Isolation of Red Beet Plant-Derived Nanovesicles, and Characterization of Their Molecular Content and Biological Activities in Human Cells
Abstract
1. Introduction
2. Results
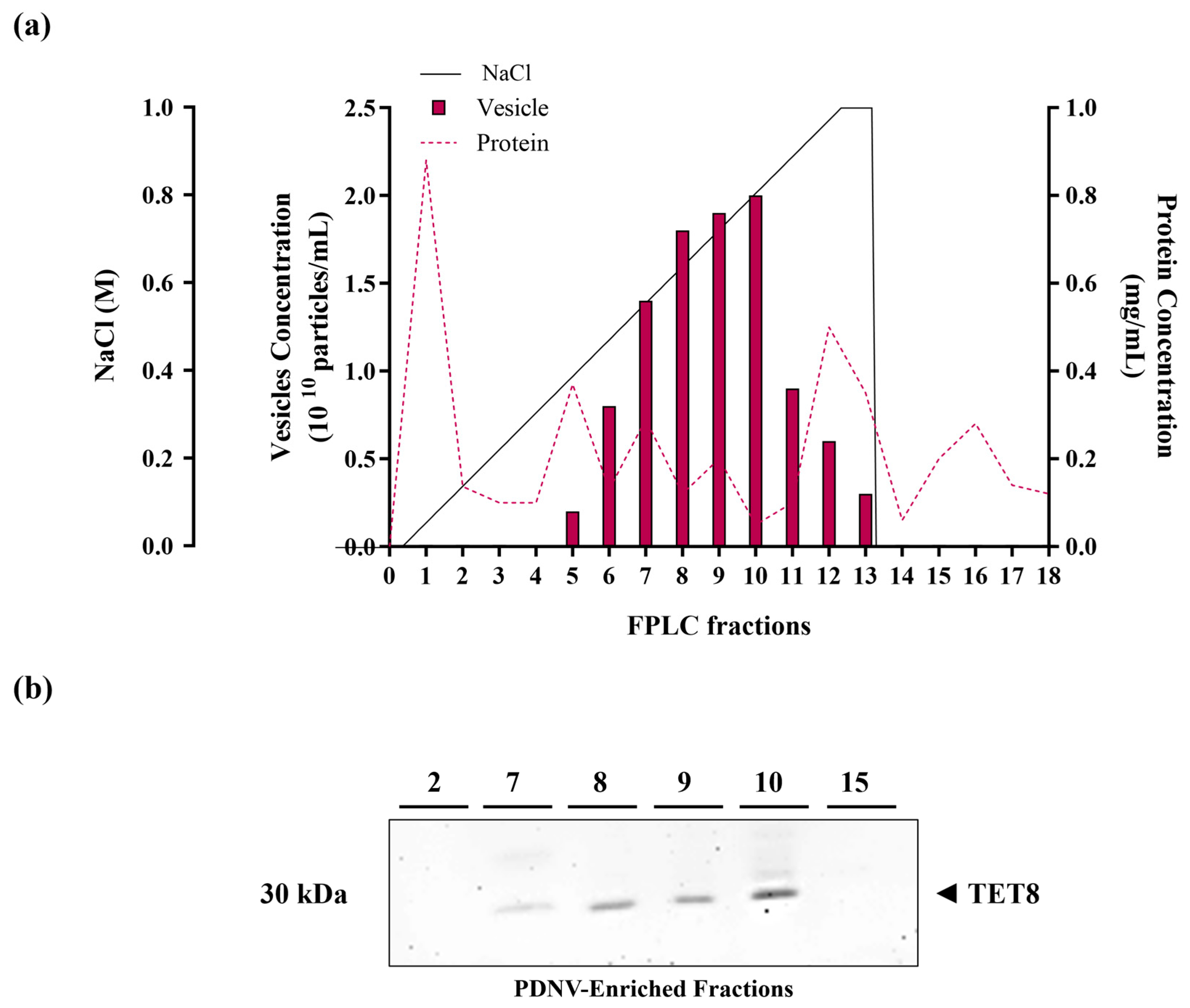
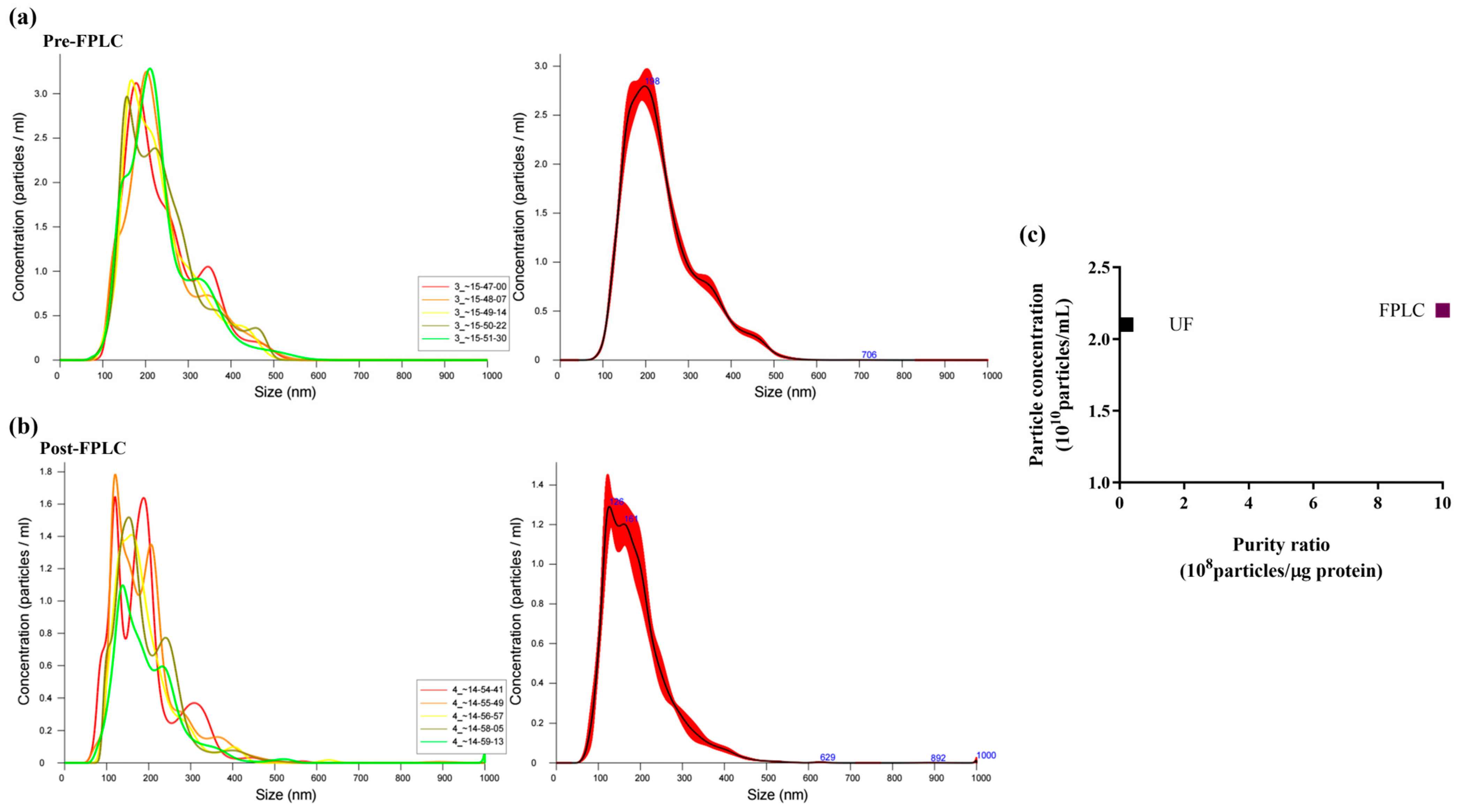
2.1. Purification by B. vulgaris PDNVs by Anion Exchange Chromatography
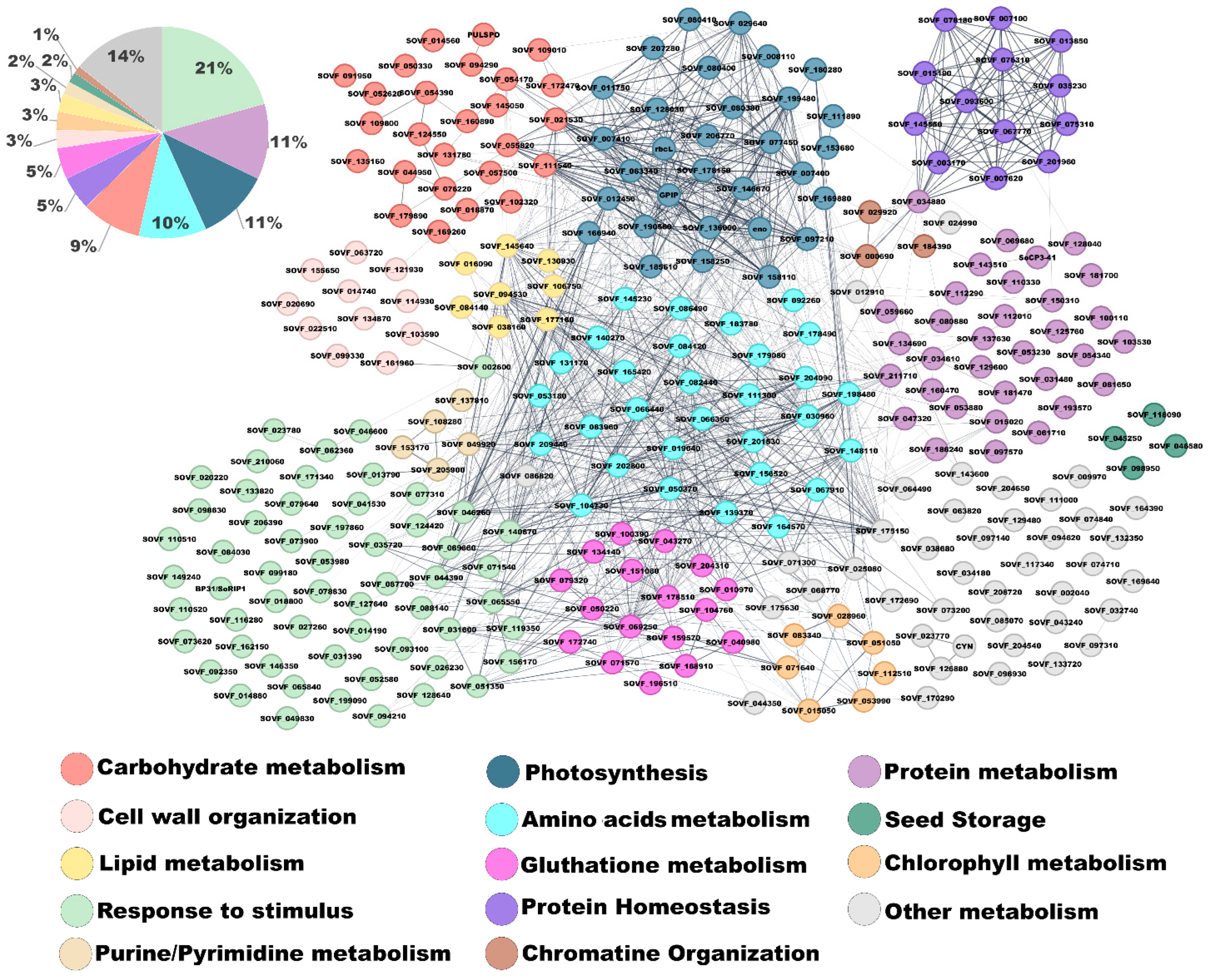
2.2. Proteomic Analysis
2.3. Lipidomic Analysis
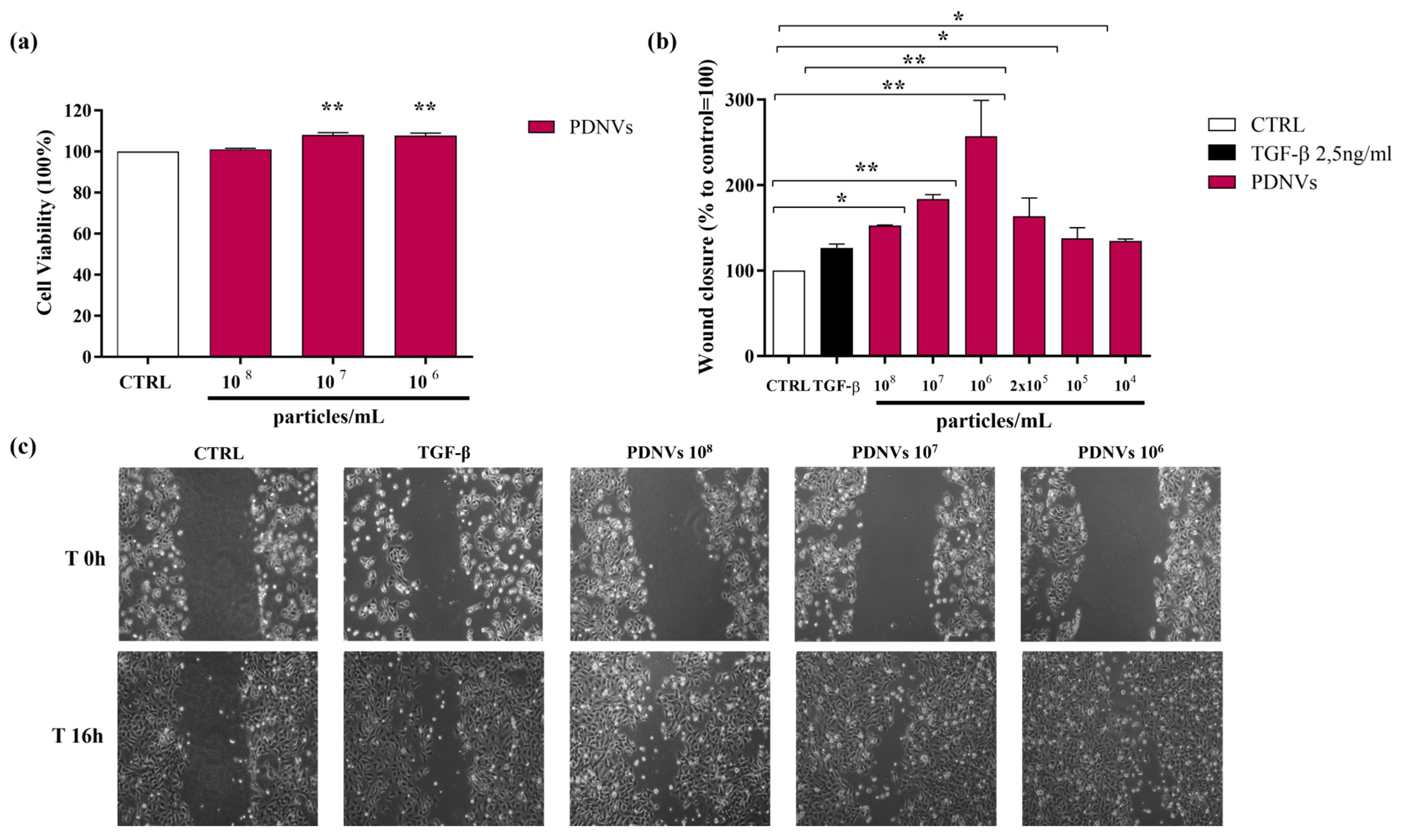
2.4. Effect of B. vulgaris PDNVs on Hakata Cell Viability
2.5. Effect of B. vulgaris PDNVs on Wound Healing in Hakata Cells
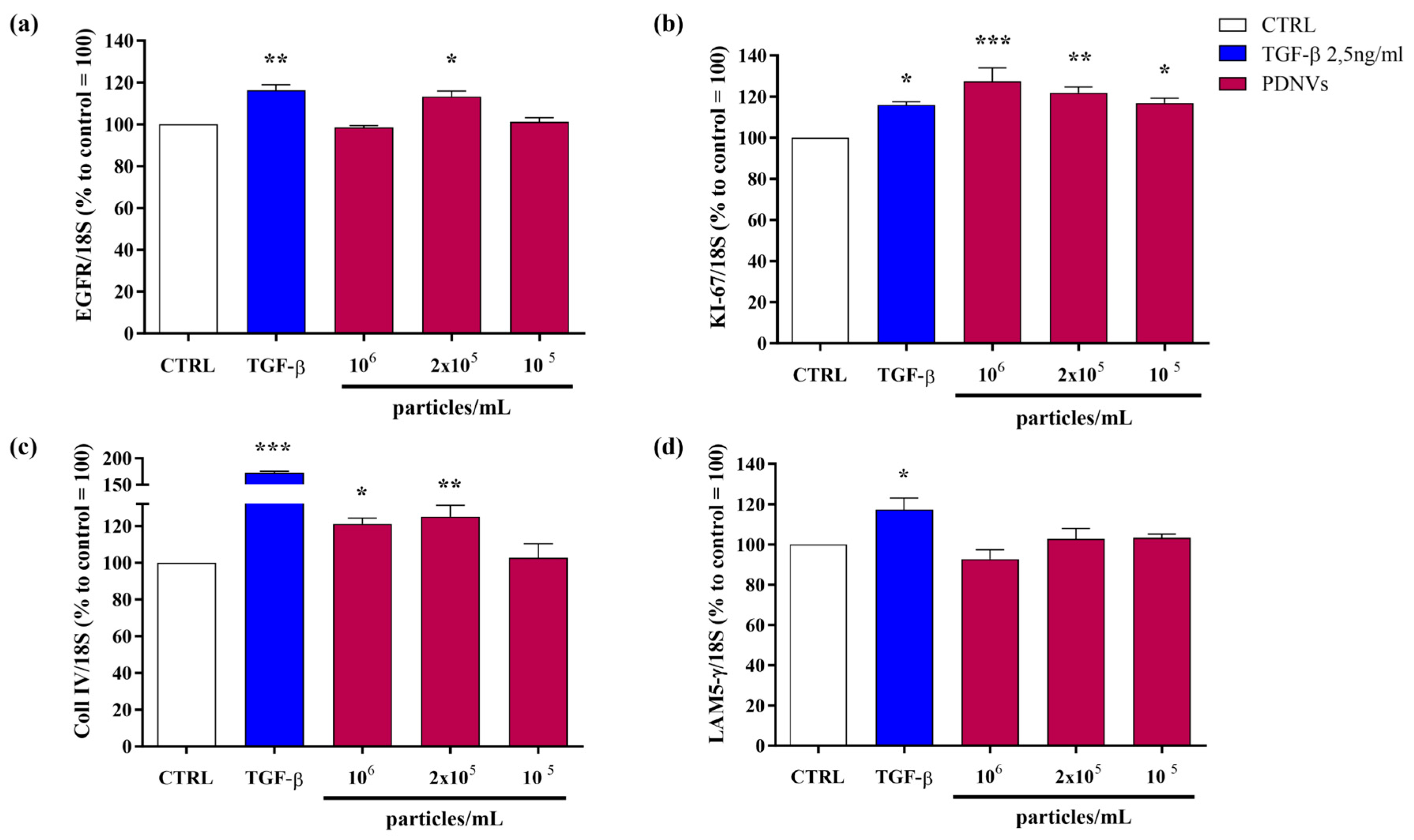
2.6. Effect of B. vulgaris PDNVs on the Transcription of Wound Healing-Related Genes in Scratched Hakata Cells
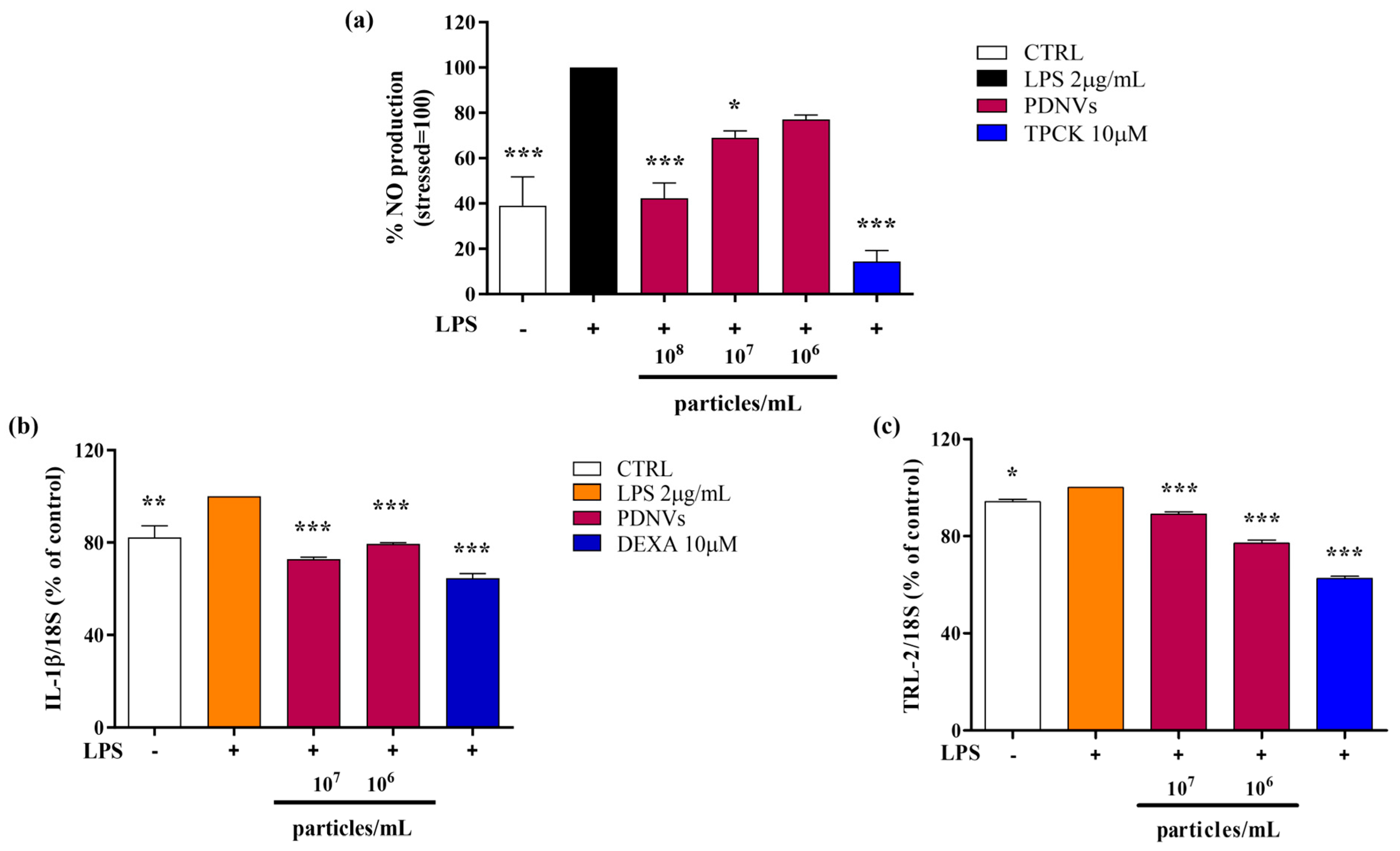
2.7. Effect of B. vulgaris PDNVs on Nitric Oxide Production in LPS-Stimulated RAW 264.7 Cells
2.8. Effect of B. vulgaris PDNVs on the Transcription of Inflammation-Related Genes in LPS-Stimulated HaCaT Cells
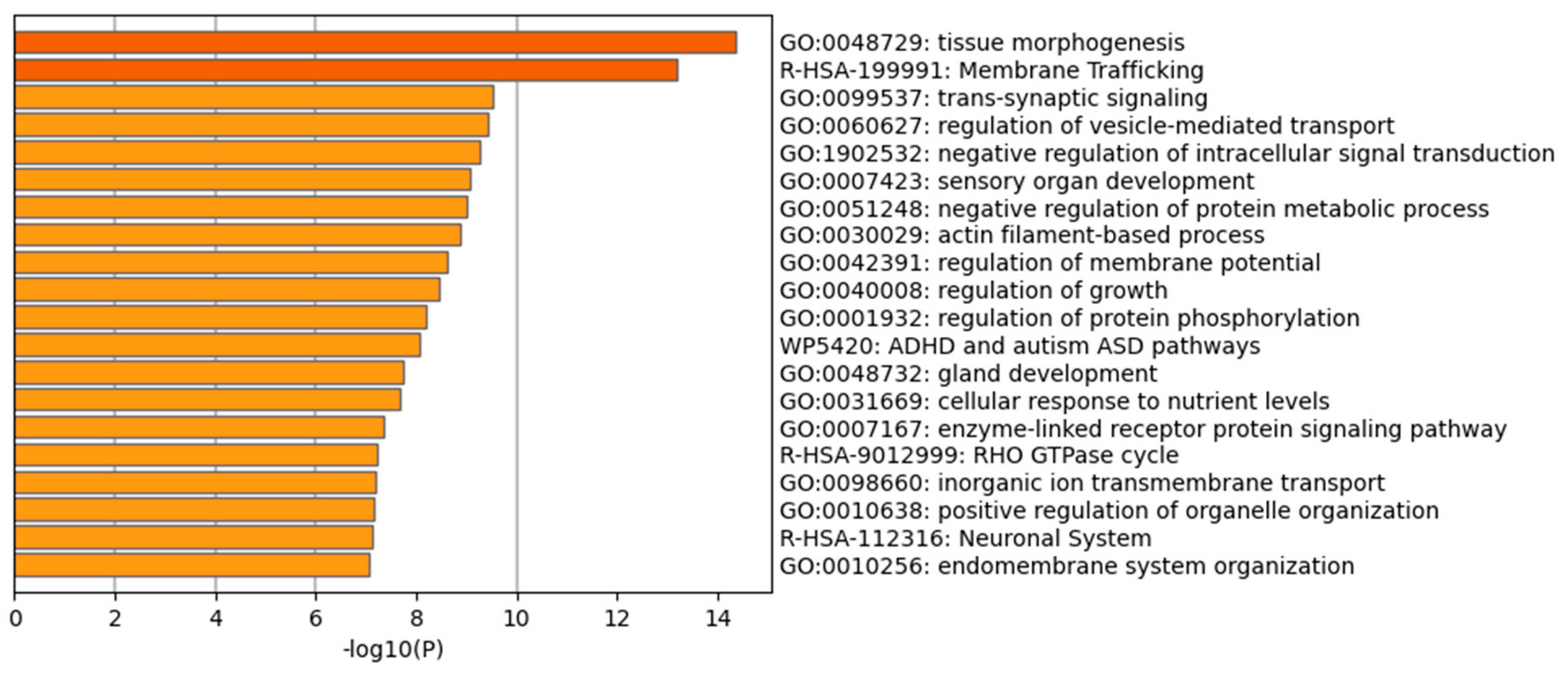
2.9. MicroRNA Analysis and Bioinformatic Prediction of Potential Human Gene Targets
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Materials and Growth Conditions
4.3. Preparation of the PDNV Extract for Anion Exchange Chromatography
4.4. Anion Exchange Chromatography Purification of PDNVs
4.5. Nanoparticle Tracking Analysis
4.6. Transmission Electron Microscopy
4.7. SDS-PAGE and Western Blotting
4.8. Protein Extraction
4.9. Proteomics
4.10. Lipid Extraction, LC-HR-MS/MS Analysis and Untargeted Lipidomics
4.11. Total RNA Extraction
4.12. Small RNA Sequencing
4.13. Bioinformatic Analysis for microRNA Identification
4.14. Skin Cell Cultures
4.15. Treatment of Skin Cell Lines with PDNVs
4.16. Cytotoxicity Assay
4.17. Scratch Assay
4.18. Analysis of Gene Expression in Scratch-Wounded HaCaT Cells
| Primers | Sequence |
| EGFR Fw | ATGTCCTCATTGCCCTCA |
| EGFR Rv | CACATCCATCTGGTACGT |
| Ki-67 Fw | CTAGAAATCGAACACCAGC |
| Ki-67 Rv | GGATTTCTGAACCTGACTC |
| Coll IV Fw | TGTGACCAGGCATAGTCA |
| Coll IV Rv | GAGCCAAAAGCTGTAAGC |
| LAM5-γ Fw | ATCAACAGGTGAGCTATGG |
| LAM5-γ Rv | CAATCTCCTGTGTCTGGAT |
4.19. Nitric Oxide Assay in LPS-Stimulated RAW 264.7 Murine Macrophages
4.20. Analysis of Inflammatory Genes Expression in LPS-Stimulated HaCaT Cells
| Primers | Sequence |
| IL-1β Fw | ATGGCAGAAGTACCTGAGCT |
| IL-1β Rv | AAGGACATGGAGAACACCAC |
| TLR2 Fw | CTGGGCAGTCTTGAACAT |
| TLR2 Rv | CATCTTTTCTGGCCACTGACA |
4.21. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PDNVs | Plant-Derived Nanovesicles |
| MDEs | Mammal-Derived Exosomes |
| HEs | Human Exosomes |
| TEM | Transmission Electron Microscopy |
| NTA | Nanoparticle Tracker Analysis |
References
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle Formation during Reticulocyte Maturation. Association of Plasma Membrane Activities with Released Vesicles (Exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar] [CrossRef]
- Théry, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, Biogenesis and Function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef]
- Baldrich, P.; Rutter, B.D.; Karimi, H.Z.; Podicheti, R.; Meyers, B.C.; Innes, R.W. Plant Extracellular Vesicles Contain Diverse Small RNA Species and Are Enriched in 10- to 17-Nucleotide “Tiny” RNAs. Plant Cell 2019, 31, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yang, J.; Yang, Y.; Liu, J.; Li, H.; Li, R.; Cao, C.; Shi, L.; Wu, W.; He, K. A Timely Review of Cross-Kingdom Regulation of Plant-Derived MicroRNAs. Front. Genet. 2021, 12, 613197. [Google Scholar] [CrossRef]
- Lee, K.W.A.; Chan, L.K.W.; Hung, L.C.; Phoebe, L.K.W.; Park, Y.; Yi, K.-H. Clinical Applications of Exosomes: A Critical Review. Int. J. Mol. Sci. 2024, 25, 7794. [Google Scholar] [CrossRef]
- Pan, B.-T.; Johnstone, R.M. Fate of the Transferrin Receptor during Maturation of Sheep Reticulocytes in Vitro: Selective Externalization of the Receptor. Cell 1983, 33, 967–978. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular Vesicles: Exosomes, Microvesicles, and Friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, S.; Naselli, F.; Fontana, S.; Monteleone, F.; Lo Dico, A.; Saieva, L.; Zito, G.; Flugy, A.; Manno, M.; Di Bella, M.A.; et al. Citrus limon—Derived Nanovesicles Inhibit Cancer Cell Proliferation and Suppress CML Xenograft Growth by Inducing TRAIL-Mediated Cell Death. Oncotarget 2015, 6, 19514–19527. [Google Scholar] [CrossRef]
- Mu, N.; Li, J.; Zeng, L.; You, J.; Li, R.; Qin, A.; Liu, X.; Yan, F.; Zhou, Z. Plant-Derived Exosome-Like Nanovesicles: Current Progress and Prospects. Int. J. Nanomed. 2023, 18, 4987–5009. [Google Scholar] [CrossRef] [PubMed]
- Sarasati, A.; Syahruddin, M.H.; Nuryanti, A.; Ana, I.D.; Barlian, A.; Wijaya, C.H.; Ratnadewi, D.; Wungu, T.D.K.; Takemori, H. Plant-Derived Exosome-like Nanoparticles for Biomedical Applications and Regenerative Therapy. Biomedicines 2023, 11, 1053. [Google Scholar] [CrossRef]
- Subha, D.; Harshnii, K.; Madhikiruba, K.G.; Nandhini, M.; Tamilselvi, K.S. Plant Derived Exosome- like Nanovesicles: An Updated Overview. Plant Nano Biol. 2023, 3, 100022. [Google Scholar] [CrossRef]
- Mu, J.; Zhuang, X.; Wang, Q.; Jiang, H.; Deng, Z.; Wang, B.; Zhang, L.; Kakar, S.; Jun, Y.; Miller, D.; et al. Interspecies Communication between Plant and Mouse Gut Host Cells through Edible Plant Derived Exosome-like Nanoparticles. Mol. Nutr. Food Res. 2014, 58, 1561–1573, Erratum in Mol. Nutr. Food Res. 2016, 60, 964. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhuang, X.; Deng, Z.-B.; Jiang, H.; Mu, J.; Wang, Q.; Xiang, X.; Guo, H.; Zhang, L.; Dryden, G.; et al. Targeted Drug Delivery to Intestinal Macrophages by Bioactive Nanovesicles Released from Grapefruit. Mol. Ther. 2014, 22, 522–534. [Google Scholar] [CrossRef]
- Wang, Q.; Zhuang, X.; Mu, J.; Deng, Z.-B.; Jiang, H.; Zhang, L.; Xiang, X.; Wang, B.; Yan, J.; Miller, D.; et al. Delivery of Therapeutic Agents by Nanoparticles Made of Grapefruit-Derived Lipids. Nat. Commun. 2013, 4, 1867. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, X.; Han, M.K.; Collins, J.F.; Merlin, D. Oral Administration of ginger-derived Nanolipids Loaded With siRNA As a Novel Approach for Efficient siRNA Drug Delivery to Treat Ulcerative Colitis. Nanomedicine 2017, 12, 1927–1943. [Google Scholar] [CrossRef]
- Gagnon, P.; Goričar, B.; Peršič, Š.; Černigoj, U.; Štrancar, A. Two New Capture Options for Improved Purification of Large mRNA. Cell Gene Ther. Insights 2020, 6, 1035–1046. [Google Scholar] [CrossRef]
- Silva, R.M.; Rosa, S.S.; Cunha, R.; da Silva, C.L.; Azevedo, A.M.; Fernandes-Platzgummer, A. Anion Exchange Chromatography-Based Platform for the Scalable Purification of Extracellular Vesicles Derived from Human Mesenchymal Stromal Cells. Sep. Purif. Technol. 2023, 310, 123238. [Google Scholar] [CrossRef]
- Zanotti, C.; Arena, S.; De Pascale, S.; Ciaravolo, V.; Ferracane, R.; Troise, A.D.; Pontecorvi, C.; Pacello, F.; Niespolo, C.; Gismondi, A.; et al. Anion Exchange Chromatography-Based Purification of Plant-Derived Nanovesicles from Brassica oleracea L.: Molecular Profiling and Bioactivity in Human Cells. Front. Bioeng. Biotechnol. 2025, 13, 1617478. [Google Scholar] [CrossRef] [PubMed]
- Sulakhiya, K.; Patel, V.; Saxena, R.; Dashore, J.; Srivastava, A.; Rathore, M. Effect of Beta vulgaris Linn. Leaves Extract on Anxiety- and Depressive-like Behavior and Oxidative Stress in Mice after Acute Restraint Stress. Phcog Res. 2016, 8, 1. [Google Scholar] [CrossRef]
- Liangsupree, T.; Multia, E.; Riekkola, M.-L. Modern Isolation and Separation Techniques for Extracellular Vesicles. J. Chromatogr. A 2021, 1636, 461773. [Google Scholar] [CrossRef] [PubMed]
- Rutter, B.D.; Innes, R.W. Extracellular Vesicles Isolated from the Leaf Apoplast Carry Stress-Response Proteins. Plant Physiol. 2017, 173, 728–741. [Google Scholar] [CrossRef]
- Kurepa, J.; Wang, S.; Li, Y.; Smalle, J. Proteasome Regulation, Plant Growth and Stress Tolerance. Plant Signal. Behav. 2009, 4, 924–927. [Google Scholar] [CrossRef]
- Lindquist, E.; Aronsson, H. Chloroplast vesicle transport. Photosynth. Res. 2018, 138, 361–371. [Google Scholar] [CrossRef]
- Ambrosone, A.; Barbulova, A.; Cappetta, E.; Cillo, F.; De Palma, M.; Ruocco, M.; Pocsfalvi, G. Plant Extracellular Vesicles: Current Landscape and Future Directions. Plants 2023, 12, 4141. [Google Scholar] [CrossRef]
- Mahdipour, E. Beta vulgaris juice contains biologically active exosome-like nanoparticles. Tissue Cell 2022, 76, 101800. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Shin, S. ExoCAS-2: Rapid and Pure Isolation of Exosomes by Anionic Exchange Using Magnetic Beads. Biomedicines 2021, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.; Mu, J.; Dokland, T.; Zhuang, X.; Wang, Q.; Jiang, H.; Xiang, X.; Deng, Z.-B.; Wang, B.; Zhang, L.; et al. Grape Exosome-like Nanoparticles Induce Intestinal Stem Cells and Protect Mice From DSS-Induced Colitis. Mol. Ther. 2013, 21, 1345–1357. [Google Scholar] [CrossRef]
- Zhang, M.; Viennois, E.; Prasad, M.; Zhang, Y.; Wang, L.; Zhang, Z.; Han, M.K.; Xiao, B.; Xu, C.; Srinivasan, S.; et al. Edible Ginger-Derived Nanoparticles: A Novel Therapeutic Approach for the Prevention and Treatment of Inflammatory Bowel Disease and Colitis-Associated Cancer. Biomaterials 2016, 101, 321–340. [Google Scholar] [CrossRef]
- Pocsfalvi, G.; Turiák, L.; Ambrosone, A.; Del Gaudio, P.; Puska, G.; Fiume, I.; Silvestre, T.; Vékey, K. Protein Biocargo of Citrus Fruit-Derived Vesicles Reveals Heterogeneous Transport and Extracellular Vesicle Populations. J. Plant Physiol. 2018, 229, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Pocsfalvi, G.; Turiák, L.; Ambrosone, A.; Del Gaudio, P.; Puska, G.; Fiume, I.; Silvestre, T.; Vékey, K. Physiochemical and Protein Datasets Related to Citrus Juice Sac Cells-Derived Nanovesicles and Microvesicles. Data Brief. 2019, 22, 251–254. [Google Scholar] [CrossRef]
- Jimenez-Jimenez, S.; Hashimoto, K.; Santana, O.; Aguirre, J.; Kuchitsu, K.; Cárdenas, L. Emerging Roles of Tetraspanins in Plant Inter-Cellular and Inter-Kingdom Communication. Plant Signal. Behav. 2019, 14, e1581559. [Google Scholar] [CrossRef]
- Urzì, O.; Raimondo, S.; Alessandro, R. Extracellular Vesicles from Plants: Current Knowledge and Open Questions. Int. J. Mol. Sci. 2021, 22, 5366. [Google Scholar] [CrossRef]
- Rodríguez De Lope, M.M.; Sánchez-Pajares, I.R.; Herranz, E.; López-Vázquez, C.M.; González-Moro, A.; Rivera-Tenorio, A.; González-Sanz, C.; Sacristán, S.; Chicano-Gálvez, E.; De La Cuesta, F. A Compendium of Bona Fide Reference Markers for Genuine Plant Extracellular Vesicles and Their Degree of Phylogenetic Conservation. J. Extracell. Vesicles 2025, 14, e70147. [Google Scholar] [CrossRef] [PubMed]
- Karamanidou, T.; Tsouknidas, A. Plant-Derived Extracellular Vesicles as Therapeutic Nanocarriers. Int. J. Mol. Sci. 2021, 23, 191. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Ren, Y.; Sayed, M.; Hu, X.; Lei, C.; Kumar, A.; Hutchins, E.; Mu, J.; Deng, Z.; Luo, C.; et al. Plant-Derived Exosomal MicroRNAs Shape the Gut Microbiota. Cell Host Microbe 2018, 24, 637–652.e8. [Google Scholar] [CrossRef]
- Xu, F.; Mu, J.; Teng, Y.; Zhang, X.; Sundaram, K.; Sriwastva, M.K.; Kumar, A.; Lei, C.; Zhang, L.; Liu, Q.M.; et al. Restoring Oat Nanoparticles Mediated Brain Memory Function of Mice Fed Alcohol by Sorting Inflammatory Dectin-1 Complex Into Microglial Exosomes. Small 2022, 18, 2105385. [Google Scholar] [CrossRef]
- Liu, N.-J.; Wang, N.; Bao, J.-J.; Zhu, H.-X.; Wang, L.-J.; Chen, X.-Y. Lipidomic Analysis Reveals the Importance of GIPCs in Arabidopsis Leaf Extracellular Vesicles. Mol. Plant 2020, 13, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, A.H.; Venkatesan, M.; Aguirre, A. Bioactive Lipid Signaling in Cardiovascular Disease, Development, and Regeneration. Cells 2020, 9, 1391. [Google Scholar] [CrossRef]
- You, J.Y.; Kang, S.J.; Rhee, W.J. Isolation of Cabbage Exosome-like Nanovesicles and Investigation of Their Biological Activities in Human Cells. Bioact. Mater. 2021, 6, 4321–4332. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Park, J.H. Isolation of Aloe Saponaria—Derived Extracellular Vesicles and Investigation of Their Potential for Chronic Wound Healing. Pharmaceutics 2022, 14, 1905. [Google Scholar] [CrossRef]
- Cui, W.-W.; Ye, C.; Wang, K.-X.; Yang, X.; Zhu, P.-Y.; Hu, K.; Lan, T.; Huang, L.-Y.; Wang, W.; Gu, B.; et al. Momordica. charantia—Derived Extracellular Vesicles-Like Nanovesicles Protect Cardiomyocytes Against Radiation Injury via Attenuating DNA Damage and Mitochondria Dysfunction. Front. Cardiovasc. Med. 2022, 9, 864188. [Google Scholar] [CrossRef]
- Sánchez-López, C.M.; Manzaneque-López, M.C.; Pérez-Bermúdez, P.; Soler, C.; Marcilla, A. Characterization and Bioactivity of Extracellular Vesicles Isolated from Pomegranate. Food Funct. 2022, 13, 12870–12882. [Google Scholar] [CrossRef]
- Şahin, F.; Koçak, P.; Güneş, M.Y.; Özkan, İ.; Yıldırım, E.; Kala, E.Y. In Vitro Wound Healing Activity of Wheat-Derived Nanovesicles. Appl. Biochem. Biotechnol. 2019, 188, 381–394. [Google Scholar] [CrossRef]
- Chen, X.; Xing, X.; Lin, S.; Huang, L.; He, L.; Zou, Y.; Zhang, X.; Su, B.; Lu, Y.; Zheng, D. Plant-Derived Nanovesicles: Harnessing Nature’s Power for Tissue Protection and Repair. J. Nanobiotechnol. 2023, 21, 445. [Google Scholar] [CrossRef]
- Xiao, J.; Feng, S.; Wang, X.; Long, K.; Luo, Y.; Wang, Y.; Ma, J.; Tang, Q.; Jin, L.; Li, X.; et al. Identification of Exosome-like Nanoparticle-Derived microRNAs from 11 Edible Fruits and Vegetables. PeerJ 2018, 6, e5186. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Zanotti, C.; Vurro, M.; Evidente, A.; Marra, M. A-costic Acid, the Main Sesquiterpenoid Isolated from Dittrichia viscosa (L) Greuter, Induces Oxidative Stress and Autophagy in Tomato. Plant Biol. 2024, 26, 1213–1222. [Google Scholar] [CrossRef]
- Kim, S.R.; Nguyen, T.V.; Seo, N.R.; Jung, S.; An, H.J.; Mills, D.A.; Kim, J.H. Comparative proteomics: Assessment of biological variability and dataset comparability. BMC Bioinform. 2015, 16, 121. [Google Scholar] [CrossRef] [PubMed]
- Tabb, D.L.; Vega-Montoto, L.; Rudnick, P.A.; Variyath, A.M.; Ham, A.J.; Bunk, D.M.; Kilpatrick, L.E.; Billheimer, D.D.; Blackman, R.K.; Cardasis, H.L.; et al. Repeatability and reproducibility in proteomic identifications by liquid chromatography-tandem mass spectrometry. J. Proteome Res. 2010, 9, 761–776. [Google Scholar] [CrossRef] [PubMed]
- Claassen, C.; Kuballa, J.; Rohn, S. Metabolomics-Based Approach for the Discrimination of Potato Varieties (Solanum tuberosum) using UPLC-IMS-QToF. J. Agric. Food Chem. 2019, 67, 5700–5709. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2010. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 3 February 2025).
- Aparicio-Puerta, E.; Lebrón, R.; Rueda, A.; Gómez-Martín, C.; Giannoukakos, S.; Jaspez, D.; Medina, J.M.; Zubkovic, A.; Jurak, I.; Fromm, B.; et al. sRNAbench and sRNAtoolbox 2019: Intuitive Fast Small RNA Profiling and Differential Expression. Nucleic Acids Res. 2019, 47, W530–W535. [Google Scholar] [CrossRef]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. miRBase: From microRNA Sequences to Function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef]
- Dai, X.; Zhuang, Z.; Zhao, P.X. psRNATarget: A Plant Small RNA Target Analysis Server (2017 Release). Nucleic Acids Res. 2018, 46, W49–W54. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape Provides a Biologist-Oriented Resource for the Analysis of Systems-Level Datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Bai, J.; Bandla, C.; García-Seisdedos, D.; Hewapathirana, S.; Kamatchinathan, S.; Kundu, D.J.; Prakash, A.; Frericks-Zipper, A.; Eisenacher, M.; et al. The PRIDE Database Resources in 2022: A Hub for Mass Spectrometry-Based Proteomics Evidences. Nucleic Acids Res. 2022, 50, D543–D552. [Google Scholar] [CrossRef]
- Yurekten, O.; Payne, T.; Tejera, N.; Amaladoss, F.X.; Martin, C.; Williams, M.; O’Donovan, C. MetaboLights: Open data re-pository for metabolomics. Nucleic Acids Res. 2024, 52, D640–D646. [Google Scholar] [CrossRef] [PubMed]


| miRNA Name | miRNA 5′-3′ Sequence | High Quality Reads |
|---|---|---|
| ath-miR159a | UUUGGAUUGAAGGGAGCUCUA | 130,619 |
| ath-miR159b-3p | UUUGGAUUGAAGGGAGCUCUU | 127,618 |
| ath-miR159c | UUUGGAUUGAAGGGAGCUCCU | 104,742 |
| ath-miR157d | UGACAGAAGAUAGAGAGCAC | 87,007 |
| ath-miR157b-5p | UUGACAGAAGAUAGAGAGCAC | 67,930 |
| ath-miR319a | UUGGACUGAAGGGAGCUCCCU | 48,439 |
| ath-miR156d-5p | UGACAGAAGAGAGUGAGCAC | 37,434 |
| ath-miR156g | CGACAGAAGAGAGUGAGCAC | 25,175 |
| ath-miR8175 | GAUCCCCGGCAACGGCGCCA | 8703 |
| ath-miR156i | UGACAGAAGAGAGAGAGCAG | 5847 |
| ath-miR319c | UUGGACUGAAGGGAGCUCCUU | 4335 |
| ath-miR168a-5p | UCGCUUGGUGCAGGUCGGGAA | 4198 |
| ath-miR167d | UGAAGCUGCCAGCAUGAUCUGG | 3749 |
| ath-miR164b-5p | UGGAGAAGCAGGGCACGUGCA | 3742 |
| ath-miR164c-5p | UGGAGAAGCAGGGCACGUGCG | 3385 |
| ath-miR167a-5p | UGAAGCUGCCAGCAUGAUCUA | 2873 |
| ath-miR166a-3p | UCGGACCAGGCUUCAUUCCCC | 2515 |
| miRNA | Target Gene | Expectation Value | Biological Function | Gene Aligned Sequence |
|---|---|---|---|---|
| ath-miR159a | NM_014906|PPM1E | 2.5 | Protein Phosphatase, Mg2+/Mn2+ Dependent 1E, inhibitor of kinase activity; affects cytoskeletal remodeling and metabolic regulation. | AUGCUCUUUGUCUUUUGUCAA |
| ath-miR159a | NM_001024455|RGAG4 | 2.5 | Retrotransposon Gag Domain Containing Protein 4; involved in the regulation of gene expression and the maintenance of genomic stability. | AUGUUUUCUGUCUUCUGUUAA |
| ath-miR157d | NM_001031848|SERPINB8 | 1.5 | Serpin B8: member of the serine protease inhibitor (serpin) family; modulates inflammation and immune responses. | AUGCUCUUUGUCUUUUGUCA |
| ath-miR157d | NM_007021|C10orf10 | 2 | DEPP1 (Decidual Protein Induced by Progesterone); regulates FOXO3-induced autophagy via increased cellular reactive oxygen species (ROS). | AUGUUUUCUGUCUUCUGUUA |
| ath-miR157d | NM_000599|IGFBP5 | 2.5 | Insulin-Like Growth Factor Binding Protein 5; regulates growth, bone metabolism, and fibrotic diseases. | UUGAUCUUUGUCUUUUGUCA |
| ath-miR319a | NM_014906|PPM1E | 2.0 | Protein Phosphatase, Mg2+/Mn2+ Dependent 1E, inhibitor of kinase activity; affects cytoskeletal remodeling and metabolic regulation. | AAGUAGUUCCCUUCAGUUCAA |
| ath-miR156d-5p | NM_001178097|C12orf74 | 2.5 | Chromosome 12 open reading frame 74, putative guanyl-nucleotide exchange factor (GEF) for Rho GTPases; involved in actin cytoskeleton organization, cell migration, and cell cycle progression. | UCGCUCACUUUCUUCUGUCC |
| ath-miR156d-5p | NM_152795|HIF3A | 2.5 | Hypoxia-Inducible Factor 3 Alpha Subunit; regulates the adaptive response to hypoxia and suppresses hypoxia-inducible gene expression. | CUGCUCGCUCUGUUUUGUCA |
| ath-miR156d-5p | NM_152387|KCTD18 | 2.5 | Potassium Channel Tetramerization Domain Containing 18; involved in cell proliferation, differentiation, apoptosis, and metabolism. | UUUCUUACUCUUUUCUGUUA |
| ath-miR156g | NM_001256272|VSX1 | 2.5 | Visual System Homeobox 1; involved in the regulation of cone opsin genes during early development. | UUUUUCACUUUCUUCUGUUG |
| ath-miR156g | NM_005737|ARL4C | 2.5 | ADP-ribosylation factor-like protein 4C; involved in vesicular trafficking and cell proliferation. | UUUUUCACUUUCUUCUGUUG |
| ath-miR156i | NM_014873|LPGAT1 | 2.0 | Lysophosphatidylglycerol Acyltransferase 1; involved in lipid metabolism. | AUGUUCUCUCUCUUCUGUUU |
| ath-miR156i | NM_001128933|SYNPO2 | 2.5 | Synaptopodin-2 a (Myopodin), actin-binding protein; regulates actin cytoskeleton through the RhoA signaling pathways. | CUGCUCUUUUUCUGCUGUCA |
| ath-miR156i | NM_001031848|SERPINB8 | 2.5 | Serpin B8: member of the serine protease inhibitor (serpin) family; regulates inflammation and immune responses. | AUGCUCUUUGUCUUUUGUCA |
| ath-miR156i | NM_006241|PPP1R2 | 2.5 | Protein Phosphatase 1 Regulatory Inhibitor Subunit 2; involved in cell division, muscle contraction, and neuronal activities. | UUUCUCUCACUCUUCUGUCA |
| ath-miR156i | NM_207645|C11orf87 | 2.5 | Neuronal Integral Membrane Protein 1. | UUGUAUUCUCUUUUCUGUCA |
| ath-miR156i | NM_014048|MKL2 | 2.5 | Myocardin-Related Transcription Factor B, transcriptional coactivator of SRF; regulates the expression of genes involved in myogenic differentiation and motility. | UGGCCCUCUUUCUUCUGUCA |
| ath-miR156i | NM_001199876|SERF2 | 2.5 | Small EDRK-Rich Factor 2; involved in cell aggregation and stress responses. | GUUCACUUUCUCUUCUGUCA |
| ath-miR319c | NM_014906|PPM1E | 2.0 | Protein Phosphatase, Mg2+/Mn2+ Dependent 1E, inhibitor of kinase activity; affects cytoskeletal remodeling and metabolic regulation. | AAGUAGUUCCCUUCAGUUCAA |
| ath-miR168a-5p | NM_145166|ZBTB47 | 2 | Putative DNA-binding transcription factor; involved in the regulation of transcription. | UCCCUGACCUGCACCAGGUGG |
| ath-miR164b-5p | NM_022492|TTC31 | 2.5 | Tetratricopeptide Repeat Domain 31; involved in Adams-Oliver Syndrome (skin and limb abnormalities). | AGCUCGUGCCCUGUUUUUCUA |
| ath-miR164b-5p | NM_014982|PCNX | 2.5 | Pecanex Homolog (PCNX1); involved in cellular signaling. | CCCAUGUGCUCUGCUUCUCUC |
| ath-miR164b-5p | NM_016257|HPCAL4 | 2.5 | Hippocalcin-Like Protein 4; involved in neuronal calcium signaling. | CCCACCUGUCCUGCUUCUCUG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanotti, C.; Troise, A.D.; Arena, S.; Renzone, G.; De Pascale, S.; Ferracane, R.; Pontecorvi, C.; Niespolo, C.; Gismondi, A.; Scaloni, A.; et al. Isolation of Red Beet Plant-Derived Nanovesicles, and Characterization of Their Molecular Content and Biological Activities in Human Cells. Int. J. Mol. Sci. 2025, 26, 11261. https://doi.org/10.3390/ijms262311261
Zanotti C, Troise AD, Arena S, Renzone G, De Pascale S, Ferracane R, Pontecorvi C, Niespolo C, Gismondi A, Scaloni A, et al. Isolation of Red Beet Plant-Derived Nanovesicles, and Characterization of Their Molecular Content and Biological Activities in Human Cells. International Journal of Molecular Sciences. 2025; 26(23):11261. https://doi.org/10.3390/ijms262311261
Chicago/Turabian StyleZanotti, Clarissa, Antonio Dario Troise, Simona Arena, Giovanni Renzone, Sabrina De Pascale, Rosalia Ferracane, Chiara Pontecorvi, Chiara Niespolo, Angelo Gismondi, Andrea Scaloni, and et al. 2025. "Isolation of Red Beet Plant-Derived Nanovesicles, and Characterization of Their Molecular Content and Biological Activities in Human Cells" International Journal of Molecular Sciences 26, no. 23: 11261. https://doi.org/10.3390/ijms262311261
APA StyleZanotti, C., Troise, A. D., Arena, S., Renzone, G., De Pascale, S., Ferracane, R., Pontecorvi, C., Niespolo, C., Gismondi, A., Scaloni, A., & Marra, M. (2025). Isolation of Red Beet Plant-Derived Nanovesicles, and Characterization of Their Molecular Content and Biological Activities in Human Cells. International Journal of Molecular Sciences, 26(23), 11261. https://doi.org/10.3390/ijms262311261






