High Sorption Efficiency of Purified Clinoptilolite-Tuff for Aflatoxins B1 and M1: A Case Study in Plant-Based Beverages and Milk
Abstract
1. Introduction
1.1. Mycotoxins with a Special Focus on Aflatoxins
1.1.1. Aflatoxin B1-Contamination of Plant-Based Beverages
1.1.2. Aflatoxin M1-Contamination of Animal Milk and Dairy Products
1.1.3. Aflatoxin Decontamination
1.2. Zeolite—Structure, Application, and Purification
1.2.1. The Application of Zeolites as Sorbents for Various Substances
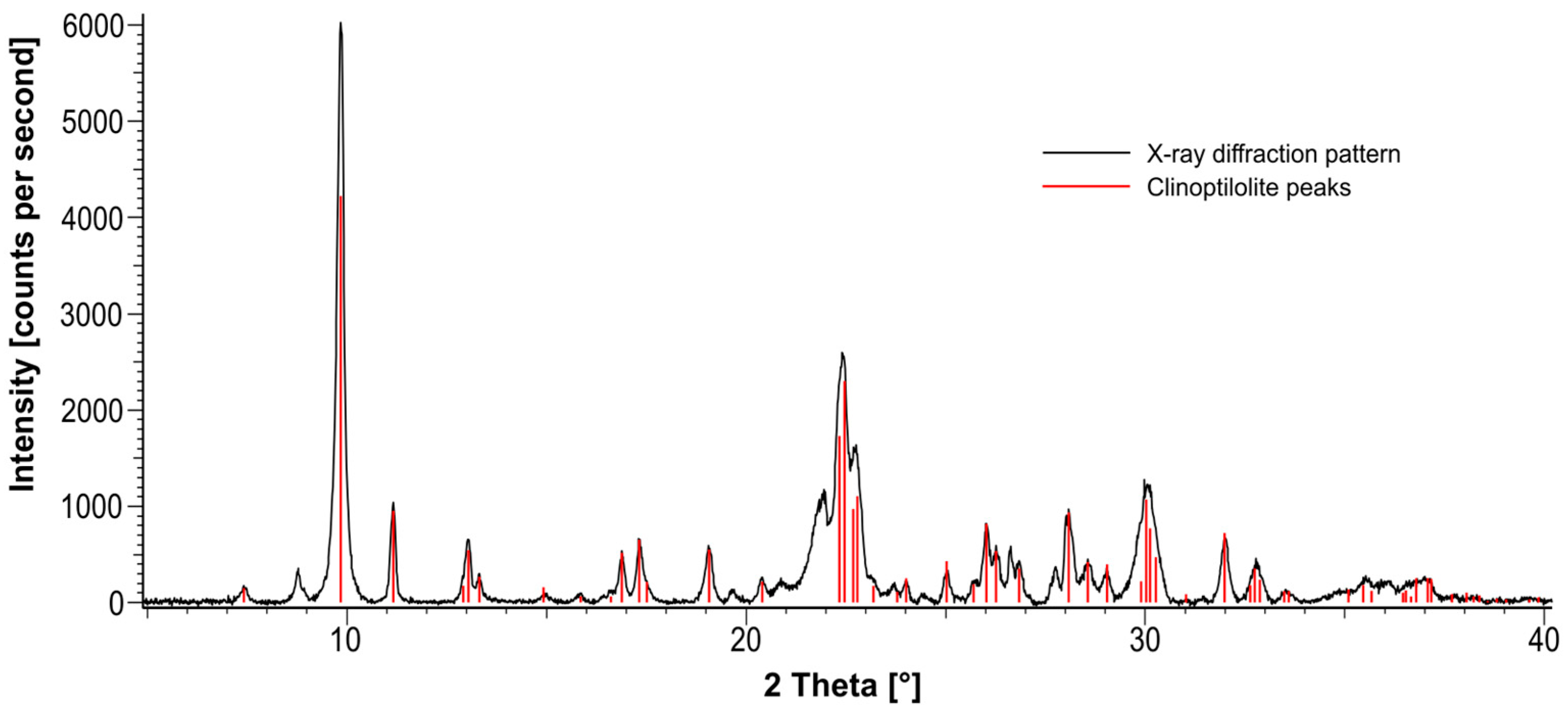
1.2.2. Clinoptilolite and Purified Clinoptilolite-Tuff (PCT)
2. Results
2.1. Analysis of AfB1 Sorption in Plant-Based Beverages by PCT
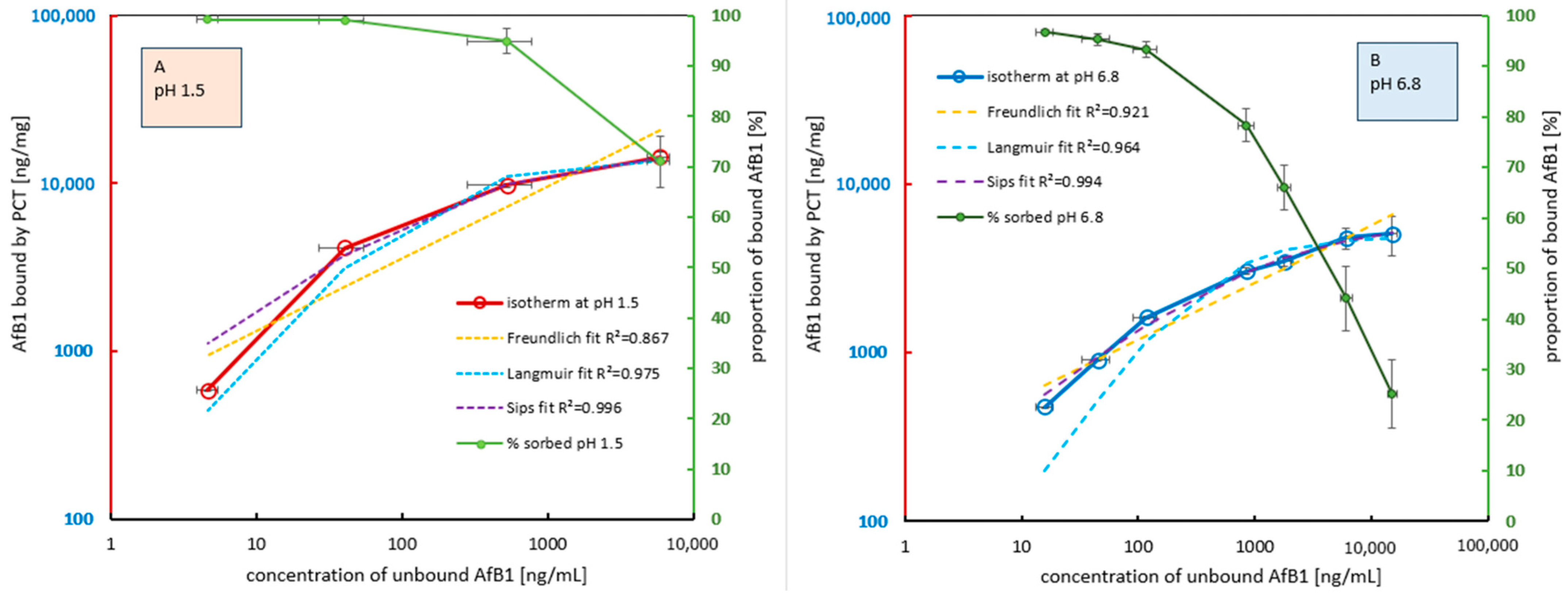
2.1.1. Saturation of PCT with AfB1 at Different pH Values Relevant to Human Digestion
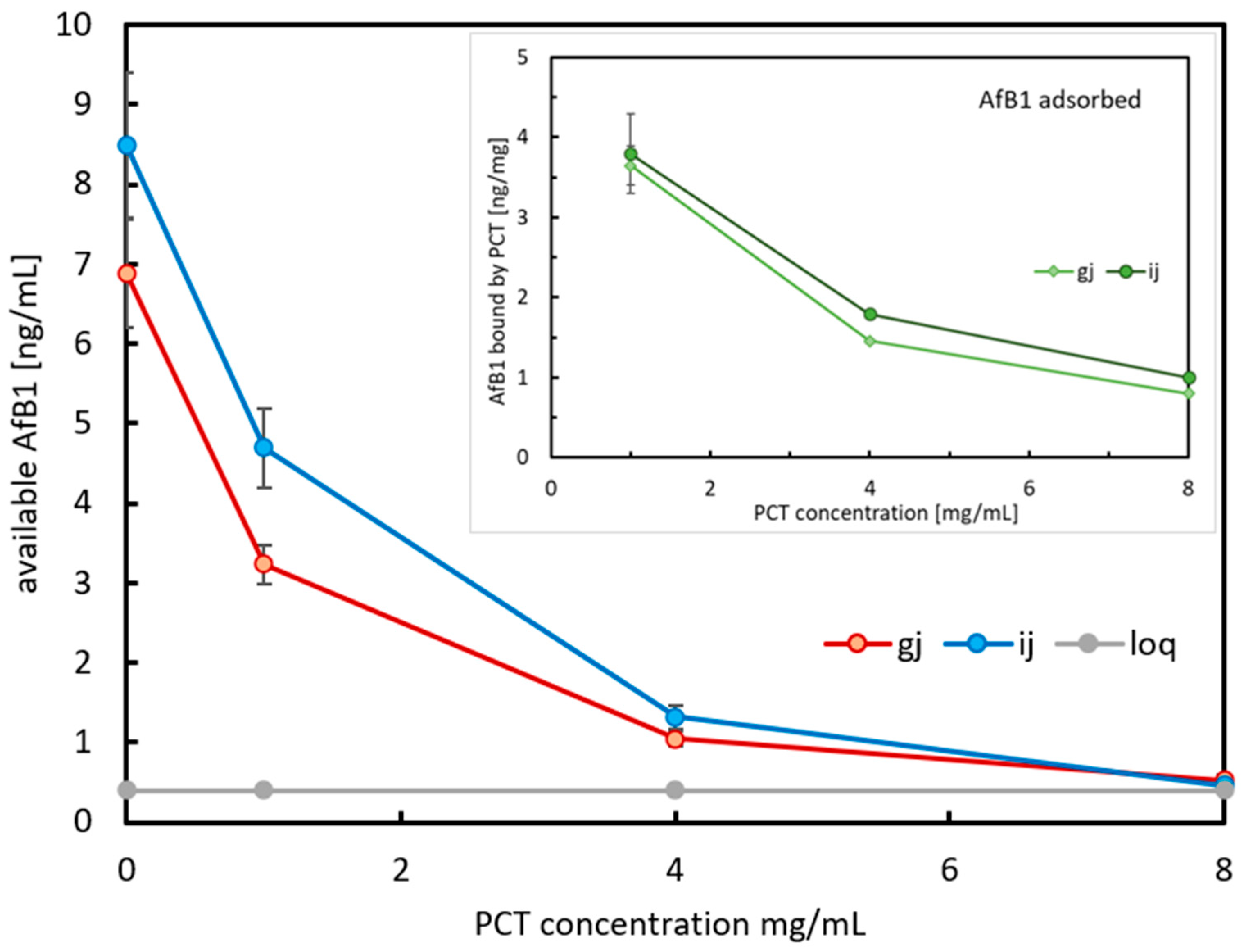
2.1.2. Sorption of AfB1 by PCT in Spiked Almond Drink During Artificial Digestion in Synthetic Gastric and Intestinal Juice
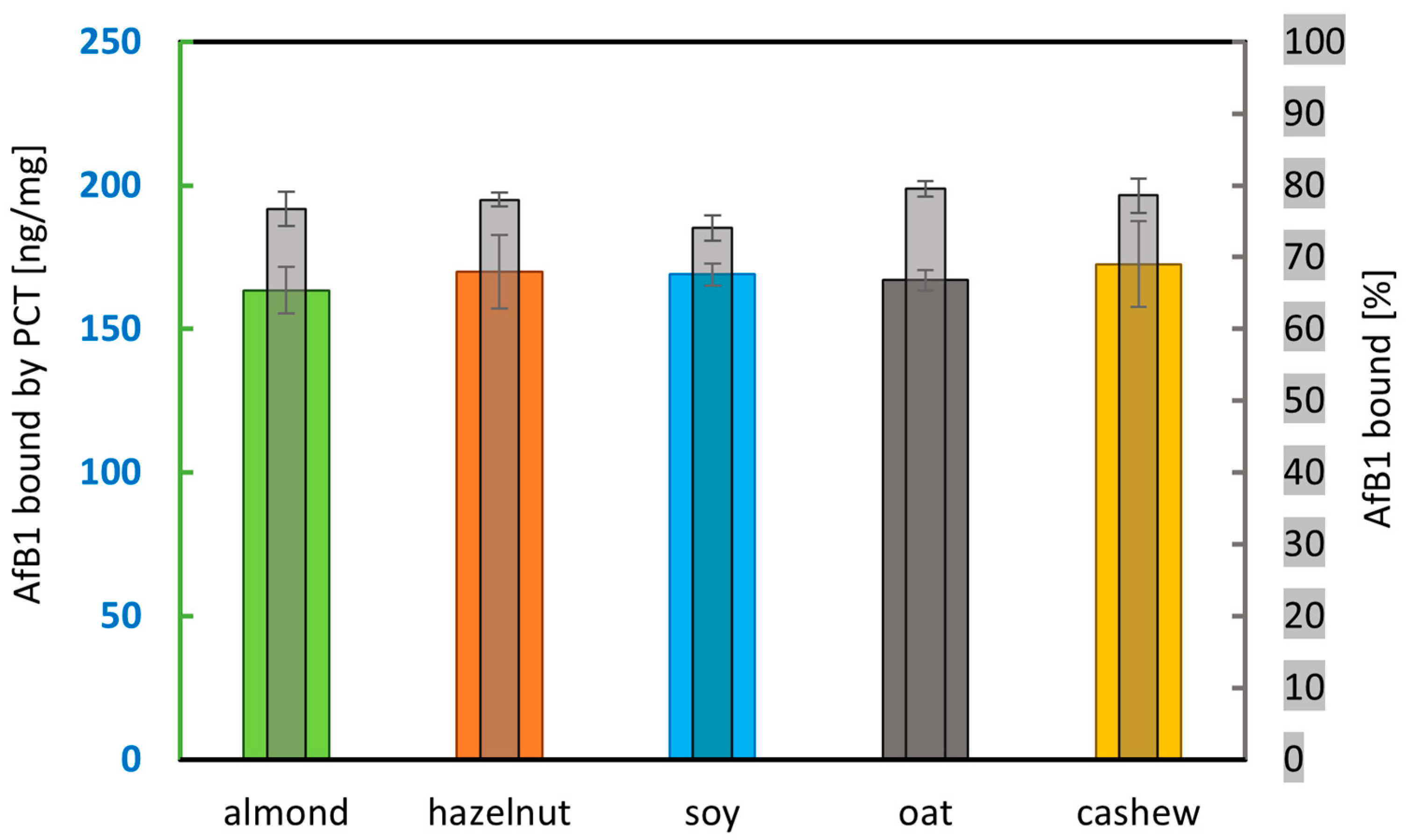
2.1.3. Sorption of Aflatoxin B1 by PCT in Selected Types of Plant-Based Beverages (Almond, Hazelnut, Soy, Oat, and Cashew Drinks) in Phosphate Buffer at pH 6.8
2.2. Adsorption of Aflatoxin M1 in Spiked Cow Milk on Purified Clinoptilolite-Tuff
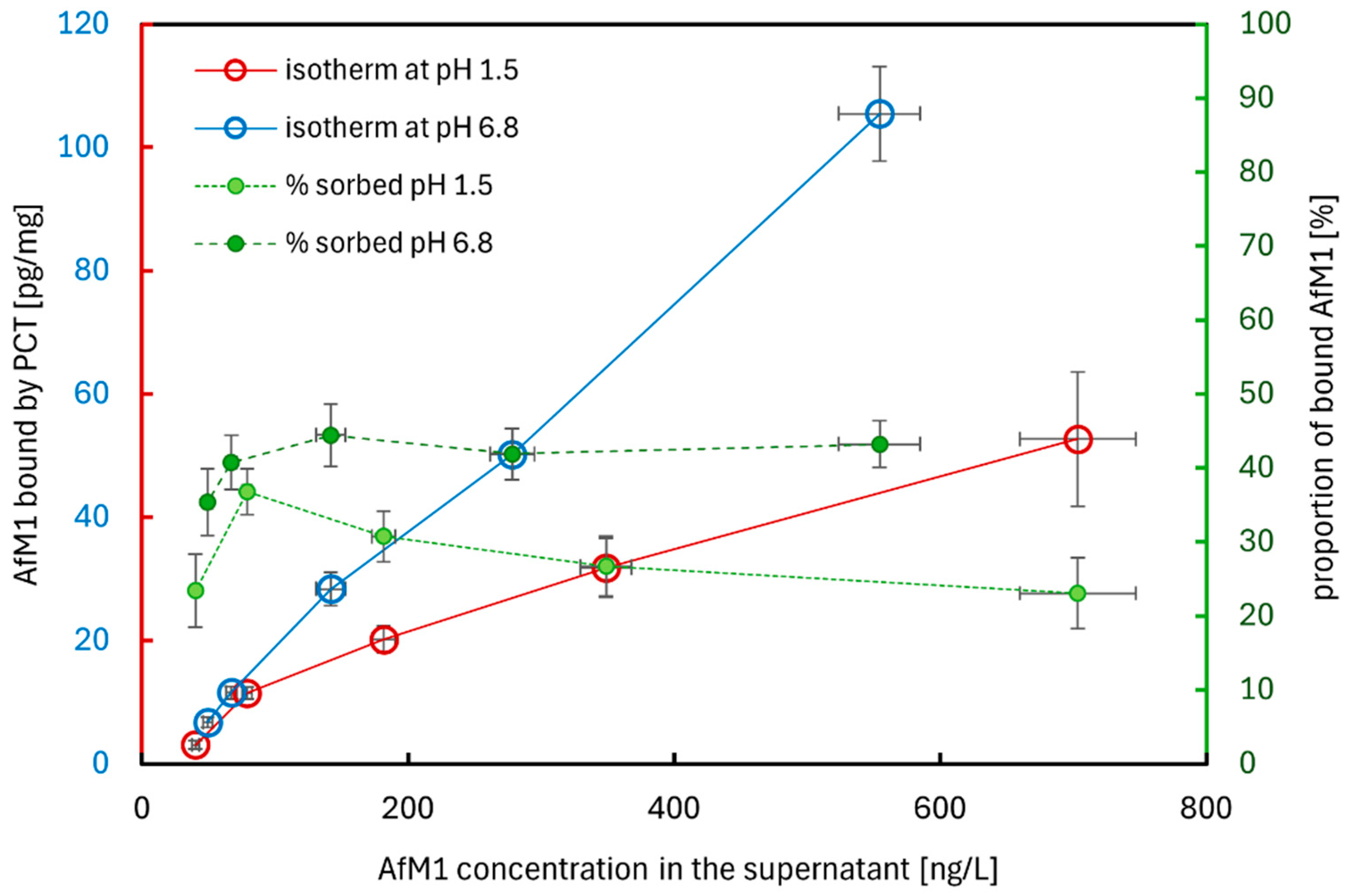
2.2.1. The Sorption of AfM1 to PCT at Different pH Values
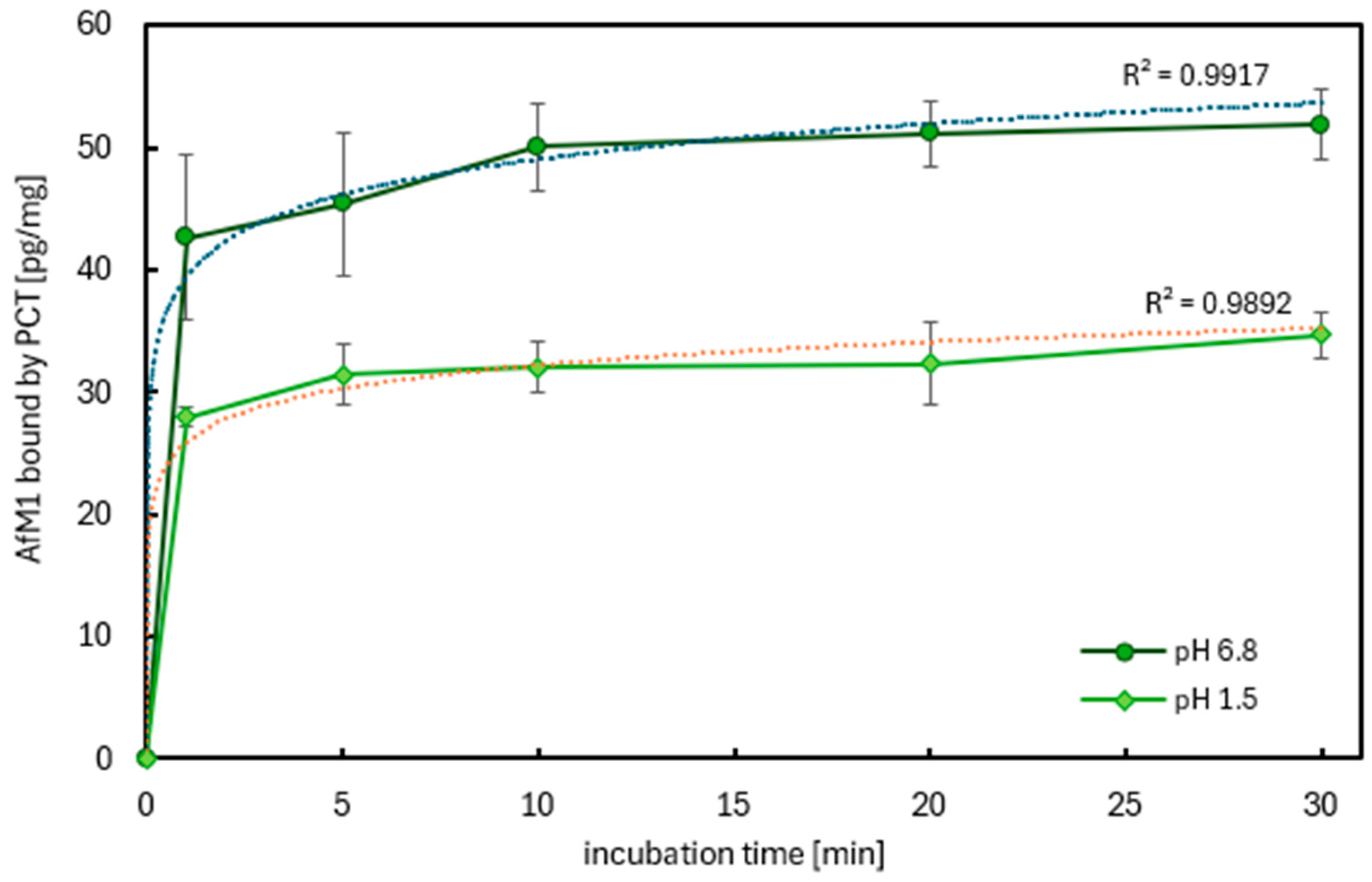
2.2.2. The Kinetics of AfM1 Sorption in Milk by PCT at Different pH Values
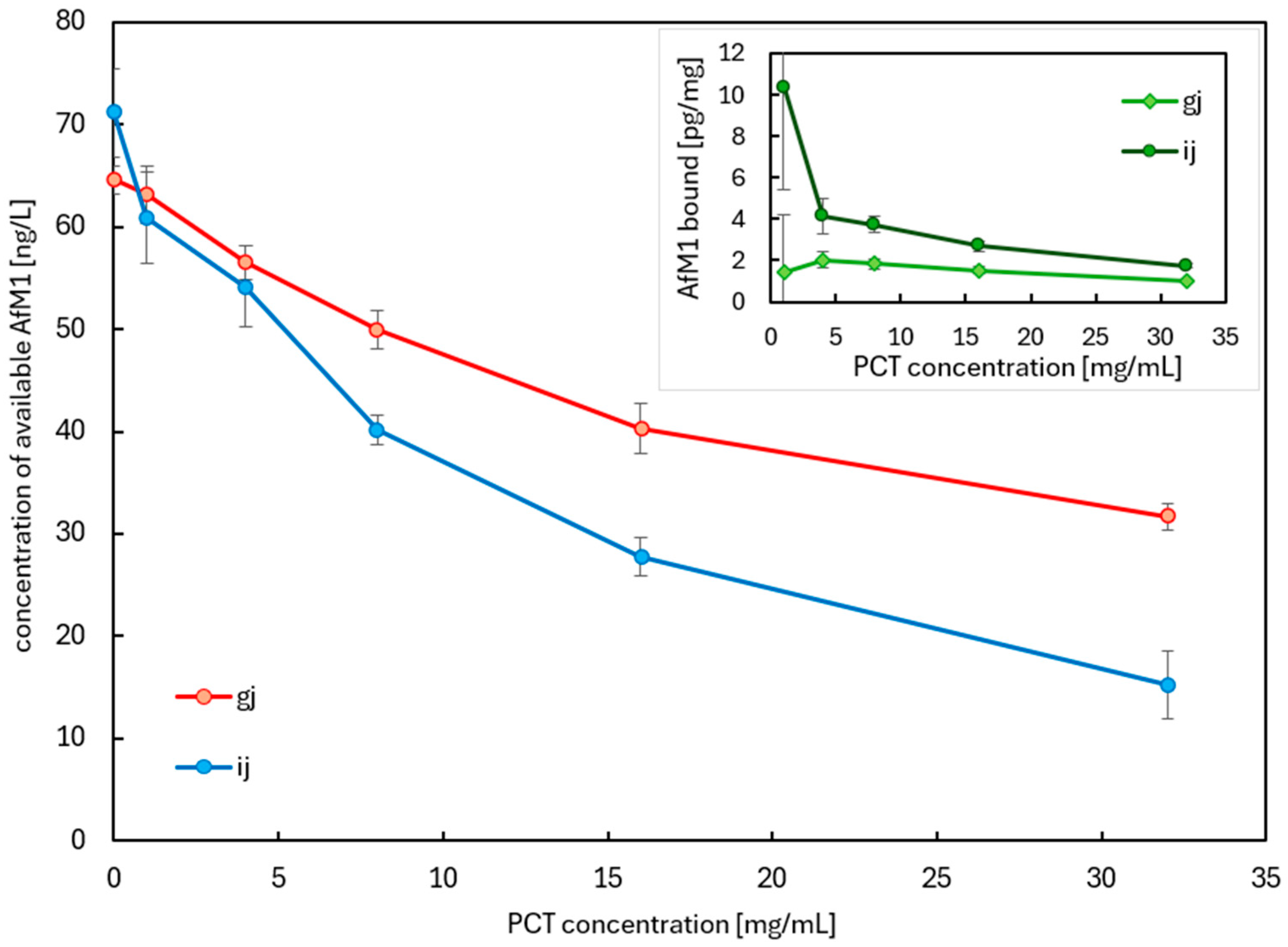
2.2.3. Adsorption of AfM1 by PCT During Simple Artificial Digestion by Stepwise Increase in pH and Desorption of AfM1 from PCT After a 4 Hour Incubation
2.2.4. PCT as an Adsorbent of AfM1 in Contaminated Cow Milk During Simulated Digestion Using Artificial Digestive Juices
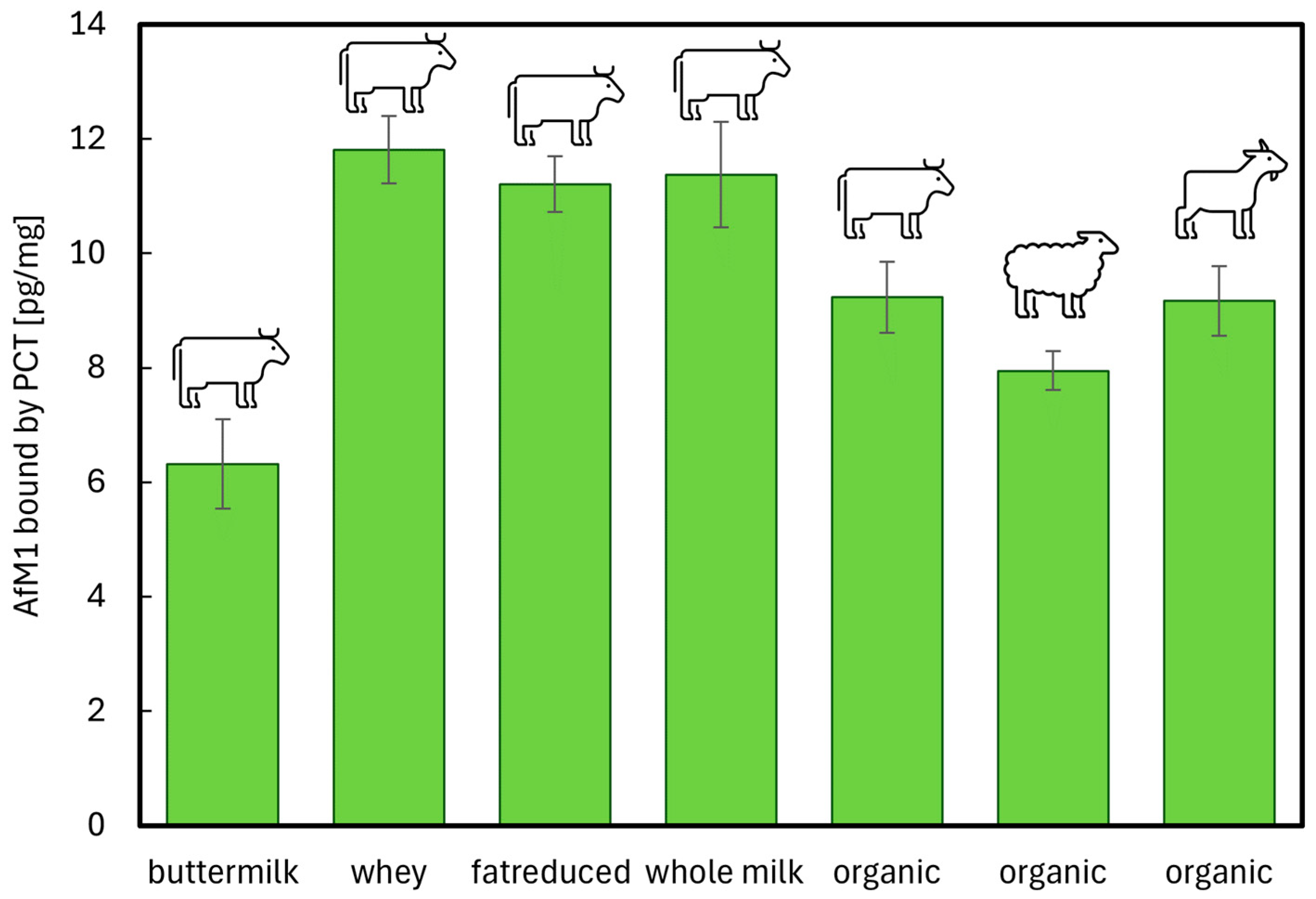
2.3. Neutralization of AfM1 by Purified Clinoptilolite-Tuff in Cow’s Dairy Products of Varying Qualities and in Milk from Sheep and Goat
Comparative Study of AfM1 Neutralization by PCT in Cow, Sheep, and Goat Milk and Dairy Products
3. Discussion
3.1. The Complexity of Mycotoxin Science
3.2. Aflatoxins as a Global Concern
3.3. Aflatoxin B1 in Plant-Based Beverages
3.4. Aflatoxin M1 in Dairy Products of Different Origins
3.5. Strategies Against Aflatoxin Exposure
3.5.1. Prevention and Avoidance
3.5.2. Detoxification/Decontamination
Chemical Mitigation of Aflatoxin Contamination
Physical Methods to Reduce the Aflatoxin Content
- Temperature
- Sorbents
Biological Methods
4. Materials and Methods
4.1. The Chemical Composition of Buffers Used in the Experiments Performed
4.2. Artificial Digestion Fluids Preparations
4.2.1. Preparation of Artificial Gastric Fluid
4.2.2. Preparation of Artificial Intestinal Fluid
4.3. Preparing the PCT Suspension
4.4. Preparing the Aflatoxin B1 and M1 Stock Solutions
4.5. Adsorption and Desorption of Aflatoxins B1 or M1 to and from Purified Clinoptilolite Tuff (PCT) in Milk and Vegetable Drinks
4.5.1. The Generation of an AfB1 Saturation Curve
| qe | … | equilibrium adsorption capacity [ng/mg] or [pg/mg]; |
| Kf | … | Freundlich constant; |
| ce | … | equilibrium adsorbate concentration in the supernatant [ng/mL]; |
| 1/n | … | heterogeneity factor [dimensionless]; |
| qmax | … | maximum adsorption capacity [ng/mg] or [pg/mg]; |
| KL | … | Langmuir constant. |
4.5.2. Gastric and Intestinal Digestion Model of AfB1 Adsorption in Almond Drink
4.5.3. Comparison of AfB1 Adsorption in Different Vegetable Drinks
4.5.4. The Generation of a AfM1 Saturation Curve
4.5.5. The Kinetics of AfM1 Adsorption to PCT at pH 1.5 and pH 6.8
4.5.6. The Creation of a Simple Digestion Model with Incremental pH for AfM1
4.5.7. PCT-Adsorption of AfM1 by Mimicking In Vivo Conditions Using Artificial Gastric and Intestinal Fluids
4.5.8. Comparative Analysis of AfM1 Sorption by PCT in Milk and Milk Products of Cow, Goat, and Sheep
4.6. Determination of Aflatoxin AfB1 and AfM1 Content via ELISA
4.6.1. Analysis of AFB1 Content by ELISA
4.6.2. Analysis of AFM1 Content by ELISA
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khaneghah, A.M.; Fakhri, Y.; Gahruie, H.H.; Niakousari, M.; Sant’Ana, A.S. Mycotoxins in cereal-based products during 24 years (1983–2017): A global systematic review. Trends Food Sci. Technol. 2019, 91, 95–105. [Google Scholar] [CrossRef]
- Phillips, T.D.; Afriyie-Gyawu, E.; Williams, J.; Huebner, H.; Ankrah, N.A.; Ofori-Adjei, D.; Jolly, P.; Johnson, N.; Taylor, J.; Marroquin-Cardona, A.; et al. Reducing human exposure to aflatoxin through the use of clay: A review. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2008, 25, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Binder, E.M.; Tan, L.M.; Chin, L.J.; Handl, J.; Richard, J. Worldwide occurrence of mycotoxins in commodities, feeds and feed ingredients. Anim. Feed Sci. Technol. 2007, 137, 265–282. [Google Scholar] [CrossRef]
- Pickova, D.; Ostry, V.; Toman, J.; Malir, F. Aflatoxins: History, significant milestones, recent data on their toxicity and ways to mitigation. Toxins 2021, 13, 399. [Google Scholar] [CrossRef]
- Sipos, P.; Peles, F.; Brassó, D.L.; Béri, B.; Pusztahelyi, T.; Pócsi, I.; Győri, Z. Physical and chemical methods for reduction in aflatoxin content of feed and food. Toxins 2021, 13, 204. [Google Scholar] [CrossRef]
- Romero-Sánchez, I.; Alonso-Núñez, I.; Gracia-Lor, E.; Madrid-Albarrán, Y. Analysis and evaluation of in vitro bioaccessibility of aflatoxins B1, B2, G1 and G2 in plant-based milks. Food Chem. 2024, 460 Pt 1, 140538. [Google Scholar] [CrossRef]
- Giovati, L.; Magliani, W.; Ciociola, T.; Santinoli, C.; Conti, S.; Polonelli, L. AFM1 in milk: Physical, biological, and prophylactic methods to mitigate contamination. Toxins 2015, 7, 4330–4349. [Google Scholar] [CrossRef]
- Commission Regulation (EU) 2023/915 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No 1881/2006 (Text with EEA Relevance), C/2023/35, OJ L 119. 5 May 2023, pp. 103–157. Available online: https://eur-lex.europa.eu/eli/reg/2023/915/oj/eng (accessed on 5 May 2025).
- Commission Regulation (EC) No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32006R1881 (accessed on 5 May 2025).
- Pavlenko, R.; Berzina, Z.; Reinholds, I.; Bartkiene, E.; Bartkevics, V. An occurrence study of mycotoxins in plant-based beverages using liquid chromatography-mass spectrometry. Toxins 2024, 16, 53. [Google Scholar] [CrossRef] [PubMed]
- Di Natale, F.; Gallo, M.; Nigro, R. Adsorbents selection for aflatoxins removal in bovine milks. J. Food Eng. 2009, 95, 186–191. [Google Scholar] [CrossRef]
- Assaf, J.C.; Nahle, S.; Chokr, A.; Louka, N.; Atoui, A.; El Khoury, A. Assorted methods for decontamination of aflatoxin M1 in milk using microbial adsorbents. Toxins 2019, 11, 304. [Google Scholar] [CrossRef]
- Sharma, A.K.; Kumar, A.; Rijal, R. Phylogenetic studies and distinction of aflatoxin-producing aspergillus species in section flavi, ochraceorosei and nidulantes: A review. Gene 2025, 937, 149151. [Google Scholar] [CrossRef]
- Summa, S.; Lo Magro, S.; Vita, V.; Franchino, C.; Scopece, V.; D’Antini, P.; Iammarino, M.; De Pace, R.; Muscarella, M. Occurrence of aflatoxin M1 in raw and processed milk: A Contribution to human exposure assessment after 12 years of investigation. Appl. Sci. 2025, 15, 853. [Google Scholar] [CrossRef]
- Bashiry, M.; Javanmardi, F.; Sadeghi, E.; Shokri, S.; Hossieni, H.; Oliveira, C.A.F.; Khaneghah, A.M. The prevalence of aflatoxins in commercial baby food products: A global systematic review, meta-analysis, and risk assessment study. Trends Food Sci. Technol. 2021, 114, 100–115. [Google Scholar] [CrossRef]
- Ramsing, R.; Santo, R.; Kim, B.F.; Altema-Johnson, D.; Wooden, A.; Chang, K.B.; Semba, R.D.; Love, D.C. Dairy and plant-based milks: Implications for nutrition and planetary health. Curr. Environ. Health Rep. 2023, 10, 291–302. [Google Scholar] [CrossRef]
- Brooker, P.G.; Anastasiou, K.; Smith, B.P.C.; Tan, R.; Cleanthous, X.; Riley, M.D. Nutrient composition of milk and plant-based milk alternatives: A cross-sectional study of products sold in Australia and Singapore. Food Res. Int. 2023, 173 Pt 2, 113475. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.S.; Costa, A.; Pozza, M.; Vamerali, T.; Niero, G.; Censi, S.; De Marchi, M. How animal milk and plant-based alternatives diverge in terms of fatty acid, amino acid, and mineral composition. npj Sci. Food 2023, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Gil-Serna, J.; Vázquez, C.; Patiño, B. Mycotoxins in functional beverages: A review. Beverages 2020, 6, 52. [Google Scholar] [CrossRef]
- Bundesinstitut für Risikobewertung. Mycotoxins in plant-based drinks: More data required results of a study by the Max Rubner Institute and their relevance for risk assessment: BfR-Opinion No. 029/2024 issued 25 June 2024. In BfR-Stellungnahmen; Bundesinstitut für Risikobewertung: Berlin, Germany, 2024; Volume 2024. [Google Scholar] [CrossRef]
- Regulation (EU) No 1308/2013 establishing a common organization of the markets in agricultural products. Off. J. Eur. Union 2013, 347, 671–854. Available online: https://eur-lex.europa.eu/eli/reg/2013/1308/oj/eng (accessed on 5 May 2025).
- Naeimipour, F.; Aghajani, J.; Kojuri, S.A.; Ayoubi, S. Useful approaches for reducing aflatoxin M1 content in milk and dairy products. Biomed. Biotechnol. Res. J. 2018, 2, 94–99. [Google Scholar] [CrossRef]
- Radonić, J.R.; Kocić Tanackov, S.D.; Mihajlović, I.J.; Grujić, Z.S.; Vojinović Miloradov, M.B.; Škrinjar, M.M.; Turk Sekulić, M.M. Occurrence of aflatoxin M1 in human milk samples in Vojvodina, Serbia: Estimation of average daily intake by babies. J. Environ. Sci. Health B 2017, 52, 59–63. [Google Scholar] [CrossRef]
- Akbar, N.; Nasir, M.; Naeem, N.; Ahmad, M.U.; Iqbal, S.; Rashid, A.; Imran, M.; Aslam Gondal, T.; Atif, M.; Salehi, B.; et al. Occurrence and seasonal variations of aflatoxin M1 in milk from Punjab, Pakistan. Toxins 2019, 11, 574. [Google Scholar] [CrossRef] [PubMed]
- Alrashedi, H.; Omer, S.; Almutairi, A.; Al-Basher, G.; Mohammed, O. Determination of aflatoxin M1 (AFM1) in dairy sheep, goats and camel milk in Hail Region, Kingdom of Saudi Arabia and evaluation of different methods reducing its concentrations in milk. Environ. Pollut. Bioavailab. 2023, 35, 2283056. [Google Scholar] [CrossRef]
- Jajić, I.; Glamočić, D.; Krstović, S.; Polovinski Horvatović, M. Aflatoxin M1 occurrence in Serbian milk and its impact on legislative. J. Hell. Vet. Med. Soc. 2019, 69, 1283–1290. [Google Scholar] [CrossRef]
- Minato, H. Characteristics and Uses of Natural Zeolites. Koatsugasu 1968, 5, 536–547. [Google Scholar]
- Mumpton, F.A. La roca magica: Uses of natural zeolites in agriculture and industry. Proc. Natl. Acad. Sci. USA 1999, 96, 3463–3470. [Google Scholar] [CrossRef]
- Eroglu, N.; Emekci, M.; Athanassiou, C. Applications of natural zeolites on agriculture and food production. J. Sci. Food Agric. 2017, 97, 3487–3499. [Google Scholar] [CrossRef]
- Hernández-Martínez, S.P.; Delgado-Cedeño, A.; Ramos-Zayas, Y.; Franco-Molina, M.A.; Méndez-Zamora, G.; Marroquín-Cardona, A.G.; Kawas, J.R. Aluminosilicates as a Double-Edged Sword: Adsorption of aflatoxin B1 and sequestration of essential trace minerals in an in vitro gastrointestinal poultry model. Toxins 2023, 15, 519. [Google Scholar] [CrossRef]
- Carraro, A.; De Giacomo, A.; Giannossi, M.L.; Medici, L.; Muscarella, M.; Palazzo, L.; Quaranta, V.; Summa, V.; Tateo, F. Clay minerals as adsorbents of aflatoxin M1 from contaminated milk and effects on milk quality. Appl. Clay Sci. 2014, 88–89, 92–99. [Google Scholar] [CrossRef]
- Moussa, A.I.; Sobeih, A.M.K.; Al-Hawary, I.I.; Elkassas, W.M.; Barakat, R. Efficacy of kaolin and bentonite clay to reduce aflatoxin M1 content in contaminated milk and effects on milk quality. Pak. Vet. J. 2020, 40, 181–186. [Google Scholar] [CrossRef]
- Mumpton, F.A.; Fishman, P.H. The application of natural zeolites in animal science and aquaculture. J. Anim. Sci. 1977, 45, 1188–1203. [Google Scholar] [CrossRef]
- Grifasi, N.; Ziantoni, B.; Fino, D.; Piumetti, M. Fundamental properties and sustainable applications of the natural zeolite clinoptilolite. Environ. Sci. Pollut. Res. 2024, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Karami-Osboo, R.; Maham, M.; Nasrollahzadeh, M. Synthesised magnetic nano-zeolite as a mycotoxins binder to reduce the toxicity of aflatoxins, zearalenone, ochratoxin A, and deoxynivalenol in barley. IET Nanobiotechnol. 2020, 14, 623–627. [Google Scholar] [CrossRef]
- Tomasevic-Canovic, M.; Milosevic, S. Tribochemical Process for Obtaining Organozeolite Adsorbent of Mycotoxins. EP 1 363 854 B1. 22 April 2009. Available online: https://patents.google.com/patent/EP1363854B1/en (accessed on 5 May 2025).
- Masimango, N.; Remacle, J.; Ramaut, J.L. The role of adsorption in the elimination of aflatoxin B1 from contaminated media. Eur. J. Appl. Microbiol. Biotechnol. 1978, 6, 101–105. [Google Scholar] [CrossRef]
- Applebaum, R.S.; Marth, E.H. Use of sulphite or bentonite to eliminate aflatoxin M1 from naturally contaminated raw whole milk. Z. Lebensm. Unters. Forch. 1982, 174, 303–305. [Google Scholar] [CrossRef]
- Tschegg, C.; Rice, A.H.N.; Grasemann, B.; Matiasek, E.; Kobulej, P.; Dzivák, M.; Berger, T. Petrogenesis of a large-scale miocene zeolite tuff in the eastern Slovak Republic: The Nižny Hrabovec open-pit clinoptilolite mine. Econ. Geol. 2019, 114, 1177–1194. [Google Scholar] [CrossRef]
- Tschegg, C.; Hou, Z.; Rice, A.H.N.; Fendrych, J.; Matiasek, E.; Berger, T.; Grasemann, B. Fault zone structures and strain localization in clinoptilolite-tuff (Nižny Hrabovec, Slovak Republic). J. Struct. Geol. 2020, 138, 104090. [Google Scholar] [CrossRef]
- Pabiś-Mazgaj, E.; Gawenda, T.; Pichniarczyk, P.; Stempkowska, A. mineral composition and structural characterization of the clinoptilolite powders obtained from zeolite-rich tuffs. Minerals 2021, 11, 1030. [Google Scholar] [CrossRef]
- Namanya, L.; Mukhokosi, E.P.; Mugampoza, E. Nanomaterials based biosensors applied for detection of aflatoxin B1 in cereals: A review. Discov. Mater. 2025, 5, 20. [Google Scholar] [CrossRef]
- Ehsanifar, M.; Rajati, R.; Gholami, A.; Reiss, J.P. Mold and Mycotoxin Exposure and Brain Disorders. J. Integr. Neurosci. 2023, 22, 137. [Google Scholar] [CrossRef]
- Hope, J. A review of the mechanism of injury and treatment approaches for illness resulting from exposure to water-damaged buildings, mold, and mycotoxins. Sci. World J. 2013, 2013, 767482. [Google Scholar] [CrossRef]
- Reháková, M.; Čuvanová, S.; Dzivák, M.; Rimár, J.; Gavaľová, Z. Agricultural and agrochemical uses of natural zeolite of the clinoptilolite type. Curr. Opin. Solid State Mater. Sci. 2004, 8, 397–404. [Google Scholar] [CrossRef]
- Glock, G. Method for the Removal of Heavy Metals. EP 2 040 837 B1. 20 June 2012. Available online: https://patentimages.storage.googleapis.com/3f/db/58/7f3549f0a6c10a/EP2040837B1.pdf (accessed on 5 May 2025).
- Haemmerle, M.; Fendrych, J.; Matiasek, E.; Tschegg, C. Adsorption and release characteristics of purified and non-purified clinoptilolite tuffs towards health-relevant heavy metals. Crystals 2021, 11, 1343. [Google Scholar] [CrossRef]
- Ranftler, C.; Röhrich, A.; Sparer, A.; Tschegg, C.; Nagl, D. Purified clinoptilolite-tuff as an efficient sorbent for gluten derived from food. Int. J. Mol. Sci. 2022, 23, 5143. [Google Scholar] [CrossRef] [PubMed]
- Ranftler, C.; Zehentner, M.; Pengl, A.; Röhrich, A.; Tschegg, C.; Nagl, D. Purified clinoptilolite-tuff as an efficient sorbent for food-derived peanut allergens. Int. J. Mol. Sci. 2024, 25, 6510. [Google Scholar] [CrossRef]
- Ranftler, C.; Nagl, D.; Sparer, A.; Röhrich, A.; Freissmuth, M.; El-Kasaby, A.; Nasrollahi Shirazi, S.; Koban, F.; Tschegg, C.; Nizet, S. Binding and neutralization of C. difficile toxins A and B by purified clinoptilolite-tuff. PLoS ONE 2021, 16, e0252211. [Google Scholar] [CrossRef] [PubMed]
- Nizet, S.; Rieger, J.; Sarabi, A.; Lajtai, G.; Zatloukal, K.; Tschegg, C. Binding and inactivation of human coronaviruses, including SARS-CoV-2, onto purified clinoptilolite-tuff. Sci. Rep. 2023, 13, 4673. [Google Scholar] [CrossRef]
- Sarabi, A.; Nizet, S.; Röhrich, A.; Tschegg, C. Unveiling the broad-spectrum virucidal potential of purified clinoptilolite-tuff. Microorganisms 2024, 12, 1572. [Google Scholar] [CrossRef]
- Council of Europe; EQDM European Directorate for the Quality of Medicines & HealthCare. European Pharmacopoeia, 10th ed.; Council of Europe: Strasbourg, France, 2019. [Google Scholar]
- Monter-Arciniega, A.; del Socorro Cruz-Cansino, N.; Ramírez-Moreno, E.; Zafra-Rojas, Q.Y.; Cervantes-Elizarrarás, A.; Ariza-Ortega, J.A. Pathologies associated to the consumption of aflatoxin M1 in dairy products. Mex. J. Med. Res. 2022, 10, 7–15. [Google Scholar] [CrossRef]
- Pożarska, A.; Karpiesiuk, K.; Kozera, W.; Czarnik, U.; Dąbrowski, M.; Zielonka, Ł. AFB1 Toxicity in Human Food and Animal Feed Consumption: A Review of Experimental Treatments and Preventive Measures. Int. J. Mol. Sci. 2024, 25, 5305. [Google Scholar] [CrossRef]
- Butovskaya, E.; Caprai, E.; Peloso, M.; Gasparini, M.; Borgia, M.; Abdul, M.E.; Candotti, P.; Menotta, S. Plant-based milk alternatives: Assessing the occurrence of chemical and microbiological contaminants in soy, oat, rice and almond beverages from Italian market. Food Control 2025, 169, 111005. [Google Scholar] [CrossRef]
- Caloni, F.; Stammati, A.; Friggè, G.; De Angelis, I. Aflatoxin M1 absorption and cytotoxicity on human intestinal in vitro model. Toxicon 2006, 47, 409–415. [Google Scholar] [CrossRef]
- Kiermeier, F.; Buchner, M. Zur Verteilung von Aflatoxin M1 auf Molke und Bruch bei der Käseherstellung. Z. Lebensm. Unters. Forch. 1977, 164, 82–86. [Google Scholar] [CrossRef]
- Fallah, A.A.; Fazlollahi, R.; Emami, A. Seasonal study of aflatoxin M1 contamination in milk of four dairy species in Yazd, Iran. Food Control 2016, 68, 77–82. [Google Scholar] [CrossRef]
- Sulaiman, S.H.; Jamaluddin, R.; Sabran, M.R. Association between urinary aflatoxin (AFM1) and dietary intake among adults in Hulu Langat District, Selangor, Malaysia. Nutrients 2018, 10, 460. [Google Scholar] [CrossRef]
- de Lima Schlösser, L.M.; Simões, C.T.; Sarturi, J.A.; da Silva, C.R.; Laber, I.F.; Franco, D.S.P.; Mallmann, C.A. Adsorption of aflatoxin B1 by different antimycotoxin additives: Bentonite, clinoptilolite, and beta-glucans extracted from yeast cell wall. Mycotoxin Res. 2024, 40, 111–121. [Google Scholar] [CrossRef]
- European Union. Directive 202/32/EC of the European Parliament and Council of 7 May 2002 on Undesirable Substance in Animal Feed; European Union: Brussels, Belgium, 2002. [Google Scholar]
- Commission Regulation (EU) No 2015/786 of 19 May 2015 Defining Acceptability Criteria for Detoxification Processes Applied to Products Intended for Animal Feed as Provided for in Directive 2002/32/EC of the European Parliament and of the Council. Off. J. Eur. Union 2015, L125, 10–14. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32015R0786 (accessed on 20 May 2025).
- Dommergues, Y.; Mangenot, F. Ecologie Microbienne Du Sol, 1st ed.; Masson et Cie, éditeurs: Paris, France, 1970; p. 796. [Google Scholar]
- Hamad, G.M.; El-Makarem, H.S.A.; Allam, M.G.; El Okle, O.S.; El-Toukhy, M.I.; Mehany, T.; El-Halmouch, Y.; Abushaala, M.M.F.; Saad, M.S.; Korma, S.A.; et al. Evaluation of the adsorption efficacy of bentonite on aflatoxin M1 Levels in contaminated milk. Toxins. 2023, 15, 107. [Google Scholar] [CrossRef]
- Samekova, K.; Firbas, C.; Irrgeher, J.; Opper, C.; Prohaska, T.; Retzmann, A.; Tschegg, C.; Meisslitzer, C.; Tchaikovsky, A.; Gouya, G.; et al. Concomitant oral intake of purified clinoptilolite tuff (G-PUR) reduces enteral lead uptake in healthy humans. Sci. Rep. 2021, 11, 14796. [Google Scholar] [CrossRef]
- Irrgeher, J.; Berger, T.; Tchaikovsky, A.; Tschegg, C.; Gouya, G.; Lechner, P.; Retzmann, A.; Opper, C.; Firbas, C.; Freissmuth, M.; et al. Enriched stable 204Pb as tracer at ultra-low levels in clinical investigations. Anal. Bioanal. Chem. 2023, 415, 255–268. [Google Scholar] [CrossRef]
- Raj, J.; Vasiljević, M.; Tassis, P.; Farkaš, H.; Bošnjak-Neumüller, J.; Männer, K. Effects of a modified clinoptilolite zeolite on growth performance, health status and detoxification of aflatoxin B1 and ochratoxin A in male broiler chickens. Br. Poult. Sci. 2021, 62, 601–610. [Google Scholar] [CrossRef]
- Alharthi, A.S.; Al Sulaiman, A.R.; Aljumaah, R.S.; Alabdullatif, A.A.; Ferronato, G.; Alqhtani, A.H.; Al-Garadi, M.A.; Al-Sornokh, H.; Abudabos, A.M. The efficacy of bentonite and zeolite in reducing aflatoxin B1 toxicity on production performance and intestinal and hepatic health of broiler chickens. Ital. J. Anim. Sci. 2022, 21, 1181–1189. [Google Scholar] [CrossRef]
- Nizet, S.; Muñoz, E.; Fiebich, B.L.; Abuja, P.M.; Kashofer, K.; Zatloukal, K.; Tangermann, S.; Kenner, L.; Tschegg, C.; Nagl, D.; et al. Clinoptilolite in dextran sulphate sodium-induced murine colitis: Efficacy and safety of a microparticulate preparation. Inflamm. Bowel Dis. 2017, 24, 54–66. [Google Scholar] [CrossRef]
- Sterba, J.H.; Sperrer, H.; Wallenko, F.; Welch, J.M. Adsorption characteristics of a clinoptilolite-rich zeolite compound for Sr and Cs. J. Radioanal. Nucl. Chem. 2018, 318, 267–270. [Google Scholar] [CrossRef]
- Welch, J.M.; Foster, M.; Sterba, J.H.; Nagl, D.; Tschegg, C. Decontamination potential of radioactively contaminated wounds with purified clinoptilolite-tuff. J. Radioanal. Nucl. Chem. 2025, 1–7. [Google Scholar] [CrossRef]
- Jiménez-Reyes, M.; Almazán-Sánchez, P.T.; Solache-Ríos, M. Radioactive waste treatments by using zeolites. A short review. J. Environ. Radioact. 2021, 233, 106610. [Google Scholar] [CrossRef]
- Lihareva, N.; Petrov, O.; Dimowa, L.; Tzvetanova, Y.; Piroeva, I.; Ublekov, F.; Nikolov, A. Ion exchange of Cs+ and Sr2+ by natural clinoptilolite from bi-cationic solutions and XRD control of their structural positioning. J. Radioanal. Nucl. Chem. 2020, 323, 1093–1102. [Google Scholar] [CrossRef]
- Deinsberger, J.; Marquart, E.; Nizet, S.; Meisslitzer, C.; Tschegg, C.; Uspenska, K.; Gouya, G.; Niederdöckl, J.; Freissmuth, M.; Wolzt, M.; et al. Topically administered purified clinoptilolite-tuff for the treatment of cutaneous wounds: A prospective, randomised phase I clinical trial. Wound Repair Regen. 2022, 30, 198–209. [Google Scholar] [CrossRef]
- Loi, M.; De Leonardis, S.; Ciasca, B.; Paciolla, C.; Mulè, G.; Haidukowski, M. Aflatoxin B1 degradation by Ery4 Laccase: From in vitro to contaminated corn. Toxins 2023, 15, 310. [Google Scholar] [CrossRef]
- Iji, S.I.; Inyang, U.E.; Etuk, B.R. Models for the development of sortpion isothems: A review. Am. J. Food Sci. Technol. 2025, 13, 27–37. [Google Scholar] [CrossRef]

| Origin | Examples |
|---|---|
| Poaceae | rice, oat, corn, spelt, teff |
| Fabaceae | soy, lupin, peanut, pea, adzuki bean, cowpea |
| Amaranthaceae | amaranth, quinoa |
| Anacardiaceae | cashew, pistachio |
| Pedaliaceae | sesame |
| Arecaceae | coconut |
| Betulaceae | hazelnut |
| Juglandaceae | walnut |
| Rosaceae | almond, apricot seed |
| Cannabaceae | hemp |
| Linaceae | flax |
| Asteraceae | sunflower |
| Method | Examples |
|---|---|
| physical | sorting, dehulling, steeping, wet or dry milling, heat treatment, irradiation (gamma, solar, UV, microwave, near-infrared), pulsed electric fields, carbon filtration, high-pressure cooking, ultrasound, plasma-treatment, electrolyzed water, pulsed light |
| chemical | adsorbents (e.g., clays), acids (organic and inorganic), bases (ammoniation), enzymes (redox-active), gases (e.g., ozonation, chlorine dioxide) |
| biological | bacteria (e.g., lactic and non-lactic acid), microfungi (e.g., yeasts), genetic engineering (e.g., generation of A. flavus lacking aflatoxin-producing ability, host-induced gene silencing), vaccination |
| Minerals | |
| Main mineral | clinoptilolite (>80 wt%) |
| Minor minerals | cristobalite, albite, orthoclase, quartz |
| Trace minerals | biotite, anorthite |
| Particle size distribution | |
| D(0.5) [µm] | 3.1 ± 0.5 |
| Cation exchange capacity | |
| [mol/kg] | >0.75 |
| Type of Plant Drink | Initial pH Value | Initial AfB1 Concentration [µg/L] | AfB1 Concentration After PCT Incubation [µg/L] | AfB1 Sorbed by PCT [%] | Free Residual AfB1 [%] |
|---|---|---|---|---|---|
| Almond | 7.83 | 213.2 | 49.7 | 76.7 | 23.3 |
| Hazelnut | 7.03 | 219.8 | 47.9 | 78.2 | 21.8 |
| Soy | 7.74 | 227.4 | 59.2 | 74.0 | 26.0 |
| Oat | 7.27 | 206.7 | 43.0 | 79.2 | 20.8 |
| Cashew | 6.96 | 216.7 | 47.0 | 78.3 | 21.7 |
| Aliquotes | Incubation Time [min] | pH Indicator Test Strips | pH Meter |
|---|---|---|---|
| control | 30 | 2.0 | 2.33 |
| PCT | 30 | 2.0 | 2.39 |
| control | 60 | 5.5 | 5.48 |
| PCT | 60 | 5.5 | 5.55 |
| control | 90 | 8.0 | 7.86 |
| PCT | 90 | 8.0 | 7.81 |
| control | 240 | 8.0 | 7.80, 7.87, 7.88, 7.87 |
| PCT | 240 | 8.0 | 7.83, 7.77, 7.83, 7.82 |
| Animal | Type of Milk Product | Fat Content [%] | Protein Content [g] |
|---|---|---|---|
| Cow | Buttermilk | 1 | 3.2 |
| Cow | Whey | <0.5 | 0.6 |
| Cow | Fat-reduced milk | 0.9 | 3.4 |
| Cow | Milk (conventional) | 3.5 | 3.3 |
| Cow | Organic milk | 3.5 | 3.4 |
| Sheep | Organic milk | 4.5 | 4.6 |
| Goat | Organic milk | 2.8 | 3.1 |
| Phosphate Buffer [pH] | KH2PO4 Solution [0.2 M] Volume [mL] | NaOH Solution [0.2 M] Volume [mL] | Final Volume [mL] |
| 6.8 | 250 | 112 | 1000 |
| Test Solution [pH] | NaCl Solution [0.2 M] Volume [mL] | HCl Solution [0.2 M] Volume [mL] | Final Volume [mL] |
| 1.5 | 250 | 207 | 1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ranftler, C.; Zehentner, M.; Tschegg, C.; Nagl, D. High Sorption Efficiency of Purified Clinoptilolite-Tuff for Aflatoxins B1 and M1: A Case Study in Plant-Based Beverages and Milk. Int. J. Mol. Sci. 2025, 26, 11265. https://doi.org/10.3390/ijms262311265
Ranftler C, Zehentner M, Tschegg C, Nagl D. High Sorption Efficiency of Purified Clinoptilolite-Tuff for Aflatoxins B1 and M1: A Case Study in Plant-Based Beverages and Milk. International Journal of Molecular Sciences. 2025; 26(23):11265. https://doi.org/10.3390/ijms262311265
Chicago/Turabian StyleRanftler, Carmen, Magdalena Zehentner, Cornelius Tschegg, and Dietmar Nagl. 2025. "High Sorption Efficiency of Purified Clinoptilolite-Tuff for Aflatoxins B1 and M1: A Case Study in Plant-Based Beverages and Milk" International Journal of Molecular Sciences 26, no. 23: 11265. https://doi.org/10.3390/ijms262311265
APA StyleRanftler, C., Zehentner, M., Tschegg, C., & Nagl, D. (2025). High Sorption Efficiency of Purified Clinoptilolite-Tuff for Aflatoxins B1 and M1: A Case Study in Plant-Based Beverages and Milk. International Journal of Molecular Sciences, 26(23), 11265. https://doi.org/10.3390/ijms262311265





