Multi-Target Botanical Complex Attenuates Cellular Senescence via Bidirectional P21/P53/SIRT1 Regulation: Dual Model Validation
Abstract
1. Introduction
2. Results
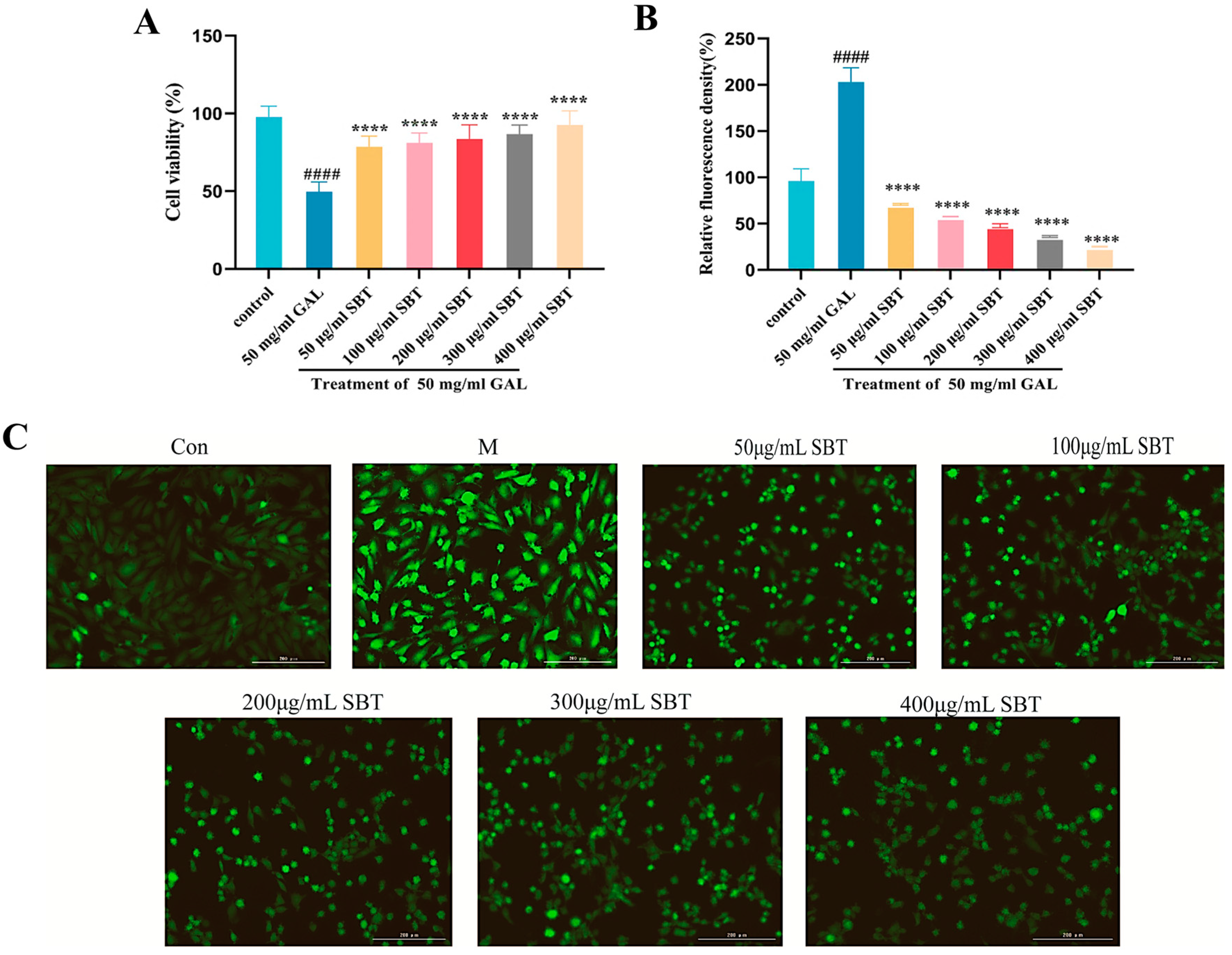
2.1. SBT Treatment Mitigates D-Galactose-Induced Oxidative Stress in H9c2 Cardiomyocytes
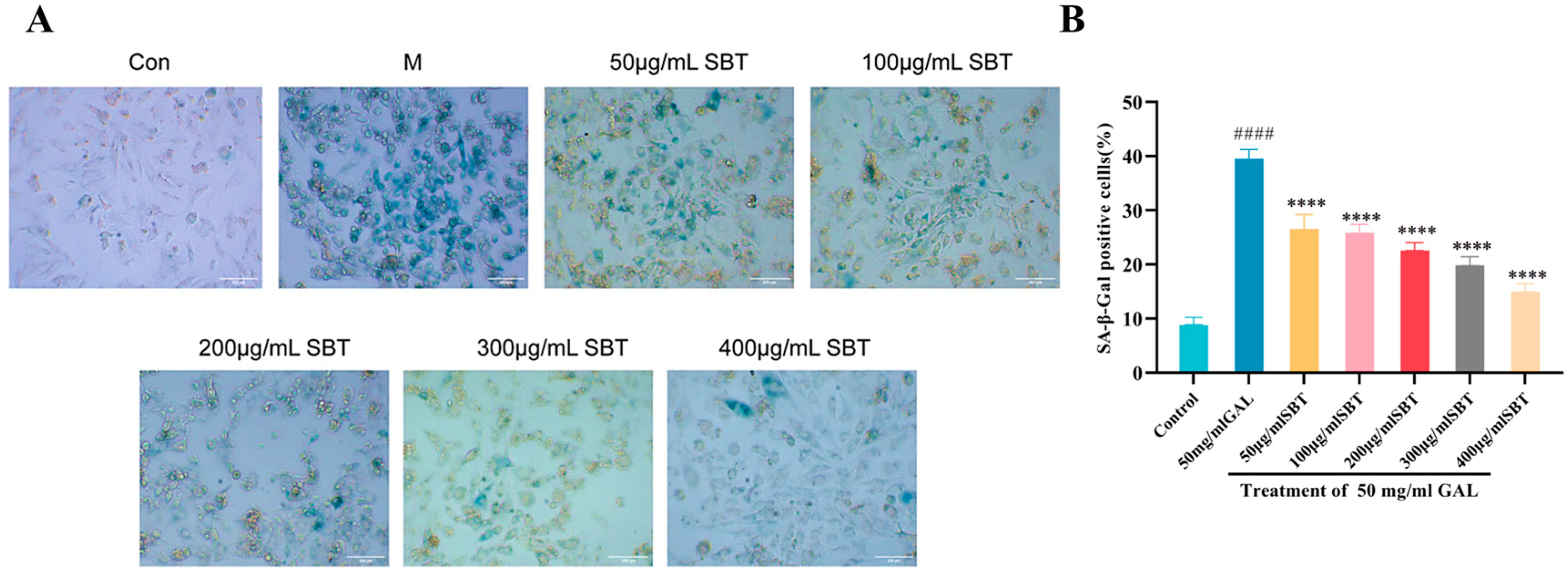
2.2. SBT Treatment Attenuates D-Galactose-Induced Cellular Senescence in H9c2 Cardiomyocytes
2.3. SBT Alleviates D-Galactose-Induced Apoptosis in H9c2 Cardiomyocytes
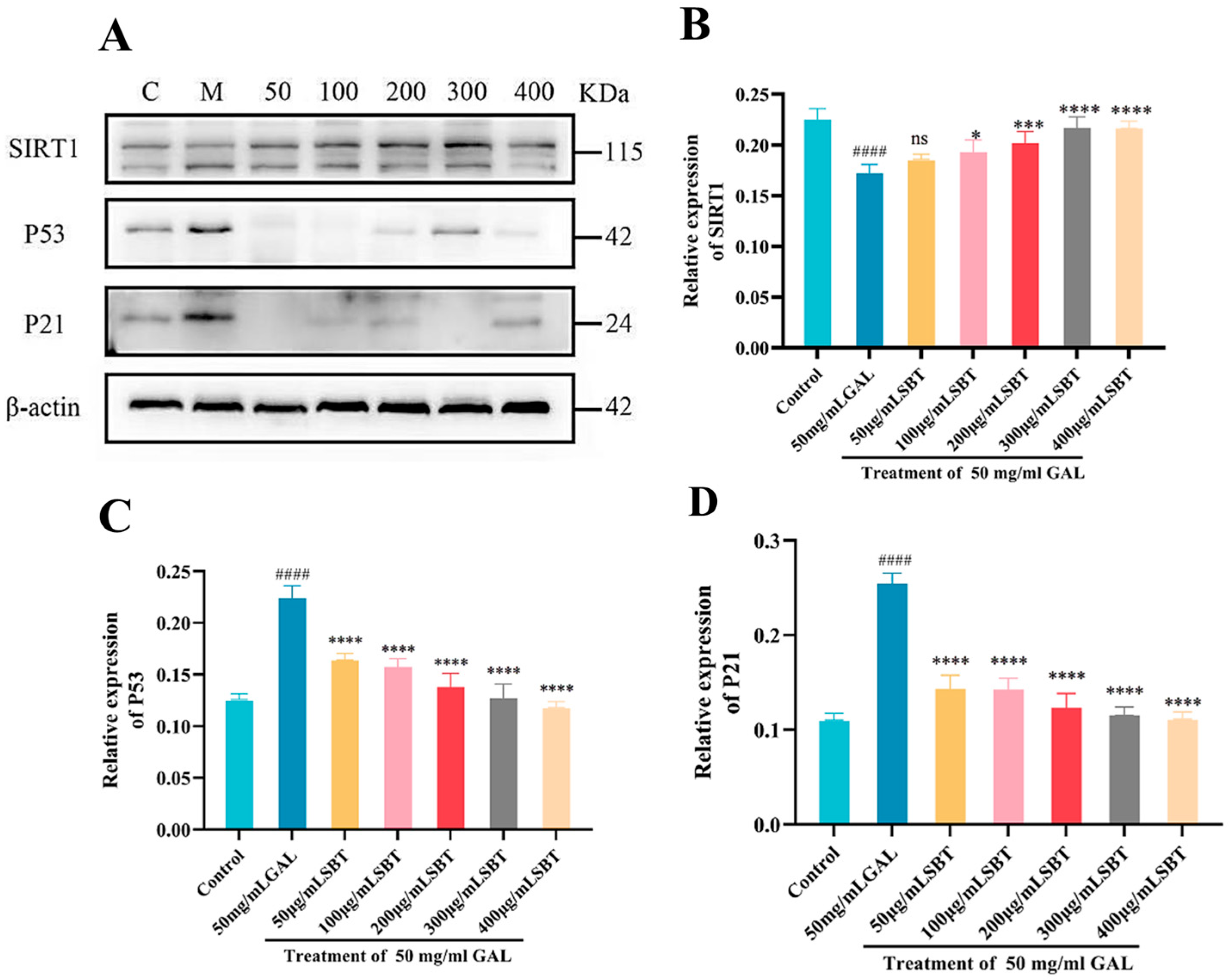
2.4. SBT Modulates the P53/P21/SIRT1 Pathway in D-Galactose-Induced H9c2 Senescent Cells
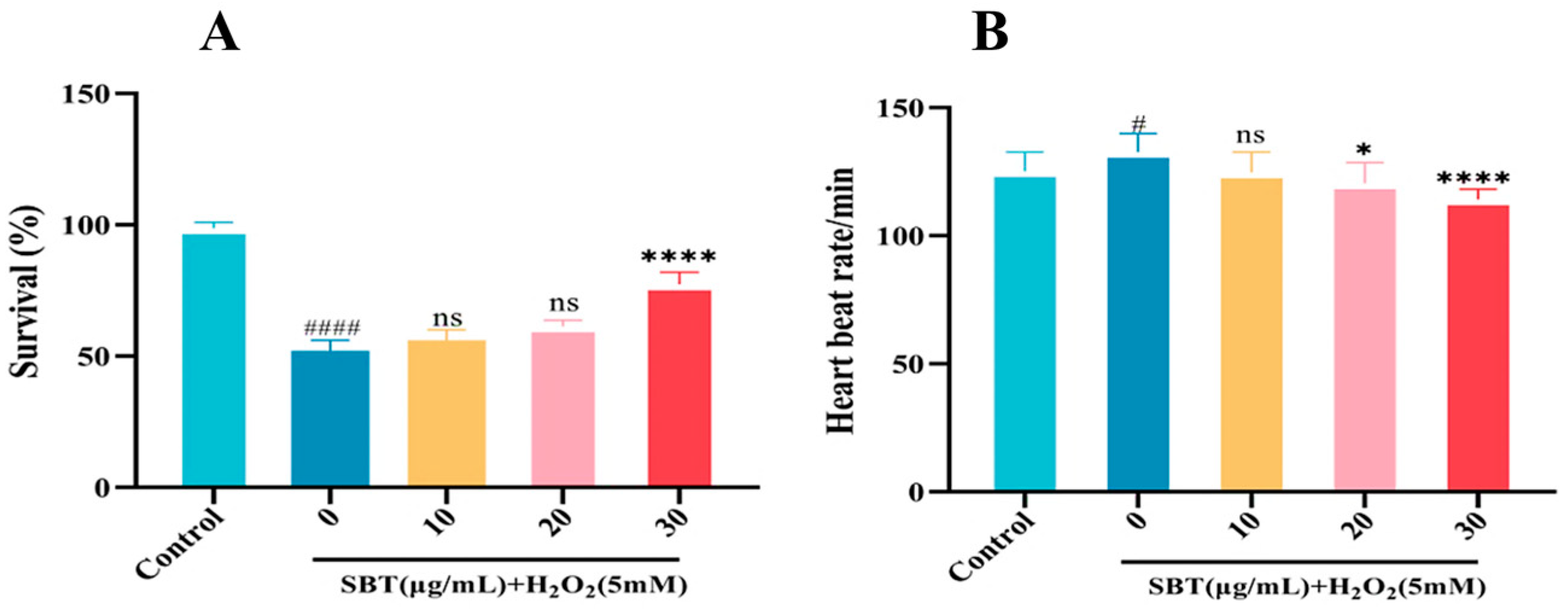
2.5. SBT Improves the Survival and Cardiac Function of H2O2-Treated Zebrafish
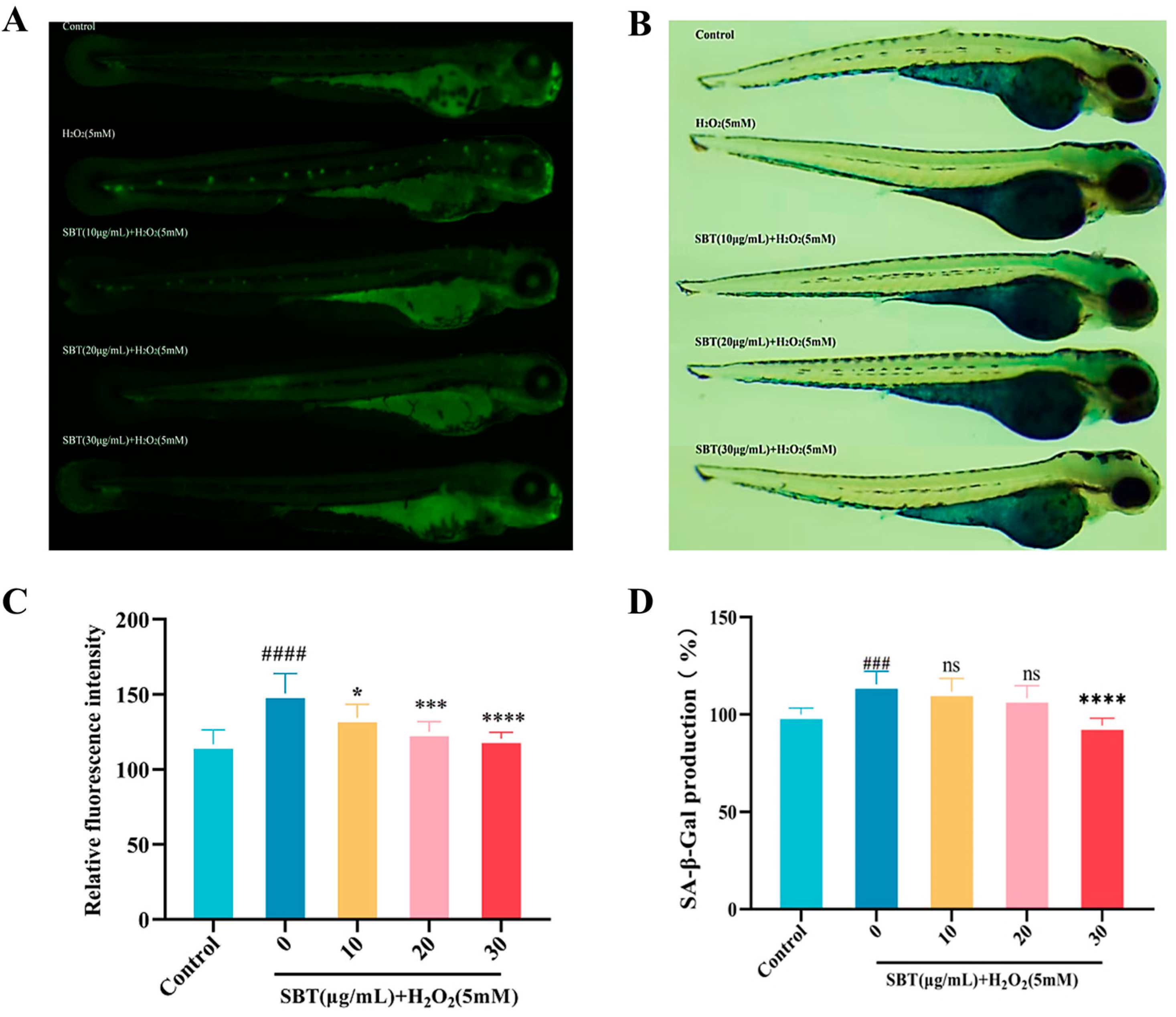
2.6. SBT Enhances the Antioxidant Defense in H2O2-Treated Zebrafish
2.7. SBT Inhibits Aging and Cell Death in Hydrogen Peroxide-Exposed Zebrafish Embryos
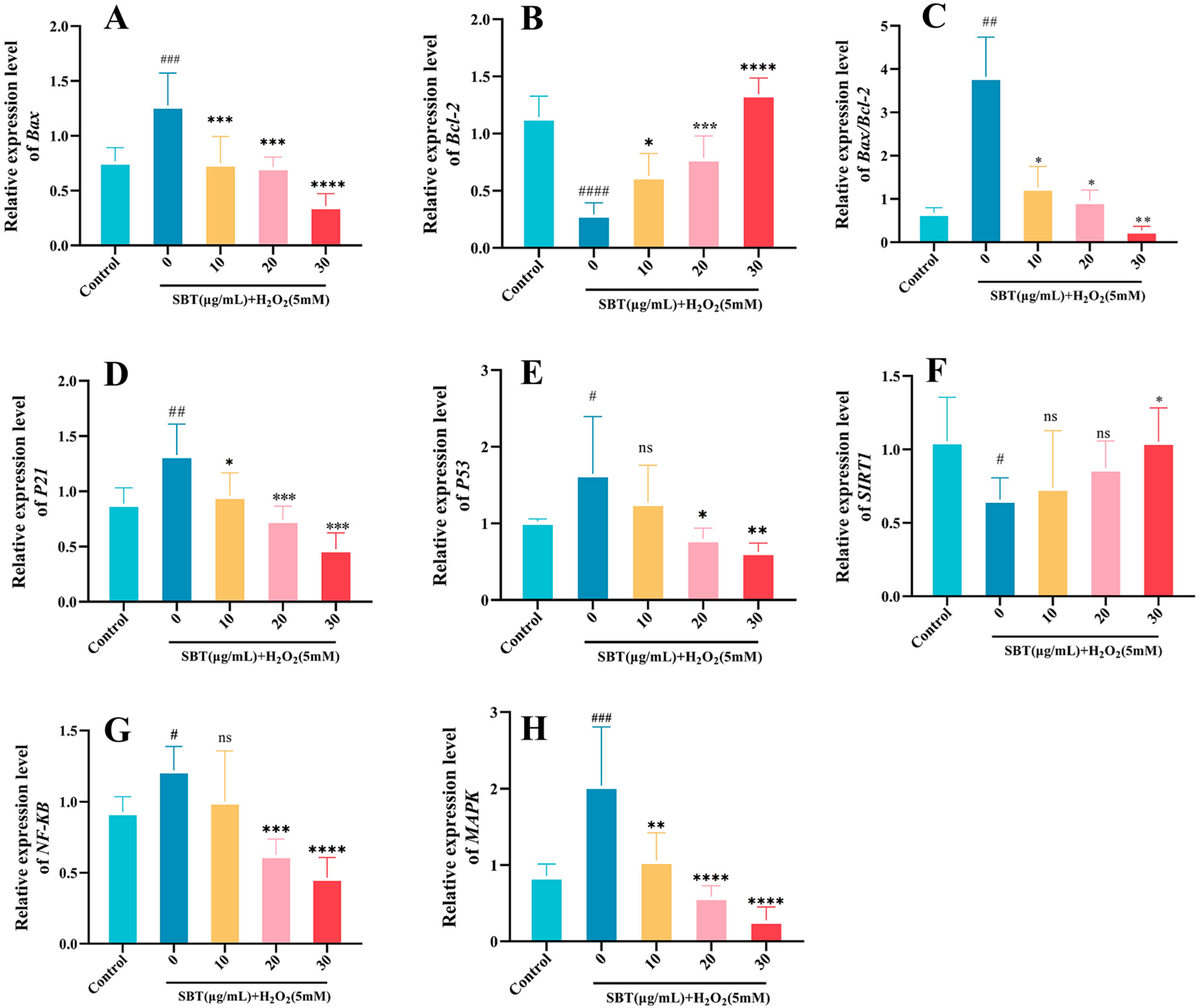
2.8. SBT Effects on P53/P21/SIRT1 Signaling Pathway
2.9. SBT-Mediated Modulation of Senescence-Associated Gene Transcription
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Drug Treatment
4.2. Cell Viability Assay H9c2
4.3. ROS Staining
4.4. SA-β-Gal Staining
4.5. TUNEL Staining
4.6. Western Blotting
4.7. Maintenance and Experimental Manipulation of Zebrafish
4.8. Survival Rate and Heartbeat Rate/Time Analysis
4.9. Measurement of Antioxidant Capacity
4.10. Acridine Orange (AO) Staining
4.11. qRT-PCR
4.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SBT | Srolo Bzhtang |
| GAL | D-galactose |
| ROS | Reactive Oxygen Species |
| SA-β-gal | Senescence-Associated β-galactosidase |
| SOD | Superoxide Dismutase |
| CAT | Catalase |
| GSH | Glutathione |
| MDA | Malondialdehyde |
| AO | Acridine Orange |
| MAPK | Mitogen-Activated Protein Kinase |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| Bax | Bcl-2-Associated X protein |
| Bcl-2 | B-cell lymphoma 2 |
| SIRT1 | Silent Information Regulator 1 |
| DMEM | Dulbecco’s Modified Eagle Medium |
| FBS | Fetal Bovine Serum |
| CCK-8 | Cell Counting Kit-8 |
| DCFH-DA | 2′,7′-Dichlorodihydrofluorescein diacetate |
| RIPA | Radioimmunoprecipitation Assay |
| SDS-PAGE | Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis |
| PVDF | Polyvinylidene Fluoride |
| HRP | Horseradish Peroxidase |
| ECL | Enhanced Chemiluminescence |
| hpf | Hours Post-Fertilization |
| TBST | Tris-Buffered Saline with Tween-20 |
| ANOVA | Analysis of Variance |
References
- Ramos, E.L.P.; Sousa Neto, I.V.; Pinto, A.P.; Cintra, D.E.; Ropelle, E.R.; Pauli, J.R.; de Freitas, E.C.; Mineo, T.W.P.; da Silva, A.S.R. Mechanisms underlying the interplay between autophagy and the inflammasome in age-related diseases: Implications for exercise immunology. Ageing Res. Rev. 2025, 110, 102821. [Google Scholar] [CrossRef]
- Sanada, F.; Hayashi, S.; Morishita, R. Targeting the hallmarks of aging: Mechanisms and therapeutic opportunities. Front. Cardiovasc. Med. 2025, 12, 1631578. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Strand, S.H.; Lee, M.R.; Saraswathibhatla, A.; van IJzendoorn, D.G.P.; Zhu, C.; Vennam, S.; Varma, S.; Hall, A.; Factor, R.E.; et al. Single-Cell Expression Analysis of Ductal Carcinoma In Situ Identifies Complex Genotypic-Phenotypic Relationships Altering Epithelial Composition. Cancer Res. 2025, 85, 2302–2319. [Google Scholar] [CrossRef]
- Yang, J.; Xu, J.; Wang, W.; Zhang, B.; Yu, X.; Shi, S. Epigenetic regulation in the tumor microenvironment: Molecular mechanisms and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 210. [Google Scholar] [CrossRef]
- Liu, B.; Qu, J.; Zhang, W.; Izpisua Belmonte, J.C.; Liu, G.H. A stem cell aging framework, from mechanisms to interventions. Cell Rep. 2022, 41, 111451. [Google Scholar] [CrossRef]
- Baechle, J.J.; Chen, N.; Makhijani, P.; Winer, S.; Furman, D.; Winer, D.A. Chronic inflammation and the hallmarks of aging. Mol. Metab. 2023, 74, 101755. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.L.; Ruan, B.; Song, P.; Fang, Z.Q.; Yue, Z.S.; Liu, J.J.; Dou, G.R.; Han, H.; Wang, L. Shear stress-induced cellular senescence blunts liver regeneration through Notch-sirtuin 1-P21/P16 axis. Hepatology 2022, 75, 584–599. [Google Scholar] [CrossRef] [PubMed]
- Azzahrani, K.; Alqahtani, F. The Tumor Suppressor p53 Downregulates p107 (RBL1) Through p21–RB/E2F Signaling and Tandem E2F Sites. Int. J. Mol. Sci. 2025, 26, 9903. [Google Scholar] [CrossRef]
- Chen, Y.; Peng, C.; Chen, J.; Chen, D.; Yang, B.; He, B.; Hu, W.; Zhang, Y.; Liu, H.; Dai, L.; et al. WTAP facilitates progression of hepatocellular carcinoma via m6A-HuR-dependent epigenetic silencing of ETS1. Mol. Cancer 2019, 18, 127. [Google Scholar] [CrossRef]
- Ashraf, S.; Biglow, L.; Dotson, J.; Tirona, M.T. Pneumonitis and cellular immunodeficiency triggered by the CDK 4/6 inhibitor Abemaciclib. Transl. Breast Cancer Res. A J. Focus. Transl. Res. Breast Cancer 2022, 3, 9. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, M.; Ge, Y.; Wang, X. SIRT1 and aging related signaling pathways. Mech. Ageing Dev. 2020, 187, 111215. [Google Scholar] [CrossRef]
- Abu Shelbayeh, O.; Arroum, T.; Morris, S.; Busch, K.B. PGC-1α Is a Master Regulator of Mitochondrial Lifecycle and ROS Stress Response. Antioxidants 2023, 12, 1075. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, H.; Zhai, X.; Gao, L.; Yang, M.; An, B.; Xia, T.; Du, G.; Li, X.; Wang, W.; et al. MELK promotes HCC carcinogenesis through modulating cuproptosis-related gene DLAT-mediated mitochondrial function. Cell Death Dis. 2023, 14, 733. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Kim, S.J.; Lei, Y.; Wang, S.; Wang, H.; Huang, H.; Zhang, H.; Tsung, A. Neutrophil extracellular traps in homeostasis and disease. Signal Transduct. Target. Ther. 2024, 9, 235. [Google Scholar] [CrossRef]
- Wei, X.; Xiong, X.; Wang, P.; Zhang, S.; Peng, D. SIRT1-mediated deacetylation of FOXO3 enhances mitophagy and drives hormone resistance in endometrial cancer. Mol. Med. 2024, 30, 147. [Google Scholar] [CrossRef]
- Lee, J.E.; Lee, H.; Baek, E.; Choi, B.; Yun, H.S.; Yoo, Y.K.; Lee, Y.S.; Song, G.J.; Cho, K.S. The role of glial and neuronal Eph/ephrin signaling in Drosophila mushroom body development and sleep and circadian behavior. Biochem. Biophys. Res. Commun. 2024, 720, 150072. [Google Scholar] [CrossRef]
- Tang, X.; Xu, Y.; Ou, N.; Tang, Y.; Chen, H. Update on late-onset hypogonadism: Current concepts, controversies, clinical diagnosis, pathogenesis, and treatment approaches. Sex. Med. Rev. 2025, 13, 652–662. [Google Scholar] [CrossRef]
- Tomasini, S.; Monteleone, E.; Altieri, A.; Margiotta, F.; Dardmeh, F.; Alipour, H.; Holm, A.; Kauppinen, S.; Panella, R. microRNA-22 Inhibition Stimulates Mitochondrial Homeostasis and Intracellular Degradation Pathways to Prevent Muscle Wasting. Int. J. Mol. Sci. 2025, 26, 9900. [Google Scholar] [CrossRef]
- Kaeberlein, T.L.; Green, A.S.; Haddad, G.; Hudson, J.; Isman, A.; Nyquist, A.; Rosen, B.S.; Suh, Y.; Zalzala, S.; Zhang, X.; et al. Evaluation of off-label rapamycin use to promote healthspan in 333 adults. GeroScience 2023, 45, 2757–2768. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jiang, B.; Shi, L.; Wang, L.; Yang, Y.; Li, Y.; Zhang, Y.; Zhu, Z.; Zhang, X.; Liu, X. The potential of natural herbal plants in the treatment and prevention of non-small cell lung cancer: An encounter between ferroptosis and mitophagy. J. Ethnopharmacol. 2025, 346, 119555. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Liang, X.; Wang, Z.; Jin, H.; Zou, L.; Yang, J. Potential Mechanism of Tibetan Medicine Liuwei Muxiang Pills against Colorectal Cancer: Network Pharmacology and Bioinformatics Analyses. Pharmaceuticals 2024, 17, 429. [Google Scholar] [CrossRef]
- Chen, T.; Su, S.; Yang, Z.; Zhang, D.; Li, Z.; Lu, D. Srolo Bzhtang reduces inflammation and vascular remodeling via suppression of the MAPK/NF-κB signaling pathway in rats with pulmonary arterial hypertension. J. Ethnopharmacol. 2022, 297, 115572. [Google Scholar] [CrossRef]
- Bian, X.; Chen, L.; Bian, X.; Li, L.; Liu, D.; Liu, S.; Xu, L.; Huo, X.; Yang, X. Protective effect of Tibetan medicine Qiwei Tiexie pills on liver injury induced by acetaminophen overdose: An integrated strategy of network pharmacology, metabolomics and transcriptomics. Phytomedicine Int. J. Phytother. Phytopharm. 2024, 123, 155221. [Google Scholar] [CrossRef]
- Wang, N.; Zhu, H. Geo-authentic Tibetan medicine: A traditional pharmacological resource for promoting human health and wellness. Front. Pharmacol. 2024, 15, 1432221. [Google Scholar] [CrossRef]
- Yang, J.; Karunarathna, S.C.; Patabendige, N.; Tarafder, E.; Lou, D.; Zhou, Y.; Hapuarachchi, K. Unveiling the Bioactive Compounds and Therapeutic Potential of Russula: A Comprehensive Review. J. Fungi 2025, 11, 341. [Google Scholar] [CrossRef]
- McMahon, S.; Spector, T.; Ramana, K.V. Significance of Macrophage-Mediated Inflammatory Response in Ocular Inflammatory Complications. Front. Biosci. (Landmark Ed.) 2025, 30, 26698. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gan, L.; Li, F.; Li, Q.; Wu, T.; Wu, Z.; Chen, P.; Scicluna, B.P.; Feng, X.; Gu, J.; et al. Tracheal epithelial cell-exosome-derived MiR-21-5p inhibits alveolar macrophage pyroptosis to resist pulmonary bacterial infection through PIK3CD-autophagy pathway. Life Sci. 2024, 336, 122340. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Chen, Y.C.; Chang, Y.C.; Hung, C.H.; Huang, C.Y.; Tsai, C.L.; Sun, C.K.; Lin, H.Y. Taurine Rescues Cancer-induced Atrophy in Human Skeletal Muscle Cells via Ameliorating the Inflammatory Tumor Microenvironment. Anticancer Res. 2024, 44, 1963–1971. [Google Scholar] [CrossRef]
- Peng, Z.; Zhao, C.; Yang, Z.; Gong, S.; Du, Z. D-galactose-induced mitochondrial oxidative damage and apoptosis in the cochlear stria vascularis of mice. BMC Mol. Cell Biol. 2023, 24, 27. [Google Scholar] [CrossRef] [PubMed]
- Shen, E.; Wu, Y.; Ye, W.; Li, S.; Zhu, J.; Jiang, M.; Hu, Z.; Cao, G.; Yi, X.; Li, F.; et al. The FGF13-Caveolin-1 Axis: A Key Player in the Pathogenesis of Doxorubicin- and D-Galactose-Induced Premature Cardiac Aging. Adv. Sci. 2025, 12, e2501055. [Google Scholar] [CrossRef]
- Jing, L.; Su, S.; Zhang, D.; Li, Z.; Lu, D.; Ge, R. Srolo Bzhtang, a traditional Tibetan medicine formula, inhibits cigarette smoke induced airway inflammation and muc5ac hypersecretion via suppressing IL-13/STAT6 signaling pathway in rats. J. Ethnopharmacol. 2019, 235, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Pu, L.; Yang, C.; Yu, L.; Li, S.; Liu, Y.; Liu, X.; Lai, X. Tibetan Medicines for the Treatment of Diabetic Nephropathy. Evid.-Based Complement. Altern. Med. ECAM 2021, 2021, 7845848. [Google Scholar] [CrossRef]
- Pan, L.; Gao, J.; Han, Y.; Shi, Y.; Tang, X.; Pu, L.; Lai, X.; Dongzhu, R.; Zhang, J.; Xiangmao, Q.; et al. The Treatment of Cholecystitis and Cholelithiasis by Tibetan Medicine. Evid.-Based Complement. Altern. Med. ECAM 2021, 2021, 9502609. [Google Scholar] [CrossRef]
- Fu, K.; Xu, M.; Zhou, Y.; Li, X.; Wang, Z.; Liu, X.; Meng, X.; Zeng, Y.; Zhang, H. The Status quo and way forwards on the development of Tibetan medicine and the pharmacological research of tibetan materia Medica. Pharmacol. Res. 2020, 155, 104688. [Google Scholar] [CrossRef]
- Yu, L.; Li, S.; Pu, L.; Yang, C.; Shi, Q.; Zhao, Q.; Meniga, S.; Liu, Y.; Zhang, Y.; Lai, X. Traditional Tibetan medicine: Therapeutic potential in rheumatoid arthritis. Front. Pharmacol. 2022, 13, 938915. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, K.; Jiang, C.; Chen, Y.; Liu, F.; Xie, M.; Yim, W.Y.; Yao, D.; Qian, X.; Chen, S.; et al. Morusin Alleviates Aortic Valve Calcification by Inhibiting Valve Interstitial Cell Senescence Through Ccnd1/Trim25/Nrf2 Axis. Adv. Sci. 2024, 11, e2307319. [Google Scholar] [CrossRef]
- Wen, Y.; Zhang, X.; Liu, H.; Ye, H.; Wang, R.; Ma, C.; Duo, T.; Wang, J.; Yang, X.; Yu, M.; et al. SGLT2 inhibitor downregulates ANGPTL4 to mitigate pathological aging of cardiomyocytes induced by type 2 diabetes. Cardiovasc. Diabetol. 2024, 23, 430. [Google Scholar] [CrossRef]
- Ke, H.; Zhang, X.; Liang, S.; Zhou, C.; Hu, Y.; Huang, Q.; Wu, J. Study on the anti-skin aging effect and mechanism of Sijunzi Tang based on network pharmacology and experimental validation. J. Ethnopharmacol. 2024, 333, 118421. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Tian, L.; Kou, W.; Cao, G.; Wang, L.; Xia, Y.; Lin, Y.; Tang, S.; Zhang, J.; Yang, H. Xiyangshen Sanqi Danshen granules attenuated D-gal-induced C57BL/6J mouse aging through the AMPK/SIRT1 signaling pathway. Phytomed. Int. J. Phytother. Phytopharm. 2025, 136, 156213. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, Y.; He, Y.; Feng, C.; Ou, H.; Yang, J.; Chen, Y.; You, F.; Shao, B.; Bao, J.; et al. Erxian decoction alleviates cisplatin-induced premature ovarian failure in rats by reducing oxidation levels in ovarian granulosa cells. J. Ethnopharmacol. 2023, 304, 116046. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, H.; Zhu, J.; Song, N.; Lu, Z.; Fang, Y.; Teng, J.; Dai, Y.; Ding, X. Inhibition of p53/miR-34a/SIRT1 axis ameliorates podocyte injury in diabetic nephropathy. Biochem. Biophys. Res. Commun. 2021, 559, 48–55. [Google Scholar] [CrossRef]
- Lv, X.; Liu, C.; Liu, S.; Li, Y.; Wang, W.; Li, K.; Hua, F.; Cui, B.; Zhang, X.; Yu, J.; et al. The cell cycle inhibitor P21 promotes the development of pulmonary fibrosis by suppressing lung alveolar regeneration. Acta Pharm. Sin. B 2022, 12, 735–746. [Google Scholar] [CrossRef]
- Tubita, A.; Lombardi, Z.; Tusa, I.; Lazzeretti, A.; Sgrignani, G.; Papini, D.; Menconi, A.; Gagliardi, S.; Lulli, M.; Dello Sbarba, P.; et al. Inhibition of ERK5 Elicits Cellular Senescence in Melanoma via the Cyclin-Dependent Kinase Inhibitor p21. Cancer Res. 2022, 82, 447–457. [Google Scholar] [CrossRef]
- Tao, P.; Zhang, H.F.; Zhou, P.; Wang, Y.L.; Tan, Y.Z.; Wang, H.J. Growth differentiation factor 11 alleviates oxidative stress-induced senescence of endothelial progenitor cells via activating autophagy. Stem Cell Res. Ther. 2024, 15, 370. [Google Scholar] [CrossRef]
- Wang, X.X.; Li, M.; Xu, X.W.; Zhao, W.B.; Jin, Y.M.; Li, L.L.; Qin, Z.H.; Sheng, R.; Ni, H. BNIP3-mediated mitophagy attenuates hypoxic-ischemic brain damage in neonatal rats by inhibiting ferroptosis through P62-KEAP1-NRF2 pathway activation to maintain iron and redox homeostasis. Acta Pharmacol. Sin. 2025, 46, 33–51. [Google Scholar] [CrossRef]
- Cui, Z.; Zhao, X.; Amevor, F.K.; Du, X.; Wang, Y.; Li, D.; Shu, G.; Tian, Y.; Zhao, X. Therapeutic application of quercetin in aging-related diseases: SIRT1 as a potential mechanism. Front. Immunol. 2022, 13, 943321. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Liang, W.; Zhang, Z.; Deng, Y.; Chen, X.; Hou, Z.; Xie, Y.; Wang, Q.; Li, Y.; et al. Microglial TMEM119 binds to amyloid-β to promote its clearance in an Aβ-depositing mouse model of Alzheimer’s disease. Immunity 2025, 58, 1830–1846.e7. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.O.; Rodriguez-Romera, A.; Reyat, J.S.; Olijnik, A.A.; Colombo, M.; Wang, G.; Wen, W.X.; Sousos, N.; Murphy, L.C.; Grygielska, B.; et al. Human Bone Marrow Organoids for Disease Modeling, Discovery, and Validation of Therapeutic Targets in Hematologic Malignancies. Cancer Discov. 2023, 13, 364–385. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, H.J. Normal Aging Induces Changes in the Brain and Neurodegeneration Progress: Review of the Structural, Biochemical, Metabolic, Cellular, and Molecular Changes. Front. Aging Neurosci. 2022, 14, 931536. [Google Scholar] [CrossRef] [PubMed]
- Frei, M.; Wirawan, R.; Wein, T.; Bracher, F. Lead Structure-Based Hybridization Strategy Reveals Major Potency Enhancement of SirReal-Type Sirt2 Inhibitors. Int. J. Mol. Sci. 2025, 26, 9855. [Google Scholar] [CrossRef] [PubMed]



| Gene Name | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| Bax | CGGCATGGCGACAGGGATG | CATAGCAGGAGACGGTGGTGATG |
| Bcl-2 | CTCCTTCTCATACTTCAGCCTCCAC | ACCTTCAATGCCTCCTCCATCTTAC |
| MAPK | GTCCTACAGCAGCACAACTTCTAC | TCAACCCACAACGAAACACTCAG |
| NF-κB | CCTGTCTGTCTGTCTGTCTGTCTG | TCGTGGTGTCGTTGCTCTTCTC |
| p21 | CCAGAGACGACACCGTTTATT | GGAAGACTGAGGAATGGATCTTT |
| p53 | CGAGCCACTGCCATCTATAA | CTGATTGCCCTCCACTCTTATC |
| SIRT1 | CGCAAAGACATCAACACGTTAG | CAGGAATCCCACAGGAAACA |
| β-actin | TCGAGCAGGAGATGGGAACC | CTCGTGGATACCGCAAGATTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Dongzhu, N.; Zhao, C.; Liang, J.; Wang, N.; Suonan, D.; Sun, S. Multi-Target Botanical Complex Attenuates Cellular Senescence via Bidirectional P21/P53/SIRT1 Regulation: Dual Model Validation. Int. J. Mol. Sci. 2025, 26, 11394. https://doi.org/10.3390/ijms262311394
Wu J, Dongzhu N, Zhao C, Liang J, Wang N, Suonan D, Sun S. Multi-Target Botanical Complex Attenuates Cellular Senescence via Bidirectional P21/P53/SIRT1 Regulation: Dual Model Validation. International Journal of Molecular Sciences. 2025; 26(23):11394. https://doi.org/10.3390/ijms262311394
Chicago/Turabian StyleWu, Jiaqin, Nanjia Dongzhu, Chengzhou Zhao, Jialin Liang, Ningbo Wang, Dengdeng Suonan, and Shengnan Sun. 2025. "Multi-Target Botanical Complex Attenuates Cellular Senescence via Bidirectional P21/P53/SIRT1 Regulation: Dual Model Validation" International Journal of Molecular Sciences 26, no. 23: 11394. https://doi.org/10.3390/ijms262311394
APA StyleWu, J., Dongzhu, N., Zhao, C., Liang, J., Wang, N., Suonan, D., & Sun, S. (2025). Multi-Target Botanical Complex Attenuates Cellular Senescence via Bidirectional P21/P53/SIRT1 Regulation: Dual Model Validation. International Journal of Molecular Sciences, 26(23), 11394. https://doi.org/10.3390/ijms262311394






