Inactivation of the High-Affinity Ca2+ Uptake System Delays the Amiodarone-Induced Ca2+ Influx in Yeast Ogataea parapolymorpha
Abstract
1. Introduction
2. Results
2.1. Inactivation of MID1 Suppresses SDS Hypersensitivity Caused by the Lack of the Pmc1 Ca2+ Ion Pump
2.2. Effects of Inactivation of MID1 and CCH1 on the SDS-Induced [Ca2+]cyt Rise in the pmc1-Δ Mutant Are the Same
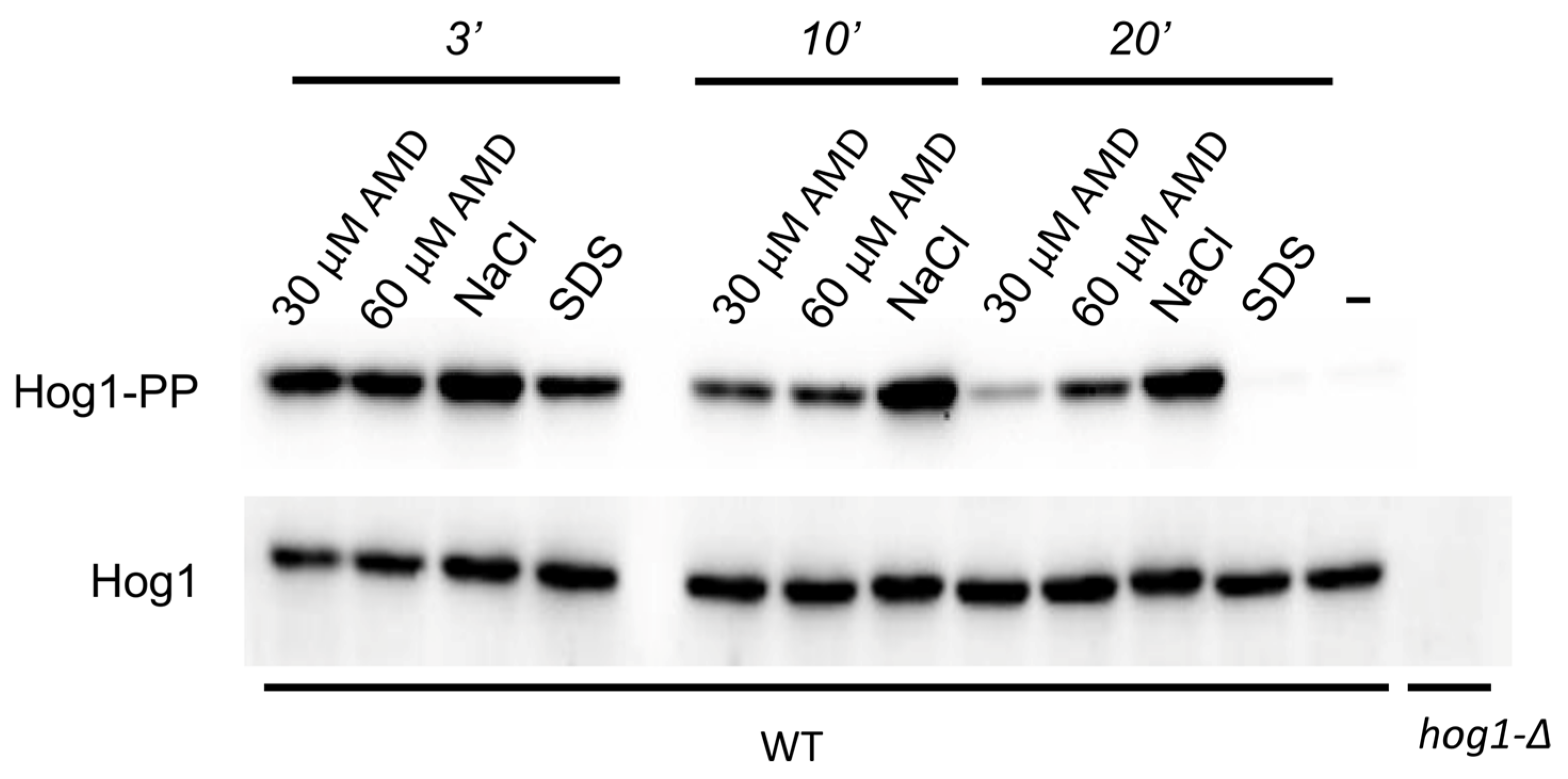
2.3. Amiodarone Causes a Rapid Increase in [Ca2+]cyt and Hog1 Activation in O. parapolymorpha
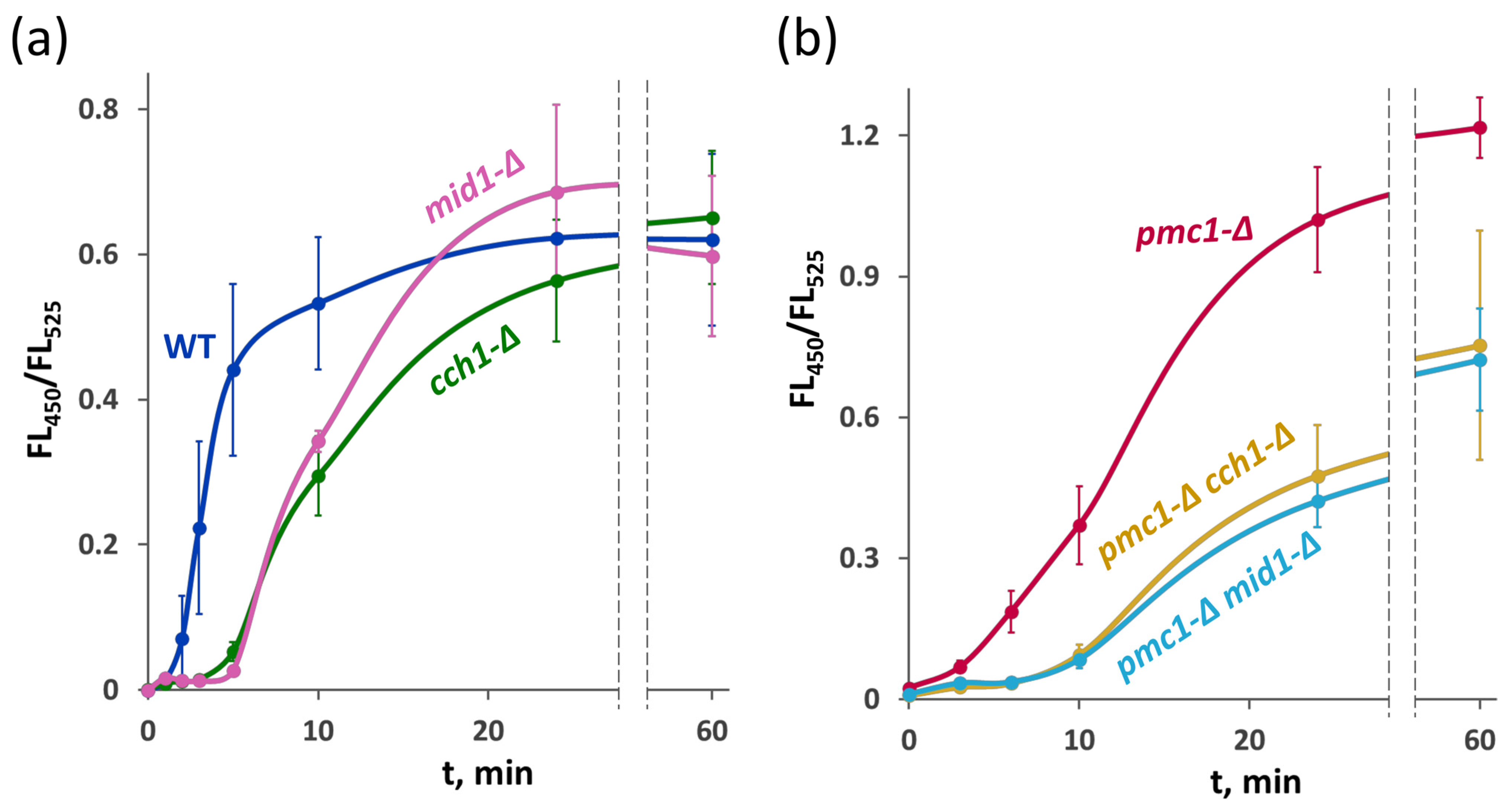
2.4. Effects of the Loss of HACS in the Strain with Wild-Type PMC1 and in the pmc1-Δ Mutant on Survival Rate on Amiodarone-Containing Medium Are Opposite
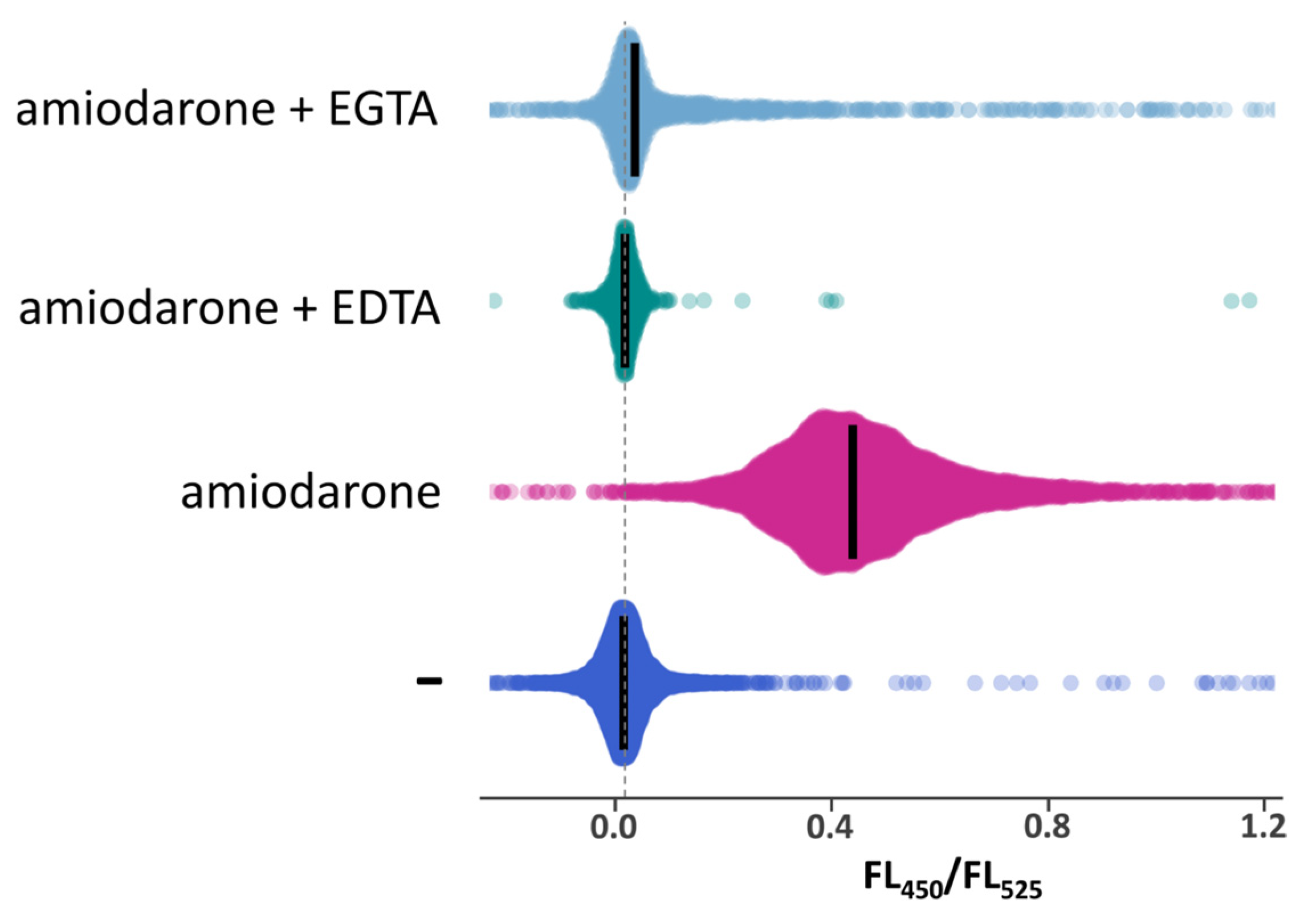
2.5. Caffeine Does Not Alleviate Effects of Amiodarone on Cell Growth and Has a Negligible Effect on the Amiodarone-Induced [Ca2+]cyt Rise
2.6. Deletion Mutations of MID1 and CCH1 Delay the Amiodarone-Induced Ca2+ Influx
3. Discussion
4. Materials and Methods
4.1. Culture Media and Yeast Transformation
4.2. Yeast Strains
4.3. Monitoring of the Cytosolic Ca2+ Concentration
4.4. Growth Inhibition and Cell Survival Assays
4.5. Analysis of Hog1 Phosphorylation
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pittman, J.K. Vacuolar Ca2+ Uptake. Cell Calcium 2011, 50, 139–146. [Google Scholar] [CrossRef]
- Dürr, G.; Strayle, J.; Plemper, R.; Elbs, S.; Klee, S.K.; Catty, P.; Wolf, D.H.; Rudolph, H.K. The Medial-Golgi Ion Pump Pmr1 Supplies the Yeast Secretory Pathway with Ca2+ and Mn2+ Required for Glycosylation, Sorting, and Endoplasmic Reticulum-Associated Protein Degradation. Mol. Biol. Cell 1998, 9, 1149–1162. [Google Scholar] [CrossRef]
- Fokina, A.V.; Chechenova, M.B.; Karginov, A.V.; Ter-Avanesyan, M.D.; Agaphonov, M.O. Genetic Evidence for the Role of the Vacuole in Supplying Secretory Organelles with Ca2+ in Hansenula Polymorpha. PLoS ONE 2015, 10, e0145915. [Google Scholar] [CrossRef] [PubMed]
- Vashist, S.; Frank, C.G.; Jakob, C.A.; Ng, D.T.W. Two Distinctly Localized P-Type ATPases Collaborate to Maintain Organelle Homeostasis Required for Glycoprotein Processing and Quality Control. Mol. Biol. Cell 2002, 13, 3955–3966. [Google Scholar] [CrossRef] [PubMed]
- Strayle, J.; Pozzan, T.; Rudolph, H.K. Steady-State Free Ca2+ in the Yeast Endoplasmic Reticulum Reaches Only 10 MicroM and Is Mainly Controlled by the Secretory Pathway Pump Pmr1. EMBO J. 1999, 18, 4733–4743. [Google Scholar] [CrossRef] [PubMed]
- Müller, E.M.; Locke, E.G.; Cunningham, K.W.; Muller, E.M.; Locke, E.G.; Cunningham, K.W. Differential Regulation of Two Ca2+ Influx Systems by Pheromone Signaling in Saccharomyces Cerevisiae. Genetics 2001, 159, 1527–1538. [Google Scholar] [CrossRef]
- Muller, E.M.; Mackin, N.A.; Erdman, S.E.; Cunningham, K.W. Fig1p facilitates Ca2+ influx and cell fusion during mating of Saccharomyces cerevisiae. J. Biol. Chem. 2003, 278, 38461–38469. [Google Scholar] [CrossRef]
- Paidhungat, M.; Garrett, S. A Homolog of Mammalian, Voltage-Gated Calcium Channels Mediates Yeast Pheromone-Stimulated Ca2+ Uptake and Exacerbates the Cdc1(Ts) Growth Defect. Mol. Cell. Biol. 1997, 17, 6339–6347. [Google Scholar] [CrossRef]
- Ghezzi, A.; Liebeskind, B.J.; Thompson, A.; Atkinson, N.S.; Zakon, H.H. Ancient Association between Cation Leak Channels and Mid1 Proteins Is Conserved in Fungi and Animals. Front. Mol. Neurosci. 2014, 7, 15. [Google Scholar] [CrossRef]
- Martin, D.C.; Kim, H.; Mackin, N.A.; Maldonado-Báez, L.; Evangelista, C.C.; Beaudry, V.G.; Dudgeon, D.D.; Naiman, D.Q.; Erdman, S.E.; Cunningham, K.W. New Regulators of a High Affinity Ca2+ Influx System Revealed through a Genome-Wide Screen in Yeast. J. Biol. Chem. 2011, 286, 10744–10754. [Google Scholar] [CrossRef]
- Courchesne, W.E. Characterization of a Novel, Broad-Based Fungicidal Activity for the Antiarrhythmic Drug Amiodarone. J. Pharmacol. Exp. Ther. 2002, 300, 195–199. [Google Scholar] [CrossRef]
- Muend, S.; Rao, R. Fungicidal Activity of Amiodarone Is Tightly Coupled to Calcium Influx. FEMS Yeast Res. 2008, 8, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Courchesne, W.E.; Ozturk, S. Amiodarone Induces a Caffeine-inhibited, MID1 -depedent Rise in Free Cytoplasmic Calcium in Saccharomyces Cerevisiae. Mol. Microbiol. 2003, 47, 223–234. [Google Scholar] [CrossRef]
- Gupta, S.S.; Ton, V.-K.; Beaudry, V.; Rulli, S.; Cunningham, K.; Rao, R. Antifungal Activity of Amiodarone Is Mediated by Disruption of Calcium Homeostasis. J. Biol. Chem. 2003, 278, 28831–28839. [Google Scholar] [CrossRef]
- Pozniakovsky, A.I.; Knorre, D.A.; Markova, O.V.; Hyman, A.A.; Skulachev, V.P.; Severin, F.F. Role of Mitochondria in the Pheromone- and Amiodarone-Induced Programmed Death of Yeast. J. Cell Biol. 2005, 168, 257–269. [Google Scholar] [CrossRef]
- Peiter, E.; Fischer, M.; Sidaway, K.; Roberts, S.K.; Sanders, D. The Saccharomyces Cerevisiae Ca2+ Channel Cch1pMid1p Is Essential for Tolerance to Cold Stress and Iron Toxicity. FEBS Lett. 2005, 579, 5697–5703. [Google Scholar] [CrossRef]
- Gelman, I.; Sharma, N.; Mckeeman, O.; Lee, P.; Campagna, N.; Tomei, N.; Baranchuk, A.; Zhang, S.; El-Diasty, M. The Ion Channel Basis of Pharmacological Effects of Amiodarone on Myocardial Electrophysiological Properties, a Comprehensive Review. Biomed. Pharmacother. 2024, 174, 116513. [Google Scholar] [CrossRef] [PubMed]
- Fokina, A.V.; Sokolov, S.S.; Kang, H.A.; Kalebina, T.S.; Ter-Avanesyan, M.D.; Agaphonov, M.O. Inactivation of Pmc1 Vacuolar Ca2+ ATPase Causes G2 Cell Cycle Delay in Hansenula Polymorpha. Cell Cycle 2012, 11, 778–784. [Google Scholar] [CrossRef]
- Kulakova, M.; Pakhomova, M.; Bidiuk, V.; Ershov, A.; Alexandrov, A.; Agaphonov, M. High-Affinity Plasma Membrane Ca2+ Channel Cch1 Modulates Adaptation to Sodium Dodecyl Sulfate-Triggered Rise in Cytosolic Ca2+ Concentration in Ogataea Parapolymorpha. Int. J. Mol. Sci. 2024, 25, 11450. [Google Scholar] [CrossRef] [PubMed]
- Cyert, M.S. Calcineurin Signaling in Saccharomyces Cerevisiae: How Yeast Go Crazy in Response to Stress. Biochem. Biophys. Res. Commun. 2003, 311, 1143–1150. [Google Scholar] [CrossRef]
- Goldman, A.; Roy, J.; Bodenmiller, B.; Wanka, S.; Landry, C.R.; Aebersold, R.; Cyert, M.S. The Calcineurin Signaling Network Evolves via Conserved Kinase-Phosphatase Modules That Transcend Substrate Identity. Mol. Cell 2014, 55, 422–435. [Google Scholar] [CrossRef]
- Kaminska, J.; Soczewka, P.; Rzepnikowska, W.; Zoladek, T. Yeast as a Model to Find New Drugs and Drug Targets for VPS13-Dependent Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5106. [Google Scholar] [CrossRef] [PubMed]
- Soczewka, P.; Kolakowski, D.; Smaczynska-de Rooij, I.; Rzepnikowska, W.; Ayscough, K.R.; Kaminska, J.; Zoladek, T. Yeast-Model-Based Study Identified Myosin- and Calcium-Dependent Calmodulin Signalling as a Potential Target for Drug Intervention in Chorea-Acanthocytosis. Dis. Model. Mech. 2019, 12, dmm036830. [Google Scholar] [CrossRef]
- Ravin, N.V.; Eldarov, M.A.; Kadnikov, V.V.; Beletsky, A.V.; Schneider, J.; Mardanova, E.S.; Smekalova, E.M.; Zvereva, M.I.; Dontsova, O.A.; Mardanov, A.V.; et al. Genome Sequence and Analysis of Methylotrophic Yeast Hansenula Polymorpha DL1. BMC Genom. 2013, 14, 837. [Google Scholar] [CrossRef] [PubMed]
- Iida, K.; Teng, J.; Cho, T.; Yoshikawa-Kimura, S.; Iida, H. Post-Translational Processing and Membrane Translocation of the Yeast Regulatory Mid1 Subunit of the Cch1/VGCC/NALCN Cation Channel Family. J. Biol. Chem. 2017, 292, 20570–20582. [Google Scholar] [CrossRef]
- Kulakova, M.V.; Karginov, A.V.; Alexandrov, A.I.; Agaphonov, M.O. The GEM-GECO Calcium Indicator Is Useable in Ogataea Parapolymorpha Yeast, but Aggravates Effects of Increased Cytosolic Calcium Levels. Int. J. Mol. Sci. 2022, 23, 10004. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Araki, S.; Wu, J.; Teramoto, T.; Chang, Y.-F.; Nakano, M.; Abdelfattah, A.S.; Fujiwara, M.; Ishihara, T.; Nagai, T.; et al. An Expanded Palette of Genetically Encoded Ca2+ Indicators. Science 2011, 333, 1888–1891. [Google Scholar] [CrossRef]
- Volkova, M.; Atamas, A.; Tsarenko, A.; Rogachev, A.; Guskov, A. Cation Transporters of Candida Albicans—New Targets to Fight Candidiasis? Biomolecules 2021, 11, 584. [Google Scholar] [CrossRef]
- Kanzaki, M.; Nagasawa, M.; Kojima, I.; Sato, C.; Naruse, K.; Sokabe, M.; Iida, H. Molecular Identification of a Eukaryotic, Stretch-Activated Nonselective Cation Channel. Science 1999, 285, 882–886, Erratum in Science 1999, 285, 1493. https://doi.org/10.1126/science.288.5470.1347. [Google Scholar] [CrossRef]
- Kuranda, K.; Leberre, V.; Sokol, S.; Palamarczyk, G.; François, J. Investigating the Caffeine Effects in the Yeast Saccharomyces Cerevisiae Brings New Insights into the Connection between TOR, PKC and Ras/CAMP Signalling Pathways. Mol. Microbiol. 2006, 61, 1147–1166. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, X.; Li, G.; Wang, X. TORC1 Signaling in Fungi: From Yeasts to Filamentous Fungi. Microorganisms 2023, 11, 218. [Google Scholar] [CrossRef]
- Hohmann, S.; Krantz, M.; Nordlander, B. Yeast Osmoregulation. Methods Enzymol. 2007, 428, 29–45. [Google Scholar] [CrossRef]
- Elhasi, T.; Blomberg, A. Caffeine Activates HOG-Signalling and Inhibits Pseudohyphal Growth in Saccharomyces Cerevisiae. BMC Res. Notes 2023, 16, 52. [Google Scholar] [CrossRef]
- Clotet, J.; Escoté, X.; Adrover, M.A.; Yaakov, G.; Garí, E.; Aldea, M.; de Nadal, E.; Posas, F. Phosphorylation of Hsl1 by Hog1 Leads to a G2 Arrest Essential for Cell Survival at High Osmolarity. EMBO J. 2006, 25, 2338–2346. [Google Scholar] [CrossRef] [PubMed]
- Asano, S.; Park, J.; Sakchaisri, K.; Yu, L.; Song, S.; Supavilai, P.; Veenstra, T.D.; Lee, K.S. Concerted Mechanism of Swe1/Wee1 Regulation by Multiple Kinases in Budding Yeast. EMBO J. 2005, 24, 2194–2204. [Google Scholar] [CrossRef] [PubMed]
- Gietz, R.D.; Schiestl, R.H. Quick and Easy Yeast Transformation Using the LiAc/SS Carrier DNA/PEG Method. Nat. Protoc. 2007, 2, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Karginov, A.V.; Alexandrov, A.I.; Kushnirov, V.V.; Agaphonov, M.O. Perturbations in the Heme and Siroheme Biosynthesis Pathways Causing Accumulation of Fluorescent Free Base Porphyrins and Auxotrophy in Ogataea Yeasts. J. Fungi 2021, 7, 884. [Google Scholar] [CrossRef]
- Agaphonov, M.O.; Trushkina, P.M.; Sohn, J.H.; Choi, E.S.; Rhee, S.K.; Ter-Avanesyan, M.D. Vectors for Rapid Selection of Integrants with Different Plasmid Copy Numbers in the Yeast Hansenula Polymorpha DL1. Yeast 1999, 15, 541–551. [Google Scholar] [CrossRef]
- Agaphonov, M.; Alexandrov, A. Self-Excising Integrative Yeast Plasmid Vectors Containing an Intronated Recombinase Gene. FEMS Yeast Res. 2014, 14, 1048–1054. [Google Scholar] [CrossRef]
- Agaphonov, M.; Romanova, N.; Choi, E.-S.; Ter-Avanesyan, M. A Novel Kanamycin/G418 Resistance Marker for Direct Selection of Transformants in Escherichia Coli and Different Yeast Species. Yeast 2010, 27, 189–195. [Google Scholar] [CrossRef]
- Giaever, G.; Chu, A.M.; Ni, L.; Connelly, C.; Riles, L.; Véronneau, S.; Dow, S.; Lucau-Danila, A.; Anderson, K.; André, B.; et al. Functional Profiling of the Saccharomyces Cerevisiae Genome. Nature 2002, 418, 387–391. [Google Scholar] [CrossRef] [PubMed]




| Source | Protein Length | Position of the Hydrophobic Regions | |
|---|---|---|---|
| N-Terminal | C-Terminal | ||
| O. parapolymorpha | 479 | 1–13 | 466–479 |
| S. cerevisiae | 548 | 1–14 | - |
| Y. lipolytica | 640 | 3–15 | 627–640 |
| Sch. pombe | 486 | 1–16 | 474–485 |
| C. neoformans | 623 | 58–73 | - |
| C. albicans | 559 | 1–13 | 546–559 |
| A. fumigatus | 642 | 14–33 | 621–638 |
| Strain | Genotype * | Source |
|---|---|---|
| DL5 | leu2 {PMAL1-GEM-GECO} | [26] |
| DL5-LC | leu2 {PMAL1-GEM-GECO} {LEU2} | [19] |
| DL5-cch1 | leu2 cch1::LEU2 {PMAL1-GEM-GECO} | [19] |
| DL5-pmc1-LC | leu2 pmc1::loxP {PMAL1-GEM-GECO} {LEU2} | [19] |
| DL5-pmc1-cch1 | leu2 pmc1::loxP cch1::LEU2 {PMAL1-GEM-GECO} | [19] |
| DL5-pmc1-mid1 | leu2 pmc1::loxP mid1::LEU2 {PMAL1-GEM-GECO} | This study |
| DL5-mid1 | leu2 mid1::LEU2 {PMAL1-GEM-GECO} | This study |
| DL5-hog1 | leu2 hog1::LEU2 {PMAL1-GEM-GECO} | [19] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulakova, M.; Pakhomova, M.; Bidiuk, V.; Agaphonov, M. Inactivation of the High-Affinity Ca2+ Uptake System Delays the Amiodarone-Induced Ca2+ Influx in Yeast Ogataea parapolymorpha. Int. J. Mol. Sci. 2025, 26, 11386. https://doi.org/10.3390/ijms262311386
Kulakova M, Pakhomova M, Bidiuk V, Agaphonov M. Inactivation of the High-Affinity Ca2+ Uptake System Delays the Amiodarone-Induced Ca2+ Influx in Yeast Ogataea parapolymorpha. International Journal of Molecular Sciences. 2025; 26(23):11386. https://doi.org/10.3390/ijms262311386
Chicago/Turabian StyleKulakova, Maria, Maria Pakhomova, Victoria Bidiuk, and Michael Agaphonov. 2025. "Inactivation of the High-Affinity Ca2+ Uptake System Delays the Amiodarone-Induced Ca2+ Influx in Yeast Ogataea parapolymorpha" International Journal of Molecular Sciences 26, no. 23: 11386. https://doi.org/10.3390/ijms262311386
APA StyleKulakova, M., Pakhomova, M., Bidiuk, V., & Agaphonov, M. (2025). Inactivation of the High-Affinity Ca2+ Uptake System Delays the Amiodarone-Induced Ca2+ Influx in Yeast Ogataea parapolymorpha. International Journal of Molecular Sciences, 26(23), 11386. https://doi.org/10.3390/ijms262311386





