Modulatory Potential of Alpinetin on Inflammation, Oxidative Stress, Apoptosis, and Mitochondrial Dynamics in a Rat Middle Cerebral Artery Occlusion Model of Ischemic Stroke
Abstract
1. Introduction
2. Results
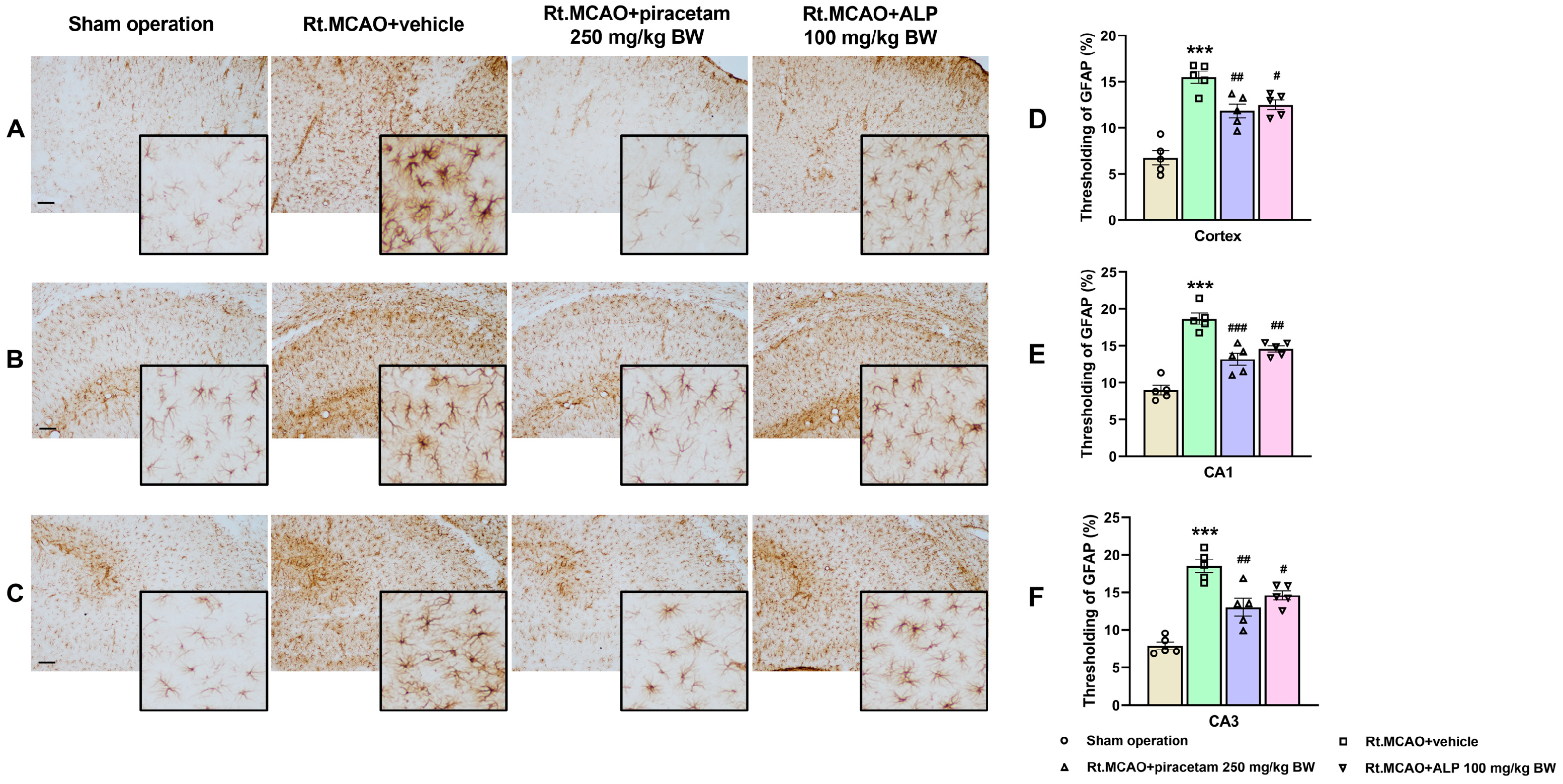
2.1. Effect of Alpinetin on Ischemic Stroke-Induced Morphological Changes in Astrocytes Within the Cortex and Hippocampus
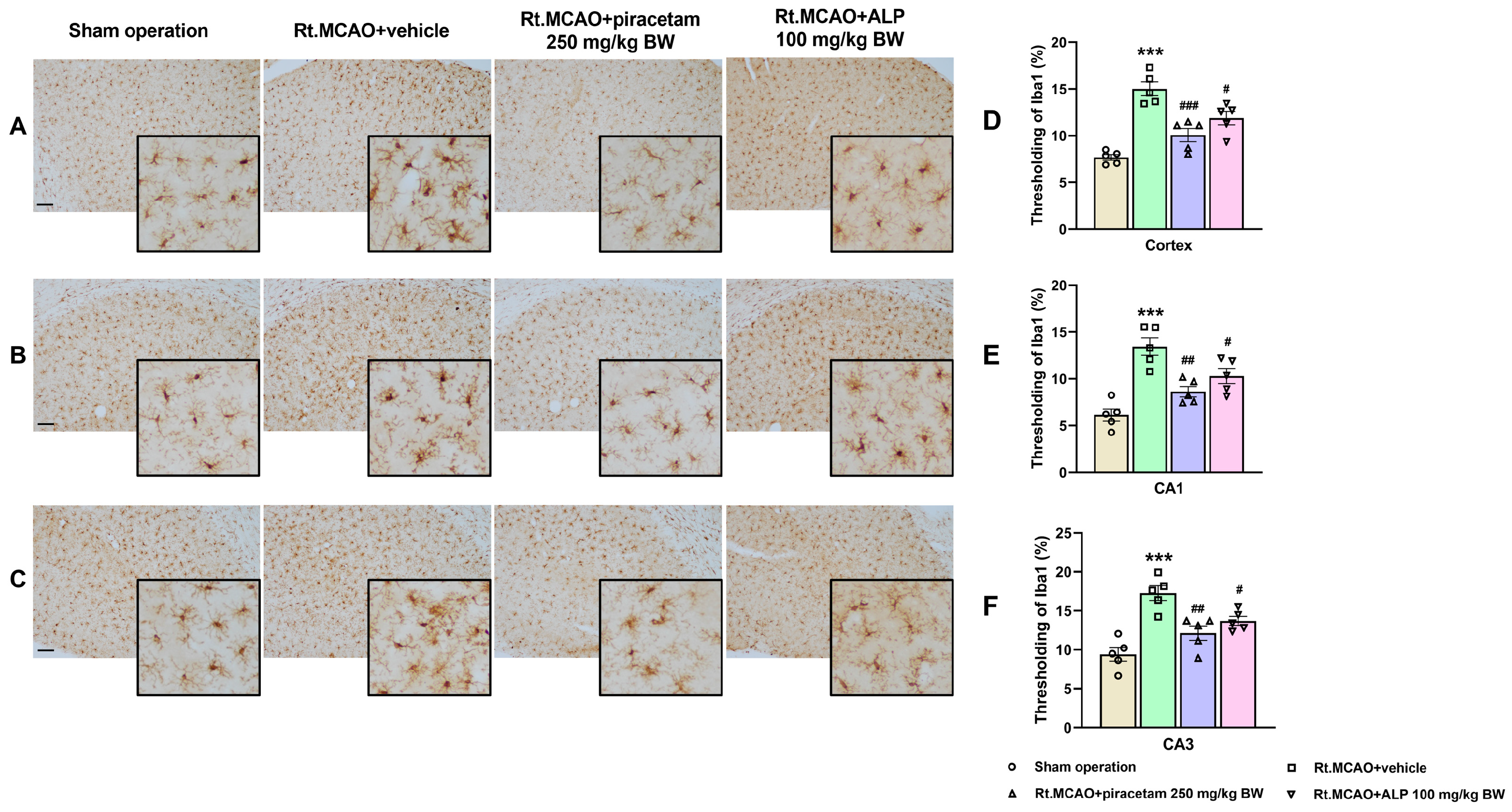
2.2. Effect of Alpinetin on Ischemic Stroke-Induced Morphological Changes in Microglia Within the Cortex and Hippocampus
2.3. Effect of Alpinetin on Akt Expression in the Cortex and CA1 of Hippocampus
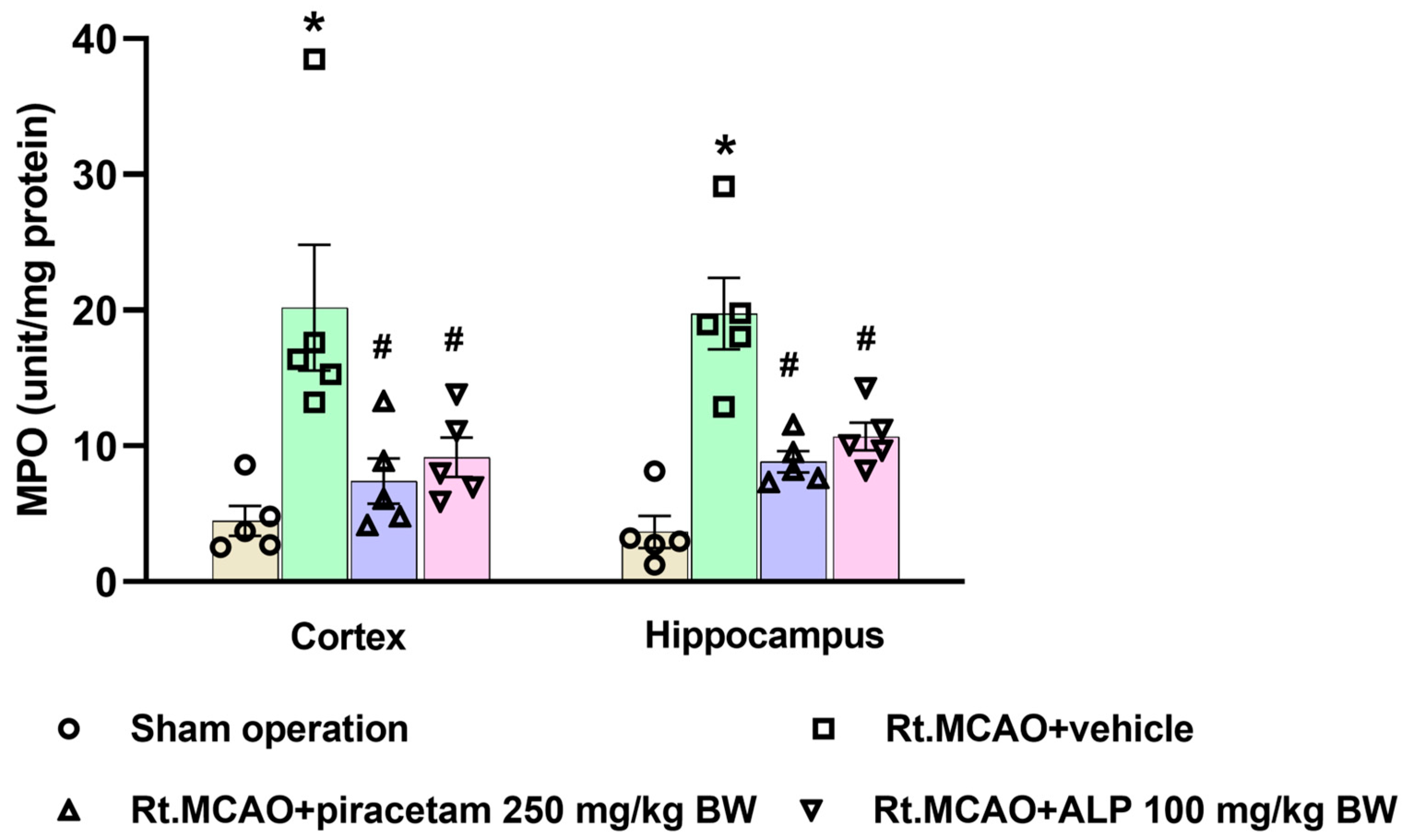
2.4. Effect of Alpinetin on Myeloperoxidase (MPO) Activity in Ischemic Brain Tissue
2.5. Effect of Alpinetin on Mitochondrial Superoxide Dismutase (MnSOD) Activity in the Cortex and Hippocampus
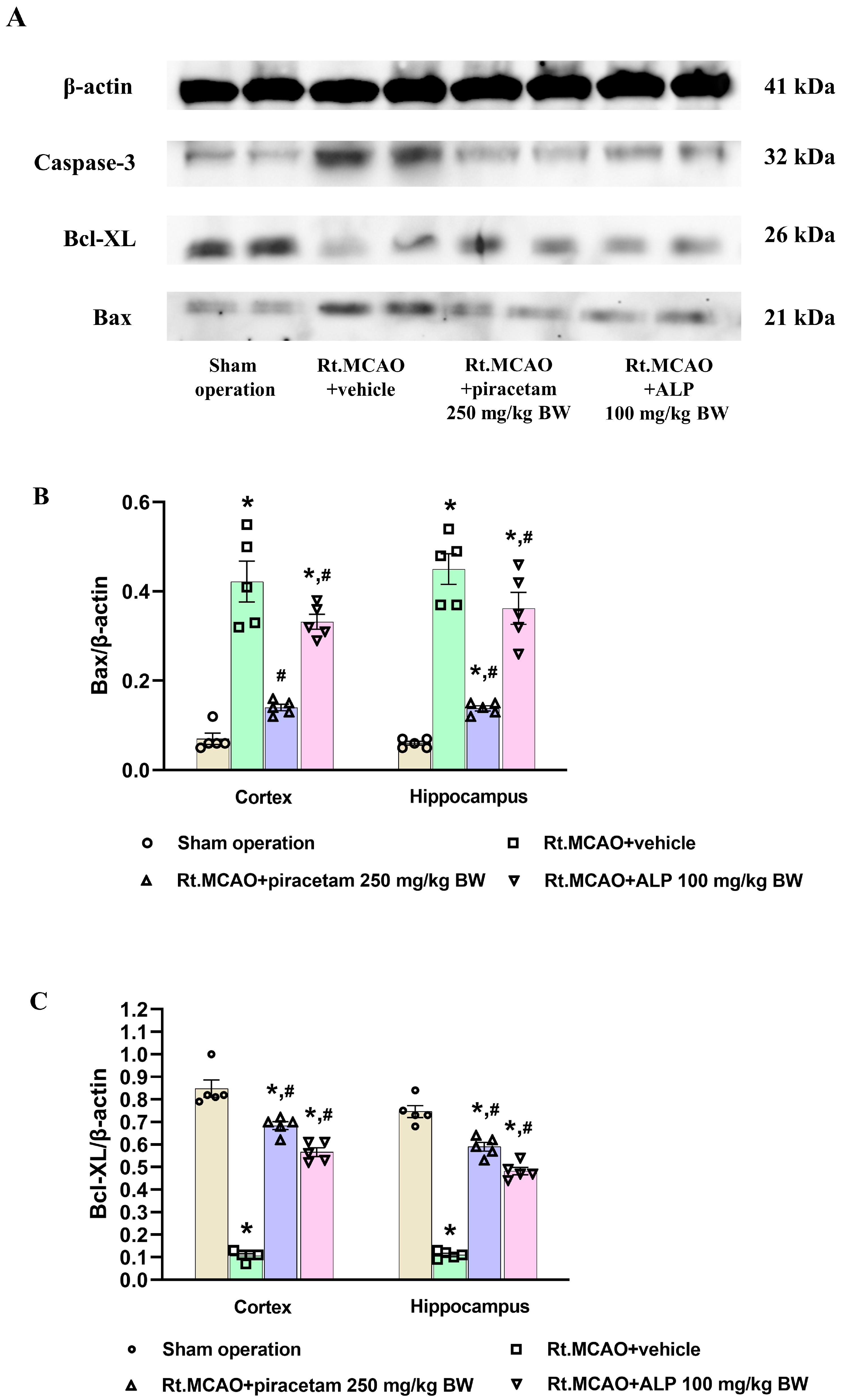
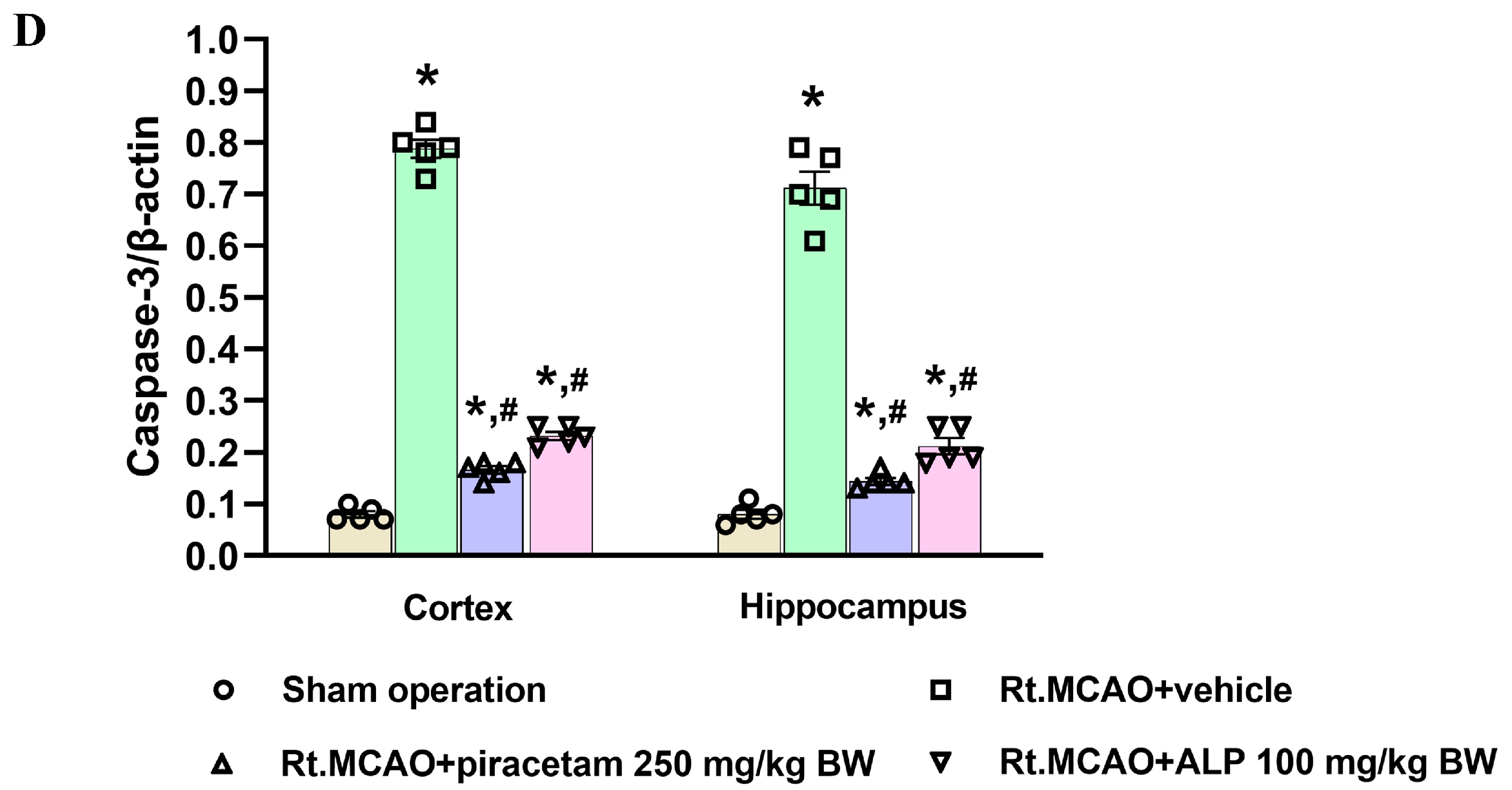
2.6. Effect of Alpinetin on Anti-Apoptotic Signaling in Rt.MCAO-Induced Brain Injury
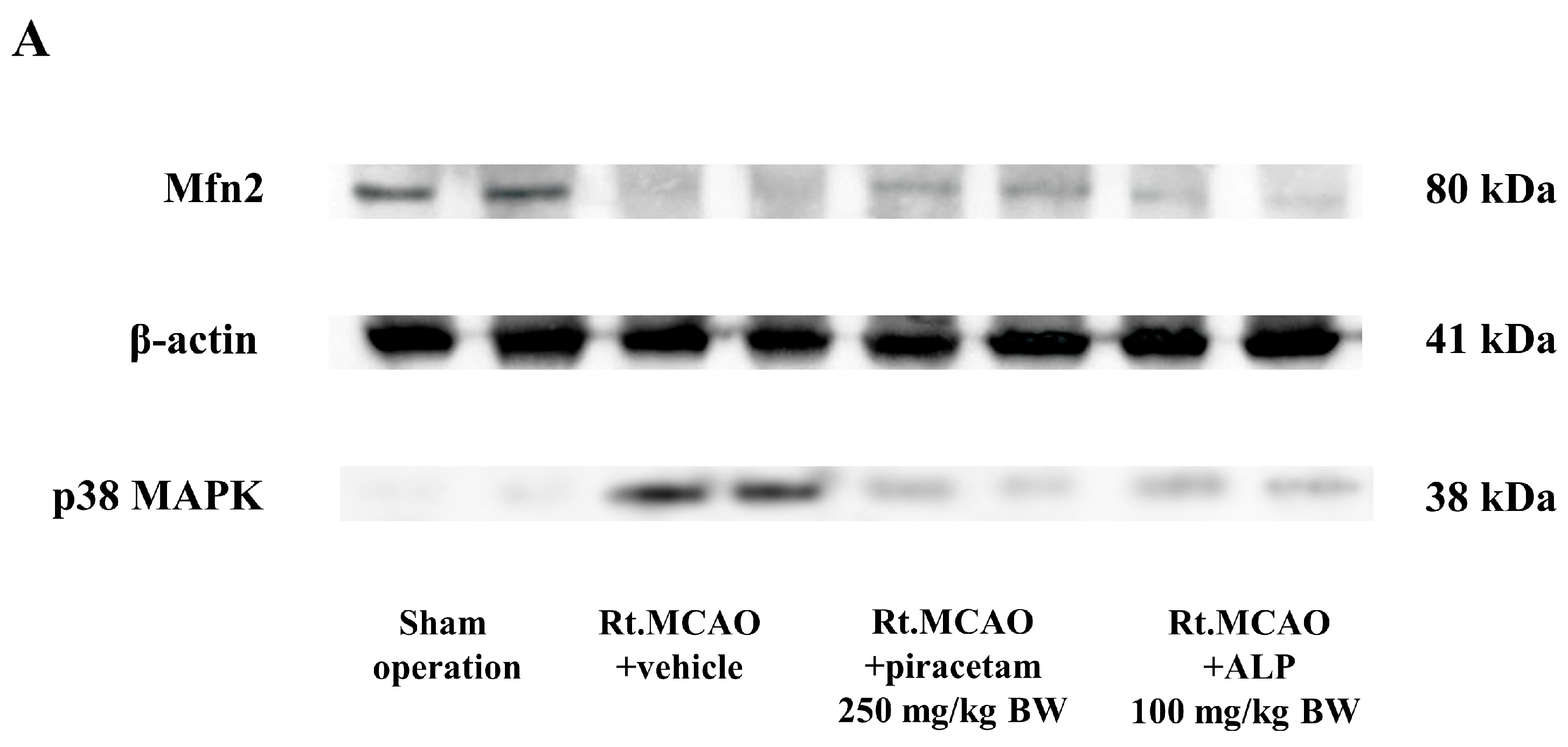
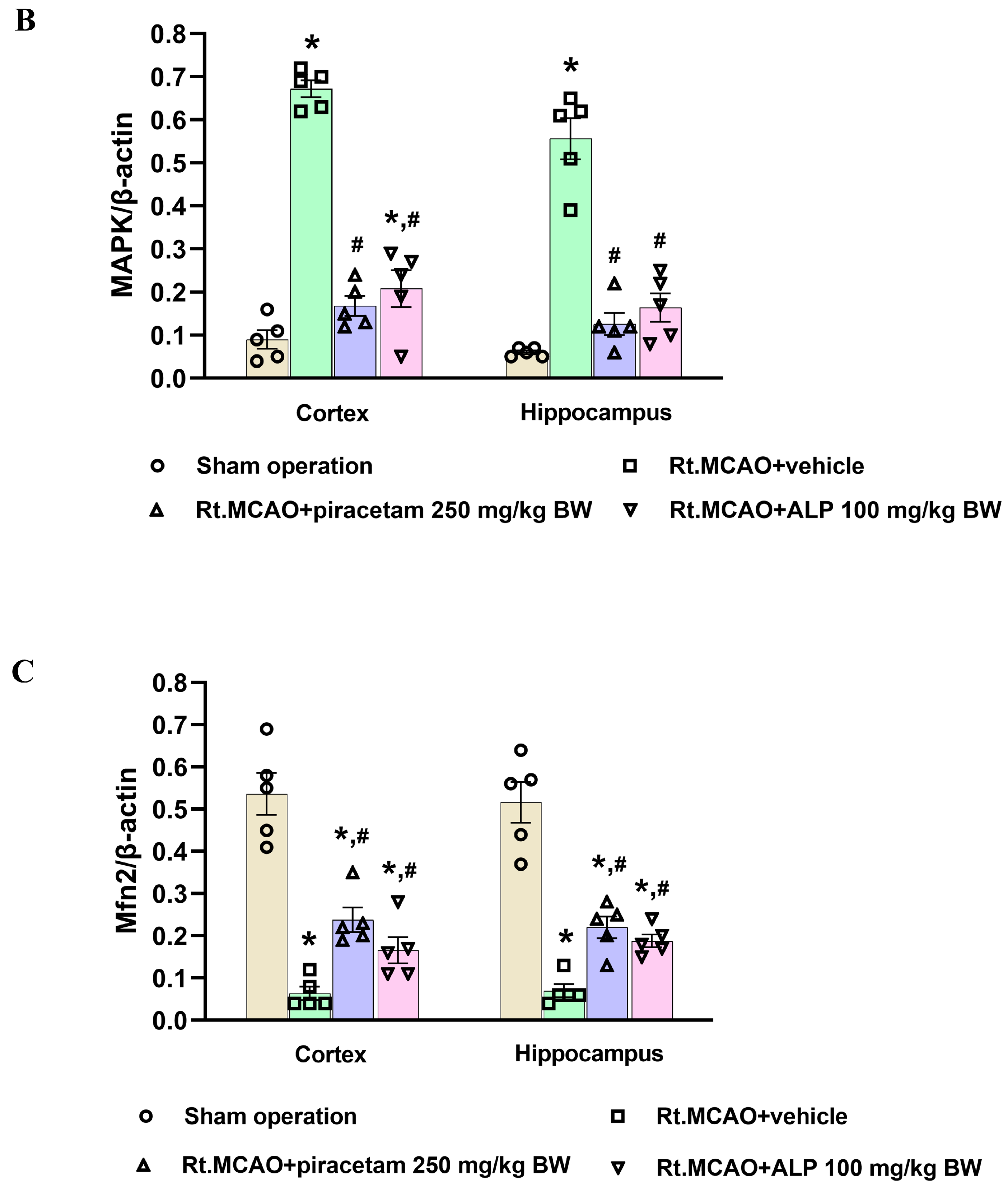
2.7. Effect of Alpinetin on p38 MAPK and Mitofusin-2 (Mfn2) Expression Following Ischemic Stroke
3. Discussion
4. Materials and Methods
4.1. Investigational Compounds
4.2. Study Design
- Sham operation group: A sham surgery was performed on these animals, but a nylon filament was not inserted. This group received no subsequent treatment.
- Rt.MCAO + vehicle group: Following the Rt.MCAO procedure, animals were given an i.p. injection of the vehicle (DMSO) at a volume of 0.5 mL. This treatment was administered daily for three consecutive days to control for the effects of the solvent.
- Rt.MCAO + piracetam (250 mg/kg BW) group: This group served as the positive control. Animals underwent Rt.MCAO and were subsequently treated with an i.p. dose of piracetam (250 mg/kg BW) once daily for three days.
- Rt.MCAO + alpinetin (ALP 100 mg/kg BW) group: This group received the Rt.MCAO procedure and was treated with alpinetin at a daily i.p. dose of 100 mg/kg BW for three days.
4.3. Right Middle Cerebral Artery Occlusion Model
4.4. Immunohistochemistry Staining for Microglia and Astrocytes
4.5. Immunofluorescence Staining of Akt
4.6. Protein Quantification
4.7. Measurement of Myeloperoxidase (MPO) Activity
4.8. Brain Mitochondrial Extraction for MnSOD Analysis
4.9. Determination of Mitochondrial Superoxide Dismutase (MnSOD) Activity
4.10. Western Blot Analysis
4.11. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, J.; Xu, A.; Zhao, Z.; Ren, B.; Gao, Z.; Fang, D.; Hei, B.; Sun, J.; Bao, X.; Ma, L.; et al. Epidemiology and future trend predictions of ischemic stroke based on the global burden of disease study 1990–2021. Commun. Med. 2025, 5, 273. [Google Scholar] [CrossRef]
- Majumder, D. Ischemic Stroke: Pathophysiology and Evolving Treatment Approaches. Neurosci. Insights 2024, 19, 26331055241292600. [Google Scholar] [CrossRef]
- Jover-Mengual, T.; Hwang, J.Y.; Byun, H.R.; Court-Vazquez, B.L.; Centeno, J.M.; Burguete, M.C.; Zukin, R.S. The Role of NF-kappaB Triggered Inflammation in Cerebral Ischemia. Front. Cell. Neurosci. 2021, 15, 633610. [Google Scholar] [CrossRef]
- Golenia, A.; Olejnik, P. The Role of Oxidative Stress in Ischaemic Stroke and the Influence of Gut Microbiota. Antioxidants 2025, 14, 542. [Google Scholar] [CrossRef]
- Shademan, B.; Avci, C.B.; Karamad, V.; Soureh, G.J.; Olia, J.B.H.; Esmaily, F.; Nourazarian, A.; Nikanfar, M. The Role of Mitochondrial Biogenesis in Ischemic Stroke. J. Integr. Neurosci. 2023, 22, 88. [Google Scholar] [CrossRef]
- Bersano, A.; Gatti, L. Pathophysiology and Treatment of Stroke: Present Status and Future Perspectives. Int. J. Mol. Sci. 2023, 24, 14848. [Google Scholar] [CrossRef]
- Radak, D.; Katsiki, N.; Resanovic, I.; Jovanovic, A.; Sudar-Milovanovic, E.; Zafirovic, S.; Mousad, S.A.; Isenovic, E.R. Apoptosis and Acute Brain Ischemia in Ischemic Stroke. Curr. Vasc. Pharmacol. 2017, 15, 115–122. [Google Scholar] [CrossRef]
- Alsbrook, D.L.; Di Napoli, M.; Bhatia, K.; Biller, J.; Andalib, S.; Hinduja, A.; Rodrigues, R.; Rodriguez, M.; Sabbagh, S.Y.; Selim, M.; et al. Neuroinflammation in Acute Ischemic and Hemorrhagic Stroke. Curr. Neurol. Neurosci. Rep. 2023, 23, 407–431. [Google Scholar] [CrossRef]
- Jin, B.; Han, Y.; Xu, F.; Wang, J.; Zhao, Y.; Liu, H.; Wang, F.; Wang, Z.; Lu, W.; Wang, M.; et al. Translatome analysis in acute ischemic stroke: Astrocytes and microglia exhibit differences in poststroke alternative splicing of expressed transcripts. FASEB J. 2024, 38, e23855. [Google Scholar] [CrossRef]
- Liang, Z.; Lou, Y.; Hao, Y.; Li, H.; Feng, J.; Liu, S. The Relationship of Astrocytes and Microglia with Different Stages of Ischemic Stroke. Curr. Neuropharmacol. 2023, 21, 2465–2480. [Google Scholar] [CrossRef]
- Dordoe, C.; Huang, W.; Bwalya, C.; Wang, X.; Shen, B.; Wang, H.; Wang, J.; Ye, S.; Wang, P.; Xiaoyan, B.; et al. The role of microglial activation on ischemic stroke: Modulation by fibroblast growth factors. Cytokine Growth Factor Rev. 2023, 74, 122–133. [Google Scholar] [CrossRef]
- Shui, X.; Chen, J.; Fu, Z.; Zhu, H.; Tao, H.; Li, Z. Microglia in Ischemic Stroke: Pathogenesis Insights and Therapeutic Challenges. J. Inflamm. Res. 2024, 17, 3335–3352. [Google Scholar] [CrossRef]
- Savchenko, E.S.; Pevzner, I.B.; Zorova, L.D.; Silachev, D.N.; Babenko, V.A.; Manskikh, V.N.; Gulyaev, M.V.; Pirogov, Y.A.; Plotnikov, E.Y.; Zorov, D.B. Changes in the Number of Neurons, Astrocytes and Microglia in the Brain after Ischemic Stroke Assessed by Immunohistochemistry and Immunoblotting. Tsitologiia 2016, 58, 534–542. [Google Scholar] [CrossRef]
- Li, Z.; Xiao, G.; Wang, H.; He, S.; Zhu, Y. A preparation of Ginkgo biloba L. leaves extract inhibits the apoptosis of hippocampal neurons in post-stroke mice via regulating the expression of Bax/Bcl-2 and Caspase-3. J. Ethnopharmacol. 2021, 280, 114481. [Google Scholar] [CrossRef]
- Kongsui, R.; Jittiwat, J. In vivo protective effects of 6-gingerol in cerebral ischemia involve preservation of antioxidant defenses and activation of anti-apoptotic pathways. Biomed. Rep. 2024, 20, 85. [Google Scholar] [CrossRef]
- Jittiwat, J.; Suksamrarn, A.; Tocharus, C.; Tocharus, J. Dihydrocapsaicin effectively mitigates cerebral ischemia-induced pathological changes in vivo, partly via antioxidant and anti-apoptotic pathways. Life Sci. 2021, 283, 119842. [Google Scholar] [CrossRef]
- Jittiwat, J.; Chonpathompikunlert, P.; Sukketsiri, W. Neuroprotective effects of Apium graveolens against focal cerebral ischemia occur partly via antioxidant, anti-inflammatory, and anti-apoptotic pathways. J. Sci. Food Agric. 2021, 101, 2256–2263. [Google Scholar] [CrossRef]
- Wang, Y.C.; Lu, Y.B.; Huang, X.L.; Lao, Y.F.; Zhang, L.; Yang, J.; Shi, M.; Ma, H.L.; Pan, Y.W.; Zhang, Y.N. Myeloperoxidase: A new target for the treatment of stroke? Neural Regen. Res. 2022, 17, 1711–1716. [Google Scholar] [CrossRef]
- Zheng, M.; Liu, Y.; Zhang, G.; Yang, Z.; Xu, W.; Chen, Q. The Applications and Mechanisms of Superoxide Dismutase in Medicine, Food, and Cosmetics. Antioxidants 2023, 12, 1675. [Google Scholar] [CrossRef]
- Supawat, A.; Palachai, N.; Jittiwat, J. Effect of galangin on oxidative stress, antioxidant defenses and mitochondrial dynamics in a rat model of focal cerebral ischemia. Biomed. Rep. 2025, 22, 10. [Google Scholar] [CrossRef]
- Li, C.; Chen, C.; Qin, H.; Ao, C.; Chen, J.; Tan, J.; Zeng, L. The Role of Mitochondrial Dynamin in Stroke. Oxid. Med. Cell. Longev. 2022, 2022, 2504798. [Google Scholar] [CrossRef]
- Zhang, X.M.; Zhang, L.; Wang, G.; Niu, W.; He, Z.; Ding, L.; Jia, J. Suppression of mitochondrial fission in experimental cerebral ischemia: The potential neuroprotective target of p38 MAPK inhibition. Neurochem. Int. 2015, 90, 1–8. [Google Scholar] [CrossRef]
- Kang, J.B.; Koh, P.O. Retinoic acid alleviates the reduction of Akt and Bad phosphorylation and regulates Bcl-2 family protein interactions in animal models of ischemic stroke. PLoS ONE 2024, 19, e0303213. [Google Scholar] [CrossRef]
- Li, J.; Lang, J.; Zeng, Z.; McCullough, L.D. Akt1 gene deletion and stroke. J. Neurol. Sci. 2008, 269, 105–112. [Google Scholar] [CrossRef]
- Kongsui, R.; Thongrong, S.; Jittiwat, J. In Vivo Neuroprotective Effects of Alpinetin Against Experimental Ischemic Stroke Damage Through Antioxidant and Anti-Inflammatory Mechanisms. Int. J. Mol. Sci. 2025, 26, 5093. [Google Scholar] [CrossRef]
- Radosavljevic, T.; Brankovic, M.; Djuretic, J.; Grujic-Milanovic, J.; Kovacic, M.; Jevtic, J.; Stankovic, S.; Samardzic, J.; Vucevic, D.; Jakovljevic, V. Alpinetin Exhibits Antioxidant and Anti-Inflammatory Effects in C57BL/6 Mice with Alcoholic Liver Disease Induced by the Lieber-DeCarli Ethanol Liquid Diet. Int. J. Mol. Sci. 2024, 26, 86. [Google Scholar] [CrossRef]
- Zhao, G.; Tong, Y.; Luan, F.; Zhu, W.; Zhan, C.; Qin, T.; An, W.; Zeng, N. Alpinetin: A Review of Its Pharmacology and Pharmacokinetics. Front. Pharmacol. 2022, 13, 814370. [Google Scholar] [CrossRef]
- Guo, Y.; Jiang, L.; Luo, S.; Hu, D.; Zhao, X.; Zhao, G.; Tang, W. Network Analysis and Basic Experiments on the Inhibition of Renal Cancer Proliferation and Migration by Alpinetin through PI3K/AKT/ mTOR Pathway. Curr. Mol. Med. 2024, 24, 134–144. [Google Scholar] [CrossRef]
- Gul, S.; Maqbool, M.F.; Zheng, D.; Li, Y.; Khan, M.; Ma, T. Alpinetin: A Dietary Flavonoid with Diverse Anticancer Effects. Appl. Biochem. Biotechnol. 2022, 194, 4220–4243. [Google Scholar] [CrossRef]
- Zhou, Y.; Ding, Y.L.; Zhang, J.L.; Zhang, P.; Wang, J.Q.; Li, Z.H. Alpinetin improved high fat diet-induced non-alcoholic fatty liver disease (NAFLD) through improving oxidative stress, inflammatory response and lipid metabolism. Biomed. Pharmacother. 2018, 97, 1397–1408. [Google Scholar] [CrossRef]
- Chen, H.; Liu, C.; Zhan, Y.; Wang, Y.; Hu, Q.; Zeng, Z. Alpinetin ameliorates bleomycin-induced pulmonary fibrosis by repressing fibroblast differentiation and proliferation. Biomed. Pharmacother. 2024, 171, 116101. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Tao, X.; Xu, J. Protective effect of Alpinetin on rats with chronic obstructive pulmonary disease. Food Sci. Nutr. 2020, 8, 6603–6611. [Google Scholar] [CrossRef]
- Lakhan, S.E.; Kirchgessner, A.; Hofer, M. Inflammatory mechanisms in ischemic stroke: Therapeutic approaches. J. Transl. Med. 2009, 7, 97. [Google Scholar] [CrossRef]
- Shen, X.Y.; Gao, Z.K.; Han, Y.; Yuan, M.; Guo, Y.S.; Bi, X. Activation and Role of Astrocytes in Ischemic Stroke. Front. Cell. Neurosci. 2021, 15, 755955. [Google Scholar] [CrossRef]
- Qin, J.; Ma, Z.; Chen, X.; Shu, S. Microglia activation in central nervous system disorders: A review of recent mechanistic investigations and development efforts. Front. Neurol. 2023, 14, 1103416. [Google Scholar] [CrossRef]
- Stence, N.; Waite, M.; Dailey, M.E. Dynamics of microglial activation: A confocal time-lapse analysis in hippocampal slices. Glia 2001, 33, 256–266. [Google Scholar] [CrossRef]
- Benko, S.; Denes, A. Microglial Inflammatory Mechanisms in Stroke: The Jury Is Still Out. Neuroscience 2024, 550, 43–52. [Google Scholar] [CrossRef]
- Ito, D.; Tanaka, K.; Suzuki, S.; Dembo, T.; Fukuuchi, Y. Enhanced expression of Iba1, ionized calcium-binding adapter molecule 1, after transient focal cerebral ischemia in rat brain. Stroke 2001, 32, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Sillerud, L.O.; Yang, Y.; Yang, L.Y.; Duval, K.B.; Thompson, J.; Yang, Y. Longitudinal monitoring of microglial/macrophage activation in ischemic rat brain using Iba-1-specific nanoparticle-enhanced magnetic resonance imaging. J. Cereb. Blood Flow Metab. 2020, 40, S117–S133. [Google Scholar] [CrossRef]
- Ozevren, H.; Deveci, E.; Tuncer, M.C. The effect of rosmarinic acid on deformities occurring in brain tissue by craniectomy method. Histopathological evaluation of IBA-1 and GFAP expressions. Acta Cir. Bras. 2020, 35, e202000406. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-kappaB in biology and targeted therapy: New insights and translational implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef]
- Carregosa, D.; Carecho, R.M.; Figueira, I.; Santos, C.N. Low-Molecular Weight Metabolites from Polyphenols as Effectors for Attenuating Neuroinflammation. J. Agric. Food Chem. 2020, 68, 1790–1807. [Google Scholar] [CrossRef]
- Garcia, G.; Nanni, S.; Figueira, I.; Ivanov, I.; McDougall, G.J.; Stewart, D.; Ferreira, R.B.; Pinto, P.; Silva, R.F.; Brites, D.; et al. Bioaccessible (poly)phenol metabolites from raspberry protect neural cells from oxidative stress and attenuate microglia activation. Food Chem. 2017, 215, 274–283. [Google Scholar] [CrossRef]
- Lin, W.; Chen, H.; Chen, X.; Guo, C. The Roles of Neutrophil-Derived Myeloperoxidase (MPO) in Diseases: The New Progress. Antioxidants 2024, 13, 132. [Google Scholar] [CrossRef]
- Chen, S.; Chen, H.; Du, Q.; Shen, J. Targeting Myeloperoxidase (MPO) Mediated Oxidative Stress and Inflammation for Reducing Brain Ischemia Injury: Potential Application of Natural Compounds. Front. Physiol. 2020, 11, 433. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, K.; Feng, Q.; Zhang, Y.; Wang, C.; Liu, Q.; Liu, X.; Wang, X.; Gao, W.; Bai, X.; et al. Alpinetin Attenuates Persistent Inflammation, Immune Suppression, and Catabolism Syndrome in a Septic Mouse Model. J. Immunol. Res. 2021, 2021, 9998517. [Google Scholar] [CrossRef]
- Liang, Y.; Shen, T.; Ming, Q.; Han, G.; Zhang, Y.; Liang, J.; Zhu, D. Alpinetin ameliorates inflammatory response in LPS-induced endometritis in mice. Int. Immunopharmacol. 2018, 62, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; He, Q.; Zheng, J.; Li, L.Y.; Hou, Y.H.; Song, F.Z. Sulforaphane improves outcomes and slows cerebral ischemic/reperfusion injury via inhibition of NLRP3 inflammasome activation in rats. Int. Immunopharmacol. 2017, 45, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Forghani, R.; Kim, H.J.; Wojtkiewicz, G.R.; Bure, L.; Wu, Y.; Hayase, M.; Wei, Y.; Zheng, Y.; Moskowitz, M.A.; Chen, J.W. Myeloperoxidase propagates damage and is a potential therapeutic target for subacute stroke. J. Cereb. Blood Flow Metab. 2015, 35, 485–493. [Google Scholar] [CrossRef]
- Liu, T.; Li, X.; Zhou, X.; Chen, W.; Wen, A.; Liu, M.; Ding, Y. PI3K/AKT signaling and neuroprotection in ischemic stroke: Molecular mechanisms and therapeutic perspectives. Neural Regen. Res. 2025, 20, 2758–2775. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Yang, S.; Chu, Y.H.; Zhang, H.; Pang, X.W.; Chen, L.; Zhou, L.Q.; Chen, M.; Tian, D.S.; Wang, W. Signaling pathways involved in ischemic stroke: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2022, 7, 215. [Google Scholar] [CrossRef]
- Gong, Z.; Guo, J.; Liu, B.; Guo, Y.; Cheng, C.; Jiang, Y.; Liang, N.; Hu, M.; Song, T.; Yang, L.; et al. Mechanisms of immune response and cell death in ischemic stroke and their regulation by natural compounds. Front. Immunol. 2023, 14, 1287857. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X.; Yang, M.; Liu, S.B. Research progress on morphology and mechanism of programmed cell death. Cell Death Dis. 2024, 15, 327. [Google Scholar] [CrossRef]
- Mansuri, M.L.; Parihar, P.; Solanki, I.; Parihar, M.S. Flavonoids in modulation of cell survival signalling pathways. Genes. Nutr. 2014, 9, 400. [Google Scholar] [CrossRef]
- Sekerdag, E.; Solaroglu, I.; Gursoy-Ozdemir, Y. Cell Death Mechanisms in Stroke and Novel Molecular and Cellular Treatment Options. Curr. Neuropharmacol. 2018, 16, 1396–1415. [Google Scholar] [CrossRef] [PubMed]
- Fluri, F.; Schuhmann, M.K.; Kleinschnitz, C. Animal models of ischemic stroke and their application in clinical research. Drug Des. Dev. Ther. 2015, 9, 3445–3454. [Google Scholar] [CrossRef]
- Liu, G.; Wang, T.; Wang, T.; Song, J.; Zhou, Z. Effects of apoptosis-related proteins caspase-3, Bax and Bcl-2 on cerebral ischemia rats. Biomed. Rep. 2013, 1, 861–867. [Google Scholar] [CrossRef]
- Gupta, R.; Ghosh, S. Putative roles of mitochondrial Voltage-Dependent Anion Channel, Bcl-2 family proteins and c-Jun N-terminal Kinases in ischemic stroke associated apoptosis. Biochim. Open 2017, 4, 47–55. [Google Scholar] [CrossRef]
- Dong, H.; Gao, X.; Li, H.; Gao, J.; Zhang, L. Protective effects of flavonoids against intracerebral and subarachnoid hemorrhage (Review). Exp. Ther. Med. 2024, 28, 350. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Qi, Y.; Tsang, S.Y. Mitochondrial Biogenesis, Mitochondrial Dynamics, and Mitophagy in the Maturation of Cardiomyocytes. Cells 2021, 10, 2463. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.M. Mitochondrial Dysfunction in Neurodegenerative Diseases. Cells 2025, 14, 276. [Google Scholar] [CrossRef]
- Meng, K.; Jia, H.; Hou, X.; Zhu, Z.; Lu, Y.; Feng, Y.; Feng, J.; Xia, Y.; Tan, R.; Cui, F.; et al. Mitochondrial Dysfunction in Neurodegenerative Diseases: Mechanisms and Corresponding Therapeutic Strategies. Biomedicines 2025, 13, 327. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Guo, S.; Tang, Y.; Mou, C.; Hu, X.; Shao, F.; Yan, W.; Wu, Q. Mitochondrial Fusion and Fission in Neuronal Death Induced by Cerebral Ischemia-Reperfusion and Its Clinical Application: A Mini-Review. Med. Sci. Monit. 2020, 26, e928651. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Li, H.; Liao, P.; Chen, L.; Pan, Y.; Zheng, Y.; Zhang, C.; Liu, D.; Zheng, M.; Gao, J. Mitochondrial dysfunction: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 124. [Google Scholar] [CrossRef]
- Bhaskar, S.; Sheshadri, P.; Joseph, J.P.; Potdar, C.; Prasanna, J.; Kumar, A. Mitochondrial Superoxide Dismutase Specifies Early Neural Commitment by Modulating Mitochondrial Dynamics. iScience 2020, 23, 101564. [Google Scholar] [CrossRef] [PubMed]
- Indo, H.P.; Yen, H.C.; Nakanishi, I.; Matsumoto, K.; Tamura, M.; Nagano, Y.; Matsui, H.; Gusev, O.; Cornette, R.; Okuda, T.; et al. A mitochondrial superoxide theory for oxidative stress diseases and aging. J. Clin. Biochem. Nutr. 2015, 56, 1–7. [Google Scholar] [CrossRef]
- Huang, H.F.; Guo, F.; Cao, Y.Z.; Shi, W.; Xia, Q. Neuroprotection by manganese superoxide dismutase (MnSOD) mimics: Antioxidant effect and oxidative stress regulation in acute experimental stroke. CNS Neurosci. Ther. 2012, 18, 811–818. [Google Scholar] [CrossRef]
- Miriyala, S.; Holley, A.K.; St Clair, D.K. Mitochondrial superoxide dismutase--signals of distinction. Anticancer. Agents Med. Chem. 2011, 11, 181–190. [Google Scholar] [CrossRef]
- Abhijit, S.; Tripathi, S.J.; Bhagya, V.; Shankaranarayana Rao, B.S.; Subramanyam, M.V.; Asha Devi, S. Antioxidant action of grape seed polyphenols and aerobic exercise in improving neuronal number in the hippocampus is associated with decrease in lipid peroxidation and hydrogen peroxide in adult and middle-aged rats. Exp. Gerontol. 2018, 101, 101–112. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Valko, R.; Liska, J.; Nepovimova, E.; Kuca, K.; Valko, M. Flavonoids and their role in oxidative stress, inflammation, and human diseases. Chem. Biol. Interact. 2025, 413, 111489. [Google Scholar] [CrossRef]
- Kicinska, A.; Jarmuszkiewicz, W. Flavonoids and Mitochondria: Activation of Cytoprotective Pathways? Molecules 2020, 25, 3060. [Google Scholar] [CrossRef] [PubMed]
- Altahrawi, A.Y.; James, A.W.; Shah, Z.A. The Role of Oxidative Stress and Inflammation in the Pathogenesis and Treatment of Vascular Dementia. Cells 2025, 14, 609. [Google Scholar] [CrossRef] [PubMed]
- Palachai, N.; Supawat, A.; Kongsui, R.; Klimaschewski, L.; Jittiwat, J. Galangin’s Neuroprotective Role: Targeting Oxidative Stress, Inflammation, and Apoptosis in Ischemic Stroke in a Rat Model of Permanent Middle Cerebral Artery Occlusion. Int. J. Mol. Sci. 2025, 26, 1847. [Google Scholar] [CrossRef]
- Tripathi, A.; Paliwal, P.; Krishnamurthy, S. Piracetam Attenuates LPS-Induced Neuroinflammation and Cognitive Impairment in Rats. Cell. Mol. Neurobiol. 2017, 37, 1373–1386. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Eliwa, D.; Alexiou, A.; Papadakis, M.; Alruwaili, M.; Batiha, G.E. The mechanistic role of piracetam in the management of vascular dementia. Behav. Brain Res. 2025, 486, 115551. [Google Scholar] [CrossRef] [PubMed]
- Kessler, J.; Thiel, A.; Karbe, H.; Heiss, W.D. Piracetam improves activated blood flow and facilitates rehabilitation of poststroke aphasic patients. Stroke 2000, 31, 2112–2116. [Google Scholar] [CrossRef]
- Zhu, Z.; Hu, R.; Li, J.; Xing, X.; Chen, J.; Zhou, Q.; Sun, J. Alpinetin exerts anti-inflammatory, anti-oxidative and anti-angiogenic effects through activating the Nrf2 pathway and inhibiting NLRP3 pathway in carbon tetrachloride-induced liver fibrosis. Int. Immunopharmacol. 2021, 96, 107660. [Google Scholar] [CrossRef]
- Pan, J.; Chen, S.; Guo, W.; Cao, S.; Shi, X.; Zhang, J.; Zhang, H.; Zhang, S. Alpinetin protects against hepatic ischemia/reperfusion injury in mice by inhibiting the NF-kappaB/MAPK signaling pathways. Int. Immunopharmacol. 2021, 95, 107527. [Google Scholar] [CrossRef]
- Hasan, S.; Khatri, N.; Rahman, Z.N.; Menezes, A.A.; Martini, J.; Shehjar, F.; Mujeeb, N.; Shah, Z.A. Neuroprotective Potential of Flavonoids in Brain Disorders. Brain Sci. 2023, 13, 1258. [Google Scholar] [CrossRef]
- Youdim, K.A.; Shukitt-Hale, B.; Joseph, J.A. Flavonoids and the brain: Interactions at the blood-brain barrier and their physiological effects on the central nervous system. Free Radic. Biol. Med. 2004, 37, 1683–1693. [Google Scholar] [CrossRef]
- Di Pietro, A.; Conseil, G.; Perez-Victoria, J.M.; Dayan, G.; Baubichon-Cortay, H.; Trompier, D.; Steinfels, E.; Jault, J.M.; de Wet, H.; Maitrejean, M.; et al. Modulation by flavonoids of cell multidrug resistance mediated by P-glycoprotein and related ABC transporters. Cell. Mol. Life Sci. 2002, 59, 307–322. [Google Scholar] [CrossRef]
- Jittiwat, J. Baihui Point Laser Acupuncture Ameliorates Cognitive Impairment, Motor Deficit, and Neuronal Loss Partly via Antioxidant and Anti-Inflammatory Effects in an Animal Model of Focal Ischemic Stroke. Evid.-Based Complement. Altern. Med. 2019, 2019, 1204709. [Google Scholar] [CrossRef] [PubMed]
- Thongrong, S.; Surapinit, S.; Promsrisuk, T.; Jittiwat, J.; Kongsui, R. Pinostrobin alleviates chronic restraint stress-induced cognitive impairment by modulating oxidative stress and the function of astrocytes in the hippocampus of rats. Biomed. Rep. 2023, 18, 20. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.J.; Walker, F.R. Strategies to improve quantitative assessment of immunohistochemical and immunofluorescent labelling. Sci. Rep. 2015, 5, 10607. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thongrong, S.; Kongsui, R.; Klimaschewski, L.; Jittiwat, J. Modulatory Potential of Alpinetin on Inflammation, Oxidative Stress, Apoptosis, and Mitochondrial Dynamics in a Rat Middle Cerebral Artery Occlusion Model of Ischemic Stroke. Int. J. Mol. Sci. 2025, 26, 11329. https://doi.org/10.3390/ijms262311329
Thongrong S, Kongsui R, Klimaschewski L, Jittiwat J. Modulatory Potential of Alpinetin on Inflammation, Oxidative Stress, Apoptosis, and Mitochondrial Dynamics in a Rat Middle Cerebral Artery Occlusion Model of Ischemic Stroke. International Journal of Molecular Sciences. 2025; 26(23):11329. https://doi.org/10.3390/ijms262311329
Chicago/Turabian StyleThongrong, Sitthisak, Ratchaniporn Kongsui, Lars Klimaschewski, and Jinatta Jittiwat. 2025. "Modulatory Potential of Alpinetin on Inflammation, Oxidative Stress, Apoptosis, and Mitochondrial Dynamics in a Rat Middle Cerebral Artery Occlusion Model of Ischemic Stroke" International Journal of Molecular Sciences 26, no. 23: 11329. https://doi.org/10.3390/ijms262311329
APA StyleThongrong, S., Kongsui, R., Klimaschewski, L., & Jittiwat, J. (2025). Modulatory Potential of Alpinetin on Inflammation, Oxidative Stress, Apoptosis, and Mitochondrial Dynamics in a Rat Middle Cerebral Artery Occlusion Model of Ischemic Stroke. International Journal of Molecular Sciences, 26(23), 11329. https://doi.org/10.3390/ijms262311329






