Accessing Altered Metabolic Profile in Acute Deep Vein Thrombosis Through Nuclear Magnetic Resonance Spectroscopy
Abstract
1. Introduction
2. Results
2.1. Baseline Characteristics of the Study Population
2.2. Acute DVT Patients Exhibit Elevated Inflammatory Cytokines
2.3. Pro-Inflammatory Cytokines Are Decreased at DVT > 6 Months, but Adhesion Molecules Are Elevated
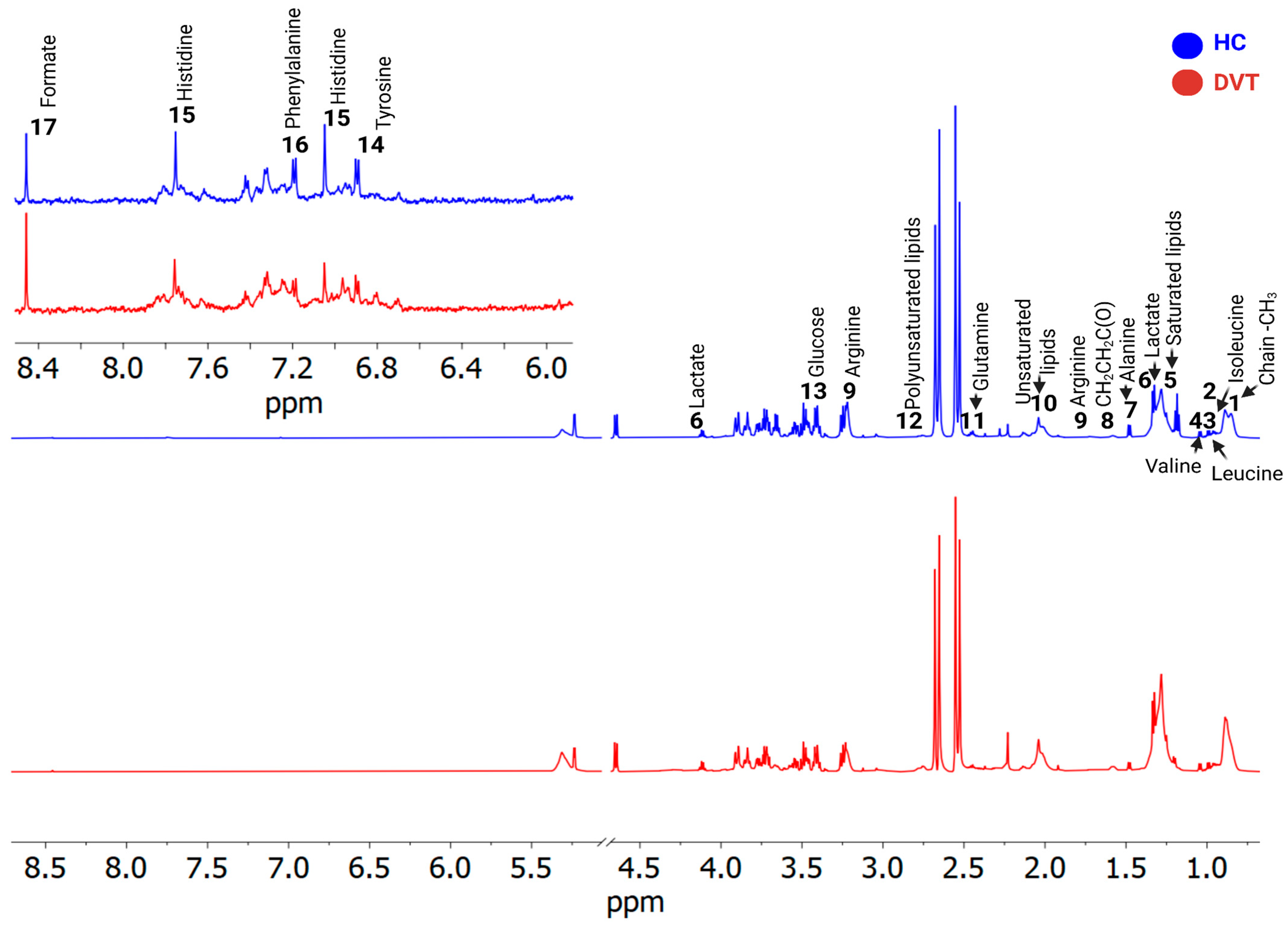
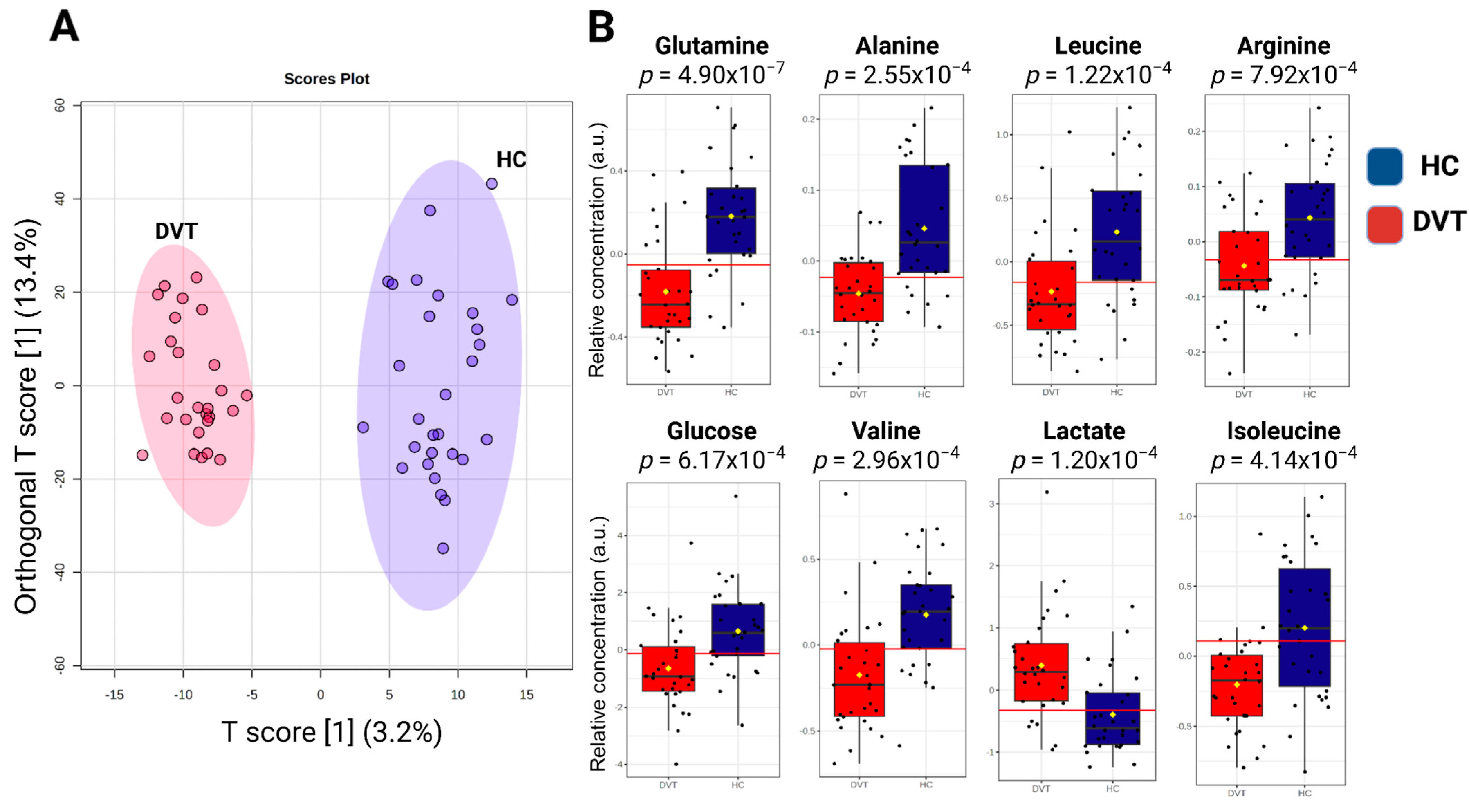
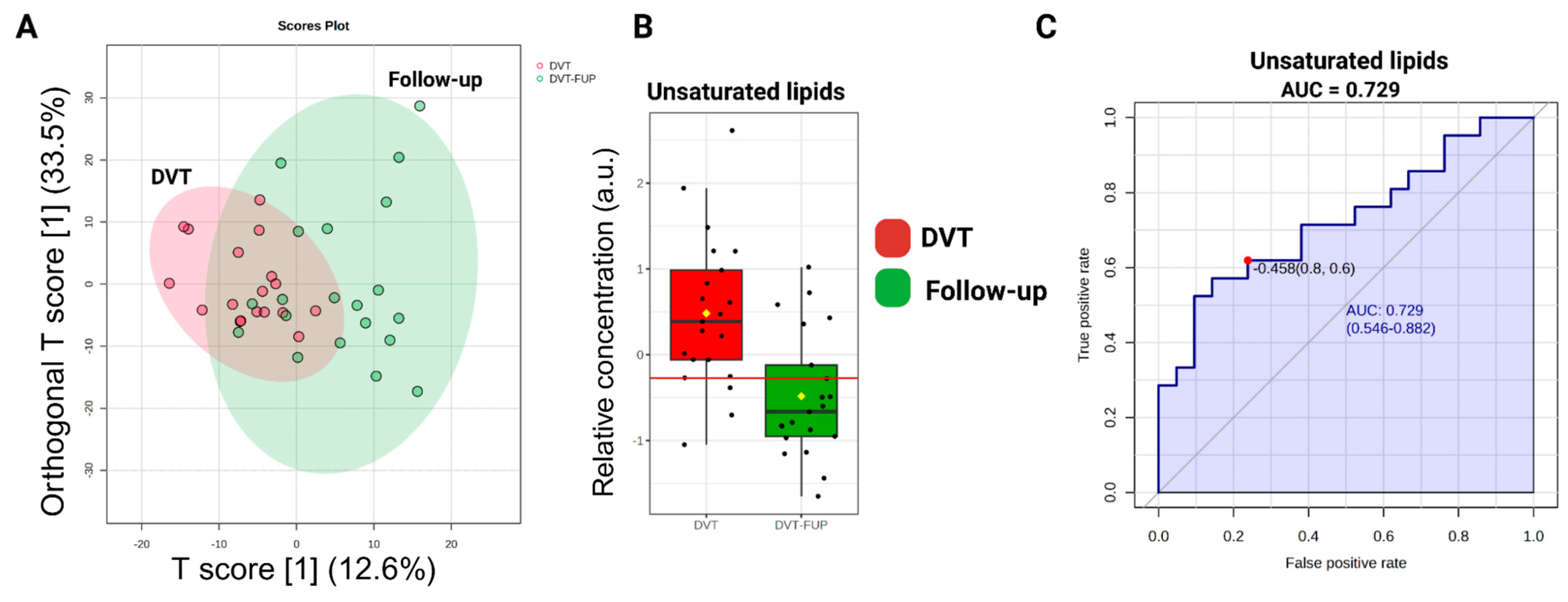
2.4. Metabolomic Data Analysis and Multivariate Statistical Analysis
3. Discussion
Limitations and Strengths of the Study
4. Methods
4.1. Study Population
4.2. Blood Collection and Sample Processing
4.3. Evaluation of Plasma Markers
4.4. H-NMR Spectroscopy Analyses
4.5. Data Analysis: NMR Data Processing and Statistics
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 1H-NMR | Proton Nuclear Magnetic Resonance |
| Ala | Alanine |
| Arg | Arginine |
| ATP | Adenosine Triphosphate |
| AUC | Area Under the Curve |
| BCAAs | Branched-Chain Amino Acids |
| BMI | Body Mass Index |
| DNA | Deoxyribonucleic Acid |
| DVT | Deep Venous Thrombosis |
| EC | Endothelial Cells |
| EDTA | Ethylenediaminetetraacetic Acid |
| Gln | Glutamine |
| HC | Healthy Controls |
| HMDB | Human Metabolome Database |
| TOCSY | Total Correlation Spectroscopy experiments |
| ICAM-1 | Intercellular Adhesion Molecule-1 |
| IFN-γ | Interferon-Gamma |
| Ile | Isoleucine |
| IL-1β | Interleukin-1 Beta |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| IL-10 | Interleukin-10 |
| Leu | Leucine |
| Luminex | Multiplex Assay Technology |
| MetaboAnalyst | Metabolomics Data Analysis Tool |
| mtDNA | Mitochondrial DNA |
| PDGF-AB/BB | Platelet-Derived Growth Factor AB/BB |
| PPP | Platelet-Poor Plasma |
| P-selectin | Platelet Activation Marker |
| ROC | Receiver Operating Characteristic |
| ROS | Reactive Oxygen Species |
| sCD40L | Soluble CD40 Ligand |
| sICAM-1 | Soluble Intercellular Adhesion Molecule-1 |
| sVCAM-1 | Soluble Vascular Cell Adhesion Molecule-1 |
| sVEGFR-2 | Soluble Vascular Endothelial Growth Factor Receptor-2 |
| TBI | Triple Resonance Broadband Inverse |
| TSP | Trimethylsilyl Propanoic Acid |
| Val | Valine |
| VTE | Venous Thromboembolism |
| VCAM-1 | Vascular Cell Adhesion Molecule-1 |
References
- Wolberg, A.S.; Rosendaal, F.R.; Weitz, J.I.; Jaffer, I.H.; Agnelli, G.; Baglin, T.; Mackman, N. Venous thrombosis. Nat. Rev. Dis. Primers 2015, 1, 15006. [Google Scholar] [CrossRef]
- Heit, J.A. Epidemiology of venous thromboembolism. Nat. Rev. Cardiol. 2015, 12, 464–474. [Google Scholar] [CrossRef]
- Beckman, M.G.; Hooper, W.C.; Critchley, S.E.; Ortel, T.L. Venous thromboembolism. A public health concern. Am. J. Prev. Med. 2010, 38, S495–S501. [Google Scholar] [CrossRef] [PubMed]
- Næss, I.A.; Christiansen, S.C.; Romundstad, P.; Cannegieter, S.C.; Rosendaal, F.R.; Hammerstrøm, J. Incidence and mortality of venous thrombosis: A population-based study. J. Thromb. Haemost. 2007, 5, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Raskob, G.; Angchaisuksiri, P.; Blanco, A.N.; Buller, H.; Gallus, A.; Hunt, B.J.; Hylek, E.M.; Kakkar, T.L.; Konstantinides, S.V.; McCumber, M.; et al. Thrombosis: A major contributor to global disease burden. Thromb. Haemost. 2014, 12, 1580–1590. [Google Scholar] [CrossRef] [PubMed]
- Rosendaal, F.R. Risk factors for venous thrombotic disease. Thromb. Haemost. 1999, 82, 610–619. [Google Scholar] [CrossRef]
- Rosendaal, F.R. Thrombosis in the young: Epidemiology and risk factors. A focus on venous thrombosis. Thromb. Haemost. 1997, 78, 1–6. [Google Scholar] [CrossRef]
- Rosendaal, F.R. Venous thrombosis: The role of genes, environment, and behavior. Hematol. Am. Soc. Hematol. Educ. Program 2005, 2005, 1–12. [Google Scholar] [CrossRef]
- Pulivarthi, S.; Gurram, M.K. Effectiveness of D-dimer as a screening test for venous thromboembolism: An update. N. Am. J. Med. Sci. 2014, 6, 491–499. [Google Scholar]
- Parakh, R.S.; Sabath, D.E. Venous thromboembolism: Role of the clinical laboratory in diagnosis and management. J. Appl. Lab. Med. 2019, 3, 870–882. [Google Scholar] [CrossRef]
- Bates, S.M.; Jaeschke, R.; Stevens, S.M.; Wells, P.S.; Stevenson, M.D.; Kearon, C.; Goodacre, S.; Schunemann, H.J.; Crowther, M.; Pauker, S.G.; et al. Diagnosis of DVT: Antithrombotic therapy and prevention of thrombosis, 9th ed. Chest 2012, 141, e351S–e418S. [Google Scholar] [CrossRef]
- Righini, M.; Van Es, J.; Den Exter, P.L.; Roy, P.M.; Verschuren, F.; Ghuysen, A.; Rutschmann, O.T.; Sanchez, O.; Jaffrelot, M.; Trinh-Duc, A.; et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: The ADJUST-PE study. J. Am. Med. Assoc. 2014, 311, 1117–1124. [Google Scholar] [CrossRef]
- Nicholson, J.; Lindon, J. Metabonomics. Nature 2008, 455, 1054–1056. [Google Scholar] [CrossRef]
- Franczyk, B.; Gluba-Brzózka, A.; Ławiński, J.; Rysz-Górzyńska, M.; Rysz, J. Metabolomic profile in venous thromboembolism (Vte). Metabolites 2021, 11, 495. [Google Scholar] [CrossRef]
- Escobar, M.Q.; Tasic, L.; da Costa, T.B.B.C.; Stanisic, D.; Montalvão, S.; Huber, S.; Annichino-Bizzacchi, J.M. Serum metabolic profiles based on nuclear magnetic resonance spectroscopy among patients with deep vein thrombosis and healthy controls. Metabolites 2021, 11, 874. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Zeleznik, O.A.; Lindström, S.; Lasky-Su, J.; Hagan, K.; Clish, C.B.; Eliassen, A.H.; Kraft, P.; Kabrhel, C. Metabolites associated with the risk of incident venous thromboembolism: A metabolomic analysis. J. Am. Heart Assoc. 2018, 7, e010317. [Google Scholar] [CrossRef] [PubMed]
- Febra, C.; Saraiva, J.; Vaz, F.; Macedo, J.; Al-Hroub, H.M.; Semreen, M.H.; Maio, R.; Gil, V.; Soares, N.; Penque, D. Acute venous thromboembolism plasma and red blood cell metabolomic profiling reveals potential new early diagnostic biomarkers: Observational clinical study. J. Transl. Med. 2024, 22, 200. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, M.C.; Worthley, L.I.G. Acute venous thromboembolism. Crit. Care Resusc. 2024, 2, 290–303. [Google Scholar] [CrossRef]
- Borow, M.; Goldson, H.J. Prevention of postoperative deep venous thrombosis and pulmonary emboli with combined modalities. Am. Surgeon 1983, 49, 599–605. [Google Scholar]
- Roumen-Klappe, E.M.; den Heijer, M.; van Uum, S.H.M.; van der Ven-Jongekrijg, J.; van der Graaf, F.; Wollersheim, H. Inflammatory response in the acute phase of deep vein thrombosis. J. Vasc. Surg. 2002, 35, 701–706. [Google Scholar] [CrossRef]
- Alhabibi, A.M.; Eldewi, D.M.; Abdel Wahab, M.; Saleh, O. Platelet-derived growth factor-beta as a new marker of deep venous thrombosis. J. Res. Med. Sci. 2019, 24, 48. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Gao, Y.; Wang, Q.; Chi, J.; Zhu, Z.; Diao, Q.; Li, X.; Wang, Z.; Qu, M.; Shi, Y. Preliminary clinical analysis and pathway study of S100A8 as a biomarker for the diagnosis of acute deep vein thrombosis. Sci. Rep. 2024, 14, 13298. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, P.; Huang, W.; Zhang, Y.; Tang, D.; Yang, T.; Guo, Y. Integrated landscape of plasma metabolism and proteome of patients with post-traumatic deep vein thrombosis. Nat. Commun. 2024, 15, 7831. [Google Scholar] [CrossRef] [PubMed]
- Rowbottom, D.G.; Keast, D.; Morton, A.R. The emerging role of glutamine as an indicator of exercise stress and overtraining. Sports Med. 1996, 21, 80–97. [Google Scholar] [CrossRef]
- Singleton, K.D.; Beckey, V.E.; Wischmeyer, P.E. Glutamine prevents activation of NF-κB and stress kinase pathways, attenuates inflammatory cytokine release, and prevents acute respiratory distress syndrome (ARDS) following sepsis. Shock 2006, 24, 583–589. [Google Scholar] [CrossRef]
- Hernandez-Lopez, R.; Chavez-Gonzalez, A.; Torres-Barrera, P.; Moreno-Lorenzana, D.; Lopez-DiazGuerrero, N.; Santiago-German, D.; Isordia-Salas, I.; Smadja, D.; Yoder, M.C.; Majluf-Cruz, A.; et al. Reduced proliferation of endothelial colony-forming cells in unprovoked venous thromboembolic disease as a consequence of endothelial dysfunction. PLoS ONE 2017, 12, e0183827. [Google Scholar] [CrossRef]
- Bittar, L.F.; da Silva, L.Q.; de Andrade Orsi, F.L.; Zapponi, K.C.S.; de Moraes Mazetto, B.; de Paula, E.V.; Montalvão, S.A.d.L.; Annichino-Bizzacchi, J.M. Increased inflammation and endothelial markers in patients with late severe postthrombotic syndrome. PLoS ONE 2020, 15, e0227150. [Google Scholar] [CrossRef]
- Xu, Y.; Jiang, H.; Li, L.; Chen, F.; Liu, Y.; Zhou, M.; Wang, J.; Jiang, J.; Li, X.; Fan, X.; et al. Branched-chain amino acid catabolism promotes thrombosis risk by enhancing tropomodulin-3 propionylation in platelets. Circulation 2020, 142, 49–64. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Zhang, B.; Lin, X.; Fu, X.; An, Y.; Zou, Y.; Wang, J.X.; Wang, Z.; Yu, T. Lactate metabolism in human health and disease. Signal Transduct. Target. Ther. 2022, 7, 305, Correction in Signal Transduct. Target. Ther. 2022, 7, 372. [Google Scholar] [CrossRef]
- Jones, T.E.; Pories, W.J.; Houmard, J.A.; Tanner, C.J.; Zheng, D.; Zou, K.; Coen, P.M.; Goodpaster, B.H.; Kraus, W.E.; Dohm, G.L. Plasma lactate as a marker of metabolic health: Implications of elevated lactate for impairment of aerobic metabolism in the metabolic syndrome. Surgery 2019, 166, 861–866. [Google Scholar] [CrossRef]
- Gupta, N.; Zhao, Y.Y.; Evans, C.E. The stimulation of thrombosis by hypoxia. Thromb. Res. 2019, 181, 77–83. [Google Scholar] [CrossRef]
- Yin, M.; Tian, S.; Huang, X.; Huang, Y.; Jiang, M. Role and mechanism of tissue plasminogen activator in venous wall fibrosis remodeling after deep venous thrombosis via the glycogen synthase kinase-3 beta signaling pathway. J. Surg. Res. 2013, 184, 1182–1195. [Google Scholar] [CrossRef]
- Maekawa, K.; Sugita, C.; Yamashita, A.; Moriguchi-Goto, S.; Furukoji, E.; Sakae, T.; Gi, T.; Hirai, T.; Asada, Y. Higher lactate and purine metabolite levels in erythrocyte-rich fresh venous thrombus: Potential markers for early deep vein thrombosis. Thromb. Res. 2019, 177, 136–144. [Google Scholar] [CrossRef]
- Morelli, V.M.; Lijfering, W.M.; Bos, M.H.A.; Rosendaal, F.R.; Cannegieter, S.C. Lipid levels and risk of venous thrombosis: Results from the MEGA-study. Eur. J. Epidemiol. 2017, 32, 669–681. [Google Scholar] [CrossRef]
- Madamanchi, N.R.; Hakim, Z.S.; Runge, M.S. Oxidative stress in atherogenesis and arterial thrombosis: The disconnect between cellular studies and clinical outcomes. J. Thromb. Haemost. 2005, 3, 254–267. [Google Scholar] [CrossRef]
- Matos, M.F.; Lourenço, D.M.; Orikaza, C.M.; Bajerl, J.A.H.; Noguti, M.A.E.; Morelli, V.M. The role of IL-6, IL-8 and MCP-1 and their promoter polymorphisms IL-6-174GC, IL-8-251AT and MCP-1-2518AG in the risk of venous thromboembolism: A case-control study. Thromb. Res. 2011, 128, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Gremmel, T.; Ay, C.; Seidinger, D.; Pabinger, I.; Panzer, S.; Koppensteiner, R. Soluble p-selectin, D-dimer, and high-sensitivity C-reactive protein after acute deep vein thrombosis of the lower limb. J. Vasc. Surg. 2011, 54, 48S–55S. [Google Scholar] [CrossRef] [PubMed]
- Shbaklo, H.; Holcroft, C.A.; Kahn, S.R. Levels of inflammatory markers and the development of the post-thrombotic syndrome. Thromb. Haemost. 2009, 101, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Kearon, C.; Ageno, W.; Cannegieter, S.C.; Cosmi, B.; Geersing, G.J.; Kyrle, P.A. Categorization of patients as having provoked or unprovoked venous thromboembolism: Guidance from the SSC of ISTH. J. Thromb. Haemost. 2016, 14, 1480–1483. [Google Scholar] [CrossRef]



| Acute DVT (n = 30) | HIs (n = 30) | p | |
|---|---|---|---|
| Age Mean (±SD) | 44 ± 12.5 | 44 ± 12.5 | 0.81 |
| Gender | |||
| Male/Female | 9 (30%)/21 (70%) | 9 (30%)/21 (70%) | 1.0 |
| BMI Median (IQR) kg/m2 | 29.9 (26.0–31.7) | 25.0 (21.3–28.2) | 0.003 |
| Comorbidities | |||
| Hypertension | 6 (20%) | 1 (3.3%) | 0.10 |
| Dyslipidemia | 3 (10%) | 3 (10%) | 1.0 |
| Hypothyroidism | 3 (10%) | 2 (6.6%) | 1.0 |
| Others * | 8 (26.6%) | 3 (10%) | NA |
| Recently SARS-CoV-2 infection | 2 (6.6%) | 0 | 0.49 |
| A previous episode of DVT More than 3 years | 6 (20%) | NA | - |
| Number of DVT events per person Median (min–max) | 2 (1–2) | NA | - |
| Family history: DVT, stroke, CVA | 12 (40%) | 4 (13.3%) | |
| 2nd degree of kinship | 6 | 3 | 0.47 |
| DVT LR/LL | 13:18 | NA | - |
| The time between symptoms and diagnosis | NA | ||
| 1–15 days | 23 (76.6%) | - | |
| 15–30 days | 7 (23.4%) | ||
| Spontaneous/provoked DVT | 11:19 | NA | - |
| Major transient risk factor | 8 (42%) | NA | - |
| Surgery with anesthesia for over 30 min | 6 | ||
| Immobilization in a hospital with acute illness (>3 days) | 2 | ||
| Minor transient risk factor | 12 (58%) | NA | - |
| Oral contraceptives and hormone replacement therapy | 9 | ||
| Trauma or fractures | 2 | ||
| Immobilization outside the hospital—reduced mobility (>3 days) | 1 | ||
| An interval of days between DVT diagnosis and blood collection Median (IQR) | 10 (1.0–30.0) | NA | - |
| Anticoagulant use | 27 (90%) | 1 (3.3%) | <0.0001 |
| Acute DVT (n = 21) | DVT > 6 Months (n = 21) | |
|---|---|---|
| BMI Mean (SD) kg/m2 | 28.3 ± 4.9 | 28.1 ± 4.8 |
| Comorbidities | ||
| Hypertension | 4 (19.0%) | 2 (9.5%) |
| Dyslipidemia | 1 (4.7%) | 2 (9.5%) |
| Hypothyroidism | 1 (4.7%) | 1 (4.7%) |
| Anticoagulant use | 20 (95.2%) | 12 (57.1%) |
| The time between acute DVT diagnosis and the second blood collection Months, mean (±SD) | NA | 7 ± 1.8 |
| PTS | NA | |
| Mild | 6 (28.5%) | |
| Moderate | 2 (9.5%) | |
| Severe | 1 (4.7%) | |
| DVT without PTS | 12 (57.1%) | |
| Recurrence of DVT | NA | 0 |
| Acute DVT n = 23 | HI n = 19 | p * | |
|---|---|---|---|
| IL-1 (pg/mL) | 6.3 (IQR 3.1–16.7) | 6.7 (IQR 3.1–10.8) | 0.79 |
| IL-6 (pg/mL) | 3.5 (IQR 1.5–8.9) | 0.3 (IQR 0.05–1.4) | 0.0001 |
| IL-8 (pg/mL) | 1.3 (IQR 0.9–1.8) | 0.9 (IQR 0.5–1.3) | 0.04 |
| TNF-α (pg/mL) | 8.9 (IQR 2.7–15.3) | 5.6 (IQR 2.5–9.0) | 0.29 |
| IFN-γ (pg/mL) | 0.9 (IQR 0.5–1.5) | 1.8 (IQR 0.6–3.7) | 0.21 |
| sCD40L (pg/mL) | 68.8 (IQR 41.6–136.9) | 52.7 (IQR 37.4–85.7) | 0.22 |
| sICAM-1 (ng/mL) | 575.5 (IQR 512.5–822.2) | 789.1 (IQR 621.5–962.8) | 0.21 |
| sVCAM-1 (ng/mL) | 1270 (IQR 1120–1550) | 1340 (IQR 1290–1620) | 0.31 |
| P-selectin (ng/mL) | 35.99 (IQR 33.7–43.5) | 34.8 (IQR 31.0–35.8) | 0.05 |
| PDGF-AB/BB (pg/mL) | 5479 (IQR 3861–7455) | 2714 (IQR 1571–4354) | 0.004 |
| Acute DVT n = 19 | DVT > 6 Months n = 19 | p * | |
|---|---|---|---|
| IL-1 (pg/mL) | 6.3 (IQR 3.1–16.7) | 6.2 (IQR 3.1–10.7) | 0.81 |
| IL-6 (pg/mL) | 3.9 (IQR 1.7–10.6) | 1.4 (IQR 0.5–2.9) | 0.09 |
| IL-8 (pg/mL) | 1.3 (IQR 0.9–1.8) | 1.0 (IQR 0.8–2.1) | 0.82 |
| TNF-α (pg/mL) | 8.9 (IQR 2.7–15.3) | 10.2 (IQR 298–13.9) | 0.88 |
| IFN-γ (pg/mL) | 0.9 (IQR 0.5–1.4) | 0.9 (IQR 0.5–4.2) | 0.79 |
| sCD40L (pg/mL) | 68.8 (47.2–137.1) | 81.8 (IQR 38.8–118.8) | 0.33 |
| sICAM-1 (pg/mL) | 575,536 (IQR 512,487–822,183) | 790,820 (IQR 601,059–966,992) | 0.0002 |
| sVCAM-1(pg/mL) | 1,270,000 (IQR 1,120,000–1,550,000) | 1,620,000 (IQR 1,280,000–1,740,000) | 0.01 |
| P-selectin (ng/mL) | 35.99 (IQR 33.7–43.5) | 33.9 (IQR 28.4–35.6) | 0.001 |
| PDGF-AB/BB (pg/mL) | 5479 (IQR 4175–7035) | 5373.5 (IQR 3468–7441.0) | 0.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Queiroz da Silva, L.; Santana da Costa, T.; Gelain Martins, L.; de Lima Montalvão, S.A.; Cares Huber, S.; Martins Silva Soares, S.; Tasic, L.; Annichino-Bizzacchi, J.M. Accessing Altered Metabolic Profile in Acute Deep Vein Thrombosis Through Nuclear Magnetic Resonance Spectroscopy. Int. J. Mol. Sci. 2025, 26, 11345. https://doi.org/10.3390/ijms262311345
Queiroz da Silva L, Santana da Costa T, Gelain Martins L, de Lima Montalvão SA, Cares Huber S, Martins Silva Soares S, Tasic L, Annichino-Bizzacchi JM. Accessing Altered Metabolic Profile in Acute Deep Vein Thrombosis Through Nuclear Magnetic Resonance Spectroscopy. International Journal of Molecular Sciences. 2025; 26(23):11345. https://doi.org/10.3390/ijms262311345
Chicago/Turabian StyleQueiroz da Silva, Letícia, Thyerre Santana da Costa, Lucas Gelain Martins, Silmara Aparecida de Lima Montalvão, Stephany Cares Huber, Sandra Martins Silva Soares, Ljubica Tasic, and Joyce Maria Annichino-Bizzacchi. 2025. "Accessing Altered Metabolic Profile in Acute Deep Vein Thrombosis Through Nuclear Magnetic Resonance Spectroscopy" International Journal of Molecular Sciences 26, no. 23: 11345. https://doi.org/10.3390/ijms262311345
APA StyleQueiroz da Silva, L., Santana da Costa, T., Gelain Martins, L., de Lima Montalvão, S. A., Cares Huber, S., Martins Silva Soares, S., Tasic, L., & Annichino-Bizzacchi, J. M. (2025). Accessing Altered Metabolic Profile in Acute Deep Vein Thrombosis Through Nuclear Magnetic Resonance Spectroscopy. International Journal of Molecular Sciences, 26(23), 11345. https://doi.org/10.3390/ijms262311345







