervous System of the Mouse Colon and the Effect of Their Activation on Spontaneous Colonic Contractions. Neurogastroenterol. Motil. 2016, 28, 705–720. [Google Scholar] [CrossRef]
- Filpa, V.; Moro, E.; Protasoni, M.; Crema, F.; Frigo, G.; Giaroni, C. Role of Glutamatergic Neurotransmission in the Enteric Nervous System and Brain-Gut Axis in Health and Disease. Neuropharmacology 2016, 111, 14–33. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.J.; Savtchouk, I. Ca 2+ Permeable AMPA Receptors Switch Allegiances: Mechanisms and Consequences. J. Physiol. 2012, 590, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.; Coca, R.; Parikh, S.; McCarthy, K.; Man, H.Y. ADAR2-Mediated Q/R Editing of GluA2 in Homeostatic Synaptic Plasticity. Sci. Signal 2025, 18, eadr1442. [Google Scholar] [CrossRef]
- Kozlova, I.; Sah, S.; Keable, R.; Leshchyns’ka, I.; Janitz, M.; Sytnyk, V. Cell Adhesion Molecules and Protein Synthesis Regulation in Neurons. Front. Mol. Neurosci. 2020, 13, 592126. [Google Scholar] [CrossRef]
- Sytnyk, V.; Leshchyns’ka, I.; Schachner, M. Neural Cell Adhesion Molecules of the Immunoglobulin Superfamily Regulate Synapse Formation, Maintenance, and Function. Trends Neurosci. 2017, 40, 295–308. [Google Scholar] [CrossRef]
- Yasuda, T.; Delbono, O.; Wang, Z.M.; Messi, M.L.; Girard, T.; Urwyler, A.; Treves, S.; Zorzato, F. JP-45/JSRP1 Variants Affect Skeletal Muscle Excitation Contraction Coupling by Decreasing the Sensitivity of the Dihydropyridine Receptor. Hum. Mutat. 2012, 34, 184. [Google Scholar] [CrossRef]
- Selman, M.; Pardo, A. Fibroageing: An Ageing Pathological Feature Driven by Dysregulated Extracellular Matrix-Cell Mechanobiology. Ageing Res. Rev. 2021, 70, 101393. [Google Scholar] [CrossRef]
- Jang, D.H.; Shin, J.W.; Shim, E.; Ohtani, N.; Jeon, O.H. The Connection between Aging, Cellular Senescence and Gut Microbiome Alterations: A Comprehensive Review. Aging Cell 2024, 23, e14315. [Google Scholar] [CrossRef]
- Hodgkinson, C.P.; Gomez, J.A.; Payne, A.J.; Zhang, L.; Wang, X.; Dal-Pra, S.; Pratt, R.E.; Dzau, V.J. Abi3bp Regulates Cardiac Progenitor Cell Proliferation and Differentiation. Circ. Res. 2014, 115, 1007–1016. [Google Scholar] [CrossRef]
- DeAguero, J.L.; McKown, E.N.; Zhang, L.; Keirsey, J.; Fischer, E.G.; Samedi, V.G.; Canan, B.D.; Kilic, A.; Janssen, P.M.L.; Delfín, D.A. Altered Protein Levels in the Isolated Extracellular Matrix of Failing Human Hearts with Dilated Cardiomyopathy. Cardiovasc. Pathol. 2017, 26, 12–20. [Google Scholar] [CrossRef]
- Godfrey, A.K.; Naqvi, S.; Chmátal, L.; Chick, J.M.; Mitchell, R.N.; Gygi, S.P.; Skaletsky, H.; Page, D.C. Quantitative Analysis of Y-Chromosome Gene Expression across 36 Human Tissues. Genome Res. 2020, 30, 860–873. [Google Scholar] [CrossRef]
- Vidaki, A.; González, D.M.; Jiménez, B.P.; Kayser, M. Male-Specific Age Estimation Based on Y-Chromosomal DNA Methylation. Aging 2021, 13, 6442–6458. [Google Scholar] [CrossRef]
- Li, G.; Wang, C.; Guan, X.; Bai, Y.; Feng, Y.; Wei, W.; Meng, H.; Fu, M.; He, M.; Zhang, X.; et al. Age-related DNA Methylation on Y Chromosome and Their Associations with Total Mortality among Chinese Males. Aging Cell 2022, 21, e13563. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, Y.; Xie, J.; Li, C.; Wang, X.; Shen, J.; Zhang, Y.; Wang, S.; Cheng, N. Regenerating Gene 1B Silencing Inhibits Colon Cancer Cell HCT116 Proliferation and Invasion. Int. J. Biol. Markers 2015, 30, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Gazi, M.A.; Alam, M.A.; Fahim, S.M.; Wahid, B.Z.; Khan, S.S.; Islam, M.O.; Hasan, M.M.; Hasan, S.M.T.; Das, S.; Mahfuz, M.; et al. Infection With Escherichia Coli Pathotypes Is Associated With Biomarkers of Gut Enteropathy and Nutritional Status Among Malnourished Children in Bangladesh. Front. Cell Infect. Microbiol. 2022, 12, 901324. [Google Scholar] [CrossRef] [PubMed]
- Dönertaş, H.M.; İzgi, H.; Kamacıoğlu, A.; He, Z.; Khaitovich, P.; Somel, M. Gene Expression Reversal toward Pre-Adult Levels in the Aging Human Brain and Age-Related Loss of Cellular Identity. Sci. Rep. 2017, 7, 5894. [Google Scholar] [CrossRef]
- Holzscheck, N.; Söhle, J.; Kristof, B.; Grönniger, E.; Gallinat, S.; Wenck, H.; Winnefeld, M.; Falckenhayn, C.; Kaderali, L. Multi-Omics Network Analysis Reveals Distinct Stages in the Human Aging Progression in Epidermal Tissue. Aging 2020, 12, 12393–12409. [Google Scholar] [CrossRef]
- Qiu, A.; Zhang, H.; Kennedy, B.K.; Lee, A. Spatio-Temporal Correlates of Gene Expression and Cortical Morphology across Lifespan and Aging. Neuroimage 2021, 224, 117426. [Google Scholar] [CrossRef]
- Sailaja, B.S.; He, X.C.; Li, L. The Regulatory Niche of Intestinal Stem Cells. J. Physiol. 2016, 594, 4827–4836. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Vries, R.G.; Snippert, H.J.; van de Wetering, M.; Barker, N.; Stange, D.E.; van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 Stem Cells Build Crypt-Villus Structures in Vitro without a Mesenchymal Niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Li, Y.; Zhao, B.; Xu, C.; Liu, Y.; Li, H.; Zhang, B.; Wang, X.; Yang, X.; Xie, W.; et al. BMP Restricts Stemness of Intestinal Lgr5+ Stem Cells by Directly Suppressing Their Signature Genes. Nat. Commun. 2017, 8, 13824. [Google Scholar] [CrossRef] [PubMed]
- Nalapareddy, K.; Nattamai, K.J.; Kumar, R.S.; Karns, R.; Wikenheiser-Brokamp, K.A.; Sampson, L.L.; Mahe, M.M.; Sundaram, N.; Yacyshyn, M.-B.; Yacyshyn, B.; et al. Canonical Wnt Signaling Ameliorates Aging of Intestinal Stem Cells. Cell Rep. 2017, 18, 2608–2621. [Google Scholar] [CrossRef]
- Igarashi, M.; Miura, M.; Williams, E.; Jaksch, F.; Kadowaki, T.; Yamauchi, T.; Guarente, L. NAD+ Supplementation Rejuvenates Aged Gut Adult Stem Cells. Aging Cell 2019, 18, e12935. [Google Scholar] [CrossRef]
- Annunziata, F.; Rasa, S.M.M.; Krepelova, A.; Lu, J.; Minetti, A.; Omrani, O.; Nunna, S.; Adam, L.; Käppel, S.; Neri, F. Paneth Cells Drive Intestinal Stem Cell Competition and Clonality in Aging and Calorie Restriction. Eur. J. Cell Biol. 2022, 101, 151282. [Google Scholar] [CrossRef]
- Liu, J.; Liu, D.; Zhang, X.; Li, Y.; Fu, X.; He, W.; Li, M.; Chen, P.; Zeng, G.; DiSanto, M.E.; et al. NELL2 Modulates Cell Proliferation and Apoptosis via ERK Pathway in the Development of Benign Prostatic Hyperplasia. Clin. Sci. 2021, 135, 1591–1608. [Google Scholar] [CrossRef]
- Aihara, K.; Kuroda, S.; Kanayama, N.; Matsuyama, S.; Tanizawa, K.; Horie, M. A Neuron-Specific EGF Family Protein, NELL2, Promotes Survival of Neurons through Mitogen-Activated Protein Kinases. Mol. Brain Res. 2003, 116, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Q.; Sun, Y.; Yang, L.; Li, Q.; Feng, Y.; Li, M.; Yin, Y.; Zheng, L.; Li, N.; Qiu, H.; et al. TSPAN32 Suppresses Chronic Myeloid Leukemia Pathogenesis and Progression by Stabilizing PTEN. Signal Transduct. Target. Ther. 2023, 8, 90. [Google Scholar] [CrossRef]
- Lombardo, S.D.; Mazzon, E.; Basile, M.S.; Campo, G.; Corsico, F.; Presti, M.; Bramanti, P.; Mangano, K.; Petralia, M.C.; Nicoletti, F.; et al. Modulation of Tetraspanin 32 (TSPAN32) Expression in T Cell-Mediated Immune Responses and in Multiple Sclerosis. Int. J. Mol. Sci. 2019, 20, 4323. [Google Scholar] [CrossRef] [PubMed]
- Luettig, J.; Rosenthal, R.; Barmeyer, C.; Schulzke, J. Claudin-2 as a Mediator of Leaky Gut Barrier during Intestinal Inflammation. Tissue Barriers 2015, 3, e977176. [Google Scholar] [CrossRef]
- Furuse, M.; Fujita, K.; Hiiragi, T.; Fujimoto, K.; Tsukita, S. Claudin-1 and -2: Novel Integral Membrane Proteins Localizing at Tight Junctions with No Sequence Similarity to Occludin. J. Cell Biol. 1998, 141, 1539–1550. [Google Scholar] [CrossRef] [PubMed]
- Nio-Kobayashi, J.; Takahashi-Iwanaga, H.; Iwanaga, T. Immunohistochemical Localization of Six Galectin Subtypes in the Mouse Digestive Tract. J. Histochem. Cytochem. 2009, 57, 41–50. [Google Scholar] [CrossRef]
- Bülck, C.; Nyström, E.E.L.; Koudelka, T.; Mannbar-Frahm, M.; Andresen, G.; Radhouani, M.; Tran, F.; Scharfenberg, F.; Schrell, F.; Armbrust, F.; et al. Proteolytic Processing of Galectin-3 by Meprin Metalloproteases Is Crucial for Host-Microbiome Homeostasis. Sci. Adv. 2023, 9, eadf4055. [Google Scholar] [CrossRef]
- Mey, A.; Leffler, H.; Hmama, Z.; Normier, G.; Revillard, J.P. The Animal Lectin Galectin-3 Interacts with Bacterial Lipopolysaccharides via Two Independent Sites. J. Immunol. 1996, 156, 1572–1577. [Google Scholar] [CrossRef]
- Kavanaugh, D.; Kane, M.; Joshi, L.; Hickey, R.M. Detection of Galectin-3 Interaction with Commensal Bacteria. Appl. Environ. Microbiol. 2013, 79, 3507–3510. [Google Scholar] [CrossRef]
- Gupta, S.K.; Masinick, S.; Garrett, M.; Hazlett, L.D. Pseudomonas Aeruginosa Lipopolysaccharide Binds Galectin-3 and Other Human Corneal Epithelial Proteins. Infect. Immun. 1997, 65, 2747–2753. [Google Scholar] [CrossRef]
- Bresalier, R.S.; Byrd, J.C.; Wang, L.; Raz, A. Colon Cancer Mucin: A New Ligand for the Beta-Galactoside-Binding Protein Galectin-3. Cancer Res. 1996, 56, 4354–4357. [Google Scholar]
- Müller, S.; Schaffer, T.; Flogerzi, B.; Fleetwood, A.; Weimann, R.; Schoepfer, A.M.; Seibold, F. Galectin-3 Modulates T Cell Activity and Is Reduced in the Inflamed Intestinal Epithelium in IBD. Inflamm. Bowel Dis. 2006, 12, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.B.; Dodd, S.; Yu, L.-G.; Subramanian, S. Serum Galectins as Potential Biomarkers of Inflammatory Bowel Diseases. PLoS ONE 2020, 15, e0227306. [Google Scholar] [CrossRef]
- Argüeso, P.; Mauris, J.; Uchino, Y. Galectin-3 as a Regulator of the Epithelial Junction: Implications to Wound Repair and Cancer. Tissue Barriers 2015, 3, e1026505. [Google Scholar] [CrossRef]
- Puthenedam, M.; Wu, F.; Shetye, A.; Michaels, A.; Rhee, K.-J.; Kwon, J.H. Matrilysin-1 (MMP7) Cleaves Galectin-3 and Inhibits Wound Healing in Intestinal Epithelial Cells. Inflamm. Bowel Dis. 2011, 17, 260–267. [Google Scholar] [CrossRef]
- Cao, Z.; Said, N.; Amin, S.; Wu, H.K.; Bruce, A.; Garate, M.; Hsu, D.K.; Kuwabara, I.; Liu, F.-T.; Panjwani, N. Galectins-3 and -7, but Not Galectin-1, Play a Role in Re-Epithelialization of Wounds. J. Biol. Chem. 2002, 277, 42299–42305. [Google Scholar] [CrossRef]
- Mauris, J.; Woodward, A.M.; Cao, Z.; Panjwani, N.; Argüeso, P. Molecular Basis for MMP9 Induction and Disruption of Epithelial Cell-Cell Contacts by Galectin-3. J. Cell Sci. 2014, 127, 3141–3148. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Zhang, W.; Wang, Y.; Qian, P.; Huang, H. Inflammation and Aging: Signaling Pathways and Intervention Therapies. Signal Transduct. Target. Ther. 2023, 8, 239. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-H.; Bae, Y.-S. Phospholipase D2 Downregulation Induces Cellular Senescence through a Reactive Oxygen Species–P53–P21 Cip1/WAF1 Pathway. FEBS Lett. 2014, 588, 3251–3258. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Park, J.-W.; Lee, J.-H.; Kim, D.-Y.; Hahm, J.-H.; Bae, Y.-S. Role of Phospholipase D in the Lifespan of Caenorhabditis Elegans. Exp. Mol. Med. 2018, 50, 1–10. [Google Scholar] [CrossRef]
- Sang, X.; Wang, Q.; Ning, Y.; Wang, H.; Zhang, R.; Li, Y.; Fang, B.; Lv, C.; Zhang, Y.; Wang, X.; et al. Age-Related Mucus Barrier Dysfunction in Mice Is Related to the Changes in Muc2 Mucin in the Colon. Nutrients 2023, 15, 1830. [Google Scholar] [CrossRef]
- Wojnacki, J.; Lujan, A.L.; Brouwers, N.; Aranda-Vallejo, C.; Bigliani, G.; Rodriguez, M.P.; Foresti, O.; Malhotra, V. Tetraspanin-8 Sequesters Syntaxin-2 to Control Biphasic Release Propensity of Mucin Granules. Nat. Commun. 2023, 14, 3710. [Google Scholar] [CrossRef]
- Wang, X.; Chen, S.; Wang, J.; Chen, Y.; Guo, Y.; Wang, Q.; Liu, Z.; Zeng, H.; Xu, C. Olfactomedin-4 Deletion Exacerbates DSS-Induced Colitis through a Matrix Metalloproteinase-9-Dependent Mechanism. Int. J. Biol. Sci. 2023, 19, 2150–2166. [Google Scholar] [CrossRef]
- Kim, Y.; Kang, B.; Kim, J.C.; Park, T.J.; Kang, H.Y. Senescent Fibroblast–Derived GDF15 Induces Skin Pigmentation. J. Investig. Dermatol. 2020, 140, 2478–2486.e4. [Google Scholar] [CrossRef]
- Conte, M.; Giuliani, C.; Chiariello, A.; Iannuzzi, V.; Franceschi, C.; Salvioli, S. GDF15, an Emerging Key Player in Human Aging. Ageing Res. Rev. 2022, 75, 101569. [Google Scholar] [CrossRef] [PubMed]
- Pence, B.D. Growth Differentiation Factor-15 in Immunity and Aging. Front. Aging 2022, 3, 837575. [Google Scholar] [CrossRef] [PubMed]
- Branca, J.J.V.; Gulisano, M.; Nicoletti, C. Intestinal Epithelial Barrier Functions in Ageing. Ageing Res. Rev. 2019, 54, 100938. [Google Scholar] [CrossRef] [PubMed]
- Thevaranjan, N.; Puchta, A.; Schulz, C.; Naidoo, A.; Szamosi, J.C.; Verschoor, C.P.; Loukov, D.; Schenck, L.P.; Jury, J.; Foley, K.P.; et al. Age-Associated Microbial Dysbiosis Promotes Intestinal Permeability, Systemic Inflammation, and Macrophage Dysfunction. Cell Host Microbe 2017, 21, 455–466.e4. [Google Scholar] [CrossRef]
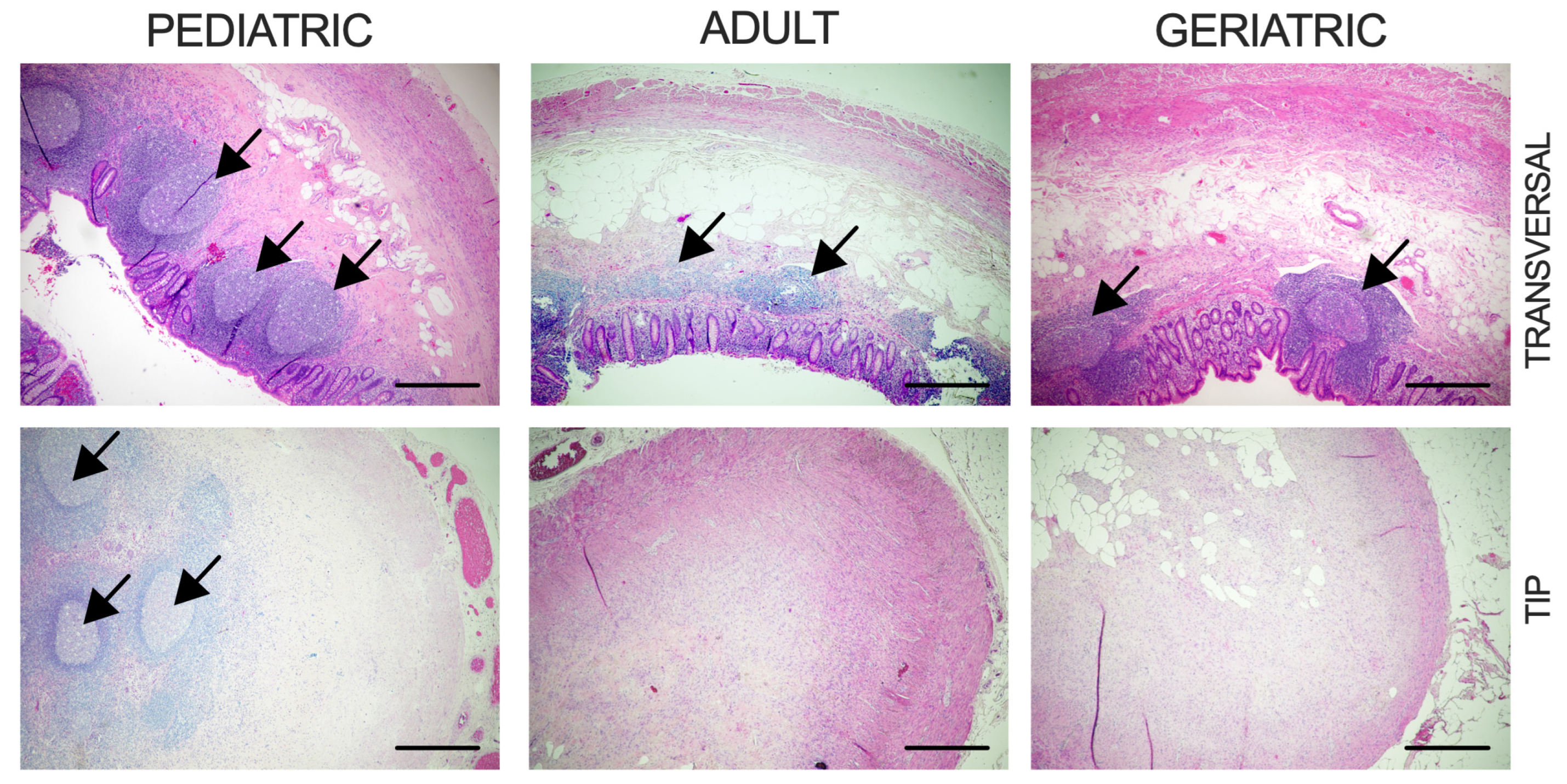

| Pediatric | Adult | Geriatric | p | |
|---|---|---|---|---|
| Age, Years | ||||
| Mean ± SD | 16 ± 2.1 | 39.5 ± 10.6 | 74.6 ± 8.5 | p < 0.0001 * |
| Sex | ||||
| Male | 0 | 4 | 4 | |
| Female | 5 | 4 | 3 | |
| BMI, kg/m2 | ||||
| Mean | 18.8 | 26 | 25.8 | pPA = 0.021 *, pPG = 0.016 *, pAG = 0.920 |
| 95% CI | 15.1–22.5 | 21.5–30.6 | 21.4–30.1 | |
| Reduction of Lymphoid Follicles | 20% (1/5) | 62.5% (5/8) | 85.7% (6/7) | pPA = 0.265, pPG = 0.072, pAG = 0.569 |
| Fibrous Obliteration of Tip | 20% (1/5) | 75% (6/8) | 71.4% (5/7) | pPA = 0.103, pPG = 0.242, pAG > 0.999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quien, D.; Korac-Prlic, J.; Vilović, K.; Pogorelić, Z.; Boric, M.; Barcot, O.; Degoricija, M. Age-Related Transcriptomic Changes in the Vermiform Appendix. Int. J. Mol. Sci. 2025, 26, 11399. https://doi.org/10.3390/ijms262311399
Quien D, Korac-Prlic J, Vilović K, Pogorelić Z, Boric M, Barcot O, Degoricija M. Age-Related Transcriptomic Changes in the Vermiform Appendix. International Journal of Molecular Sciences. 2025; 26(23):11399. https://doi.org/10.3390/ijms262311399
Chicago/Turabian StyleQuien, Damir, Jelena Korac-Prlic, Katarina Vilović, Zenon Pogorelić, Matija Boric, Ognjen Barcot, and Marina Degoricija. 2025. "Age-Related Transcriptomic Changes in the Vermiform Appendix" International Journal of Molecular Sciences 26, no. 23: 11399. https://doi.org/10.3390/ijms262311399
APA StyleQuien, D., Korac-Prlic, J., Vilović, K., Pogorelić, Z., Boric, M., Barcot, O., & Degoricija, M. (2025). Age-Related Transcriptomic Changes in the Vermiform Appendix. International Journal of Molecular Sciences, 26(23), 11399. https://doi.org/10.3390/ijms262311399







