Dysregulated Resolution of Inflammation After Respiratory Viral Infections: Molecular Pathways Linking Neuroinflammation to Post-Viral Neuropathic Pain—A Narrative Review
Abstract
1. Introduction
2. Material and Methods
3. Molecular Mechanisms of Dysregulated Inflammation Resolution
3.1. Defective Efferocytosis
3.2. Persistent Activation of Macrophages and Glial Cells
3.3. Dysregulation of Specialized Pro-Resolving Mediators (SPMs)
3.3.1. Lipoxins
3.3.2. Resolvins
3.3.3. Protectins
3.3.4. Maresins
3.4. Mitochondrial Dysfunction
3.5. Summary of Key Mechanistic Insights
4. Discussion
4.1. Integration of Mechanisms with Other Chronic Inflammatory Diseases
4.2. Therapeutic and Clinical Perspectives
4.3. Prevention Through Vaccination: Insights into Neuroinflammation and Pathways to Resolution
4.4. Potential for Predictive Algorithms and Personalized Approaches
5. Predictive Frameworks and the Conceptual Basis for a Resolution Failure Index (RFI)
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADAM17 | A disintegrin and metalloproteinase 17 |
| AMPK | AMP-activated protein kinase |
| APPPS1 | Amyloid Precursor Protein/Presenilin-1 mouse model |
| ATP | Adenosine triphosphate |
| Axl | Axl receptor tyrosine kinase |
| BBB | Blood–brain barrier |
| BDNF | Brain-derived neurotrophic factor |
| BCL-2 | B-cell lymphoma 2 |
| CGRP | Calcitonin gene-related peptide |
| cf-mtDNA | Cell-free mitochondrial DNA |
| COX-2 | Cyclooxygenase-2 |
| CRP | C-reactive protein |
| DAMPs | Damage-associated molecular patterns |
| DAP12 | DNAX-activating protein of 12 kDa |
| DHA | Docosahexaenoic acid |
| DRG | Dorsal root ganglion |
| EPA | Eicosapentaenoic acid |
| ERK | Extracellular signal-regulated kinase |
| GFAP | Glial fibrillary acidic protein |
| GPR32 | G protein-coupled receptor 32 |
| HMGB1 | High mobility group box 1 |
| hs-CRP | High-sensitivity C-reactive protein |
| Iduna | Induced DNA damage response protein 1 |
| IFN-λ | Interferon lambda |
| IL | Interleukin |
| iNOS | Inducible nitric oxide synthase |
| LGR6 | Leucine-rich repeat-containing G protein-coupled receptor 6 |
| LOX | Lipoxygenase |
| LPA | Lysophosphatidic acid |
| LPS | Lipopolysaccharide |
| LXA4, LXB4 | Lipoxin A4, Lipoxin B4 |
| MAPK | Mitogen-activated protein kinase |
| MaR1, MaR2 | Maresin 1, Maresin 2 |
| MCTR | Maresin conjugates in tissue regeneration |
| MDA | Malondialdehyde |
| MerTK | Mer receptor tyrosine kinase |
| MFG-E8 | Milk fat globule epidermal growth factor 8 |
| mtROS | Mitochondrial reactive oxygen species |
| NAC | N-acetylcysteine |
| NET | Neutrophil extracellular trap |
| Neuro-PASC | Neurologic Post-Acute Sequelae of SARS-CoV-2 infection |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NLRP3 | NOD-like receptor family pyrin domain-containing 3 |
| NP | Neuropathic pain |
| NPD1 | Neuroprotectin D1 |
| Nrf2 | Nuclear factor erythroid 2–related factor 2 |
| PCTR1 | Protectin conjugate in tissue regeneration 1 |
| PD1, PDX | Protectin D1, Protectin DX |
| PI3K | Phosphoinositide 3-kinase |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| PS | Phosphatidylserine |
| Rac1 | Ras-related C3 botulinum toxin substrate 1 |
| RA | Rheumatoid arthritis |
| RAGE | Receptor for advanced glycation end-products |
| RFI | Resolution Failure Index |
| ROS | Reactive oxygen species |
| RSV | Respiratory Syncytial Virus |
| RvD1, RvD5, RvE1 | Resolvin D1, Resolvin D5, Resolvin E1 |
| RXRα | Retinoid X receptor alpha |
| SOCS3 | Suppressor of cytokine signaling 3 |
| MS | Multiple Sclerosis |
| SPM, SPMs | Specialized pro-resolving mediator(s) |
| STAT6 | Signal transducer and activator of transcription 6 |
| TGF-β | Transforming growth factor beta |
| TG | Trigeminal ganglion |
| TREM2 | Triggering receptor expressed on myeloid cells 2 |
| TRPV1 | Transient receptor potential vanilloid 1 |
| UCP2 | Uncoupling protein 2 |
References
- Chiang, N.; Serhan, C.N. Specialized Pro-Resolving Mediator Network: An Update on Production and Actions. Essays Biochem. 2020, 64, 443–462. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.M.; Soliman, M.; Shah, S.S.H.; Baig, H.A.; Gouda, N.S.; Alenezi, B.T.; Alenezy, A.; Hegazy, A.M.S.; Jan, M.; Eltom, E.H. Molecular Dynamics of Inflammation Resolution: Therapeutic Implications. Front. Cell Dev. Biol. 2025, 13, 1600149. [Google Scholar] [CrossRef]
- Panigrahy, D.; Gilligan, M.M.; Serhan, C.N.; Kashfi, K. Resolution of Inflammation: An Organizing Principle in Biology and Medicine. Pharmacol. Ther. 2021, 227, 107879. [Google Scholar] [CrossRef]
- Futokoro, R.; Hijioka, M.; Arata, M.; Kitamura, Y. Lipoxin A4 Receptor Stimulation Attenuates Neuroinflammation in a Mouse Model of Intracerebral Hemorrhage. Brain Sci. 2022, 12, 162. [Google Scholar] [CrossRef]
- Popa, E.; Popa, A.E.; Poroch, M.; Poroch, V.; Ungureanu, M.I.; Slanina, A.M.; Bacusca, A.; Coman, E.A. The Molecular Mechanisms of Cognitive Dysfunction in Long COVID: A Narrative Review. Int. J. Mol. Sci. 2025, 26, 5102. [Google Scholar] [CrossRef] [PubMed]
- Ellul, M.A.; Benjamin, L.; Singh, B.; Lant, S.; Michael, B.D.; Easton, A.; Kneen, R.; Defres, S.; Sejvar, J.; Solomon, T. Neurological Associations of COVID-19. Lancet Neurol. 2020, 19, 767–783. [Google Scholar] [CrossRef]
- Pașa, V.; Popa, E.; Poroch, M.; Cosmescu, A.; Bacusca, A.I.; Slanina, A.M.; Ceasovschih, A.; Stoica, A.; Petroaie, A.; Ungureanu, M.; et al. The “Viral” Form of Polyarteritis Nodosa (PAN)—A Distinct Entity: A Case Based Review. Medicina 2023, 59, 1162. [Google Scholar] [CrossRef]
- Costa, V.V.; Resende, F.; Melo, E.M.; Teixeira, M.M. Resolution Pharmacology and the Treatment of Infectious Diseases. Br. J. Pharmacol. 2024, 181, 917–937. [Google Scholar] [CrossRef]
- Valente, M.; Dentoni, M.; Bellizzi, F.; Kuris, F.; Gigli, G.L. Specialized Pro-Resolving Mediators in Neuroinflammation: Overview of Studies and Perspectives of Clinical Applications. Molecules 2022, 27, 4836. [Google Scholar] [CrossRef]
- Sodero, G.; Gentili, C.; Mariani, F.; Pulcinelli, V.; Valentini, P.; Buonsenso, D. Procalcitonin and Presepsin as Markers of Infectious Respiratory Diseases in Children: A Scoping Review of the Literature. Children 2024, 11, 350. [Google Scholar] [CrossRef] [PubMed]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA—A Scale for the Quality Assessment of Narrative Review Articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Kallaste, A.; Kisand, K.; Aart, A.; Salumets, A.; Kisand, K.; Peterson, P.; Lember, M. Long COVID and Biomarker Dysregulation—A Shift Toward Immune Exhaustion? Medicina 2025, 61, 996. [Google Scholar] [CrossRef]
- Ge, Y.; Huang, M.; Yao, Y. Efferocytosis and Its Role in Inflammatory Disorders. Front. Cell Dev. Biol. 2022, 10, 839248. [Google Scholar] [CrossRef]
- Shen, D.; Li, H.; Zhou, R.; Liu, M.; Yu, H.; Wu, D.-F. Pioglitazone Attenuates Aging-Related Disorders in Aged Apolipoprotein E Deficient Mice. Exp. Gerontol. 2018, 102, 101–108. [Google Scholar] [CrossRef]
- Mehrotra, P.; Ravichandran, K.S. Drugging the Efferocytosis Process: Concepts and Opportunities. Nat. Rev. Drug Discov. 2022, 21, 601–620. [Google Scholar] [CrossRef] [PubMed]
- Nishi, C.; Yanagihashi, Y.; Segawa, K.; Nagata, S. MERTK Tyrosine Kinase Receptor Together with TIM4 Phosphatidylserine Receptor Mediates Distinct Signal Transduction Pathways for Efferocytosis and Cell Proliferation. J. Biol. Chem. 2019, 294, 7221–7230. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Wang, X.; Xu, M.; Bai, J.; Yu, H.; Zhang, L. PI3K/AKT Signaling Pathway: Molecular Mechanisms and Therapeutic Potential in Depression. Pharmacol. Res. 2024, 206, 107300. [Google Scholar] [CrossRef]
- Kiyatkin, A.; van Alderwerelt van Rosenburgh, I.K.; Klein, D.E.; Lemmon, M.A. Kinetics of Receptor Tyrosine Kinase Activation Define ERK Signaling Dynamics. Sci. Signal. 2020, 13, eaaz5267. [Google Scholar] [CrossRef]
- Ma, M.; Jiang, W.; Zhou, R. DAMPs and DAMP-Sensing Receptors in Inflammation and Diseases. Immunity 2024, 57, 752–771. [Google Scholar] [CrossRef]
- Zizzo, G.; Cohen, P.L. The PPAR-γ Antagonist GW9662 Elicits Differentiation of M2c-like Cells and Upregulation of the MerTK/Gas6 Axis: A Key Role for PPAR-γ in Human Macrophage Polarization. J. Inflamm. 2015, 12, 36. [Google Scholar] [CrossRef]
- Popa, E.; Zugun-Eloae, F.; Zlei, M.; Jitaru, D.; Pintilie, O.M.; Coman, A.E.; Traian, M.; Ungureanu, D.A.; Carasevici, E. Flow Cytometry Analysis of Pparα Receptors in Metabolic Syndrome/Studiul Receptorilor Pparα Prin Metoda Citometriei În Flux În Sindromul Metabolic. Rev. Romana Med. Lab. 2014, 22, 427–438. [Google Scholar] [CrossRef]
- Zheng, W.; Zhou, Z.; Guo, X.; Zuo, X.; Zhang, J.; An, Y.; Zheng, H.; Yue, Y.; Wang, G.; Wang, F. Efferocytosis and Respiratory Disease. Int. J. Mol. Sci. 2023, 24, 14871. [Google Scholar] [CrossRef]
- Ma, Y.; Kemp, S.; Yang, X.; Wu, M.H.; Yuan, S.Y. Cellular Mechanisms Underlying the Impairment of Macrophage Efferocytosis. Immunol. Lett. 2023, 254, 41–53. [Google Scholar] [CrossRef]
- Lauber, K.; Keppeler, H.; Munoz, L.E.; Koppe, U.; Schröder, K.; Yamaguchi, H.; Krönke, G.; Uderhardt, S.; Wesselborg, S.; Belka, C.; et al. Milk Fat Globule-EGF Factor 8 Mediates the Enhancement of Apoptotic Cell Clearance by Glucocorticoids. Cell Death Differ. 2013, 20, 1230–1240. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, W.; Xu, Y.; Wu, D.; Gao, Z.; Zhou, J.; Qian, H.; He, B.; Wang, G. Extracellular HMGB1 Impairs Macrophage-Mediated Efferocytosis by Suppressing the Rab43-Controlled Cell Surface Transport of CD91. Front. Immunol. 2022, 13, 767630. [Google Scholar] [CrossRef]
- Chen, Y.; Kou, Y.; Ni, Y.; Yang, H.; Xu, C.; Fan, H.; Liu, H. Microglia Efferocytosis: An Emerging Mechanism for the Resolution of Neuroinflammation in Alzheimer’s Disease. J. Neuroinflamm. 2025, 22, 96. [Google Scholar] [CrossRef]
- Bohan, D.; Ert, H.V.; Ruggio, N.; Rogers, K.J.; Badreddine, M.; Briseño, J.A.A.; Elliff, J.M.; Chavez, R.A.R.; Gao, B.; Stokowy, T.; et al. Phosphatidylserine Receptors Enhance SARS-CoV-2 Infection. PLoS Pathog. 2021, 17, e1009743. [Google Scholar] [CrossRef]
- Rizzi, M.; Tonello, S.; D’Onghia, D.; Sainaghi, P.P. Gas6/TAM Axis Involvement in Modulating Inflammation and Fibrosis in COVID-19 Patients. Int. J. Mol. Sci. 2023, 24, 951. [Google Scholar] [CrossRef]
- Lekshmi, V.S.; Asha, K.; Sanicas, M.; Asi, A.; Arya, U.M.; Kumar, B. PI3K/Akt/Nrf2 Mediated Cellular Signaling and Virus-Host Interactions: Latest Updates on the Potential Therapeutic Management of SARS-CoV-2 Infection. Front. Mol. Biosci. 2023, 10, 1158133. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Mukherjee, A.; Nongthomba, U. Before the “Cytokine Storm”: Boosting Efferocytosis as an Effective Strategy against SARS-CoV-2 Infection and Associated Complications. Cytokine Growth Factor Rev. 2022, 63, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Yuan, C.; Yang, S.; Ma, Z.; Li, W.; Mao, L.; Jiao, P.; Liu, W. The Role of Reactive Oxygen Species in Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-COV-2) Infection-Induced Cell Death. Cell Mol. Biol. Lett. 2024, 29, 138. [Google Scholar] [CrossRef]
- Vora, S.M.; Lieberman, J.; Wu, H. Inflammasome Activation at the Crux of Severe COVID-19. Nat. Rev. Immunol. 2021, 21, 694–703. [Google Scholar] [CrossRef]
- Montilla, A.; Zabala, A.; Calvo, I.; Bosch-Juan, M.; Tomé-Velasco, I.; Mata, P.; Koster, M.; Sierra, A.; Kooistra, S.M.; Soria, F.N.; et al. Microglia Regulate Myelin Clearance and Cholesterol Metabolism after Demyelination via Interferon Regulatory Factor 5. Cell Mol. Life Sci. 2025, 82, 131. [Google Scholar] [CrossRef]
- Zhang, S.; Weinberg, S.; DeBerge, M.; Gainullina, A.; Schipma, M.; Kinchen, J.M.; Ben-Sahra, I.; Gius, D.R.; Yvan-Charvet, L.; Chandel, N.S.; et al. Efferocytosis Fuels Requirements of Fatty Acid Oxidation and the Electron Transport Chain to Polarize Macrophages for Tissue Repair. Cell Metab. 2019, 29, 443–456.e5. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wang, R.; Zhu, H.; Wen, B.; Xu, L.; Huang, Y. Biomarker Signatures in Time-Course Progression of Neuropathic Pain at Spinal Cord Level Based on Bioinformatics and Machine Learning Analysis. Biomolecules 2025, 15, 1254. [Google Scholar] [CrossRef] [PubMed]
- Hwang, M.; Savarin, C.; Kim, J.; Powers, J.; Towne, N.; Oh, H.; Bergmann, C.C. Trem2 Deficiency Impairs Recovery and Phagocytosis and Dysregulates Myeloid Gene Expression during Virus-Induced Demyelination. J. Neuroinflamm. 2022, 19, 267. [Google Scholar] [CrossRef]
- Wang, Y.; Cella, M.; Mallinson, K.; Ulrich, J.D.; Young, K.L.; Robinette, M.L.; Gilfillan, S.; Krishnan, G.M.; Sudhakar, S.; Zinselmeyer, B.H.; et al. TREM2 Lipid Sensing Sustains the Microglial Response in an Alzheimer’s Disease Model. Cell 2015, 160, 1061–1071. [Google Scholar] [CrossRef]
- Podleśny-Drabiniok, A.; Marcora, E.; Goate, A.M. Microglial Phagocytosis: A Disease-Associated Process Emerging from Alzheimer’s Disease Genetics. Trends Neurosci. 2020, 43, 965–979. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Chen, Y.; Grajales-Reyes, G.; Colonna, M. TREM2 Dependent and Independent Functions of Microglia in Alzheimer’s Disease. Mol. Neurodegener. 2022, 17, 84. [Google Scholar] [CrossRef]
- Coman, A.E.; Ceasovschih, A.; Petroaie, A.D.; Popa, E.; Lionte, C.; Bologa, C.; Haliga, R.E.; Cosmescu, A.; Slănină, A.M.; Bacușcă, A.I.; et al. The Significance of Low Magnesium Levels in COVID-19 Patients. Medicina 2023, 59, 279. [Google Scholar] [CrossRef]
- Koutsiaris, A.G.; Karakousis, K. Long COVID Mechanisms, Microvascular Effects, and Evaluation Based on Incidence. Life 2025, 15, 887. [Google Scholar] [CrossRef]
- Colonna, M.; Wang, Y. TREM2 Variants: New Keys to Decipher Alzheimer Disease Pathogenesis. Nat. Rev. Neurosci. 2016, 17, 201–207. [Google Scholar] [CrossRef]
- Mecca, C.; Giambanco, I.; Donato, R.; Arcuri, C. Microglia and Aging: The Role of the TREM2–DAP12 and CX3CL1-CX3CR1 Axes. Int. J. Mol. Sci. 2018, 19, 318. [Google Scholar] [CrossRef]
- Polara, R.; Ganesan, R.; Pitson, S.M.; Robinson, N. Cell Autonomous Functions of CD47 in Regulating Cellular Plasticity and Metabolic Plasticity. Cell Death Differ. 2024, 31, 1255–1266. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, Q.; Xiao, W.; Zhao, Y.; Pi, J.; Xu, H.; Zhao, H.; Xu, J.; Evans, C.E.; Jin, H. Advances in Anti-Tumor Treatments Targeting the CD47/SIRPα Axis. Front. Immunol. 2020, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Ma, L.; Deng, D.; Zhang, T.; Han, L.; Xu, F.; Huang, S.; Ding, Y.; Chen, X. M2 Macrophage Polarization: A Potential Target in Pain Relief. Front. Immunol. 2023, 14, 1243149. [Google Scholar] [CrossRef]
- Liu, W.; Taso, O.; Wang, R.; Bayram, S.; Graham, A.C.; Garcia-Reitboeck, P.; Mallach, A.; Andrews, W.D.; Piers, T.M.; Botia, J.A.; et al. Trem2 Promotes Anti-Inflammatory Responses in Microglia and Is Suppressed under pro-Inflammatory Conditions. Hum. Mol. Genet. 2020, 29, 3224–3248. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Langmead, C.J.; Riddy, D.M. New Advances in Targeting the Resolution of Inflammation: Implications for Specialized Pro-Resolving Mediator GPCR Drug Discovery. ACS Pharmacol. Transl. Sci. 2020, 3, 88–106. [Google Scholar] [CrossRef]
- Spiera, R.; Kuwana, M.; Khanna, D.; Hummers, L.; Frech, T.M.; Stevens, W.; Matucci-Cerinic, M.; Kafaja, S.; Distler, O.; Jun, J.-B.; et al. Efficacy and Safety of Lenabasum, a Cannabinoid Type 2 Receptor Agonist, in a Phase 3 Randomized Trial in Diffuse Cutaneous Systemic Sclerosis. Arthritis Rheumatol. 2023, 75, 1608–1618. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Mohan, M.; Bose, M.; Brennan, E.P.; Kiriazis, H.; Deo, M.; Nowell, C.J.; Godson, C.; Cooper, M.E.; Zhao, P.; et al. Lipoxin A4 Improves Cardiac Remodeling and Function in Diabetes-Associated Cardiac Dysfunction. Cardiovasc. Diabetol. 2024, 23, 413. [Google Scholar] [CrossRef]
- Kobayashi, D.; Kiguchi, N.; Saika, F.; Kishioka, S.; Matsuzaki, S. Insufficient Efferocytosis by M2-like Macrophages as a Possible Mechanism of Neuropathic Pain Induced by Nerve Injury. Biochem. Biophys. Res. Commun. 2020, 525, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Kalinski, A.L.; Yoon, C.; Huffman, L.D.; Duncker, P.C.; Kohen, R.; Passino, R.; Hafner, H.; Johnson, C.; Kawaguchi, R.; Carbajal, K.S.; et al. Analysis of the Immune Response to Sciatic Nerve Injury Identifies Efferocytosis as a Key Mechanism of Nerve Debridement. eLife 2020, 9, e60223. [Google Scholar] [CrossRef]
- Soliman, E.; Leonard, J.; Basso, E.K.G.; Gershenson, I.; Ju, J.; Mills, J.; de Jager, C.; Kaloss, A.M.; Elhassanny, M.; Pereira, D.; et al. Efferocytosis Is Restricted by Axon Guidance Molecule EphA4 via ERK/Stat6/MERTK Signaling Following Brain Injury. J. Neuroinflamm. 2023, 20, 256. [Google Scholar] [CrossRef]
- Wanke, F.; Gutbier, S.; Rümmelin, A.; Steinberg, M.; Hughes, L.D.; Koenen, M.; Komuczki, J.; Regan-Komito, D.; Wagage, S.; Hesselmann, J.; et al. Ligand-Dependent Kinase Activity of MERTK Drives Efferocytosis in Human iPSC-Derived Macrophages. Cell Death Dis. 2021, 12, 538. [Google Scholar] [CrossRef]
- Ruan, S.; Jia, R.; Hu, L.; Liu, Y.; Tian, Q.; Jiang, K.; Xia, X.; Tao, X.; Liu, W.-T.; Pan, Y.; et al. Ozone Promotes Macrophage Efferocytosis and Alleviates Neuropathic Pain by Activating the AMPK/Gas6-MerTK/SOCS3 Signaling Pathway. Front. Immunol. 2024, 15, 1455771. [Google Scholar] [CrossRef]
- Totolici, I.; Pascu, A.; Poroch, V.; Mosoiu, D. The Impact of Ozone Therapy on Antioxidant Status and Quality of Life in Palliative Care—Exploratory Study. Rev. Chim. 2017, 68, 2416–2421. [Google Scholar] [CrossRef]
- Cai, X.; Shi, Y.; Dai, Y.; Wang, F.; Chen, X.; Li, X. Baicalin Clears Inflammation by Enhancing Macrophage Efferocytosis via Inhibition of the RhoA/ROCK Signaling Pathway and Regulating Macrophage Polarization. Int. Immunopharmacol. 2022, 105, 108532. [Google Scholar] [CrossRef]
- Grossini, E.; Concina, D.; Rinaldi, C.; Russotto, S.; Garhwal, D.; Zeppegno, P.; Gramaglia, C.; Kul, S.; Panella, M. Association Between Plasma Redox State/Mitochondria Function and a Flu-Like Syndrome/COVID-19 in the Elderly Admitted to a Long-Term Care Unit. Front. Physiol. 2021, 12, 707587. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-J.; Kim, B.-M.; Ahn, Y.-H.; Choi, J.H.; Choi, Y.-H.; Kang, J.L. STAT6 Signaling Mediates PPARγ Activation and Resolution of Acute Sterile Inflammation in Mice. Cells 2021, 10, 501. [Google Scholar] [CrossRef] [PubMed]
- Noonong, K.; Chatatikun, M.; Surinkaew, S.; Kotepui, M.; Hossain, R.; Bunluepuech, K.; Noothong, C.; Tedasen, A.; Klangbud, W.K.; Imai, M.; et al. Mitochondrial Oxidative Stress, Mitochondrial ROS Storms in Long COVID Pathogenesis. Front. Immunol. 2023, 14, 1275001. [Google Scholar] [CrossRef]
- Soliman, A.M.; Barreda, D.R. The Acute Inflammatory Response of Teleost Fish. Dev. Comp. Immunol. 2023, 146, 104731. [Google Scholar] [CrossRef] [PubMed]
- Yurdagul, A.; Subramanian, M.; Wang, X.; Crown, S.B.; Ilkayeva, O.R.; Darville, L.; Kolluru, G.K.; Rymond, C.C.; Gerlach, B.D.; Zheng, Z.; et al. Macrophage Metabolism of Apoptotic Cell-Derived Arginine Promotes Continual Efferocytosis and Resolution of Injury. Cell Metab. 2020, 31, 518–533.e10. [Google Scholar] [CrossRef]
- Merlin, J.; Ivanov, S.; Dumont, A.; Sergushichev, A.; Gall, J.; Stunault, M.; Ayrault, M.; Vaillant, N.; Castiglione, A.; Swain, A.; et al. Non-Canonical Glutamine Transamination Sustains Efferocytosis by Coupling Redox Buffering to Oxidative Phosphorylation. Nat. Metab. 2021, 3, 1313–1326. [Google Scholar] [CrossRef]
- Adame-García, S.R.; Cervantes-Villagrana, R.D.; Orduña-Castillo, L.B.; del Rio, J.C.; Gutkind, J.S.; Reyes-Cruz, G.; Taylor, S.S.; Vázquez-Prado, J. cAMP-Dependent Activation of the Rac Guanine Exchange Factor P-REX1 by Type I Protein Kinase A (PKA) Regulatory Subunits. J. Biol. Chem. 2019, 294, 2232–2246. [Google Scholar] [CrossRef]
- Paul, B.D.; Lemle, M.D.; Komaroff, A.L.; Snyder, S.H. Redox Imbalance Links COVID-19 and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Proc. Natl. Acad. Sci. USA 2021, 118, e2024358118. [Google Scholar] [CrossRef]
- Xu, S.; Li, H.; Ai, Z.; Guo, R.; Cheng, H.; Wang, Y. Exploring Viral Neuropathic Pain: Molecular Mechanisms and Therapeutic Implications. PLoS Pathog. 2024, 20, e1012397. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Dai, X.; Chen, J.; Zhao, J.; Xu, M.; Zhang, L.; Yang, B.; Zhang, W.; Rocha, M.; Nakao, T.; et al. STAT6/Arg1 Promotes Microglia/Macrophage Efferocytosis and Inflammation Resolution in Stroke Mice. JCI Insight 2019, 4, e131355. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.G.; Fonken, L.K.; Annis, J.L.; Watkins, L.R.; Maier, S.F. Stress Disinhibits Microglia via Down-Regulation of CD200R: A Mechanism of Neuroinflammatory Priming. Brain Behav. Immun. 2018, 69, 62–73. [Google Scholar] [CrossRef]
- Song, E.; Zhang, C.; Israelow, B.; Lu-Culligan, A.; Prado, A.V.; Skriabine, S.; Lu, P.; Weizman, O.-E.; Liu, F.; Dai, Y.; et al. Neuroinvasion of SARS-CoV-2 in Human and Mouse Brain. J. Exp. Med. 2021, 218, e20202135. [Google Scholar] [CrossRef]
- Zang, H.; Hu, Y.; Ji, X.; Chen, Y.; He, X.; Wan, L.; Yao, W.; Zhang, C.; Zhu, C.; Liu, T. LXR-β Regulates Microglial Efferocytosis and Neuroinflammation in CPSP via STAT6 Activation. Brain Behav. Immun. 2025, 130, 106089. [Google Scholar] [CrossRef]
- Fattori, V.; Pinho-Ribeiro, F.A.; Staurengo-Ferrari, L.; Borghi, S.M.; Rossaneis, A.C.; Casagrande, R.; Verri, W.A. The Specialised Pro-resolving Lipid Mediator Maresin 1 Reduces Inflammatory Pain with a Long-lasting Analgesic Effect. Br. J. Pharmacol. 2019, 176, 1728–1744. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, Y.; Zhu, J.; Zou, M.; Zhang, Y.; Wu, H.; Jin, T. Specialized Pro-Resolving Lipid Mediators: A Key Player in Resolving Inflammation in Autoimmune Diseases. Sci. Bull. 2025, 70, 778–794. [Google Scholar] [CrossRef] [PubMed]
- Bo, C.; Liu, X.; Liu, Y.; Xu, L.; Huang, Q. Resolvin D1 Accelerates Resolution of Neuroinflammation by Inhibiting Microglia Activation through the BDNF/TrkB Signaling Pathway. Eur. J. Med. Res. 2025, 30, 189. [Google Scholar] [CrossRef]
- Chiang, N.; Libreros, S.; Norris, P.C.; de la Rosa, X.; Serhan, C.N. Maresin 1 Activates LGR6 Receptor Promoting Phagocyte Immunoresolvent Functions. J. Clin. Investig. 2019, 129, 5294–5311, Erratum in J. Clin. Investig. 2019, 129, 5294–5311. [Google Scholar] [CrossRef]
- Serhan, C.N. Novel Pro-Resolving Lipid Mediators in Inflammation Are Leads for Resolution Physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef]
- Martini, A.C.; Berta, T.; Forner, S.; Chen, G.; Bento, A.F.; Ji, R.-R.; Rae, G.A. Lipoxin A4 Inhibits Microglial Activation and Reduces Neuroinflammation and Neuropathic Pain after Spinal Cord Hemisection. J. Neuroinflamm. 2016, 13, 75. [Google Scholar] [CrossRef]
- Zhang, T.; Hao, H.; Zhou, Z.-Q.; Zeng, T.; Zhang, J.-M.; Zhou, X.-Y. Lipoxin A4 Inhibited the Activation of Hepatic Stellate Cells -T6 Cells by Modulating Profibrotic Cytokines and NF-κB Signaling Pathway. Prostaglandins Other Lipid Mediat. 2020, 146, 106380. [Google Scholar] [CrossRef]
- Nargis, T.; Muralidharan, C.; Enriquez, J.R.; Wang, J.E.; Kaylan, K.B.; Chakraborty, A.; Pratuangtham, S.; Figatner, K.; Nelson, J.B.; May, S.C.; et al. 12-Lipoxygenase Inhibition Delays Onset of Autoimmune Diabetes in Human Gene Replacement Mice. JCI Insight 2024, 9, e185299. [Google Scholar] [CrossRef]
- Zamora, A.; Nougué, M.; Verdu, L.; Balzan, E.; Draia-Nicolau, T.; Benuzzi, E.; Pujol, F.; Baillif, V.; Lacazette, E.; Morfoisse, F.; et al. 15-Lipoxygenase Promotes Resolution of Inflammation in Lymphedema by Controlling Treg Cell Function through IFN-β. Nat. Commun. 2024, 15, 221. [Google Scholar] [CrossRef]
- Recchiuti, A.; Mattoscio, D.; Isopi, E. Roles, Actions, and Therapeutic Potential of Specialized Pro-Resolving Lipid Mediators for the Treatment of Inflammation in Cystic Fibrosis. Front. Pharmacol. 2019, 10, 252. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-García, S.; Jaén, R.I.; Lozano-Rodríguez, R.; Avendaño-Ortiz, J.; Pascual-Iglesias, A.; Hurtado-Navarro, L.; López-Collazo, E.; Boscá, L.; Prieto, P. Lipoxin A4 Levels Correlate with Severity in a Spanish COVID-19 Cohort: Potential Use of Endogenous pro-Resolving Mediators as Biomarkers. Front. Immunol. 2025, 15, 1509188. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Xie, Y.; Shi, C.; Ma, J.; Wang, Y.; Qiao, L.; Li, K.; Sun, T. Lipoxin A4 Inhibits NLRP3 Inflammasome Activation in Rats With Non-Compressive Disc Herniation Through the JNK1/Beclin-1/PI3KC3 Pathway. Front. Neurosci. 2020, 14, 799, Erratum in Front. Neurosci. 2020, 14, 608184. [Google Scholar] [CrossRef]
- Irún, P.; Gracia, R.; Piazuelo, E.; Pardo, J.; Morte, E.; Paño, J.R.; Boza, J.; Carrera-Lasfuentes, P.; Higuera, G.A.; Lanas, A. Serum Lipid Mediator Profiles in COVID-19 Patients and Lung Disease Severity: A Pilot Study. Sci. Rep. 2023, 13, 6497. [Google Scholar] [CrossRef]
- Gomes-da-Silva, N.C.; Xavier-de-Britto, I.; Soares, M.A.G.; Yoshihara, N.M.A.; Ilem Özdemir, D.; Ricci-Junior, E.; Fechine, P.B.A.; Alencar, L.M.R.; de Oliveira Henriques, M.D.G.M.; Barja-Fidalgo, T.C.; et al. Nanostructured Lipoxin A4: Understanding Its Biological Behavior and Impact on Alzheimer’s Disease (Proof of Concept). Pharmaceutics 2025, 17, 649. [Google Scholar] [CrossRef]
- Ferreira, M.V.; Jesus, C.H.A.; Bonfim da Costa, J.P.; Oliveira, G.; Liebl, B.; Verri Junior, W.; Zanoveli, J.M.; Cunha, J.M. da Aspirin-Triggered Lipoxin A4 Reduces Neuropathic Pain and Anxiety-like Behaviours in Male Diabetic Rats: Antinociceptive Enhancement by Cannabinoid Receptor Agonists. Eur. J. Pharmacol. 2025, 989, 177254. [Google Scholar] [CrossRef] [PubMed]
- Baral, P.K.; Amin, M.T.; Rashid, M.M.O.; Hossain, M.S. Assessment of Polyunsaturated Fatty Acids on COVID-19-Associated Risk Reduction. Rev. Bras. Farmacogn. 2022, 32, 50–64. [Google Scholar] [CrossRef]
- Chi, J.; Cheng, J.; Wang, S.; Li, C.; Chen, M. Promising Anti-Inflammatory Tools: Biomedical Efficacy of Lipoxins and Their Synthetic Pathways. Int. J. Mol. Sci. 2023, 24, 13282. [Google Scholar] [CrossRef]
- Das, U.N. Can Bioactive Lipids Inactivate Coronavirus (COVID-19)? Arch. Med. Res. 2020, 51, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Kanuri, S. Repercussions of Microglial Efferocytosis on Neurodegeneration in Alzheimer’s Disease (AD): A Double-Edged Sword and Perplexing Factor Warranting Scrutiny in AD Research. Egypt. J. Neurol. Psychiatry Neurosurg. 2024, 60, 81. [Google Scholar] [CrossRef]
- Liu, C.; Fan, D.; Lei, Q.; Lu, A.; He, X. Roles of Resolvins in Chronic Inflammatory Response. Int. J. Mol. Sci. 2022, 23, 14883. [Google Scholar] [CrossRef]
- Libreros, S.; Shay, A.E.; Nshimiyimana, R.; Fichtner, D.; Martin, M.J.; Wourms, N.; Serhan, C.N. A New E-Series Resolvin: RvE4 Stereochemistry and Function in Efferocytosis of Inflammation-Resolution. Front. Immunol. 2021, 11, 631319. [Google Scholar] [CrossRef]
- Gracia Aznar, A.; Moreno Egea, F.; Gracia Banzo, R.; Gutierrez, R.; Rizo, J.M.; Rodriguez-Ledo, P.; Nerin, I.; Regidor, P.-A. Pro-Resolving Inflammatory Effects of a Marine Oil Enriched in Specialized Pro-Resolving Mediators (SPMs) Supplement and Its Implication in Patients with Post-COVID Syndrome (PCS). Biomedicines 2024, 12, 2221. [Google Scholar] [CrossRef]
- Recchiuti, A.; Patruno, S.; Mattoscio, D.; Isopi, E.; Pomilio, A.; Lamolinara, A.; Iezzi, M.; Pecce, R.; Romano, M. Resolvin D1 and D2 Reduce SARS-CoV-2-Induced Inflammatory Responses in Cystic Fibrosis Macrophages. FASEB J. 2021, 35, e21441. [Google Scholar] [CrossRef]
- Yasmeen, N.; Selvaraj, H.; Lakhawat, S.S.; Datta, M.; Sharma, P.K.; Jain, A.; Khanna, R.; Srinivasan, J.; Kumar, V. Possibility of Averting Cytokine Storm in SARS-COV 2 Patients Using Specialized pro-Resolving Lipid Mediators. Biochem. Pharmacol. 2023, 209, 115437. [Google Scholar] [CrossRef]
- Pang, J.; Xin, P.; Kong, Y.; Wang, Z.; Wang, X. Resolvin D2 Reduces Chronic Neuropathic Pain and Bone Cancer Pain via Spinal Inhibition of IL-17 Secretion, CXCL1 Release and Astrocyte Activation in Mice. Brain Sci. 2023, 13, 152. [Google Scholar] [CrossRef] [PubMed]
- Chiang, N.; Sakuma, M.; Rodriguez, A.R.; Spur, B.W.; Irimia, D.; Serhan, C.N. Resolvin T-Series Reduce Neutrophil Extracellular Traps. Blood 2022, 139, 1222–1233. [Google Scholar] [CrossRef] [PubMed]
- de Diego, C.; Lasierra, A.B.; López-Vergara, L.; Torralba, L.; Ruiz de Gopegui, P.; Lahoz, R.; Abadía, C.; Godino, J.; Cebollada, A.; Jimeno, B.; et al. What Is the Actual Relationship between Neutrophil Extracellular Traps and COVID-19 Severity? A Longitudinal Study. Respir. Res. 2024, 25, 48. [Google Scholar] [CrossRef] [PubMed]
- Nesman, J.I.; Chen, O.; Luo, X.; Ji, R.-R.; Serhan, C.N.; Hansen, T.V. A New Synthetic Protectin D1 Analog 3-Oxa-PD1n-3 DPA Reduces Neuropathic Pain and Chronic Itch in Mice. Org. Biomol. Chem. 2021, 19, 2744–2752. [Google Scholar] [CrossRef]
- Walker, K.H.; Krishnamoorthy, N.; Brüggemann, T.R.; Shay, A.E.; Serhan, C.N.; Levy, B.D. Protectins PCTR1 and PD1 Reduce Viral Load and Lung Inflammation During Respiratory Syncytial Virus Infection in Mice. Front. Immunol. 2021, 12, 704427. [Google Scholar] [CrossRef]
- Navarini, L.; Vomero, M.; Currado, D.; Berardicurti, O.; Biaggi, A.; Marino, A.; Bearzi, P.; Corberi, E.; Rigon, A.; Arcarese, L.; et al. The Specialized Pro-Resolving Lipid Mediator Protectin D1 Affects Macrophages Differentiation and Activity in Adult-Onset Still’s Disease and COVID-19, Two Hyperinflammatory Diseases Sharing Similar Transcriptomic Profiles. Front. Immunol. 2023, 14, 1148268. [Google Scholar] [CrossRef]
- Das, U.N. Bioactive Lipid-Based Therapeutic Approach to COVID-19 and Other Similar Infections. Arch. Med. Sci. 2023, 19, 1327–1359. [Google Scholar] [CrossRef]
- Fortin, N.; Hénaut, M.; Goyette, N.; Maltais, R.; Sancéau, J.-Y.; Marette, A.; Poirier, D.; Abed, Y.; Boivin, G. A Protectin DX (PDX) Analog with in Vitro Activity against Influenza A(H1N1) Viruses. J. Med. Virol. 2024, 96, e29484. [Google Scholar] [CrossRef] [PubMed]
- Hartling, I.; Cremonesi, A.; Osuna, E.; Lou, P.H.; Lucchinetti, E.; Zaugg, M.; Hersberger, M. Quantitative Profiling of Inflammatory and Pro-Resolving Lipid Mediators in Human Adolescents and Mouse Plasma Using UHPLC-MS/MS. Clin. Chem. Lab. Med. 2021, 59, 1811–1823. [Google Scholar] [CrossRef] [PubMed]
- Dalli, J.; Chiang, N.; Serhan, C.N. Elucidation of Novel 13-Series Resolvins That Increase with Atorvastatin and Clear Infections. Nat. Med. 2015, 21, 1071–1075. [Google Scholar] [CrossRef] [PubMed]
- Zahoor, I.; Nematullah, M.; Ahmed, M.E.; Fatma, M.; Sajad, M.; Ayasolla, K.; Cerghet, M.; Palaniyandi, S.; Ceci, V.; Carrera, G.; et al. Maresin-1 Promotes Neuroprotection and Modulates Metabolic and Inflammatory Responses in Disease-Associated Cell Types in Preclinical Models of Multiple Sclerosis. J. Biol. Chem. 2025, 301, 108226. [Google Scholar] [CrossRef]
- Yang, T.; Xu, G.; Newton, P.T.; Chagin, A.S.; Mkrtchian, S.; Carlström, M.; Zhang, X.-M.; Harris, R.A.; Cooter, M.; Berger, M.; et al. Maresin 1 Attenuates Neuroinflammation in a Mouse Model of Perioperative Neurocognitive Disorders. Br. J. Anaesth. 2019, 122, 350–360. [Google Scholar] [CrossRef]
- Wei, J.; Su, W.; Zhao, Y.; Wei, Z.; Hua, Y.; Xue, P.; Zhu, X.; Chen, Y.; Chen, G. Maresin 1 Promotes Nerve Regeneration and Alleviates Neuropathic Pain after Nerve Injury. J. Neuroinflamm. 2022, 19, 32. [Google Scholar] [CrossRef]
- Lopes, R.V.; Baggio, D.F.; Ferraz, C.R.; Bertozzi, M.M.; Saraiva-Santos, T.; Verri Junior, W.A.; Chichorro, J.G. Maresin-2 Inhibits Inflammatory and Neuropathic Trigeminal Pain and Reduces Neuronal Activation in the Trigeminal Ganglion. Curr. Res. Neurobiol. 2023, 4, 100093. [Google Scholar] [CrossRef]
- Frigerio, F.; Pasqualini, G.; Craparotta, I.; Marchini, S.; van Vliet, E.A.; Foerch, P.; Vandenplas, C.; Leclercq, K.; Aronica, E.; Porcu, L.; et al. N-3 Docosapentaenoic Acid-Derived Protectin D1 Promotes Resolution of Neuroinflammation and Arrests Epileptogenesis. Brain 2018, 141, 3130–3143. [Google Scholar] [CrossRef]
- Li, Y.; Wang, N.; Ma, Z.; Wang, Y.; Yuan, Y.; Zhong, Z.; Hong, Y.; Zhao, M. Lipoxin A4 Protects against Paraquat-induced Acute Lung Injury by Inhibiting the TLR4/MyD88-mediated Activation of the NF-κB and PI3K/AKT Pathways. Int. J. Mol. Med. 2021, 47, 86. [Google Scholar] [CrossRef]
- Molnar, T.; Lehoczki, A.; Fekete, M.; Varnai, R.; Zavori, L.; Erdo-Bonyar, S.; Simon, D.; Berki, T.; Csecsei, P.; Ezer, E. Mitochondrial Dysfunction in Long COVID: Mechanisms, Consequences, and Potential Therapeutic Approaches. GeroScience 2024, 46, 5267–5286. [Google Scholar] [CrossRef] [PubMed]
- Karim, A.; Muhammad, T.; Iqbal, M.S.; Qaisar, R. Elevated Plasma CAF22 Are Incompletely Restored Six Months after COVID-19 Infection in Older Men. Exp. Gerontol. 2023, 171, 112034. [Google Scholar] [CrossRef]
- Medini, H.; Zirman, A.; Mishmar, D. Immune System Cells from COVID-19 Patients Display Compromised Mitochondrial-Nuclear Expression Co-Regulation and Rewiring toward Glycolysis. iScience 2021, 24, 103471. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Cao, Y.; Zhang, H.; Wang, Z.; Man, C.H.; Yang, Y.; Chen, L.; Xu, S.; Yan, X.; Zheng, Q.; et al. COVID-19 Metabolism: Mechanisms and Therapeutic Targets. MedComm 2022, 3, e157. [Google Scholar] [CrossRef]
- Maes, M.; Kubera, M.; Uytterhoeven, M.; Vrydags, N.; Bosmans, E. Increased Plasma Peroxides as a Marker of Oxidative Stress in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). Med. Sci. Monit. 2011, 17, SC11–SC15. [Google Scholar] [CrossRef]
- Maes, M.; Mihaylova, I.; Kubera, M.; Uytterhoeven, M.; Vrydags, N.; Bosmans, E. Increased 8-Hydroxy-Deoxyguanosine, a Marker of Oxidative Damage to DNA, in Major Depression and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Neuro Endocrinol. Lett. 2009, 30, 715–722. [Google Scholar]
- Peacock, B.N.; Gherezghiher, T.B.; Hilario, J.D.; Kellermann, G.H. New Insights into Lyme Disease. Redox Biol. 2015, 5, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Vernon, S.D.; Whistler, T.; Cameron, B.; Hickie, I.B.; Reeves, W.C.; Lloyd, A. Preliminary Evidence of Mitochondrial Dysfunction Associated with Post-Infective Fatigue after Acute Infection with Epstein Barr Virus. BMC Infect. Dis. 2006, 6, 15. [Google Scholar] [CrossRef]
- Raijmakers, R.P.H.; Jansen, A.F.M.; Keijmel, S.P.; ter Horst, R.; Roerink, M.E.; Novakovic, B.; Joosten, L.A.B.; van der Meer, J.W.M.; Netea, M.G.; Bleeker-Rovers, C.P. A Possible Role for Mitochondrial-Derived Peptides Humanin and MOTS-c in Patients with Q Fever Fatigue Syndrome and Chronic Fatigue Syndrome. J. Transl. Med. 2019, 17, 157. [Google Scholar] [CrossRef]
- Popa, E.; Tetia, T.; Poroch, M.; Ungureanu, M.; Cosmescu, A.; Barbacariu, L.; Slanina, A.M.; Bacusca, A.; Petroae, A.; Novac, O.; et al. The Effects of the COVID-19 Pandemic on Mental Health: A Web-Based Study Among Romanian Adults. Cureus 2022, 14, e31331. [Google Scholar] [CrossRef]
- Gilroy, D.W. Resolving Inflammation. Nat. Rev. Immunol. 2021, 21, 620–621. [Google Scholar] [CrossRef]
- Heneka, M.T.; van der Flier, W.M.; Jessen, F.; Hoozemanns, J.; Thal, D.R.; Boche, D.; Brosseron, F.; Teunissen, C.; Zetterberg, H.; Jacobs, A.H.; et al. Neuroinflammation in Alzheimer Disease. Nat. Rev. Immunol. 2025, 25, 321–352. [Google Scholar] [CrossRef]
- Owlett, L.D.; Karaahmet, B.; Le, L.; Belcher, E.K.; Dionisio-Santos, D.; Olschowka, J.A.; Elliott, M.R.; O’Banion, M.K. Gas6 Induces Inflammation and Reduces Plaque Burden but Worsens Behavior in a Sex-Dependent Manner in the APP/PS1 Model of Alzheimer’s Disease. J. Neuroinflamm. 2022, 19, 38. [Google Scholar] [CrossRef]
- Julliard, W.A.; Myo, Y.P.A.; Perelas, A.; Jackson, P.D.; Thatcher, T.H.; Sime, P.J. Specialized Pro-Resolving Mediators as Modulators of Immune Responses. Semin. Immunol. 2022, 59, 101605. [Google Scholar] [CrossRef] [PubMed]
- Marchand, N.E.; Choi, M.Y.; Oakes, E.G.; Cook, N.R.; Stevens, E.; Gomelskaya, N.; Kotler, G.; Manson, J.E.; Lasky-Su, J.; Mora, S.; et al. Over-the-Counter Fish Oil Supplementation and Pro-Resolving and Pro-Inflammatory Lipid Mediators in Rheumatoid Arthritis. Prostaglandins Leukot. Essent. Fat. Acids 2023, 190, 102542. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.-J.; Li, D.-Y.; Liu, D.-Q.; Sun, J.; Zhang, L.-Q.; Wu, J.-Y.; Song, F.-H.; Zhou, Y.-Q.; Mei, W. Dimethyl Fumarate Attenuates Pain Behaviors in Osteoarthritis Rats via Induction of Nrf2-Mediated Mitochondrial Biogenesis. Mol. Pain 2022, 18, 17448069221124920. [Google Scholar] [CrossRef] [PubMed]
- Kooij, G.; Troletti, C.D.; Leuti, A.; Norris, P.C.; Riley, I.; Albanese, M.; Ruggieri, S.; Libreros, S.; van der Pol, S.M.A.; van het Hof, B.; et al. Specialized Pro-Resolving Lipid Mediators Are Differentially Altered in Peripheral Blood of Patients with Multiple Sclerosis and Attenuate Monocyte and Blood-Brain Barrier Dysfunction. Haematologica 2020, 105, 2056–2070. [Google Scholar] [CrossRef]
- Liotti, F.; Marotta, M.; Melillo, R.M.; Prevete, N. The Impact of Resolution of Inflammation on Tumor Microenvironment: Exploring New Ways to Control Cancer Progression. Cancers 2022, 14, 3333. [Google Scholar] [CrossRef]
- Teixeira-Santos, L.; Martins, S.; Sousa, T.; Albino-Teixeira, A.; Pinho, D. The Pro-Resolving Lipid Mediator Maresin 1 Ameliorates Pain Responses and Neuroinflammation in the Spared Nerve Injury-Induced Neuropathic Pain: A Study in Male and Female Mice. PLoS ONE 2023, 18, e0287392. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Z.; Chen, L.; Sun, J.; Kwan, K.Y.H.; Jones, M.; Li, Y.M.; Hu, Y.; Wang, X.; Makvandi, P.; et al. Synergistic Modulating of Mitochondrial Transfer and Immune Microenvironment to Attenuate Discogenic Pain. Adv. Sci. 2025, 12, 2500128. [Google Scholar] [CrossRef]
- Baggio, D.F.; da Luz, F.M.R.; Lopes, R.V.; Ferreira, L.E.N.; Araya, E.I.; Chichorro, J.G. Sex Dimorphism in Resolvin D5-Induced Analgesia in Rat Models of Trigeminal Pain. J. Pain 2023, 24, 717–729. [Google Scholar] [CrossRef]
- Demuth, L.; Ohm, M.; Michaelsen-Preusse, K.; Schulze, K.; Riese, P.; Guzmán, C.A.; Korte, M.; Hosseini, S. Influenza Vaccine Is Able to Prevent Neuroinflammation Triggered by H7N7 IAV Infection. Front. Pharmacol. 2023, 14, 1142639. [Google Scholar] [CrossRef]
- Hellgren, F.; Rosdahl, A.; Cerveira, R.A.; Lenart, K.; Ols, S.; Gwon, Y.-D.; Kurt, S.; Delis, A.M.; Joas, G.; Evander, M.; et al. Modulation of Innate Immune Response to mRNA Vaccination after SARS-CoV-2 Infection or Sequential Vaccination in Humans. JCI Insight 2024, 9, e175401. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, C.S.; Navarro Ramil, L.; Bieligk, J.; Meineke, R.; Rimmelzwaan, G.; Käufer, C.; Richter, F. Intravenous SARS-CoV-2 Spike Protein Induces Neuroinflammation and Alpha-Synuclein Accumulation in Brain Regions Relevant to Parkinson’s Disease. Brain Behav. Immun. 2025, 129, 102–123. [Google Scholar] [CrossRef]
- Zelkoski, A.E.; Lu, Z.; Sukumar, G.; Dalgard, C.; Said, H.; Alameh, M.-G.; Mitre, E.; Malloy, A.M.W. Ionizable Lipid Nanoparticles of mRNA Vaccines Elicit NF-κB and IRF Responses through Toll-like Receptor 4. NPJ Vaccines 2025, 10, 73. [Google Scholar] [CrossRef] [PubMed]
- Ismail, I.I.; Salama, S. A Systematic Review of Cases of CNS Demyelination Following COVID-19 Vaccination. J. Neuroimmunol. 2022, 362, 577765. [Google Scholar] [CrossRef]
- Bahramy, M.A.; Hashempour, Z.; Shahriarirad, R. Chronic Inflammatory Demyelinating Polyneuropathy Following COVID-19 Vaccination: A Case Report and Literature Review. BMC Neurol. 2024, 24, 262. [Google Scholar] [CrossRef] [PubMed]
- Primicerio, G.C.; Bille, M.B.; Lund, E.L.; Birk, S. Small Fiber Neuropathy Following COVID-19 Vaccination: A Case Series. J. Neurol. Sci. 2025, 474, 123536. [Google Scholar] [CrossRef]
- Kim, K.H.; Kim, S.-H.; Park, N.Y.; Hyun, J.-W.; Kim, H.J. Onset of Various CNS Inflammatory Demyelination Diseases Following COVID-19 Vaccinations. Mult. Scler. Relat. Disord. 2022, 68, 104141. [Google Scholar] [CrossRef]
- Patone, M.; Handunnetthi, L.; Saatci, D.; Pan, J.; Katikireddi, S.V.; Razvi, S.; Hunt, D.; Mei, X.W.; Dixon, S.; Zaccardi, F.; et al. Neurological Complications after First Dose of COVID-19 Vaccines and SARS-CoV-2 Infection. Nat. Med. 2021, 27, 2144–2153. [Google Scholar] [CrossRef]
- Rinaldi, V.; Bellucci, G.; Buscarinu, M.C.; Reniè, R.; Marrone, A.; Nasello, M.; Zancan, V.; Nistri, R.; Palumbo, R.; Salerno, A.; et al. CNS Inflammatory Demyelinating Events after COVID-19 Vaccines: A Case Series and Systematic Review. Front. Neurol. 2022, 13, 1018785. [Google Scholar] [CrossRef]
- Harris, K.; Ling, Y.; Bukhbinder, A.S.; Chen, L.; Phelps, K.N.; Cruz, G.; Thomas, J.; Kim, Y.; Jiang, X.; Schulz, P.E. The Impact of Routine Vaccinations on Alzheimer’s Disease Risk in Persons 65 Years and Older: A Claims-Based Cohort Study Using Propensity Score Matching. J. Alzheimers Dis. 2023, 95, 703–718. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Shi, J.; Fu, G.; Cao, Z.; Qiu, R.; Zhao, T.; Zhang, J.; Lan, Y.; Guan, J.; Zhao, K.; et al. DNA Vaccine Targeting Betacoronavirus Spike Protein Blocks Neuroinvasion and Neuroinflammation in Swine via Dual Antiviral-Immunomodulatory Action. NPJ Vaccines 2025, 10, 187. [Google Scholar] [CrossRef] [PubMed]
- Aderinto, N.; Abraham, I.C.; Olatunji, G.; Kokori, E.; Ashinze, P.; Babawale, E.A.; Alabi, B.O.; Opeyemi, O.D.; Babalola, A.E.; Oluwapelumi, A.I.; et al. Vaccine Based Approaches for the Prevention and Treatment of Neurological Disease. Curr. Treat. Options Neurol. 2025, 27, 16. [Google Scholar] [CrossRef]
- Antel, R.; Whitelaw, S.; Gore, G.; Ingelmo, P. Moving towards the Use of Artificial Intelligence in Pain Management. Eur. J. Pain Lond. Engl. 2025, 29, e4748. [Google Scholar] [CrossRef]
- Allwright, M.; Karrasch, J.F.; O’Brien, J.A.; Guennewig, B.; Austin, P.J. Machine Learning Analysis of the UK Biobank Reveals Prognostic and Diagnostic Immune Biomarkers for Polyneuropathy and Neuropathic Pain in Diabetes. Diabetes Res. Clin. Pract. 2023, 201, 110725. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.-C.; Ming, X.-P.; Cai, W.-Y.; Hu, Y.-F.; Hao, B.; Wu, J.-H.; Tuohuti, A.; Chen, X. Development and Validation of a Prognostic Model for Assessing Long COVID Risk Following Omicron Wave—A Large Population-Based Cohort Study. Virol. J. 2024, 21, 123. [Google Scholar] [CrossRef]
- Solís-Tarazona, L.R.; Reddam, S.; Gil-Perotín, S. Use of Individual Measure and Z-Scores to Monitor Disease Course in Relapsing Multiple Sclerosis: A 1-Year Prospective Study in a Single Center (P5-3.012). Neurology 2023, 100, 3332. [Google Scholar] [CrossRef]
- National Institutes of Health (NIH). NIH RECOVER Makes Long COVID Data Easier to Access. Available online: https://www.nih.gov/news-events/news-releases/nih-recover-makes-long-covid-data-easier-access (accessed on 5 October 2025).
- Paithankar, V.; Devnani, D.; Nimburkar, T.A. A Review Article on “AI-Guided Discovery of Novel Anti-Inflammatory Agents for Cancer Therapy: A New Era in Drug Development. ” Intell. Hosp. 2025, 1, 100007. [Google Scholar] [CrossRef]
- Gudin, J.; Mavroudi, S.; Korfiati, A.; Hurwitz, P. Personalized Pain Therapy: An Artificial Intelligence (AI) Method and Web-Tool to Predict Patient Response to OTC Topical Analgesics. Anesth. Pain Res. 2021, 5, 1–11. [Google Scholar] [CrossRef]
- Froicu, E.-M.; Onicescu, O.-M.; Creangă-Murariu, I.; Dascălu, C.; Gafton, B.; Afrăsânie, V.-A.; Alexa-Stratulat, T.; Marinca, M.-V.; Pușcașu, D.-M.; Miron, L.; et al. Modeling Pain Dynamics and Opioid Response in Oncology Inpatients: A Retrospective Study with Application to AI-Guided Analgesic Strategies in Colorectal Cancer. Medicina 2025, 61, 1741. [Google Scholar] [CrossRef] [PubMed]
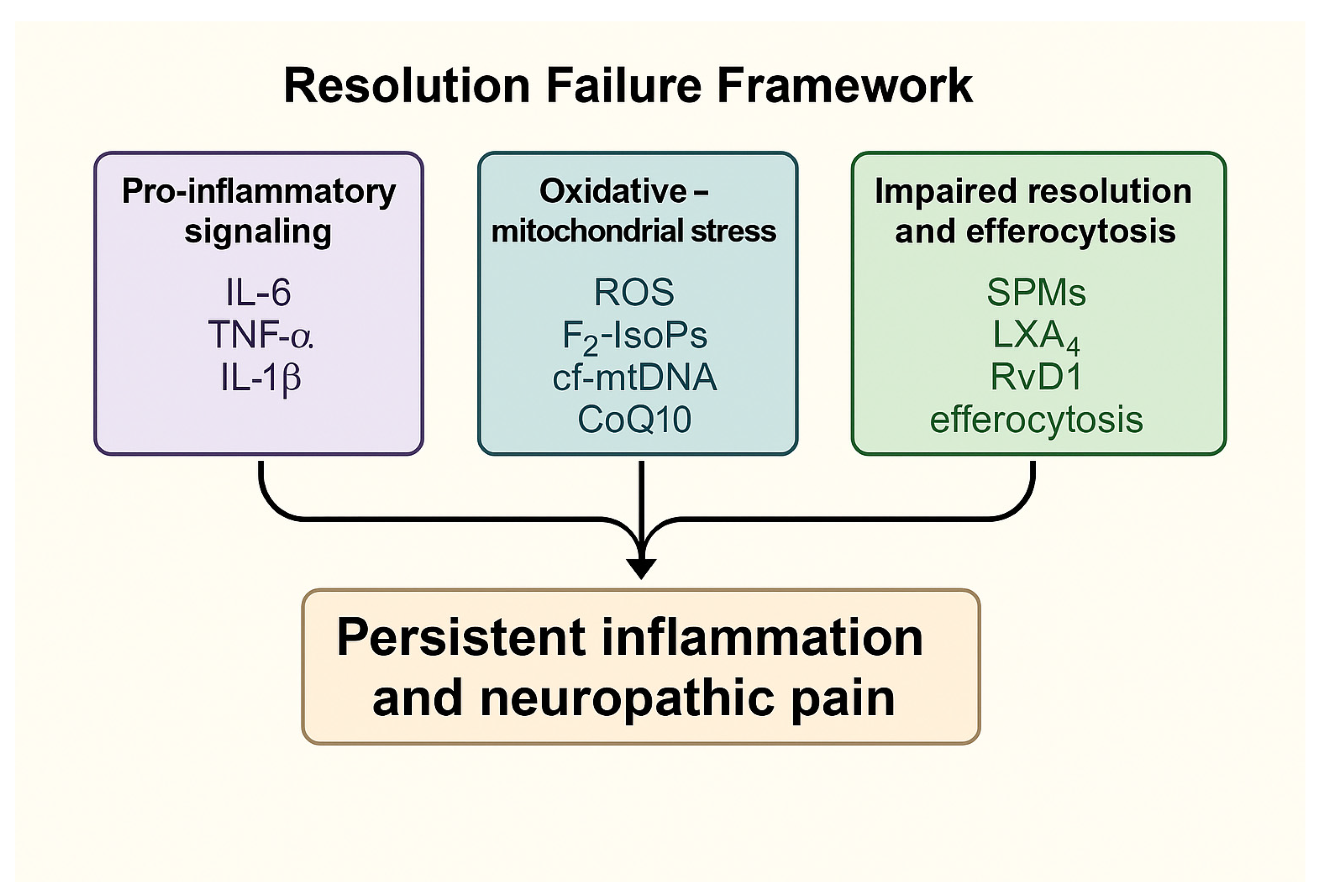
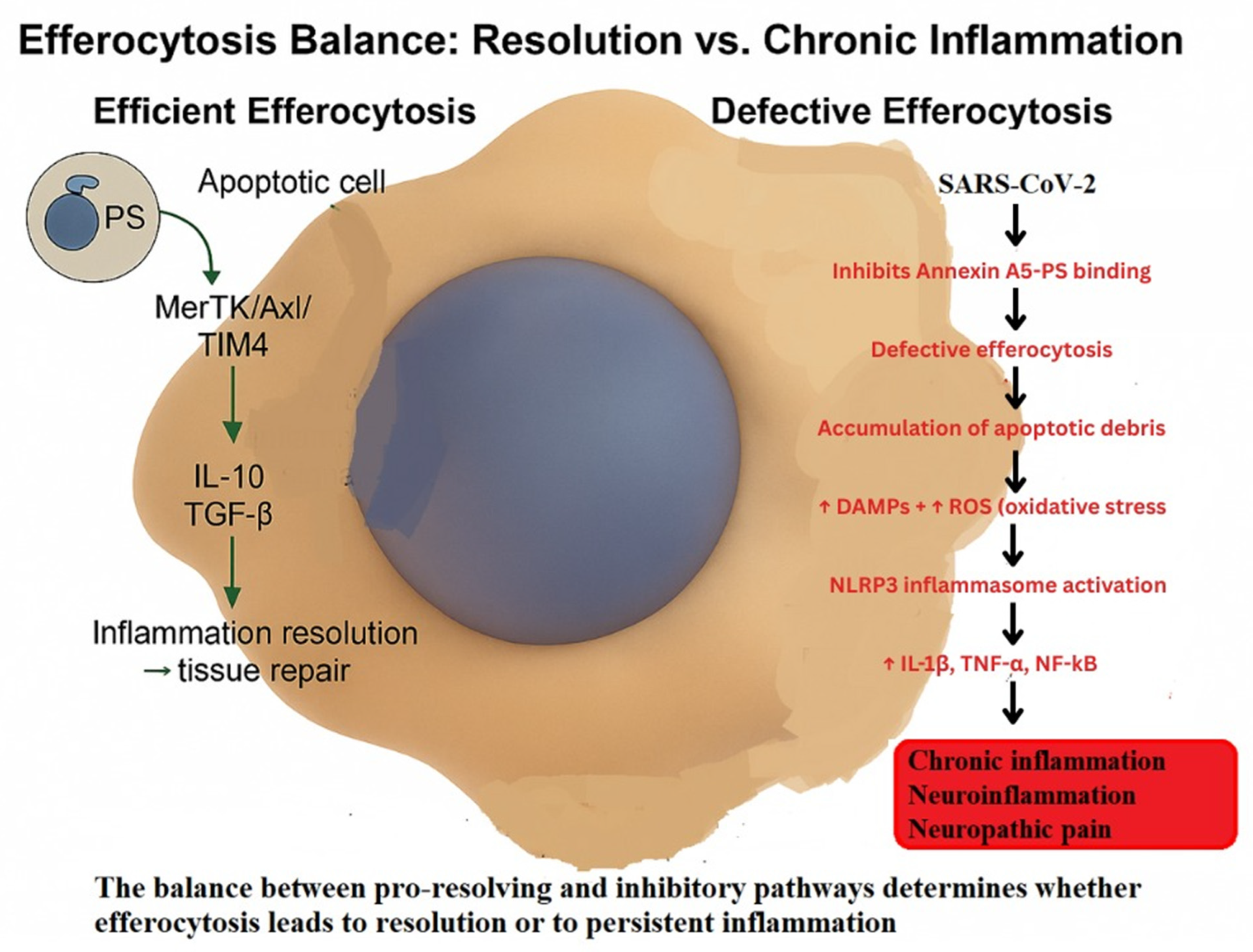
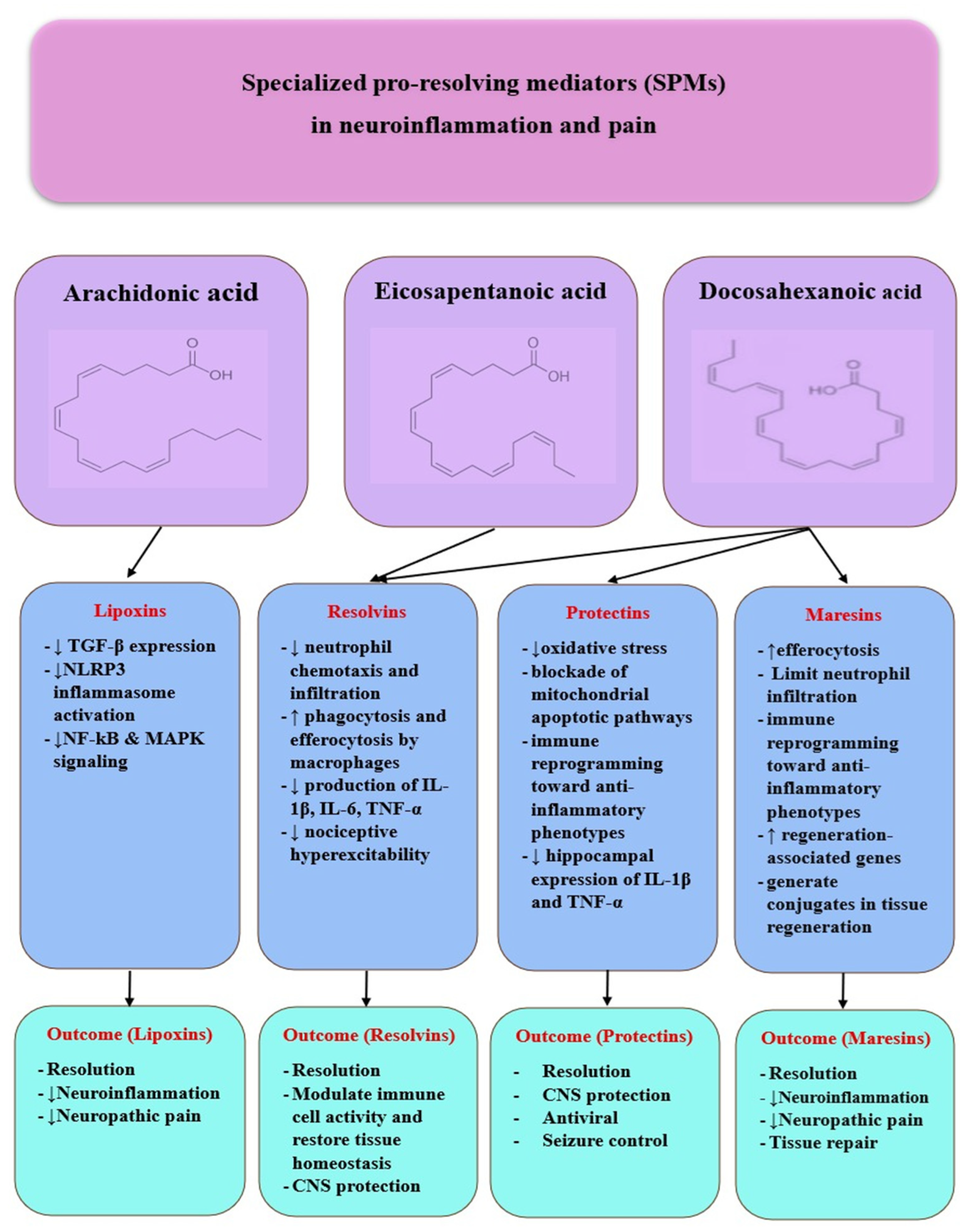

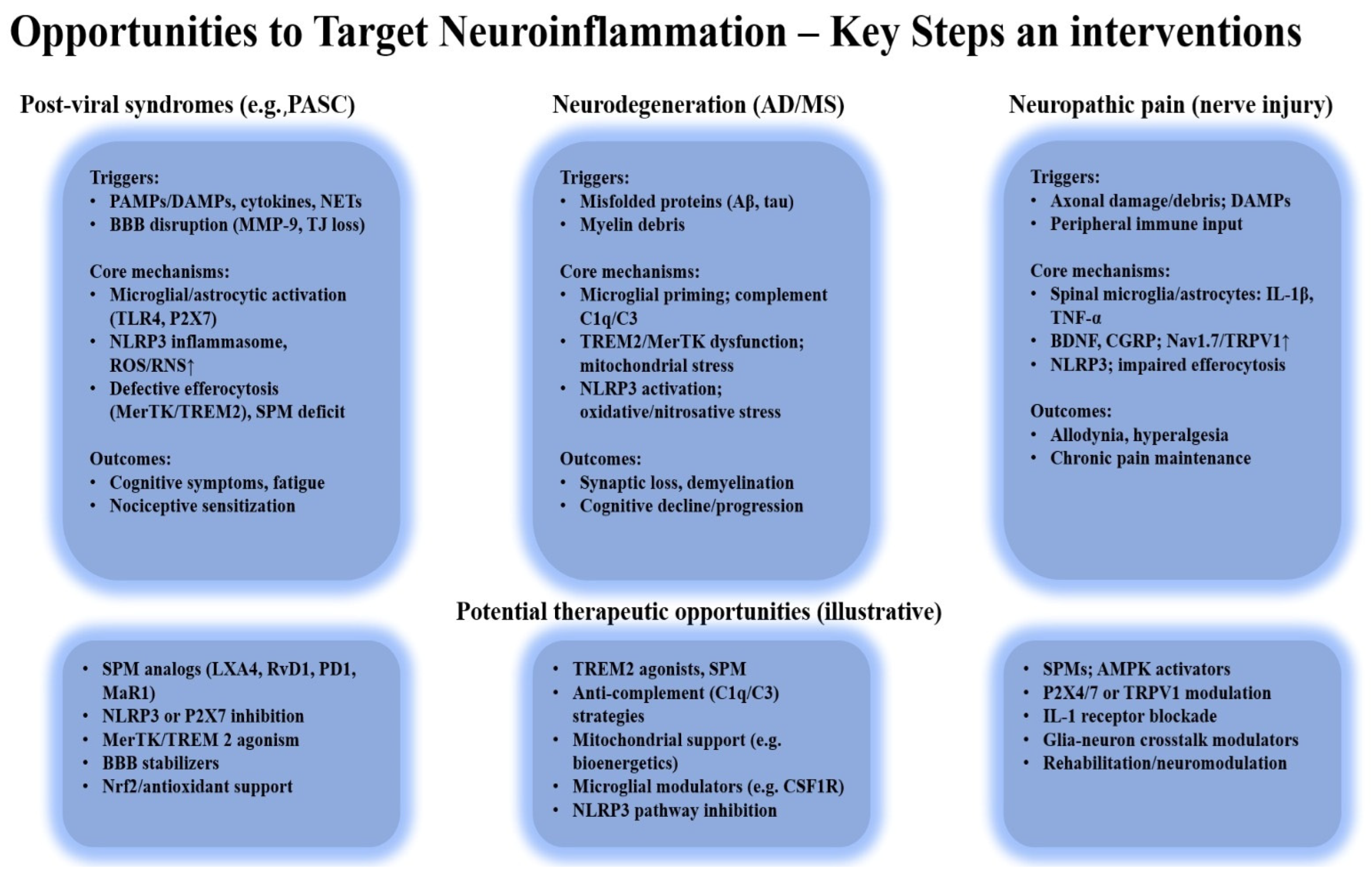
| Author (Year) | Model | Mechanism Studied | Outcome (Persistent Activation) |
|---|---|---|---|
| Cai et al. (2019) [67] | Ischemic stroke (mouse) | Loss of STAT6/Arg1 signaling → impaired efferocytosis | Accumulation of apoptotic neurons, ↑ infarct size, neuroinflammation |
| Frank et al. (2018) [68] | Stress-induced (mouse) | ↓ CD200R expression → microglial disinhibition | Microglial priming, exaggerated inflammatory responses, chronic glial activation |
| Kalinski et al. (2020) [52] | Sciatic nerve injury (mouse) | Macrophage efferocytosis of apoptotic leukocytes | Anti-inflammatory microenvironment, ↓ cytokine release, prevention of neuropathic pain |
| Kobayashi et al. (2020) [51] | Peripheral nerve injury (mouse) | ↓ MerTK in M2 macrophages → defective efferocytosis | Sustained NF-κB activation, DAMP release, chronic nociceptive hypersensitivity |
| Soliman et al. (2023) [53] | Cortical injury (mouse) | EphA4 inhibits ERK/STAT6/MerTK signaling | Defective debris clearance, sustained microglial inflammasome activation |
| Song et al. (2021) [69] | SARS-CoV-2 infection (mouse) | Viral interference with apoptotic clearance | Persistent microglial activation, ↑ IL-6 and TNF-α |
| Wang et al. (2015) [37] | Alzheimer’s disease (APPPS1 mice) | TREM2 lipid sensing → efferocytosis of apoptotic neurons and amyloid debris | Defective efferocytosis → impaired microglial response, chronic neuroinflammation, accelerated neurodegeneration |
| Wanke et al. (2021) [54] | Murine and human macrophages | MerTK kinase activity inhibition | NLRP3 activation, unresolved inflammation |
| Zang et al. (2025) [70] | Thalamic hemorrhage (rat, CPSP model) | LXR-β activation → ↑ MerTK/Axl/CD36 via p-STAT6 | Enhanced efferocytosis, ↓ neuroinflammation, alleviated post-stroke central pain |
| SPM Family | Biosynthesis and Receptors | Core Mechanisms | Neuroinflammation/Neuropathic Pain | Post-Viral/COVID-19 Relevance |
|---|---|---|---|---|
| Lipoxins (LXA4, LXB4) | Arachidonic acid via 15-LOX/5-LOX; main receptor ALX/FPR2 [75]. | Inhibits NLRP3, NF-κB, MAPK; lowers TNF-α/IL-1β/IL-6; supports epithelial repair [75]. | LXA4 reduces glial activation and neuropathic pain; ALX/FPR2 signaling dampens microglial reactivity; nano-LXA4 improves cognition in neurodegeneration (summarized from preclinical studies). | ICU COVID-19 cohorts: low LXA4 despite severe disease; other SPMs rise but remain insufficient → impaired resolution [75,92]. |
| Resolvins (RvD/E/T) | From EPA/DHA via 5/12/15-LOX; aspirin-acetylated COX-2 yields AT-resolvins; receptors ChemR23, ALX/FPR2, GPR32 [75]. | Reduce neutrophil chemotaxis; increase phagocytosis/efferocytosis; lower IL-1β/IL-6/TNF-α; promote M2 polarization [75]. | Limit microglial/astrocytic activation; constrain inflammasome; protect cognition in models (overview and synthesis). | Severe COVID-19: altered SPM profiles (↑RvE1, MaR2, RvD5; low LXA4), still inadequate resolution—candidate biomarkers/targets [75]. |
| Protectins (PD1/NPD1/PDX) | DHA-derived (15-LOX); receptors include ALX/FPR2 [9]. | Decrease oxidative stress and mitochondrial apoptosis; increase Iduna (DNA repair); stabilize BBB; promote neuro/angiogenesis [9]. | PD1n-3 DPA lowers hippocampal IL-1β/TNF-α and reduces seizures; ALX/FPR2 and ChemR23 upregulated in epileptogenic astrocytes [109]. | RSV: PCTR1/PD1 reduce viral load and lung inflammation, restore IFN-λ, induce cathelicidin [99]). COVID-19: higher plasma PD1 in critical illness associated with M2 polarization/IL-10 [100]. |
| Maresins (MaR1/MaR2/MCTR) | DHA → 12-LOX in macrophages; receptors LGR6, ALX/FPR2 [74,104]. | Enhance efferocytosis and tissue-repair programs; limit neutrophil influx/cytokines; MCTR couple clearance with regeneration [74,104]. | MaR1 mitigates perioperative neuroinflammation/cognitive decline [110]; promotes axonal regrowth; dampens spinal glia and TRPV1/PI3K-AKT-mTOR [107]; provides long-lasting analgesia via NF-κB/CGRP control [71]. MaR2 (intrathecal) reduces orofacial nociception, prevents postoperative hyperalgesia, and reverses trigeminal NP by suppressing c-Fos and NF-κB+/CGRP+ TG neurons [108]. | Post-COVID syndrome: 12-week SPM-enriched marine oil increased 14-HDHA/17-HDHA/18-HEPE and improved fatigue/dyspnea—supporting translational potential [105]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popa, A.E.; Popa, E.; Dramba, T.; Coman, E.A.; Poroch, M.; Ungureanu, M.; Bacusca, A.; Slanina, A.M.; Bacaoanu, G.; Poroch, V. Dysregulated Resolution of Inflammation After Respiratory Viral Infections: Molecular Pathways Linking Neuroinflammation to Post-Viral Neuropathic Pain—A Narrative Review. Int. J. Mol. Sci. 2025, 26, 11383. https://doi.org/10.3390/ijms262311383
Popa AE, Popa E, Dramba T, Coman EA, Poroch M, Ungureanu M, Bacusca A, Slanina AM, Bacaoanu G, Poroch V. Dysregulated Resolution of Inflammation After Respiratory Viral Infections: Molecular Pathways Linking Neuroinflammation to Post-Viral Neuropathic Pain—A Narrative Review. International Journal of Molecular Sciences. 2025; 26(23):11383. https://doi.org/10.3390/ijms262311383
Chicago/Turabian StylePopa, Andrei Emilian, Elena Popa, Tatiana Dramba, Elena Adorata Coman, Mihaela Poroch, Monica Ungureanu, Agnes Bacusca, Ana Maria Slanina, Gema Bacaoanu, and Vladimir Poroch. 2025. "Dysregulated Resolution of Inflammation After Respiratory Viral Infections: Molecular Pathways Linking Neuroinflammation to Post-Viral Neuropathic Pain—A Narrative Review" International Journal of Molecular Sciences 26, no. 23: 11383. https://doi.org/10.3390/ijms262311383
APA StylePopa, A. E., Popa, E., Dramba, T., Coman, E. A., Poroch, M., Ungureanu, M., Bacusca, A., Slanina, A. M., Bacaoanu, G., & Poroch, V. (2025). Dysregulated Resolution of Inflammation After Respiratory Viral Infections: Molecular Pathways Linking Neuroinflammation to Post-Viral Neuropathic Pain—A Narrative Review. International Journal of Molecular Sciences, 26(23), 11383. https://doi.org/10.3390/ijms262311383





