Functional Characterization of VS-186B, a Novel HDAC Inhibitor with Anticancer Activity
Abstract
1. Introduction
2. Results
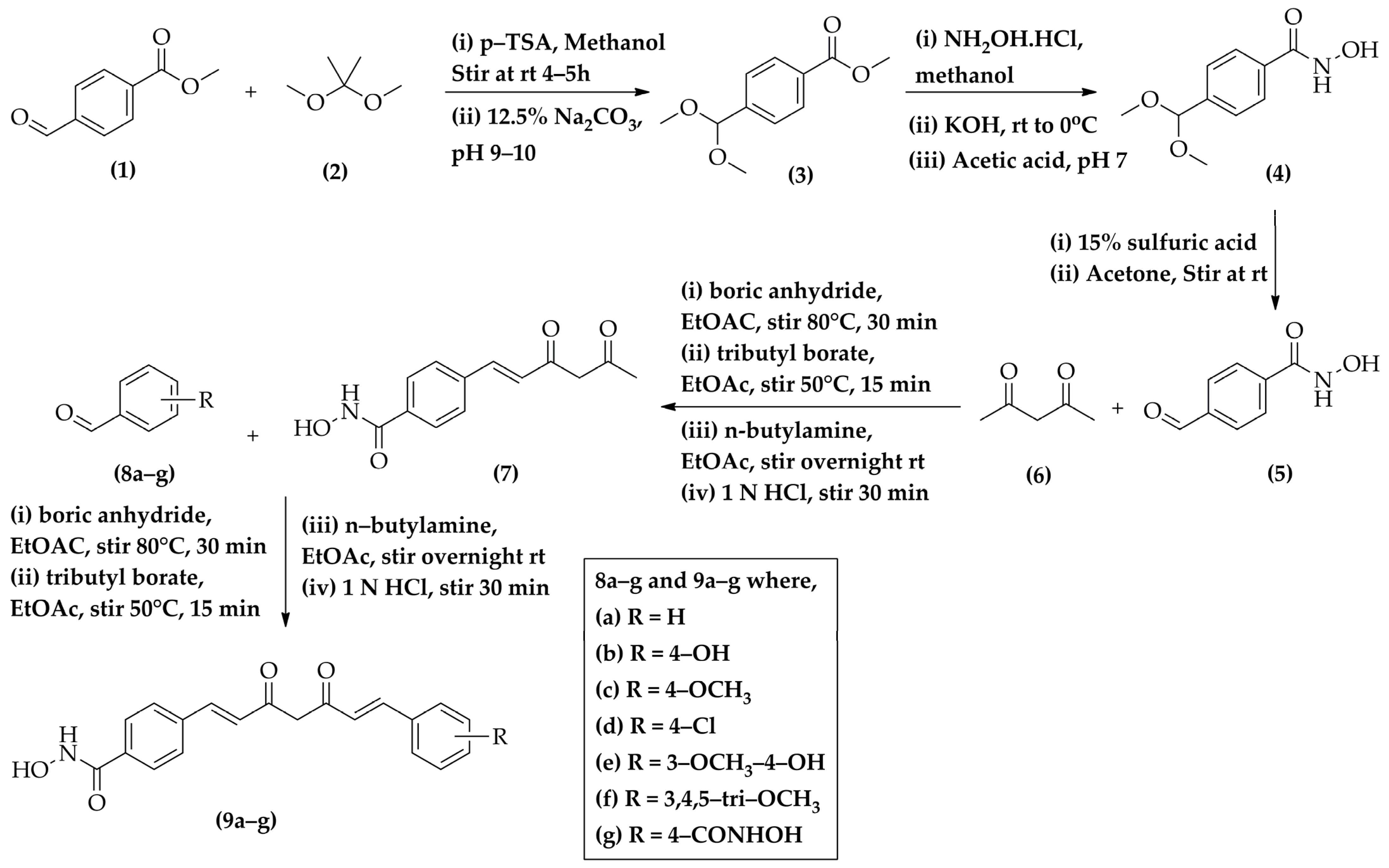
2.1. Chemistry
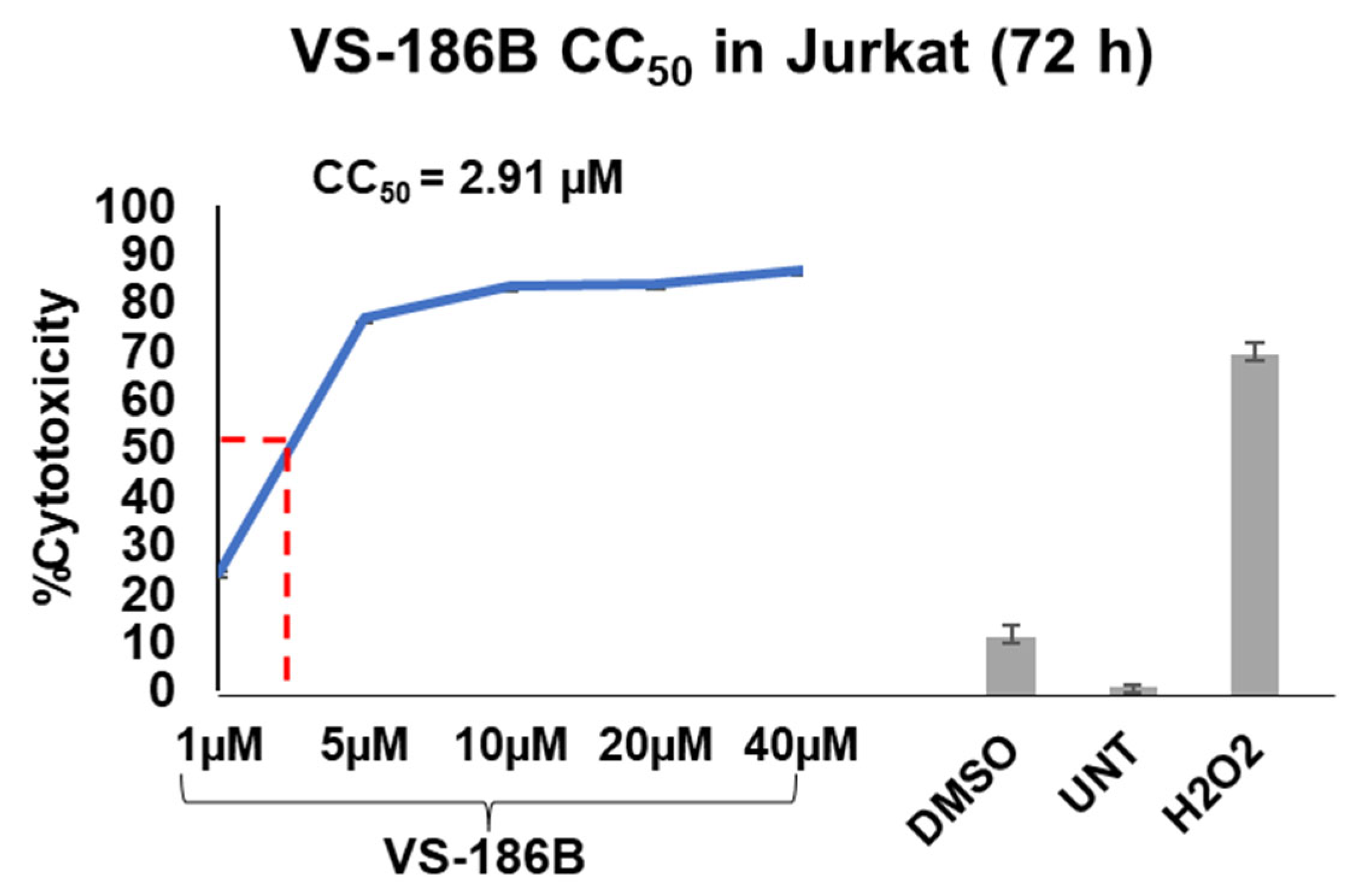
2.2. VS-186B Exhibits Potent Cytotoxicity and Selectivity Against Hematological and Solid Tumor Cell Lines
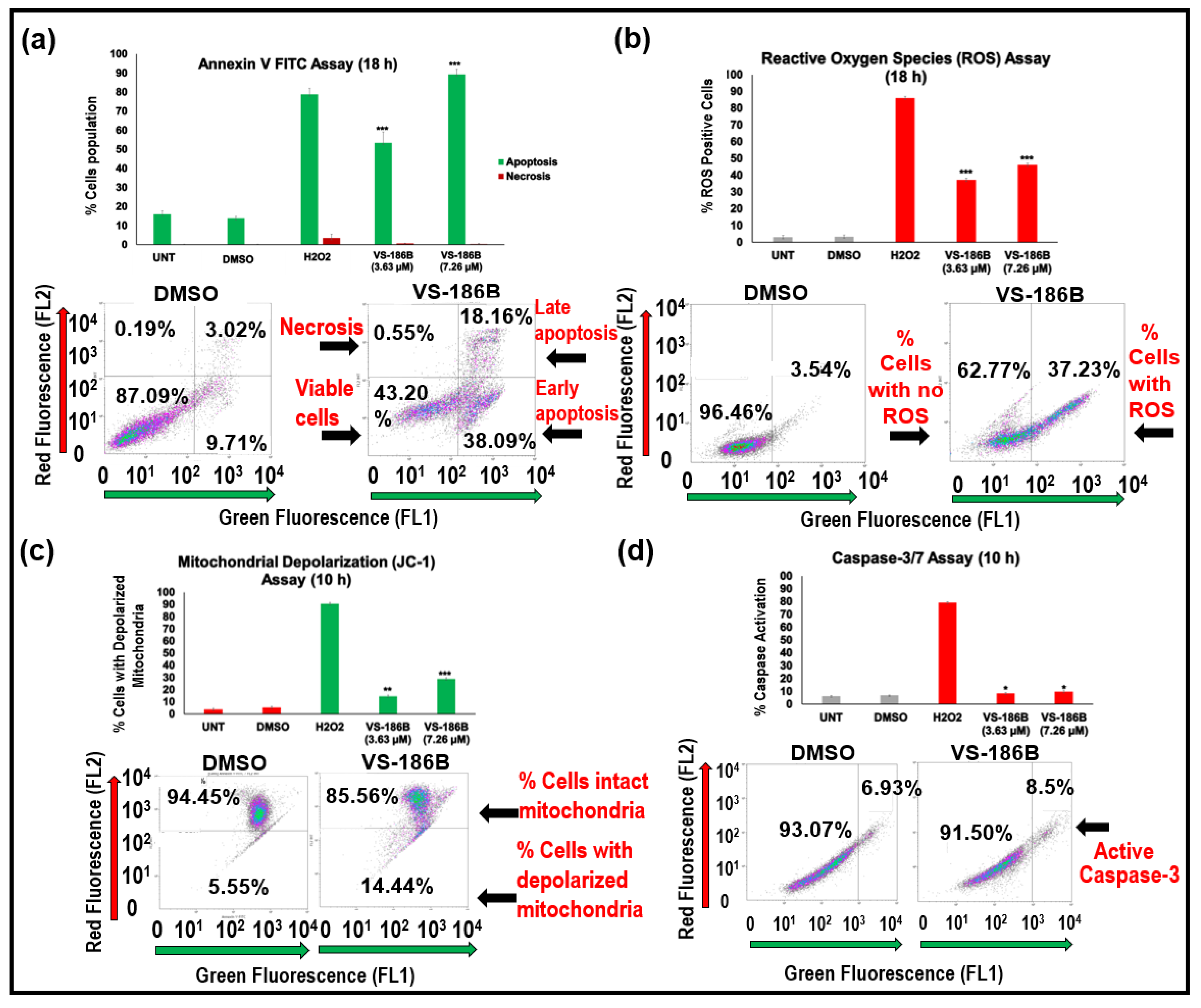
2.3. VS-186B Induces Intrinsic Apoptotic Cell Death
2.4. VS-186B Does Not Alter Cell Cycle Progression
2.5. VS-186B Displays Similar Transcriptional Profile to Known HDAC Inhibitors
2.6. VS-186B Inhibits HDAC Enzymatic Activity in a Dose-Dependent Manner
3. Discussion
4. Materials and Methods
4.1. Chemicals and Instruments
4.2. General Procedure for Synthesis of Methyl 4-(Dimethoxymethyl)benzoate (3)
4.3. General Procedure for the Synthesis of 4-(Dimethoxymethyl)-N-hydroxybenzamide (4)
4.4. General Procedure for the Synthesis of 4-Formyl-N-hydroxybenzamide (5)
4.5. General Procedure for the Synthesis of 4-(3,5-Dioxohex-1-en-1-yl)-N-hydroxybenzamide (7)
4.6. General Procedure for the Synthesis of N-Hydroxy-4-((1E,6E)-7-(substitutedphenyl)-3,5-dioxohepta-1,6-dien-1-yl)benzamide (9a–g)
4.6.1. N-Hydroxy-4-((1E,3Z,6E)-3-hydroxy-5-oxo-7-phenylhepta-1,3,6-trien-1-yl)benzamide (9a/VS-186C)
4.6.2. N-Hydroxy-4-((1E,3Z,6E)-3-hydroxy-7-(4-hydroxyphenyl)-5-oxohepta-1,3,6-trien-1-yl)benzamide (9b/VS-186B)
4.6.3. N-Hydroxy-4-((1E,6E)-7-(4-methoxyphenyl)-3,5-dioxohepta-1,6-dien-1-yl)benzamide (9c/VS-183A)
4.6.4. 4-((1E,6E)-7-(4-Chlorophenyl)-3,5-dioxohepta-1,6-dien-1-yl)-N-hydroxybenzamide (9d/VS-183D)
4.6.5. N-Hydroxy-4-((1E,3Z,6E)-3-hydroxy-7-(4-hydroxy-3-methoxyphenyl)-5-oxohepta-1,3,6-trien-1-yl)benzamide (9e/VS-186A)
4.6.6. N-Hydroxy-4-((1E,3Z,6E)-3-hydroxy-5-oxo-7-(3,4,5-trimethoxyphenyl)hepta-1,3,6-trien-1-yl)benzamide (9f/VS-186E)
4.6.7. 4,4′-((1E,3Z,6E)-3-Hydroxy-5-oxohepta-1,3,6-triene-1,7-diyl)bis(N-hydroxybenzamide) (9g/VS-169B)
4.7. Cell Culture
4.8. Differential Nuclear Staining Assay
4.9. Annexin V FITC Assay
4.10. Reactive Oxygen Species (ROS) Assay
4.11. JC-1 Mitochondrial Depolarization Assay
4.12. Caspase-3/7 Assay
4.13. Cell Cycle Analysis
4.14. Transcriptomic Analysis
4.15. Connectivity Map (CMap) Analysis
4.16. HDAC Inhibition Assay
4.17. Selective Cytotoxic Index (SCI) Calculation
4.18. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HDACs | histone deacetylases |
| DNS | Differential Nuclear Staining |
| RNA | Ribonucleic acid |
| SCI | Selective Cytotoxicity Index |
| ROS | Reactive oxygen species |
| JC-1 | 5,5′,6,6′-Tetrachloro-1,1′,3,3′-tetraethylbenzimidazolocarbocyanine iodide |
| CMap | Connectivity Map |
| DNA | Deoxyribonucleic acid |
| SAHA | Vorinostat |
| FDA | Food and Drug Administration |
| NCEs | new chemical entities |
| IUPAC | International Union of Pure and Applied Chemistry |
| GF254 | SILICA GEL GF 254 |
| TLC | thin-layer chromatography |
| FTIR | Fourier Transform Infrared Spectroscopy |
| NMR | Nuclear Magnetic Resonance |
| DMSO-d6 | Deuterated dimethyl sulfoxide |
| CDCl3 | deuterated chloroform |
| TMS | Tetramethylsilane |
| HR-MS | High-Resolution Mass Spectrometry |
| HCl | Hydrochloric acid |
| DMF | Dimethylformamide |
| EtOH | Ethanol |
| KBr | Potassium bromide |
| RPMI | Roswell Park Memorial Institute |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| FBS | fetal bovine serum |
| CO2 | Carbon dioxide |
| PI | Propidium Iodide |
| H2O2 | hydrogen peroxide |
| FITC | Fluorescein Isothiocyanate |
| DCFDA | 6-carboxy-2′,7′-dichlorodihydrofluorescein diacetate |
| PBS | Phosphate-Buffered Saline |
| CC50 | 50% cytotoxic concentration |
| IC50 | half-maximal inhibitory concentration |
| mol | Mole |
| mL | Milli Litre |
| min | Minute |
| °C | Degree Celsius |
| b.p. | Boiling point |
| m.p. | Melting point |
| rt | Room temperature |
| pH | potential of Hydrogen |
| w/v | weight by volume |
| mM | Milli molar |
| % | Percentage |
| ppm | Parts per million |
| h | Hour |
| µM | Micro molar |
| SD | Standard deviation |
| TSA | Trichostatin A |
| EMT | epithelial-to-mesenchymal transition |
| HIA | high gastrointestinal absorption |
| CNS | Central Nervous system |
| P-gp | P-glycoprotein |
References
- Paro, P.D.R.; Grossniklaus, P.D.U.; Santoro, D.R.; Wutz, P.D.A. Epigenetics and Cancer. In Introduction to Epigenetics [Internet]; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Jenke, R.; Reßing, N.; Hansen, F.K.; Aigner, A.; Büch, T. Anticancer Therapy with HDAC Inhibitors: Mechanism-Based Combination Strategies and Future Perspectives. Cancers 2021, 13, 634. [Google Scholar] [CrossRef] [PubMed]
- Morrison, O.; Thakur, J. Molecular Complexes at Euchromatin, Heterochromatin and Centromeric Chromatin. Int. J. Mol. Sci. 2021, 22, 6922. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Seto, E. HDACs and HDAC Inhibitors in Cancer Development and Therapy. Cold Spring Harb. Perspect. Med. 2016, 6, a026831. [Google Scholar] [CrossRef]
- Lv, Z.; Ji, T.; Liu, J.; Sun, X.; Liang, H. Synthetic Approaches and Clinical Applications of Representative HDAC Inhibitors for Cancer Therapy: A Review. Eur. J. Med. Chem. 2025, 283, 117185. [Google Scholar] [CrossRef]
- Karagiannis, D.; Rampias, T. HDAC Inhibitors: Dissecting Mechanisms of Action to Counter Tumor Heterogeneity. Cancers 2021, 13, 3575. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Su, R.; Liu, J.; Chen, K.; Wu, C.; Sun, P.; Sun, T. Tubulin/HDAC Dual-Target Inhibitors: Insights from Design Strategies, SARs, and Therapeutic Potential. Eur. J. Med. Chem. 2025, 281, 117022. [Google Scholar] [CrossRef]
- Juthani, R.; Punatar, S.; Mittra, I. New Light on Chemotherapy Toxicity and Its Prevention. BJC Rep. 2024, 2, 41. [Google Scholar] [CrossRef]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Adv. Pharm. Bull. 2017, 7, 339–348. [Google Scholar] [CrossRef]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer Chemotherapy and beyond: Current Status, Drug Candidates, Associated Risks and Progress in Targeted Therapeutics. Genes Dis. 2023, 10, 1367–1401, Erratum in Genes Dis. 2024, 11, 101211. [Google Scholar] [CrossRef]
- Saeed, M.E.M.; Yücer, R.; Dawood, M.; Hegazy, M.-E.F.; Drif, A.; Ooko, E.; Kadioglu, O.; Seo, E.-J.; Kamounah, F.S.; Titinchi, S.J.; et al. In Silico and In Vitro Screening of 50 Curcumin Compounds as EGFR and NF-κB Inhibitors. Int. J. Mol. Sci. 2022, 23, 3966. [Google Scholar] [CrossRef]
- Mazumder, K.; Aktar, A.; Roy, P.; Biswas, B.; Hossain, M.E.; Sarkar, K.K.; Bachar, S.C.; Ahmed, F.; Monjur-Al-Hossain, A.S.M.; Fukase, K. A Review on Mechanistic Insight of Plant Derived Anticancer Bioactive Phytocompounds and Their Structure Activity Relationship. Molecules 2022, 27, 3036. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Hegde, M.; Parama, D.; Girisa, S.; Kumar, A.; Daimary, U.D.; Garodia, P.; Yenisetti, S.C.; Oommen, O.V.; Aggarwal, B.B. Role of Turmeric and Curcumin in Prevention and Treatment of Chronic Diseases: Lessons Learned from Clinical Trials. ACS Pharmacol. Transl. Sci. 2023, 6, 447–518. [Google Scholar] [CrossRef]
- Kelkel, M.; Jacob, C.; Dicato, M.; Diederich, M. Potential of the Dietary Antioxidants Resveratrol and Curcumin in Prevention and Treatment of Hematologic Malignancies. Molecules 2010, 15, 7035–7074. [Google Scholar] [CrossRef]
- Tiwari, H.; Rao, M.V. Curcumin Supplementation Protects from Genotoxic Effects of Arsenic and Fluoride. Food Chem. Toxicol. 2010, 48, 1234–1238. [Google Scholar] [CrossRef]
- Allegra, A.; Mirabile, G.; Ettari, R.; Pioggia, G.; Gangemi, S. The Impact of Curcumin on Immune Response: An Immunomodulatory Strategy to Treat Sepsis. Int. J. Mol. Sci. 2022, 23, 14710. [Google Scholar] [CrossRef] [PubMed]
- Razali, N.S.C.; Lam, K.W.; Rajab, N.F.; Jamal, A.R.A.; Kamaluddin, N.F.; Chan, K.M. Curcumin Piperidone Derivatives Induce Anti-Proliferative and Anti-Migratory Effects in LN-18 Human Glioblastoma Cells. Sci. Rep. 2022, 12, 13131. [Google Scholar] [CrossRef]
- Dei Cas, M.; Ghidoni, R. Dietary Curcumin: Correlation between Bioavailability and Health Potential. Nutrients 2019, 11, 2147. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Smart, J.D.; Pannala, A.S. Recent Developments in Formulation Design for Improving Oral Bioavailability of Curcumin: A Review. J. Drug Deliv. Sci. Technol. 2020, 60, 102082. [Google Scholar] [CrossRef]
- Chakraborti, S.; Dhar, G.; Dwivedi, V.; Das, A.; Poddar, A.; Chakraborti, G.; Basu, G.; Chakrabarti, P.; Surolia, A.; Bhattacharyya, B. Stable and Potent Analogues Derived from the Modification of the Dicarbonyl Moiety of Curcumin. Biochemistry 2013, 52, 7449–7460. [Google Scholar] [CrossRef]
- Kaur, K.; Al-Khazaleh, A.K.; Bhuyan, D.J.; Li, F.; Li, C.G. A Review of Recent Curcumin Analogues and Their Antioxidant, Anti-Inflammatory, and Anticancer Activities. Antioxidants 2024, 13, 1092. [Google Scholar] [CrossRef] [PubMed]
- Teli, S.K.; Suvarna, V.M.; Abhyankar, A.N. Monocarbonyl Modifications of Curcumin: An Overview of Structure–Activity Relationship Study as Anti-Infective Agents. Chem. Pap. 2025, 79, 5593–5607. [Google Scholar] [CrossRef]
- Le, V.K.H.; Pham, T.P.D.; Truong, D.H. Delivery Systems for Vorinostat in Cancer Treatment: An Updated Review. J. Drug Deliv. Sci. Technol. 2021, 61, 102334. [Google Scholar] [CrossRef]
- Squarzoni, A.; Scuteri, A.; Cavaletti, G. HDACi: The Columbus’ Egg in Improving Cancer Treatment and Reducing Neurotoxicity? Cancers 2022, 14, 5251. [Google Scholar] [CrossRef] [PubMed]
- Robles-Escajeda, E.; Das, U.; Ortega, N.M.; Parra, K.; Francia, G.; Dimmock, J.R.; Varela-Ramirez, A.; Aguilera, R.J. A Novel Curcumin-like Dienone Induces Apoptosis in Triple-Negative Breast Cancer Cells. Cell. Oncol. 2016, 39, 265–277. [Google Scholar] [CrossRef]
- Rodrigues, D.A.; Ferreira-Silva, G.À.; Ferreira, A.C.S.; Fernandes, R.A.; Kwee, J.K.; Sant’Anna, C.M.R.; Ionta, M.; Fraga, C.A.M. Design, Synthesis, and Pharmacological Evaluation of Novel N-Acylhydrazone Derivatives as Potent Histone Deacetylase 6/8 Dual Inhibitors. J. Med. Chem. 2016, 59, 655–670. [Google Scholar] [CrossRef] [PubMed]
- Khan, O.; La Thangue, N.B. HDAC Inhibitors in Cancer Biology: Emerging Mechanisms and Clinical Applications. Immunol. Cell Biol. 2012, 90, 85–94. [Google Scholar] [CrossRef]
- Minchinton, A.I.; Tannock, I.F. Drug Penetration in Solid Tumours. Nat. Rev. Cancer 2006, 6, 583–592. [Google Scholar] [CrossRef]
- Saggar, J.K.; Yu, M.; Tan, Q.; Tannock, I.F. The Tumor Microenvironment and Strategies to Improve Drug Distribution. Front. Oncol. 2013, 3, 154. [Google Scholar] [CrossRef]
- Choi, I.-K.; Strauss, R.; Richter, M.; Yun, C.-O.; Lieber, A. Strategies to Increase Drug Penetration in Solid Tumors. Front. Oncol. 2013, 3, 193. [Google Scholar] [CrossRef] [PubMed]
- Simsek, H.; Klotzsch, E. The Solid Tumor Microenvironment—Breaking the Barrier for T Cells. BioEssays 2022, 44, 2100285. [Google Scholar] [CrossRef]
- Sato, H.; Hara, T.; Meng, S.; Tsuji, Y.; Arao, Y.; Saito, Y.; Sasaki, K.; Kobayashi, S.; Doki, Y.; Eguchi, H.; et al. Multifaced Roles of Desmoplastic Reaction and Fibrosis in Pancreatic Cancer Progression: Current Understanding and Future Directions. Cancer Sci. 2023, 114, 3487–3495. [Google Scholar] [CrossRef]
- Tripathi, M.; Billet, S.; Bhowmick, N.A. Understanding the Role of Stromal Fibroblasts in Cancer Progression. Cell Adhes. Migr. 2012, 6, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhang, Y.; Dai, H.; Han, B. Epithelial–Mesenchymal Transition-Mediated Tumor Therapeutic Resistance. Molecules 2022, 27, 4750. [Google Scholar] [CrossRef] [PubMed]
- Lema, C.; Varela-Ramirez, A.; Aguilera, R.J. Differential Nuclear Staining Assay for High-Throughput Screening to Identify Cytotoxic Compounds. Curr. Cell. Biochem. 2011, 1, 1–14. [Google Scholar] [PubMed]
- Varela-Ramirez, A.; Costanzo, M.; Carrasco, Y.P.; Pannell, K.H.; Aguilera, R.J. Cytotoxic Effects of Two Organotin Compounds and Their Mode of Inflicting Cell Death on Four Mammalian Cancer Cells. Cell Biol. Toxicol. 2011, 27, 159–168. [Google Scholar] [CrossRef] [PubMed]
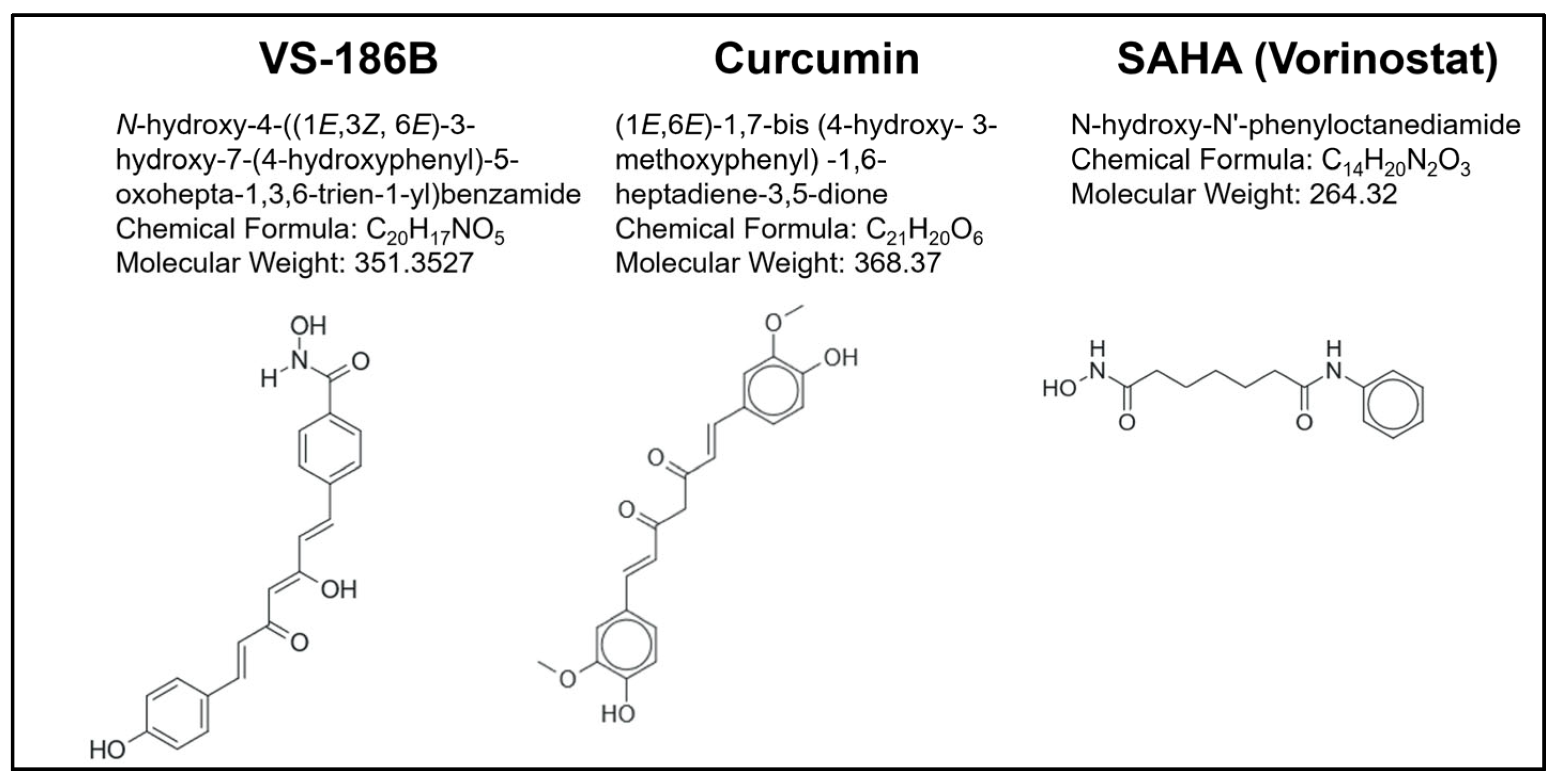



| CC50 Values in Hematological Cancer Cell Lines at 48 h (µM) * | ||||
|---|---|---|---|---|
| Cell Line | Cell Type | VS-186B | Curcumin | Vorinostat |
| CEM | T-Lymphoblast | 3.18 ± 0.25 | 16.80 ± 2.88 | 7.55 ± 3.13 |
| Ramos | B-Lymphocyte | 4.17 ± 1.35 | 9.09 ± 1.48 | 5.16 ± 1.78 |
| Jurkat | T-Lymphocyte | 4.86 ± 0.15 | 28.96 ± 2.72 | 16.82 ± 1.66 |
| HL-60 | Leukemia | 4.91 ± 0.22 | 13.53 ± 1.24 | 11.36 ± 1.46 |
| NALM6 | Precursor B-Cell Leukemia | 5.00 ± 0.70 | 7.12 ± 0.40 | 25.95 ± 2.60 |
| KMS-11 | Multiple Myeloma | 7.31 ± 1.03 | 6.72 ± 2.79 | 16.82 ± 1.66 |
| MM.1S | Multiple Myeloma | 8.52 ± 0.47 | 6.59 ± 0.29 | 14.26 ± 1.07 |
| CC50 Values in Hematological Cancer Cell Lines at 72 h (µM) | ||||
| Cell Line | Cell Type | VS-186B | Curcumin | Vorinostat |
| Jurkat | T-Lymphocyte | 2.91 ± 0.50 | 19.01 ± 1.39 | 9.32 ± 0.31 |
| CEM | T-Lymphoblast | 4.55 ± 0.73 | 20.63 ± 1.63 | 7.92 ± 2.06 |
| MM.1S | Multiple Myeloma | 4.71 ± 0.11 | 5.65 ± 0.35 | 12.29 ± 1.27 |
| CC50 Values in Adherent Cancer Cell Lines at 72 h (µM) | ||||
| Cell Line | Cell Type | VS-186B | Curcumin | Vorinostat |
| MCF-7 | ER+ Breast Cancer | 3.18 ± 0.25 | 16.80 ± 2.88 | 7.55 ± 3.13 |
| T-47D | ER+ Breast Cancer | 5.00 ± 0.70 | 7.12 ± 0.40 | 25.95 ± 2.6 |
| MDA-MB-231-LM2-4 | TNBC | 4.17 ± 1.35 | 9.09 ± 1.48 | 5.16 ±1.78 |
| MDA-MB-231 | TNBC | 6.99 ± 0.15 | 21.29 ± 0.90 | 22.85 ± 0.27 |
| MDA-MB-468 | TNBC | 8.53 ± 0.58 | 24.77 ± 0.52 | 24.21 ± 0.40 |
| HEP G2 | Liver Cancer | 4.86 ± 0.15 | 28.96 ± 2.72 | 16.82 ± 1.66 |
| A549 | Lung Cancer | 7.29 ± 0.44 | 26.02 ± 1.61 | 8.58 ± 0.15 |
| PANC-1 | Pancreatic Cancer | 8.85 ± 0.90 | 20.25 ± 3.21 | 5.22 ± 0.50 |
| Caco-2 | Colorectal Cancer | 22.19 ± 2.07 | 24.14 ± 1.42 | 5.09 ± 0.69 |
| CC50 Values of VS-186B in Non-Cancerous Cells at 72 h | ||
|---|---|---|
| Cell Line | Cell Type | VS-186B (µM) * |
| Hs27 | Foreskin fibroblast | 46.82 ± 1.26 |
| MCF 10A | Breast epithelial | 4.37 ± 0.73 |
| SCI of VS-186B in Cancer Cells at 72 h | ||
| Cell Line | Cell Type | VS-186B (µM) * |
| CEM | T-Lymphoblast | 10.29 |
| Jurkat | T-Lymphocyte | 16.08 |
| MM.1S | Multiple Myeloma | 9.94 |
| MCF-7 | Breast Cancer (ER+) | 1.37 |
| T-47D | Breast Cancer (ER+) | 0.87 |
| MDA-MB-231-LM2-4 | Triple Negative Breast Cancer (TNBC) | 1.04 |
| MDA-MB-231 | TNBC | 0.62 |
| MDA-MB-468 | TNBC | 0.51 |
| HEP G2 | Liver Cancer | 9.63 |
| A549 | Lung Cancer | 6.42 |
| PANC-1 | Pancreatic Cancer | 5.29 |
| Caco-2 | Colorectal Cancer | 2.10 |
| Rank | Score | Name | Description |
|---|---|---|---|
| 1 | 99.15 | THM-I-94 | HDAC inhibitor |
| 2 | 99.08 | WT-171 | HDAC inhibitor |
| 3 | 99.08 | ISOX | HDAC inhibitor |
| 4 | 99.01 | Trichostatin-A | HDAC inhibitor |
| 5 | 99.01 | Pyroxamide | HDAC inhibitor |
| 6 | 99.01 | Droxinostat | HDAC inhibitor |
| 7 | 99.01 | Belinostat | HDAC inhibitor |
| 8 | 99.00 | Scriptaid | HDAC inhibitor |
| 9 | 98.98 | Givinostat | HDAC inhibitor |
| 10 | 98.98 | NCH-51 | HDAC inhibitor |
| 11 | 98.98 | Vorinostat | HDAC inhibitor |
| 12 | 98.94 | HC-toxin | HDAC inhibitor |
| 13 | 98.94 | APHA-compound-8 | HDAC inhibitor |
| 14 | 98.87 | Dacinostat | HDAC inhibitor |
| 15 | 98.66 | Panobinostat | HDAC inhibitor |
| 16 | 98.66 | BI-2536 | PLK inhibitor |
| 17 | 98.52 | Apicidin | HDAC inhibitor |
| 18 | 98.33 | NVP-AUY922 | HSP inhibitor |
| 19 | 98.24 | Piperlongumine | Glutathione transferase inhibitor |
| 20 | 98.10 | HSP90-inhibitor | HSP inhibitor |
| Code | IR (cm−1) | 1H-NMR (δ ppm) | 13C-NMR (δ ppm) | HRMS Calc. Mass (Obs. Mass) -MS (ESI) m/z |
|---|---|---|---|---|
| 9a (VS186C) | 3289, 3113, 1708, 1643, 1428, 1189, 1165 | 6.19 (s, 1H, enolic OH), 6.94–7.04 (m, 2H, ethylene), 7.41–7.44 (m, 3H, Ar, J = 12), 7.62–7.65 (m, 2H, Ar), 7.71–7.79 (m, 7H, Ar), 9.08 (s, 1H, NH), 11.28 (s, 1H, OH) | 184.58, 182.88, 164.03, 141.31, 139.66, 129.57, 129.54, 128.99, 128.82, 128.00, 126.30, 124.92, 102.71 | 335.1158 (334.1088) |
| 9b (VS186B) | 3109, 2850, 1707, 1605, 1522, 1346, 1197, 1105 | 6.10 (s, 1H, enolic OH), 6.70–7.00 (m, 4H, Ar), 7.53–7.63 (m, 4H, Ar), 7.71–7.82 (m, 5H, Ar), 9.08 (s, 1H, NH), 10.08 (s, 1H, Ar-OH), 11.28 (s, 1H, OH) | 191.52, 186.12, 181.06, 164.04, 163.84, 160.59, 142.08, 138.82, 137.92, 134.20, 132.64, 131.15, 128.95, 128.68, 128.55, 127.97, 126.26, 126.18, 121.41, 116.47, 116.36, 102.38 | 351.1107 (350.1026) |
| 9c (VS183A) | 3280, 3028, 2914, 1694, 1624, 1498, 1281, 1183, 1142, 1114 | 3.79 (s, 3H, OCH3), 6.14 (s, 1H, enolic OH), 6.83 (d, 1H, J = 12), 6.98–7.02 (m, 3H, Ar), 7.59–7.64 (m, 2H, Ar), 7.70 (d, 2H, Ar, J = 8), 7.74–7.79 (m, 4H, Ar), 7.86 (s, 1H, Ar), 9.08 (s, 1H, NH), 11.29 (s, 1H, OH) | 185.78, 181.69, 163.93, 161.60, 141.69, 139.23, 137.78, 130.84, 130.27, 128.75, 127.81, 127.23, 126.22, 122.46, 115.08, 102.38, 55.89 | 365.1263 (364.1147) |
| 9d (VS183D) | 3394, 3106, 3046, 2849, 1707, 1605, 1198, 1105 | 6.18 (s, 1H, enolic OH), 6.95–7.05 (m, 2H, Ar), 7.50 (d, 2H, Ar, J = 4), 7.61–7.66 (m, 2H, Ar), 7.75–7.79 (m, 7H, Ar), 9.10 (s, 1H, NH), 11.30 (s, 1H, OH) | 184.02, 183.24, 164.00, 139.80, 139.74, 135.39, 134.14, 130.63, 129.59, 128.84, 127.99, 126.29, 125.62, 102.86 | 369.0768 (368.0645) |
| 9e (VS186A) | 3304, 3019, 2938, 1636, 1570, 1286, 1159 | 3.84 (s, 3H, OCH3), 6.15 (s, 1H, enolic OH), 6.81–6.84 (m, 1H, Ar), 6.90–7.05 (m, 2H, Ar), 7.19 (d, 1H, Ar, J = 8), 7.35 (s, 1H, Ar), 7.59–7.63 (m, 2H, Ar), 7.77–7.82 (m, 6H, Ar), 9.12 (s, 1H, NH), 9.74 (s, 1H, Ar-OH), 11.32 (s, 1H, OH) | 186.09, 181.35, 164.12, 150.34, 148.83, 142.58, 138.92, 134.40, 128.80, 127.94, 126.86, 124.28, 121.69, 116.31, 112.00, 102.31, 56.00 | 381.1212 (380.1132) |
| 9f (VS186E) | 3010, 2967, 1683, 1586, 1234, 1127 | 3.67 (s, 3H, OCH3), 3.80 (s, 6H, OCH3), 6.15 (s, 1H, enolic OH), 6.86–7.06 (m, 4H, Ar), 7.52–7.64 (m, 2H), 7.71–7.77 (m, 5H, Ar), 9.08 (s, 1H, NH), 11.28 (s, 1H, OH) | 199.73, 184.82, 182.52, 176.23, 163.94, 153.65, 141.70, 140.06, 137.81, 130.73, 128.79, 128.53, 127.98, 127.95, 126.32, 124.21, 106.54, 102.50, 102.39, 60.68, 56.56 | 425.1475 (424.1395) |
| 9g (VS169B) | 3533, 3157, 3026, 2961, 1708, 1613, 1420, 1206, 1160 | 6.23 (s, 1H, enolic OH), 7.09 (d, 2H, ethylene, J = 16), 7.70 (d, 2H, Ar, J = 16), 7.81 (s, 9H, Ar), 9.13 (s, 2H, NH), 11.32 (s, 2H, OH) | 183.09, 163.48, 139.42, 137.20, 133.90, 129.82, 128.33, 127.45, 125.80, 102.43 | 394.1165 (393.1084) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez-Michael, L.A.; Sudarshan, V.; Elias, A.; Gutierrez, D.A.; Lopez-Saenz, J.A.; Pena-Zacarias, J.; Torres, G.C.; Varela-Ramirez, A.; Kumar, S.; Karki, S.S.; et al. Functional Characterization of VS-186B, a Novel HDAC Inhibitor with Anticancer Activity. Int. J. Mol. Sci. 2025, 26, 11354. https://doi.org/10.3390/ijms262311354
Sanchez-Michael LA, Sudarshan V, Elias A, Gutierrez DA, Lopez-Saenz JA, Pena-Zacarias J, Torres GC, Varela-Ramirez A, Kumar S, Karki SS, et al. Functional Characterization of VS-186B, a Novel HDAC Inhibitor with Anticancer Activity. International Journal of Molecular Sciences. 2025; 26(23):11354. https://doi.org/10.3390/ijms262311354
Chicago/Turabian StyleSanchez-Michael, Laura A., Vijayalakshmi Sudarshan, Allison Elias, Denisse A. Gutierrez, Jose A. Lopez-Saenz, Jaqueline Pena-Zacarias, Gabriela C. Torres, Armando Varela-Ramirez, Sujeet Kumar, Subhas S. Karki, and et al. 2025. "Functional Characterization of VS-186B, a Novel HDAC Inhibitor with Anticancer Activity" International Journal of Molecular Sciences 26, no. 23: 11354. https://doi.org/10.3390/ijms262311354
APA StyleSanchez-Michael, L. A., Sudarshan, V., Elias, A., Gutierrez, D. A., Lopez-Saenz, J. A., Pena-Zacarias, J., Torres, G. C., Varela-Ramirez, A., Kumar, S., Karki, S. S., & Aguilera, R. J. (2025). Functional Characterization of VS-186B, a Novel HDAC Inhibitor with Anticancer Activity. International Journal of Molecular Sciences, 26(23), 11354. https://doi.org/10.3390/ijms262311354











