Age-Related Changes in Neuron–Microglia Interaction Mediated by Fractalkine Under Inflammatory Conditions
Abstract
1. Introduction
1.1. Ageing-Related Changes in Neuroinflammation and Microglia Reactivity
1.2. Ageing-Related Changes in TGFβ Signalling
1.3. Age-Related Changes in Neuron-Glia Interactions
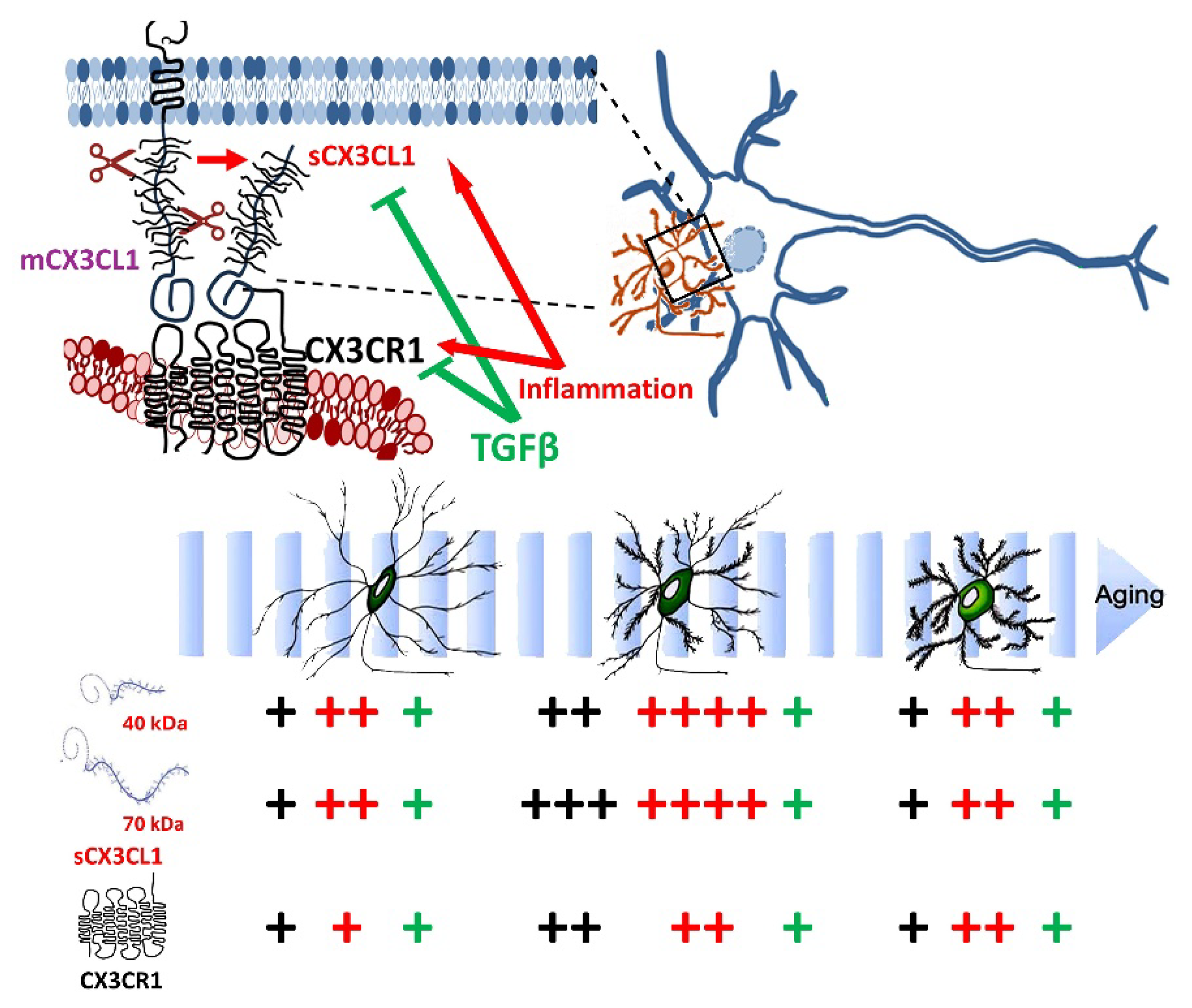
1.4. Regulation of Microglia by CX3CL1
1.5. Regulation of Microglia by CX3CL1 in Ageing
2. Results
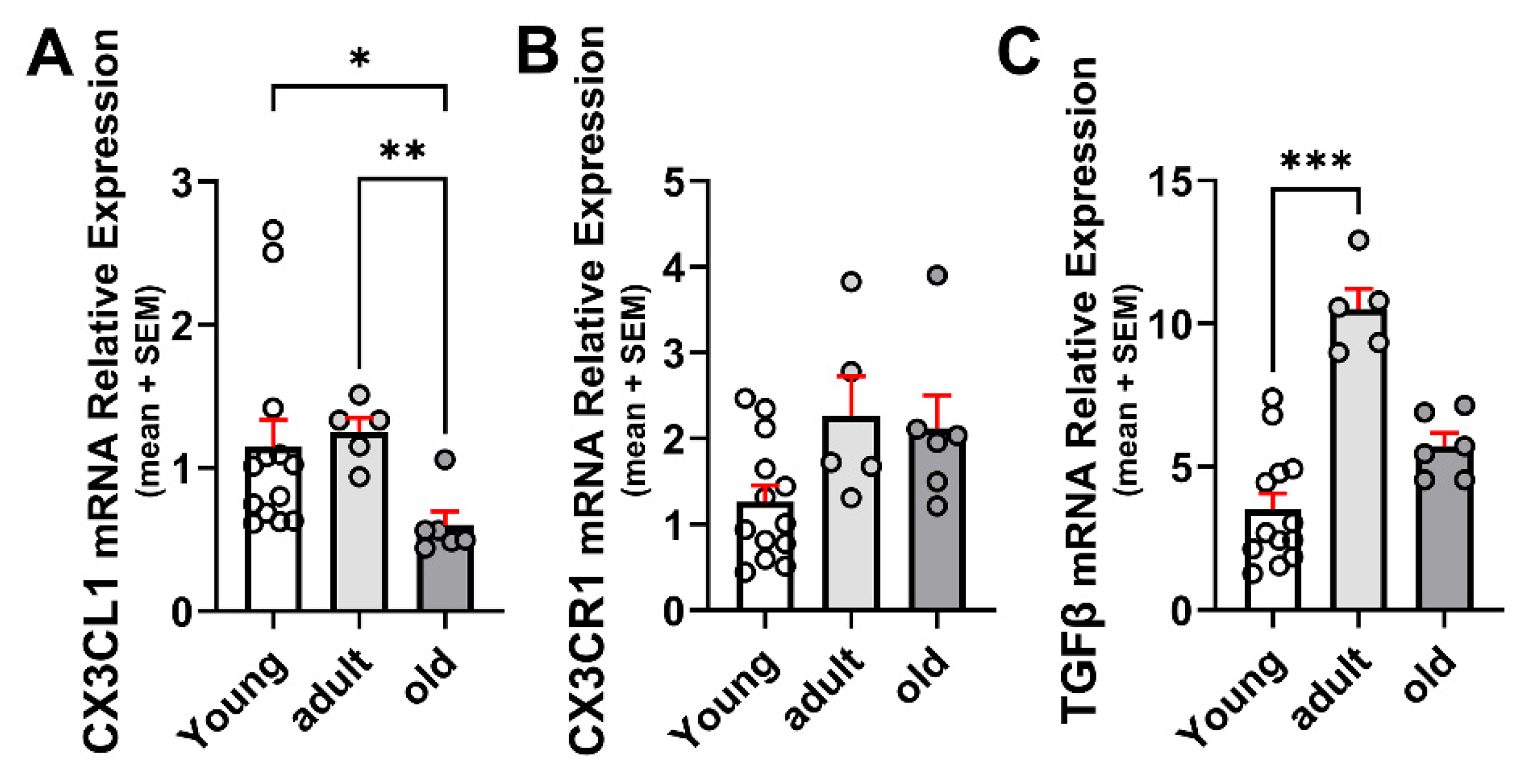
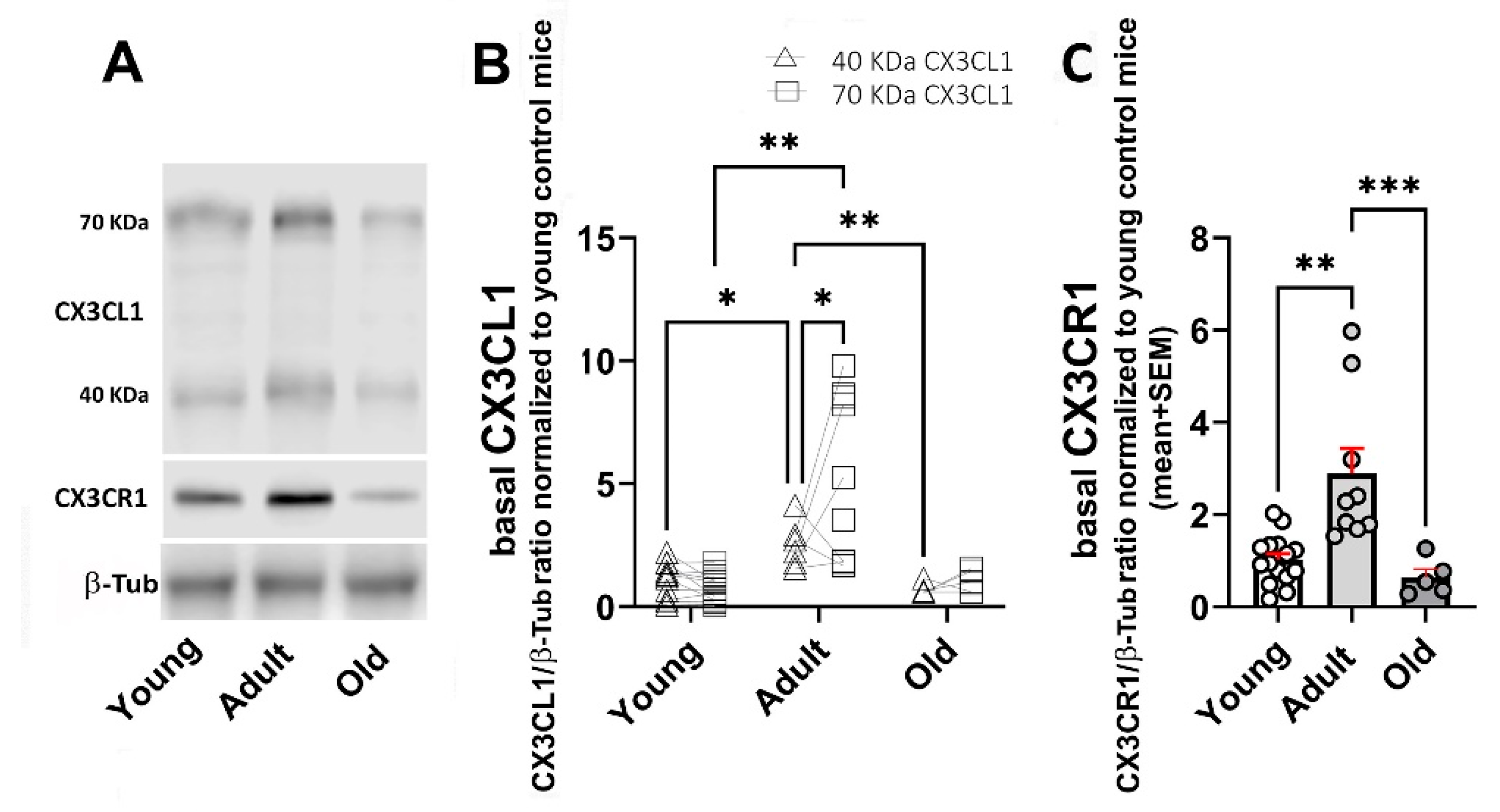
2.1. Age-Related Changes for CX3CL1, CX3CR1, and TGFβ
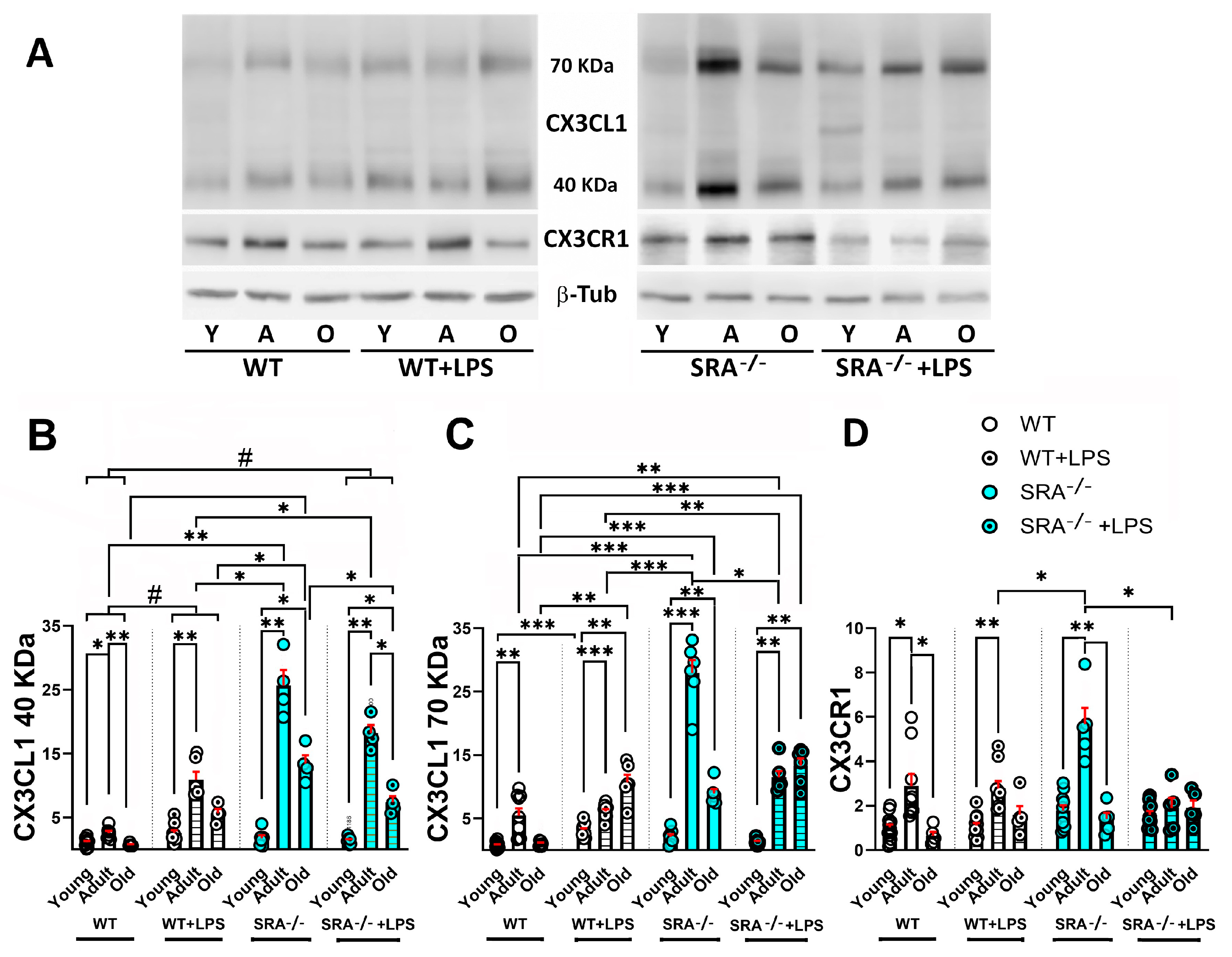
2.2. Age-Dependent Changes on the Regulation of mRNAs for CX3CL1, CX3CR1, and TGFβ by Inflammatory Stimulation
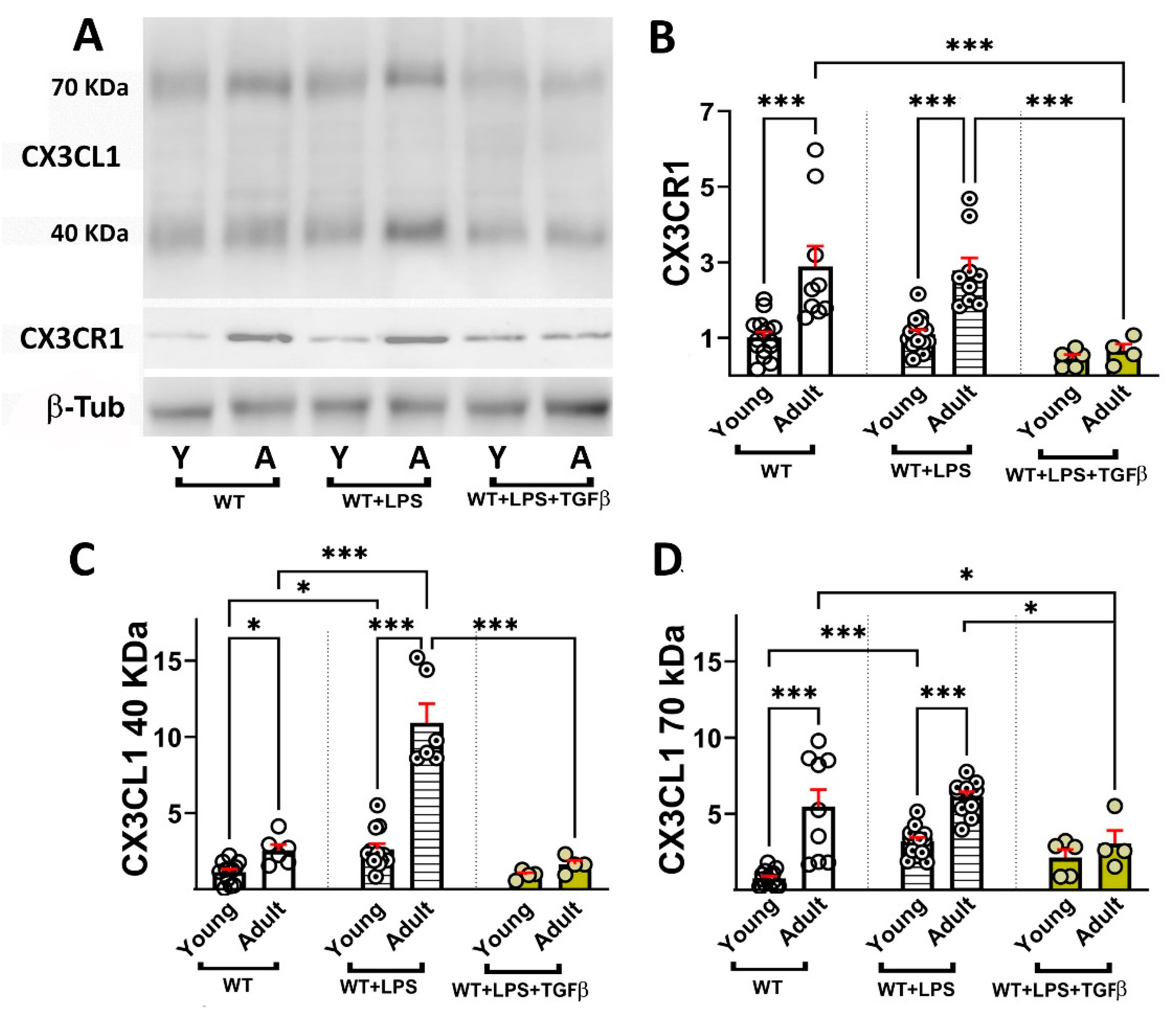
2.3. Age-Dependent Changes in sCX3CL1 and CX3CR1 in Response to Inflammation and TGFβ
3. Discussion
4. Materials and Methods
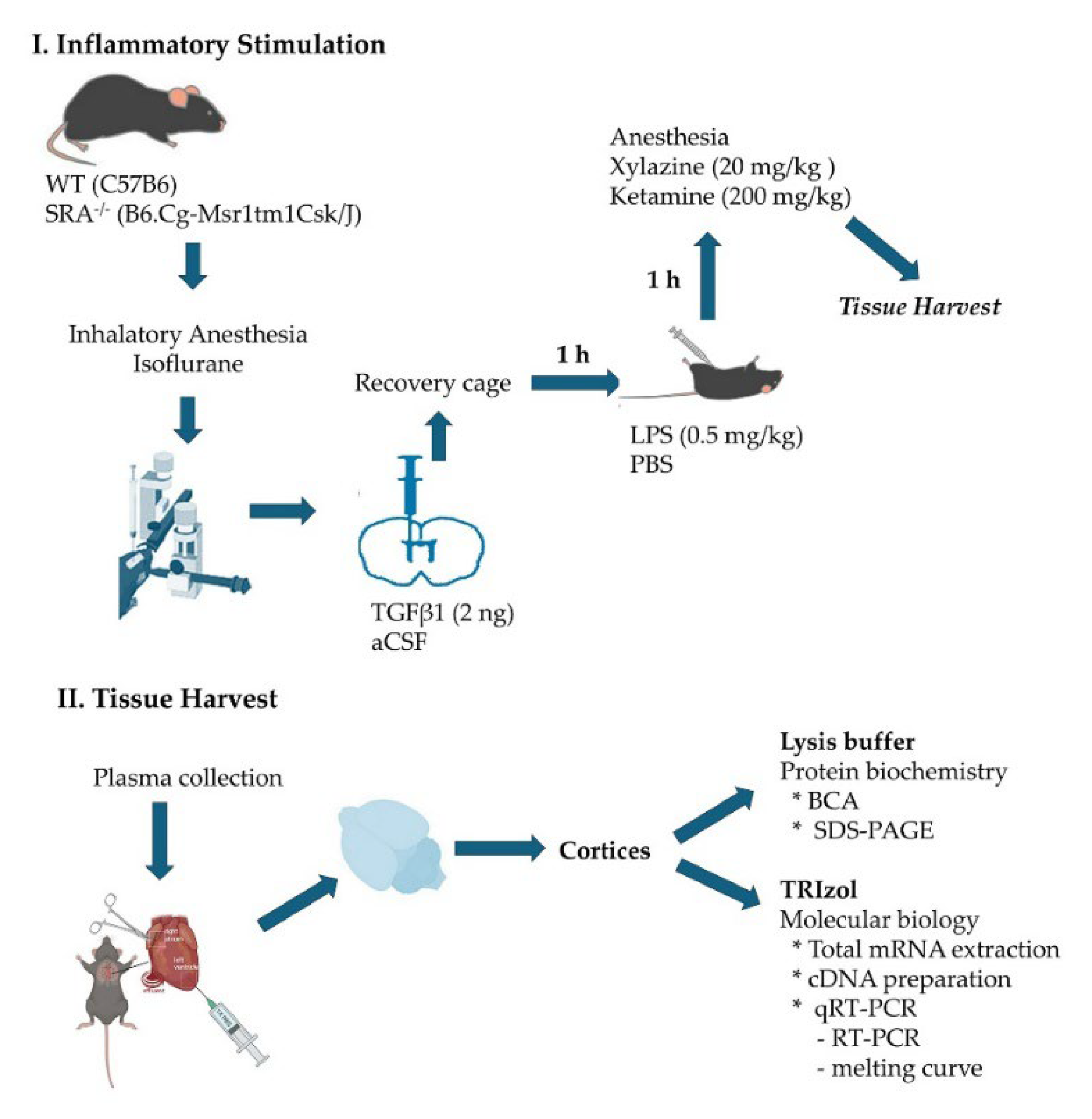
4.1. Animals and Animal Protocols
- Stereotaxic intracerebroventricular TGFβ injection
- Generation of acute systemic inflammation
- Obtention of the tissue
4.2. Western Blot
4.2.1. Protein Quantification
4.2.2. Western Blot Analysis
4.3. RNA Isolation and Real-Time Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR) Assays
4.4. Statistical Analysis
5. Conclusions
- Ageing-related changes resulted in the highest levels of CX3CL1 and TGFβ in adults, with a significant decrease in old mice; CX3CR1 WB showed a similar effect, although there were no differences at the mRNA level.
- LPS administered acutely induced a conspicuous increase in CX3CL1 and CX3CR1 in adult mice, and the response in SRA−/− mice was even more robust.
- TGFβ reduced the levels of CX3CL1, CX3CR1, and TGFβ induced by the acute treatment with LPS, although CX3CL1 mRNA was potentiated by TGFβ.
- Ageing effects on the TGFβ level can serve as a key regulator of neuroinflammation, being involved in augmented inflammatory activation.
- CX3CL1 serves regulatory functions in the activation of microglia and neuroinflammation. Modifications in membrane-associated and soluble CX3CL1 in ageing, as well as in CX3CR1, are interesting candidates for understanding ageing-associated changes in the neural regulation and neurotoxicity of microglia.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAV | Adeno-associated virus |
| Aβ | β amyloid |
| aCSF | Artificial cerebrospinal fluid |
| AD | Alzheimer’s disease |
| ALS | Amyotrophic lateral sclerosis |
| BBB | Blood–brain barrier |
| β-Tub | Beta-tubulin |
| CNS | Central nervous system |
| CX3CL1 | Fractalkine |
| CX3CR1 | Fractalkine receptor |
| ERK | Extracellular signal-regulated kinase |
| ICV | Intracerebroventricular |
| IFNγ | Interferon γ |
| IL1β | Interleukin 1β |
| IL6 | Interleukin 6 |
| IL10 | Interleukin 10 |
| i.p. | Intra peritoneal |
| i.t. | Intrathecal |
| LPS | Lipopolysaccharide |
| MAPKs | Mitogen-activated protein kinases |
| MCP1 | Monocyte chemoattractant protein-1 |
| mCX3CL1 | Membrane-bound fractalkine |
| MKP-1 | MAP kinase fosfatase-1 |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| mRNA | Messenger ribonucleic acid |
| NO | Nitric oxide |
| Nrf2 | Nuclear factor erythroid related factor 2 |
| PBS | Phosphate-buffered saline |
| PD | Parkinson’s disease |
| PI3K | Phosphatidylinositol 3-kinase |
| rAAV | Recombinant adeno-associated virus |
| ROS | Reactive oxygen species |
| RT | Reverse transcriptase |
| RT-qPCR | Reverse Transcription Quantitative Polymerase Chain Reaction |
| sCX3CL1 | Soluble fractalkine |
| SN | Substantia nigra |
| SNpc | Substantia nigra pars compacta |
| SPARC | Secreted protein acidic and rich in cysteine |
| SRA | Scavenger receptor type A |
| SRA−/− | Scavenger receptor type A knockout |
| TGFβ | Transforming growth factor β |
| TNFα | Tumour necrosis factor α |
| WT | Wild type |
References
- Schmauck-Medina, T.; Moliere, A.; Lautrup, S.; Zhang, J.; Chlopicki, S.; Madsen, H.B.; Cao, S.; Soendenbroe, C.; Mansell, E.; Vestergaard, M.B.; et al. New hallmarks of ageing: A 2022 Copenhagen ageing meeting summary. Aging 2022, 14, 6829–6839. [Google Scholar] [CrossRef]
- Darweesh, S.K.L.; Wolters, F.J.; Ikram, M.A.; de Wolf, F.; Bos, D.; Hofman, A. Inflammatory markers and the risk of dementia and Alzheimer’s disease: A meta-analysis. Alzheimers Dement. 2018, 14, 1450–1459. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Chavakis, T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat. Rev. Immunol. 2021, 21, 426–440. [Google Scholar] [CrossRef]
- von Bernhardi, R.; Eugenin, J. Aging Microglia and Their Impact in the Nervous System. Adv. Neurobiol. 2024, 37, 379–395. [Google Scholar] [CrossRef]
- Cherry, J.D.; Olschowka, J.A.; O’Banion, M.K. Neuroinflammation and M2 microglia: The good, the bad, and the inflamed. J. Neuroinflamm. 2014, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- Norden, D.M.; Godbout, J.P. Review: Microglia of the aged brain: Primed to be activated and resistant to regulation. Neuropathol. Appl. Neurobiol. 2013, 39, 19–34. [Google Scholar] [CrossRef]
- Das, R.; Chinnathambi, S. Microglial priming of antigen presentation and adaptive stimulation in Alzheimer’s disease. Cell. Mol. Life Sci. 2019, 76, 3681–3694. [Google Scholar] [CrossRef] [PubMed]
- Hickman, S.; Izzy, S.; Sen, P.; Morsett, L.; El Khoury, J. Microglia in neurodegeneration. Nat. Neurosci. 2018, 21, 1359–1369. [Google Scholar] [CrossRef] [PubMed]
- Conboy, M.J.; Conboy, I.M.; Rando, T.A. Heterochronic parabiosis: Historical perspective and methodological considerations for studies of aging and longevity. Aging Cell 2013, 12, 525–530. [Google Scholar] [CrossRef]
- Villeda, S.A.; Plambeck, K.E.; Middeldorp, J.; Castellano, J.M.; Mosher, K.I.; Luo, J.; Smith, L.K.; Bieri, G.; Lin, K.; Berdnik, D.; et al. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat. Med. 2014, 20, 659–663. [Google Scholar] [CrossRef]
- Cerpa, W.; Ramos-Fernandez, E.; Inestrosa, N.C. Modulation of the NMDA Receptor Through Secreted Soluble Factors. Mol. Neurobiol. 2016, 53, 299–309. [Google Scholar] [CrossRef]
- Johnson-Venkatesh, E.M.; Umemori, H. Secreted factors as synaptic organizers. Eur. J. Neurosci. 2010, 32, 181–190. [Google Scholar] [CrossRef]
- Schafer, D.P.; Lehrman, E.K.; Stevens, B. The “quad-partite” synapse: Microglia-synapse interactions in the developing and mature CNS. Glia 2013, 61, 24–36. [Google Scholar] [CrossRef]
- Antignano, I.; Liu, Y.; Offermann, N.; Capasso, M. Aging microglia. Cell. Mol. Life Sci. 2023, 80, 126. [Google Scholar] [CrossRef]
- Cornejo, F.; Vruwink, M.; Metz, C.; Munoz, P.; Salgado, N.; Poblete, J.; Andrés, M.E.; Eugenín, J.; von Bernhardi, R. Scavenger Receptor-A deficiency impairs immune response of microglia and astrocytes potentiating Alzheimer’s disease pathophysiology. Brain Behav. Immun. 2018, 69, 336–350. [Google Scholar] [CrossRef]
- Di Micco, R.; Krizhanovsky, V.; Baker, D.; d’Adda di Fagagna, F. Cellular senescence in ageing: From mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 2021, 22, 75–95. [Google Scholar] [CrossRef]
- Amelimojarad, M.; Amelimojarad, M.; Cui, X. The emerging role of brain neuroinflammatory responses in Alzheimer’s disease. Front. Aging Neurosci. 2024, 16, 1391517. [Google Scholar] [CrossRef]
- Tichauer, J.E.; Flores, B.; Soler, B.; Eugenin-von Bernhardi, L.; Ramirez, G.; von Bernhardi, R. Age-dependent changes on TGFbeta1 Smad3 pathway modify the pattern of microglial cell activation. Brain Behav. Immun. 2014, 37, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, M.; Anusuyadevi, M.; Aigner, K.M.; Unger, M.S.; Kniewallner, K.M.; de Sousa, D.M.B.; Altendorfer, B.; Mrowetz, H.; Bogdahn, U.; Aigner, L. TGF-beta Signaling: A Therapeutic Target to Reinstate Regenerative Plasticity in Vascular Dementia? Aging Dis. 2020, 11, 828–850. [Google Scholar] [CrossRef] [PubMed]
- Vidovic, N.; Spittau, B. Microglial Transforming Growth Factor-beta Signaling in Alzheimer’s Disease. Int. J. Mol. Sci. 2024, 25, 3090. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Molina, R.; Flores, B.; Orellana, J.A.; von Bernhardi, R. Modulation of interferon-gamma-induced glial cell activation by transforming growth factor beta1: A role for STAT1 and MAPK pathways. J. Neurochem. 2012, 123, 113–123. [Google Scholar] [CrossRef] [PubMed]
- von Bernhardi, R.; Cornejo, F.; Parada, G.E.; Eugenin, J. Role of TGFbeta signaling in the pathogenesis of Alzheimer’s disease. Front. Cell. Neurosci. 2015, 9, 426. [Google Scholar] [CrossRef]
- Flores, B.; von Bernhardi, R. Transforming growth factor beta1 modulates amyloid beta-induced glial activation through the Smad3-dependent induction of MAPK phosphatase-1. J. Alzheimers Dis. 2012, 32, 417–429. [Google Scholar] [CrossRef]
- Derynck, R.; Zhang, Y.E. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 2003, 425, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.; Attisano, L. The TGFbeta superfamily signaling pathway. Wiley Interdiscip. Rev. Dev. Biol. 2013, 2, 47–63. [Google Scholar] [CrossRef] [PubMed]
- von Bernhardi, R.; Eugenin-von Bernhardi, L.; Eugenin, J. Microglial cell dysregulation in brain aging and neurodegeneration. Front. Aging Neurosci. 2015, 7, 124. [Google Scholar] [CrossRef]
- Preininger, M.K.; Kaufer, D. Blood-Brain Barrier Dysfunction and Astrocyte Senescence as Reciprocal Drivers of Neuropathology in Aging. Int. J. Mol. Sci. 2022, 23, 6217. [Google Scholar] [CrossRef]
- Lloyd-Burton, S.M.; York, E.M.; Anwar, M.A.; Vincent, A.J.; Roskams, A.J. SPARC regulates microgliosis and functional recovery following cortical ischemia. J. Neurosci. 2013, 33, 4468–4481. [Google Scholar] [CrossRef]
- Goldberg, E.L.; Shaw, A.C.; Montgomery, R.R. How Inflammation Blunts Innate Immunity in Aging. Interdiscip. Top. Gerontol. Geriatr. 2020, 43, 1–17. [Google Scholar] [CrossRef]
- Lee, S.; Xu, G.; Jay, T.R.; Bhatta, S.; Kim, K.W.; Jung, S.; Landreth, G.E.; Ransohoff, R.M.; Lamb, B.T. Opposing effects of membrane-anchored CX3CL1 on amyloid and tau pathologies via the p38 MAPK pathway. J. Neurosci. 2014, 34, 12538–12546. [Google Scholar] [CrossRef]
- Mecca, C.; Giambanco, I.; Donato, R.; Arcuri, C. Microglia and Aging: The Role of the TREM2-DAP12 and CX3CL1-CX3CR1 Axes. Int. J. Mol. Sci. 2018, 19, 318. [Google Scholar] [CrossRef]
- Ostuni, M.A.; Hermand, P.; Saindoy, E.; Guillou, N.; Guellec, J.; Coens, A.; Hattab, C.; Desuzinges-Mandon, E.; Jawhari, A.; Iatmanen-Harbi, S.; et al. CX3CL1 homo-oligomerization drives cell-to-cell adherence. Sci. Rep. 2020, 10, 9069. [Google Scholar] [CrossRef]
- Rivas-Fuentes, S.; Salgado-Aguayo, A.; Arratia-Quijada, J.; Gorocica-Rosete, P. Regulation and biological functions of the CX3CL1-CX3CR1 axis and its relevance in solid cancer: A mini-review. J. Cancer 2021, 12, 571–583. [Google Scholar] [CrossRef]
- Tsou, C.L.; Haskell, C.A.; Charo, I.F. Tumor necrosis factor-alpha-converting enzyme mediates the inducible cleavage of fractalkine. J. Biol. Chem. 2001, 276, 44622–44626. [Google Scholar] [CrossRef]
- Hundhausen, C.; Schulte, A.; Schulz, B.; Andrzejewski, M.G.; Schwarz, N.; von Hundelshausen, P.; Winter, U.; Paliga, K.; Reiss, K.; Saftig, P.; et al. Regulated shedding of transmembrane chemokines by the disintegrin and metalloproteinase 10 facilitates detachment of adherent leukocytes. J. Immunol. 2007, 178, 8064–8072. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.K.; Yip, P.K.; Malcangio, M. The liberation of fractalkine in the dorsal horn requires microglial cathepsin S. J. Neurosci. 2009, 29, 6945–6954. [Google Scholar] [CrossRef]
- Jones, B.A.; Beamer, M.; Ahmed, S. Fractalkine/CX3CL1: A potential new target for inflammatory diseases. Mol. Interv. 2010, 10, 263–270. [Google Scholar] [CrossRef]
- Szepesi, Z.; Manouchehrian, O.; Bachiller, S.; Deierborg, T. Bidirectional Microglia-Neuron Communication in Health and Disease. Front. Cell. Neurosci. 2018, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.L.; Holman, D.W.; Klein, R.S. Chemokines in the balance: Maintenance of homeostasis and protection at CNS barriers. Front. Cell. Neurosci. 2014, 8, 154. [Google Scholar] [CrossRef]
- Eugenin, J.; Eugenin-von Bernhardi, L.; von Bernhardi, R. Age-dependent changes on fractalkine forms and their contribution to neurodegenerative diseases. Front. Mol. Neurosci. 2023, 16, 1249320. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, S.; Devi, M.H.; Chakrabarty, S.; Paylar, B.; Pradhan, A.; Thaker, M.; Ayyadhury, S.; Manavalan, A.; Olsson, P.-E.; Pramanik, G.; et al. Microglia signaling in health and disease—Implications in sex-specific brain development and plasticity. Neurosci. Biobehav. Rev. 2024, 165, 105834. [Google Scholar] [CrossRef]
- Galan-Ganga, M.; Garcia-Yague, A.J.; Lastres-Becker, I. Role of MSK1 in the Induction of NF-kappaB by the Chemokine CX3CL1 in Microglial Cells. Cell. Mol. Neurobiol. 2019, 39, 331–340. [Google Scholar] [CrossRef]
- Castro-Sanchez, S.; Garcia-Yague, A.J.; Kugler, S.; Lastres-Becker, I. CX3CR1-deficient microglia show impaired signalling of the transcription factor NRF2: Implications in tauopathies. Redox Biol. 2019, 22, 101118. [Google Scholar] [CrossRef]
- Li, R.; Jia, Z.; Zhu, H. Regulation of Nrf2 Signaling. React. Oxyg. Species (Apex) 2019, 8, 312–322. [Google Scholar] [CrossRef]
- Trougakos, I.P. Nrf2, stress and aging. Aging 2019, 11, 5289–5291. [Google Scholar] [CrossRef]
- Simon, E.; Obst, J.; Gomez-Nicola, D. The Evolving Dialogue of Microglia and Neurons in Alzheimer’s Disease: Microglia as Necessary Transducers of Pathology. Neuroscience 2019, 405, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Triolo, M.; Fadic, R.; von Bernhardi, R. Microglial Cell Dysregulation in the Aged Brain and Neurodegeneration. In Handbook of Neurotoxicity; Kostrzewa, R.M., Ed.; Springer Nature Switzerland AG: Chams, Switzerland, 2021. [Google Scholar] [CrossRef]
- Beltrán-Castillo, S.; von Bernhardi, R.; Eugenín, J. The impact of aged microglia on D-serine-regulated glutamatergic transmission. In Factors Affecting Neurological Aging: Genetics, Neurology, Behavior, and Diet; Martin, C., Preedy, V.R., Rajendram, R., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2021; pp. 227–236. ISBN 978-0-12-818369-4. [Google Scholar] [CrossRef]
- Bertollini, C.; Ragozzino, D.; Gross, C.; Limatola, C.; Eusebi, F. Fractalkine/CX3CL1 depresses central synaptic transmission in mouse hippocampal slices. Neuropharmacology 2006, 51, 816–821. [Google Scholar] [CrossRef]
- Ragozzino, D.; Di Angelantonio, S.; Trettel, F.; Bertollini, C.; Maggi, L.; Gross, C.; Charo, I.F.; Limatola, C.; Eusebi, F. Chemokine fractalkine/CX3CL1 negatively modulates active glutamatergic synapses in rat hippocampal neurons. J. Neurosci. 2006, 26, 10488–10498. [Google Scholar] [CrossRef]
- Maggi, L.; Scianni, M.; Branchi, I.; D’Andrea, I.; Lauro, C.; Limatola, C. CX(3)CR1 deficiency alters hippocampal-dependent plasticity phenomena blunting the effects of enriched environment. Front. Cell. Neurosci. 2011, 5, 22. [Google Scholar] [CrossRef]
- Rogers, J.T.; Morganti, J.M.; Bachstetter, A.D.; Hudson, C.E.; Peters, M.M.; Grimmig, B.A.; Weeber, E.J.; Bickford, P.C.; Gemma, C. CX3CR1 deficiency leads to impairment of hippocampal cognitive function and synaptic plasticity. J. Neurosci. 2011, 31, 16241–16250. [Google Scholar] [CrossRef]
- Sheridan, G.K.; Wdowicz, A.; Pickering, M.; Watters, O.; Halley, P.; O’Sullivan, N.C.; Mooney, C.; O’Connell, D.J.; O’Connor, J.J.; Murphy, K.J. CX3CL1 is up-regulated in the rat hippocampus during memory-associated synaptic plasticity. Front. Cell. Neurosci. 2014, 8, 233. [Google Scholar] [CrossRef]
- Zujovic, V.; Schussler, N.; Jourdain, D.; Duverger, D.; Taupin, V. In vivo neutralization of endogenous brain fractalkine increases hippocampal TN alpha and 8-isoprostane production induced by intracerebroventricular injection of LPS. J. Neuroimmunol. 2001, 115, 135–143. [Google Scholar] [CrossRef]
- Mizuno, T.; Kawanokuchi, J.; Numata, K.; Suzumura, A. Production and neuroprotective functions of fractalkine in the central nervous system. Brain Res. 2003, 979, 65–70. [Google Scholar] [CrossRef]
- Zujovic, V.; Benavides, J.; Vige, X.; Carter, C.; Taupin, V. Fractalkine modulates TNF-alpha secretion and neurotoxicity induced by microglial activation. Glia 2000, 29, 305–315. [Google Scholar] [CrossRef]
- Cardona, A.E.; Pioro, E.P.; Sasse, M.E.; Kostenko, V.; Cardona, S.M.; Dijkstra, I.M.; Huang, D.; Kidd, G.; Dombrowski, S.; Dutta, R.; et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat. Neurosci. 2006, 9, 917–924. [Google Scholar] [CrossRef]
- Lyons, A.; McQuillan, K.; Deighan, B.F.; O’Reilly, J.A.; Downer, E.J.; Murphy, A.C.; Watson, M.; Piazza, A.; O’cOnnell, F.; Griffin, R.; et al. Decreased neuronal CD200 expression in IL-4-deficient mice results in increased neuroinflammation in response to lipopolysaccharide. Brain Behav. Immun. 2009, 23, 1020–1027. [Google Scholar] [CrossRef]
- Wynne, A.M.; Henry, C.J.; Huang, Y.; Cleland, A.; Godbout, J.P. Protracted downregulation of CX3CR1 on microglia of aged mice after lipopolysaccharide challenge. Brain Behav. Immun. 2010, 24, 1190–1201. [Google Scholar] [CrossRef]
- Chen, P.; Zhao, W.; Guo, Y.; Xu, J.; Yin, M. CX3CL1/CX3CR1 in Alzheimer’s Disease: A Target for Neuroprotection. BioMed Res. Int. 2016, 2016, 8090918. [Google Scholar] [CrossRef]
- Lastres-Becker, I.; Innamorato, N.G.; Jaworski, T.; Rabano, A.; Kugler, S.; Van Leuven, F.; Cuadrado, A. Fractalkine activates NRF2/NFE2L2 and heme oxygenase 1 to restrain tauopathy-induced microgliosis. Brain 2014, 137 Pt 1, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Finneran, D.J.; Nash, K.R. Neuroinflammation and fractalkine signaling in Alzheimer’s disease. J. Neuroinflamm. 2019, 16, 30. [Google Scholar] [CrossRef] [PubMed]
- Angelopoulou, E.; Paudel, Y.N.; Shaikh, M.F.; Piperi, C. Fractalkine (CX3CL1) signaling and neuroinflammation in Parkinson’s disease: Potential clinical and therapeutic implications. Pharmacol. Res. 2020, 158, 104930. [Google Scholar] [CrossRef]
- Nash, K.R.; Moran, P.; Finneran, D.J.; Hudson, C.; Robinson, J.; Morgan, D.; Bickford, P.C. Fractalkine over expression suppresses alpha-synuclein-mediated neurodegeneration. Mol. Ther. 2015, 23, 17–23. [Google Scholar] [CrossRef]
- Castro-Sanchez, S.; Garcia-Yague, A.J.; Lopez-Royo, T.; Casarejos, M.; Lanciego, J.L.; Lastres-Becker, I. Cx3cr1-deficiency exacerbates alpha-synuclein-A53T induced neuroinflammation and neurodegeneration in a mouse model of Parkinson’s disease. Glia 2018, 66, 1752–1762. [Google Scholar] [CrossRef]
- Morganti, J.M.; Nash, K.R.; Grimmig, B.A.; Ranjit, S.; Small, B.; Bickford, P.C.; Gemma, C. The soluble isoform of CX3CL1 is necessary for neuroprotection in a mouse model of Parkinson’s disease. J. Neurosci. 2012, 32, 14592–14601. [Google Scholar] [CrossRef] [PubMed]
- Pawelec, P.; Ziemka-Nalecz, M.; Sypecka, J.; Zalewska, T. The Impact of the CX3CL1/CX3CR1 Axis in Neurological Disorders. Cells 2020, 9, 2277. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Sun, B.; Zhou, Y.; Kauppinen, T.M.; Halabisky, B.; Wes, P.; Ransohoff, R.M.; Gan, L. CX3CR1 protein signaling modulates microglial activation and protects against plaque-independent cognitive deficits in a mouse model of Alzheimer disease. J. Biol. Chem. 2011, 286, 32713–32722. [Google Scholar] [CrossRef]
- Godoy, B.; Murgas, P.; Tichauer, J.; Von Bernhardi, R. Scavenger receptor class A ligands induce secretion of IL1beta and exert a modulatory effect on the inflammatory activation of astrocytes in culture. J. Neuroimmunol. 2012, 251, 6–13. [Google Scholar] [CrossRef]
- Murgas, P.; Cornejo, F.A.; Merino, G.; von Bernhardi, R. SR-A regulates the inflammatory activation of astrocytes. Neurotox. Res. 2014, 25, 68–80. [Google Scholar] [CrossRef]
- Xin, W.; Pan, Y.; Wei, W.; Gerner, S.T.; Huber, S.; Juenemann, M.; Butz, M.; Bähr, M.; Huttner, H.B.; Doeppner, T.R. TGF-beta1 Decreases Microglia-Mediated Neuroinflammation and Lipid Droplet Accumulation in an In Vitro Stroke Model. Int. J. Mol. Sci. 2023, 24, 17329. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, N.; Feng, W.; Liu, X.; Wu, Q.; Chen, J.; Jiao, X.; Ning, X.; Qi, Z.; Xu, Z.; et al. Soluble TGF-beta decoy receptor TGFBR3 exacerbates Alzheimer’s disease pathology by modifying microglial function. Glia 2024, 72, 2201–2216. [Google Scholar] [CrossRef]
- Kimura, K.; Subramanian, A.; Yin, Z.; Khalilnezhad, A.; Wu, Y.; He, D.; Dixon, K.O.; Chitta, U.K.; Ding, X.; Adhikari, N.; et al. Immune checkpoint TIM-3 regulates microglia and Alzheimer’s disease. Nature 2025, 641, 718–731. [Google Scholar] [CrossRef]
- Behl, T.; Kaur, G.; Sehgal, A.; Bhardwaj, S.; Singh, S.; Buhas, C.; Judea-Pusta, C.; Uivarosan, D.; Munteanu, M.A.; Bungau, S. Multifaceted Role of Matrix Metalloproteinases in Neurodegenerative Diseases: Pathophysiological and Therapeutic Perspectives. Int. J. Mol. Sci. 2021, 22, 1413. [Google Scholar] [CrossRef]
- Hernandez-Espinosa, D.R.; Medina-Ruiz, G.I.; Scrabis, M.G.; Thathiah, A.; Aizenman, E. Proinflammatory microglial activation impairs in vitro cortical tissue repair via zinc-dependent ADAM17 cleavage of the CSF-1 receptor. J. Neurochem. 2025, 169, e16239. [Google Scholar] [CrossRef]
- Lively, S.; Schlichter, L.C. The microglial activation state regulates migration and roles of matrix-dissolving enzymes for invasion. J. Neuroinflamm. 2013, 10, 75. [Google Scholar] [CrossRef]
- Nakanishi, H. Cathepsin regulation on microglial function. Biochim. Biophys. Acta Proteins Proteom. 2020, 1868, 140465. [Google Scholar] [CrossRef] [PubMed]
- von Bernhardi, R.; Eugenin, J. Ageing-related changes in the regulation of microglia and their interaction with neurons. Neuropharmacology 2025, 265, 110241. [Google Scholar] [CrossRef]
- Chen, S.; Luo, D.; Streit, W.J.; Harrison, J.K. TGF-beta1 upregulates CX3CR1 expression and inhibits fractalkine-stimulated signaling in rat microglia. J. Neuroimmunol. 2002, 133, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.; Sharma, A.; Kumar, D.; Asthana, M.K.; Lalhlenmawia, H.; Kumar, A.; Bhattacharyya, S. Promising protein biomarkers in the early diagnosis of Alzheimer’s disease. Metab. Brain Dis. 2022, 37, 1727–1744. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Li, S.; Maurer, K.; Zhang, Z.; Petri, M.; Sullivan, K.E. Enhancer RNA and NFkappaB-dependent P300 regulation of ADAMDEC1. Mol. Immunol. 2018, 103, 312–321. [Google Scholar] [CrossRef]
- Wendt, W.; Lubbert, H.; Stichel, C.C. Upregulation of cathepsin S in the aging and pathological nervous system of mice. Brain Res. 2008, 1232, 7–20. [Google Scholar] [CrossRef]
- Brifault, C.; Gilder, A.S.; Laudati, E.; Banki, M.; Gonias, S.L. Shedding of membrane-associated LDL receptor-related protein-1 from microglia amplifies and sustains neuroinflammation. J. Biol. Chem. 2017, 292, 18699–18712. [Google Scholar] [CrossRef]
- Chen, C.D.; Tung, T.Y.; Liang, J.; Zeldich, E.; Tucker Zhou, T.B.; Turk, B.E.; Abraham, C.R. Identification of cleavage sites leading to the shed form of the anti-aging protein klotho. Biochemistry 2014, 53, 5579–5587. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, J.; Tran, H.; Verbeek, M.M.; Reiss, K.; Estus, S.; Bu, G. LRP1 shedding in human brain: Roles of ADAM10 and ADAM17. Mol. Neurodegener. 2009, 4, 17. [Google Scholar] [CrossRef]
- Bertoldi, K.; Cechinel, L.R.; Schallenberger, B.; Meireles, L.; Basso, C.; Lovatel, G.A.; Bernardi, L.; Lamers, M.L.; Siqueira, I.R. Aging process alters hippocampal and cortical secretase activities of Wistar rats. Behav. Brain Res. 2017, 317, 374–381. [Google Scholar] [CrossRef]
- Park, S.K.; Kim, K.; Page, G.P.; Allison, D.B.; Weindruch, R.; Prolla, T.A. Gene expression profiling of aging in multiple mouse strains: Identification of aging biomarkers and impact of dietary antioxidants. Aging Cell 2009, 8, 484–495. [Google Scholar] [CrossRef]
- Finneran, D.; Li, Q.; Subbarayan, M.S.; Joly-Amado, A.; Kamath, S.; Dengler, D.G.; Gordon, M.N.; Jackson, M.R.; Morgan, D.; Bickford, P.C.; et al. Concentration and proteolysis of CX3CL1 may regulate the microglial response to CX3CL1. Glia 2023, 71, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Keren-Shaul, H.; Spinrad, A.; Weiner, A.; Matcovitch-Natan, O.; Dvir-Szternfeld, R.; Ulland, T.K.; David, E.; Baruch, K.; Lara-Astaiso, D.; Toth, B.; et al. A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell 2017, 169, 1276–1290.e1217. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.S.; Koh, S.H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Streit, W.J.; Khoshbouei, H.; Bechmann, I. Dystrophic microglia in late-onset Alzheimer’s disease. Glia 2020, 68, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Fryatt, G.L.; Ghorbani, M.; Obst, J.; Menassa, D.A.; Martin-Estebane, M.; Muntslag, T.A.; Olmos-Alonso, A.; Guerrero-Carrasco, M.; Thomas, D.; et al. Replicative senescence dictates the emergence of disease-associated microglia and contributes to Abeta pathology. Cell Rep. 2021, 35, 109228. [Google Scholar] [CrossRef]
- Zhou, Y.; Song, W.M.; Andhey, P.S.; Swain, A.; Levy, T.; Miller, K.R.; Poliani, P.L.; Cominelli, M.; Grover, S.; Gilfillan, S.; et al. Human and mouse single-nucleus transcriptomics reveal TREM2-dependent and TREM2-independent cellular responses in Alzheimer’s disease. Nat. Med. 2020, 26, 131–142, Erratum in Nat. Med. 2020, 26, 981. [Google Scholar] [CrossRef]
- Balusu, S.; Praschberger, R.; Lauwers, E.; De Strooper, B.; Verstreken, P. Neurodegeneration cell per cell. Neuron 2023, 111, 767–786. [Google Scholar] [CrossRef] [PubMed]
- Lau, V.; Ramer, L.; Tremblay, M.E. An aging, pathology burden, and glial senescence build-up hypothesis for late onset Alzheimer’s disease. Nat. Commun. 2023, 14, 1670. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, A.; Mentlein, R. Glial cross-talk by transmembrane chemokines CX3CL1 and CXCL16. J. Neuroimmunol. 2008, 198, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]

| Mice | Age (m) | Number Mice |
|---|---|---|
| Mus Musculus/C57B6/–WT | 3–7 | 65 |
| Mus Musculus/C57B6/–WT | 12–15 | 48 |
| Mus Musculus/C57B6/–WT | >20 | 29 |
| Mus Musculus /B6.Cg-Msr1tm1Csk/J/–SRA−/− | 3–7 | 20 |
| Mus Musculus/B6.Cg-Msr1tm1Csk/J/–SRA−/− | 12–15 | 12 |
| Mus Musculus/B6.Cg-Msr1tm1Csk/J/–SRA−/− | >20 | 12 |
| Antibody Name | Manufacturer Info | Concentration |
|---|---|---|
| α-CX3CL1, rabbit | 14-7186-81 Invitrogen, Waltham, MA, USA | 1:1000 |
| α-CX3CR1 rabbit | 14-6093-81, Invitrogen, Waltham, MA, USA | 1:1000 |
| α-β3 tubulin, mouse | SC-80005, Santa Cruz, Dallas, TX, USA | 1:1000 |
| Goat α-Rabbit IgG, H&L Chain | 401315, Calbiochem, Darmstadt, Germany | 1:5000 |
| Goat α-Mouse IgG, H&L Chain | 401215, Calbiochem, Darmstadt, Germany | 1:10,000 |
| Primer | Sequence | Amplification Product Length |
|---|---|---|
| CX3CL1-fw | AACCAGTTGTAGGCCTGAGC | 129 bp |
| CX3CL1-rev | CACATTCTGCTCTGGGAGGG | |
| CX3CR1-fw | CCCCTTTATCTACGCCTTTGC | 180 bp |
| CX3CR1-rev | CCATCTCCCTCGCTTGTGT | |
| TGFβ-fw | CTATGCTAAAGAGGTCACCCG | 123 bp |
| TGFβ-rev | ACTGCTTCCCGAATGTCTG | |
| β-actin-fw | GATGACCCAGATCATGTTTG | 292 bp |
| β-actin-rev | CTTCTCTTTGATGTCACGCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
von Bernhardi, R.; Cortes, F.; Narea, C.; Metz, C.; Godoy, G.; Eugenin, J. Age-Related Changes in Neuron–Microglia Interaction Mediated by Fractalkine Under Inflammatory Conditions. Int. J. Mol. Sci. 2025, 26, 11378. https://doi.org/10.3390/ijms262311378
von Bernhardi R, Cortes F, Narea C, Metz C, Godoy G, Eugenin J. Age-Related Changes in Neuron–Microglia Interaction Mediated by Fractalkine Under Inflammatory Conditions. International Journal of Molecular Sciences. 2025; 26(23):11378. https://doi.org/10.3390/ijms262311378
Chicago/Turabian Stylevon Bernhardi, Rommy, Franchesca Cortes, Claudia Narea, Claudia Metz, Gaston Godoy, and Jaime Eugenin. 2025. "Age-Related Changes in Neuron–Microglia Interaction Mediated by Fractalkine Under Inflammatory Conditions" International Journal of Molecular Sciences 26, no. 23: 11378. https://doi.org/10.3390/ijms262311378
APA Stylevon Bernhardi, R., Cortes, F., Narea, C., Metz, C., Godoy, G., & Eugenin, J. (2025). Age-Related Changes in Neuron–Microglia Interaction Mediated by Fractalkine Under Inflammatory Conditions. International Journal of Molecular Sciences, 26(23), 11378. https://doi.org/10.3390/ijms262311378







