1. Introduction
Ulcerative colitis (UC) is a complex and multifactorial disease characterized by the interaction of genetic, environmental, and immunological factors. Its pathogenesis is associated with the dysbiosis of gut microbiota, leading to increased intestinal permeability and chronic inflammation, for which non-pathogenic enteric bacteria play a primary role in the phenotype of the UC and the severity of intestinal inflammation. Therefore, UC appears to arise from the disruption of the homeostatic equilibrium between the host’s mucosal immune system and the enteric microbiota, leading to an inappropriate immune response against non-pathogenic commensal bacteria [
1,
2]. During the various treatment processes for UC, conventional treatments, including 5-aminosalicylic acid (5-ASA), glucocorticoids, and biological agents, have failed or come with significant side effects for a substantial proportion of patients with UC. 5-ASA intolerance is associated with a risk of adverse clinical outcomes and intestinal microbial ecology dysbiosis in patients with UC; its common clinical adverse events include diarrhea, fever and rash [
3], and more serious events such as renal toxicity, liver dysfunction, pancreatitis, pericarditis, pneumonia, severe skin reactions, etc. [
4]. Glucocorticoid can induce osteoporosis (GIOP) in patients [
5]; biological agents such as monoclonal antibodies infliximab, which work as antitumor necrosis factor (TNF) agents, may increase the risk of infection in patients and the risk of certain malignant tumors (such as lymphoma); and responses to infliximab therapy are highly variable among individuals, too. Formation of antidrug antibodies (ADAs) due to the failure of anti-TNF therapy is another thorny issue, especially in children, as the available alternative treatment options are limited [
6]. However, in patients with UC who have undergone ileal pouch-anal anastomosis but continue to experience intermittent symptoms of pouchitis, probiotics are recommended to prevent recurrence [
7]. The role of probiotics in the prevention of UC is a meaningful concept that should be explored.
Meanwhile, the balance of gut microbiota is crucial in UC, owing to its function in modulating the immune system and maintaining intestinal homeostasis. Dysbiosis, or an imbalance in the gut microbiota, is a hallmark of UC, characterized by a decrease in beneficial bacteria and an increase in pathogenic bacteria. Therefore, there is a growing interest in exploring probiotics, such as
Lacticaseibacillus rhamnosus, as a safer and more natural therapeutic option to help restore this balance [
8].
Lacticaseibacillus rhamnosus has been shown to benefit human health, based on numerous studies, and its advantages include immune modulation, diarrhea prevention, allergy reduction, cholesterol levels regulation, and the ability to maintain the microbiota balance, etc. [
9,
10,
11,
12].
Lacticaseibacillus rhamnosus has also been extensively studied for its potential therapeutic effects across a range of health conditions. One of the most notable applications is in the treatment of gastrointestinal disorders, such as UC [
13]. Different strains of
Lacticaseibacillus rhamnosus may have distinct mechanisms of action in treating UC. For example,
Lacticaseibacillus rhamnosus L34 can attenuate colitis severity through gut microbiota modulation and by decreasing the secretion of pro-inflammatory cytokines, while
Lacticaseibacillus rhamnosus GG (LGG) suppresses colitis in a STING-dependent manner in Ly6C+ monocytes [
14]. However, the specific mechanisms by which other strains exert their effects are still not fully understood.
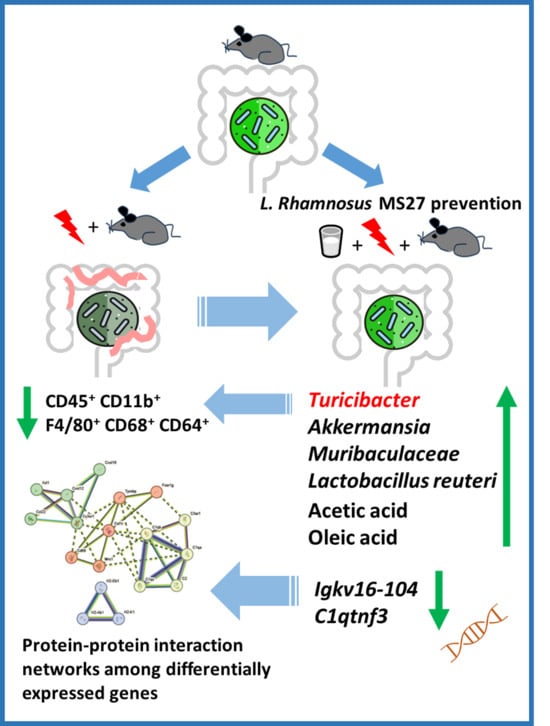
In this study, we explored the role of L. rhamnosus MS27 d in alleviating inflammatory phenotypes and also explored the comprehensive characteristics of L. rhamnosus MS27 and its effectiveness of action via the perspective of microbiomics and untargeted transcriptomics. Through multi-omics association analysis and microbiota–immune axis elucidation, new research targets will be established for advancing more in-depth mechanism analysis on L. rhamnosus MS27.
3. Discussion
With the discovery of interactions between microorganisms and the host, it has become increasingly evident that the intestinal microbiota plays a crucial role in the pathogenesis and progression of inflammatory bowel disease (IBD), particularly for ulcerative colitis. However, the exact mechanisms underlying their mode of action remain to be fully elucidated [
18,
19]. Evidence from several strains indicates their critical role in regulating epithelial barrier function, achieved through the stimulation of tight junctions between epithelial cells, preservation of epithelial cell integrity, and induction of mucus production [
20]. The capacity to modulate the host immune system is also considered to play a significant role, including the stimulation of antimicrobial defensins, interaction with local host immune cells to regulate cytokine production, and even providing benefits at distant sites [
21]. Among these,
Lacticaseibacillus rhamnosus, as a probiotic species, plays a significant role in supporting the treatment of inflammatory diseases; it achieves this by restoring the intestinal microbiota, improving intestinal barrier function, and reducing the levels of pro-inflammatory cytokines, among others. These effects are particularly relevant to the management of ulcerative colitis (UC) [
14,
22].
In terms of alleviating colitis induced by DSS,
L. rhamnosus MS27 significantly reduced the decline of weight gain ratio; reduced the decline of colon length; reduced the amount of inflammatory cell infiltration; and showed a good effect in inhibiting colon inflammation.
L. rhamnosus MS27 belongs to a group of porcine-derived probiotics isolated from the porcine saliva. As an animal model, a pig’s digestive physiology and metabolic processes are similar to that of a human’s. By applying novel molecular techniques to assess the intestinal microbiome, we are able to compare the similarity of representative bacterial species between pigs and humans [
23]. Thus, pig-derived
L. rhamnosus probiotics should be safe for humans, too. Due to differences in growth environment, the probiotics derived from pigs may have better antibacterial and anti-inflammatory properties. Our results show that
L. rhamnosus MS27 is sensitive to carbenicillin, erythromycin, and tetracycline, and is moderately sensitive to chloramphenicol, clindamycin, and penicillin, and also has obvious inhibitory effects on common pathogenic bacteria, such as
Staphylococcus aureus,
Escherichia coli, and
Salmonella. Its effects on inhibiting pathogens are consistent with the previous research results [
24], which together further confirms its safety. Of course, its safety for human use still is worthy of further research and verification before it can be applied in clinical practice.
L. rhamnosus MS27 has a strong acid tolerance, and it can survive when the pH value drops to 3.23. Moreover, the number of viable bacteria remains at a high level within 24 h. These characteristics ensured that the oral formulation can smoothly pass through the stomach. However, L. rhamnosus MS27 has no pili, and whether it is a transient bacterial strain or a colonizing bacterial strain remains to be further investigated. In this study, analysis of the bacterial community of the intestinal chyme indicated that L. rhamnosus increased significantly in the LR+DSS group compared to the CON group and DSS group. It demonstrated L. rhamnosus MS27 could survive in the gastrointestinal tract, providing health benefits and proving the effectiveness of its oral administration.
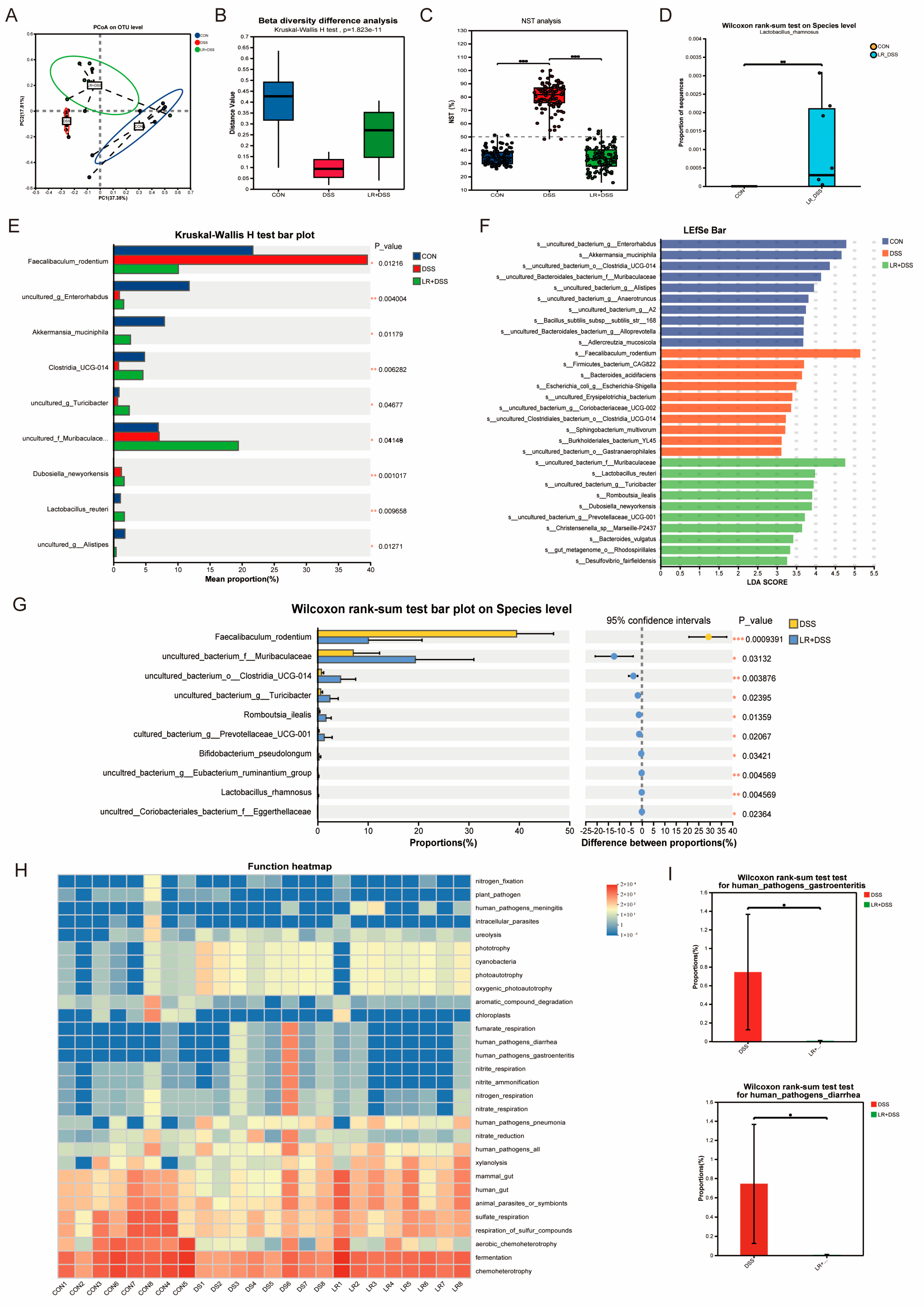
L. rhamnosus MS27 significantly improved the intestinal microbial flora, which is regarded as an important way for it to exert beneficial effects. In this study, L. rhamnosus MS27 effectively influenced these microbial abundance changes, such as Muribaculaceae, Akkermansia, Limosilactobacillus reuteri, Turicibacter, and Alistipes. Among these, the Muribaculaceae bacteria is an anaerobic bacterium, and its quantity significantly increased with L. rhamnosus MS27 addition, which indirectly indicates that L. rhamnosus MS27 has beneficial effects on the recovery of barrier integrity in the colon.
To date, members of the family
Muribaculaceae have attracted considerable attention due to their diverse roles in maintaining host health and are regarded as a promising “next-generation probiotic.”
Muribaculaceae belongs to one of the dominant bacterial genera in the intestinal symbiotic flora and is mainly colonized in the intestinal tract of mammals. The
Muribaculaceae family possesses the core metabolic capability to degrade a variety of complex polysaccharides. It can utilize dietary fiber interventions, such as inulin, resistant starch, and soluble fiber, to enhance the production of short-chain fatty acids. Additionally, members of this family are capable of encoding O-polysaccharide hydrolases and sialidases. The O-polysaccharidease is the most important enzyme for degrading mucin proteins, while the sialidase can cleave the sialic acid and sulfate residues at the terminal O-polysaccharide end of mucin proteins [
25].
Akkermansia muciniphila is another famous “next-generation probiotic”. It can promote intestinal mucus secretion, helping to maintain the dynamic balance of intestinal mucus and regulating the intestinal mucosal barrier function, playing a crucial role in metabolic regulation and immune responses in the body.
Akkermansia muciniphila can utilize mucin-derived sugars and degrade proteins of the intestinal mucus layer, such as mucin MUC2, as a carbon sources, thereby promoting host secretion of new mucus and enhancing gut barrier function [
26,
27].
Limosilactobacillus reuteri is a probiotic bacterium that resides in various human body sites, including the gastrointestinal tract, urinary tract, skin, and breast milk.
Limosilactobacillus reuteri is capable of producing antibacterial molecules, including ethanol, reuterin, and organic acids, which inhibit the colonization of pathogenic microorganisms and contribute to the modulation of the host’s symbiotic microbial community [
28].
Limosilactobacillus reuteri has been demonstrated to reduce the production of pro-inflammatory cytokines and enhance the differentiation and function of regulatory T cells. Moreover, it contributes to reinforcement of the intestinal barrier by improving the integrity of the colonic epithelial barrier through the upregulation of tight junction proteins, such as ZO-1, Occludin, and Claudin4 [
29]. Over the past few decades, the abundance of
Limosilactobacillus reuteri in humans has declined; this is associated with an increased incidence of inflammatory diseases during the same period. This correlation suggests that
Limosilactobacillus reuteri may represent a promising strategy for the prevention and/or treatment of inflammatory diseases [
30]. Clinical trials have further demonstrated that specific strains, such as
Limosilactobacillus reuteri ATCC 6475, can alleviate intestinal inflammation in patients with ulcerative colitis [
31].
Turicibacter participates in the modification of bile acids and host lipids metabolism differentially with strain-specific bsh genes [
32].
Turicibacter can down-regulate the bile signaling expression in the liver [
32].
Alistipes can produce hippuric acid to boost intestinal urate excretion via enhancing the binding of peroxisome-proliferator-activated receptor γ (PPARγ) to the promoter of ATP-binding cassette subfamily G member 2 (ABCG2) and maintain uric acid homeostasis [
33]. These results collectively demonstrate that
L. rhamnosus MS27 plays a role in modifying the structure and function of the intestinal microbiota to enhance the body’s response to inflammation, thereby restoring intestinal health.
Understanding the molecular mechanisms underlying the probiotic effects of
L. rhamnosus MS27 is also essential for optimizing its use. The potential molecular mechanisms of
Lacticaseibacillus rhamnosus on treatment of UC emphasize its ability to modulate the immune response, which is believed to be related to the bacterial pili, especially the spa CBA gene cluster encoding the pili [
34]. The main strain of
Lacticaseibacillus rhamnosus LGG is well-known and its surface is covered with pili, but
L. rhamnosus MS27 has no pili structures, which means that
L. rhamnosus MS27 has its own specific regulatory mechanism. In this study, we conducted a transcriptome–phenotype analysis to investigate the changes in the transcriptome after supplementing
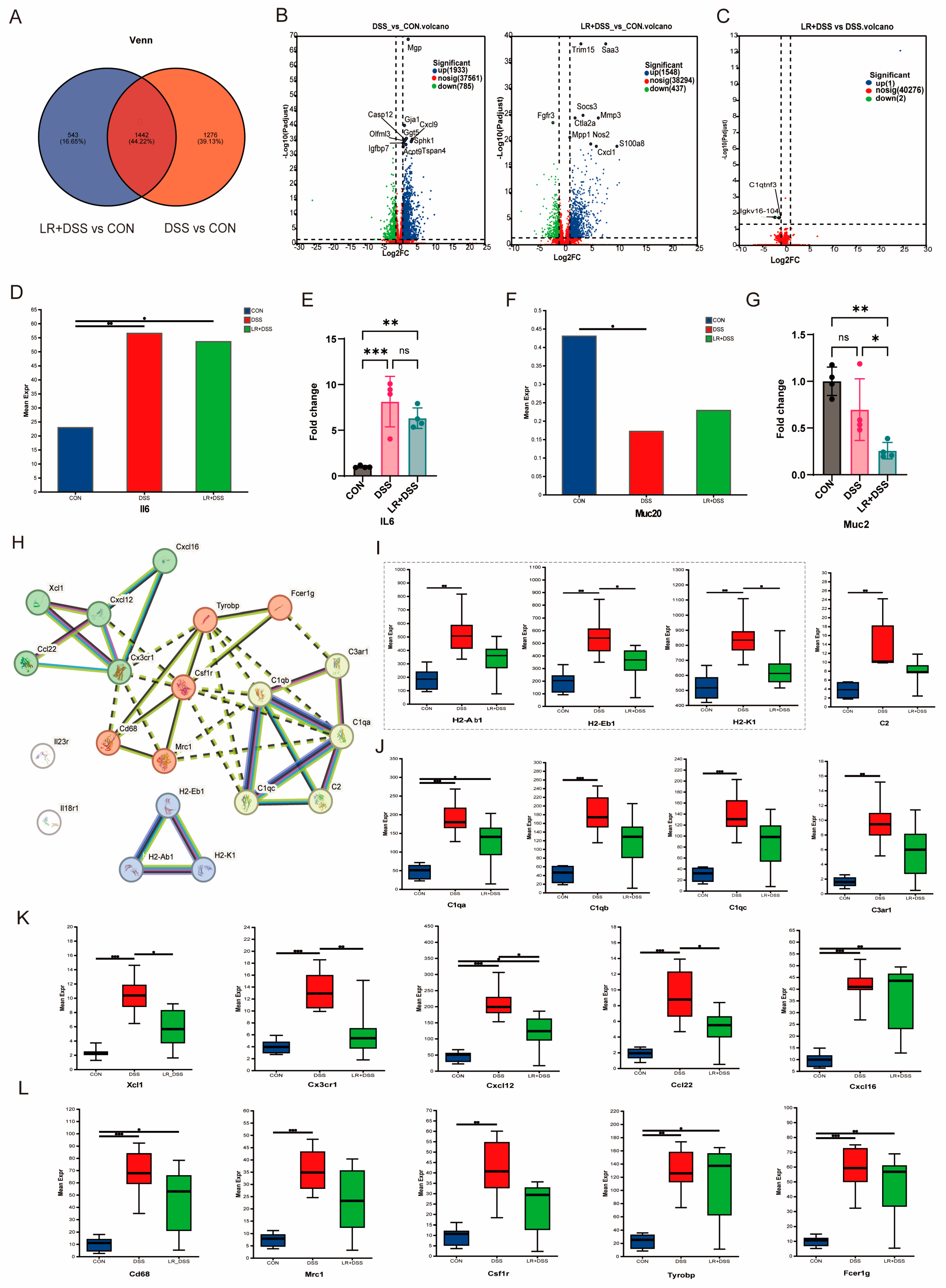
L. rhamnosus MS27 and the possible pathways in the interaction of immune-related proteins. Firstly, our results show that adding
L. rhamnosus MS27 before the DSS processing, compared with the DSS alone, has a significant impact on the expression of two genes,
Igkv16-104 and
C1qtnf3. Under the condition of inflammatory bowel disease, the expression of
Igkv16-104 is related to immune regulation and it should connect with
IL6 expression [
35]. In inflammatory bowel diseases (IBDs) and other inflammatory disorders, the level of
IL-6 usually increases. Intestinal inflammation should stimulate macrophages/Th17 to secrete
IL-6, which then activates the JAK-STAT3 pathway of B cell, thereby upregulating the Igκ enhancer expression (like
Igkv16-104) and promoting antibody secretion, thereby exacerbating inflammation or tissue damage. Our results showed that the expression of
IL6 experienced a certain degree of decline after
L. rhamnosus MS27 addition, but it did not drop significantly like
Igkv16-104 did. These results demonstrated that the decline of
Igkv16-104 should have another trigger mechanism.
C1q tumor necrosis factor related protein 3 (
C1qtnf3) is a member of the C1q family that is upregulated under inflammatory conditions. Studies have indicated that
C1qtnf3 is involved in inflammatory responses and may play a role in the regulation of immune cell migration and activation, much like how
C1qtnf3 was upregulated during the remodeling of subcutaneous adipose tissue and promoted macrophage chemotaxis and M1-like polarization. This suggests that
C1qtnf3 may play a significant role in inflammation and immune regulation [
36]. Following
L. rhamnosus MS27 treatment, the expression of
36 was downregulated, which may be attributed to the effect of
L. rhamnosus MS27 in regulating the balance of the intestinal microbiota and further contributing to the inhibition of inflammatory responses.
L. rhamnosus MS27 may serve as a potential therapeutic strategy for the prevention and treatment of inflammatory diseases. However, the immunomodulatory role of
C1qtnf3 in the process of tissue remodeling merits further investigation in the future.
To investigate the potential mechanism of action of
L. rhamnosus MS27, we further analyzed the genes that exhibited significant differential expressions between the
L. rhamnosus MS27 + DSS treatment group and the DSS treatment group, as well as those that demonstrated 9*-protein interaction relationships. These are
H2Ab1,
H2Eb1,
H2k1;
C1qa,
C1qb,
C1qc,
C3ar1,
C2;
Xcl1,
Cx3cr1,
Cxcl12,
Ccl22,
Cxcl16; and
Cd68,
Mrc1,
Csf1r,
Tyrobp,
Fcer1g, which are different gene groups and mainly have immunity functions, antigen presentation, and immune cell mark, et al. The results show that preventive supplementation of
L. rhamnosus MS27 also has a regulatory effect on the upregulation results of these genes induced solely by DSS, especially for
H2Ab1,
H2Eb1, and
H2k1. They all belong to the MHC, corresponding to the
HLA genes in humans. Herein,
H2-Ab1 and
H2-Eb1 are both belong to the MHC II class that present antigens to CD4+ T cells, however,
H2-Ab1 primarily regulates Th1/Th17 inflammatory responses, whereas
H2-Eb1 mainly influences immune tolerance.
H2-K1 belongs to MHC I class antigen and can be presented in CD8+ T cells to mediate cytotoxic immunity.
H2-Ab1 and
H2-Eb1 jointly form the MHC II class heterodimer (α + β), which affects the activation of CD4+ T cells [
37], while H2-K1 independently presents endogenous antigens (such as viral proteins) to CD8+ T cells, regulating cellular immunity [
38]. The upregulated expression of
H2-Ab1 exerts pro-inflammatory effects, such as promoting Th1/Th17 responses, thereby exacerbating autoimmune colitis in the DSS model [
37]. When
H2-Eb1 upregulates expression, certain haplotypes (such as
H2-Eb1d) promote the secretion of IL-17 and exacerbate intestinal inflammation [
31], while the increased expression of
H2-K1 enhanced the killing ability of CD8+ T cells, exerting a protective effect in viral infections (such as influenza) and tumor immunity [
39]. Based on these analyses, further examination of the impact of probiotics on immune phenotypes will be the next pressing question.
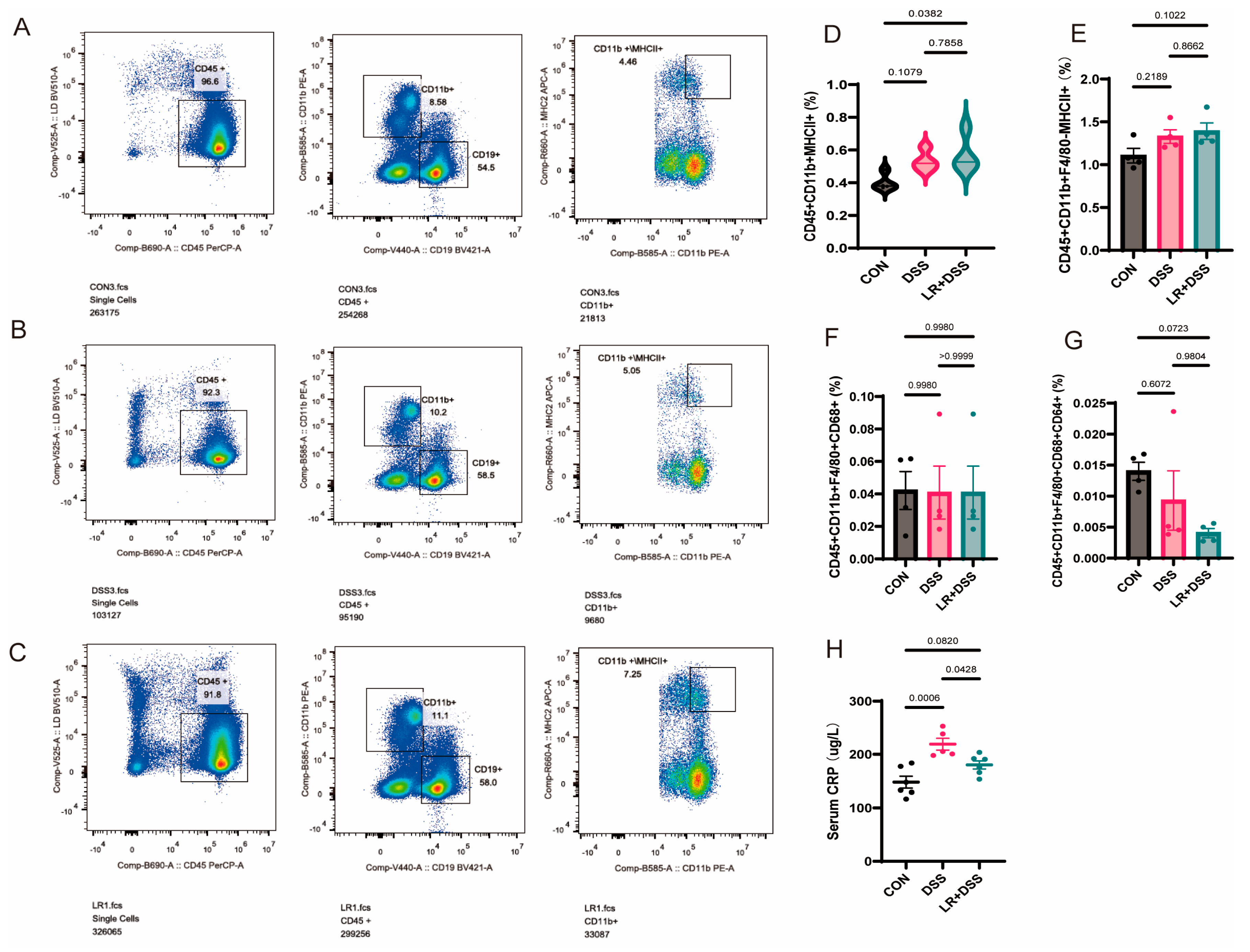
Disorders of the autoimmune system cause the intestinal lesions from UC to continue affecting other organs, leading to extraintestinal manifestations. After 14 days of probiotic treatment, its effects should not only work in the intestine but also reach the spleen via the lymphatic system or blood system, after which its intensity will weaken but the scope of its influence will expand. In order to further explore the mechanism of L. rhamnosus MS27, we further analyzed the changes in immune cells of the spleen.
The analysis results of splenic immune cells indicated that an increase happened in CD45
+CD11b
+MHCII
+, but no changes happened in CD45
+ CD11b
+ F4/80
− MHC II
+ cells and CD45
+ CD11b
+ F4/80
+ CD68
+ cells, and there is a non-significant decline in CD45
+ CD11b
+ F4/80
+ CD68
+ CD64
+ cells. Overall, these results are consistent with the characteristics of “distal fine-tuning” of the spleen. Firstly, CD45
+CD11b
+MHCII
+ is a mixed gate of innate immune cells derived from bone marrow, which expresses antigen-presenting molecules and mainly includes the classical CD11b
+ dendritic cells (cDC2) and inflammatory monocytes (precursors of Mo-DCs that are upregulating MHC II). Via further analysis, the number of CD45
+ CD11b
+ F4/80
− MHC II
+ cells, which are predominantly classical dendritic cells (cDC2), proved that cDC2 cells are stable, and while MS27 mainly affected the quantity of inflammatory monocytes, it perhaps plays a role in cytokine production, flexible antigen-presenting and innate-adaptive immune responses modulation, and tissue repair to inflammation resolution [
14,
40]. Secondly, CD45
+ CD11b
+ F4/80
+ CD68
+ CD64
+ cells were gated as mature macrophage populations with high phagocytic and high Fc-γ receptor-mediated effector functions, mainly biased towards the M1 functional state [
41]. Their decrease indirectly corroborates the improvement of the inflammatory state of the body, and the reduction in serum CRP concentration also supports this point. These results jointly supported the alleviation of the inflammatory response of MS27.
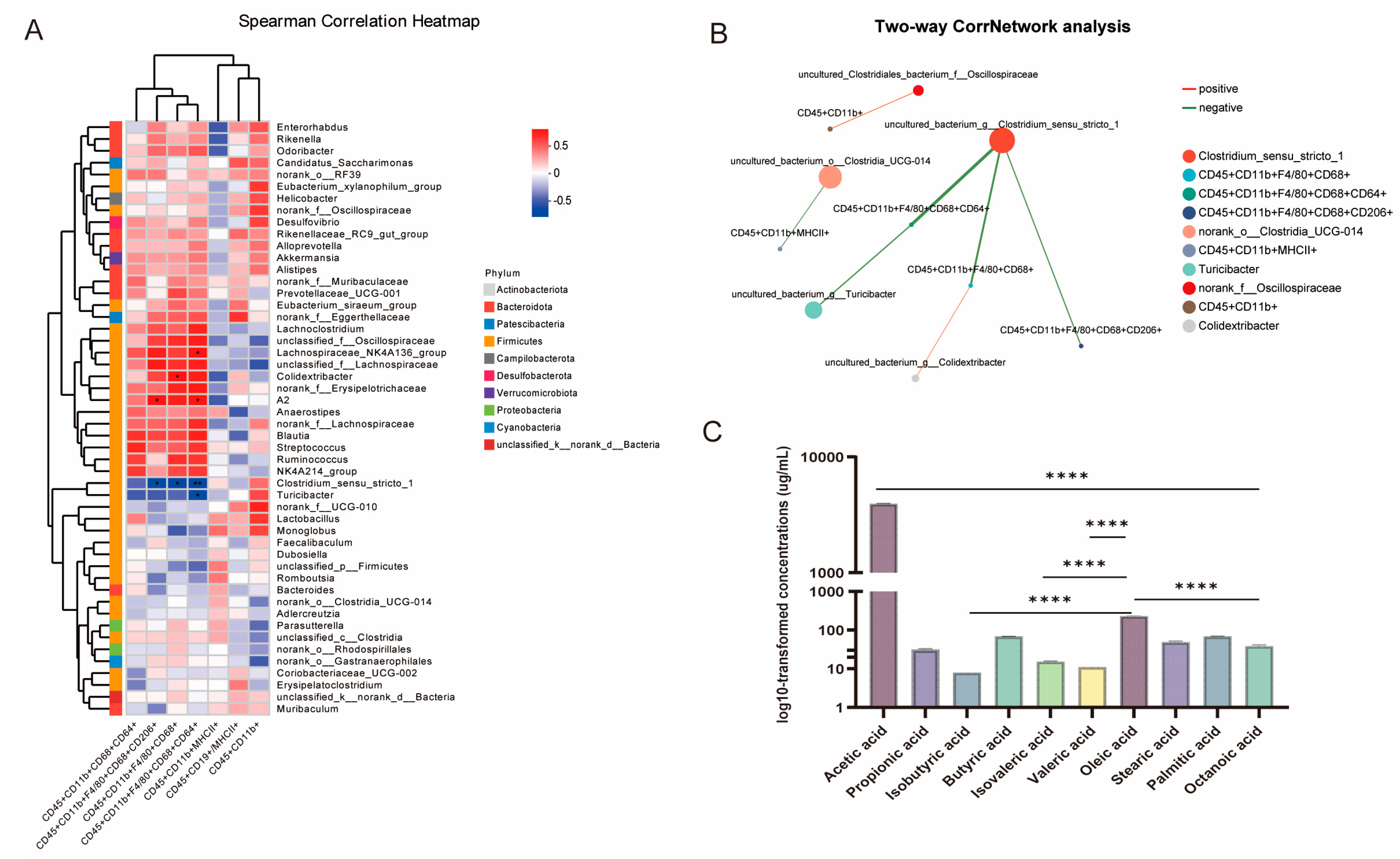
In combination with the regulatory effects of MS27 on intestinal microecology, its function of improving the immune system of the body may be associated with certain specific microorganisms. Among these,
Turicibacter warrants particular attention. In our results,
Turicibacter significantly increased in MS27 preventive treatment groups, in contrast with DSS treatment alone, and
Turicibacter is significantly negatively related to CD45
+ CD11b
+ F4/80
+ CD68
+ CD64
+. Thus, the anti-inflammatory effects of MS27 might be related to improvements in
Turicibacter abundance. It can also produce conjugated linoleic acid to inhibit inflammasomes, and hence is helpful for inflammation alleviation [
32]. We also explored whether MS27 could produce beneficial metabolic fatty acids to enhance its anti-inflammatory effects. Based on examination of the content of SCFAs and FAs of MS27, we found that acetic acid was its main volatile fatty acid product and oleic acid was the main fatty acid product. Here, acetic acid could work as a raw material for the synthesis of butyric acid to perform its beneficial function [
42] and oleic acid is helpful for restoring the intestinal barrier to alleviate inflammation [
43].
4. Materials and Methods
4.1. Strain Source and Identification
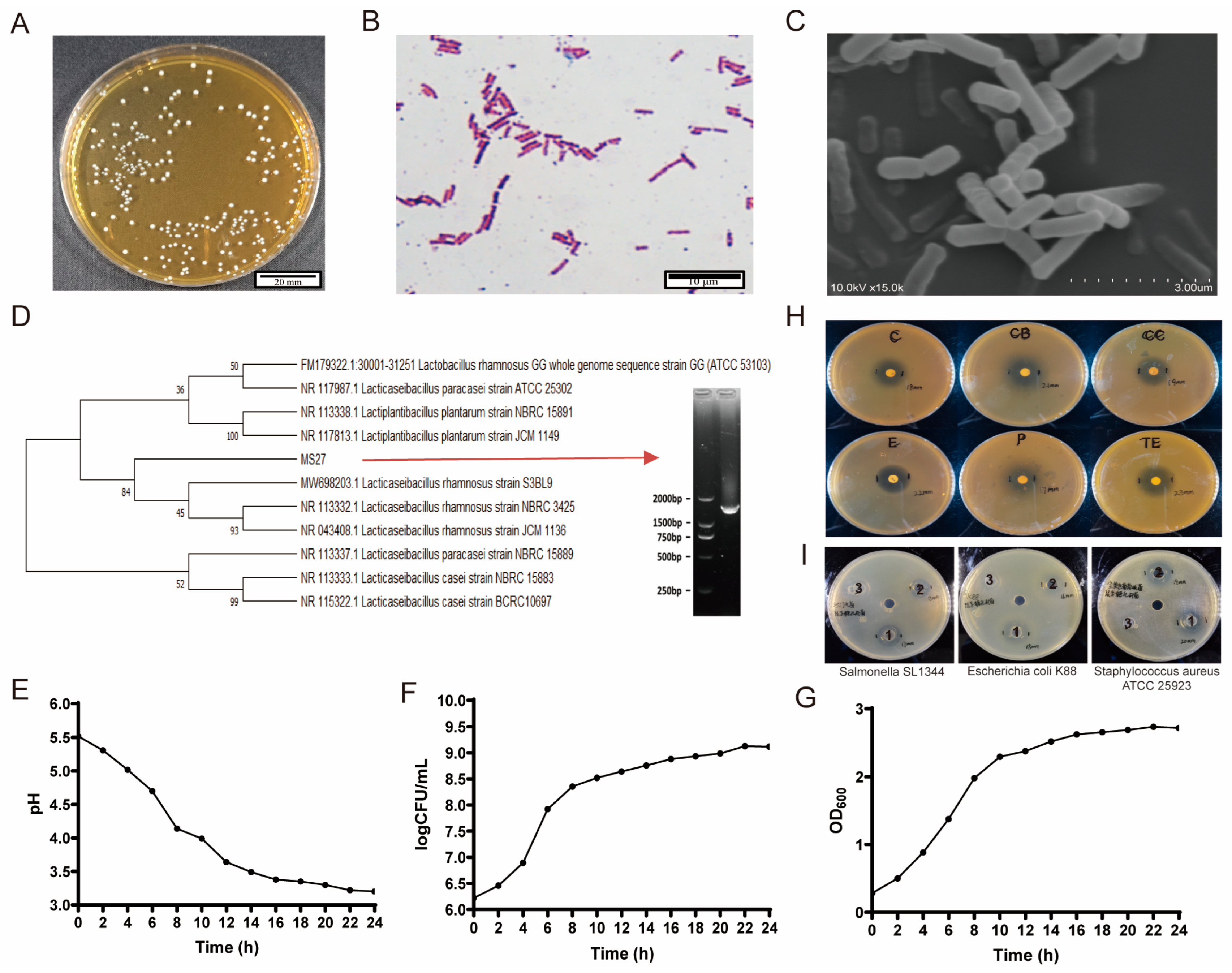
L. rhamnosus MS27 was isolated from oral saliva of Bama miniature pigs. Sterile swabs were used to collect oral mucus, placed in sterile centrifuge tubes, collected and filtered through sterile gauze, then inoculated at a concentration of 1% into 10 mL of Lacticaseibacillus selective medium. Cultivate anaerobically at 37 °C to 45 °C for 24 h to 36 h. Observe the colony morphology, pick out the oval-shaped strains with obvious calcium solubilization reaction, and conduct three streak purifications. After purification, sequence and identify the strains. Single colony was cultured on de Man, Rogosa, and Sharpe (MRS) solid medium plates at 37 °C for 24 h to purify them and this process was repeated for further purification. The Gram staining process proved that this strain is a Gram-positive, non-spore type intestinal symbiotic bacterium.
Using bacteria genomic DNA kit (CWBIO, Beijing, China) to extract total DNA of purified strains and amplifying them by PCR amplification using 27F (AGAGTTTGATCMTGGCTCAG) and 1492R (GGTTACCTTGTTACGACTT) universal primers [
44] conducted in 50 μL reactions system, a product of 1500 base pairs in size was obtained. The sequencing results of the 16S rRNA of strain were compared with the NBCI database and the 16S rRNA gene sequences with similar homologies were selected from GenBank. Furthermore, the phylogenetic tree was constructed using MEGA 11.0. The sequence alignment was constructed using the Neighbor-joining method, and the self-expansion method (Bootstrap) was used for verification. The number of repetitions was 2000 times, and confirmed that this strain is a new strain of
Lacticaseibacillus rhamnosus; it was named
Lacticaseibacillus rhamnosus MS27 (
L. rhamnosus MS27). It was stored in the China General Microbiological Culture Collection Center (CGMCC No. 27412).
4.2. Growth and Survival Curves, pH Tolerance Curves
Activated purified strains were incubated in MRS broth at 37 °C and then were tested on OD600 nm values to express viable bacteria numbers every 2 h within 24 h. Furthermore, they were incubated with different pH (0, 2.5, 3.0, and 4.0) of sterile phosphate-buffered saline (PBS) (Gibco, Brooklyn, NY, USA) at 37 °C for 0, 1, 2, 3, 4 h to quantify viable bacterial counts using the plate colony counting method. The viable bacterial count at 0 h was used as the control to calculate the survival rate of
L. rhamnosus MS27 in different pH conditions. This method follows our previous procedure [
45]. Prism 10.0 was carried out to analyze data and create growth curves, survival curves, and pH curves.
4.3. Biochemical Identification and Antibiotic Sensitivity Analysis
Prepare L. rhamnosus MS27 that has been purified and can grow into single colonies and take single colonies from the plate with an inoculation needle and inoculate them into the biochemical identification tubes of Lacticaseibacillus (No. SHBG13, Hopebio, Qingdao, China). Cultivate them according to the methods in the manual and determine the reaction conditions of the MS27 strain to sucrose, lactose, maltose, inulin, raffinose, esculin, salicin, and mannitol, respectively.
The drug resistances of the strains were evaluated by conducting drug sensitivity analysis using representative types of antibiotics. The main steps are as follows: penicillins (penicillin, carbenicillin), macrolides (erythromycin), tetracyclines (tetracycline), aminoglycosides (chloramphenicol), and lincosamides (clindamycin). Logarithmic growth phase L. rhamnosus MS27 was inoculated at a 1% ratio onto MRS solid medium at 45 °C to 55 °C and left to stand for 20 min. After the medium solidified, the drug sensitivity test paper was picked up with forceps and placed in the center of the solid plate, gently pressed down, and then incubated at 37 °C for 48 h. The growth of the strains was observed. Inhibition zone diameters of less than 15 mm were labeled as resistant (R), those between 16 mm and 20 mm were moderately sensitive (I), and those greater than 20 mm were sensitive (S).
4.4. Antibacterial Property of Pathogenic Bacteria
To explore the ability to inhibit pathogenic bacteria in vitro, pick out L. rhamnosus MS27 from the solid plate, inoculate it into MRS liquid medium, and ferment and culture at 37 °C for 36 h at 100 r/min. The effects of bacterial liquid, fermentation supernatant, and bacterial cells on common pathogenic bacteria in livestock and poultry were detected, respectively. The bacterial liquid was centrifuged at 4 °C and 5000 rpm for 10 min, and the supernatant was obtained after filtering the membrane. The bacterial cells were precipitated and re-resuspended with an equal volume of sterile physiological saline. Escherichia coli K88, Staphylococcus aureus ATCC 25923, and Salmonella typhimurium SL1344 growing on solid plates were picked and inoculated into 30 mL of sterilized liquid medium. After culturing at 37 °C for 12 h, the concentration was adjusted with sterile normal saline, and the pathogenic bacteria were diluted to 1.0 × 108 CFU/mL for later use. Place Oxford cups on plain AGAR plates. Cool the sterilized solid LB mehhdium to 45–55 °C. Mix 20 mL of solid LB medium +200 μL of diluted pathogen solution evenly and pour it onto the plates. After cooling and solidification, take out the Oxford cups and add 150 μL of sample to each well. Let it stand at 4 °C for 2–4 h and then incubate at 37 °C for 24 h.
4.5. Scanning Electron Microscope (SEM) Observation
We performed samples according to the China Agricultural University Bio-ultrastructure analysis Lab’s rules for bacteria sample preparation. After liquid enrichment culture of the L. rhamnosus MS27 strain, we removed the supernatant carefully by 2000 rpm, 5 min centrifugation. Resuspended the bacteria in 2 mL 2.5% glutaraldehyde to fix them for at least 2 h. Then we transferred them to a 4 °C environment for storage. Before observation, the fixed L. rhamnosus MS27 strain samples need to be vacuum-dried and dehydrated, then the dried powder should be transferred to the sample platform with conductive glue. Gold should be sprayed onto the samples by sputtering to enhance their conductivity. After that, the prepared samples were observed in S3400N SEM (Hitachi, Ltd., Tokyo, Japan).
4.6. Animal Experimental Protocol and Sample Collection
The C57BL/6 mice used in this trial are laboratory animals from Beijing HFK Bio-Technology. Co., Ltd, Beijng, China. Before the formal trial begins, a three-day acclimatization period is implemented to enable the animals to adapt to the environment. After that, the trial period is 14 days and the gavage was performed every 2 days. Eight-week-old 24 C57BL/6 male mice were randomly divided into three groups, as follows: the control group (CON, gavage with 200 µL normal saline every 2 days on 1–14 d), Dextran Sulfate Sodium Salt (DSS) group (gavage with 200 µL normal saline every 2 days on 1–14 d and drink 3% DSS on 7–14 d), and L. rhamnosus MS27+DSS group (LR+DSS, gavage with 200 µL 1 × 108 CFU/mL L. rhamnosus MS27 every 2 days from 1 to 14 d and drink 3% DSS on 7–14 d). Each group has eight replicates.
During the experimental time, daily weight was recorded every day. Based on these data, weight gain and the weight gain ratio (weight gain to the original body weight) were calculated. All mice were selected from each group for sampling on the 14th day. Obtain the weight of the liver and calculate the liver index (liver weight to body weight). Liver Bloods were collected and centrifuged at low temperature to obtain serum, then were stored at −20 °C for subsequent experiments. Separated total colon to measure its length and then collected colon sample formalin fixation for histological sections and HE staining. In addition, keeping collected colon tissue with liquid nitrogen treated immediately for RNA extraction and sequencing. Collected colon chyme in 1.5 mL sterile centrifuge tube and stored at −80 °C for subsequent 16S rRNA and metabolomic sequencing. All experimental procedures complied with relevant management regulations of Animal Protection and Utilization Committee of China Agricultural University (CAU20171015-3).
4.7. RNA Extraction, Reverse Transcription, and Gene Expression Analysis by Quantitative Real-Time PCR
Total colon tissue RNA was extracted using TRIzol® Reagent following the manufacturer’s instructions. Subsequently, RNA was quantified using the ND-2000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). Only high-quality RNA samples were used with OD260/280 ratios ranging from 1.8 to 2.2, RIN values greater than 6.5, and concentrations exceeding 10 ng/μL.
Subsequently, RNA was reverse transcribed into cDNA at 42 °C for 60 min using random hexamer primers and the RevertAid RT Kit (Cat. No. EP0441, Thermo Fisher Scientific, Wilmington, DE, USA), following the manufacturer’s instructions. Real-time PCR was performed to assess the relative expression levels of the target genes. RT-qPCR reactions were performed with tenfold-diluted cDNA using the PowerUp SYBR Green Master Mix (No. Q712, Vazyme, Nanjing, China). Each reaction was prepared in a 20 μL reaction mixture containing 10 μL of Power SYBR Green master mix, 0.3 μM of each primer adjusted to the designated final concentration, and 5 μL of diluted cDNA. The thermal cycling protocol was as follows: initial incubation at 50 °C for 2 min, followed by denaturation at 95 °C for 2 min, then 40 cycles of denaturation at 95 °C for 15 s, and annealing/extension at 60 °C for 1 min with fluorescence detection. A final melt curve analysis was performed, consisting of incubation at 95 °C for 15 s, cooling to 60 °C for 1 min, and a temperature ramp to 95 °C at a rate of 0.15 °C/s.
4.8. 16S rRNA Microbiome Analysis (Second-Generation OTU)
Total microbial genomic DNA was extracted from fecal samples using the E.Z.N.A.® DNA Kit (Omega Bio-tek, Norcross, GA, USA) following the manufacturer’s instructions. The quality and concentration of the extracted DNA were assessed by 1.0% agarose gel electrophoresis and using a NanoDrop 2000 spectrophotometer (Thermo Scientific, DE, USA), and the DNA was stored at −80 °C until further use. The hypervariable V3–V4 region of the bacterial 16S rRNA gene was amplified using the primer pairs 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) on a T100 Thermal Cycler PCR system (BIO-RAD, Hercules, CA, USA). The PCR reaction mixture consisted of 4 μL of 5× Fast Pfu buffer, 2 μL of 2.5 mM dNTPs, 0.8 μL of each primer (5 μM), 0.4 μL of Fast Pfu polymerase, 10 ng of template DNA, and ddH2O to a final volume of 20 µL. The PCR amplification program included an initial denaturation at 95 °C for 3 min, followed by 27 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 45 s, with a final extension at 72 °C for 10 min, and a holding step at 4 °C. The resulting PCR products were excised from a 2% agarose gel, purified using the PCR Clean-Up Kit (YuHua, Shanghai, China) according to the manufacturer’s instructions, and quantified using a Qubit 4.0 fluorometer (Thermo Fisher Scientific, Wilmington, DE, USA).
During the Illumina sequencing step, purified amplicons were pooled in equimolar concentrations and subjected to paired-end sequencing on an Illumina Nextseq 2000 platform (Illumina, San Diego, CA, USA) following the standard protocols provided by Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China). The raw sequencing reads were submitted to the NCBI Sequence Read Archive (SRA) database under Accession Number PRJNA1276053. Raw FASTQ files were de-multiplexed using a custom Perl script, quality-filtered with fastp version 0.19.6 [
46], and merged using FLASH version 1.2.7 [
47]. Subsequently, the processed sequences were clustered into operational taxonomic units (OTUs) at a 97% sequence similarity threshold using UPARSE 11.0 and UNOISE3 11.0 [
48,
49]. The most abundant sequence within each OTU was selected as the representative sequence.
Bioinformatic analysis of the gut microbiota was carried out using the Majorbio Cloud platform (
https://cloud.majorbio.com, 30 March 2025) [
50]. Based on the OTU information, rarefaction curves and alpha diversity indices, including the number of observed OTUs, Chao1 richness estimator, Shannon diversity index, and Good’s coverage, were calculated using Mothur v1.30.1 [
51]. The similarity among microbial communities in different samples was assessed using principal coordinate analysis (PCoA) based on Bray–Curtis dissimilarity with the Vegan v2.5-3 package. The PERMANOVA test was conducted to evaluate the proportion of variation attributable to treatment effects and its statistical significance, using the same package. Linear discriminant analysis (LDA) effect size (LEfSe) [
52] was applied to identify bacterial taxa (from phylum to genus level) that were significantly enriched across different groups (LDA score > 2,
p < 0.05).
4.9. Eukaryotes Reference Transcriptome Analysis
After RNA extraction and library preparation, the RNA-seq transcriptome library was prepared following SMART-Seq_V4 Ultra Low Input RNA Kit for Sequencing from Clontech (San Diego, CA, USA) using 10 ng of total RNA. Reverse transcription (one-strand synthesis) was performed first, and RNA with a poly Atail (major mRNA) was reverse transcribed using Oligo (dT) primer. Three cytosine (C) residues are added to the 3′ end of the cDNA strand because of the use of a special active reverse transcriptase (MMLVRT) for reverse transcription. Two strands of cDNA were synthesized with the use of TSO (template-switching oligo) primers, thereby replacing RNA complementary to one strand of cDNA. Then, the cDNA was expanded to ng level by PCR amplification. DNA disruption using a modified, highly active Tn5 transposase was performed while the linker was added to both ends of the cDNA. After the last PCR amplification, the machine was ready for sequencing. Following quantification using Qubit 4.0, the sequencing libraries were prepared and subjected to paired-end sequencing (PE150) on either the NovaSeq X Plus platform using the NovaSeq Reagent Kit or the DNBSEQ-T7 platform using the DNBSEQ-T7 RS Reagent Kit (FCL PE150), version 3.0.
The raw paired-end reads were trimmed and subjected to quality control using FastQC [
49] with default parameters. Subsequently, the cleaned reads were aligned to the reference genome in strand-specific mode using HISAT2 [
53]. The mapped reads from each sample were assembled using StringTie [
54] through a reference-based approach. To identify differentially expressed genes (DEGs) between the two sample groups, transcript expression levels were calculated based on the transcripts per million (TPM) method. Gene abundance quantification was performed using RSEM [
55]. Differential expression analysis was carried out using either DESeq2 [
56] or DEGseq [
57], as appropriate. DEGs with |log2FC| ≥ 1 and FDR < 0.05 (DESeq2) or FDR < 0.001 (DEGseq) were considered to be significantly different expressed genes. In addition, functional enrichment analysis including GO and KEGG were performed to identify which DEGs were significantly enriched in GO terms and metabolic pathways at Bonferroni-corrected
p-value < 0.05 compared with the whole-transcriptome background. GO functional enrichment analysis and KEGG pathway enrichment analysis were performed using Goatools and the Python (3.10) SciPy package, respectively. All RNA-seq data have been submitted to the GSA database under the following accession numbers: PRJNA1283609.
4.10. Flow Cytometry Experiment
Following the separation of the spleen, it was aseptically minced into small fragments using sterile scissors and subsequently dissociated through a 70 μm cell strainer with the aid of sterile forceps. A single-cell suspension was generated using a mouse spleen lymphocyte isolation kit (Cat#P8860, Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) according to the manufacturer’s protocol. The cell concentration of the suspension was adjusted to 108–109 cells/mL based on the spleen volume.
The CytoPeak R1 3-laser 14-color flow cytometer (Dakewe Biotech Co., Ltd., Shenzhen, China) was used to detect the CD 45 (PerCP/Cyanine5.5 Anti-Mouse CD45, Cat#E-AB-F1136J, Elabscience, Wuhan, China), CD64 (PE/Cyanine7 Anti-Mouse CD64/FcγRI Antibody[X54-5/7.1], Cat#E-AB-F1186UH, Elabscience, China), F4/80 (Elab Fluor® Violet 610 Anti-Mouse F4/80 Antibody[CI:A3-1], Cat#E-AB-F0995T, Elabscience, China), MHC II (APC Anti-Mouse MHC II Antibody[M5/114], Cat#E-AB-F0990UE, Elabscience, China), CD206 (FITC Anti-Mouse CD206/MMR Antibody[C068C2], Cat#E-AB-F1135C, Elabscience, China), CD19 (Elab Fluor® Violet 450 Anti-Mouse CD19 Antibody, Cat#E-AB-F0986UQ, Elabscience, China), CD11b (PE CD11b anti-mouse, Cat#E-AB-F1081D, Elabscience, China) and CD68 (CoraLite® Plus 750 Anti-Mouse CD68 Rabbit Recombinant Antibody, Cat#CL750-98029, Proteintech, Wuhan, China).
4.11. Detection of Volatile Fatty Acids and Fatty Acids
After 12 h of
L. rhamnosus MS27 culturing, the supernatant was taken after centrifugation at 2000 rpm to detect the SCFAs via an iron chromatographic method as previously reported [
45]. 1 mL of supernatant was diluted with dd H
2O (1:50) and filtered with 0.22 μm filter. Finally, it was injected into a high-performance ion chromatograph (ICS 3000 Dionex, Sunnyvale, CA, USA) to detect the content of acetic acid, propionic acid, isobutyric acid, butyric acid, isovaleric acid, valeric acid, oleic acid, stearic acid, palmitic acid, and octanoic acid.
4.12. Detection of Serum CRP Concentration
Serum CRP concentration was measured by high-sensitivity ELISA (Cat# SEKM-0059, Beijing Solarbio Science & Technology Co., Ltd.) and expressed as µg L−1. The limit of detection was 0.1 mg L−1. n = 8.
4.13. Statistical Analysis
All the graphs and analyses were carried out by GraphPad prism 10.0. The results were shown on mean ± SEM. Statistical significance was considered at p < 0.05.